Introduction
Treatment of large segmental bone defects (LSBD)
remains a major challenge in orthopedics. Increasing evidence
suggests that tissue-engineered bones (TEB) seeded with osteogenic
cells, such as mesenchymal stem cells (MSCs), are promising
alternatives to conventional approaches, such as autologous or
allograft bone grafting for repairing LSBD (1-3).
However, TEB are not widely adopted in practice, due to a variety
of technical and logistical challenges and the subsequent high cost
caused by the need to maintain viability during manufacturing and
transportation. In addition, highly-trained technicians are
required, inhibiting large-scale production of TEB (4).
The role of MSCs in TEB has previously been
challenged; it was reported that MSCs in TEB were almost
undetectable 30 days after transplantation and, eventually, almost
all newly-formed bones originated from the host (5-7).
Another study suggested that MSCs helped create a favorable
microenvironment for tissue regeneration instead of contributing to
the cellular contents directly (8).
MSCs have been shown to secrete a variety of growth factors,
chemokines and osteogenic factors, such as C-C motif chemokine
ligand 5 (CXCL5), CXCL8 and stromal cell-derived factor 1 (SDF-1)
(9). These factors played critical
roles in modulating bone repair, including inflammation,
angiogenesis, bone formation and remodeling (10). The present study reported a novel
strategy, functional TEB (fTEB), to harness the benefit of these
factors in TEB by disengaging from viable cells. fTEB were obtained
by freeze-drying TEB fabricated with human umbilical cord MSCs
(hUC-MSCs) and demineralized bone matrix (DBM) scaffolds.
Conditioned media (CM) were prepared from fTEB and their effects on
the proliferation, differentiation and migration of host human bone
marrow MSCs (hBMSCs) were further assessed.
Materials and methods
Cell isolation and culture
hUC-MSCs and hBMSCs were isolated, cultured and
characterized, as previously described (11). All protocols involving human
subjects were approved by the Ethics Committee of the 960th
Hospital of PLA (Jinan, China; approval no. JNZY201603), with all
subjects providing informed consent. Between January and December
2016, hUC-MSCs were obtained from the umbilical cords of five
volunteers (25-35 years old) who underwent a full-term pregnancy
(38-40 weeks). hBMSCs were isolated from iliaccrest bone marrow
aspirates of five healthy volunteers. Exclusion criteria included
malignant tumor or accompanied systemic diseases, such as
rheumatoid arthritis, systematic lupus erythematosus and diabetes.
Cells derived from these sources were cultured in DMEM/F12
(dilution, 1:1; Cytiva) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/ml of penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). Cells at passage 3-4 were obtained
for use.
Preparation of fTEB
Allogenic DBM scaffolds (Beijing Datsing Bio-Tech
Co., Ltd.; Fig. 1A) with a volume
porosity of 76±8.5% (pore size, 200-800 µm) were cut into blocks
(1x1x0.5 cm; 0.417 g/block) and submerged in DMEM/F12 medium
overnight at 4˚C. TEB were fabricated by dropwise instillation of
an aliquot (20 µl) of single-cell suspension of hUC-MSCs (density,
1x107/ml) onto two opposite surfaces of the scaffolds.
After 2 h of incubation at room temperature, media were added and
replaced every 2 days. The seeding efficiency was assayed at 10 h
by calculating the ratio of the number of cells retained in the
scaffold to the number of cells seeded. The number of retained
cells in the scaffold was defined as the difference between the
cells seeded and the residual cells in the medium. hUC-MSCs
retained on the scaffolds were visualized on days 5 and 10, by
scanning electron microscopy (SEM; Hitachi, Ltd.). In addition, the
cytoskeletons and nuclei of hUC-MSCs were stained with 25 mg/ml
rhodamine-labelled phalloidin (Biotium, Inc.) for 1 h and 10 mg/ml
DAPI (Merck KGaA) for 15 min at room temperature, respectively.
Samples were then subjected to confocal laser scanning microscopy
(Leica Microsystems GmbH). To fabricate fTEB, TEB were washed three
times with PBS, immersed in PBS for 2 h and frozen at -80˚C for 2
h. Samples were then freeze-dried using a deep-hypothermic
lyophilizer (Modulyo®; Thermo Fisher Scientific, Inc.)
for 4 h and stored at -80˚C.
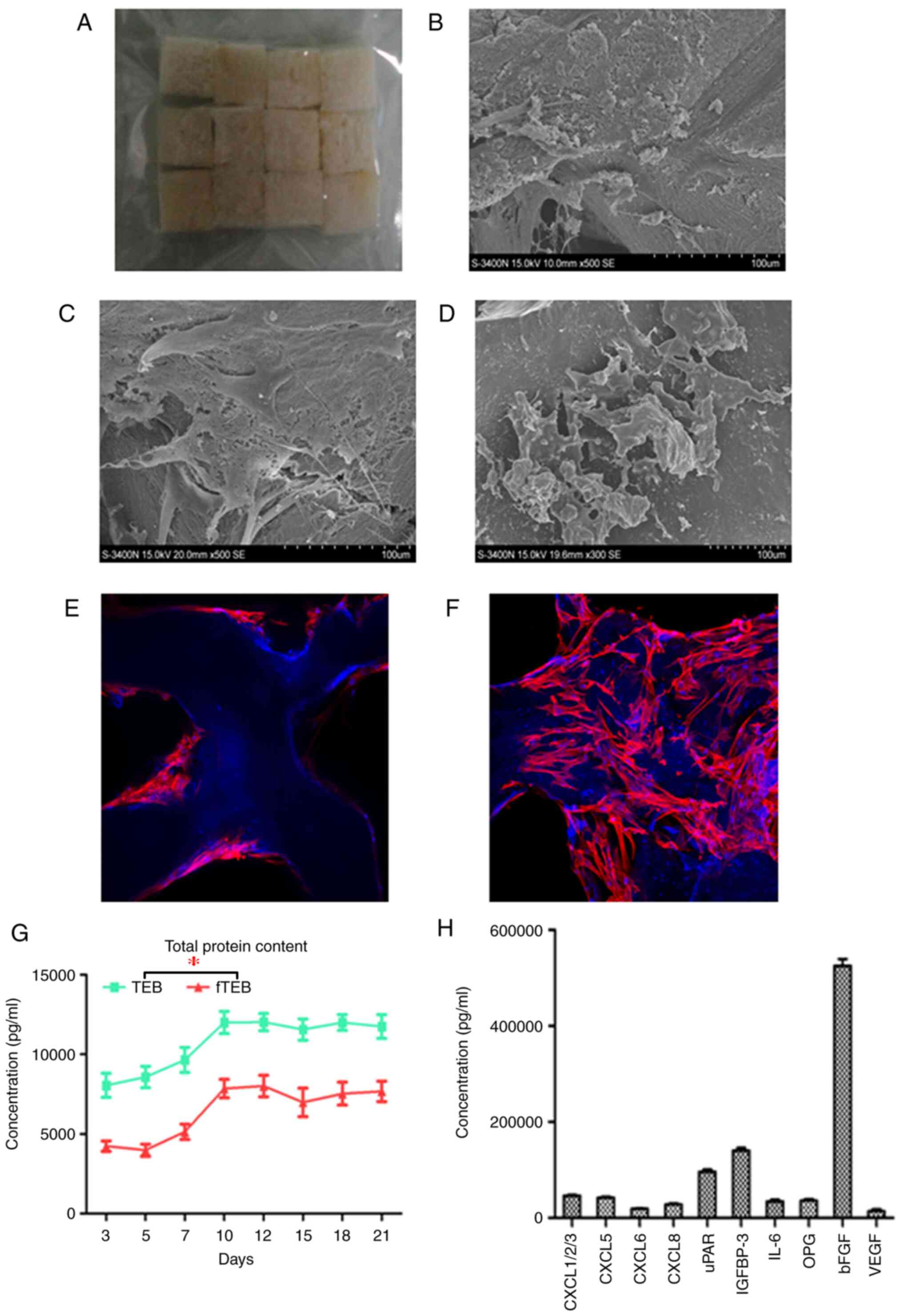 | Figure 1Adhesion and growth of cells on DBM
scaffolds. (A) Gross image of DBM scaffolds. (B) A total of 3 days
after seeding, cells adhered to the inner surfaces of the scaffolds
with dense extracellular matrix deposition (magnification x100).
(C) After 10 days of culture, cells were tightly interwoven and
stacked on top of one another (magnification x100). (D) In fTEB
obtained on day 10, the exposed cellular contents had been retained
at the pore surfaces. (E and F) Confocal microscopy further
confirmed that cells were tightly attached to the scaffolds after 3
and 10 days (magnification x100). (G) Total protein contents of TEB
and fTEB over 10 days. *P<0.001 (H) The top 10
cytokines identified in fTEB (>10 ng/ml). DBM, demineralized
bone matrix; TEB, tissue-engineered bones; fTEB, functional TEB;
CXCL, C-C motif chemokine ligand; uPAR, urokinase type plasminogen
activator receptor; IGFBP-3, insulin-like growth factor binding
protein 3; IL-6, interleukin 6; OPG, osteoprotegerin; bFGF, basic
fibroblast growth factor. |
Preparation of total protein content
and CM
In total, 1 cm3 fTEB were used for
protein extraction. Samples were homogenized in liquid nitrogen.
Total proteins were extracted using RIPA Buffer (Beyotime Institute
of Biotechnology) and protein concentrations were determined using
a BCA Protein Assay kit (Beyotime Institute of Biotechnology). CM
were prepared by dissolving total protein extracted from 1
cm3 fTEB in 10 ml DMEM/F12 complete media. CM of TEB and
DBM were also prepared using the same method.
Quantitative human cytokine array
A customized Quantibody® human cytokine
array (QAH-CUSTOM; RayBiotech, Inc.) was performed for 49
cytokines. The cytokines of interest are presented in Table SI. The fTEB-CM (100 µl) was assayed
according to the manufacturer's instructions. Fluorescent signals
were detected using a GenePix™ 4000B laser scanner (Molecular
Devices, LLC). Data were extracted and analyzed using RayBio
QAM-TH17-1software (RayBiotech, Inc.).
Migration assay
Migration assays were performed in Transwell inserts
(8-µm pores; Corning, Inc.). Briefly, 700 µl fTEB-CM was added to
the lower chamber. A total of 2x104 hBMSCs in 200 µl
serum-free medium (Cytiva) were seeded in the upper chamber and
allowed to migrate for 48 h at 37˚C. Next, cells in the upper
chamber (non-migrating cells) were removed using a cotton wool
swab. Migrated cells in the lower chamber were washed with PBS,
fixed with 4% paraformaldehyde (Wuhan Boster Biological Technology,
Ltd.), stained with DAPI for 15 min at room temperature and
observed under a fluorescent microscope (Olympus Corporation). The
number of migrated cells was counted from 10 random high-power
fields (magnification x100) and the mean taken. TEB-CM, DBM-CM and
DMEM/F12 containing recombinant human SDF-1 (20 ng/ml) served as
controls. Migration assays were performed on five batches of hBMSCs
from different donors.
Proliferation assay
The proliferation of hBMSCs was evaluated by Cell
Counting Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.).
hBMSCs were harvested and resuspended with different CMs (fTEB-CM,
TEB-CM and DBM-CM) and basic culture media (BCM) at a density of
1x105 cells/ml. A total of 0.1-ml aliquots of hBMSC
suspension was seeded in 24-well plates. At each time point (0, 12,
24, 48, 72 and 96 h), CCK-8 reagent was added and the absorbance
was measured at 450 nm using a microplate reader. The survival of
hBMSCs was represented as the optical density (OD) value of each
well. The growth curve of cells was plotted and population doubling
time (PDT) was calculated using the following formula: PDT=h of
exponential phase/[(logN2-logN1)/log2], where N1 is the number of
cells at the beginning of the exponential growth phase and N2 is
the number of cells at the end of the exponential growth phase.
Evaluation of osteogenic
differentiation
Briefly, hBMSCs were seeded into 6-well plates
(density, 5x103/cm2). Osteogenic
differentiation was induced by incubation with different media,
including fTEB-CM, TEB-CM and DBM-CM, and osteogenic medium (OM;
Cytiva). After 14 days, the osteogenic differentiation potential
was verified by Alizarin Red staining (Cytiva) according to the
manufacturer's instructions. Osteogenic genes and proteins were
analyzed by reverse transcription (RT) semi-quantitative PCR and
western blot (WB) analysis, respectively.
RT semi-quantitative PCR
Cells were obtained and total RNA was extracted
using Qiagen RNeasy Mini kits (Qiagen AB). cDNA was prepared from 1
µg total RNA using cDNA synthesis kits (Promega Corporation)
according to the manufacturer's instructions. RT semi-quantitative
PCR was performed using SYBR ExScript RT-PCR kits (PerfectRealTime;
Takara Bio, Inc.) 18s ribosomal RNA served as the internal control.
The primers used are presented in Table
I.
 | Table IReverse transcription
semi-quantitative PCR primers. |
Table I
Reverse transcription
semi-quantitative PCR primers.
| | Primer (5'-3') |
|---|
| Gene | Forward | Reverse |
|---|
| ALP |
CCCACAATGTGGACTACCT |
GAAGCCTTTGGGGTTCTTC |
| RUNX2 |
CGGAGTGGACGAGGCAAGAG |
TGAGGAATGCGCCCTAAATC |
| OC |
CACTCCTCGCCCTATTGGCC |
CCTCCTGCTTGGACACAAAG |
| 18s rRNA |
GTAACCCGTTGAACCCCATT |
CCATCCAATCGGTAGTAGCG |
WB analysis
Cells were collected, lysed with SDS lysis buffer
[100 mM Tris (Ph 8.0), 10% glycerol and 1% SDS; Beyotime Institute
of Biotechnology] on ice and then centrifuged at 10,000 x g for 1
min at 4˚C. The supernatant was obtained, centrifuged at 10,000 x g
for 1 min at 4˚C and stored at -20˚C. Each SDS-PAGE (Beyotime
Institute of Biotechnology) lane was loaded with 20 µg protein. The
gel electrophoresis conditions were polyacrylamide (10%,v/v), 80 V
and 90 min. The gel was then transferred onto PVDF membranes (250
mA for 60 min; EMD Millipore). After blocking with 5% skimmed milk
for 1 h at room temperature, the membranes were incubated overnight
at 4˚C with the primary antibodies, including anti-RUNX family
transcription factor 2 (RUNX2; cat. no. ab236639; 1:1,000; Abcam),
anti-alkaline phosphatase (ALP; cat. no. ab154100; 1:2,000; Abcam),
anti-amyloid fibrils (OC; cat. no. ab133612; 1:4,000; Abcam) and
β-actin (cat. no. ab8227; 1:4,000; Abcam). After washing with TBST
(Beyotime Institute of Biotechnology) under oscillation for 15 min
for 3 times, the peroxidase-conjugated secondary antibody (cat. no.
AP132P; 1:4,000; SouthernBiotech) was added and incubated at room
temperature for 1 h. The membrane was washed with TBST for 15 min
for 3 times and signals were then detected by ECL (Kirkegaard &
Perry Laboratories Inc.). When the bands were developed, images
were captured and ImageJ V1.8.0 (NIH) used for analysis.
Statistical analysis
All values are expressed as means ± SD. Statistical
analysis was performed using SPSS 13.0 software (SPSS, Inc.). The
results of migration, proliferation and differentiation assays
among groups were compared using one-way ANOVA with the Bonferroni
correction as a post hoc test, where P<0.0083 was considered to
indicate a statistically significant difference. The results of
cytokine antibody arrays were analyzed by one-way ANOVA with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
hUC-MSCs in TEB
The mean efficiency of cell seeding was 82.4±3.3%
(n=10). On day 3 after cell seeding, hUC-MSCs spread and attached
well on the pore surfaces of the scaffolds, with dense
extracellular matrix deposition (Fig.
1B). With the proliferation of hUC-MSCs and production of the
extracellular matrix, cells were stacked and superimposed over each
other on day 10 (Fig. 1C). Confocal
microscopy further confirmed that hUC-MSCs were tightly attached to
the scaffolds after 3 (Fig. 1E) and
10 (Fig. 1F) days. It was revealed
by SEM that although hUC-MSCs were devitalized by freeze-drying,
the remnant cellular contents were retained on the pore surfaces of
fTEB (Fig. 1D).
The majority of proteins are retained
following freeze-drying
To assess the effect of lyophilization on the
protein loss of TEB, the protein content was monitored following
cell seeding. The protein contents of TEB and the derived fTEB
increased over time and peaked on day 12. Thus, fTEB prepared 12
days after cell seeding were harvested for subsequent use. At any
given time-point, the protein content of TEB was significantly
higher than that of fTEB (P<0.05). However, the mean retention
rate from 10 samples, which was defined as the ratio between
protein contents of fTEB and TEB harvested following a 10-day
culture was 64.0% (60.4-66.7%; Fig.
1G and H).
Certain cytokines in fTEB might be
responsible for its biological function
To identify the proteins accounting for the
functions of fTEB, a customized Quantibody® human
cytokine array (QAH-CUSTOM; RayBiotech, Inc.) was performed. The
levels of the majority of proteins of interest were significantly
higher in fTEB, as compared with DBM (Fig. 1H). Only those with concentrations of
>10 ng/ml were selected and presented in Fig. 1H. The maximal concentration occurred
in basic fibroblast growth factor (bFGF) with a mean of 525.42
ng/ml in fTEB (n=4).
fTEB shows a marked capability in
inducing hBMSC migration
To investigate the hBMSC-recruiting capacity of
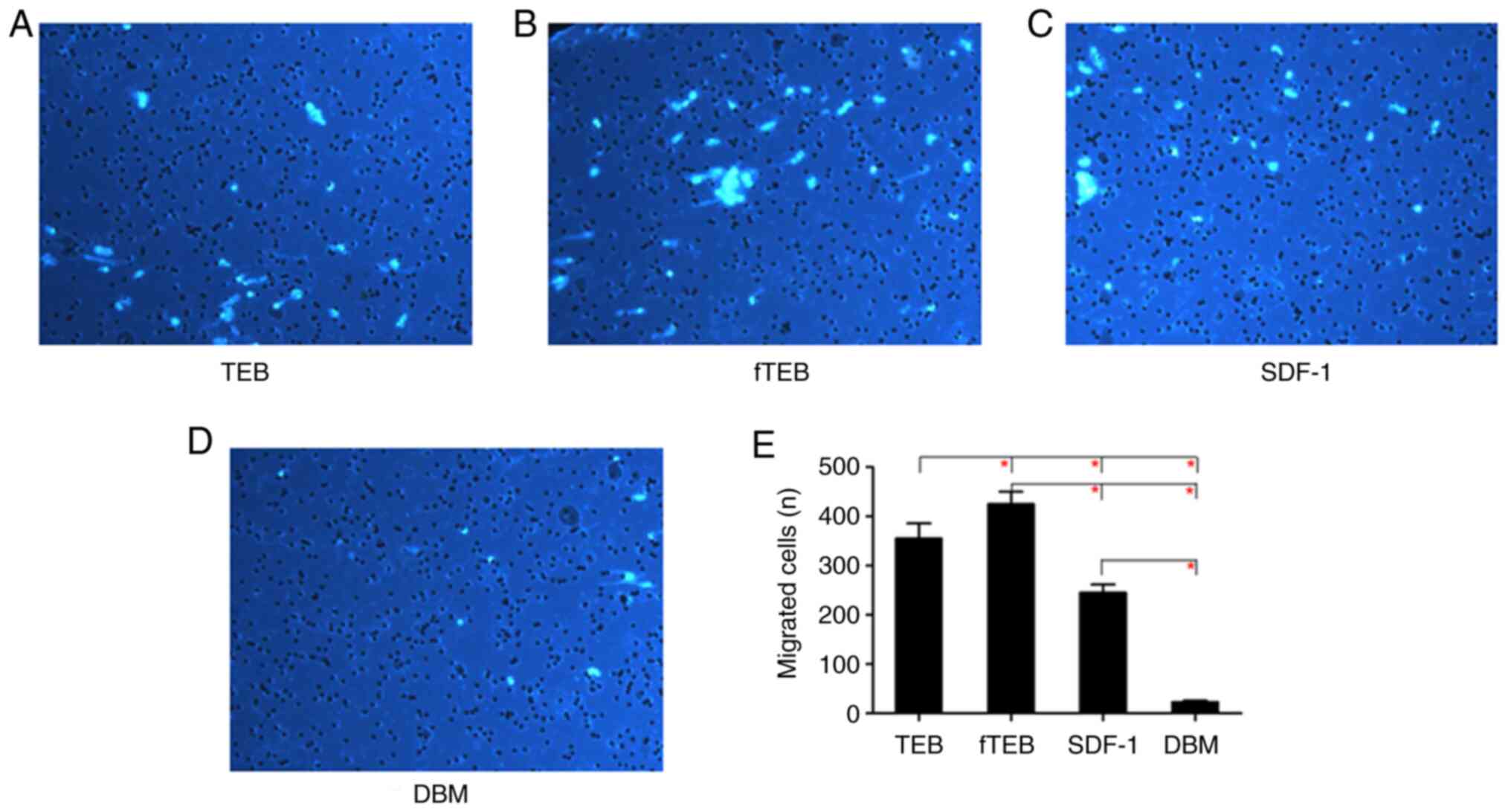
fTEB, migration assays were performed. As demonstrated in Fig. 2, the chemotactic activity of fTEB
was significantly higher than that of TEB, SDF-1 and DBM
(P<0.0083).
fTEB exerts positive effects on the
proliferation of hBMSCs
To evaluate the biological role of fTEB in the
proliferation of hBMSCs, cell growth kinetics were evaluated. CCK-8
assays demonstrated that, after 24 h of culture, fTEB-CM and TEB-CM
promoted the proliferation of hBMSCs, as evidenced by the increased
OD values when compared to the BCM and DBM-CM (Fig. 3). The proliferation-promoting
capacity of fTEB-CM was not significantly different to TEB-CM
(P>0.05) at all time points but 96 h. According to the growth
curve, hBMSCs cultured with fTEB-CM and TEB-CM displayed a similar
growth pattern, with a lag stage and an extensive log phase lasting
~2 and ~4 days, respectively. The subsequent platform phase
occurred at day 8. Moreover, the growth rates of hBMSCs cultured
with BCM and DBM-CM were significantly lower than those of the
fTEB-CM and TEB-CM groups (P<0.05). In addition, no apparent
difference in the mean PDT was observed between fTEB-CM (28.24±6.51
h) and TEB-CM (26.68±4.32 h; P>0.05). However, both were
significantly lower than BCM (36.69±8.92 h) and DBM-CM
(123.52±13.50 h; P<0.05).
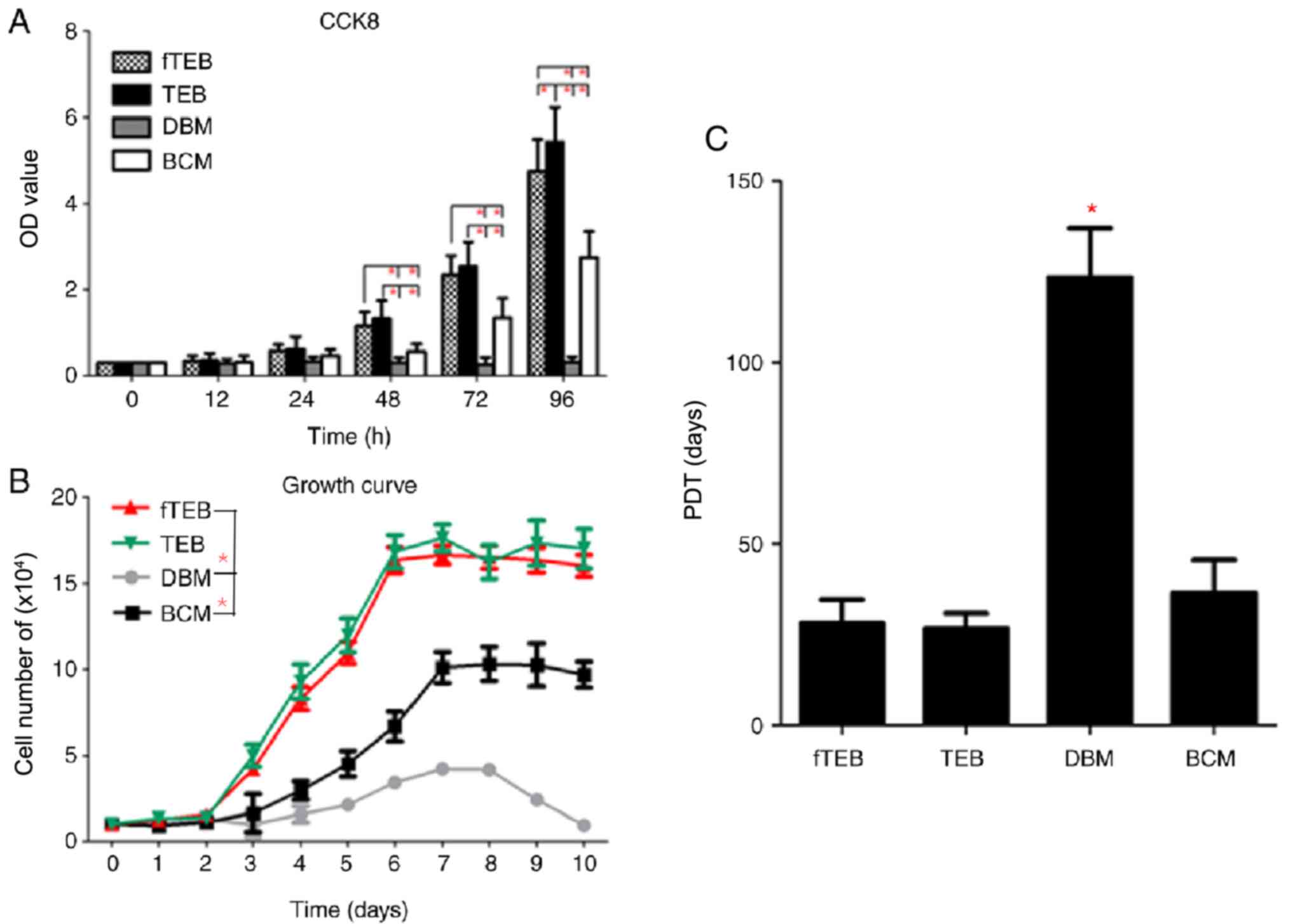 | Figure 3Proliferation assays. (A) fTEB-CM
showed comparable pro-proliferation effects on hBMSCs in TEB-CM.
(B) Growth curves of cells treated with fTEB, TEB-CM, DBM and BCM.
(C) Mean PDT of cells treated with fTEB-CM, TEB-CM, DBM or BCM.
*P<0.0083. TEB, tissue-engineered bones; fTEB,
functional TEB; CM, conditioned media; BCM, basic culture media;
PDT, population doubling time; hBMSCs, human bone marrow
mesenchymal stem cells; DBM, demineralized bone matrix. |
fTEB exhibit comparable osteogenic
capacity to TEB
Microscopic examination of Alizarin Red staining
showed clear crimson deposits indicative of mineralization
following incubation with fTEB-CM, TEB-CM and OM, but not with
DBM-CM (Fig. 4A-D). The expression
levels of osteocyte markers were semi-quantitatively evaluated by
RT-PCR (Fig. 4E). ALP mRNA
expression levels were similar in cells treated with fTEB-CM and
TEB-CM, but significantly higher than those in OM-treated cells
(P<0.05). OM induced a higher OC mRNA expression level than
fTEB-CM and TEB-CM (P<0.05). In addition, the difference between
RUNX2 mRNA expression levels in the fTEB-CM, TEB-CM and OM groups
were not significantly different (P>0.05). A similar expression
pattern was also observed at the protein level (Fig. 4F). In combination, these results
demonstrated that fTEB were not significantly different to TEB with
regard to inducing osteogenic differentiation of hBMSCs.
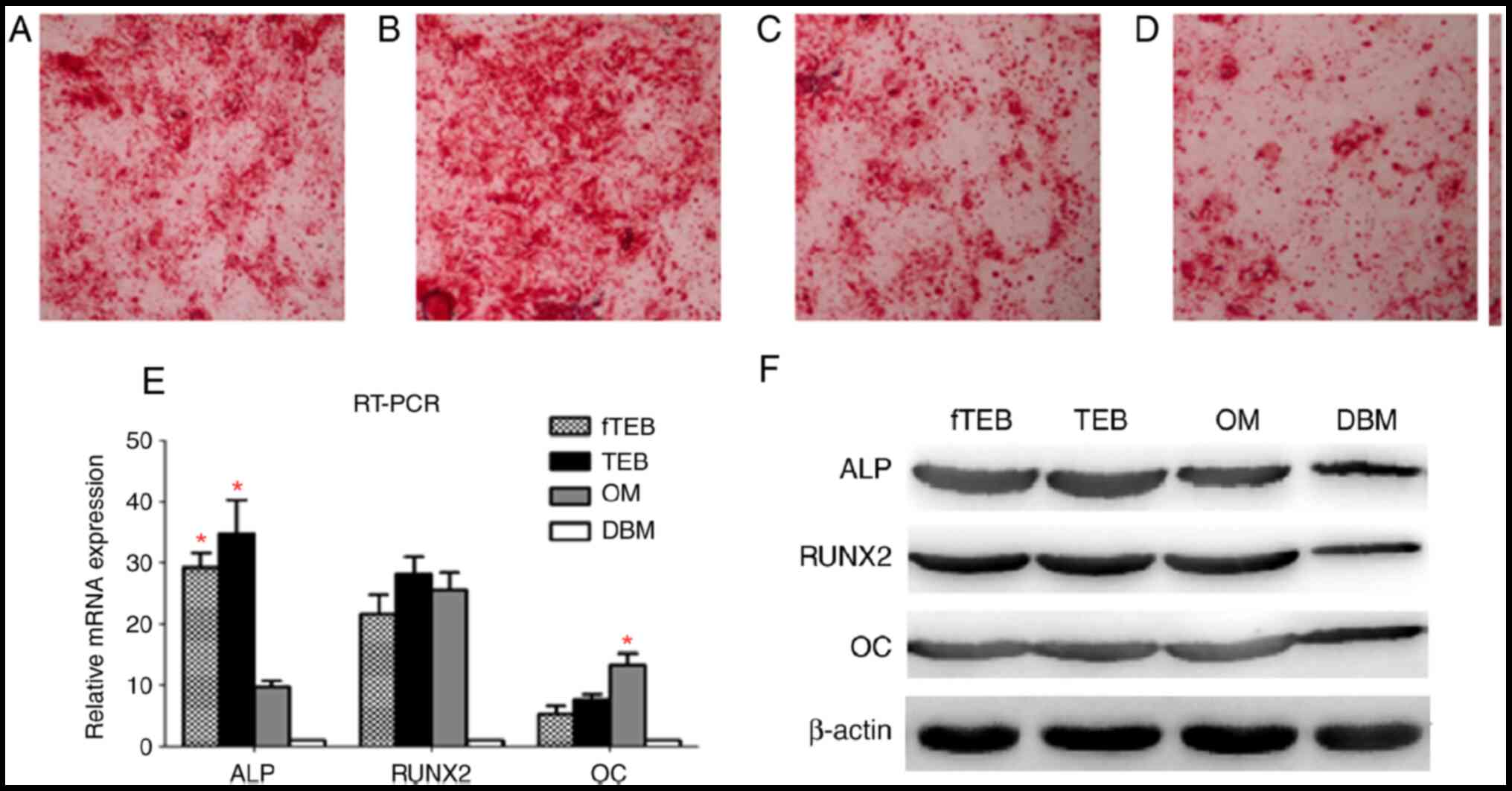 | Figure 4Comparison of osteogenic
differentiation between hBMSCs treated with different CM. (A-D)
Microscopic examination of Alizarin Red staining (magnification
x40). (E) ALP, RUNX2 and OC mRNA expression levels in cells treated
with different CM (n=5). ALP mRNA expression levels were similar in
cells treated with fTEB-CM and TEB-CM, but significantly higher
than those in OM-treated cells (*P<0.05). OM induced
a higher OC mRNA expression level than fTEB-CM and TEB-CM
(*P<0.05). In addition, the difference between RUNX2
mRNA expression levels in the fTEB-CM, TEB-CM and OM groups were
not significantly different (P>0.05). (F) Protein expression of
ALP, RUNX2 and OC (n=5). Bars represent the mean ± SD. hBMSCs,
human bone marrow mesenchymal stem cells; ALP, alkaline
phosphatase; RUNX2, RUNX family transcription factor 2; OC,
anti-amyloid fibrils; OM, osteogenic medium; TEB, tissue-engineered
bones; fTEB, functional TEB; CM, conditioned media. |
Discussion
The application of TEB in the clinical setting has
been limited by several crucial hurdles, such as the requirement of
long-term in vitro cell culture, high cost, safety concerns
and logistical challenges (8). In
the present study, a novel TEB device, fTEB, which is based on the
concept that implanted MSCs aid bone repair via paracrine modes,
rather than direct osteogenic differentiation, was introduced
(12-14).
Briefly, fTEB were prepared by freeze-drying TEB with allogenic
hUC-MSCs. Using a deep-hypothermic lyophilizer, cells were
devitalized while most protein content was retained. It is
important to note that if the major contributing components were
retained the aforementioned obstacles in the application of TEB
could be greatly mitigated (15).
The role of exogenous MSCs within TEB in bone repair
remains unclear. Previous studies indicate that, upon implantation,
exogenous MSCs recruit host stem cells and modulate their
biological behaviors viacytokines (5-7).
Moreover, it is widely accepted that the mobilization and homing of
endogenous progenitor cells (such as MSCs) to the injury site is a
prerequisite for tissue regeneration (16). Our previous study identified a
series of chemokines associated with the recruitment of host MSCs
mediated by donor MSCs (9). In the
present study, the MSC-recruiting capacity of fTEB was similar to
that of TEB, suggesting that most of the functional
chemoattractants were retained following freeze-drying. It was
found that growth-regulated oncogene (GRO; 46.08 ng/ml), CXCL5
(41.89 ng/ml), CXCL6 (18.89 ng/ml) and CXCL8 (27.89 ng/ml) might
contribute to the MSC-recruiting capacity of fTEB. The GRO subgroup
belongs to the CXCL8 cytokine family and consists of three members,
CXCL1/GRO-α, CXCL2/GRO-β and CXCL3/GRO-γ (17). By binding CXCR2, a cell-surface
cognate receptor constitutively expressed by hBMSCs, these
chemokines play crucial roles in cell mobilization and homing
(18,19). Notably, CXCR2 is not only a
co-receptor of the CXCL8 family of cytokines, but also binds to
CXCL5 and CXCL6(20). Downstream
signaling pathways, including the PI3K-Akt and mTOR signaling
pathways, are activated by CXCR2, thus regulating cell migration
(18). In addition, it is worth
noting that the recruiting capacity of fTEB-CM was more potent than
that of a well-defined MSC chemoattractant, SDF-1, which was
demonstrated in the present study.
During bone defect repair, implanted TEB generate
trophic factors to modulate the surrounding microenvironment and
promote tissue regeneration (21).
In the present study, it was found that fTEB-CM exerted comparable
effects on the proliferation and osteogenic differentiation of
hBMSCs to those of TEB-CM. This was further supported by the
cytokine array, as the concentrations of certain growth factors
associated with cell proliferation and differentiation were high in
fTEB-CM. According to the literature, bFGF appears to be the main
facilitator of cell proliferation (21). To further support this notion, the
proliferation-promoting effect of bFGF on MSCs had been
well-recognized in previous studies (22-25)
and, in the present study, a high concentration of bFGF was
observed in fTEB-CM (higher than the concentration used in previous
studies) (22-25).
Conversely, identifying a predominant factor accounting for the
enhanced osteogenic differentiation was challenging. Insulin-like
growth factor-binding protein 3, interleukin 6 and vascular
endothelial growth factor (VEGF) have all been shown to have the
ability to potentiate osteogenic differentiation (26-28).
In addition, bFGF has been reported to promote osteogenic
differentiation in the presence of bone morphogenetic protein 2
(BMP-2) (23), BMP-2 and VEGF
(29) or BMP-4/7(30), but to inhibit osteogenic
differentiation on its own or with EGF (31). These results further complicated the
compound effects induced by cytokine combinations. The regulation
of osteogenic repair involves multiple factors, including cells and
various cytokines. Currently, the integration of factors into DBM
has been limited to no more than three factors. However, there are
49 known factors in fTEB, indicating its comprehensive and
synergetic effects on osteogenesis.
It must be noted that the present study had certain
limitations. The immunogenicity of fTEB was not evaluated.
Furthermore, freeze-drying damaged the cell membrane, which may
have exposed immunogenic intracellular proteins to the immune
system. Additionally, the in vivo safety and efficacy of fTEB were
not investigated in the present study. Obtaining an understanding
of this issue is critical to the further development of fTEB and
relevant animal studies are ongoing. Finally, this was a
preliminary study, the next step will be to verify the key
cytokines modulating the biological behaviors of MSCs and elucidate
the correlation between them.
Thus, the present study introduced a prototype of an
fTEB device, which could easily be fabricated by freeze-drying
matured TEB with a widely available deep-hypothermic lyophilizer.
These results collectively supported the hypothesis that compared
to TEB, fTEB may be more economical and easier to manufacture.
However, further validation of the efficacy and safety of fTEB is
warranted in future studies.
Supplementary Material
The 49 bioactive factors in fTEB.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the China
Postdoctoral Science Foundation (grant. no. 2015M572713), the
Natural Science Foundation of China (grant. no. 81971762) and the
President Funding of the 960th Hospital of PLA (grant. no.
2013ZD02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and JX contributed equally to this work. ZC and
JX made substantial contributions to acquisition, analysis and
interpretation of data. XY was responsible for the conception and
design of the study and the drafting and writing of this
manuscript. All authors had read and approved the final manuscript.
All authors confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All protocols involving human subjects were approved
by the Ethics Committee of the 960th Hospital of PLA (approval. no.
JNZY201603). All patients were asked to sign an informed consent
statement for publication.
Patient consent for publication
All patients agreed to publish the data and
associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Quarto R, Mastrogiacomo M, Cancedda R,
Kutepov SM, Mukhachev V, Lavroukov A, Kon E and Marcacci M: Repair
of large bone defects with the use of autologous bone marrow
stromal cells. New Engl J Med. 344:385–386. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu JZ, Qin H, Wang XQ, Zhou Q, Luo F, Hou
TY and He QY: Repair of large segmental bone defects using bone
marrow stromal cells with demineralized bone matrix. Orthop Surg.
1:34–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kitoh H, Kitakoji T, Tsuchiya H, Katoh M
and Ishiguro N: Distraction osteogenesis of the lower extremity in
patients with achondroplasia/hypochondroplasia treated with
transplantation of culture-expanded bone marrow cells and
platelet-rich plasma. J Pediatr Orthop. 27:629–634. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xing J, Lu Y, Cui Y, Zhu X, Luo F, Xie Z,
Wu X, Deng M, Xu J and Hou T: A standardized and
quality-controllable protocol of constructing individual
tissue-engineered grafts applicable to treating large bone defects.
Tissue Eng Part C Methods. 25:137–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tasso R, Augello A, Boccardo S, Salvi S,
Carida M, Postiglione F, Fais F, Truini M, Cancedda R and Pennesi
G: Recruitment of a host's osteoprogenitor cells using exogenous
mesenchymal stem cells seeded on porous ceramic. Tissue Eng Part A.
15:2203–2212. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tortelli F, Tasso R, Loiacono F and
Cancedda R: The development of tissue-engineered bone of different
origin through endochondral and intramembranous ossification
following the implantation of mesenchymal stem cells and
osteoblasts in a murine model. Biomaterials. 31:242–249.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Becquart P, Cambon-Binder A, Monfoulet LE,
Bourguignon M, Vandamme K, Bensidhoum M, Petite H and
Logeart-Avramoglou D: Ischemia is the prime but not the only cause
of human multipotent stromal cell death in tissue-engineered
constructs in vivo. Tissue Eng Part A. 18:2084–2094.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Amini AR, Laurencin CT and Nukavarapu SP:
Bone tissue engineering: Recent advances and challenges. Crit Rev
Biomed Eng. 40:363–408. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xing J, Hou T, Jin H, Luo F, Change Z, Li
Z, Xie Z and Xu J: Inflammatory microenvironment changes the
secretory profile of mesenchymal stem cells to recruit mesenchymal
stem cells. Cell Physiol Biochem. 33:905–919. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Habib HS, Halawa TF and Atta HM:
Therapeutic applications of mesenchymal stroma cells in pediatric
diseases: Current aspects and future perspectives. Med Sci Monit.
17:RA233–RA239. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hou T, Xu J, Wu X, Xie Z, Luo F, Zhang Z
and Zeng L: Umbilical cord Wharton's Jelly: A new potential cell
source of mesenchymal stromal cells for bone tissue engineering.
Tissue Eng Part A. 15:2325–2334. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Horwitz EM, Prockop DJ, Fitzpatrick LA,
Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz
RE and Brenner MK: Transplantability and therapeutic effects of
bone marrow-derived mesenchymal cells in children with osteogenesis
imperfecta. Nat Med. 5:309–313. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Horwitz EM, Gordon PL, Koo WK, Marx JC,
Neel MD, McNall RY, Muul L and Hofmann T: Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth in
children with osteogenesis imperfecta: Implications for cell
therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li W, Liu Y, Zhang P, Tang Y, Zhou M,
Jiang W, Zhang X, Wu G and Zhou Y: Tissue-engineered bone
immobilized with human adipose stem cells-derived exosomes promotes
bone regeneration. ACS Appl Mater Interfaces. 10:5240–5254.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen FM, Wu LA, Zhang M, Zhang R and Sun
HH: Homing of endogenous stem/progenitor cells for in situ tissue
regeneration: Promises, strategies, and translational perspectives.
Biomaterials. 32:3189–3209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen HW, Chen HY, Wang LT, Wang FH, Fang
LW, Lai HY, Chen HH, Lu J, Hung MS, Cheng Y, et al: Mesenchymal
stem cells tune the development of monocyte-derived dendritic cells
toward a myeloid-derived suppressive phenotype through
growth-regulated oncogene chemokines. J Immunol. 190:5065–5077.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ringe J, Strassburg S, Neumann K, Endres
M, Notter M, Burmester GR, Kaps C and Sittinger M: Towards in situ
tissue repair: Human mesenchymal stem cells express chemokine
receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with
CXCL8 but not CCL2. J Cell Biochem. 101:135–146. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Joukhadar J, Nevers T and Kalkunte S: New
frontiers in reproductive immunology research: Bringing bedside
problems to the bench. Exp Rev Clin Immunol. 7:575–577.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li F, Whyte N and Niyibizi C:
Differentiating multipotent mesenchymal stromal cells generate
factors that exert paracrine activities on exogenous MSCs:
Implications for paracrine activities in bone regeneration. Biochem
Biophys Res Commun. 426:475–479. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramasamy R, Tong CK, Yip WK, Vellasamy S,
Tan BC and Seow HF: Basic fibroblast growth factor modulates cell
cycle of human umbilical cord-derived mesenchymal stem cells. Cell
Prolif. 45:132–139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rose LC, Fitzsimmons R, Lee P, Krawetz R,
Rancourt DE and Uludag H: Effect of basic fibroblast growth factor
in mouse embryonic stem cell culture and osteogenic
differentiation. J Tissue Eng Regen Med. 7:371–382. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Dighe PA, Viswanathan P, Mruthunjaya AK
and Seetharam RN: Effect of bFGF on HLA-DR expression of human bone
marrow-derived mesenchymal stem cells. J Stem Cells. 8:43–57.
2013.PubMed/NCBI
|
|
25
|
Kim TH, Kim JJ and Kim HW: Basic
fibroblast growth factor-loaded, mineralized biopolymer-nanofiber
scaffold improves adhesion and proliferation of rat mesenchymal
stem cells. Biotechnol Lett. 36:383–390. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen L, Jiang W, Huang J, He BC, Zuo GW,
Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, et al: Insulin-like
growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic
differentiation and bone formation. J Bone Miner Res. 25:2447–2459.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Fukuyo S, Yamaoka K, Sonomoto K, Oshita K,
Okada Y, Saito K, Yoshida Y, Kanazawa T, Minami Y and Tanaka Y:
IL-6-accelerated calcification by induction of ROR2 in human
adipose tissue-derived mesenchymal stem cells is STAT3 dependent.
Rheumatology (Oxford). 53:1282–1290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berendsen AD and Olsen BR: How vascular
endothelial growth factor-A (VEGF) regulates differentiation of
mesenchymal stem cells. J Histochem Cytochem. 62:103–108.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bai Y, Li P, Yin G, Huang Z, Liao X, Chen
X and Yao Y: BMP-2, VEGF and bFGF synergistically promote the
osteogenic differentiation of rat bone marrow-derived mesenchymal
stem cells. Biotechnol Lett. 35:301–308. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuan S, Pan Q, Fu CJ and Bi Z: Effect of
growth factors (BMP-4/7 & bFGF) on proliferation &
osteogenic differentiation of bone marrow stromal cells. Indian J
Med Res. 138:104–110. 2013.PubMed/NCBI
|
|
31
|
Hu F, Wang X, Liang G, Lv L, Zhu Y, Sun B
and Xiao Z: Effects of epidermal growth factor and basic fibroblast
growth factor on the proliferation and osteogenic and neural
differentiation of adipose-derived stem cells. Cell Reprogram.
15:224–232. 2013.PubMed/NCBI View Article : Google Scholar
|