Introduction
Incontinence-associated dermatitis (IAD) is
inflammation of the skin caused by long-term exposure of the
perineum or other skin to urine or feces (1). It is clinically manifested as
erythema, with or without erosion or secondary infection (2). It widely occurs in bedridden patients,
the elderly and critically ill patients (3,4). Risk
factors for IAD include incontinence chemical irritants (such as
proteases and lipases that digest intestinal enzymes), changes in
skin surface pH and associated micro-organisms (such as fungal
infections caused by Candida albicans), repeated skin
cleansing activities, and occluded perineal environment (such as
the use of airtight nursing pads) and mechanical factors such as
friction (5). IAD is recognized as
a risk factor for pressure ulcers, causing serious inconvenience
and pain to patients (6).
Therefore, the development of preventive and therapeutic methods
for IAD is urgently needed.
Currently, the prevention and treatment of IAD
include: i) Correcting the causes of diarrhea and incontinence; ii)
reducing urine and stool irritation; iii) correct cleaning,
moisturizing and skin care; iv) maintaining the skin pH; and v)
regular observation and evaluation (7-9).
Since the presence of high moisture and corrosive enzymes in the
intestinal juice can cause destructive damage to the skin, leading
to peeling and erosion of the cortex, barrier products such as
petrolatum, polydimethylsiloxane and zinc oxide ointment are
necessary to protect patients with IAD (10). Although several studies on the
prevention and treatment of IAD have been conducted, there is
significant heterogeneity and low comparability between results
(11-13).
It is difficult for clinical medical staff to determine the
relative performance of these barrier materials. Indeed, due to
deficiencies in knowledge and clinical evidence, product selection
is still a challenge faced by clinical medical staff when
preventing and managing IAD. The aim of the present study was to
identify a simple and easy-to-replicate rat model of IAD, and to
study the effect of zinc oxide, painless skin protective film and
silicone dressing on the healing of IAD and to provide experimental
evidence for the treatment of IAD.
Materials and methods
Animals
63 male Sprague-Dawley rats (weight, 150-220 g) aged
7-8 weeks were provided by The Jinhua Center of Laboratory Animals
(Zhejiang, China). Animal experiments were carried out in The
Jinhua Food and Drug Inspection and Testing Research Institute
(Zhejiang, China). Before the experiment, all rats were adaptively
fed for one week. All procedures were performed following the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. This study was
approved by The Ethics Committee of The School of Medicine, Jinhua
Polytechnic (approval no. 2019018).
Construction of an IAD model
In an initial experiment, 9 rats were randomly
divided into three groups according to a random number table: i)
Control group; ii) trypsin-induced IAD (model) group; and iii)
synthetic urine combined with trypsin-induced IAD (joint model)
group. The artificial urine was generated as follows: i) 25 g urea
(purity >99%); ii) 9 g sodium chloride (>99%); iii) 3 g
ammonium chloride (>99.9%); iv) 3 g sodium sulfite (>98%); v)
2.5 g anhydrous disodium hydrogen orthophosphate (>99%); and vi)
2 g creatinine (>99%). These were dissolved in 1,000 ml
Ultrapure water prepared using a Direct Q5 purification system (EMD
Millipore). Subsequently, 25% ammonium hydroxide solution was added
into 25 ml of the above mixture, thus resulting in synthetic urine
solution with 1% ammonium hydroxide. Sodium hydroxide was added
into 4 g/100 ml trypsin liquid or synthetic urine combined with
trypsin, thus adjusting pH to 7.5-8.5.
The selected area on the back of the rat was covered
with a cotton ball containing the trypsin solution or the synthetic
urine with trypsin and adhesive tape (3M company) and fixed with an
elastic bandage. The cotton ball was maintained for 4 days. Once a
day in the morning and afternoon, 5 ml corresponding solution was
added to the cotton ball of the rat to keep the cotton ball moist
and continuously covered the back of the rat. IAD scores and skin
pH values were examined every day. After 4 days, the bandage was
removed and the severity of IAD was observed, including the size of
the dermatitis occurrence area and the IAD score of severity. The
rats were anesthetized using an intraperitoneal injection of sodium
pentobarbital. All procedures were strictly in line with The Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health. The control group was treated with cotton
balls soaked in saline.
Assessment of the IAD model
According to the IAD severity assessment tool scale
described by Borchert et al (14), the rats were observed and evaluated
once a day before and after the intervention for 4 days.
Furthermore, the degree of recovery from dermatitis was also
evaluated after the intervention according to the severity of the
rash and missing skin. IAD was evaluated based on the degree of
skin redness in the back, skin loss and rash. The scoring criteria
were as follows: i) None, 0 point; ii) erythema, 1 point; iii)
edema, 2 points; iv) papule, 3 points; and v) erosion and
superficial ulcer, 4 points.
Animal groups
After successful modeling, 54 rats were randomly
divided into 9 groups according to the random number table (n=6 in
each group): i) Control group; ii) trypsin model group; iii) model
+ zinc oxide group; iv) model + painless skin protective film
group; v) model + silicon dressing group; vi) synthetic urine
combined with trypsin model group (joint model group); vii) joint
model + zinc oxide (Guangzhou Baiyunshan Pharmaceutical Co., Ltd.)
group; viii) joint model + painless skin protective film (3M
company) group; and ix) joint model + silicone dressing (Jiangsu
Youchuang Biomedical Technology Co., Ltd.) group.
For rats in the zinc oxide group, zinc oxide
ointment was evenly applied >1 cm away from the urine- and
feces-contaminated skin, twice a day. For rats in the painless skin
protective film group, the skin protective film was sprayed twice a
day. The distance was 10-15 cm from the urine- and
stool-contaminated skin and l cm beyond the urine and stool
contaminated skin. The painless skin protective film was applied on
the affected skin. For rats in the silicone dressing group, the
silicone dressing was applied twice a day to the affected skin.
IAD severity assessment and skin pH test were
performed for each group every 4 days after applying the zinc
oxide, protective film or silicon dressing intervention. After the
rats had been anesthetized by intraperitoneal injection of
pentobarbital sodium (60 mg/kg), blood samples were collected from
the eyeball. All rats were then sacrificed by intraperitoneal
injection of an overdose of pentobarbital sodium (200 mg/kg). Skin
tissue samples were then collected.
Hematoxylin and eosin (H&E)
staining
Fresh skin tissue samples were fixed in 4%
paraformaldehyde (Wuhan Google Biotechnology Co., Ltd.) for 24 h at
4˚C. After dehydration and paraffin embedding, tissue sections were
cut to 4-µm thickness. After the paraffin sections were
deparaffinized, H & E staining was performed. The sections were
stained with hematoxylin (cat. no. B600020; ProteinTech Group) at
37˚C for 5 min and 0.5% eosin solution (Sigma-Aldrich; Merck KGaA)
at 37˚C for 2 min. After dehydration, the sections were mounted
with neutral gum and placed under a light microscope (BX53; Olympus
Corporation) for observation.
Immunohistochemistry
The skin tissue sections were fixed with 4%
paraformaldehyde overnight at 4˚C and paraffin-embedded. The tissue
sections were cut to 4 µm thick and blocked with goat serum
(Beyotime Institute of Biotechnology) for 30 min at room
temperature. The sections were incubated with anti-major
histocompatibility complex class II (MHC-II) antibody (1:200; cat.
no. ab23990; Abcam) overnight at 4˚C, then with HRP-conjugated goat
anti-mouse secondary antibody (1:200; cat. no. K5007; Dako; Agilent
Technologies) for 30 min at room temperature. After the sections
were stained with DAB for 5-10 min and the nuclei were
counterstained by hematoxylin for 3-5 min. After dehydration and
transparency, the sections were mounted with neutral gum (cat. no.
G1403; Sangon Biotech Co., Ltd.). Finally, images were acquired and
analyzed under an light microscope (Olympus Corporation). The
Image-Pro Plus 6.0 (Media Cybernetics, Inc.) image processing
system was used to measure the optical density of MHC-Ⅱ protein in
each group.
Western blot analysis
The tissue specimens were extracted with 200 µl RIPA
lysis buffer (Beyotime Institute of Biotechnology) at 4˚C for 30
min. After centrifugation at 12,000 x g for 10 min at 4˚C, the
supernatant was collected and stored at -80˚C. The BCA
quantification kit was utilized for evaluating the protein
concentration. A total of 30 µg protein was loaded per lane and the
samples were separated by 12% polyacrylamide gel electrophoresis,
then transferred to a PVDF membrane. The membrane was blocked with
5% skimmed milk powder for 2 h at room temperature. The membrane
was then incubated with primary antibodies against MHC-II (1:1,000;
cat. no. ab23990; Abcam), β-actin (1:1,000; cat. no. ab179467;
Abcam), NF-κB/p65 (Ser727; 1:1,000; cat. no. 10745-1-AP;
ProteinTech group), phosphorylated (p)-NF-κB p65 (Ser536; 1:1,000;
cat. no. 3033T; Cell Signaling Technology, Inc.), p-STAT1 (1:1,000;
cat. no. ab126598; Abcam), STAT1 (1:1,000; cat. no. 10144-2-AP;
ProteinTech group) and GAPDH (1:1,000; cat. no. 60004-1-Ig;
ProteinTech group) overnight at 4˚C, then incubated with
HRP-conjugated goat anti-mouse secondary antibody (1:5,000; cat.
no. SA00001-1; ProteinTech group) at room temperature for 1.5 h.
ECL Plus Luminescence Kit (Beyotime Institute of Biotechnology) was
used to visualize protein bands. ImageJ software (version 1.48;
National Institutes of Health) was used to quantify protein
expression levels.
ELISA
The blood samples from rat eyeball were allowed to
stand for 30 min at room temperature. After centrifugation at 3,500
x g for 15 min at 4˚C, the serum samples were obtained. IFN-γ (cat.
no. RK00199), IL-1β (cat. no. RK00009), IL-2 (cat. no. RK00010) and
TNF-α (cat. no. RK00029) ELISA kits (all from ABclonal Biotech Co.,
Ltd.) were separately used to quantify IFN-γ, IL-1β, IL-2 and TNF-α
levels in serum.
Statistical analysis
SPSS 18.0 software was used for statistical
analysis. The data were expressed as the mean ± standard deviation.
For IAD severity, Kruskal-Wallis followed by Dunn's post hoc test
was performed. For all other variables, one-way ANOVA followed by
Tukey's post hoc test was used. P<0.05 was used to indicate a
statistically significant difference.
Results
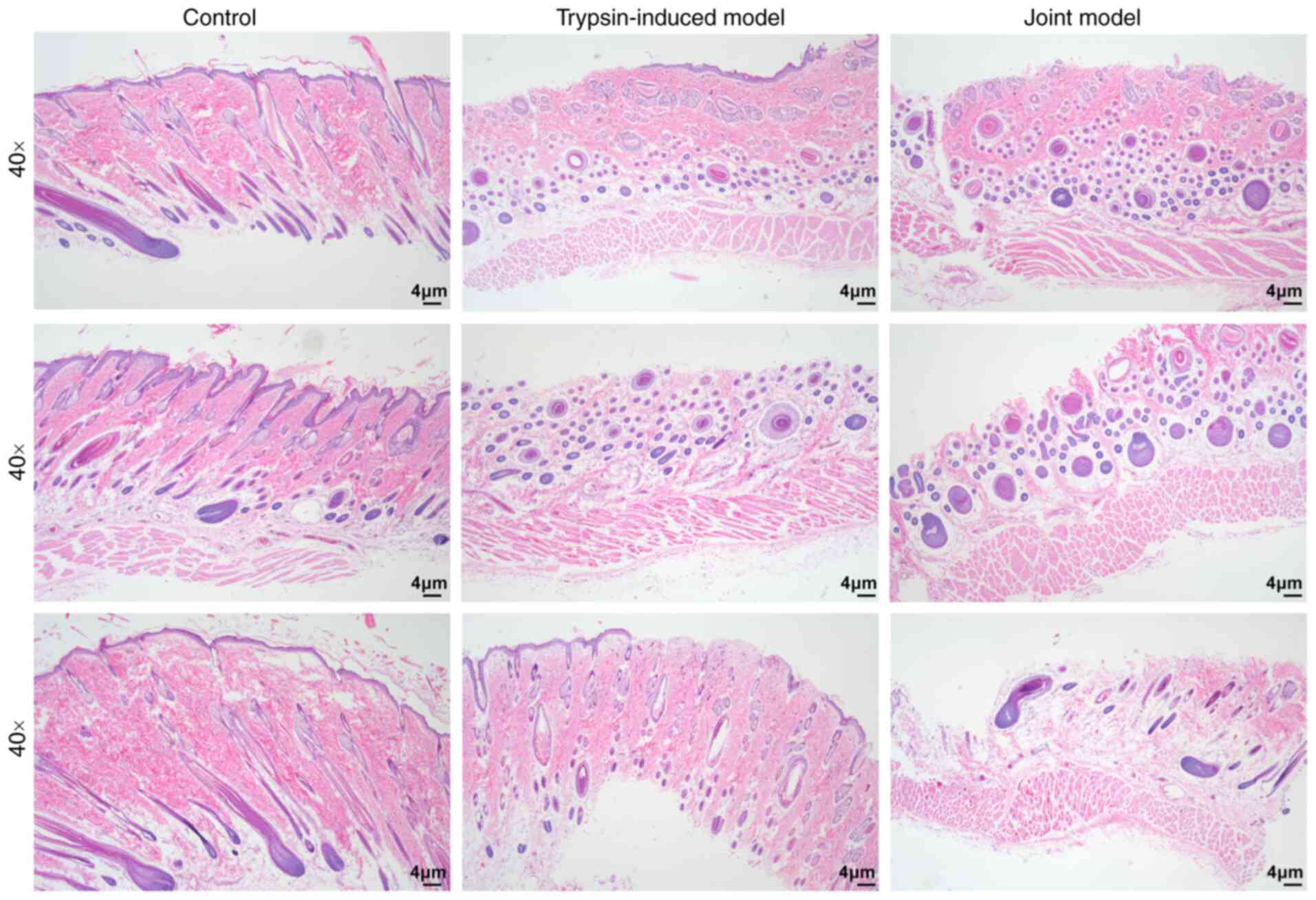
Construction of IAD models
In the present study, trypsin-induced IAD and
synthetic urine combined with trypsin-induced IAD models were
constructed. H&E staining results showed that the skin
structure of rats in the control group was complete and clear. The
skin tissue structure of the rats in the trypsin-induced model
group and the synthetic urine combined with the trypsin model
(joint model) group was severely damaged (Fig. 1).
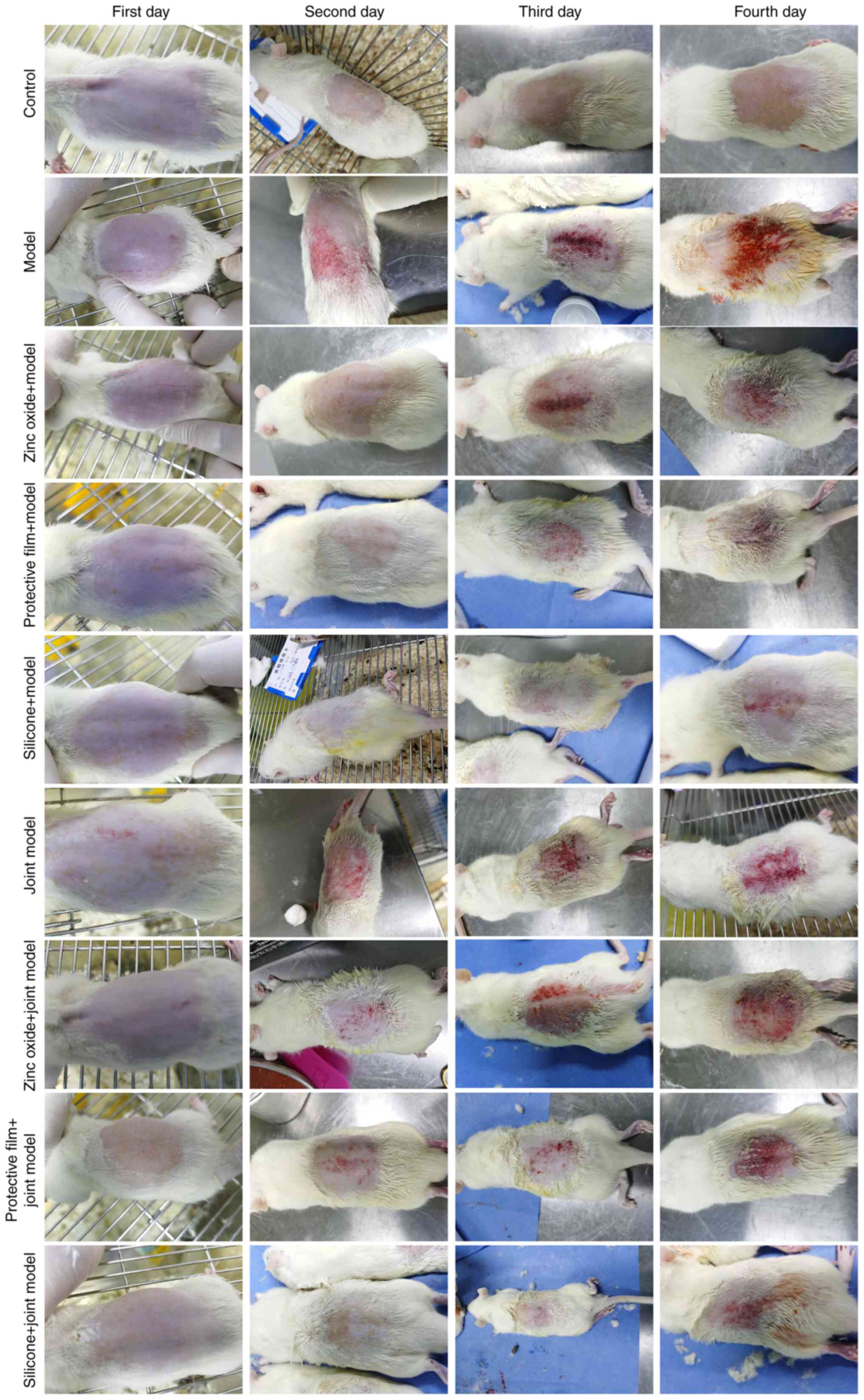
Zinc oxide, painless skin protective
film and silicone dressing decrease IAD score and pH of IAD
rats
A total of 54 rats were randomly divided into nine
groups, as described in Materials and methods. All rats were
carefully observed each day (Fig.
2). The present study found that there was significant
dermatitis in IAD rats compared to rats in the control group.
However, after treatment with zinc oxide, painless skin protective
film or silicone dressing, the dermatitis of IAD rats was
significantly ameliorated. Following treatment for 4 days, the IAD
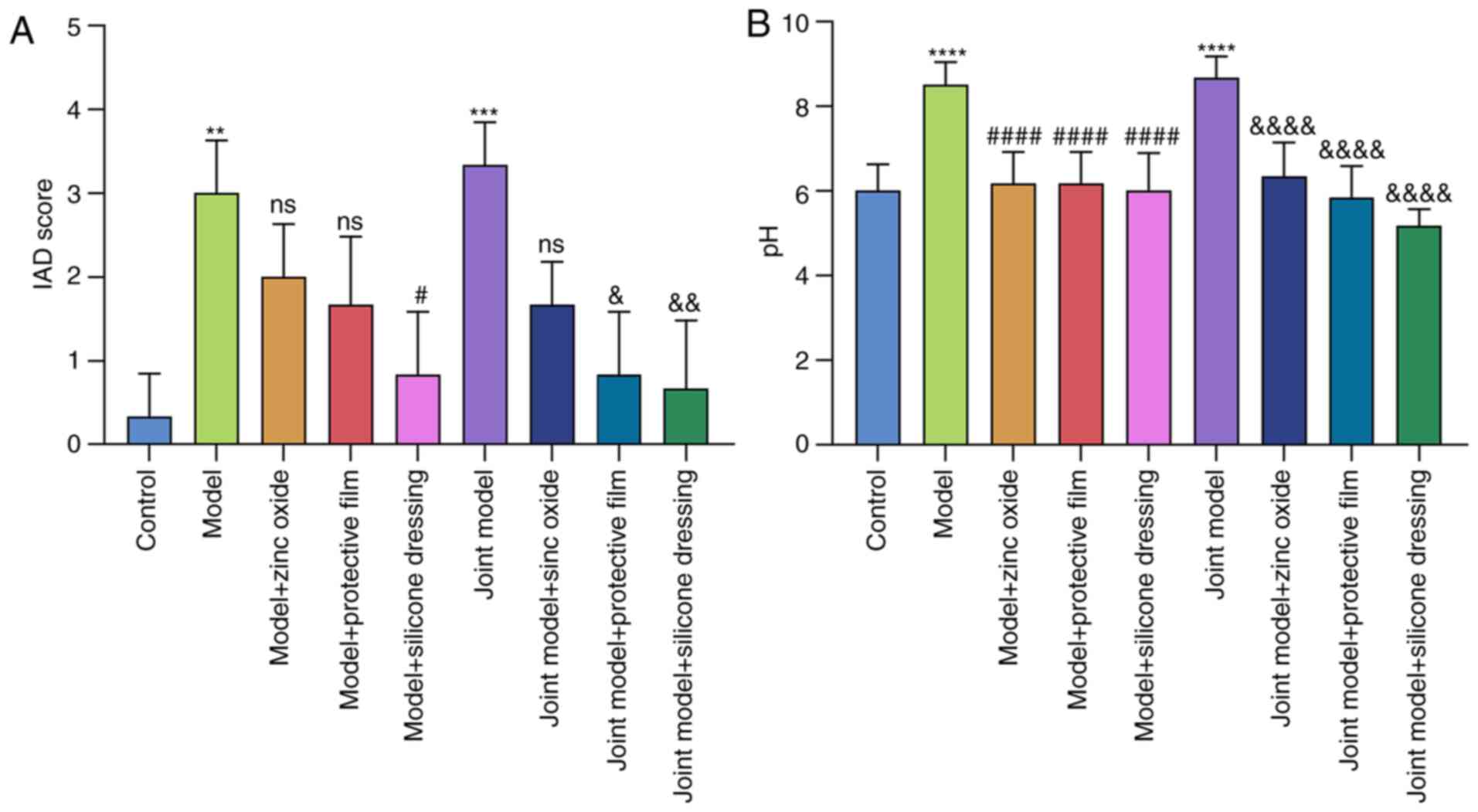
scores and pH values of the rats were obtained. As shown in
Fig. 3A, compared with rats in the
control group (average IAD score=0.333), the severity of dermatitis
in IAD rats induced by trypsin (model group; average IAD score=3;
P<0.01) or artificial urine combined with trypsin (joint model
group; average IAD score=3.333; P<0.001) was significantly
increased. Compared with the model group, dermatitis significantly
improved after 4 days of treatment with silicone dressing (average
IAD score; P<0.05). However, zinc oxide (average IAD score=2;
P>0.05) and painless skin protective film (average IAD
score=1.667; P>0.05) did not reduce the severity of dermatitis.
Compared with the joint model group, dermatitis significantly
improved after 4 days of treatment with painless skin protective
film (the average value of IAD=0.833; P<0.05) or silicone
dressing (average IAD score=0.667; P<0.01), but not with zinc
oxide (average IAD score=1.667; P>0.05). The therapeutic effect
of silicon dressing was the largest of the three interventions.
Furthermore, the pH of rat skin was also tested
(Fig. 3B). In model and joint model
groups, the pH value of rat skin was significantly higher than that
of the control group, and the difference was statistically
significant (both P<0.0001). Treatment with zinc oxide, painless
skin protective film or silicone dressing significantly reduced the
pH value of the skin in both models (all P<0.0001).
Zinc oxide, painless skin protective
film and silicone dressing improve dermatitis in IAD rat
models
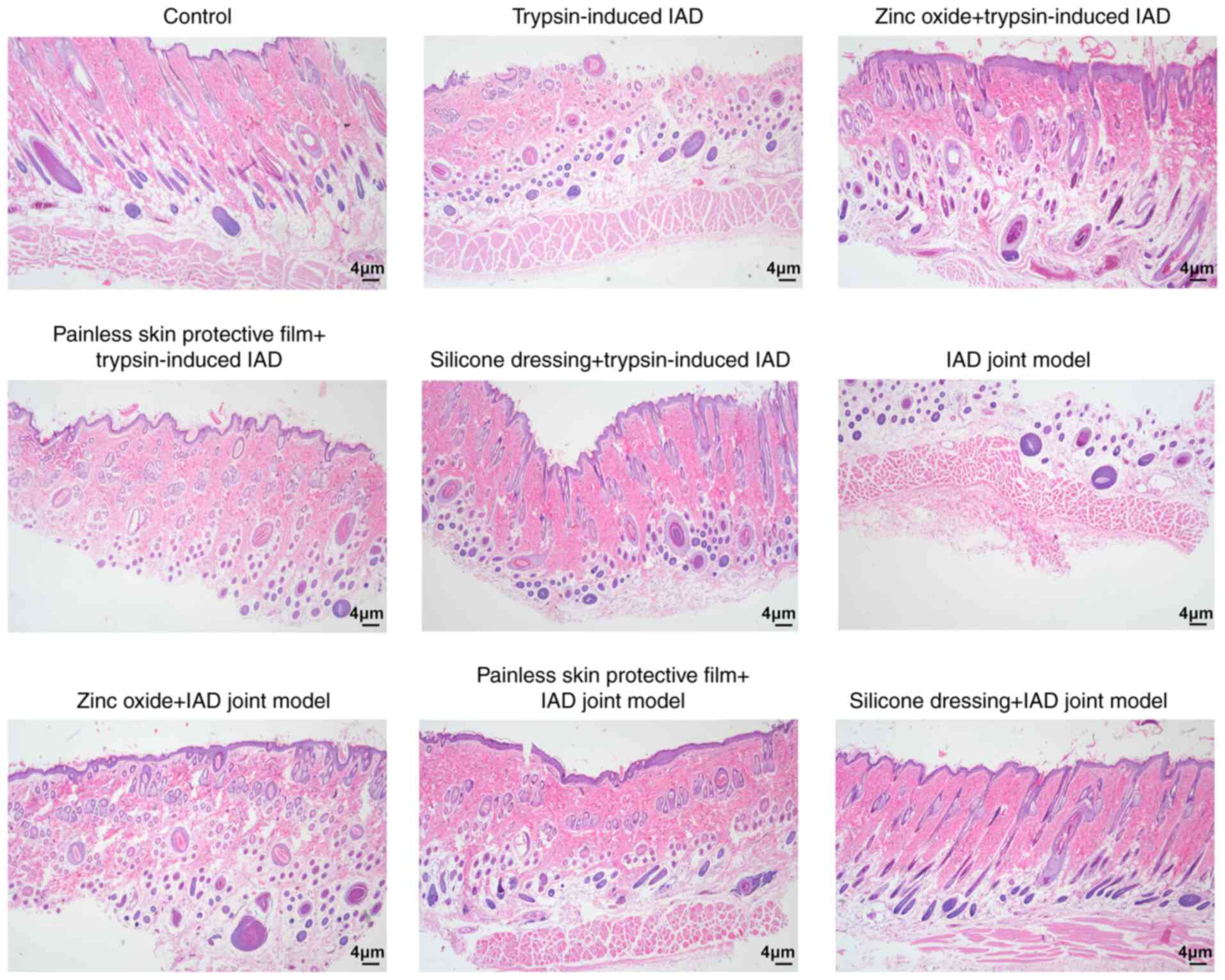
After treatment with zinc oxide, painless skin
protective film or silicone dressing for 4 days, skin tissue
samples were taken for observation. H&E staining was used to
detect the pathological changes associated with dermatitis. As
shown in Fig. 4, compared with rats
in the control group, the skin tissue structure in both model
groups was severely damaged. After 4 days of treatment with zinc
oxide, painless skin protective film or silicone dressing, the skin
tissues showed distinct epidermal and dermal structures; however,
each group still had different degrees of hair follicle damage and
edema. Among them, rats in the silicone dressing treatment group
displayed the best recovery.
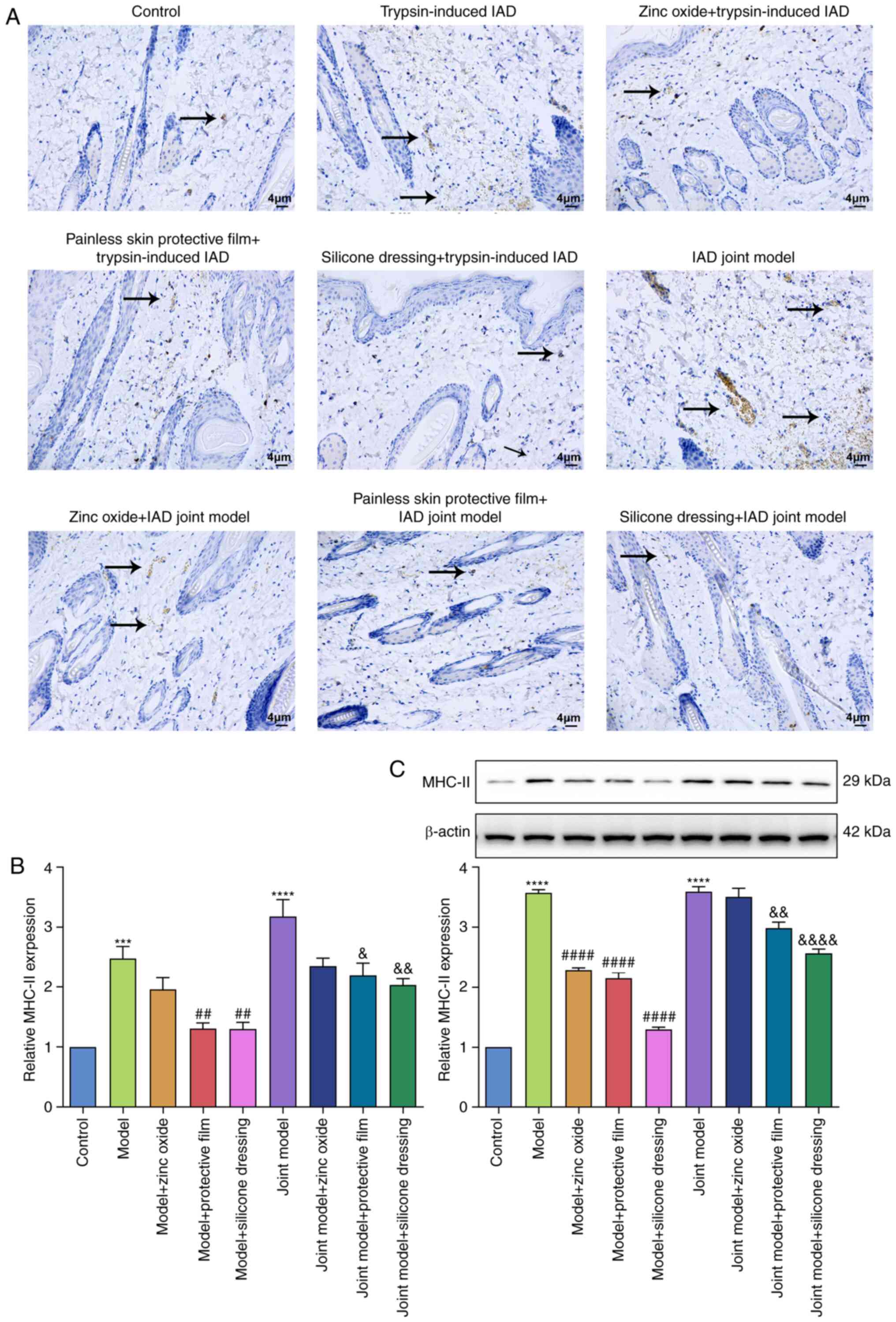
Zinc oxide, painless skin protective
film and silicone dressing lower expression of MHC-II in IAD rat
models
Immunohistochemistry and western blot experiments
were utilized to detect the expression of MHC-Ⅱ in rat skin
tissues. In Fig. 5A-C, there was
almost no expression of MHC-II in the skin tissues of rats in the
control group. The expression of MHC-Ⅱ in the skin tissues of the
trypsin model group and the artificial urine combined with trypsin
model group were significantly increased compared to the control
group. Furthermore, the expression of MHC-Ⅱ in the combined model
group was slightly higher than that of the trypsin model group.
Compared with the model group, the expression of MHC-Ⅱ in the zinc
oxide, painless skin protective film, and silicone treatment groups
all decreased to varying degrees, and the decrease was the most
significant in the silicone dressing treatment group.
Zinc oxide, painless skin protective
film and silicone dressing do not decrease the NF-κB/p-NF-κB and
STAT1/p-STAT1 ratios in IAD rat models
Western blotting was used to detect the expression
of NF-κB (p65), p-NF-κB, STAT1 and p-STAT1 in rat skin tissue
(Fig. 6A). Compared with the
control group, the expression levels of NF-κB (P<0.0001;
Fig. 6B), p-NF-κB (P<0.0001;
Fig. 6C), STAT1 (P<0.0001;
Fig. 6D) and p-STAT1 (P<0.0001;
Fig. 6E) were higher in the skin
tissues of the trypsin model group and the artificial urine
combined with the trypsin model group. However, compared to the
control group, no significant differences in NF-κB/p-NF-κB ratio
(Fig. 6F) or STAT1/p-STAT1 ratio
(Fig. 6G) were observed in the
trypsin model group or the artificial urine combined with the
trypsin model group, suggesting that phosphorylation levels of
NF-κB and STAT1 were not affected in the two IAD models. After
treatment with painless skin protective film (P<0.001 or
P<0.01) or silicone dressing (both P<0.0001), the expression
of NF-κB significantly decreased in the skin tissues of the trypsin
model and the artificial urine combined with the trypsin model
(Fig. 6B). However, zinc oxide did
not change NF-κB expression in the skin tissues of the IAD models.
p-NF-κB levels in the skin tissues of the trypsin model and the
combined model was significantly lowered by painless skin
protective film (both P<0.001) and silicone dressing (both
P<0.0001). Moreover, zinc oxide decreased the expression of
p-NF-κB in the skin tissues of the trypsin model (P<0.05), but
not the combined model (Fig. 6C).
STAT1 and p-STAT1 expression in the skin tissues of IAD rats was
significantly reduced by zinc oxide, painless skin protective film
and silicone dressing (Fig. 6D and
E). Among them, the reduction
levels of NF-κB, p-NF-κB, STAT1 and p-STAT1 were the most
significant in the silicon dressing treatment group. However, no
significant differences were found in p-NF-κB/NF-κB ratio (Fig. 6F) or p-STAT1/STAT1 ratio (Fig. 6G) in any group, suggesting that zinc
oxide, painless skin protective film and silicone dressing did not
affect the phosphorylation levels of NF-κB and STAT1 in IAD rat
models.
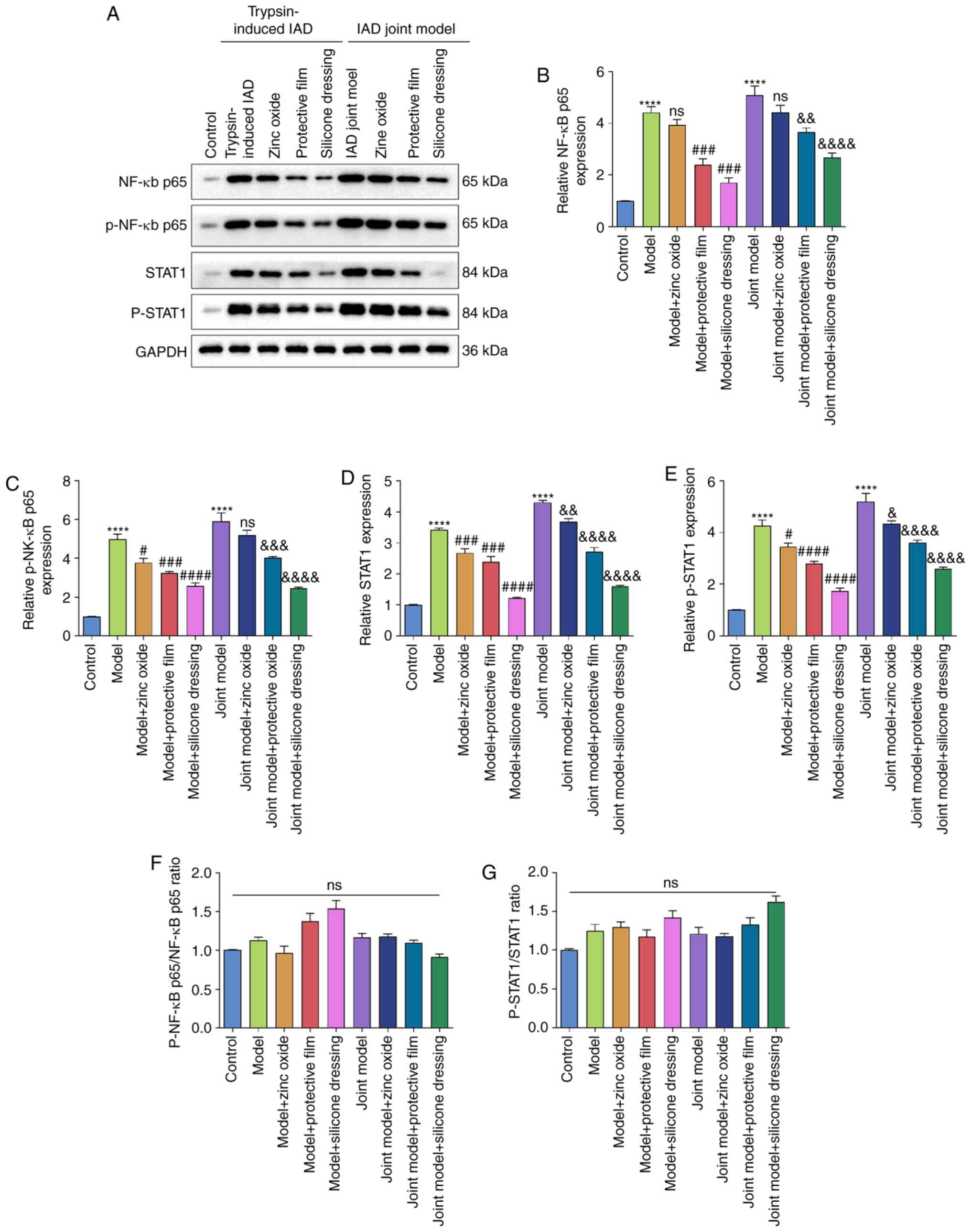 | Figure 6Western blot analysis of the
expression of NF-κB/p65, p-NF-κB/p65, STAT1 and p-STAT1 in the skin
tissue of rats from the control, trypsin-induced IAD model and
synthetic urine combined with trypsin-induced IAD model groups. (A)
Representative images of western blots of each group. (B) NF-κB
p65, (C) p-NF-κB p65, (D) STAT1 and (E) p-STAT1 expression levels
were quantified according to their gray values. (F) p-NF-κB/p65 to
NF-κB/p65 ratio. (G) p-STAT1/STAT1 ratio.
****P<0.0001 vs. control group;
#P<0.05, ###P<0.001,
####P<0.0001 vs. model group;
&P<0.05, &&P<0.01,
&&&P<0.001,
&&&&P<0.0001 vs. joint model group.
IAD, incontinence-associated dermatitis; ns, not significant; p-,
phosphorylated. |
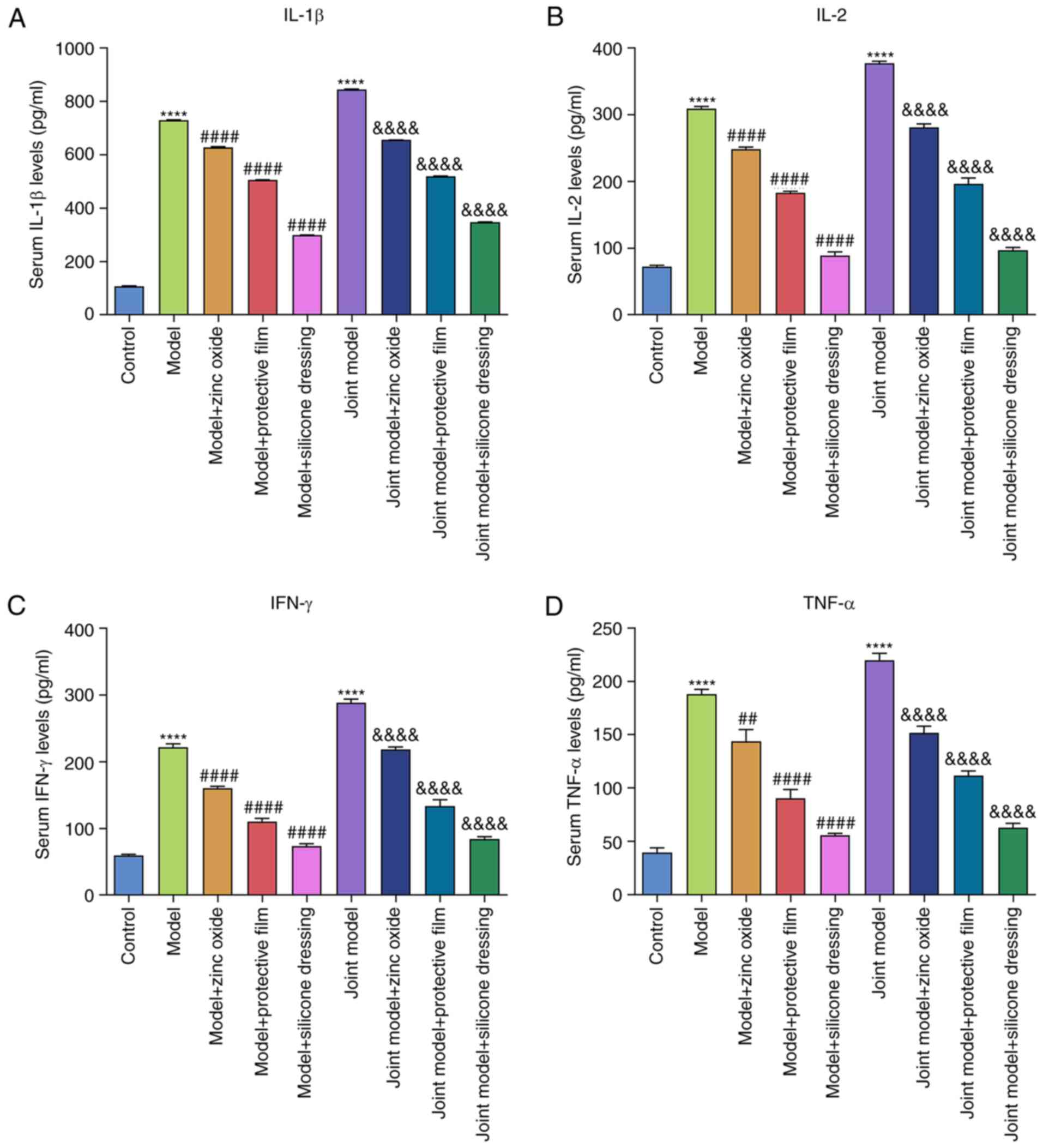
Zinc oxide, painless skin protective
film and silicone dressing decrease IFN-γ, IL-1β, IL-2 and TNF-α
levels in serum samples of IAD rats
ELISA was performed to examine the serum levels of
IFN-γ, IL-1β, IL-2 and TNF-α. Compared with the control group,
serum IL-1β (Fig. 7A), IL-2
(Fig. 7B), IFN-γ (Fig. 7C) and TNF-α (Fig. 7D) levels were significantly elevated
in both model groups (all P<0.0001). After treatment with zinc
oxide, painless skin protective film and silicone dressing, their
serum levels were significantly reduced in IAD models.
Discussion
IAD has a high incidence in the community, nursing
homes and long-term care institutions, as well as in the clinical
departments and intensive care units of hospitals. It has severely
reduced the patients' quality of life and increased the economic
burden of patients (15).
Therefore, the development of preventive and therapeutic methods
for IAD is urgently needed. In the present study, rat models of IAD
were established using two methods (trypsin model and synthetic
urine combined with trypsin model). The effects of zinc oxide,
painless skin protective film and silicone dressing on these IAD
models were then examined.
There are still very few animal models of IAD. As
early as 2011, Minematsu et al (16) used agar gel to soak the soles of the
feet of aged rats, which provides a potential method to construct
IAD animal models. Been et al (17) established an IAD model by immersion
and fixation of 1% pancreatin on the back of rats. Mugita et
al (18) applied
protease-containing agarose gel to the back skin of male SD rats
for 4 h, followed by inoculation with Pseudomonas aeruginosa
for 30 min. Wen et al (19)
established an IAD model on the back of guinea pigs by using
different concentrations of pancreatin solution, confirming that
increased pancreatic juice concentration may lead to more severe
skin reactions and higher IAD scores. In addition, Biçer et
al (20) performed an ileostomy
in rats to establish an ileostomy-related dermatitis model and used
ozone to treat the resulting dermatitis.
The search for a simple and easy-to-replicate IAD
animal model requires continual exploration. The skin is the body's
protective layer that provides an important anatomical barrier to
pathogens, irritants, moisture loss and environmental threats.
Incontinence can damage the integrity of the epidermis due to
excessive water, high pH and the presence of enzymes in the stool,
leading to inflammation and pain and increasing the risk of
infection (21). The cortex is
affected by the external environment, causing the cells to actively
release related inflammatory mediators (10,22).
The keratinocytes in the cortex become increasingly less elastic,
the cells shrink and the barrier function of the cortex is also
weakened (23). Skin related
damages such as loss, erosion, exudation and blisters appear in the
cortex, which can be accompanied by bacterial or fungal infections
(24). In addition, long-term skin
contact with urine or feces causes excessive moisture on the cortex
to overwhelm the stratum corneum cortex structure, and exhibit
excessive hydration or maceration (25). When the digestive enzymes present in
the feces encounter the urea present in the urine, they are used to
produce repeated cycles of chemical stimulation, leading to
inflammation and decomposition of the cortex (9,26). In
the present study, two methods were used to construct the IAD rat
model. H&E staining confirmed the establishment of dermatitis
in both models. These two IAD models are simple to implement and
are worth studying further.
IAD is one of the most common problems in clinical
nursing. There is no uniform treatment standard for IAD, but
choosing the right skin care program is key to identifying one. In
the present study, after 4 days of treatment with zinc oxide,
painless skin protective film or silicone dressing, dermatitis was
significantly relieved and skin pH was significantly reduced.
H&E staining results indicated that the skin tissues
significantly recovered following treatment. Park (27) found that silicone dressings can
effectively reduce the incidence of pressure ulcers in patients
with IAD and improve the symptoms of dermatitis. In the present
study, silicone dressing had the most visible therapeutic effect on
IAD. MHC-Ⅱ plays an important role in the pathogenesis of
dermatitis (27-29).
Inhibiting the expression of NF-κB p65, p-NF-κB p65, STAT1 and
p-STAT1 can effectively reduce the severity of dermatitis (30-32).
Compared with the control group, the expression of MHC-Ⅱ, NF-κB
p65, p-NF-κB p65, STAT1 and p-STAT1 in the skin tissues of the
model groups increased significantly. After treatment, the
expression of the above proteins was significantly reduced. The
reduction in the silicone dressing group was the most apparent.
These results indicated that zinc oxide, painless skin protective
film and silicone dressings can effectively inhibit
inflammation-related pathways in IAD, thereby improving the
symptoms of dermatitis. Further studies are needed to examine the
molecular mechanisms of zinc oxide, painless skin protective film
or silicone dressings in the treatment of IAD.
In conclusion, zinc oxide, painless skin protective
film and silicone dressings significantly ameliorated dermatitis in
rats with IAD. Among these treatment modalities, silicone dressings
exhibited the best therapeutic effects. Thus, these intervention
methods warrant further validation.
Acknowledgements
Not applicable.
Funding
Funding: This work was funded by The Basic Public Welfare
Research Program of Zhejiang Province (grant no. LGD20C040002) and
The Science and Technology Project of Jinhua City in China (grant
no. 2019-4-074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD conceived and designed the study. GC and LH
conducted most of the experiments and data analysis and wrote the
manuscript. YC, SZ and LZ conducted a number of experiments and
data analysis, and contributed to the writing and revision of the
manuscript. MD and GC confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
The School of Medicine, Jinhua Polytechnic (approval no.
2019018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beele H, Smet S, Van Damme N and Beeckman
D: Incontinence-associated dermatitis: Pathogenesis, contributing
factors, prevention and management options. Drugs Aging. 35:1–10.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Koudounas S, Bader DL and Voegeli D:
Knowledge gaps in the etiology and pathophysiology of
incontinence-associated dermatitis: A scoping review. J Wound
Ostomy Continence Nurs. 47:388–395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raepsaet C, Fourie A, Van Hecke A,
Verhaeghe S and Beeckman D: Management of incontinence-associated
dermatitis: A systematic review of monetary data. Int Wound J.
18:79–94. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Y, Leng M, Guo J, Duan J and Wang Z:
The effectiveness of faecal collection devices in preventing
incontinence-associated dermatitis in critically ill patients with
faecal incontinence: A systematic review and meta-analysis. Aust
Crit Care. 34:103–112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lichterfeld-Kottner A, El Genedy M,
Lahmann N, Blume-Peytavi U, Büscher A and Kottner J: Maintaining
skin integrity in the aged: A systematic review. Int J Nurs Stud.
103(103509)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barakat-Johnson M, Basjarahil S, Campbell
J, Cunich M, Disher G, Geering S, Ko N, Lai M, Leahy C, Leong T, et
al: Implementing best available evidence into practice for
incontinence-associated dermatitis in Australia: A multisite
multimethod study protocol. J Tissue Viability. 30:67–77.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gates BP, Vess J, Long MA and Johnson E:
Decreasing incontinence-associated dermatitis in the surgical
intensive care unit: A quality improvement project. J Wound Ostomy
Continence Nurs. 46:327–331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kottner J, Hahnel E, El Genedy M, Neumann
K and Balzer K: Enhancing SKIN health and safety in aged CARE
(SKINCARE Trial): A study protocol for an exploratory
cluster-randomized pragmatic trial. Trials. 20(302)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Phipps L, Gray M and Call E: Time of onset
to changes in skin condition during exposure to synthetic urine: A
prospective study. J Wound Ostomy Continence Nurs. 46:315–320.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Acton C, Ivins N, Bainbridge P and
Browning P: Management of incontinence-associated dermatitis
patients using a skin protectant in acute care: A case series. J
Wound Care. 29:18–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Van Damme N, Van Hecke A, Himpens A,
Verhaeghe S and Beeckman D: Design and psychometric testing of the
attitude towards the prevention of incontinence-associated
dermatitis instrument (APrIAD). Int Wound J. 16:492–502.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gray M: Context for practice: Prevention
of pressure injury and incontinence-associated dermatitis. J Wound
Ostomy Continence Nurs. 44:406–408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kayser SA, Phipps L, VanGilder CA and
Lachenbruch C: Examining prevalence and risk factors of
incontinence-associated dermatitis using the international pressure
ulcer prevalence survey. J Wound Ostomy Continence Nurs.
46:285–290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Borchert K, Bliss DZ, Savik K and
Radosevich DM: The incontinence-associated dermatitis and its
severity instrument: Development and validation. J Wound Ostomy
Continence Nurs. 37:527–535. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arnold-Long M and Johnson E: Epidemiology
of incontinence-associated dermatitis and intertriginous dermatitis
(Intertrigo) in an acute care facility. J Wound Ostomy Continence
Nurs. 46:201–206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Minematsu T, Yamamoto Y, Nagase T, Naito
A, Takehara K, Iizaka S, Komagata K, Huang L, Nakagami G, Akase T,
et al: Aging enhances maceration-induced ultrastructural alteration
of the epidermis and impairment of skin barrier function. J
Dermatol Sci. 62:160–168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Been RA, Bernatchez SF, Conrad-Vlasak DM,
Asmus RA, Ekholm BP and Parks PJ: In vivo methods to evaluate a new
skin protectant for loss of skin integrity. Wound Repair Regen.
24:851–859. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mugita Y, Minematsu T, Huang L, Nakagami
G, Kishi C, Ichikawa Y, Nagase T, Oe M, Noguchi H, Mori T, et al:
Histopathology of incontinence-associated skin lesions: Inner
tissue damage due to invasion of proteolytic enzymes and bacteria
in macerated rat skin. PLoS One. 10(e0138117)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wen Z, Zhu W, Liu Q, Zhang H, Mei B and
Shen M: Development of an animal model for inducing various degrees
of severity of incontinence-associated dermatitis. J Wound Ostomy
Continence Nurs. 44:578–582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Biçer Ş, Sayar İ, Gürsul C, Işık A, Aydın
M, Peker K and Demiryilmaz İ: Use of ozone to treat ileostomy
dermatitis in an experimental rat model. Med Sci Monit. 22:757–765.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hoedl M and Eglseer D: Which
characteristics of fecal incontinence predispose
incontinence-associated dermatitis? A classification and regression
tree analysis. Adv Skin Wound Care. 34:103–108. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koudounas S, Mugita Y, Minematsu T,
Nakagami G, Weller C and Sanada H: Does the presence of bacterial
urinary infection contribute to the development of
incontinence-associated dermatitis? A scoping review. J Tissue
Viability. 30:256–261. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hödl M, Blanař V, Amir Y and Lohrmann C:
Association between incontinence, incontinence-associated
dermatitis and pressure injuries: A multisite study among
hospitalised patients 65 years or older. Australas J Dermatol.
61:e144–e146. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Coyer F, Campbell J and Doubrovsky A:
Efficacy of incontinence-associated dermatitis intervention for
patients in intensive care: An open-label pilot randomized
controlled trial. Adv Skin Wound Care. 33:375–382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tay C, Yuh AS, Sheau Lan EL, Ong CE,
Aloweni F and Lopez V: Development and validation of the
incontinence associated dermatitis knowledge, attitude and practice
questionnaire. J Tissue Viability. 29:244–251. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Werth SL and Justice R: Prevalence of
moisture-associated skin damage in an acute care setting: Outcomes
from a quality improvement project. J Wound Ostomy Continence Nurs.
46:51–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park KH: The effect of a silicone border
foam dressing for prevention of pressure ulcers and
incontinence-associated dermatitis in intensive care unit patients.
J Wound Ostomy Continence Nurs. 41:424–429. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aquino M and Rosner G: Systemic contact
dermatitis. Clin Rev Allergy Immunol. 56:9–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kolesnik M, Franke I, Lux A, Quist SR and
Gollnick HP: Eczema in psoriatico: An important differential
diagnosis between chronic allergic contact dermatitis and psoriasis
in palmoplantar localization. Acta Derm Venereol. 98:50–58.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gil TY, Kang YM, Eom YJ, Hong CH and An
HJ: Anti-atopic dermatitis effect of seaweed fulvescens extract via
inhibiting the STAT1 pathway. Mediators Inflamm.
2019(3760934)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang BY, Cheng YG, Liu Y, Liu Y, Tan JY,
Guan W, Guo S and Kuang HX: Datura Metel L. Ameliorates
imiquimod-induced psoriasis-like dermatitis and inhibits
inflammatory cytokines production through TLR7/8-MyD88-NF-κB-NLRP3
Inflammasome pathway. Molecules. 24(2157)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Irrera N, Vaccaro M, Bitto A, Pallio G,
Pizzino G, Lentini M, Arcoraci V, Minutoli L, Scuruchi M, Cutroneo
G, et al: BAY 11-7082 inhibits the NF-κB and NLRP3 inflammasome
pathways and protects against IMQ-induced psoriasis. Clin Sci
(Lond). 131:487–498. 2017.PubMed/NCBI View Article : Google Scholar
|