Introduction
Gestational hypertension is a common obstetric
disease, occurring in 5-10% of pregnancies (1). Maternal death induced by gestational
hypertension accounts for 10-16% of all pregnancy-associated
mortalities and gestational hypertension is the second leading
cause of maternal death in China (1). Hypertension, proteinuria and edema are
the main symptoms of this condition. Gestational hypertension may
be induced by maternal, placental and fetal factors, such as
abnormal invasion of trophoblasts, abnormal immune regulation,
endothelial cell damage, genetic factors and nutritional factors
(2). No single factor can explain
the complex etiology and mechanism of gestational hypertension
(3). In the treatment of
gestational hypertension, blood pressure must be lowered and severe
preeclampsia and eclampsia must be prevented (4).
Metoprolol succinate is a selective β1 receptor
blocker. The dose required for its action on cardiac β1 receptors
is lower than that required for its action on peripheral blood
vessels and bronchi β2 receptors (5). Metoprolol succinate doesn't activate β
receptors on membranes. Metoprolol succinate can attenuate the
effect of catecholamines associated with physiological and
psychological loads, and reduce the heart rate, cardiac output and
blood pressure (5). Bisoprolol
fumarate is also a highly selective β1 adrenoceptor antagonist,
without intrinsic sympathomimetic and membrane stabilization
activities. Bisoprolol fumarate has a high affinity for β1
receptors of bronchi and vascular smooth muscle, which leads to
vasodilation and lower blood pressure (5). Metoprolol succinate and bisoprolol
fumarate are widely used in the treatment of hypertension (5). In addition, during pregnancy, estrogen
upregulates ten-eleven translocation 1 (TET1) expression in uterine
arteries, which activates demethylation and subsequently increases
calcium-activated potassium channel subunit β1 (KCNMB1) expression
(6). KCNMB1 is associated with
hypertension (7). Fumaric acid and
succinic acid are known to inhibit TET (8). Therefore, the present study
hypothesized that fumaric acid and succinic acid may exhibit
therapeutic effects on gestational hypertension, and the present
study was performed for preliminary confirmation.
The TET1 gene is a research hotspot at present for
its special role in DNA methylation (9). TET1 is a hydroxymethylase, originally
discovered as a fusion protein of histone H3K4 methyltransferase
(10). TET1 can bind to the CpG
region in the genome, which hinders catalytic activity of DNA
methyltransferase and activates gene transcription (11). In addition, TET1 can recruit
polycomb repressive complex 2 to the promoter region of a target
gene, which ultimately suppressed the expression of the target gene
(12). The TET protein family
participates in the whole embryonic development process. When TET1
and TET3 are knocked-out at the same time, the transcriptome
diversity increases during early embryonic development (13). Growth defects, increased mortality
and developmental retardation are identified in the offspring of
paternal mice following TET1-knockdown (14). BKCa channels are involved in the
regulation of cellular functions, including neurotransmitter
release, hormone secretion, heart rate, vascular stress resistance
and smooth muscle tension, for their multiple regulatory
characteristics. BKCa is composed of an ion-mediated α subunit and
different β subunits. The β1 subunit is predominantly expressed in
smooth muscle cells and it affects the behavior of these cells
(15). Mutations in the KCNMBl gene
encoding the β1 subunit are associated with heart rate variability
and baroreflex function (16).
Variation of the KCNMBl gene loci decreases arterial impedance
(17). KCNMBl may serve an
important role in the development and progression of hypertension
(18).
Therefore, the present study established a rat
gestational hypertension model and investigated whether fumaric
acid and succinic acid exhibit therapeutic effects on gestational
hypertension and whether these effects are mediated by TET1 and
KCNMBl.
Materials and methods
Materials and animals
Nω-Nitro-L-arginine methyl ester hydrochloride
(L-NAME; cat. no. 51298-65.5) and dimethyl fumarate (DMF; cat. no.
C1821194) were purchased from Shanghai Aladdin Biochemical
Technology Co., Ltd. Succinic acid (cat. no. ZN1113EA14) was
obtained from Shanghai Yuanye Bio-Technology Co., Ltd. The rat
urinary protein kit (cat. no. CO35-2) was obtained from Nanjing
Jiancheng Bioengineering Institute. The bicinchoninic acid (BCA)
kit (cat. no. CW0014), diaminobenzidine (DAB) kit (cat. no.
CW0125), TRIzol reagent (cat. no. CW0580), Ultrapure RNA extraction
kit (cat. no. CW0581), HiFiScript cDNA synthesis kit (cat. no.
CW2569) and ULtraSYBR Mixture (cat. no. CW0957) were purchased from
CoWin Biosciences Co., Ltd. (CWBIO). RIPA lysis buffer (cat. no.
C1053) was purchased from Applygen Technologies, Inc.
SuperSignal® west pico chemiluminescent substrate (cat.
no. RJ239676) was obtained from Thermo Fisher Scientific, Inc. The
polyvinylidene fluoride (PVDF) membrane (cat. no. IPVH00010) was
obtained from EMD Millipore. Mouse anti-GAPDH monoclonal antibody
(cat. no. TA-08; 1:2,000), horseradish peroxidase (HRP) conjugated
goat anti-mouse IgG (H+L) (cat. no. ZB-2305; 1:2,000) and HRP
conjugated goat anti-rabbit IgG (H+L) (cat. no. ZB-2301; 1:2,000)
were purchased from OriGene Technologies, Inc. Rabbit anti-TET1
polyclonal antibody (cat. no. DF6428; 1:500) and rabbit anti-KCNMB1
polyclonal antibody (cat. no. DF-9301; 1:1,000) were purchased from
Affinity Biosciences. Rabbit anti-KCNMB1 polyclonal antibody (cat,
no. bs-7689R; 1:1,000) was obtained from BIOSS.
In total, 35 female (three months-old, 250-280 g)
and 20 male (three months-old, 400-500 g) Sprague-Dawley rats were
obtained from the Hunan SJA Laboratory Animal Co., Ltd. [license
no. SCXK(Xiang)2016-0002]. All rats were provided with free access
to food and water and kept in conditions of 22-25˚C under a 12-h
light/dark cycle. During the experimental phase, animal well-being
was monitored by individual ventilated caging system (SmartRack,
BioZone). Unpregnant rats and male rats after mating was completed
and the fetuses after birth were fed normally and sacrificed at the
end of the whole experiment. The experiments did not affect normal
activity and eating behavior of rats. Rats were sacrificed after
anesthesia. The study protocol was reviewed and approved by the
Ethics Committee of the First Affiliated Hospital of Guiyang
University of Chinese Medicine.
Gestational hypertension model
establishment
Overnight, female and male rats were placed in a
cage at a ratio of 2:1 to induce pregnancy. Formation of vaginal
plug was observed the next morning. A total of 30 female rats were
impregnated. Rats with vaginal plug were immediately placed in
separate cages and day 0 of pregnancy was record. Blood pressure
was measured on day 9 of pregnancy using a constant temperature
noninvasive blood pressure meter (XH200; Beijing Zhongshidichuang
Co., Ltd.). The rats were then randomly grouped (n=6). Drugs were
administrated by gavage at 10 ml/kg q.o.d, from day 10 of pregnancy
to the birth of the fetus. Concentrations of DMF and succinic acid
were 2.5 and 40 mg/ml, respectively. Rats in the control and model
groups were not treated with drugs. Subcutaneous injection of
L-NAME into the back was performed continuously at 125 mg/kg q.o.d.
for 5 days from day 14 of pregnancy to induce gestational
hypertension. Blood pressure was measured again on day 19 of
pregnancy. Finally, rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate at 350 mg/kg. No signs of
peritonitis were observed. Urine was collected by abdominal
compression and punctio vesicae. After the fetus was born,
rats were sacrificed by cervical dislocation and the placenta was
collected. The urine and a portion of the placenta were stored at
-80˚C. Another portion of the placenta was fixed in 4%
paraformaldehyde at room temperature for 24 h and stored at room
temperature.
Experimental grouping
A total of 30 pregnant rats were divided into five
groups (n=6): i) Control; ii) model; iii) DMF; iv) succinic acid;
and v) DMF + succinic acid. Rats without any treatment served as
the control group. Rats in the model group received L-NAME
injection but did not receive any drug treatment. Rats that had
gestational hypertension and were treated with DMF were assigned as
the DMF group. Rats that had gestational hypertension and were
treated with succinic acid were assigned as the succinic acid
group. Rats that had gestational hypertension and were treated with
both DMF and succinic acid were assigned as the DMF + succinic acid
group. In the DMF + succinic acid group, the doses of the two drugs
were halved.
Urinary protein determination
Urinary protein was determined using a urinary
protein kit, according to the manufacturer's protocol. Briefly, 50
µl water, protein standard solution (563 mg/l) and the urine sample
were added into a blank well, standard well and test well,
respectively. Coomassie brilliant blue solution (3 ml) was added
into each well, followed by mixing. After 5 min, the absorbance
(optical density value) was measured at 595 nm using a microplate
reader (RT-6100; Rayto Life and Analytical Sciences Co., Ltd.).
Hematoxylin and eosin (H&E)
staining
Tissues were fixed in 4% paraformaldehyde at room
temperature, washed with running water, dehydrated in 70, 80 and
90% ethanol solution, immersed in an equivalent mixture of absolute
ethanol and xylene for 15 min, and transparentized in xylene twice
for 15 min each time until transparency. After immersing in an
equivalent mixture of xylene and paraffin for 15 min and then in
paraffin twice for 50-60 min each time, the tissues were embedded
in paraffin and cut into slices, 4-µm thick. Subsequently, the
slices were baked, dewaxed, hydrated and then stained with
hematoxylin solution for 3 min. Sections were then differentiated
in alcoholic hydrochloric acid for 15 sec, washed slightly, blued
in bluing buffer for 15 sec, washed with running water, stained in
eosin solution at room temperature for 3 min. After staining
sections were washed with running water, dehydrated,
transparentized, mounted with neutral resin and examined under a
light microscope (CKX41; Olympus Corporation).
Immunohistochemical analysis
Tissue slices were prepared as mentioned above and
baked at 65˚C for 2 h, immersed in xylene twice for 10 min each,
and successively incubated in 100, 100, 95 and 80% ethanol and
water for 5 min each time. Subsequently, the slices were incubated
in citrate buffer and heated for 2 min in a high-pressure cooker.
The slices were then cooled naturally, rinsed with PBS, incubated
in fresh 3% hydrogen peroxide at room temperature for 10 min and
washed with PBS. After the excess PBS was absorbed by absorbent
papers, 5% bovine serum albumin (Beijing Solarbio Science &
Technology, Co., Ltd.) was added dropwise onto the slices, which
were then incubated at 37˚C for 30 min. After excess blocking
buffer was absorbed by absorbent papers, the slices were incubated
with primary antibody buffer (rabbit anti-TET1 polyclonal antibody,
rabbit anti-KCNMB1 polyclonal antibody) at 4˚C overnight. Following
washing with PBS three times, sections were incubated with the
secondary antibody buffer (horseradish peroxidase-conjugated goat
anti-rabbit IgG) at 37˚C for 30 min. Then sections were rinsed with
PBS, developed for 5-10 min using a DAB kit, rinsed with PBS,
stained in hematoxylin solution at room temperature for 3 min.
Alcoholic hydrochloric acid was used to differentiate and after
that sections were blued in bluing buffer, rinsed with water,
dehydrated, transparentized, mounted and examined under a light
microscope (CKX41; Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tissue RNA was extracted using TRIzol reagent and RT
to cDNA was conducted at 42˚C using the HiFiScript cDNA synthesis
kit according to their manufacturer's protocol. Table I presents the primer sequences. The
PCR reaction was composed of 1 µl cDNA/DNA, 1 µl forward primer, 1
µl reverse primer, 12.5 µl ULtraSYBR Mixture and 9.5 µl RNase free
dH2O. Reaction parameters included pre-denaturation at
95˚C for 10 min, denaturation at 95˚C for 10 sec, annealing at
58.5˚C for 30 sec, elongation at 72˚C for 30 sec (40 cycles).
Analysis parameters of the dissociation curve included 15 sec at
95˚C, 1 min at 58.5˚C, 15 sec at 95˚C, 15 sec at 58.5˚C and 15 sec
at 58.5˚C, and measured stepwise from 95˚C, every 0.5˚C. Products
were examined using a RT-qPCR detection system (CFX Connect™;
Bio-Rad Laboratories, Inc.). GAPDH served as an internal control.
Relative levels of genes were calculated using a 2-ΔΔCq
method (19).
 | Table IThe sequences of primers. |
Table I
The sequences of primers.
| Primers | Sequences
(5'-3') | Product length
(bp) |
|---|
| TET1 | F:
AAACGGAAGTCAAAACCCC | 140 |
| | R:
CCGAAGAGCCATTGTAAACC | |
| KCNMB1 | F:
AACATCAAGGACCAGGAAGAG | 129 |
| | R:
TTGGTTTTGATCCCGAGTG | |
| GAPDH | F:
GCAAGTTCAACGGCACAG | 141 |
| | R:
CGCCAGTAGACTCCACGAC | |
Western blotting
Tissues were lysed in RIPA lysis buffer at 4˚C for
30 min and then centrifuged at 9,000 x g and 4˚C for 10 min. The
supernatant was collected carefully to obtain total protein.
Protein concentration was determined using a BCA kit. Subsequently,
the protein (24 µg) was denatured and separated by 12% SDS-PAGE for
1-2 h. The protein was transferred to a PVDF membrane by a wet
method for 30-50 min, which was then blocked with 3% skimmed milk
at room temperature for 1 h and incubated with primary antibody
buffer at 4˚C overnight. After washing, the membrane was incubated
with secondary antibody buffer at room temperature for 1-2 h,
incubated with chemiluminescent substrate and examined on a gel
imaging system (ChemiDoc™ XRS+; Bio-Rad Laboratories, Inc.). Gray
values were analyzed using Quantity One software (v4.62; Bio-Rad
Laboratories, Inc.). GAPDH served as an internal control.
Statistical analysis
Data are presented as the mean ± standard deviation.
Every experiment was repeated three times. Data were statistically
analyzed using one-way analysis of variance followed by Tukey's
post-hoc test with SPSS 19.0 software (IBM, Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of a gestational
hypertension model
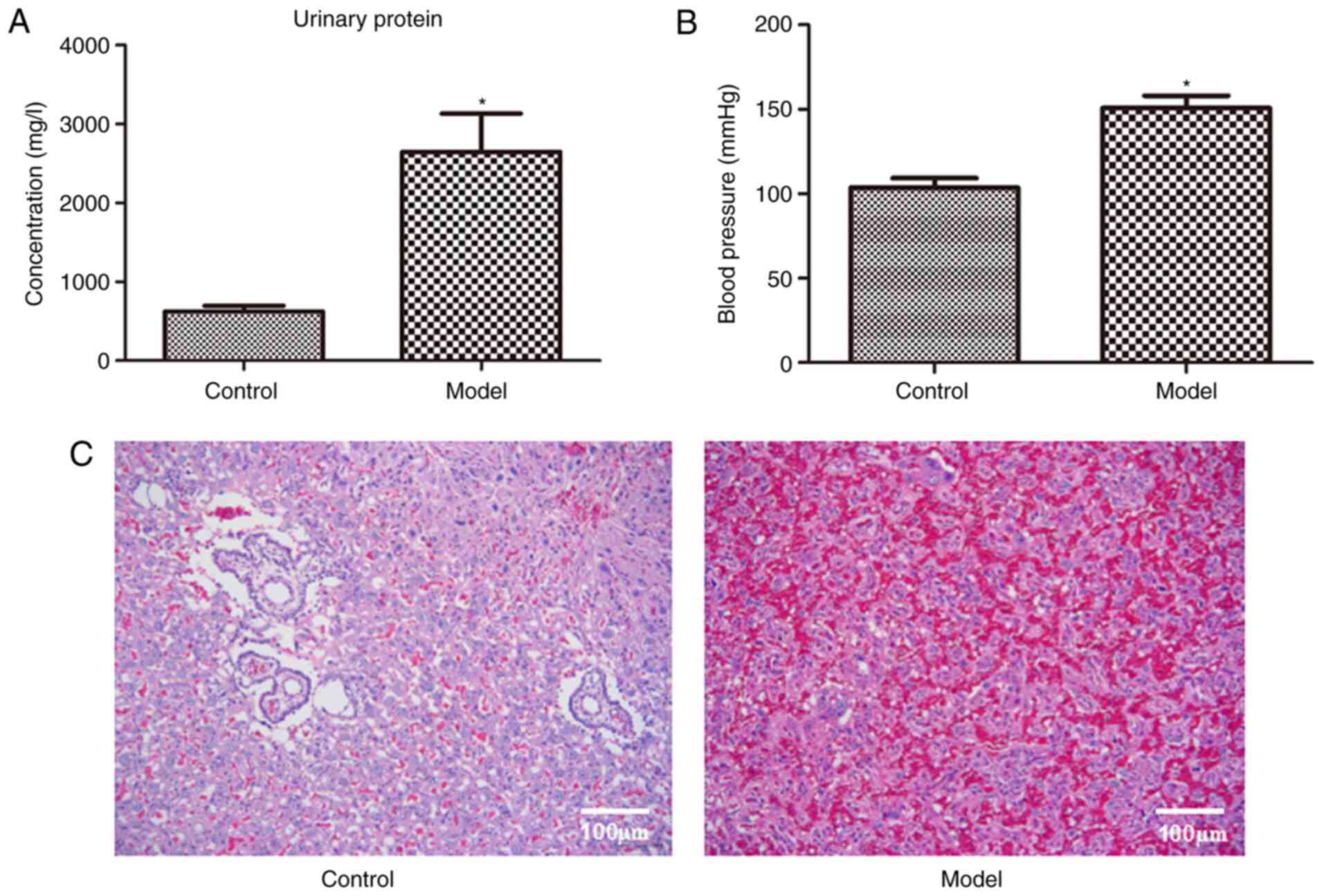
Fig. 1 presents
urinary protein (Fig. 1A), blood
pressure (Fig. 1B) and H&E
staining images of the placenta (Fig.
1C) in the control and model rats. Compared with the control
rats, urinary protein and blood pressure in the model rats
increased significantly (P<0.05; Fig. 1A and B). As presented in Fig. 1C, placental cells in the control
rats were arranged in an orderly fashion. However, in the model
rats, decidual cellular edema of the placenta and vacuolar
degeneration were observed, and the intervascular membrane was
obviously thicker with a large amount of fibrin deposition. These
results indicate that the gestational hypertension model was
successfully established.
Urinary protein and blood
pressure
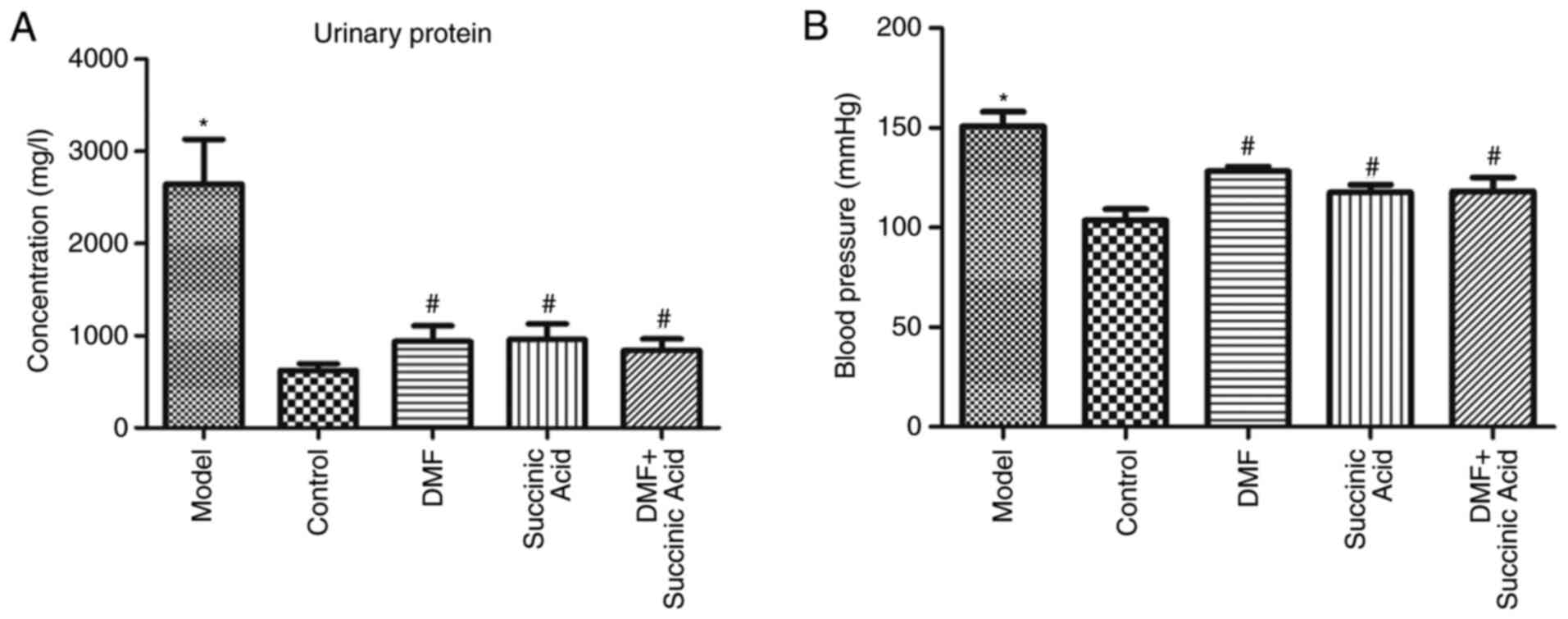
Fig. 2 presents the
urinary protein (Fig. 2A) and blood
pressure (Fig. 2B) of rats in
various groups. Compared with the control group, urinary protein
and blood pressure in the model group increased significantly
(P<0.05). However, compared with the model group, urinary
protein and blood pressure in the DMF, succinic acid and DMF +
succinic acid groups decreased significantly (P<0.05).
H&E staining
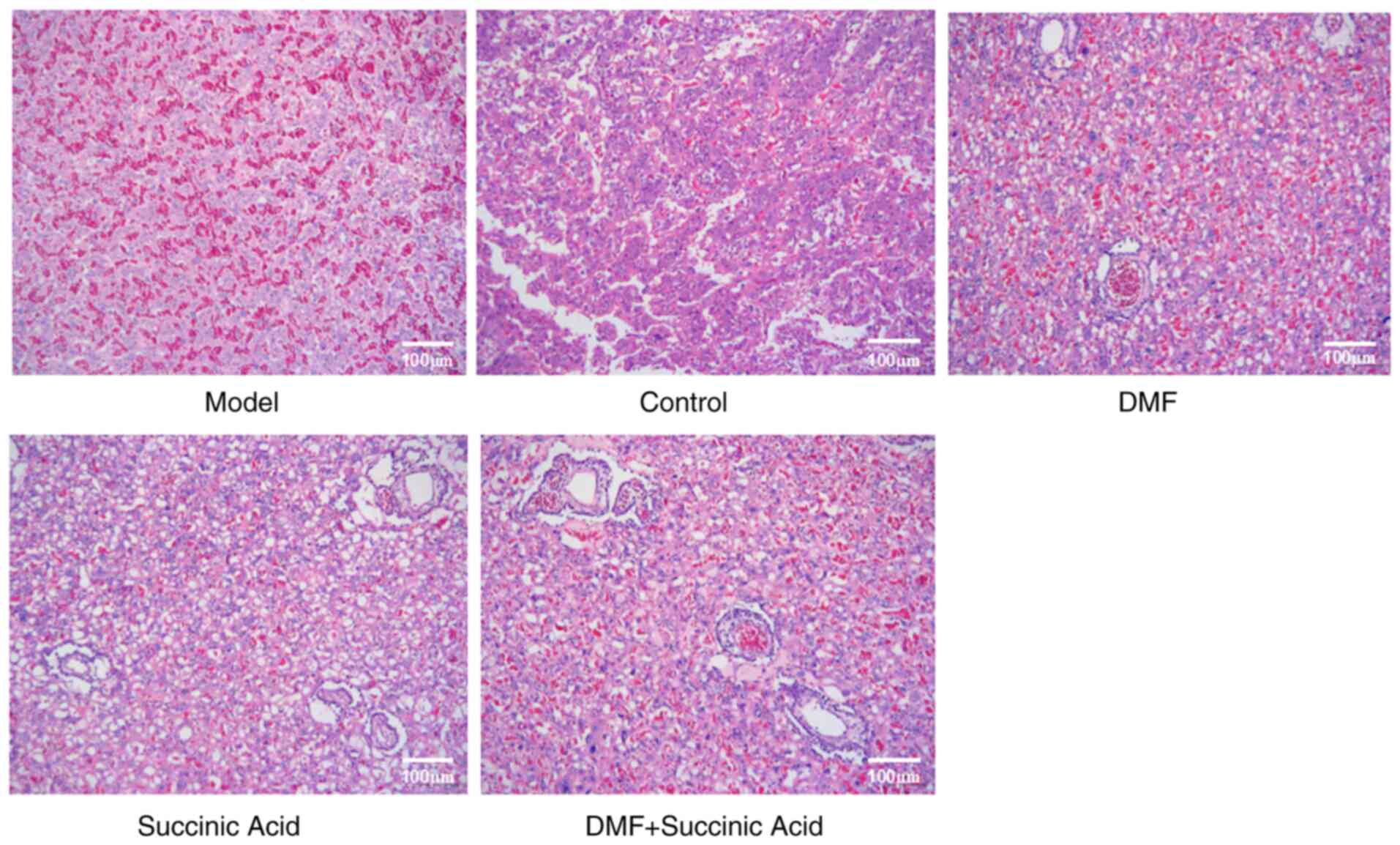
Fig. 3 presents
H&E staining images of the placenta in the rats of various
groups. Placental cells were arranged in an orderly manner in the
control group. However, decidual cellular edema of the placenta,
vacuolar degeneration, thicker intervascular membranes and a large
amount of fibrin deposition were identified in the model group.
Notably, compared with the model group, the edema, vacuoles and
fibrin deposition were markedly reduced in the DMF, succinic acid
and DMF + succinic acid groups.
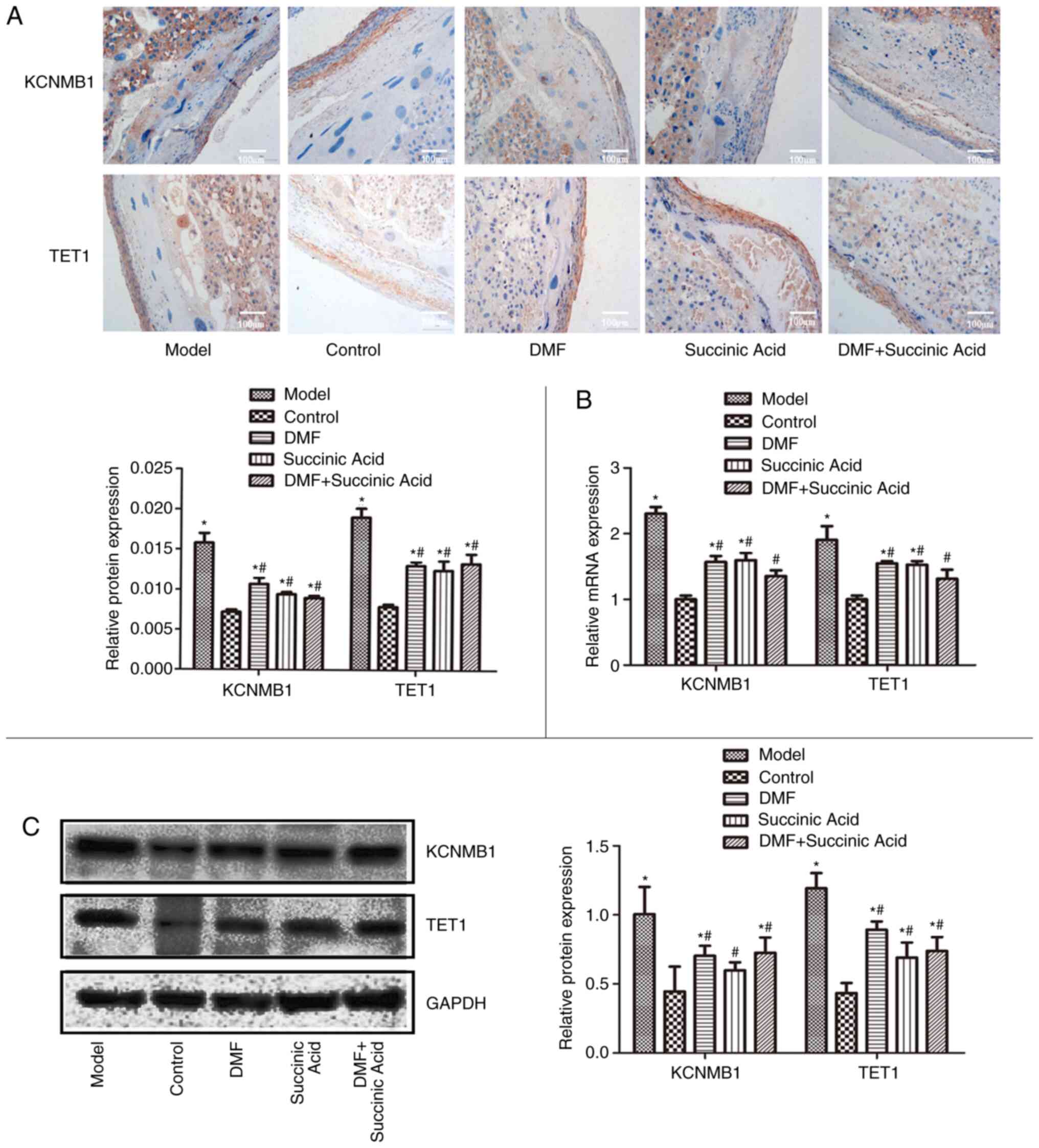
Levels of TET1 and KCNMB1
mRNA and protein levels of TET1 and KCNMB1 in the
placenta of various groups were examined by immunohistochemical
analysis (Fig. 4A), RT-qPCR
(Fig. 4B) and western blotting
(Fig. 4C). The protein of interest
is brown in the immunohistochemical images. Immunohistochemical
analysis, RT-qPCR and western blot demonstrated similar results.
The levels of TET1 and KCNMB1 were significantly increased in the
model groups compared with the control group (P<0.05). However,
compared with the model group, their levels were significantly
downregulated in the DMF, succinic acid and DMF + succinic acid
groups (P<0.05).
Discussion
At present, therapies for gestational hypertension
are far from satisfactory. Establishment of an animal model
provides a good basis for the investigation of treatments due to
the relatively short gestation period in animals. However, the
short gestation period and requirement of reserving enough time to
treat the disease makes it difficult to establish a model. In the
present study, a gestational hypertension model was established by
subcutaneous injection of L-NAME. Results of urinary protein and
blood pressure measurements demonstrated that urinary protein and
blood pressure increased in the model rats. Furthermore,
pathological examinations revealed the decidual cellular edema of
the placenta, vacuolar degeneration, thicker intervascular
membranes and a large amount of fibrin deposition in the model
rats. These results confirmed the successful establishment of a
gestational hypertension model. L-NAME is an inhibitor of nitric
oxide (NO) synthase and can effectively inhibit NO synthesis. NO is
an important active substance in maintaining homeostasis in humans
and animals. A previous study reported that the NO level in
pregnancy is higher than that in the normal state and inhibition of
NO synthesis will lead to elevated blood pressure, proteinuria and
symptoms of preeclampsia (20). The
results of the present study agreed with this previous study.
Elevated blood pressure may be a result of an enhanced response of
the vascular system in pregnant rats to angiotensin E and
norepinephrine by inhibiting NO synthesis (21).
The present study identified that urinary protein
and blood pressure decreased, and placental cells in pregnant rats
were improved during gestational hypertension when fumaric acid and
succinic acid were administrated. These results suggest that
fumaric acid and succinic acid exerted therapeutic effects on
gestational hypertension. Non-toxic side effects of fumaric acid
and succinic acid in rats are well recognized in previous reports
(22,23); therefore, the present study did not
perform experiments to confirm their non-toxic side effects in
normal non-pregnant female rats.
The TET protein family participates in the whole
embryonic development process. When TET1 and TET3 are knocked-out
at the same time, the transcriptome diversity increases during
early embryonic development (13).
Growth defects, increased mortality and developmental retardation
are identified in the offspring of paternal mice following
TET1-knockout (14). TET1
expression is upregulated during fetal growth, suggesting that TET1
gene may regulate early fetal growth (24). Numerous studies have reported that
KCNMB1 reduces the probability of a BKCa channel being open and
instantaneous outward potassium current, which weakens the negative
feedback inhibitory effect of the BKCa channel and consequently
leads to depolarization of the cell membrane, contraction of
vascular smooth muscle and elevation of blood pressure (18,25).
Correlation analysis between KCNMB1 mutation and hypertension
demonstrated that KCNMB1 mutation results in increased sensitivity
of the BK channel to calcium ions, which makes blood vessels easier
to relax (26). Therefore, the
KCNMB1 channel has a negative feedback effect on the contraction of
vascular smooth muscle (26).
During pregnancy, estrogen upregulates TET1 expression in uterine
arteries, which activates demethylation and subsequently increases
KCNMB1 expression (6). KCNMB1 is
associated with hypertension (7).
The present results revealed that expression levels of TET1 and
KCNMB1 increase during gestational hypertension. This is consistent
with the aforementioned reports that TET1 expression is elevated
during pregnancy and also suggests that KCNMB1 is associated with
hypertension. Fumaric acid and succinic acid are known to inhibit
TET (8). In the present study, the
expression of TET1 and KCNMB1 decreased following the
administration of fumaric acid and succinic acid, suggesting that
fumaric acid and succinic acid downregulate the expression of TET1
and KCNMB1.
The absence of histological scoring data was a
limitation of the current study. In future studies, promoters or
inhibitors will be used to investigate the regulatory relationship
between KCNMB1 and TET1 in the treatment of gestational
hypertension by fumaric acid and succinic acid, and detect some
other preeclampsia-related marker genes/proteins. Additionally, in
the present study there were no significant differences for all
results between the three treatment groups. This may be due to
dosage or other reasons. In the DMF + succinic acid group, the
doses of the two drugs were halved, respectively. The reasons
underlying the non-significant differences between the three
treatment groups is uncertain and requires further experiments to
clarify; therefore this will be an aim of future studies.
In conclusion, fumaric acid and succinic acid may
treat gestational hypertension by downregulating the expression of
KCNMB1 and TET1. Although this requires further confirmation by
larger-scale experiments and clinical trials, these results may
offer interesting and effective knowledge for the development of
drugs to treat gestational hypertension.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Guizhou
Provincial Science and Technology Foundation [grant no.
QianKeHeLHZi(2016)7510].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and DD designed the study, analyzed the data and
wrote the paper. YZ, FZ, HJ, DX and DD performed the study and
collected data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Ethics Committee of the First Affiliated Hospital of Guiyang
University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang YA, Chughtai AA, Farquhar CM, Pollock
W, Lui K and Sullivan EA: Increased incidence of gestational
hypertension and preeclampsia after assisted reproductive
technology treatment. Fertil Steril. 105:920–926.e2.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Egeland GM, Klungsøyr K, Øyen N, Tell GS,
Næss Ø and Skjærven R: Preconception cardiovascular risk factor
differences between gestational hypertension and preeclampsia:
Cohort Norway study. Hypertension. 67:1173–1180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hua X, Zhang J, Guo Y, Shen M, Gaudet L,
Janoudi G, Walker M and Wen SW: Effect of folic acid
supplementation during pregnancy on gestational
hypertension/preeclampsia: A systematic review and meta-analysis.
Hypertens Pregnancy. 35:447–460. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hromadnikova I, Kotlabova K, Hympanova L
and Krofta L: Gestational hypertension, preeclampsia and
intrauterine growth restriction induce dysregulation of
cardiovascular and cerebrovascular disease associated microRNAs in
maternal whole peripheral blood. Thromb Res. 137:126–140.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ripley TL and Saseen JJ: β-blockers: A
review of their pharmacological and physiological diversity in
hypertension. Ann Pharmacother. 48:723–733. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hu XQ, Dasgupta C, Chen M, Xiao D, Huang
X, Han L, Yang S, Xu Z and Zhang L: Pregnancy reprograms
large-conductance Ca2+-activated K+ channel
in uterine arteries: Roles of ten-eleven translocation
methylcytosine dioxygenase 1-mediated active demethylation.
Hypertension. 69:1181–1191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han YY, Wang LJ, Zhang L, Zhang WW, Ma KT,
Li L and Si JQ: Association between potassium channel SNPs and
essential hypertension in Xinjiang Kazak Chinese patients. Exp Ther
Med. 14:1999–2006. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Laukka T, Mariani CJ, Ihantola T, Cao JZ,
Hokkanen J, Kaelin WG Jr, Godley LA and Koivunen P: Fumarate and
succinate regulate expression of hypoxia-inducible genes via TET
enzymes. J Biol Chem. 291:4256–4265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wiehle L, Raddatz G, Musch T, Dawlaty MM,
Jaenisch R, Lyko F and Breiling A: Tet1 and Tet2 protect DNA
methylation canyons against hypermethylation. Mol Cell Biol.
36:452–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choudhury SR, Cui Y, Lubecka K, Stefanska
B and Irudayaraj J: CRISPR-dCas9 mediated TET1 targeting for
selective DNA demethylation at BRCA1 promoter. Oncotarget.
7:46545–46556. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thomson JP, Ottaviano R, Unterberger EB,
Lempiäinen H, Muller A, Terranova R, Illingworth RS, Webb S, Kerr
AR, Lyall MJ, et al: Loss of Tet1 associated
5-hydroxymethylcytosine is concomitant with aberrant promoter
hypermethylation in liver cancer. Cancer Res. 76:3097–3108.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Somineni HK, Zhang X, Biagini Myers JM,
Kovacic MB, Ulm A, Jurcak N, Ryan PH, Khurana Hershey GK and Ji H:
Ten-eleven translocation 1 (TET1) methylation is associated with
childhood asthma and traffic-related air pollution. J Allergy Clin
Immunol. 137:797–805.e5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu X, Girardo D, Govek EE, John K, Mellén
M, Tamayo P, Mesirov JP and Hatten ME: Role of Tet1/3 genes and
chromatin remodeling genes in cerebellar circuit formation. Neuron.
89:100–112. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pei YF, Tao R, Li JF, Su LP, Yu BQ, Wu XY,
Yan M, Gu QL, Zhu ZG and Liu BY: TET1 inhibits gastric cancer
growth and metastasis by PTEN demethylation and re-expression.
Oncotarget. 7:31322–31335. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Du C, Zheng Z, Li D, Chen L, Li N, Yi X,
Yang Y, Guo F, Liu W, Xie X, et al: BKCa promotes growth and
metastasis of prostate cancer through facilitating the coupling
between αvβ3 integrin and FAK. Oncotarget. 7:40174–40188.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Friedman D, Kannan K, Faustin A, Shroff S,
Thomas C, Heguy A, Serrano J, Snuderl M and Devinsky O: Cardiac
arrhythmia and neuroexcitability gene variants in resected brain
tissue from patients with sudden unexpected death in epilepsy
(SUDEP). NPJ Genom Med. 3(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Díaz-Villamarín X, Dávila-Fajardo CL,
Gónzalez-Medina MD, Soto-Pino MJ, Sánche-Gómez E, Acuña A,
Gómez-Martín A, Martínez-González LJ, Casas-Hidalgo I and
Cabeza-Barrera J: PKP-022 The KCNMB1 (A >G) (RS703505) genetic
variant and the efficacy of tocilizumab in rheumatoid arthritis
patients. Eur J Hosp Pharm Sci Pract. 23 (Suppl 1)(A188)2016.
|
|
18
|
Hu XQ, Chen M, Dasgupta C, Xiao D, Huang
X, Yang S and Zhang L: Chronic hypoxia upregulates DNA
methyltransferase and represses large conductance
Ca2+-activated K+ channel function in ovine
uterine arteries. Biol Reprod. 96:424–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jin S, Teng X, Xiao L, Xue H, Guo Q, Duan
X, Chen Y and Wu Y: Hydrogen sulfide ameliorated L-NAME-induced
hypertensive heart disease by the Akt/eNOS/NO pathway. Exp Biol Med
(Maywood). 242:1831–1841. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bogodvid TK, Andrianov VV, Muranova LN and
Gainutdinov KL: Influence of nonspecific inhibitor of NO-synthase
L-NAME on electric characteristics of premotor interneurons of
terrestrial snails. Bionanoscience. 8:884–887. 2018.
|
|
22
|
Godbole SS, Kaul R, D'Souza SF and
Nadkarni GB: Immobilization of fumarase by entrapment of rat liver
mitochondria in polyacrylamide gel using gamma rays. Biotechnol
Bioeng. 25:217–224. 1983.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kirchgessner M, Roth FX, Tschierschwitz A
and Grassmann E: Nutritive effect of fumaric acid on growth and
body composition of rats. Z Tierphysiol Tierernähr Futtermittelkd.
47:175–186. 1982.PubMed/NCBI(In German).
|
|
24
|
Kang J, Lienhard M, Pastor WA, Chawla A,
Novotny M, Tsagaratou A, Lasken RS, Thompson EC, Surani MA, Koralov
SB, et al: Simultaneous deletion of the methylcytosine oxidases
Tet1 and Tet3 increases transcriptome variability in early
embryogenesis. Proc Natl Acad Sci USA. 112:E4236–E4245.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang GX, Fu HJ, Liu TW, Li JL, Shen Y and
Qian J: Effects of atorvastatin on VSMC apoptosis in rabbits with
atherosclerosis and its molecular mechanism. Chin J Arterioscler.
25:463–466. 2017.
|
|
26
|
Wang LJ, Zhang WW, Qian YF, Zhang L, Zhao
L, Ma KT, Li L and Si JQ: Association between KCNMB1 polymorphism
and essential hypertension in Xinjiang Kazak population. J Xian
Jiaotong Univ. 37:78–81. 2016.
|