Introduction
One of the most important changes in the 5th edition
of the 2019 WHO classification of tumors of the digestive system is
the classification system of neuroendocrine neoplasms (NENs). NENs
arise in different organs and epithelial tissues and include
numerous entities with variate etiologies, clinical and
morphological aspects, and different evolutions (1). Over the years, the variable
classification methods used to evaluate these tumors generated a
considerable amount of confusion regarding the terminology and
histology of NENs.
In November 2017, a dedicated consensus meeting was
held in Lyon at the International Agency for Research on Cancer
(IARC), under the auspices of the WHO Classification of Tumors
Group (2). The consensus conference
established a unitary classification system for NENs, that was
later published in the 2018 WHO classification guide.
This novel system designed two new categories:
neuroendocrine tumors that are well-differentiated (NETs) and were
initially described as carcinoid tumors of the gastrointestinal
tract and a second category for neuroendocrine carcinomas (NECs)
that are poorly differentiated and, although they positive for most
neuroendocrine markers, have a poor prognosis and evolution
(3).
The actual classification and the tumor grading
algorithm are very similar to the one discussed in the 2017 WHO
classification of pancreatic NENs. Therefore, a new category was
generated, the well-differentiated grade 3 NETs of the digestive
system. The distinction between NETs and NECs with large cells
(LCNEC) and small cells (SCNEC) consists of different morphological
characteristics (1,4). The presence of both components, low
and high grade, in a unique tumor is a strong argument in favor of
naming NETs as grade 3 well-differentiated NETs. Mixed tumors, in
which each component, neuroendocrine and non-neuroendocrine,
represents more than 30 percent of the tumor cells, are termed
mixed neuroendocrine-non-neuroendocrine tumors or MiNENs (2).
Over the last 2-3 years, genetic studies have shown
that the genetic mutations in neuroendocrine tumor cells with
extra-pancreatic origin (especially in those of the
gastrointestinal tract) are very similar to those of the pancreas.
In gastrointestinal NECs, TP53 and RB1 mutations are frequently
encountered, similar to pancreatic and pulmonary NECs, but absent
in NETs. MEN1, DAXX and ATRX mutations are
characteristic of well-differentiated NETs (5,6).
Aside from the morphological and molecular aspects,
G3 NETs and NECs also differ from a clinical point of view.
Platinum-containing chemotherapy is successfully used in NECs,
sometimes with noteworthy results in SCNECs. Despite this aspect,
the observation was stated that some patients do not respond to
this therapy but have longer survival and better outcome than
patients that were responsive to platinum-based chemotherapy. This
subgroup of patients was subsequently diagnosed with G3 NETs
(1).
Gastroenteropancreatic NENs are characterized by the
overexpression of somatostatin receptors (SSTRs) 2 and 5, a family
of G protein-coupled receptors present in neuroendocrine cells
(7,8). Somatostatin is a cyclic neuropeptide
that is ubiquitously expressed in humans, acting as an inhibitor of
exocrine and endocrine secretions on target organs. It exerts its
biological effects by binding to five specific high-affinity
receptors on the cell surface (9).
Somatostatin then activates the second messenger system with a wide
range of actions: inhibition of adenylate cyclase, activation of
calcium channels, stimulation of phosphotyrosine phosphatase or
MAPK kinase activity (10). The
expression of these markers is the foundation for somatostatin
analogue therapy.
Adenocarcinoma ex-goblet cell carcinoid (AGCC), a
term proposed by Klimstra et al (1) and Tang et al (11) or mixed goblet cell
carcinoid-adenocarcinoma (12) is
an enigmatic entity, an amphicrine tumor with glandular/mucinous
and neuroendocrine differentiation (at least, focal
differentiation). Tang et al revealed that these tumors are
adenocarcinomas or AGCC, but not NECs (11). It seems that focal immunoreactions
to chromogranin A (CgA) and other neuroendocrine markers support
this hypothesis (12-14).
In the current WHO classification, these neoplasms are classified
as goblet cell adenocarcinomas (15).
Currently, only a few studies have followed
immunohistochemical (IHC) SSTR expression in gastrointestinal NENs,
although immunohistochemistry allows precise cellular localization
of SSTRs. In this study, the aims were to evaluate the IHC
expression of SSTR2 and SSTR5 in gastrointestinal NENs, MiNENs and
AGCCs, as well as to correlate the expression of these markers with
clinical and morphological factors that impact the overall
prognosis, outcome and treatment of the patients.
Patients and methods
Patient data
The retrospective study included 76 patients with
gastrointestinal NENs confirmed by histology and
immunohistochemistry at the Pathology Laboratory of Timis County
Emergency Clinical Hospital (Timisoara, Romania) from January 2008
to December 2018. The cases were selected according to
histopathological diagnosis and tissue material available for
pathological evaluation and IHC reactions. In 5 cases, the examined
tissue material was not sufficient to perform the required number
of sections for IHC investigation. Our study batch consisted of 52
gastrointestinal tumors (endoscopic biopsies, specimens of
polypectomy and surgical samples) and 19 cases of liver metastases
in the absence of evident primary disease. The median age of the
patients (37 men and 34 women) was 59.9 years. Patient clinical and
pathological characteristics are summarized in Table I.
 | Table IClinicopathological characteristics
of the patients with gastrointestinal NENs, MiNENs and AGCCs. |
Table I
Clinicopathological characteristics
of the patients with gastrointestinal NENs, MiNENs and AGCCs.
| Clinicopathological
characteristics | No. | % |
|---|
| Sex | | |
|
Male | 37 | 52.1 |
|
Female | 34 | 47.9 |
| Age at diagnosis
(years) | | |
|
<20 | 2 | 2.8 |
|
20-29 | 1 | 1.4 |
|
30-39 | 3 | 4.2 |
|
40-49 | 7 | 9.9 |
|
50-59 | 17 | 23.95 |
|
60-69 | 24 | 33.8 |
|
≥70 | 17 | 23.95 |
| Tumor location | | |
|
Stomach | 10 | 14.1 |
|
Duodenum | 2 | 2.8 |
|
Small
intestine | 10 | 14.1 |
|
Appendix | 6 | 8.5 |
|
Right
colon | 11 | 15.5 |
|
Left colon
(including rectum) | 13 | 18.3 |
|
Hepatic
metastasis | 19 | 26.7 |
| Tumor grade | | |
|
G1 | 28 | 39.4 |
|
G2 | 21 | 29.6 |
|
G3 | 22 | 31 |
| Tumor type | | |
|
NET | 52 | 73.2 |
|
NET G1 | 27 | 38 |
|
NET G2 | 18 | 25.4 |
|
NET G3 | 7 | 9.8 |
|
NEC | 12 | 17 |
|
SCNEC | 3 | 4.25 |
|
LCNEC | 9 | 12.7 |
|
MiNEN | 3 | 4.25 |
|
AGCCs | 4 | 5.6 |
|
AGCC G1 | 1 | 1.4 |
|
AGCC G2 | 1 | 1.4 |
|
AGCC G3 | 2 | 2.8 |
The study was conducted in accordance with
Declaration of Helsinki, in compliance with good clinical practice,
and was approved by the Ethics Committee of ‘Pius Brinzeu’
Emergency Clinical County Hospital and Victor Babes University of
Medicine and Pharmacy Timisoara (no. 20 b/2015 extended in 2019).
Written informed consent was obtained from each patient included in
our study.
Histological and IHC
interpretation
Tumors were reclassified according to the 2019 WHO
classification (Table II). IHC
stains were performed in all 71 cases. The specimens were fixed in
10% neutral-buffered formalin for a maximum of 24 h at room
temperature, paraffin-embedded and sectioned at 3- to 4-µm. IHC
staining was performed with a Leica Bond-Max, which is an automatic
and continuous access slide-staining system that simultaneously
processes IHC protocols, using a Bond Polymer Refine Detection Kit
(Leica Biosystems Newcastle). Ki67 (clone MM1, catalog no. PA0118),
CgA (clone 5H7, catalog no. PA0515), synaptophysin (Syn) (clone
27G12, catalog no. PA0299) and p53 (clone DO-7, catalog no. PA0057)
antibodies from Leica with ready-to-use (RTU) kits following the
manufacturer protocols were used. For the IHC detection of SSTR2
and SSTR5 antibodies, the following protocol was performed: tissues
were deparaffinized and pre-treated with the Epitope Retrieval
Solution 1 at 98˚C for 20 min. Specimens were then incubated with
the primary antibody for 30 min at a dilution of 1:150 for SSTR2
(clone UMB1, Abcam, catalog no. ab134152) and 1:125 for SSTR5
(clone UMB4, Abcam, catalog no. ab109495), followed by
visualization with a Leica Bond Polymer Refine Detection kit for 20
min at room temperature. Finally, the sections were washed in water
and counterstained with hematoxylin. Appropriate negative and
positive controls were generated with satisfactory staining. The
slides were examined under the Eclipse E200 Nikon microscope.
 | Table IIClassification and grading criteria
for NENs of the gastrointestinal tract (1). |
Table II
Classification and grading criteria
for NENs of the gastrointestinal tract (1).
| Terminology |
Differentiation | Grade | Mitotic
ratea | Ki-67
indexb |
|---|
| NET, G1 |
Well-differentiated | Low | <2 | <3% |
| NET, G2 | | Intermediate | 2-20 | 3-20% |
| NET, G3 | | High | >20 | >20% |
| SCNEC | Poorly
differentiated | High | >20 | >20% |
| LCNEC | | | >20 | >20% |
| MiNEN | Well or poorly
differentiated | Variable | Variable | Variable |
Cases were scored as focal or diffuse positive for
cytoplasmic staining of the tumor cells with CgA and Syn
antibodies. The proliferation index Ki-67 represents the percentage
of cells with nuclear expression of a total of 500 tumoral cells in
hot-spots. In biopsies where only a small number of tumor cells
were present, all tumor cells were counted. Tumors with a mitotic
rate >20% and a Ki-67 proliferation index of >20% were IHC
evaluated for the expression of p53. The immunoexpression was
considered positive if intense nuclear staining was present in
>25% of the tumor cells. Strongly positive p53 was considered
abnormal and indicated mutations in the TP53 gene (16,17).
The expression of SSTR2 was evaluated according to
the system proposed by Volante et al (18). Therefore, the absence of the IHC
expression was graded with 0, a cytoplasmic expression, either
focal or diffuse, with 1, and the presence of a membranous
reactivity in <50% of the tumor cells (irrespective of the
presence of cytoplasmic staining) with a score 2. The membranous
expression in >50% of tumor cells (not taking the cytoplasmic
staining into consideration) was scored as 3. The cases that scored
0 and 1 were considered negative, and those with 2 and 3 were
positive. The immunoreactions for SSTR5 were negative if <10% of
the tumor cells presented cytoplasmic or membranous staining, and
positive if >10% had a cytoplasmic or membranous reactivity.
Statistical analysis
Statistical analyses were performed with GraphPad
Prism 9.0 (GraphPad Software, Inc.). Descriptive statistics of
qualitative data such as patient general data, site, grade and type
are expressed as numbers and percentages. The results of SSTR2 and
SSTR5 expression analysis were compared in terms of various
clinicopathological data, including tumor location, grade, type and
stage. Statistical evaluation was performed utilizing χ2
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
According to the new WHO classification of
2019(1), 52 well-differentiated
NETs, 12 NECs with small or large cells, 3 MiNENs and 4 cases of
AGCC were included in the study (Table
I). The median age of the patients (37 men and 34 women) was
59.9 years. Although the incidence of NETs increases significantly
after the age of 50, 3 patients with NET were under 30 years of
age. In addition, 19 metastatic NETs that were diagnosed after
hepatic surgery or for which core biopsy was performed were
identified. The neuroendocrine nature of the tumors that were
included herein was confirmed by documenting a positive focal or
diffuse expression for at least one neuroendocrine marker (CgA or
Syn).
Primary NENs were more frequently diagnosed in the
left colon including the rectum (18.3%), the right colon (15.5%)
and in the small intestine (14.1%); however, the most frequent
tumors were hepatic metastasis of NEN (26.7%) (Table I). The majority of NENs were
well-differentiated tumors (39.4%), although in 6 cases, hepatic
metastasis was present at the time of the diagnosis (Table I).
In the 2019 WHO classification, both
well-differentiated G3 NETs and G3 NECs are characterized by a
mitotic rate of >20% and a Ki-67 proliferation index of >20%.
The histological examination of the NECs revealed solid sheets or
trabeculae of large cells with pale eosinophilic cytoplasm,
vesicular, pleomorphic nuclei with large nucleoli and a high
mitotic rate (LCNEC) or areas of small cells with scant cytoplasm
and hyperchromatic nuclei (SCNEC), tumor necrosis and occasional
desmoplastic stroma. Although well-differentiated G3 NETs are
difficult to identify on histology alone, distinct areas of
organoid pattern of tumor cells and foci of tumor cells with
relatively monomorphic nuclei are highly suggestive of this type of
tumor. To correctly diagnose and classify these tumors,
immunoreaction for p53 was performed. Tumors with >25% intensely
positive cells for the above-mentioned marker were classified as G3
NEC. Two cases of MiNEN were identified in the left colon and one
with a gastric location. According to the 2019 WHO recommendations,
1 case of G1 adenocarcinoma in association with LCNEC and 2 cases
of G2 adenocarcinoma with G2 well-differentiated NETs were
identified. Additionally, 4 cases of AGCCs were included in the
study. According to the most recent WHO classification system, 2
cases of appendicular G1 and G2 AGCC and 2 cases of G3 AGCC in the
cecum/right colon were identified.
SSTR2/SSTR5 expression and
clinicomorphological factors
The immunoreactions for SSTR2 demonstrated a
membranous expression of variable intensity in 47 cases (66.2%)
(Table III; Fig. 1). The cytoplasmic expression was
found in 6 cases (8.5%) that were scored 1 (negative). No case with
nuclear SSTR2 expression occurred.
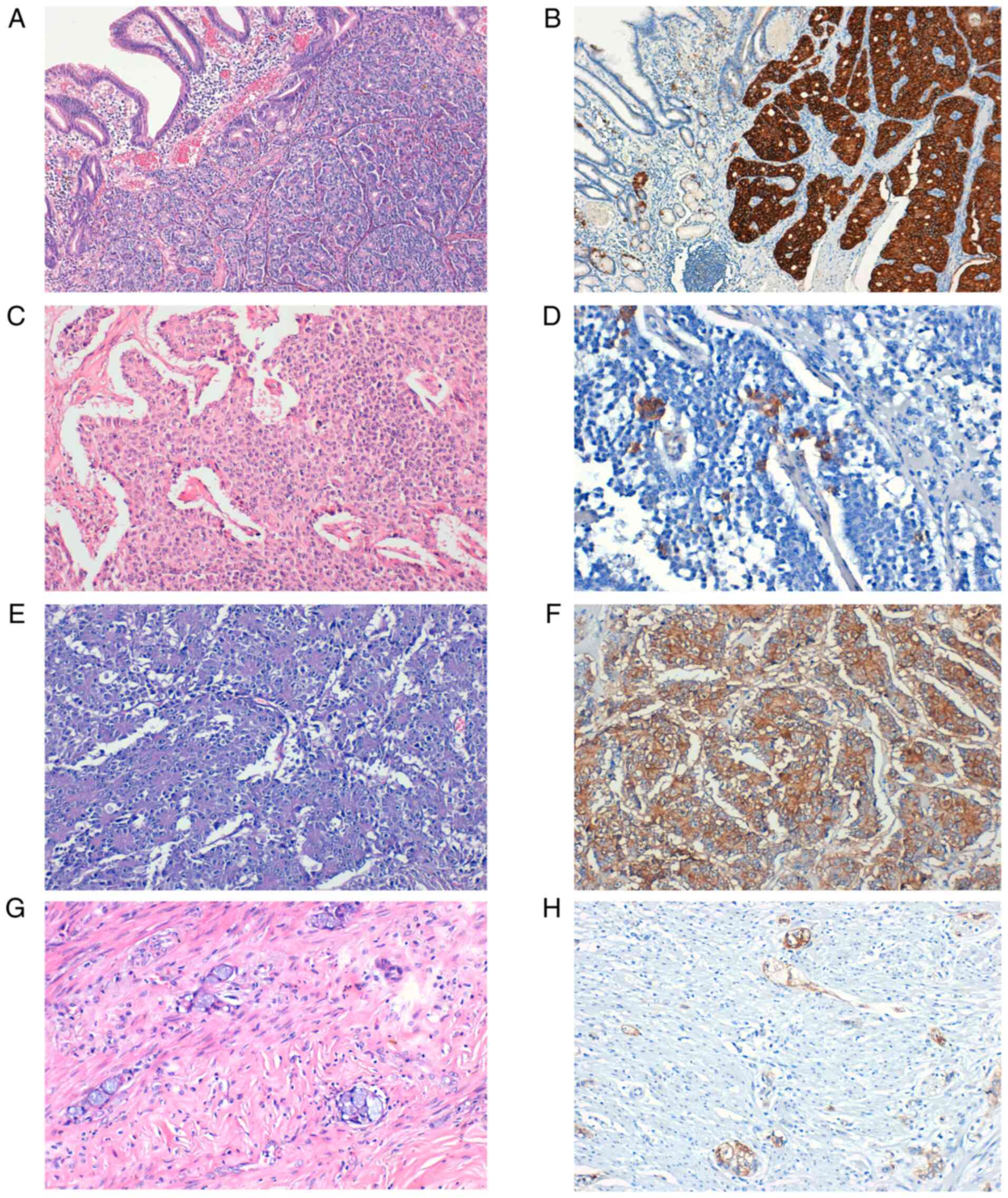 | Figure 1Expression of SSTR2 in NETs. (A)
Well-differentiated NET, G2. (B) Strong SSTR2 positive staining
(score 3), magnification x10. (C) Well-differentiated NET, G2. (D)
SSTR2 positive staining (score 2), magnification x40. (E) LCNEC.
(F) Strong SSTR2-positive staining (score 3), magnification x20.
(G) Appendiceal AGCC. (H) SSTR2-positive staining (score 3), x20
magnification. SSTR, somatostatin receptor; G, grade; NET,
neuroendocrine tumor; LCNEC, large cell neuroendocrine carcinoma;
AGCC, adenocarcinoma ex-goblet cell carcinoid. |
 | Table IIIThe correlation between SSTR2/SSTR5
expression and clinicomorphological factors. |
Table III
The correlation between SSTR2/SSTR5
expression and clinicomorphological factors.
| Clinicopathological
factors | No. | SSTR2+
cases n (%) | SSTR5+
cases n (%) |
|---|
| Tumor location | | | |
|
Stomach | 10 | 8(80) | 3(30) |
|
Duodenum | 2 | 0 (0) | 0 (0) |
|
Small
intestine | 10 | 10(100) | 5(50) |
|
Appendix | 6 | 6(100) | 1 (16.7) |
|
Right
colon | 11 | 5 (45.5) | 3 (27.3) |
|
Left colon
(including rectum) | 13 | 7 (53.8) | 5 (38.5) |
|
Hepatic
metastases | 19 | 11 (57.9) | 3 (15.8) |
| Tumor grading | | | |
|
G1 | 28 | 27 (96.4) | 10 (35.7) |
|
G2 | 21 | 5 (71.4) | 7 (33.3) |
|
G3 | 22 | 5 (22.7) | 3 (13.6) |
| Tumor type | | | |
|
NET | 52 | 39(75) | 17 (32.7) |
|
NET G1 | 27 | 26 (96.3) | 10(37) |
|
NET G2 | 18 | 12 (66.7) | 6 (33.3) |
|
NET G3 | 7 | 1 (14.3) | 1 (14.3) |
|
NEC | 12 | 4 (33.3) | 2 (16.7) |
|
MiNEN | 3 | 2 (66.7) | 1 (33.3) |
|
AGCCs | 4 | | |
|
AGCC G1 +
G2 | 2 | 2(100) | 0 (0) |
|
AGCC G3 | 2 | 0 (0) | 0 (0) |
| Tumor stage | | | |
|
I | 7 | 7(100) | 3 (42.9) |
|
II | 9 | 9(100) | 4 (44.4) |
|
III | 23 | 13 (56.5) | 8 (34.8) |
|
IV | 22 | 12 (54.5) | 3 (13.6) |
In total, 28.2% of the tumors presented a positive
cytoplasmic or membranous expression for SSTR5 (20 cases), with
variable intensity (Fig. 2).
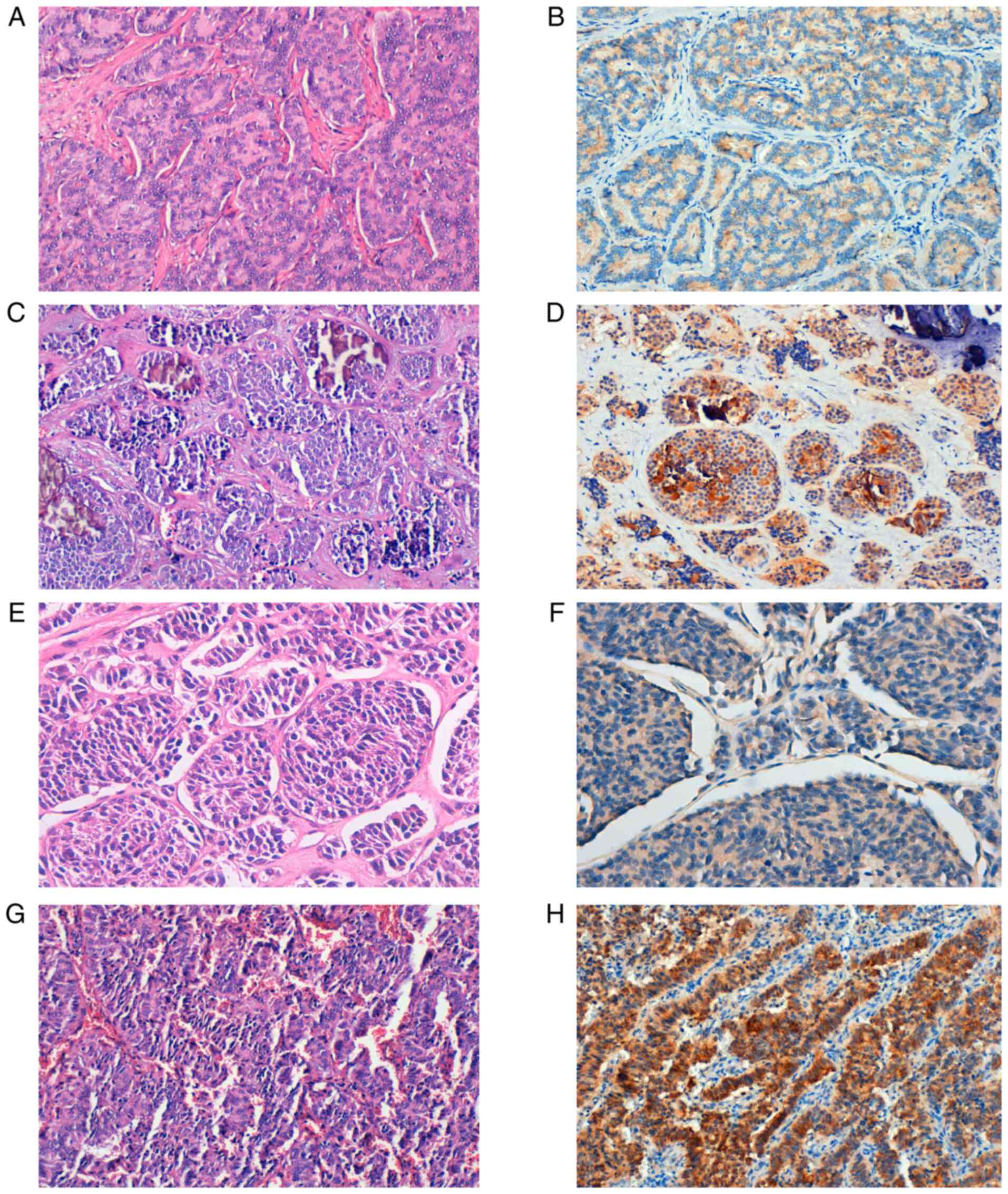 | Figure 2Expression of SSTR5 in NETs. (A)
Well-differentiated NET, G1. (B) SSTR5-positive staining,
magnification x20. (C) Well-differentiated NET, G2 with
microcalcifications. (D) SSTR5-positive staining, magnification
x20. (E) Well-differentiated NET, G3. (F) Weak SSTR5-positive
staining, magnification x40. (G) LCNEC. (H) Strong SSTR5-positive
staining, x20 magnification. SSTR, somatostatin receptor; G, grade;
NET, neuroendocrine tumor; LCNEC, large cell neuroendocrine
carcinoma. |
To simplify the interpretation, NEC, MiNEN and AGCC
were considered together in the final analysis (Table IV). NETs and NECs that showed a
positive staining for SSTR2, were more frequently found in the
small intestine (100% of the cases), appendix (100% of the cases)
and the stomach (66,66% of the cases). In addition, 50% of the
tumors in the small intestine were also positive for SSTR5. Well-
and moderately differentiated tumors exhibited significantly more
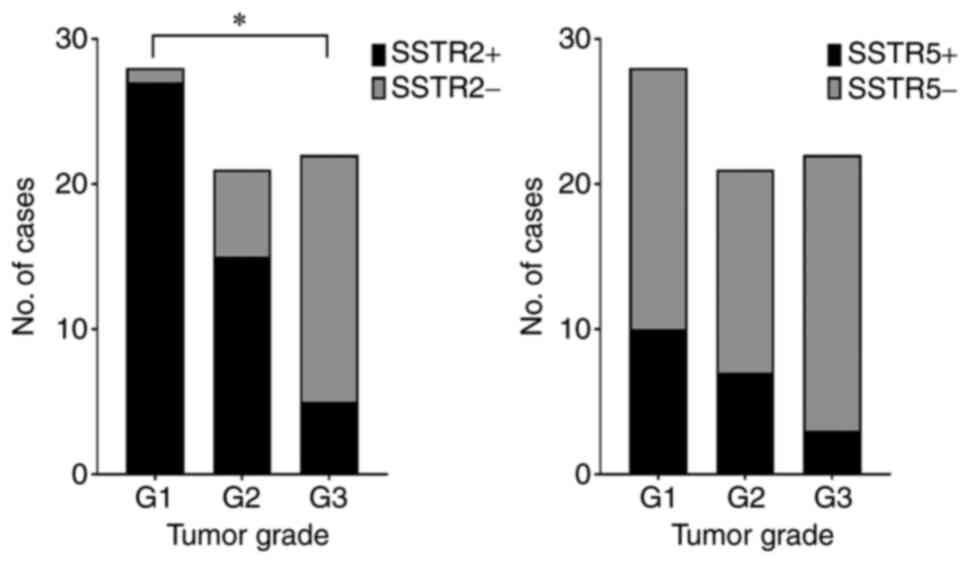
common SSTR2 in comparison with G3 tumors (P<0.0001; Fig. 3). In the present study group, 33.3%
of NECs and 14.3% of well-differentiated G3 NETs expressed SSTR2
(Table III). Both cases of
low-grade AGCC were SSTR2-positive. Poorly differentiated tumors
(NEC+MiNEN+AGCC) had significantly lower SSTR2 and SSTR5 expression
compared with NETs (P=0.0130 and P=0.0437, respectively).
 | Table IVSSTR2 and SSTR5 expression and
clinicopathological characteristics. |
Table IV
SSTR2 and SSTR5 expression and
clinicopathological characteristics.
| Clinicopathological
characteristic | No. | SSTR2+
cases n (%) | χ2
value | P-value | STR5+
cases n (%) | χ2
value | P-value |
|---|
| Tumor location | | | | | | | |
|
Stomach +
duodenum | 12 | 8 (66.66) | 1.252 | 0.869 | 3(25) | Non-valid | |
|
Small
intestine | 10 | 10(100) | | | 5(50) | | |
|
Appendix | 6 | 6(100) | | | 1 (16.7) | | |
|
Colon | 24 | 12(50) | | | 8 (33.33) | | |
|
Hepatic
metastases | 19 | 11 (57.9) | | | 3 (15.8) | | |
| Tumor grade | | | | | | | |
|
G1 | 28 | 27 (96.4) | 30.27 | <0.0001 | 10 (35.7) | 3.361 | 0.1863 |
|
G2 | 21 | 15 (71.4) | | | 7 (33.3) | | |
|
G3 | 22 | 5 (22.7) | | | 3 (13.6) | | |
| Tumor type | | | | | | | |
|
NET | 52 | 39(75) | 6.174 | 0.0130 | 17 (32.7) | 4.068 | 0.0437 |
|
NEC+MiNEN+AGCC | 19 | 8 (42.10) | | | 3 (11.53) | | |
| Tumor stage | | | | | | | |
|
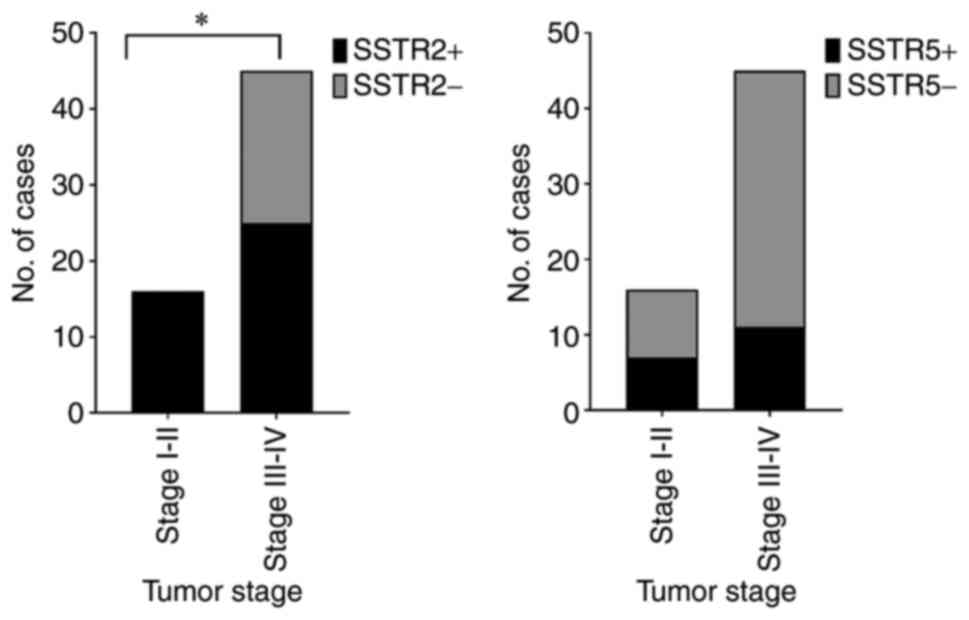
I+II | 16 | 16(100) | 3.253 | 0.0011 | 7 (43.8) | 2.115 | 0.1459 |
|
III+IV | 45 | 25 (55.6) | | | 11 (24.4) | | |
The expression rate of SSTR2 in tumors of stage I
and II was 100%, which was more frequent than tumors of stage III
and IV (55.6%; P=0.0011; Fig. 4).
NETs and NECs were diagnosed late, in the advanced stages of the
disease (stage III, 37.7% and stage IV, 36.1% vs. stage I, 11.5%
and stage II, 14.8%).
Discussion
NENs represent a heterogeneous group of tumors, with
various clinical presentations due to hormonal secretion from the
tumor cells. Patients with neuroendocrine carcinomas (NENs) often
exhibit flushing, diarrhea, bronchospasm, or suffer from
cardiovascular/valvular disease (19,20).
The new category of G3 neuroendocrine tumor (NET) was first
introduced in the 2017 WHO classification of pancreatic tumors and
later extended to all gastrointestinal NENs in 2019 (G1, G2 and G3
well-differentiated NEN) (1).
Epidemiological studies indicated that the incidence
of NENs has been on the increase, especially for cases diagnosed in
the early, asymptomatic stages of the disease. The increase of
incidence may be due to earlier detection with the increased use of
endoscopy (20). According to
national cancer registries in Western Europe and the US National
Cancer Institute, Surveillance, Epidemiology and End Results, the
most significant increase in incidence was found for gastric and
rectal NENs. The small intestinal and cecal NENs showed only a
discrete increase. The overall estimated annual incidence of
gastroenteropancreatic NENs is between 3.6 and 3.9 per 100,000
population (21-23).
In line with these findings, the present study demonstrated that
the most common locations for primary NENs and mixed neuroendocrine
non-neuroendocrine neoplasm (MiNENs) were the left colon and rectum
(18.3%).
A significant increase was observed in NENs
incidence in patients older than 50 years. The patients were often
diagnosed between 60 and 69 years (33.8%), although 3 patients
presented at a young age (2 patients aged 19 years and 1 patient
was 25 years of age). Although endoscopy is an investigation
frequently used in our hospital, the study revealed that most NENs,
MiNENs and adenocarcinoma ex-goblet cell carcinoids (AGCCs) were
diagnosed and surgically removed in the late stages of the disease
(stage III, 37.7% and stage IV, 36.1% compared with stage I, 11.5%
and stage II, 14.8%).
NETs located in the stomach, duodenum (excepting
gastrinomas), pancreas and rectum, with sizes ≤10 mm and considered
G1 in the WHO classification, are considered by some authors
‘early’ NETs. These patients have an excellent prognosis.
Endoscopic mucosal or submucosal resection is recommendable ‘early
NETS’ (some authors include tumors that are <20 mm), with no
invasion of the muscularis propria (pT1) and no vascular invasion
(V0, L0) (24). In the current
study group, only three such tumors were diagnosed after
polypectomy and classified as well-differentiated G1 NETs, stage
I.
In the case of localized NENs, it is recommendable
to choose surgery adapted to tumor type, size, or multifocality of
the lesion. Hepatic metastases of NENs require a multidisciplinary
approach and are very challenging for surgeons, pathologists,
radiologists, oncologists and nuclear medicine physicians (20,25).
SSTR positron emission tomography/computed
tomography (PET/CT) using 68Ga-labeled somatostatin analogs is an
important method of evaluating the SSTR status in NENs (26,27).
At present, few studies have assessed the IHC expression of SSTR in
NENs, although IHC allows the correct identification of molecular
targets in tumor cells and evaluation of different targeted
therapeutic approaches. Patients with SSTR-positive tumors that are
both advanced and aggressive are optimal candidates for peptide
receptor radionuclide therapy (PRRT), increasing the overall
survival rate and quality of life (28).
In the present study, the IHC expression for SSTR2
was predominantly membranous, complete or incomplete, in 47 cases
(66.2%), and 28.2% of the tumors (20 cases) being positive for
SSTR5, with a cytoplasmic or membranous expression of variable
intensity. In addition, the co-expression of the two markers in 17
cases (23.9%) was identified much more often in well-differentiated
G1 (58.8%) and G2 (29.4%) NETs.
NETs and NECs that have a positive expression for
SSTR2 are strongly correlated with locations such as the small
bowel (100% of the cases), appendix (100% of cases) and stomach
(80% of cases). In addition, 50% of tumors from the small intestine
are positive for SSTR5.
Some studies reported that SSTR2 and SSTR5
expression showed an inverse correlation with neuroendocrine tumor
grade. Low to intermediate-grade tumors had increased SSTR
expression compared with high-grade tumors (29,30).
In line with these studies, our results showed that
well-differentiated G1 and G2 NETs express SSTR2 and SSTR5 more
frequently compared to high-grade NETs (96.3 and 66.7% compared
with 14.3%). A significant decrease was observed in SSTR2
expression with increasing malignancy in gastrointestinal NENs
(P<0.0001).
In the current study group 33.3% of NECs expressed
SSTR2 compared to 14.3% of the well-differentiated G3 NETs. Two
cases of LCNECs presented a positive IHC expression for SSTR2 and
SSTR5. Although our results are noteworthy, recent studies revealed
the importance of systemic treatment such as somatostatin analogs,
chemotherapy, PRRT or ‘sandwich’ chemo-PRRT (31,32).
Both cases of appendiceal low-grade AGCCs expressed
SSTR2. Although these tumors have a neuroendocrine differentiation
indicated by the positive expression of at least one neuroendocrine
marker (predominantly focal), the latest WHO classification lists
these tumors as carcinomas and not NETs (33). Scientific data are scarce despite
some studies proving the severe outcome of patients with AGCCs.
According to NANETS 2020 consensus guidelines (34) in limited disease, appendectomy is
advised for tumors <1 cm. Right hemicolectomy with lymph node
dissection is advised for tumors between 1 and 2 cm or >2 cm.
For advanced cases, there are no guidelines, although some authors
recommend the association of surgery and treatment with everolimus
and octreotide LAR.
By analyzing the immunoreactivity for SSTR2 and
SSTR5 according to tumor stage (1,35), it
was clear that the markers showed more frequently positive
reactions in the earlier stages of the disease (100% for SSTR2 and
43.8% for SSTR5) in comparison with advanced stages (55.6% for
SSTR2 and 24.4% for SSTR5). SSTR2 expression was significantly more
often positive in the first two stages of the disease (P=0.0011).
However, other studies reveal contradictory data. Srirajaskanthan
et al (30) found an
association between the SSTR2 expression and TNM stage I and II,
but not between SSTR5 expression and tumor stage. Other studies
showed no connection between SSTR expression and tumor location,
functional status, and TNM staging. However, those previous studies
mainly focused on particular types of gastroenteropancreatic NENs
or only on pancreatic tumors (36-39).
The current study has some limitations due to the
reduced number of some particular neoplasms, including G3 NETs and
MiNENs. Another drawback of the present study is the absence of
clinical follow-up and survival information, but there is no single
cancer registry in Romania. Despite these limitations, a large
number of heterogenic gastrointestinal tumors were included. In
addition, each tumor was re-evaluated according to the latest WHO
classification, monoclonal antibodies were used and the staining
protocols for SSTR2 and SSTR5 were optimized thoroughly in our
laboratory. The results allow us to make firm conclusions regarding
the association between SSTR expression and clinical and
pathological characteristics of patients with gastrointestinal
NENs.
In summary, immunohistochemistry is a useful and
reliable method for the detection of SSTRs in gastrointestinal NENs
and MiNENs, allowing the determination of the SSTR profile in the
clinical setting. Immunohistochemical expression of SSTRs varied
considerably depending on the tumor location, with higher values of
SSTR2 in NENs originating from the small bowel, appendix, and
stomach. SSTR2 expression is frequent in a range of NENs, MiNENs,
and AGCCs. The positive expression of SSTR2 in NECs and AGCCs
provides evidence for the usefulness of somatostatin analog
treatment associated with surgery and chemotherapy. SSTR2 and SSTR5
IHC markers significantly correlate with tumor grade and tumor
stage and can be considered potential prognostic factors. In our
opinion, the evaluation of IHC expression of SSTRs should be
encouraged in all NENs, MiNENs, and the particular group of
AGCCs.
Acknowledgements
Not applicable.
Funding
Funding: No funding is received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
OP contributed to the conception of the study,
collected, analyzed and interpreted data from the literature and
critically revised the manuscript. SMT contributed to the
conception of the study, performed the literature research, drafted
the manuscript and is responsible for confirming the authenticity
of all the raw data. SP contributed to the conception of the study,
performed the literature research, drafted the manuscript and is
responsible for confirming the authenticity of all the raw data.
ADP contributed to the interpretation of the data from the
literature, collected, analyzed and interpretated the data
corresponding to the patient and critically revised the manuscript.
RAB contributed to the interpretation of the data from the
literature and critically revised the manuscript. MC collected,
analyzed and interpretated the data corresponding to the patient
and critically revised the manuscript. AAP performed the literature
research, selected the included studies, analyzed and interpretated
the data and drafted the manuscript. ALCD contributed to the
conception of the study, performed the literature research,
selected the included studies, analyzed and interpretated the data
and drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with
Declaration of Helsinki, in compliance with good clinical practice,
and was approved by the Ethics Committee of ‘Pius Brinzeu’
Emergency Clinical County Hospital and Victor Babes University of
Medicine and Pharmacy Timisoara (no. 20 b/2015 extended in 2019).
Written informed consent was obtained from each patient included in
our study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest or competing interests.
Authors' information
Oana Popa: ORCID: 0000-0003-3883-2438; Sorina Maria
Taban: ORCID: 0000-0002-3971-2756; Stelian Pantea: ORCID:
0000-0002-6048-6909; Andrei Dorel Plopeanu: ORCID:
0000-0002-4900-4809; Robert Alexandru Barna: ORCID:
0000-0003-4634-969X; Marioara Cornianu: ORCID: 0000-0001-5675-5339;
Anca-Ariana Pascu: ORCID: 0000-0002-0027-1674; Alis Liliana Carmen
Dema: ORCID: 0000-0003-0767-2718.
References
|
1
|
Klimstra DS, Kloppel G, La Rosa S and
Rindi G: Digestive System Tumours. In: WHO Classification of
Tumours. 5th edition. Vol 1. IARC, Lyon, 2019.
|
|
2
|
Rindi G, Klimstra DS, Abedi-Ardekani B,
Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M,
El-Naggar AK, et al: A common classification framework for
neuroendocrine neoplasms: An international agency for research on
cancer (IARC) and World Health Organization (WHO) expert consensus
proposal. Mod Pathol. 31:1770–1786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Klöppel G: Neuroendocrine neoplasms:
Dichotomy, origin and classifications. Visc Med. 33:324–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cockburn A and Rege TA: Gastrointestinal
neuroendocrine lesions. In: Fenoglio-Preiser' Gastrointestinal
Pathology. 4th edition. Wolters Kluwer, Alphen aan den Rijn,
pp3300-3512, 2017.
|
|
5
|
Mafficini A and Scarpa A: Genetics and
epigenetics of gastroenteropancreatic neuroendocrine neoplasms.
Endocr Rev. 40:506–536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crona J and Skogseid B: GEP-nets update:
Genetics of neuroendocrine tumors. Eur J Endocrinol. 174:R275–R290.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cakir M, Dworakowska D and Grossman A:
Somatostatin receptor biology in neuroendocrine and pituitary
tumours: Part 1-Molecular pathways. J Cell Mol Med. 14:2570–2584.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Klomp MJ, Dalm SU, de Jong M, Feelders RA,
Hofland J and Hofland LJ: Epigenetic regulation of somatostatin and
somatostatin receptors in neuroendocrine tumors and other types of
cancer. Rev Endocr Metab Disord: Oct 21, 2020 (Epub ahead of
print).
|
|
9
|
Barbieri F, Bajetto A, Pattarozzi A, Gatti
M, Würth R, Thellung S, Corsaro A, Villa V, Nizzari M and Florio T:
Peptide receptor targeting in cancer: The somatostatin paradigm.
Int J Pept. 2013(926295)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hankus J and Tomaszewska R: Neuroendocrine
neoplasms and somatostatin receptor subtypes expression. Nucl Med
Rev Cent East Eur. 19:111–117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang LH, Shia J, Soslow RA, Dhall D, Wong
WD, O'Reilly E, Qin J, Paty P, Weiser MR, Guillem J, et al:
Pathologic classification and clinical behavior of the spectrum of
goblet cell carcinoid tumors of the appendix. Am J Surg Pathol.
32:1429–1443. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taggart MW, Abraham SC, Overman MJ,
Mansfield PF and Rashid A: Goblet cell carcinoid tumor, mixed
goblet cell carcinoid-adenocarcinoma, and adenocarcinoma of the
appendix: Comparison of clinicopathologic features and prognosis.
Arch Pathol Lab Med. 139:782–790. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hristov AC, Young RH, Vang R, Yemelyanova
AV, Seidman JD and Ronnett BM: Ovarian metastases of appendiceal
tumors with goblet cell carcinoidlike and signet ring cell
patterns: A report of 30 cases. Am J Surg Pathol. 31:1502–1511.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Reid MD, Basturk O, Shaib WL, Xue Y, Balci
S, Choi HJ, Akkas G, Memis B, Robinson BS, El-Rayes BF, et al:
Adenocarcinoma ex-goblet cell carcinoid (appendiceal-type crypt
cell adenocarcinoma) is a morphologically distinct entity with
highly aggressive behavior and frequent association with
peritoneal/intra-abdominal dissemination: An analysis of 77 cases.
Mod Pathol. 29:1243–1253. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Misdraji J, Carr NJ and Pai Rk:
Appendiceal goblet cell adenocarcinoma. In: WHO Classification of
Tumours. 5th edition. Vol 1. IARC, Lyon, pp149-151, 2019.
|
|
16
|
Murnyák B and Hortobágyi T:
Immunohistochemical correlates of TP53 somatic mutations in cancer.
Oncotarget. 7:64910–64920. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nielsen K, Binderup T, Langer SW, Kjaer A,
Knigge P, Grøndahl V, Melchior L, Federspiel B and Knigge U: P53,
Somatostatin receptor 2a and Chromogranin A immunostaining as
prognostic markers in high grade gastroenteropancreatic
neuroendocrine neoplasms. BMC Cancer. 20(27)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Volante M, Brizzi MP, Faggiano A, La Rosa
S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C,
et al: Somatostatin receptor type 2A immunohistochemistry in
neuroendocrine tumors: A proposal of scoring system correlated with
somatostatin receptor scintigraphy. Mod Pathol. 20:1172–1182.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vinik AI and Chaya C: Clinical
presentation and diagnosis of neuroendocrine tumors. Hematol Oncol
Clin North Am. 30:21–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang R, Zheng-Pywell R, Chen HA, Bibb JA,
Chen H and Rose JB: Management of gastrointestinal neuroendocrine
tumors. Clin Med Insights Endocrinol Diabetes.
12(1179551419884058)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ito T, Sasano H, Tanaka M, Osamura RY,
Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, et al:
Epidemiological study of gastroenteropancreatic neuroendocrine
tumors in Japan. J Gastroenterol. 45:234–243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
united states. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hofland J, Kaltsas G and de Herder WW:
Advances in the diagnosis and management of well-differentiated
neuroendocrine neoplasms. Endocr Rev. 41:371–403. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scherübl H and Cadiot G: Early
gastroenteropancreatic neuroendocrine tumors: Endoscopic therapy
and surveillance. Visc Med. 33:332–338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lewis MA and Hobday TJ: Treatment of
neuroendocrine tumor liver metastases. Int J Hepatol.
2012(973946)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hendifar AE, Ramirez RA, Anthony LB and
Liu E: Current practices and novel techniques in the diagnosis and
management of neuroendocrine tumors of unknown primary. Pancreas.
48:1111–1118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Calabrò D, Argalia G and Ambrosini V: Role
of PET/CT and therapy management of pancreatic neuroendocrine
tumors. Diagnostics (Basel). 10(1059)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bartsch DK and Scherübl H: Neuroendocrine
tumors of the gastrointestinal tract. Visc Med. 33:321–322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Righi L, Volante M, Tavaglione V, Billè A,
Daniele L, Angusti T, Inzani F, Pelosi G, Rindi G and Papotti M:
Somatostatin receptor tissue distribution in lung neuroendocrine
tumours: A clinicopathologic and immunohistochemical study of 218
‘clinically aggressive’ cases. Ann Oncol. 21:548–555.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Srirajaskanthan R, Watkins J, Marelli L,
Khan K and Caplin ME: Expression of somatostatin and dopamine 2
receptors in neuroendocrine tumours and the potential role for new
biotherapies. Neuroendocrinology. 89:308–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parghane RV, Ostwal V, Ramaswamy A,
Bhandare M, Chaudhari V, Talole S, Shrikhande SV and Basu S:
Long-term outcome of 'Sandwich' chemo-PRRT: A novel treatment
strategy for metastatic neuroendocrine tumors with both FDG- and
SSTR-avid aggressive disease. Eur J Nucl Med Mol Imaging.
48:913–923. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Basu S, Parghane RV, Kamaldeep and
Chakrabarty S: Peptide receptor radionuclide therapy of
neuroendocrine tumors. Semin Nucl Med. 50:447–464. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nagtegaal ID, Klimstra DS and Washington
MK: Tumours of the appendix. Digestive System Tumours. WHO
Classification of Tumours, 5th Edition, Edited by WHO
Classification of Tumours Editorial Board, 135-156, 2019.
|
|
34
|
Kunz PL, Reidy-Lagunes D, Anthony LB,
Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK,
Klimstra DS, et al: Consensus guidelines for the management and
treatment of neuroendocrine tumors. Pancreas. 42:557–577.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC (eds), et al: AJCC Cancer Staging Manual (8th edition).
Springer International Publishing, New York, NY, 2017.
|
|
36
|
Papotti M, Bongiovanni M, Volante M, Allìa
E, Landolfi S, Helboe L, Schindler M, Cole SL and Bussolati G:
Expression of somatostatin receptor types 1-5 in 81 cases of
gastrointestinal and pancreatic endocrine tumors. A correlative
immunohistochemical and reverse-transcriptase polymerase chain
reaction analysis. Virchows Arch. 440:461–475. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
van Adrichem RC, Kamp K, van Deurzen CH,
Biermann K, Feelders RA, Franssen GJ, Kwekkeboom DJ, Hofland LJ and
de Herder WW: Is there an additional value of using somatostatin
receptor subtype 2a immunohistochemistry compared to somatostatin
receptor scintigraphy uptake in predicting gastroenteropancreatic
neuroendocrine tumor response? Neuroendocrinology. 103:560–566.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Okuwaki K, Kida M, Mikami T, Yamauchi H,
Imaizumi H, Miyazawa S, Iwai T, Takezawa M, Saegusa M, Watanabe M,
et al: Clinicopathologic characteristics of pancreatic
neuroendocrine tumors and relation of somatostatin receptor type 2A
to outcomes. Cancer. 119:4094–4102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Y, Wang W, Jin K, Fang C, Lin Y, Xue
L, Feng S, Zhou Z, Shao C, Chen M, et al: Somatostatin receptor
expression indicates improved prognosis in gastroenteropancreatic
neuroendocrine neoplasm, and octreotide long-acting release is
effective and safe in Chinese patients with advanced
gastroenteropancreatic neuroendocrine tumors. Oncol Lett.
13:1165–1174. 2017.PubMed/NCBI View Article : Google Scholar
|