Introduction
Pancreatic cancer (PC) is a digestive system
malignancy and the most common pathologic type is pancreatic ductal
adenocarcinoma (PDAC). In 2018, an estimated 458,918 patients were
newly diagnosed with PDAC worldwide, accounting for ~2.5% of the
total new cases in all cancer types, and ~432,242 PDAC-associated
mortalities occurred, accounting for ~4.5% of the total
cancer-associated deaths (1,2).
Furthermore, PC is often diagnosed at an advanced stage and the
only treatment available is palliative care, such as chemotherapy
and radiotherapy (3). PC is not
sensitive to palliative treatment during the late stage in the
majority of cases (3). Complete
surgical resection is the only effective method for the treatment
of PDAC (4). However, only 15-20%
of patients with PDAC may have the opportunity to undergo curative
surgical tumor resection, while the remaining patients can only
receive adjuvant therapies. In this case, adjuvant therapies refer
to chemotherapy or radiation therapy alone, as extensive metastasis
and advanced tumor stage means surgery is no longer an option
(5).
Gemcitabine is a first-line chemotherapy drug
approved by the Food and Drug Administration for PDAC (6,7). The
development of gemcitabine resistance during chemotherapy affects
the prognosis of advanced PDAC and has become an increasingly
common phenomenon (8). Although
PDAC is not sensitive to chemotherapy, gemcitabine may still
alleviate the symptoms of patients with PDAC and prolong their
survival time (9). However, it has
been reported that PDAC is gradually becoming resistant to
gemcitabine and its efficacy declines (10,11).
As patients with advanced PDAC cannot be treated with surgery, they
may only be treated with gemcitabine chemotherapy; however, a
considerable number of patients develop resistance. Therefore, it
is important to predict whether patients will develop gemcitabine
resistance through certain methods.
Circulating tumor cells (CTCs) may be used as a
predictor of advanced metastasis of malignant tumors (12). Previous studies have indicated that
the amount of CTCs in the blood may predict the prognosis of breast
cancer and PC (13-15).
PC subtypes with CTCs are more aggressive and metastatic (16,17).
In addition, an increase in the number of CTCs in the portal vein
blood may reduce the survival of patients with PDAC (18,19).
CTCs have been determined to be an independent prognosticfactor of
PDAC; however, the relationship between CTCs and gemcitabine
resistance has been rarely studied (20). In the present study, it was
hypothesized that CTCs may be used as an independent prognostic
factor for patients with PDAC and to be related to gemcitabine
resistance in patients with advanced PC. The immunomagnetic
microsphere used for sorting CTC in this study were EpCAM (12). The purpose of the present study was
to test the above hypothesis by detecting changes in CTCs during
PDAC treatment.
Materials and methods
Patients and clinical samples
The clinicopathological factors and blood samples in
the present study were obtained from 87 patients with advanced PDAC
who underwent chemotherapy with gemcitabine between June 2013 and
June 2017 at the Second Affiliated Hospital of Jiaxing University
(Jiaxing, China). The patient inclusion criteria were as follows:
i) Accurate pathological diagnosis of primary PDAC; ii) complete
clinicopathological and follow-up data; iii) lack of opportunity of
surgery and tumor-node-metastasis (TNM) stage of III or IV. The
patient exclusion criteria were as follows: i) The patient had
undergone surgery and chemotherapy prior to admission; ii) the
diagnosis was not clear; iii) presence of more than two primary
tumors.
All patients were tested with serum tumor markers
prior to chemotherapy, such as carcinoembryonic antigen (CEA) and
carbohydrate antigen 199 (CA199). Patients were classified and
staged based on the TNM classification for PDAC established by the
Union for International Cancer Control (21). The clinicopathological data are
provided in Table I. The present
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Jiaxing University (Jiaxing, China) and performed in
accordance with the Declaration of Helsinki. Informed consent was
obtained from each participant. All patients had advanced PC and no
chance of surgery. Endoscopic ultrasound-guided fine-needle
aspiration was used for pathological examination and 5 patients had
squamous cell carcinoma. CT and MRI were used to examine vascular
invasion.
 | Table ICharacteristics of patients with
advanced pancreatic cancer (n=87). |
Table I
Characteristics of patients with
advanced pancreatic cancer (n=87).
| Parameter | Value |
|---|
| Age, years | 61.8 (45-86) |
| Sex
(male/female) | 50/37 |
| Pathological type
(adenocarcinoma/squamous cell carcinoma) | 79/8 |
| Serum CA199, U/l
(positive/negative) | 52/35 |
| Serum CEA, U/l
(positive/negative) | 24/53 |
| TNM stage
(III/IV) | 40/47 |
| Tumor location
(head and neck/tail) | 65/22 |
| Tumor size, cm | 3.9 (2-7) |
Chemotherapy and clinical
evaluation
All 87 patients were treated with gemcitabine as a
first-line chemotherapy drug. Each patient was treated with
gemcitabine 1,000 mg/m2 administered as an intravenous
drip for 30 min. This medication was given on the 1st, 8th and 15th
days of the course of treatment with a rest period for 14 days and
every course of treatment lasted 28 days. This was repeated until
progression or adverse reactions of chemotherapy became
intolerable. All 87 patients received chemotherapy for >8 weeks.
As the evaluation standard for adverse reactions, the grading
standard (CTCAE3.0) established by the National Cancer Center of
the United States was used (22).
Progression-free survival (PFS) was defined as the time from the
date of the diagnosis to the date of progression or death. Overall
survival (OS) was defined as the time from the date of the
diagnosis to the date of death or the last follow-up
examination.
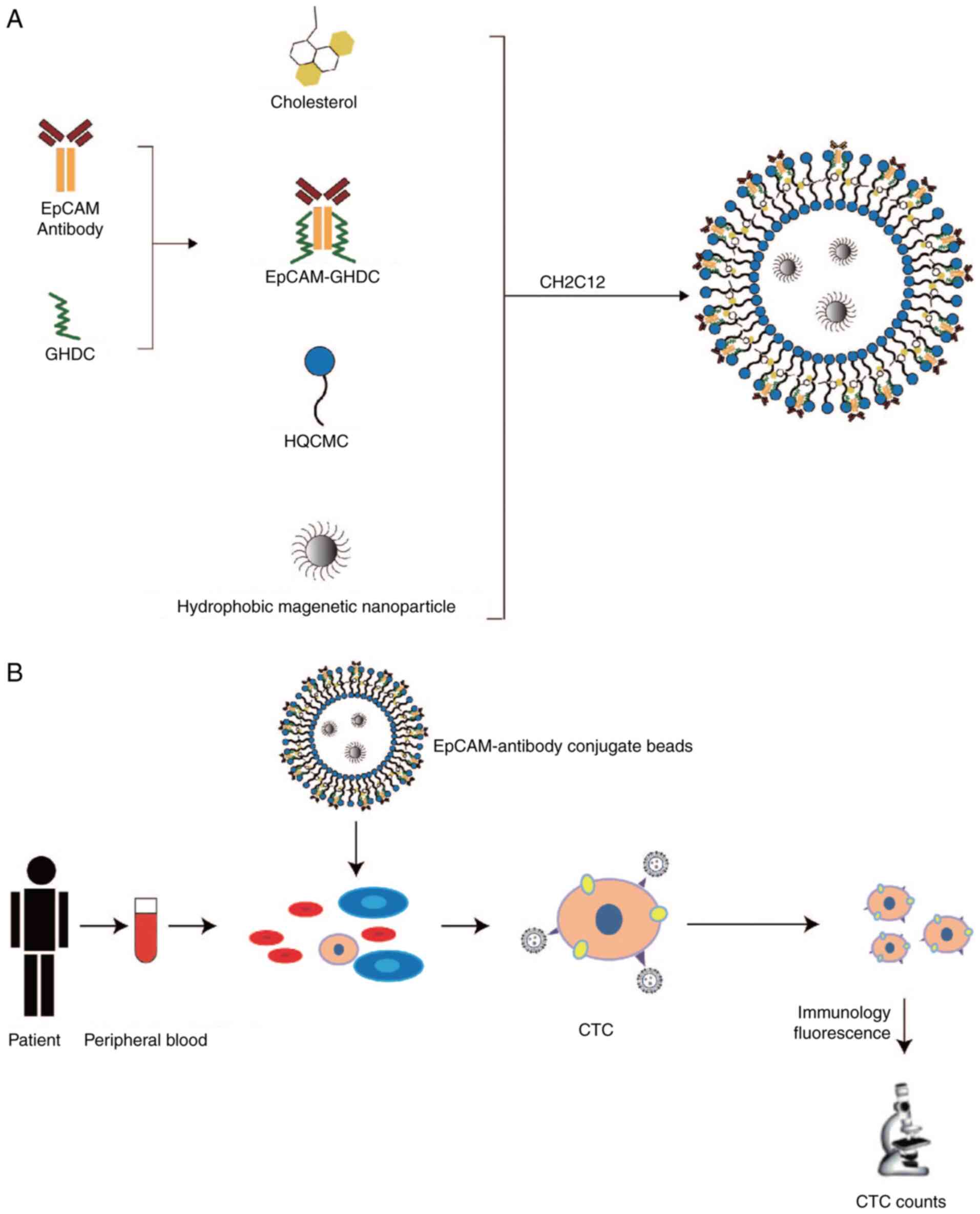
Preparation of immunomagnetic
microspheres
To perform modification of epithelial cell adhesion
molecule (EpCAM) by glycidylhexadecylamine (GHDC), 57.1 µg of EpCAM
(cat. no. ab223582; Abcam), dissolved 100 µg of GHDC (cat. no.
30-CG-049; Huzhou Liyuan Medical Laboratory Co., Ltd.) and 3.0 ml
PBS (pH 7.4) was reacted with magnetic stirring at 4˚C overnight.
The molecules to be retained in the dialysis bag with a molecular
weight of 8,000-14,000 Da were used the next day for 12 h and the
buffer (PBS) was changed every 2 h. The antibody derivative
EpCAM-GHDC obtained by dialysis and lyophilization was weighed.
GHDC, immunomagnetic microspheres and dialysis bags were purchased
from Huzhou Liyuan Medical Laboratory Co., Ltd. The preparation
procedure of EpCAM immunomagnetic microspheres is displayed in
Fig. 1A. During the preparation of
immunomagnetic microspheres, GHDC was able to interact with
transferrin antibodies. Hexadecyl-quaternized (carboxymethyl)
chitosans (cat. no. 30-CG-050; Huzhou Liyuan Medical Laboratory
Co., Ltd.) increased the grafting quantities of magnetic
microspheres and antibodies through reactive groups and exerted
emulsification, dispersion and surface activation functions
(23,24).
Characterization of EpCAM magnetic
spheres
The size distribution and zeta potential of the
EpCAM magnetic spheres were measured by a Zetasizer (Nano-ZS 90;
Malvern Instruments, Ltd.). The molecular morphology of folic acid
magnetic spheres was determined via atomic force microscopy
(BioScope SPM; Bruker Corporation). The magnetic properties of
EpCAM magnetic spheres were measured via a vibrating sample
magnetometer (Model 7407; Lake Shore Cryotronics, Inc.) and the
ultraviolet absorption spectrum of EpCAM magnetic spheres was
measured using a UV-2501PC UV-Vis spectrophotometer (Shimadzu
Corporation).
CTC detection
The CTC detection procedure for PC is displayed in
Fig. 1B. EpCAM immunomagnetic
microspheres, prepared according to Fig. 1A, were added to the peripheral
blood of patients with PDAC and the captured CTCs were identified
and counted by immunofluorescence. Peripheral blood (4 ml) from 87
patients with advanced PDAC was collected in anticoagulation tubes
prior to any treatment, and subsequently, 14 ml of red blood cell
lysate from the same patient was added within 2 h. The sample was
mixed gently by pipetting, placed in a refrigerator at 4˚C for 15
min and then centrifuged at 111 x g for 5 min at 4˚C. The
supernatant was discarded and to the cell pellet, 10 ml buffer [PBS
500 ml + EDTA 0.375 g + BSA (Thermo Fisher Scientific, Inc.) 2.5 g]
was added using the Nextctc FS100 Nano microfluidic chip (Wuxi Nao
Biomedical Co., Ltd.). Obtained CTCs were evenly poured onto the
slide. Sections were fixed with 2% paraformaldehyde for 10 min at
room temperature and 0.25% Triton X-100 for 10 min. Subsequently,
sections were washed with PBS for 15 min (three times for 5 min),
and incubated with 2% BSA for 30 min at room temperature to block
nonspecific binding. The sample was then incubated for 20 min at
room temperature with EpCAM antibody (1:200 dilution; cat. no.
ab223582; Abcam), cytokeratin (CK)-18 antibody (1:200 dilution;
cat. no. ab181597; Abcam) or CD45 antibody (1:200 dilution; cat.
no. SAB4502541; Sigma-Aldrich; Merck KGaA). Subsequently, the
samples were rinsed with PBS for 15 min (3 times for 5 min). The
sections were incubated with DyLight 488-conjugated donkey
anti-mouse IgG (H+L) (1:200 dilution; cat. no. ab150105; Abcam) and
anti-rabbit IgG (1:200 dilution; cat. no. ab150073; Abcam) for 2 h
at room temperature. Subsequently, the sections were washed with
PBS for 15 min, mounted with mounting medium and examined under a
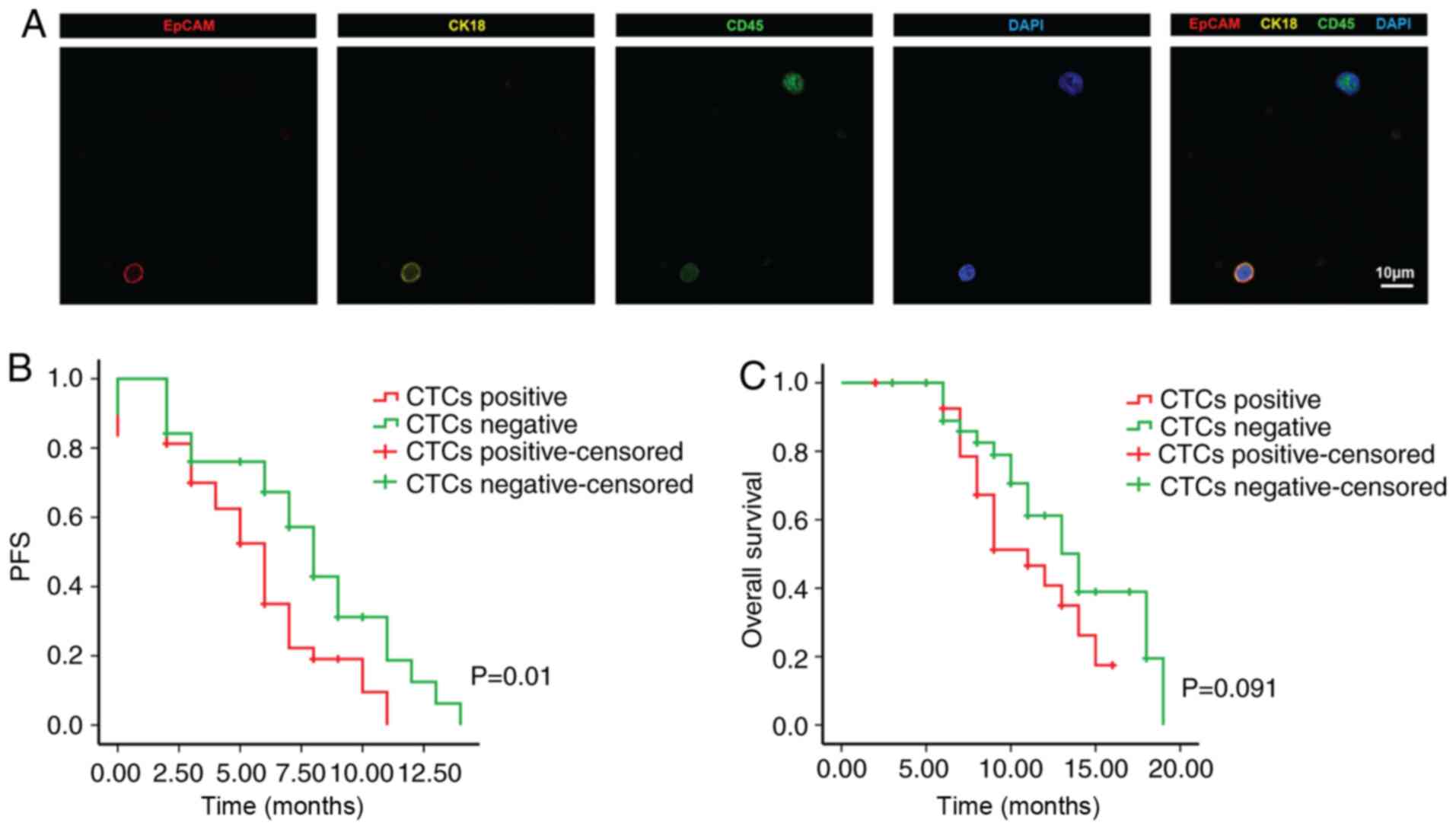
fluorescence microscope (Olympus BX51; Olympus Corporation). CTCs
were characterized by lacking CD45 expression and expressing EpCAM.
CK immunocytofluorescence staining was also assessed on detected
CTCs.
PFS follow-up
The 87 patients with advanced PDAC were followed up.
The deadline for follow-up was June 1, 2017. Patients who had
regular follow-up visits were followed up by outpatient clinics,
while those who were not able to be followed up on time were
followed up by telephone. The PFS rate was defined as the
proportion of patients with no disease progression from enrollment
to follow-up. The patients were divided into CTC-positive (≥1 cell)
and CTC-negative groups according to their circulating immune cell
test results.
Statistical analysis
SPSS statistical analysis software 22.0 (IBM Corp.)
was used for statistical analysis. The χ2 test and
t-test were utilized to determine the association between the
presence of CTCs with chemotherapy adverse reactions,
chemotherapeutic efficacy of gemcitabine and different clinical
characteristics (if the sample size was <40 or the minimum
theoretical frequency was <1, Fisher's exact test was used). For
the OS and PFS analysis, Kaplan-Meier curves were drawn and the
log-rank test was applied to compare differences between
CTC-positive and CTC-negative patients. Univariate analysis of the
relationship between CTCs and clinicopathological characteristics
was performed by the χ2 test/t-test. Multivariate
analysis was conducted with multivariate logistic regression using
the Cox proportional hazards model for analysis. P<0.05 was
considered to indicate statistical significance.
Results
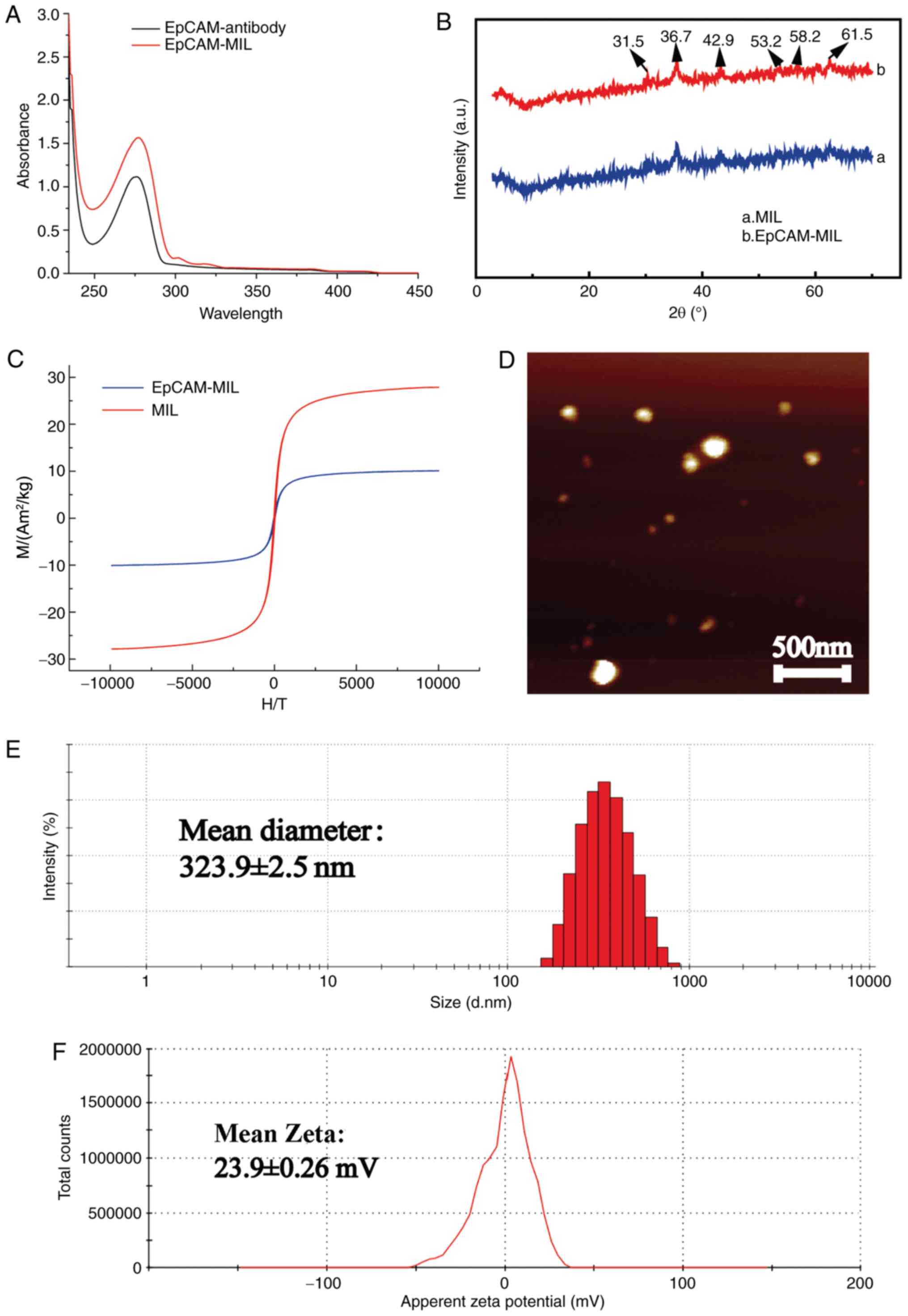
Characterization and performance
evaluation of immunomagnetic microspheres
In order to examine the performance of the
constructed CTC capture system, a series of functional tests were
used to analyze the characteristics of the magnetic beads
constructed. The UV spectrum proved that the EpCAM antibody had a
broad absorption peak at 278 nm (Fig.
2A). For EpCAM-magnetic beads (MIL), an absorption peak was
present at 281 nm. This indicated that EpCAM was indeed attached to
the surface of the magnetic spheres. The UV spectrum of EpCAM-MIL
had diffraction peaks at 31.5, 36.7, 42.9, 53.2, 58.2 and 61.5˚,
respectively, which corresponded to the (219), (312), (401), (420),
(509) and (439) crystal plane structures of
Fe3O4, respectively (Fig. 2B). The above results indicated that
the magnetic beads were composed of Fe3O4 and
that EpCAM was successfully attached to the surface of the magnetic
beads. The immunomagnetic beads exhibited the crystal
characteristics of magnetic nanoparticles. The magnetization curve
indicated that the Fe3O4-MIL and EpCAM-MIL
had no hysteresis at room temperature, but were superparamagnetic,
and the magnetizing curve was displayed at 302 Kelvin. In addition,
these results also proved that the saturation magnetization of the
MIL was 27.75 Am2/kg and the synthetic saturated
magnetization of EpCAM-MIL was 10.03 Am2/kg, indicating
the saturation magnetization of the magnetic beads on the surface
of the antibody and protein coating was immunomagnetic (Fig. 2C). The atomic force microscopy
image of EpCAM-MIL illustrated that the size of the EpCAM-MIL was
spherical and no agglomeration was present, which indicated that
the microspheres had good stability and shape (Fig. 2D). As presented in Fig. 2E, the particle size test results of
EpCAM-MIL suggested that the size of the spheres was ~400 nm and
the average particle size was 323.9 nm, which indicated
liposome-like vesicle properties. At the same time, the zeta
potential analysis results of EpCAM-MIL suggested that the zeta
potential was +23.9 mV (Fig. 2F).
In summary, the magnetic beads prepared in the present study have
smaller particle size and higher stability than those described in
a in previous study (25).
Detection results of CTCs and efficacy
of chemotherapy
The present study included 87 patients with PDAC
treated between June 2013 and June 2017. As indicated in Table I, the age ranged between 45 and 86
years (mean age, 61.8 years), the cohort contained 50 males and 37
females, 22 tumors were located in the tail of the pancreas and 65
cases were located in the head and neck of the pancreas. Among
them, 49 patients (56%, 49/87) had one or more CTCs/4 ml blood
detected, with CTC numbers ranging from 2 to 298 (mean ± standard
deviation, 109.2±71.6) (Fig. 3A).
The Kaplan-Meier plots for PFS and OS for patients with PC were
drawn and it was indicated that CTC positivity was associated with
poor PFS compared with CTC negativity (P=0.01). However, the OS
rate of CTC-positive patients was not significantly different from
that of CTC-negative patients (P=0.091; Fig. 3B and C).
Relationship between CTCs and
clinicopathological characteristics of patients with advanced
PC
The relationship between CTCs and the
clinicopathological characteristics of 87 patients with advanced
PDAC was analyzed by the χ2 test and presented in
Table II. Univariate analyses
demonstrated that CTCs were closely associated with vascular
invasion (P<0.001), TNM stage (P=0.005) and liver metastasis
(P=0.005). However, there was no significant difference between
CTCs and other clinical parameters, including age, sex, symptoms,
tumor size, tumor location, pathological type, lymph node
metastasis, neurological invasion, CA199 and CEA. Multivariate
regression analysis indicated that vascular invasion (P<0.001)
and liver metastasis (P=0.002) were independent predictors of
CTCs.
 | Table IIAssociation between CTC status and
clinicopathological characteristics of patients with pancreatic
cancer. |
Table II
Association between CTC status and
clinicopathological characteristics of patients with pancreatic
cancer.
| | Peripheral blood
CTCs | | Multivariate
analysis |
|---|
| Clinical
characteristic | Positive
(n=49) | Negative
(n=38) | Univariate analysis
P-value | OR (95% CI) | P-value |
|---|
| Sex | | | 0.612 | | |
|
Male | 27 | 23 | | | |
|
Female | 22 | 15 | | | |
| Age, years | 61.29±9.00 | 62.61±8.57 | 0.491 | | |
| Symptoms | | | 0.069 | | |
|
Present | 25 | 12 | | | |
|
Absent | 24 | 26 | | | |
| Tumor location | | | 0.489 | | |
|
Head and
neck | 38 | 27 | | | |
|
Tail | 11 | 11 | | | |
| Tumor size, cm | 4.00
(3.00,5.00) | 3.00
(3.00,4.00) | 0.203 | | |
| Pathological
type | | | 1.000a | | |
|
Adenocarcinoma | 44 | 35 | | | |
|
Squamous
cell carcinoma | 5 | 3 | | | |
| Lymph node
metastasis | | | 0.163 | | |
|
Present | 16 | 18 | | | |
|
Absent | 33 | 20 | | | |
| Vascular
invasion | | | <0.001 | Reference 57.321
(7.138-460.297) | <0.001 |
|
Present | 37 | 9 | | | |
|
Absent | 12 | 29 | | | |
| Neurological
invasion | | | 0.093 | | |
|
Present | 32 | 18 | | | |
|
Absent | 17 | 20 | | | |
| TNM stage | | | 0.005 | Reference 2.202
(0.411-11.804) | 0.357 |
|
III | 16 | 24 | | | |
|
IV | 33 | 14 | | | |
| Liver
metastasis | | | 0.005 | Reference 27.285
(3.380-220.272) | 0.002 |
|
Present | 29 | 11 | | | |
|
Absent | 20 | 27 | | | |
| CA199 | | | 0.450 | | |
|
Normal | 18 | 17 | | | |
|
Elevated | 31 | 21 | | | |
| CEA | | | 0.463 | | |
|
Normal | 37 | 26 | | | |
|
Elevated | 12 | 12 | | | |
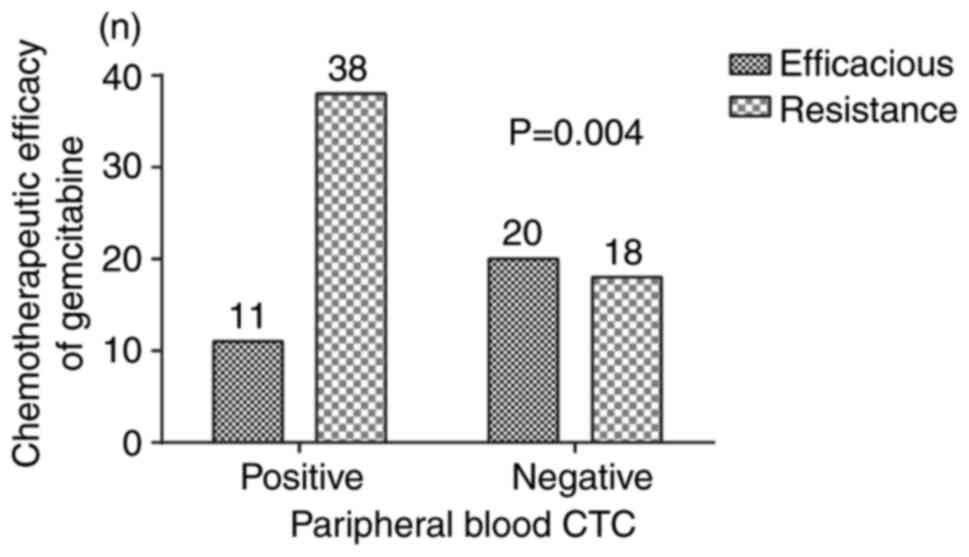
Relationship between chemotherapy
effect and CTCs
According to the analysis, 77.5% of the CTC-positive
patients were resistant to gemcitabine, while 47.4% of CTC-negative
patients developed resistance to gemcitabine. The detailed data for
the association between peripheral blood CTC and chemotherapeutic
efficacy of gemcitabine are displayed in Fig. 4, which indicated that the efficacy
of gemcitabine was also affected by CTCs (χ2=8.501,
P=0.004). The sensitivity and specificity of CTC detection for
gemcitabine resistance was calculated as follows: Sensitivity,
67.86% and specificity, 64.52%. However, there was no significant
association between the chemotherapy effect and other clinical
parameters, including age, sex, symptoms, tumor size, tumor
location, CA-199, CEA, liver metastasis and TNM stage (Table III).
 | Table IIIAssociation between efficacy of
chemotherapy and CTCs. |
Table III
Association between efficacy of
chemotherapy and CTCs.
| | Chemotherapeutic
efficacy of gemcitabine | |
|---|
| Clinical
characteristic | Efficacious
(n=31) | Resistance
(n=56) | t or
χ2 | P-value |
|---|
| Sex | | | 0.287 | 0.592 |
|
Male | 19 | 31 | | |
|
Female | 12 | 25 | | |
| Age, years | 65.83±10.94 | 68.64±10.88 | -1.007 | 0.318 |
| Symptoms | | | 0.007 | 0.934 |
|
Present | 13 | 24 | | |
|
Absent | 18 | 32 | | |
| Tumor location | | | 0.187 | 0.666 |
|
Head and
neck | 24 | 41 | | |
|
Tail | 7 | 15 | | |
| Tumor size, cm | 3.45±1.36 | 4.31±1.78 | -1.158 | 0.251 |
| CA-199, U/l | | | 0.001 | 0.974 |
|
≥37 | 21 | 31 | | |
|
<37 | 10 | 25 | | |
| CEA, U/l | | | 0.05 | 0.822 |
|
≥5 | 9 | 15 | | |
|
<5 | 22 | 41 | | |
| Liver
metastasis | | | 2.653 | 0.103 |
|
Present | 17 | 23 | | |
|
Absent | 11 | 32 | | |
| TNM stage | | | 1.024 | 0.312 |
|
III | 12 | 28 | | |
|
IV | 19 | 28 | | |
| CTC status | | | 8.501 | 0.004 |
|
Positive | 11 | 38 | | |
|
Negative | 20 | 18 | | |
Relationship between chemotherapy
adverse reactions and CTCs
All 87 patients with advanced PDAC were able to
tolerate adverse reactions to gemcitabine chemotherapy and no
chemotherapy-related death occurred. The major adverse reactions
were digestive tract reactions, myelosuppression and flu-like
symptoms. The incidence of thrombocytopenia in the CTC-negative and
-positive groups was 57.8 and 18.4%, respectively, and that in the
CTC-negative group was significantly higher than that in
the-positive group (χ2=14.58, P<0.001), but other
adverse reactions, including digestive tract reactions,
myelosuppression, anemia, liver damage and flu-like symptoms were
not associated with CTCs (Table
IV).
 | Table IVAssociation between adverse reactions
to chemotherapy and CTCs. |
Table IV
Association between adverse reactions
to chemotherapy and CTCs.
| | CTC status | |
|---|
| Chemotherapy
adverse reaction | Positive
(n=49) | Negative
(n=38) | t or
χ2 | P-value |
|---|
| Nausea, emesis | 27 | 17 | 0.920 | 0.338 |
| Diarrhea | 5 | 3 | 0.000 | 1.000a |
| Leukocytopenia | 23 | 17 | 0.042 | 0.838 |
|
Thrombocytopenia | 9 | 22 | 14.580 | <0.001 |
| Anemia | 9 | 6 | 0.100 | 0.752 |
| Hepatic function
damage | 8 | 7 | 0.066 | 0.798 |
| Rash | 4 | 3 | 0.000 | 1.000a |
| Flu-like
symptoms | 27 | 18 | 0.513 | 0.474 |
Discussion
In the present study, it was confirmed that patients
with advanced PDAC with positive CTCs have poor prognosis and short
survival. Furthermore, CTC-positive patients with advanced PDAC had
a higher ratio of resistance to gemcitabine and lower efficacy of
chemotherapy. The use of CTC count statistics and related research
is of great significance for the dynamic monitoring of PDAC
clinical samples (26,27).
PDAC is the fourth leading cause of death worldwide.
The lack of early symptoms and screening usually results in late
diagnosis and poor prognosis. CTCs have been a promising novel
biomarker in solid tumors. Over the past two decades, >100
articles have been published on this topic. Most of the studies
evaluated the use of CTCs as a prognostic marker and its
association with the survival of patients with PDAC (28). Patients with advanced PDAC may
exhibit multiple complications associated with distant metastasis
(29,30). The present study indicated that the
positive rate of peripheral blood CTCs in 87 patients with advanced
PDAC was 56%. Han et al (17) combined nine articles in a
meta-analysis, revealing a CTC-positive rate of 43% in 623 patients
with PDAC. The meta-analysis suggested that CTC-positive patients
with pancreatic cancer exhibited worse levels of PFS and OS,
compared with CTC-negative patients (17). Of note, the CTC data of patients
with metastatic PDAC using the CellSearch® system
indicated that the detection rate of CTCs is ~50% (18-20).
The higher CTC-positive rate in the present study was likely due to
the patients having advanced PDAC and the limited sample size. This
still indicated that the self-assembled lipid beads used had a good
CTC capture ratio.
Pancreatic adenocarcinoma has a moderate response to
gemcitabine-based chemotherapy, which is the most widely used
monotherapy for PDAC. Tadros et al (31) discovered a marked increase in
gemcitabine resistance in patients with pancreatic cancer following
the orlistat-induced inhibition of fatty acid biosynthesis. Using
the Cancer Genome Atlas dataset, Tadros et al indicated that
fatty acid biosynthetic pathway manipulation may help overcome the
stress and regulation of gemcitabine in PDAC (31). Furthermore, Shukla et al
(32) declared that targeting
HIF-1 cells or de novo pyrimidine biosynthesis, combined
with gemcitabine, may significantly reduce the tumor burden and
decrease the expression of transketolase and cytidine triphosphate
synthase 1. In addition, Mehla and Singh (33) revealed that a glycolytic subtype
indicates poor survival in patients with PDAC, whereas the
holesterogenic subtype correlates with more favorable outcomes,
potentially due to a higher energy expenditure.
The detection of CTCs may be of important clinical
value for the prognosis of PC. The purpose of the present study was
to evaluate the role of CTCs in recurrence, metastasis and
treatment efficacy by detecting the differences in CTCs between
patients with PDAC. Most previous studies have explored the
association between CTC detection and PC diagnosis. Both Earl et
al (34) and Liu et al
(35) reported that CTCs are a
promising marker for the management of patients with PDAC; however,
the correlation between CTCs and gemcitabine resistance in patients
with PDAC has remained largely elusive. The present study not only
confirmed that CTCs are a prognostic marker in patients with
advanced PC undergoing chemotherapy, but also that CTC-positive
patients with PC are more likely to develop gemcitabine resistance.
In the present study, all 87 patients with advanced PDAC received
gemcitabine monotherapy. Among them, 56 patients were resistant to
gemcitabine and the drug resistance rate was 64%. The resistance
rate of gemcitabine in CTC-positive patients with PDAC was as high
as 77.6% (28/39). Previous studies have indicated that the
resistance rate of patients with PDAC to gemcitabine is gradually
increasing, and the efficacy of gemcitabine is also reduced by
>20%, compared to the results established ~10 years prior
(5,6). The present clinical study confirmed
that positivity for CTCs prior to chemotherapy in patients with PC
indicates drug resistance, but the mechanisms have remained
elusive. Based on the combination of results of previous studies,
it may be hypothesized that epithelial to mesenchymal transition
(EMT) may be associated with changes in CTCs and gemcitabine
resistance (16,36). This is a process associated with
the separation of cancer cells from the primary tumor, which may
lead to CTCs that metastasize, contributing to cancer progression.
Thus, the number of CTCs can be used as an indicator for cancer
progression and its degree of malignancy. The further the cancer
has progressed, the worse the prognosis, and the poorer the
efficacy of gemcitabine. Although the expression of EpCAM was
detected and the relevant literature was reviewed, it was
determined that the mechanism of action underlying CTCs in
pancreatic cancer may be associated with EMT. In the present study,
the method used was not able to detect the expression of E-cadherin
and vimentin due to the use of peripheral blood primary cells of
patients for CTC detection. Furthermore, peripheral blood samples
cannot easily be stored for long periods of time, thus, relevant
substances in the blood are lost over time. This is also the
difficulty of CTC detection at present. Previous methods have also
failed to do this, for example, Xie et al (37) used an in vivo
CellCollector® method to detect the number of CTC in
patients. In the future, more convenient and sensitive testing
methods will be applied (37).
Previous studies have indicated that gemcitabine combined with
nab-paclitaxel chemotherapy may optimize the chemotherapy effect of
PDAC and prolong the survival time (38,39).
Therefore, improving the sensitivity of PDAC cells to gemcitabine
and combined chemotherapy may improve the chemotherapy effect and
prolong the survival time of patients. In the present study, the
median PFS was 8.0 months in CTC-positive patients compared to 7.0
months in CTC-negative patients. However, OS did not differ
significantly between CTC-positive and CTC-negative patients with
PDAC. In general, the median survival time of PDAC is low, but a
minority of patients with PDAC have undergone complete surgical
resection, so their survival time is particularly long (40). In those patients eligible for
surgery, the cancer was at an early stage without metastasis, and
the associated prognosis was improved. Furthermore, the present
study determined that patients with advanced PDAC with CTCs were
less likely to develop thrombocytopenia after receiving
gemcitabine, but the reason for this remains elusive.
The present study has certain limitations that are
worth mentioning. Due to the limited number of patients included,
the results of related studies should also be considered. The
present study is a retrospective study and the results obtained
require to be verified by larger prospective studies. In addition,
the patients of the present study were not monitored for CTCs after
treatment due to cost considerations. In subsequent studies, a
comparative study evaluating CTCs prior to and after treatment may
be performed.
In conclusion, CTC-positive patients with PC are
more likely to develop gemcitabine resistance, and these patients
have poor PFS and low incidence of thrombocytopenia. Thus, CTCs may
be considered as a prognostic marker for chemotherapy in patients
with advanced PC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical and
Health Science & Technology Planning Project of Zhejiang
Province (grant no. 2019KY219) and the Science and Technology
Planning Project of Jiaxing City (grant no. 2018AY32003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZZ was responsible for project design, obtaining
funding and resources, and conceptualization. XW was also
responsible for project conceptualization. FC was also responsible
for obtaining resources. XW, LH and XY were responsible for
drafting the manuscript. LH conceived and designed the CTC
extraction experiment, analyzed the experimental data, wrote the
results and discussion in the manuscript, and generated Figures 1 and 3, and Table
II. In addition, LH made a significant contrution to manuscript
revision. XY made substantial contributions to acquisition of data.
HX was responsible for data curation, statistical analysis and
experimentation. HY was responsible for carrying out the
experiments. HX, FC and JF were responsible for performing the
experiments. ZS made substantial contributions to analysis and
interpretation of data, and producing figures and tables of
analysis results. All authors confirm the authenticity of the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Jiaxing University (Jiaxing,
China). Informed consent was obtained from each study
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rothenberg ML, Moore MJ, Cripps MC,
Andersen JS, Portenoy RK, Burris HA III, Green MR, Tarassoff PG,
Brown TD, Casper ES, et al: A phase II trial of gemcitabine in
patients with 5-FU-refractory pancreas cancer. Ann Oncol.
7:347–353. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mao Y, Xi L, Li Q, Wang S, Cai Z, Zhang X
and Yu C: Combination of PI3K/Akt pathway inhibition and Plk1
depletion can enhance chemosensitivity to gemcitabine in pancreatic
carcinoma. Transl Oncol. 11:852–863. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Di Marco M, Di Cicilia R, Macchini M,
Nobili E, Vecchiarelli S, Brandi G and Biasco G: Metastatic
pancreatic cancer: Is gemcitabine still the best standard
treatment? (review). Oncol Rep. 23:1183–1192. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Loos M, Kleeff J, Friess H and Büchler MW:
Surgical treatment of pancreatic cancer. Ann N Y Acad Sci.
1138:169–180. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bidard FC, Pierga JY, Soria JC and Thiery
JP: Translating metastasis-related biomarkers to the
clinic-progress and pitfalls. Nat Rev Clin Oncol. 10:169–179.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thalgott M, Heck MM, Eiber M, Souvatzoglou
M, Hatzichristodoulou G, Kehl V, Krause BJ, Rack B, Retz M,
Gschwend JE, et al: Circulating tumor cells versus objective
response assessment predicting survival in metastatic
castration-resistant prostate cancer patients treated with
docetaxel chemotherapy. J Cancer Res Clin Oncol. 141:1457–1464.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giuliano M, Giordano A, Jackson S, De
Giorgi U, Mego M, Cohen EN, Gao H, Anfossi S, Handy BC, Ueno NT, et
al: Circulating tumor cells as early predictors of metastatic
spread in breast cancer patients with limited metastatic
dissemination. Breast Cancer Res. 16(440)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barriere G, Riouallon A, Renaudie J,
Tartary M and Rigaud M: Mesenchymal characterization: Alternative
to simple CTC detection in two clinical trials. Anticancer Res.
32:3363–3369. 2012.PubMed/NCBI
|
|
14
|
Arnoletti JP, Zhu X, Almodovar AJ,
Veldhuis PP, Sause R, Griffith E, Corpus G, Chang JC, Fanaian N and
Litherland SA: Portal venous blood circulation supports
immunosuppressive environment and pancreatic cancer circulating
tumor cell activation. Pancreas. 46:116–123. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95(e3407)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Effenberger KE, Schroeder C, Hanssen A,
Wolter S, Eulenburg C, Tachezy M, Gebauer F, Izbicki JR, Pantel K
and Bockhorn M: Improved risk stratification by circulating tumor
cell counts in pancreatic cancer. Clin Cancer Res. 24:2844–2850.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Han L, Chen W and Zhao Q: Prognostic value
of circulating tumor cells in patients with pancreatic cancer: A
meta-analysis. Tumour Biol. 35:2473–2480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kurihara T, Itoi T, Sofuni A, Itokawa F,
Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, et
al: Detection of circulating tumor cells in patients with
pancreatic cancer: A preliminary result. J Hepatobiliary Pancreat
Surg. 15:189–195. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dotan E, Alpaugh RK, Ruth K, Negin BP,
Denlinger CS, Hall MJ, Astsaturov I, McAleer C, Fittipaldi P,
Thrash-Bingham C, et al: Prognostic significance of MUC-1 in
circulating tumor cells in patients with metastatic pancreatic
adenocarcinoma. Pancreas. 45:1131–1135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ko AH, Scott J, Tempero MA and Park JW:
Detection and significance of circulating tumor cells (CTC) in
patients with metastatic pancreatic cancer (PC) receiving systemic
therapy. J Clin Oncol. 25 (Suppl 18)(S4596)2007.
|
|
21
|
Allen PJ, Kuk D, Castillo CF, Basturk O,
Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide
V, He J, et al: Multi-institutional validation study of the
American joint commission on cancer (8th edition) changes for T and
N staging in patients with pancreatic adenocarcinoma. Ann Surg.
265:185–191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liang X, Li X, Chang J, Duan Y and Li Z:
Properties and evaluation of quaternized chitosan/lipid cation
polymeric liposomes for cancer-targeted gene delivery. Langmuir.
29:8683–8693. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liang X, Tian H, Luo H, Wang H and Chang
J: Novel quaternized chitosan and polymeric micelles with
cross-linked ionic cores for prolonged release of minocycline. J
Biomater Sci Polym Ed. 20:115–131. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen J, Chen L, Du S, Wu J, Quan M, Yin H,
Wu Y, Ye X, Liang X and Jiang H: High sensitive detection of
circulating tumor cell by multimarker lipid magnetic nanoparticles
and clinical verifications. J Nanobiotechnology.
17(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Timme-Bronsert S, Bronsert P, Werner M,
Kulemann B and Höppner J: Circulating tumor cells in pancreatic
cancer: Results of morphological and molecular analyses and
comparisons with the primary tumor. Pathologe. 39 (Suppl
2):S311–S314. 2018.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
27
|
Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li
ZH and Liu YM: Molecular detection of epithelial-mesenchymal
transition markers in circulating tumor cells from pancreatic
cancer patients: Potential role in clinical practice. World J
Gastroenterol. 25:138–150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Martini V, Timme-Bronsert S,
Fichtner-Feigl S, Hoeppner J and Kulemann B: Circulating tumor
cells in pancreatic cancer: Current perspectives. Cancers (Basel).
11(1659)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ducreux M, Cuhna AS, Caramella C,
Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van
Laethem JL, Conroy T, et al: Cancer of the pancreas: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 26 (Suppl 5):v56–v68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shukla SK, Purohit V, Mehla K, Gunda V,
Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et
al: MUC1 and HIF-1alpha signaling crosstalk induces anabolic
glucose metabolism to impart gemcitabine resistance to pancreatic
cancer. Cancer Cell. 32:71–87.e7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mehla K and Singh PK: Metabolic subtyping
for novel personalized therapies against pancreatic cancer. Clin
Cancer Res. 26:6–8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Earl J, Garcia-Nieto S, Martinez-Avila JC,
Montans J, Sanjuanbenito A, Rodríguez-Garrote M, Lisa E, Mendía E,
Lobo E, Malats N, et al: Circulating tumor cells (CTC) and KRAS
mutant circulating free DNA (cfDNA) detection in peripheral blood
as biomarkers in patients diagnosed with exocrine pancreatic
cancer. BMC Cancer. 15(797)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu H, Sun B, Wang S, Liu C, Lu Y, Li D
and Liu X: Circulating tumor cells as a biomarker in pancreatic
ductal adenocarcinoma. Cell Physiol Biochem. 42:373–382.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
El Amrani M, Corfiotti F, Corvaisier M,
Vasseur R, Fulbert M, Skrzypczyk C, Deshorgues AC, Gnemmi V,
Tulasne D, Lahdaoui F, et al: Gemcitabine-induced
epithelial-mesenchymal transition-like changes sustain
chemoresistance of pancreatic cancer cells of mesenchymal-like
phenotype. Mol Carcinog. 58:1985–1997. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie N, Hu Z, Tian C, Xiao H, Liu L, Yang
X, Li J, Wu H, Lu J, Gao J, et al: In vivo detection of CTC and CTC
plakoglobin status helps predict prognosis in patients with
metastatic breast cancer. Pathol Oncol Res. 26:2435–2442.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Von Hoff DD, Ramanathan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kimura K, Amano R, Nakata B, Yamazoe S,
Hirata K, Murata A, Miura K, Nishio K, Hirakawa T, Ohira M and
Hirakawa K: Clinical and pathological features of five-year
survivors after pancreatectomy for pancreatic adenocarcinoma. World
J Surg Oncol. 12(360)2014.PubMed/NCBI View Article : Google Scholar
|