Introduction
Thermal injury remains a challenging disease and a
leading cause of death. The analysis of the pathological lesions
found in burn wounds showed an increase in vascular permeability at
the site of injury (1). The
responsible mechanisms are not yet well understood and can involve
numerous factors (2). Some authors
have described a possible role for matrix metalloproteinases (MMPs)
in endothelial hyperpermeability (1,3-5).
The effects of MMPs are not necessarily limited to the wound site,
thus it is difficult to assess their precise role in the dynamics
of the burn wound (4).
Wound healing involves complex processes that have
been carefully researched over time. Some studies have described
time-dependent increases in proteinase concentrations in burn wound
fluid (6). High levels of
proteinases (MMP-2 and MMP-9) have been found in wound fluid from
chronic wounds but not in those produced by other types of injuries
(7,8). Several studies have shown that the
plasmatic levels of MMP-9 are higher after burn injuries (7-10).
MMP-9 appears early after burn injury, and its level rises after 48
h (9,11). MMP-2 can also show higher levels in
burn wounds but to a lesser extent than in other injuries (7,9). In
burn wounds, with a typical chronic evolution, degradation products
can be found but it is not clear which of the tissue proteinases
are responsible for local repair (10). Another possible explanation could be
the degradation of the adhesion molecules and growth factors by the
local proteinases (12-14).
Moreover, the expression of different tissue proteases in burn
wounds could influence the prognosis (15). As a confirmation, some authors have
associated increased MMP-9 concentrations with delayed healing of
burned skin (16).
MMPs cannot be separated from their natural
inhibitors (tissue inhibitors of metalloproteinases or TIMPs)
(17). These maintain a balance
that contributes to local tissue homeostasis (18-20).
Independent roles for TIMPs have also been described (21). The inhibitor effect of TIMP-2 on
burn-induced vascular hyperpermeability was demonstrated on some
tissues, even in the presence of high MMP-9 levels (22). Serum concentrations of TIMP-1 reach
a peak 2 days after a severe burn (23). TIMP-1 is known to inhibit the
catalytic activity of MMP-9 in a 1:1 stoichiometric relationship
(24,25).
Since the role of MMP-9 and TIMP-1 in early
inflammation associated with a burn injury is poorly understood, we
aimed to investigate the dynamics of MMP-9, TIMP-1, and the
MMP-9/TIMP-1 ratio in the early shock phase of burn-injured
patients.
Patients and methods
Patients and study protocol. This study
included 25 adult patients with thermal burns (16 males/9 females,
mean age 49.40±17.55 years) admitted to the Clinical Emergency
Hospital for Plastic, Reconstructive, and Burns Surgery in
Bucharest, between 2018 and 2019. The study was conducted
respecting the principles outlined in the Declaration of Helsinki
and has been approved by the Clinical Emergency Hospital for
Plastic, Reconstructive, and Burns Surgery Ethics Committee
(approval no. 6627/04.10.2017).
The severity of the burn trauma is given by the burn
depth and burn size. Burn size has been estimated by reference to
the total body surface area (TBSA). Inclusion criteria were: i)
Thermal burns with a TBSA affected by the burn of <25%; ii) deep
second-degree and third-degree burns; iii) patient age over 18
years. All patients received routine treatment. They were
investigated upon admission and after 2 and 7 days. Exclusion
criteria were as follows: i) Patients with age under 18 years; ii)
chronic heart failure or renal failure; iii) cancer; iv) primary
and secondary immunodeficiency diseases; v) previously treated with
systemic corticosteroids affecting the body's inflammatory response
to burns; vi) treatment with doxycycline, known as an MMP inhibitor
(3).
In addition, 30 healthy subjects (19 males/11
females, mean age 49.70±8.04 years) were randomly selected from the
individuals who volunteered for general routine health evaluation.
The exclusion criteria that were applied to the patients with
thermal burns were also used to select the volunteers. Written
consents were obtained from patients or their legal representatives
and volunteers.
Blood sample collection and
processing
Blood was drawn into BD Vacutainer®
SST™ serum separation tubes purchased from Becton,
Dickinson and Company as soon as possible after the admission of
patients with burn wounds at the emergency department and in the
following 2 and 7 days. The serum samples were obtained by blood
clotting for 30 min at room temperature and centrifugation at 2,500
x g for 15 min. Serum samples were then immediately aliquoted into
labelled cryo-vials and frozen at -70˚C until analysis.
Detection of serum MMP-9 and TIMP-1 by
ELISA
The quantitative determination of human MMP-9 (cat.
no. DMP900) and TIMP-1 (cat. no. DTM100) was performed using ELISA
kits purchased from R&D Systems Inc. Because contamination may
lead to falsely elevated serum concentrations due to the MMP-9 and
TIMP-1 presence in saliva, protective measures were required to
prevent contamination during the test. Three samples of known
concentration were tested 20 times on one plate to assess
intra-assay precision and in 40 separate assays to assess
inter-assay precision. The values of the inter-assay imprecision
study were similar to those of the intra-assay study with CVs
ranging from 2 to 6.9%. The within CVs for MMP-9 were 2.0% at a
mean concentration of 83.3 ng/ml and 2.9% at a mean concentration
of 1,100 ng/ml (for low- and high-concentration patient samples).
CVs for the TIMP-1 were ~4.5%. The MMP-9/TIMP-1 ratio was
calculated according to the following formula: MMP-9 (ng/ml)/TIMP-1
(ng/ml). All assays were performed in duplicate according to the
manufacturers' recommendations and in such a way minimized any
effects of repeated freeze-thaw cycles.
Statistical analysis
Patient data processing was performed using
Microsoft Office Excel 2007 SP2 (including Data Analysis Tools).
Statistical analysis was conducted using SPSS version 25 software
(IBM Corp.). The difference between the serum concentrations
measured in dynamics for MMP-9 and TIMP-1 was analyzed by one-way
ANOVA test with Bonferroni correction, comparing their evolution
during the 7-day monitoring period from the initial burn injury.
The Anderson-Darling, Shapiro-Wilk, and Kolmogorov-Smirnov tests
were also used to verify the data obtained after preliminary
analysis and to check the consistency of the group. The correlation
between investigated biomarkers was assessed using Spearman's
correlation coefficient for TIMP-1 with data that did not have a
normal distribution and Pearson's correlation coefficient for MMP-9
and MMP-9/TIMP-1 ratio with normally distributed data. For all
tests, the significance level for statistical analysis was set at
P-values <0.05.
Results
After a post-injury accelerated
increase, MMP-9 remains at the same serum level
Data from the analysis of the sera collected from
patients with burn injury, at different time intervals, compared to
the healthy controls are summarized in Table I. Highly significant differences in
the levels of MMP-9, TIMP-1, and MMP-9/TIMP-1 ratios were observed
among patients with burn wounds and healthy controls. The
circulating level of MMP-9 was significantly higher throughout the
7-day monitoring period compared with the healthy controls
(P<0.001). MMP-9 increased immediately post-injury (a 6.25-fold
increase compared to the controls) and remained on a plateau
throughout the monitoring period as shown in Table I.
 | Table ISerum biomarkers in patients with skin
burn injury (N=25) and healthy controls (N=30). |
Table I
Serum biomarkers in patients with skin
burn injury (N=25) and healthy controls (N=30).
| | Patients with burn
injuries | |
|---|
| Variables | P1 | P2 | P3 | Control | P-valueb | P-valuec | P-valued |
|---|
| MMP-9
(ng/ml)a | 1,744.2±811.9 | 1,659.6±521.4 | 1,744.9±677.1 | 279.3±72.4 |
<0.001e |
<0.001e |
<0.001e |
| TIMP-1
(ng/ml)a | 269.9±93.6 | 386.8±334.9 | 534.7±381.2 | 166.7±45.5 |
<0.001e |
<0.001e |
<0.001e |
|
MMP-9/TIMP-1a | 6.5±2.6 | 5.5±2.6 | 4.1±2.2 | 1.7±0.3 |
<0.001e |
<0.001e |
<0.001e |
TIMP-1 is constantly increased
In contrast to MMP-9, the biomarker TIMP-1 showed a
steady increase during the study period. Thus, after 2 days from
burn injury TIMP-1 increased by 30.04% and after 7 days by 49.52%
(Table I). On the other hand, the
increase in serum TIMP-1 concentration immediately post-injury was
not as high as that of MMP-9 (1.62-fold vs. 6.25-fold).
MMP-9/TIMP-1 ratio linearly
decreases
Furthermore, the time course of MMP-9/TIMP-1
followed the inverse dynamics of TIMP-1, starting from a value of
the ratio measured at admission 3.82-fold higher than the one
observed in the healthy volunteers. The MMP-9/TIMP-1 ratio
decreased during the monitoring period as follows: 2-days
post-injury by 15.38% and 7-days post-injury by 36.92%.
Pearson correlations between serum
levels of MMP-9 measured in dynamics
To check the consistency of the statistical
analysis, a Pearson or a Spearman test was performed for
consecutive harvest time. Pearson test was chosen for normally
distributed data (MMP-9 and MMP-9/TIMP-1 ratio), while the Spearman
test was chosen for the abnormal ones (TIMP-1). As shown in
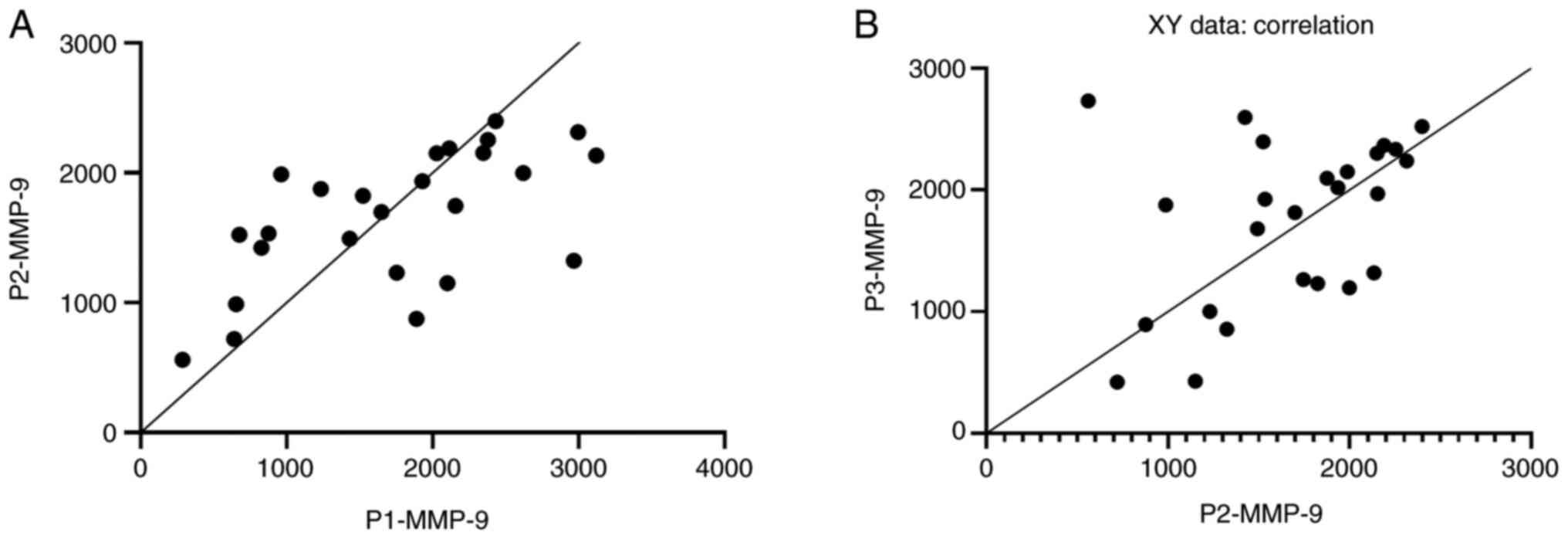
Fig. 1A and B, the serum MMP-9 concentrations measured
immediately post-injury were positively correlated with the values
recorded two days later (r=0.605, P=0.0013), values which in turn
were positively correlated with those measured 7 days post-injury
(r=0.407, P=0.04).
Spearman correlations between serum
levels of TIMP-1 measured in dynamics
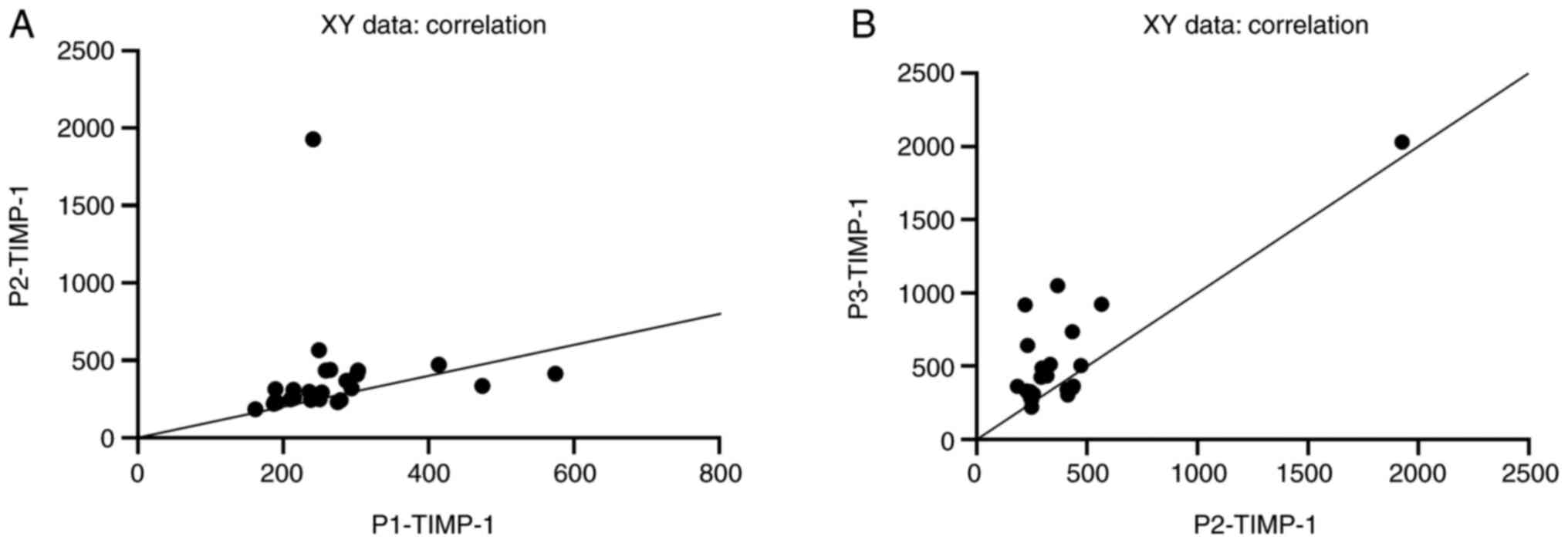
The Spearman test showed a significant correlation
between TIMP-1 concentrations at different times after burn injury
(admission vs. 2 days post-injury: rP1-P2=0.59, P=0.0017
and 2 days post-injury vs. 7 days post-injury:
rP2-P3=0.39, P=0.04) (Fig.
2A and B).
Pearson correlations between
MMP-9/TIMP-1 ratios calculated in dynamics
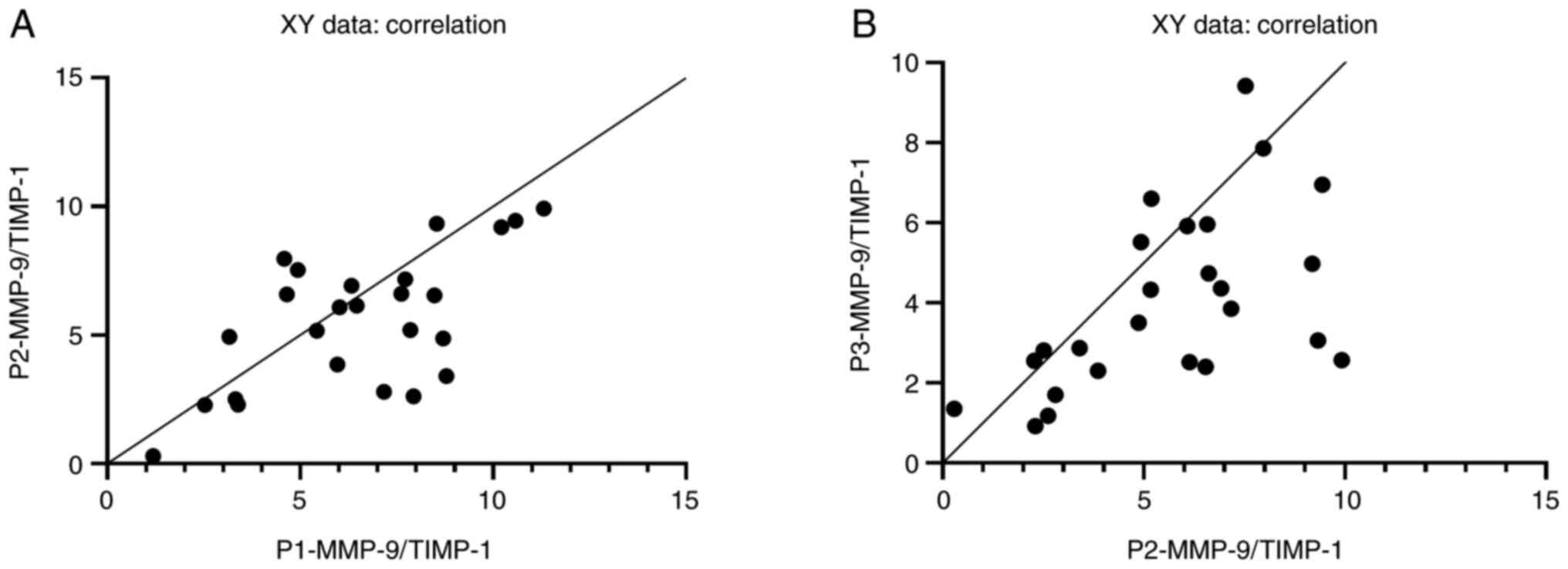
Furthermore, comparing the ratio of the two
biomarkers measured at different time post-injury, the Pearson's
correlation coefficient was as follows: Admission vs. 2 days
post-injury (rP1-P2=0.63, P=0.0006) and 2 days
post-injury vs. 7 days post-injury (rP2-P3=0.56,
P=0.0035) (Fig. 3A and B).
Discussion
The main findings of the present study are: i) MMP-9
increased immediately post-injury (a 6.25-fold increase compared to
controls) and remained on a plateau throughout the monitoring
period; ii) TIMP-1 showed a steady increase during the study
period: 2 days post-injury by 30.04% and 7 days post-injury by
49.52%; iii) the MMP-9/TIMP-1 ratio followed the inverse dynamics
of TIMP-1 with a steady decrease: 2 days post-injury by 15.38% and
7-days post-injury by 36.92%; iv) the dynamics of MMP-9, TIMP-1 and
the MMP-9/TIMP-1 ratio was demonstrated by the correlation between
the values measured at different time-points after burning
injury.
The MMP-9 and TIMP-1 biomarkers were investigated in
dynamics over 7 days. We considered that the study interval of 7
days is enough to detect both ascending and descending inflammatory
responses, the 7-day period being the time interval during which
the post-injury systemic inflammatory response syndrome usually
occurs (11,12,26,27).
It should be noted that none of the patients enrolled in our study
had septic complications during the monitoring period.
Burn injuries can trigger tissue changes that can
explain the variation in the level of different biochemical markers
that can be recorded both locally or systemically. Some events
observed in burn wounds such as vascular hyperpermeability have
been associated with matrix metalloproteinase (MMP) release after
trauma (1,3,6,7,9,27).
Because it is unknown whether the serum level of MMP-9 is a
consequence of these destructions or a local response to thermal
damage, we decided to follow its dynamics. In line with Hästbacka
et al (26), our results
showed an accelerated increase in MMP-9 serum concentration
immediately post-injury. Serum MMP-9 level was 6.25-fold higher
than that measured for the healthy controls, probably due to the
rapid release of MMP-9 from neutrophil granulocytes within minutes
to hours after the triggering insult (11). In contrast to other publications
(11,23) describing a decrease between days 4
to 6, in our study, after the early increase, the MMP-9 level
maintained on a plateau throughout the monitoring period. If the
correlation between the values measured at the patients' admission
and after 2 days was highly statistically significant (r=0.605,
P=0.0013), the one calculated between the measured values after 2
and 7 days did not have the same statistical power (r=0.407,
P=0.04). It is obvious, that MMP-9 does not occur as a simple local
response to thermal damage. It is more than that. In burn trauma,
the physiological balance is disturbed. The statistical power
decrease after 7 days post-injury can be explained by the imbalance
between MMP-9 and its tissue inhibitor, MMP-9 staying on a plateau,
and TIMP-1 having an upward trend. Our results showing an
increasing trend for TIMP-1 are in contrast to other studies
(11,23) that have found significantly higher
TIMP-1 concentrations in the plasma of patients with TBSA affected
by a burn of <20% relative to healthy controls, with a median
time to peak TIMP-1 concentration at 2.09 days. Given that a
moderate increase in the MMP-9 serum concentration is beneficial,
the epithelialization rate being partially dependent on the
presence of collagen (13,16), MMP-9 levels remaining constant
during the 7-day monitoring period could be associated with a
better-quality scar. Because none of the patients enrolled in our
study had septic complications during the 7-day monitoring period,
the maintaining/decrease of MMP-9 serum levels may be an indicator
for better survival.
Dynamic changes in circulating levels of MMP-9 and
TIMP-1 demonstrate their involvement in the early response after
thermal injury. As can be seen from Table I, at all-time points the differences
between serum MMP-9 and its inhibitor TIMP-1 concentrations in the
study group compared to the control group were statistically
significant. The time-course of the MMP-9/TIMP-1 ratio followed the
inverse dynamics of TIMP-1 starting from a ratio value measured at
admission 3.82-fold higher than the one observed in the healthy
volunteers. The constant linear decrease in the MMP-9/TIMP-1 ratio
during the 7-day monitoring period (decrease by 14.28% in 2 days
and decrease by 38.43% in 7 days from the measured value from
admission) may represent an indicator of an effective healing
process without hypertrophic scars and keloids for patients with a
TBSA affected by the thermal burn <25%. As a confirmation, the
comparison of the MMP-9/TIMP-1 ratio in dynamics showed a
statistically significant correlation both between the values
measured at hospitalization and those measured after 2 days
(r=0.63, P=0.0006) and between those measured after 2 and 7 days,
respectively (r=0.56, P=0.0035), much better than those obtained
for MMP-9 and TIMP-1 individually.
Healing processes after thermal burn evolve, as
evidenced by clinical practice. The factors that influence local
changes are not fully known, but our results have shown that serum
proteinases may be responsible for tissue remodeling. In this
regard, monitoring of the MMP-9/TIMP-1 ratio may provide
information concerning local changes over time, starting from the
moment of the triggering insult, ultimately indicating the need for
surgery or other therapeutic approaches.
The most apparent weakness of our study is related
to the small number of patients included. Despite the small sample
size, three aspects need to be mentioned: i) The study group
matched the control group in terms of number, sex distribution, and
age; ii) all the patients included in the study had a TBSA affected
by the thermal burn <25%; and iii) none of the patients enrolled
in our study had septic complications during the monitoring
period.
In conclusion, the results of the present study
demonstrated the MMP-9 and TIMP-1 involvement in the early response
after thermal injury. Our findings suggest that the MMP-9/TIMP-1
ratio may provide information on local changes over time, starting
from the triggering insult, and may be considered a predictive
biomarker of burn evolutivity. Further investigations are needed to
confirm these findings.
Acknowledgements
Not applicable.
Funding
Funding: This research did not receive any specific grant from
any funding agency in the public, commercial or not-for-profit
sector.
Availability of data and materials
All data generated or analyzed during this study are
included in the manuscript.
Authors' contributions
AES and AZCA conceived and planned the experiments.
Experiments were performed by AES. AZCA, FLF, and MMS performed
statistical analysis of the results. AES, AZCA, MMS, MG, RH, FLF
and DCG contributed to the interpretation of the results. AES took
the lead in writing the manuscript. All authors provided critical
feedback and helped shape the research, analysis, and manuscript.
All authors read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
The study conformed to the principles outlined in
the Declaration of Helsinki and was approved by the Clinical
Emergency Hospital for Plastic, Reconstructive, and Burns Surgery
ethics committee (approval no. 6627/04.10.2017). Written informed
consent was obtained from enrolled patients or their legal
representatives and volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Adina Elena Stanciu: ORCID ID: https://orcid.org/0000-0002-9494-6686.
References
|
1
|
Wiggins-Dohlvik K, Han MS, Stagg HW,
Alluri H, Shaji CA, Oakley RP, Davis ML and Tharakan B: Melatonin
inhibits thermal injury-induced hyperpermeability in microvascular
endothelial cells. J Trauma Acute Care Surg. 77:899–905.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Demling RH: The burn edema process:
Current concepts. J Burn Care Rehabil. 26:207–227. 2005.PubMed/NCBI
|
|
3
|
Stagg HW, Whaley JG, Tharakan B, Hunter
FA, Jupiter D, Little DC, Davis ML, Smythe WR and Childs EW:
Doxycycline attenuates burn-induced microvascular
hyperpermeability. J Trauma Acute Care Surg. 75:1040–1046.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rodriguez D, Morrison CJ and Overall CM:
Matrix metalloproteinases: What do they not do? New substrates and
biological roles identified by murine models and proteomics.
Biochim Biophys Acta. 1803:39–54. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pantea Stoian A, Mitrofan G, Colceag F,
Suceveanu AI, Hainarosie R, Pituru S, Diaconu CC, Timofte D,
Nitipir C, Poiana C and Serafinceanu C: Oxidative stress in
diabetes. A model of complex thinking applied in medicine. Rev
Chim. 69:2515–2519. 2018.
|
|
6
|
Young PK and Grinnell F: Metalloproteinase
activation cascade after burn injury: A longitudinal analysis of
the human wound environment. J Invest Dermatol. 103:660–664.
1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wysocki AB, Staiano-Coico L and Grinnell
F: Wound fluid from chronic leg ulcers contains elevated levels of
metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 101:64–68.
1993.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McCarty SM and Percival SL: Proteases and
delayed wound healing. Adv Wound Care (New Rochelle). 2:438–447.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dasu MR, Spies M, Barrow RE and Herndon
DN: Matrix metalloproteinases and their tissue inhibitors in
severely burned children. Wound Repair Regen. 11:177–180.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lang TC, Zhao R, Kim A, Wijewardena A,
Vandervord J, Xue M and Jackson CJ: A Critical update of the
assessment and acute management of patients with severe burns. Adv
Wound Care (New Rochelle). 8:607–633. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nagy B, Szélig L, Rendeki S, Loibl C,
Rézmán B, Lantos J, Bogár L and Csontos C: Dynamic changes of
matrix metalloproteinase 9 and tissue inhibitor of
metalloproteinase 1 after burn injury. J Crit Care. 30:162–166.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Plichta JK, Holmes CJ, Gamelli RL and
Radek KA: Local burn injury promotes defects in the epidermal lipid
and antimicrobial peptide barriers in human autograft skin and burn
margin: Implications for burn wound healing and graft survival. J
Burn Care Res. 38:e212–e226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo HF, Ali RM, Hamid RA, Chang SK, Rahman
MH, Zainal Z and Khaza'ai H: Temporal changes in the cell
population and wound healing-related gene expression in deep
partial-thickness burn wound model. Biomed Dermatol. 4(5)2020.
|
|
14
|
Suceveanu AI, Mazilu L, Katsiki N, Parepa
I, Voinea F, Pantea-Stoian A, Rizzo M, Botea F, Herlea V, Serban D
and Suceveanu AP: NLRP3 inflammasome biomarker-could be the new
tool for improved cardiometabolic syndrome outcome. Metabolites.
10(448)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stoian AP, Razvan H, Pietrosanu C, Rusescu
A, Andronache LF, Paunica S, Balalau C and Pituru TS: Modern
concepts in non-surgical esthetics; a review. J Mind Med Sci.
6:190–195. 2019.
|
|
16
|
Simonetti O, Lucarini G, Cirioni O, Zizzi
A, Orlando F, Provinciali M, Di Primio R, Giacometti A and Offidani
A: Delayed wound healing in aged skin rat models after thermal
injury is associated with an increased MMP-9, K6 and CD44
expression. Burns. 39:776–787. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Georgescu EF, Mogoantă SŞ, Costache A,
Pârvănescu V, Totolici BD, Pătraşcu Ş and Stănescu C: The
assessment of matrix metalloproteinase-9 expression and
angiogenesis in colorectal cancer. Rom J Morphol Embryol.
56:1137–1144. 2015.PubMed/NCBI
|
|
18
|
Murphy G: Tissue inhibitors of
metalloproteinases. Genome Biol. 12(233)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Raeeszadeh-Sarmazdeh M, Do LD and Hritz
BG: Metalloproteinases and their inhibitors: Potential for the
development of new therapeutics. Cells. 9(1313)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stanciu AE, Zamfir-Chiru-Anton A, Stanciu
MM, Stoian AP, Jinga V, Nitipir C, Bucur A, Pituru TS, Arsene AL,
Dragoi CM, et al: Clinical significance of serum melatonin in
predicting the severity of oral squamous cell carcinoma. Oncol
Lett. 19:1537–1543. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: Metalloproteinase-independent
biological activities. Sci Signal. 1(re6)2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wiggins-Dohlvik K, Oakley RP, Han MS,
Stagg HW, Alluri H, Shaji CA, Davis ML and Tharakan B: Tissue
inhibitor of metalloproteinase-2 inhibits burn-induced derangements
and hyperpermeability in microvascular endothelial cells. Am J
Surg. 211:197–205. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ulrich D, Noah EM, von Heimburg D and
Pallua N: TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for
skin fibrosis in patients following severe burn trauma. Plast
Reconstr Surg. 111:1423–1431. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stanciu AE: Cytokines in heart failure.
Adv Clin Chem. 93:63–113. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hästbacka J, Fredén F, Hult M, Bergquist
M, Wilkman E, Vuola J, Sorsa T, Tervahartiala T and Matrix F:
Matrix metalloproteinases-8 and -9 and tissue inhibitor of
metalloproteinase-1 in burn patients. A prospective observational
study. PLoS One. 10(e0125918)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lorente L, Martin MM, Labarta L, Diaz C,
Solé-Violán J, Blanquer J, Orbe J, Rodriguez JA, Jimenez A,
Borreguero-Leon JM, et al: Matrix metalloproteinase-9, -10, and
tissue inhibitor of matrix metalloproteinases-1 blood levels as
biomarkers of severity and mortality in sepsis. Crit Care.
13(R158)2009.PubMed/NCBI View
Article : Google Scholar
|