Introduction
Bacterial vaginosis (BV) is a common chronic
microbial genital tract infection in women that is characterized by
vaginal pH elevation, malodorous discharge and inflammation
(1,2). The female vagina is colonized by a
variety of microbes, which serve important roles in maintaining
vaginal health (3,4). Disruptions in the vaginal microbiota
can lead to BV by promoting infection from several species of
pathogens, including Gardnerella vaginalis (G.
vaginalis) and gram-negative anaerobes, such as
Prevotella, Mobiluncus and Bacteroides species
(5,6). An increasing number of studies have
demonstrated that G. vaginalis a major class of bacteria
that can cause BV (7,8). However, the underlying mechanism by
which G. vaginalis induces BV remain unclear.
Nucleotide-binding oligomerization domain, leucine
rich repeat, pyrin (NLRP) and HIN domaintein and absent in melanoma
2 are considered to be major members of the inflammasome family
(9). The NLRP3 inflammasome
consists of NLRP3-containing protein 3 (NLRP3), apoptosis
associated speck-like protein (ASC) and pro-caspase-1, which is an
important mediator of immune responses and can be activated by a
variety of stimuli, including pathogens and tissue damage (10,11).
Once NLRP3 is activated, it causes the cleavage of pro-caspase-1
into active caspase-1, which can induce the secretion of
proinflammatory cytokines, such as IL-1β and IL-18, in turn causing
pyroptotic cell death (12,13). Schroder and Tschopp (12) previously reported that monocytes and
macrophages are commonly present in the lamina propria of vaginal
tissues, which not only secrete proinflammatory cytokines but can
also regulate the formation of various inflammasome complexes
(NLRP1 and NLRP3) under the action of various microbial stressors.
In addition, a study has suggested that G. vaginalis
frequently infects the vagina and can activate the NLRP3
inflammasome, leading to BV (14).
However, whether G. vaginalis induces BV by activating the
NLRP3 inflammasome remains unclear.
Therefore, in the present study, the underlying
mechanisms by which G. vaginalis induces BV were explored.
Specifically, the effect of G. vaginalis infection on NLRP3
inflammasome-mediated pyroptosis in macrophages was investigated.
The results of the present study may provide a promising
therapeutic direction for BV.
Materials and methods
Bacterial culture
G. vaginalis (GV; type strain no. KCTC5096)
was supplied by the Korean Collection for Type Cultures (Daejon,
Korea). GV was incubated in the brain heart infusion broth
(Becton-Dickinson and Company) supplemented with yeast extract (1%;
Beyotime Institute of Biotechnology), maltose (0.1%; Beyotime
Institute of Biotechnology), glucose (0.1%, Sigma-Aldrich; Merck
KGaA) and horse serum (10%; Beyotime Institute of Biotechnology) at
37˚C for 36 h under anaerobic conditions (controlled atmosphere
composed of 10% carbon dioxide, 10% helium and 80% nitrogen in an
anaerobic jar). GV was then suspended in PBS for subsequent in
vitro experiments.
Primary culture of mouse peritoneal
macrophages and THP-1 monocyte cell culture
The primary culture of mouse peritoneal macrophages
was established as described previously (15). A total of 36 female ICR mice (8
weeks old, 25±1 g) were supplied by Beijing Vital River Laboratory
Animal Technology Co., Ltd. The mice were housed in wire cages
under climate-controlled conditions (humidity, 5±10%; temperature,
20-22°C; 12 h light/dark cycle) and received ad
libitum access to standard laboratory chow and water. The mice
were intraperitoneally injected with 2 ml 4% thioglycolate
(Sigma-Aldrich; Merck KGaA). After 3 days, all mice were sacrificed
by cervical dislocation before the peritoneal cavity fluid was
collected and pooled together. This sample was then centrifuged at
3,000 x g at 4˚C for 10 min and the cells collected, washed with
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) and
suspended in RPMI-1640 medium supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Sigma-Aldrich; Merck-KGaA). Subsequently, the cells were cultured
for 20 h at 37˚C and the adherent cells were defined as macrophages
(16). The present study was
approved by the committee of ethical animal care and use of
laboratory animals of Shandong Provincial Third Hospital (approval
no. DWKYLL-2020001).
THP-1 monocyte cells were obtained from the American
Type Culture Collection and cultured in RPMI 1640 medium containing
10% FBS, 50 µM 2-mercaptoethanol and 1% penicillin/streptomycin at
37˚C in 5% CO2. The THP-1 monocytes were differentiated
by treating the cells with 100 nM phorbol 12-myristate 13-acetate
(Thermo Fisher Scientific, Inc.) for 24 h at 37˚C.
For treatment, the primary mouse macrophages and
THP-1 cells were respectively seeded at 2x106 cells/well
into six-well plates before they were treated with either fresh
medium, medium containing 1 µg/ml LPS (Sigma-Aldrich; Merck KGaA),
medium containing GV at a multiplicity of infection of 10 or medium
containing both LPS and GV, at 37˚C for 12 h.
Cell viability assay
Cell viability was detected using a Cell Counting
Kit-8 (CCK-8) assay kit (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's protocols. The macrophages and
THP-1 cells were seeded into 96-well plates at a density of
1x104 cells/well. After exposure to LPS and GV, CCK-8
(10 µl) was added into each well and the plates were incubated at
37˚C for 1 h in the dark. Finally, the absorbance was measured
using a microplate reader at a wavelength of 450 nm.
LDH level analysis
The activity of lactate dehydrogenase (LDH) in the
culture medium of THP-1 cells and macrophages was analyzed using a
colorimetric assay (cat. no. A020-1-2; Nanjing Jiancheng
Bioengineering Institute) by following the manufacturer's
protocols. The absorbance at 490 nm was determined with a
microplate reader.
Detection of reactive oxygen species
(ROS)
The fluorescent probe dichloro-dihydro-fluorescein
diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA) was used to measure
intracellular ROS levels by following the manufacturer's protocols.
Briefly, the macrophages and THP-1 cells (5x105
cells/ml) were incubated with 40 µM DCFH-DA in serum-free medium
for 30 min in the dark at 37˚C and then washed with PBS three
times. Finally, the fluorescence of the cells was examined by using
flow cytometry (FACSCalibur; BD Biosciences) at excitation and
emission wavelengths of 488 and 525 nm. The data were analyzed
using FlowJo software (version v7.6.5; FlowJo LLC).
Measurement of caspase-1 activity
Caspase-1 activity in the culture supernatant of
THP-1 cells and macrophages was measured using a caspase-1 activity
assay kit (cat. no. C1102; Beyotime Institute of Biotechnology) by
following the manufacturer's protocols. Absorbance at 405 nm was
determined for each well with a microplate reader.
ELISA
The levels of TNF-α (mouse cat. no. PMTA00B; human
cat. no. PDTA00D), IL-1β (mouse cat. no. PMLB00C; human cat. no.
PDLB50) and IL-18 (mouse cat. no. DY7625-05; human cat. no.
DY318-05) in the culture supernatant of macrophages and THP-1 cells
were detected by using their corresponding ELISA kits (all from
R&D systems, Inc.) according to the protocols of the
manufacturer.
Calcein-AM/propidium Iodide (PI)
staining assay
Calcein-AM/PI double staining (cat. no. C2015M;
Beyotime Institute of Biotechnology) was used to quantify the
number of living and dead cells as a cell death assay. Briefly, the
macrophages and THP-1 cells (5x105 cells/ml) were mixed
with 1X assay buffer and then stained with 2 µM calcein-AM and 4.5
µM PI per well for 30 min at 37˚C. The percentage of positive cells
was counted and the image were scanned with a fluorescence
microscope (magnification, x100). The number of PI positive cells
represented pyroptosis, which was calculated using the following
equation: PI positive cells (%)=(PI positive cells/Calcein-AM
positive cells) x100%.
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was applied to extract total RNA from the
macrophages and THP-1 cells. The synthesis of cDNA was performed
using the PrimeScript II 1st Strand cDNA Synthesis kit (cat. no.
6210A; Takara Bio, Inc.). The conditions for RT were as follows:
25˚C for 5 min, 55˚C for 20 min and 85˚C for 5 min. qPCR was
performed using SYBR® Premix Ex Taq™ (cat. no. RR42LR;
Takara Bio, Inc.) in the ABI StepOne Plus™ RT-PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Primers were supplied
by Shanghai GenePharma Co., Ltd. The sequences were as follows:
NLRP3 forward, 5'-GCTGGTCTTGAATTCCTCA-3' and reverse,
5'-GGCACACGGATGAGTCTTT-3'; ASC forward, 5'-CCAAATGCTTCCCCCATCCT-3'
and reverse, 5'-GCCCTTTGGTACATGCCTCT-3'; caspase-1 forward,
5'-CTACTTCCCTGAATGCTTGGC-3' and reverse, 5'-GCTCCTGGGTTTGTCCACTC-3'
and GAPDH forward, 5'-GCACCGTCAAGGCTGAGAAC-3' and reverse,
5'-GCCTTCTCCATGGTGGTGAA-3'. The thermocycling conditions for qPCR
were follows: Initial denaturation at 95˚C for 3 min, followed by
40 cycles of 95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 30 sec.
GAPDH was used as the internal reference. The relative gene
expression levels were calculated using the 2-ΔΔCq
method (17).
Western blot analysis
Total protein was extracted from macrophages and
THP-1 cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Subsequently, the protein concentrations were
determined by a BCA protein Assay kit (Roche Diagnostics). Protein
(50 µg) was separated by 10% SDS-PAGE and then transferred onto
PVDF membranes. After blocking for 2 h in 5% skim milk at room
temperature, the membranes were incubated overnight at 4˚C with the
following primary antibodies: Rabbit monoclonal antibody against
NLRP3 (cat. no. 15101; 1:500; Cell Signaling Technology, Inc.),
rabbit monoclonal antibody against ASC (cat. no. ab47092; 1:1,000,
Abcam), rabbit polyclonal antibody against pro-caspase-1 (cat. no.
22915-1-AP; 1:1,000, Proteintech Group, Inc.), rabbit polyclonal
antibody against caspase-1 (cat. no. 22915-1-AP; p10; 1:500;
Proteintech Group, Inc.) and rabbit polyclonal antibody against
GAPDH (cat. no. 10494-1-AP; 1:2,000, Proteintech Group, Inc.).
After washing with PBST three times, the membranes were incubated
with the horseradish peroxidase-conjugated secondary antibodies
(cat. no. SA00001-2; 1:5,000; Proteintech Group, Inc.) for 2 h at
room temperature. Finally, Proteins were visualized using the
Immobilon Western Chemiluminescent HRP substrate (cat. no.
WBKLS0500; EMD Millipore), and then the blots were analyzed using
the ChemiDoc XRS system (Bio-Rad Laboratories, Inc.). The density
of the protein bands was quantified using ImageJ software (version
V1.8.0; National Institutes of Health).
Statistical analysis
All statistical analyses were performed using the
SPSS 22.0 Statistical Software (IBM Corp.). The results were
presented as the mean ± standard error of the mean. One-way ANOVA
followed by Tukey's post hoc test was used to compare differences
among the treatment groups. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was performed
at least three times.
Results
GV inhibits cell viability and
promotes LDH release from primary mouse macrophages and THP-1
cells
In the present study, treatment of LPS was used as
the positive control for NLRP3-mediated inflammasome activation
(14). To explore the effect of GV
on cellular physiology, cell viability and LDH release from
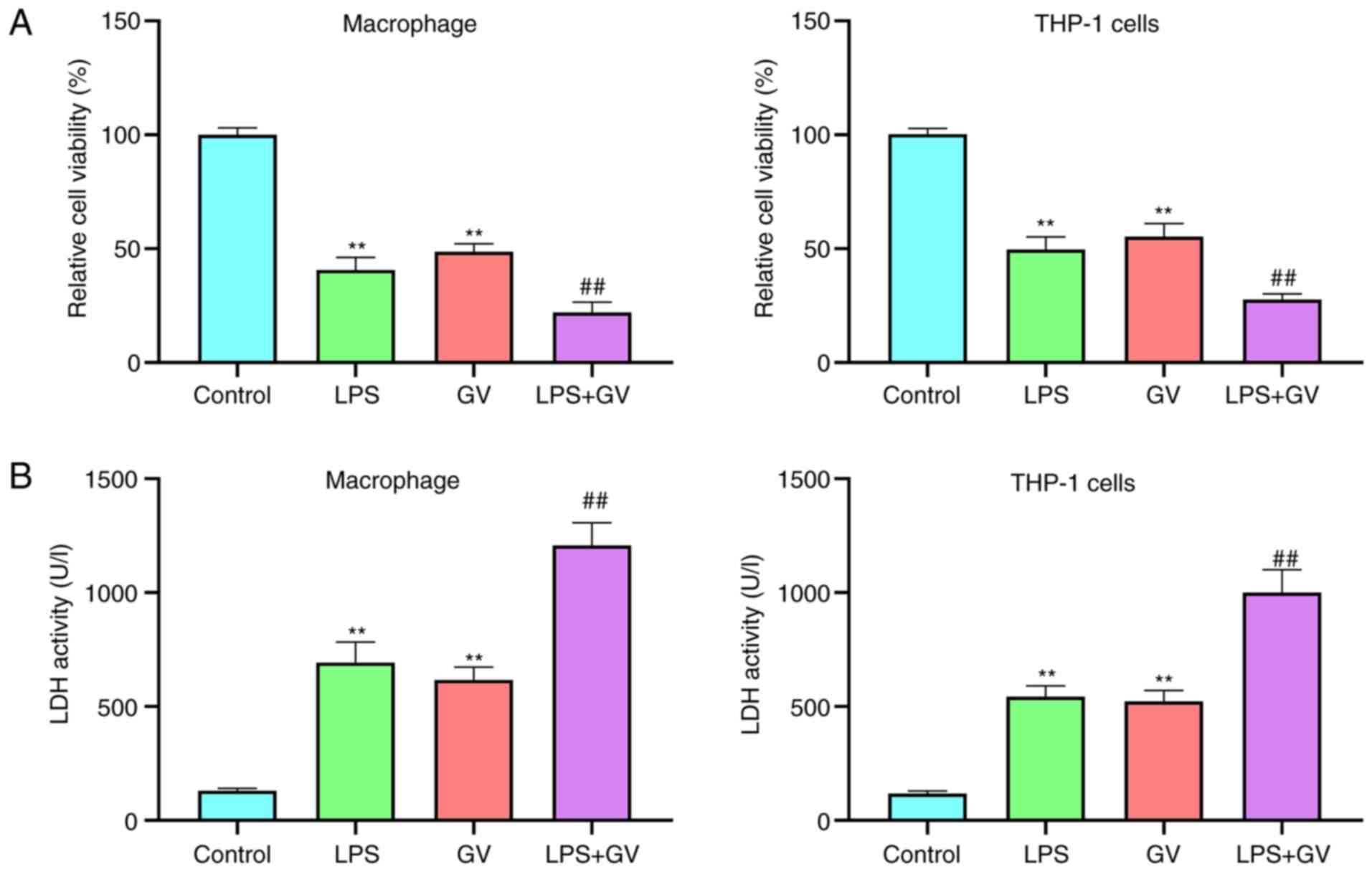
macrophages and THP-1 cells were measured. Results from the CCK-8
assay revealed that the viability of macrophages and THP-1 cells
was significantly decreased in the LPS or GV groups compared with
that in the Control group (P<0.01; Fig. 1A). Compared with the LPS or GV
groups, cell viability was significantly reduced in the LPS + GV
group (P<0.01; Fig. 1A). In
addition, LDH activities in the cell culture supernatant of the LPS
or GV groups were significantly higher compared with those in the
Control group (P<0.01; Fig. 1B).
However, LDH activities in the cell culture supernatant of the LPS
or GV groups were both significantly lower than those in the LPS +
GV group (P<0.01; Fig. 1B).
These results suggested that GV induced macrophage and THP-1 cell
injury.
GV promotes the production of
proinflammatory cytokines in primary mouse macrophages and THP-1
cells
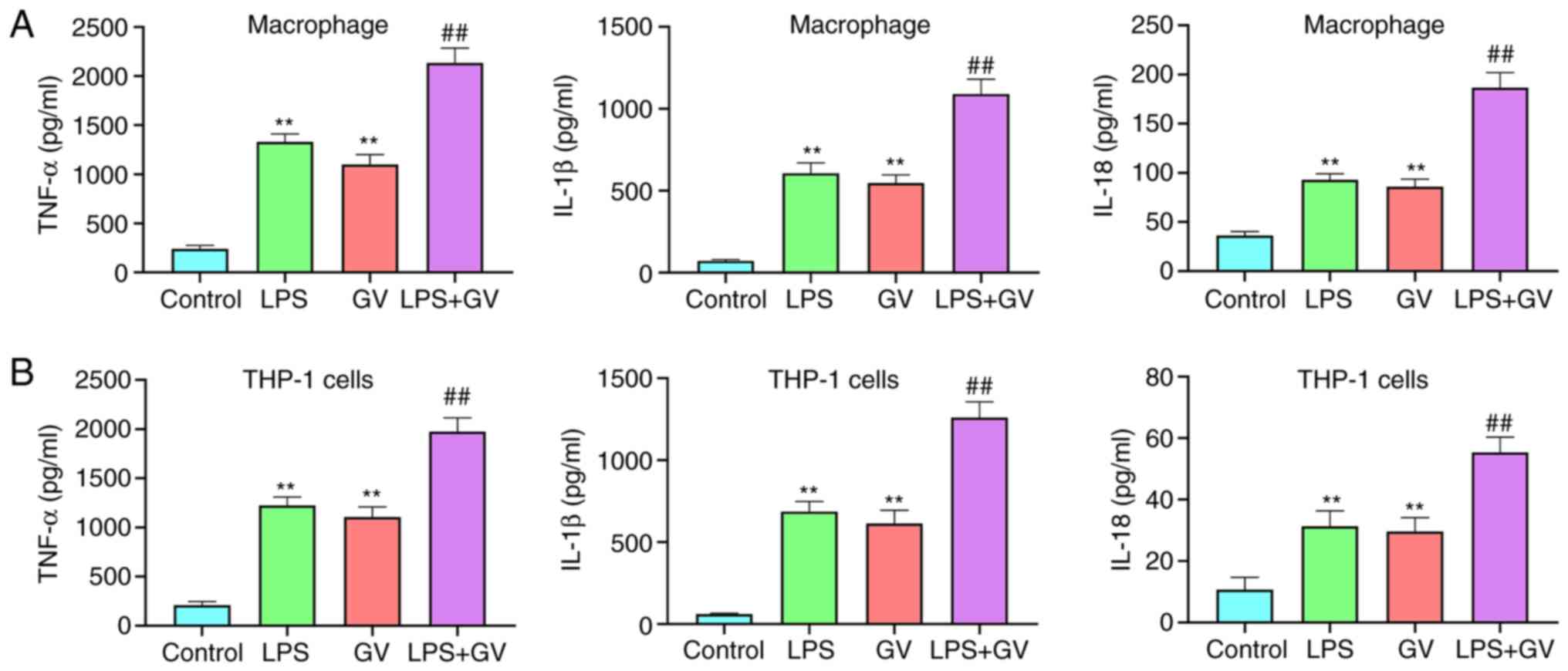
As presented in Fig.
2A and B, pretreatment with LPS
or GV significantly increased the levels of TNF-α, IL-1β and IL-18
in the macrophages and THP-1 cells compared with those in the
Control group (P<0.01). In addition, co-treatment with both LPS
and GV significantly promoted the levels of TNF-α, IL-1β and IL-18
in macrophages and THP-1 cells compared with those in the LPS or GV
groups (P<0.01). These data suggested that GV can promote the
production of proinflammatory cytokines in macrophages and THP-1
cells.
GV promotes the production of ROS in
primary mouse macrophages and THP-1 cells
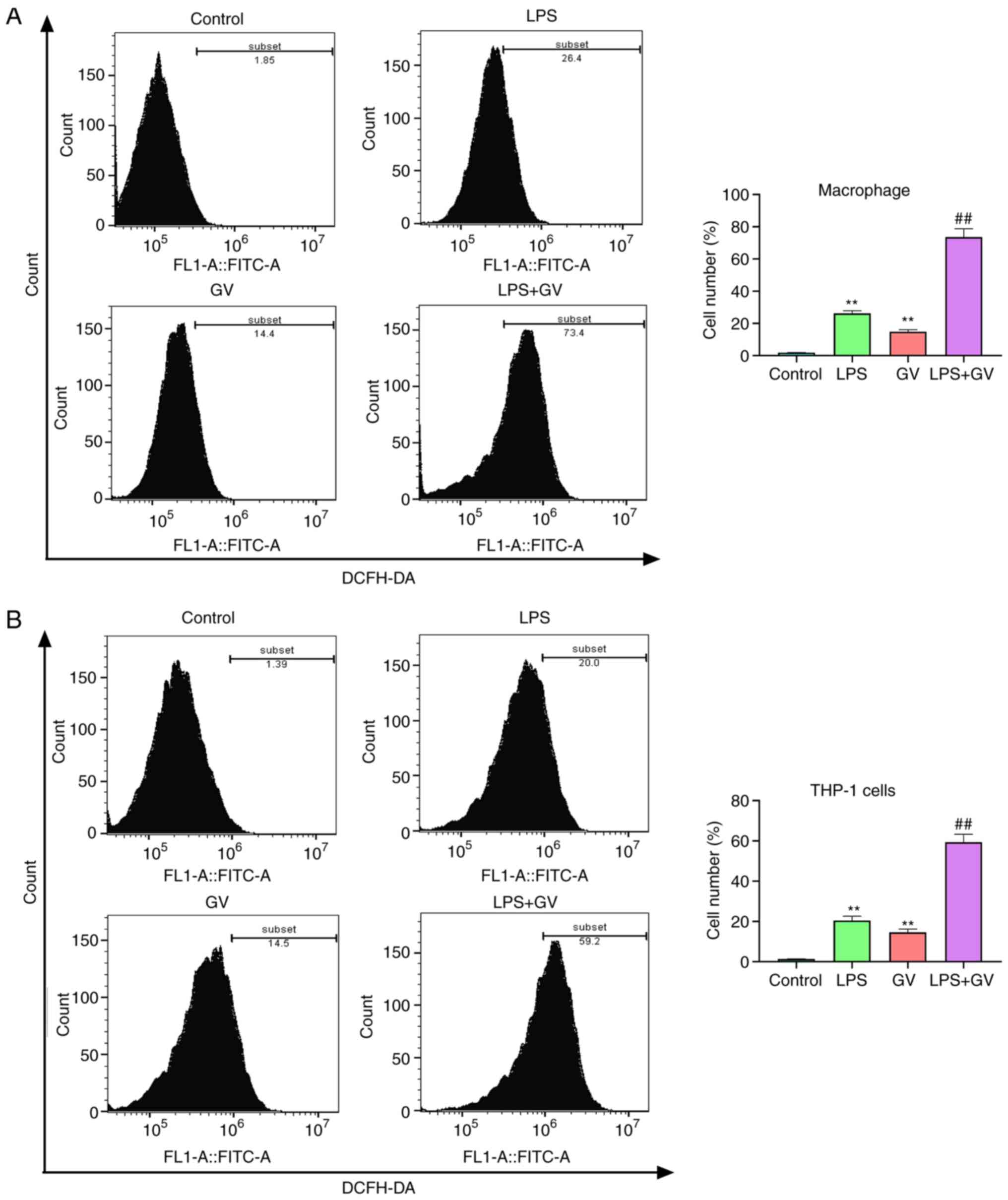
ROS production has been reported to be one of the
mechanisms responsible for NLRP3 inflammasome activation and
pyroptosis (18,19). According to Fig. 3A and B, treatment with LPS or GV significantly
elevated the levels of ROS in macrophages and THP-1 cells compared
with those in the Control group (P<0.01). In addition, combined
treatment with both LPS and GV significantly increased ROS amounts
in macrophages and THP-1 cells relative to those in the LPS or GV
groups (P<0.01). These results aforementioned suggested that GV
can promote the production of ROS in macrophages and THP-1
cells.
GV promotes the pyroptosis of
macrophages and THP-1 cells
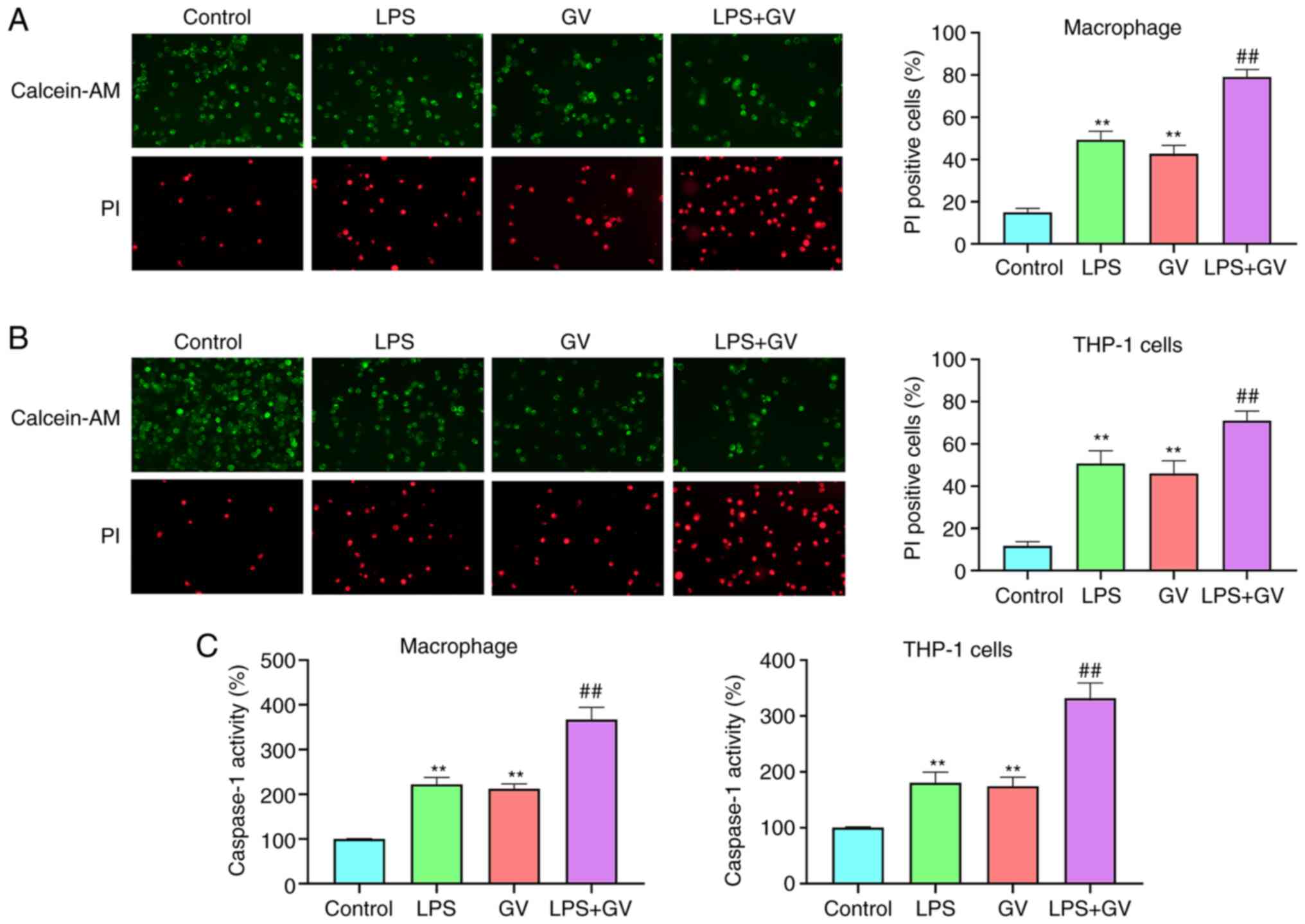
To assess if GV-induced injury of macrophages and
THP-1 cells was associated with caspase-1 activation-mediated
pyroptosis, caspase-1 activity was measured and cells positive for
pyroptosis were detected by calcein-AM/PI double staining. LPS or
GV treatment significantly facilitated pyroptotic cell death
relative to that in the Control group (P<0.01; Fig. 4A and B). Furthermore, combined LPS and GV
treatment significantly increased pyroptotic cell death in
macrophages and THP-1 cells compared with that in the LPS or GV
groups (P<0.01; Fig. 4A and
B). Caspase-1 activity in the LPS
or GV groups was also found to be significantly higher compared
with that in the Control group (P<0.01; Fig. 4C), which in turn was significantly
lower compared with that in the LPS + GV group (P<0.01; Fig. 4C). These results indicated that GV
can promote the pyroptosis of macrophages and THP-1 cells.
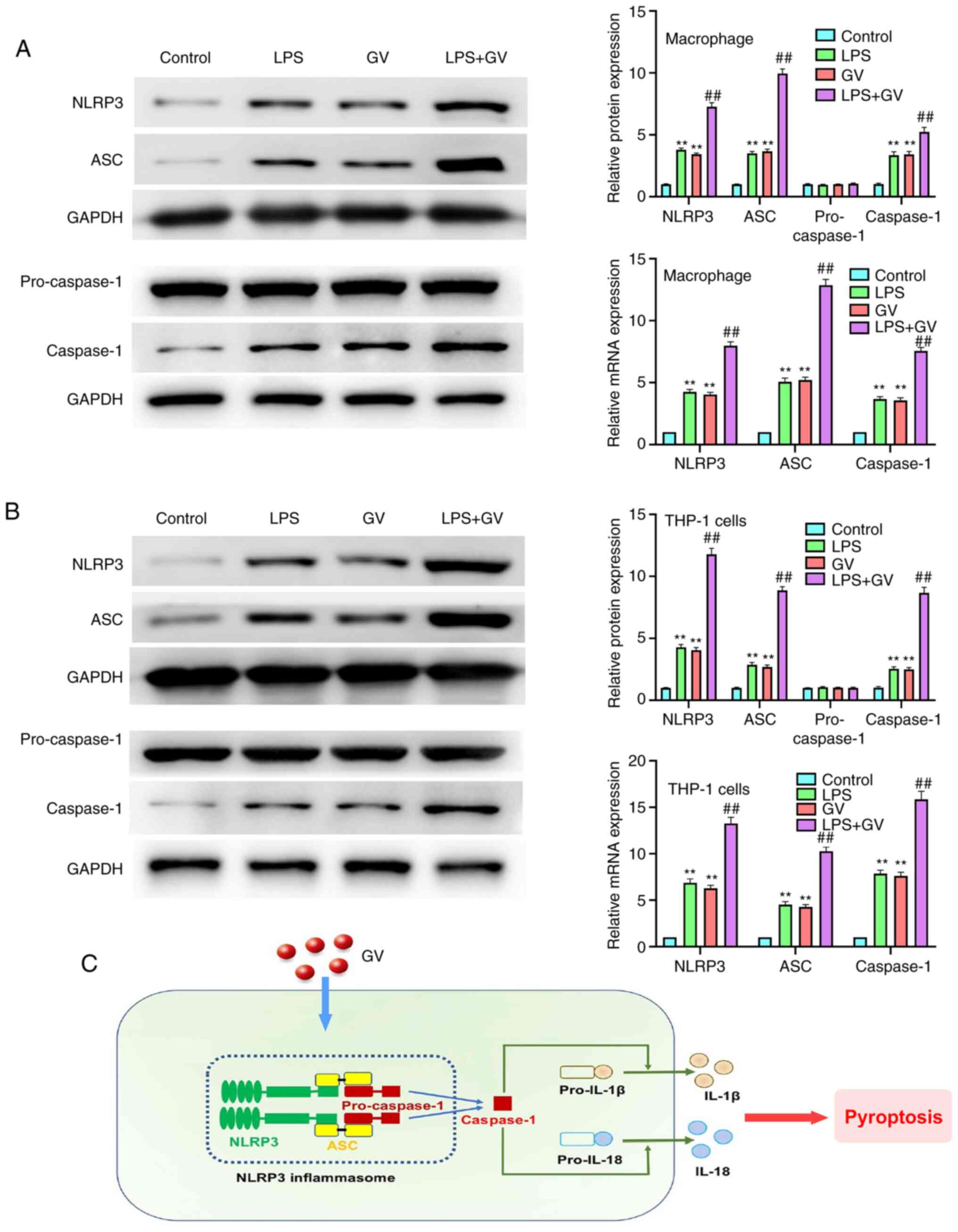
GV promotes the activation of NLRP3
inflammasomes in primary mouse macrophages and THP-1 cells
As presented in Fig.
5A and B, the protein and mRNA
expression of NLRP3, ASC and caspase-1 in macrophages and THP-1
cells were significantly increased in the LPS or GV groups compared
with those in the Control group (P<0.01). By contrast, the
protein and mRNA expression of NLRP3, ASC and caspase-1 in the LPS
or GV groups were significantly lower compared with those in the
LPS + GV group (P<0.01). However, no changes in the
pro-caspase-1 protein expression levels were observed. All these
observations suggested that GV can promote the activation of NLRP3
inflammasomes in macrophages and THP-1 cells.
Discussion
BV is an inflammatory vaginal disease that is caused
by the displacement of lactic-acid producing Lactobacillus
spp. of bacteria with anaerobic bacteria, including G.
vaginalis, Prevotella spp., Sneathia spp. and
Atopobium vaginae (20).
Previous studies have documented that G. vaginalis infection
is present in all cases of BV, which can contribute to BV
pathogenesis (21,22). At present, although antimicrobials
are commonly used for treating BV, they do not prevent its
recurrence. In addition, a growing body of evidence suggests that
resistant G. vaginalis can arise from long-term
antimicrobial treatment (23).
Therefore, there is a demand for investigating the underlying
mechanism by which G. vaginalis induces BV. In the present
study, a novel mechanism was revealed, by which G. vaginalis
can activate the NLRP3/ASC/caspase-1 pathway in macrophages and
THP-1 cells to induce pyroptosis (Fig.
5C).
A number studies have suggested previously that the
level of cytokines and chemokines, such as TNF-α and IL-1β, is
upregulated in the vaginal fluid of women with BV (24,25).
Mechanisms underlying the host immune response to key BV-associated
bacteria have also been investigated using in vitro or in
vivo models (26,27). G. vaginalis and Atopobium
vaginae have both been reported to amplify proinflammatory
responses to Trichomonas vaginalis (28). Macrophages and monocytes are
commonly present in the lamina propria of vaginal tissues, which
have been shown to regulate the expression of innate immune
response receptors (12,29). During G. vaginalis infection,
proinflammatory cytokines are secreted from monocytes and
macrophages (14). The present
study confirmed that GV can significantly promote the production of
TNF-α, IL-1β and IL-18 in primary mouse macrophages and THP-1
cells. This is consistent with a previous study performed by
Anahtar et al (30), who
reported that Sneathia spp. can induce IL-1β and IL-18, but
not TNF-α, in vaginal epithelial monolayer cultures. Large
quantities of ROS are produced during the inflammatory process,
which aggravate cell injury and cell death (31). In the present study, GV was found to
promote the production of ROS in macrophages and THP-1 cells.
Previously, several studies have demonstrated that the production
of ROS is an important mechanism responsible for pyroptosis and
NLRP3 inflammasome activation (18,19).
Pyroptosis is a unique form of programmed cell death that is
mediated by inflammasome and caspase-1 activation (32,33).
Caspase-1-mediated pyroptosis has been suggested to serve an
important role in bacterial- and inflammation-related diseases,
including Trichomonas vaginalis infection and myocardial infarction
(34). In the present study, the
effects of GV on pyroptosis were assessed by Calcein-AM/PI double
staining, which revealed that GV promoted caspase-1
activation-mediated pyroptosis in macrophages and THP-1 cells.
Activation of caspase-1 during pyroptosis requires a
protein platform called inflammasomes (35). Among the inflammasome complexes
currently known, the NLRP3 inflammasome has been extensively
characterized and consists of NLRP3, ASC and pro-caspase-1
(36,37). Upon activation, NLRP3 recruits ASC
and induces the activation of caspase-1, thereby promoting the
secretion of proinflammatory cytokines to ultimately induce
pyroptotic cell death (38). Over
the past decade, the NLRP3 inflammasome is garnering attention due
to its reported involvement in the development of a variety of
diseases, including knee osteoarthritis, atherosclerosis and
mastitis (39). The NLRP3
inflammasome can be activated by a number of stimuli, including
bacterial, viral and mitochondrial damage (40). In addition, macrophages and
monocytes in the lamina propria of vaginal tissue can not only
secrete proinflammatory cytokines, but also regulate the formation
of various inflammasome complexes in response to multiple microbial
stimuli (12). In the present
study, to investigate if G. vaginalis can induce BV by
activating the NLRP3 inflammasome, the expression levels of NLRP3,
ASC, pro-caspase-1 and caspase-1 were measured by western blotting
and RT-qPCR. It was revealed that pretreatment with GV
significantly increased the protein and mRNA expression of NLRP3,
ASC and caspase-1 in macrophages and THP-1 cells. This supports the
notion that GV can promote the activation of NLRP3 inflammasomes in
macrophages and THP-1 cells, which is a novel pathological
mechanism by which G. vaginalis can mediate BV. However,
there are limitations in the present study. Only the impact of
G. vaginalis on the activation of the canonical inflammasome
was examined. In further experiments, the effects of G.
vaginalis on the activation status of non-canonical
inflammasomes in macrophages and THP-1 cells should be
assessed.
In conclusion, the present study demonstrated that
G. vaginalis can induce BV by promoting NLRP3
inflammasome-mediated pyroptosis. The findings of the present study
may provide a promising therapeutic direction for BV.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NX made substantial contributions to conception and
design. NX, TY and TC prepared the experimental materials,
performed the experiments, interpreted and analyzed the data, and
performed statistical analysis. TC revised and approved the final
version of the manuscript. NX, TY and TC confirmed the authenticity
of all the raw data. All authors read and approved the manuscript
and agree to be accountable for all aspects of the work in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Committee of
Ethical Animal Care and Use of Laboratory Animals of Shandong
Provincial Third Hospital (approval no. DWKYLL-2020001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joo HM, Hyun YJ, Myoung KS, Ahn YT, Lee
JH, Huh CS, Han MJ and Kim DH: Lactobacillus johnsonii
HY7042 ameliorates Gardnerella vaginalis-induced vaginosis
by killing Gardnerella vaginalis and inhibiting NF-κB
activation. Int Immunopharmacol. 11:1758–1765. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hawes SE, Hillier SL, Benedetti J, Stevens
CE, Koutsky LA, Wolner-Hanssen P and Holmes KK: Hydrogen
peroxide-producing lactobacilli and acquisition of vaginal
infections. J Infect Dis. 174:1058–1063. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ravel J, Gajer P, Abdo Z, Schneider GM,
Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO,
et al: Vaginal microbiome of reproductive-age women. Proc Natl Acad
Sci USA. 108 (Suppl 1):S4680–S4687. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pybus V and Onderdonk AB: Microbial
interactions in the vaginal ecosystem, with emphasis on the
pathogenesis of bacterial vaginosis. Microb Infect. 1:285–292.
1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sobel JD: Bacterial vaginosis. Ann Rev
Med. 51:349–356. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mendling W: Vaginal microbiota. Adv Exp
Med Biol. 902:83–93. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sobel JD: Vaginal infections in adult
women. Med Clin North Am. 74:1573–1602. 1990.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gergova RT, Strateva TV and Mitov IG:
Gardnerella vaginalis-associated bacterial vaginosis in
Bulgarian women. Braz J Infect Dis. 17:313–318. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Broz P: Inflammasomes: Intracellular
detection of extracellular bacteria. Cell research. 26:859–860.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Broz P and Dixit VM: Inflammasomes:
Mechanism of assembly, regulation and signalling. Nat Rev Immunol.
16:407–420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang T, Lang X, Xu C, Wang X, Gong T, Yang
Y, Cui J, Bai L, Wang J, Jiang W and Zhou R: CLICs-dependent
chloride efflux is an essential and proximal upstream event for
NLRP3 inflammasome activation. Nat Commun. 8(202)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Latz E: The inflammasomes: Mechanisms of
activation and function. Curr Opin Immunol. 22:28–33.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vick EJ, Park HS, Huff KA, Brooks KM,
Farone AL and Farone MB: Gardnerella vaginalis triggers
NLRP3 inflammasome recruitment in THP-1 monocytes. J Reprod
Immunol. 106:67–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schleicher U and Bogdan C: Generation,
culture and flow-cytometric characterization of primary mouse
macrophages. Methods Mol Biol. 531:203–224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jang SE, Jeong JJ, Choi SY, Kim H, Han MJ
and Kim DH: Lactobacillus rhamnosus HN001 and Lactobacillus
acidophilus La-14 attenuate Gardnerella vaginalis-infected
bacterial vaginosis in mice. Nutrients. 9(531)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li
XG, Li M, Wu YS, Li BL, Song WB, et al: Heatstroke induces liver
injury via IL-1β and HMGB1-induced pyroptosis. J Hepatol.
63:622–633. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Muzny CA, Łaniewski P, Schwebke JR and
Herbst-Kralovetz MM: Host-vaginal microbiota interactions in the
pathogenesis of bacterial vaginosis. Curr Opin Infect Dis.
33:59–65. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McDuffie RS Jr, Kunze M, Barr J, Wolf D,
Sze CI, Shikes R, Sherman M and Gibbs RS: Chronic intrauterine and
fetal infection with Gardnerella vaginalis. Am J Obstet
Gynecol. 187:1263–1266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hashemi FB, Ghassemi M, Roebuck KA and
Spear GT: Activation of human immunodeficiency virus type 1
expression by Gardnerella vaginalis. J Infect Dis.
179:924–930. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Workowski KA and Bolan GA: Centers for
Disease Control and Prevention. Sexually transmitted diseases
treatment guidelines, 2015. MMWR Recomm Rep. 64:1–137.
2015.PubMed/NCBI
|
|
24
|
Hedges SR, Barrientes F, Desmond RA and
Schwebke JR: Local and systemic cytokine levels in relation to
changes in vaginal flora. J Infect Dis. 193:556–562.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Zariffard MR, Novak RM, Lurain N, Sha BE,
Graham P and Spear GT: Induction of tumor necrosis factor- alpha
secretion and toll-like receptor 2 and 4 mRNA expression by genital
mucosal fluids from women with bacterial vaginosis. J Infect Dis.
191:1913–1921. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Gilbert NM, Lewis WG, Li G, Sojka DK,
Lubin JB and Lewis AL: Gardnerella vaginalis and
prevotella bivia trigger distinct and overlapping phenotypes
in a mouse model of bacterial vaginosis. J Infect Dis.
220:1099–1108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim DE, Kim JK, Han SK, Jang SE, Han MJ
and Kim DH: Lactobacillus plantarum NK3 and
Bifidobacterium longum NK49 alleviate bacterial vaginosis
and osteoporosis in mice by suppressing NF-κB-linked TNF-α
expression. J Med Food. 22:1022–1031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Łaniewski P, Gomez A, Hire G, So M and
Herbst-Kralovetz MM: Human three-dimensional endometrial epithelial
cell model to study host interactions with vaginal bacteria and
Neisseria gonorrhoeae. Infect Immun. 85:e01049–16.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen R, Richter HE, Clements RH, Novak L,
Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR,
Smythies LE and Smith PD: Macrophages in vaginal but not intestinal
mucosa are monocyte-like and permissive to human immunodeficiency
virus type 1 infection. J Virol. 83:3258–3267. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Anahtar MN, Byrne EH, Doherty KE, Bowman
BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A,
Sabatini ME, et al: Cervicovaginal bacteria are a major modulator
of host inflammatory responses in the female genital tract.
Immunity. 42:965–976. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li C, Wang X, Kuang M, Li L, Wang Y, Yang
F and Wang G: UFL1 modulates NLRP3 inflammasome activation and
protects against pyroptosis in LPS-stimulated bovine mammary
epithelial cells. Mol Immunol. 112:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019(8151836)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Danelishvili L and Bermudez LE: Analysis
of pyroptosis in bacterial infection. Methods Mol Biol. 1004:67–73.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tschopp J and Schroder K: NLRP3
inflammasome activation: The convergence of multiple signalling
pathways on ROS production? Nat Rev Immunol. 10:210–215.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miao EA, Leaf IA, Treuting PM, Mao DP,
Dors M, Sarkar A, Warren SE, Wewers MD and Aderem A:
Caspase-1-induced pyroptosis is an innate immune effector mechanism
against intracellular bacteria. Nat Immunol. 11:1136–1142.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sagulenko V, Thygesen SJ, Sester DP, Idris
A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill
JM, et al: AIM2 and NLRP3 inflammasomes activate both apoptotic and
pyroptotic death pathways via ASC. Cell Death Differ. 20:1149–1160.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao LR, Xing RL, Wang PM, Zhang NS, Yin
SJ, Li XC and Zhang L: NLRP1 and NLRP3 inflammasomes mediate
LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol Med Rep.
17:5463–5469. 2018.PubMed/NCBI View Article : Google Scholar
|