Introduction
Diminished ovarian reserve (DOR) refers to abnormal
ovarian function caused by various factors prior to the age of 40
years and results in associated symptoms, which is the occult
abnormal ovarian function period prior to ovarian failure
amenorrhea (1,2). Without any timely and effective
intervention, the ovaries of patients with DOR may gradually shrink
within 1 to 6 years, leading to premature ovarian failure (POF)
(3).
At present, the effect of androgens on follicular
development and female reproduction is under active investigation.
Androgens, including dehydroepiandrosterone (DHEA), DHEA-sulfate
(DHEA-S), androstenedione (A4), testosterone (T) and
dihydrotestosterone (DHT), are important hormones in the female
endocrine and reproductive systems. DHEA, DHEA-S and A4 must be
bioconverted to T or DHT to have a physiological role (4,5). It
has been suggested that the androgen effect is generated when
androgens are converted to estradiols under the action of aromatase
in granulosa cells; the produced estradiols then act on estradiols
receptors. Further studies indicated that androgen itself
positively affects follicular development and T or DHT will
eventually bind to the androgen receptor (AR) to activate
downstream signaling pathways, such as follicle-stimulating hormone
receptor (FSHR) and microRNA (miRNA/miR)-125b to induce the
corresponding biological effect (6,7).
Moxibustion is a Traditional Chinese Medicine (TCM)
therapy. It uses Folium Artemisiae argyi to produce
moxibustion materials, generating heat upon combustion to stimulate
acupoints or specific body surface sites, thereby achieving disease
prevention and treatment. Increasing evidence has demonstrated that
moxibustion is able to improve ovarian function (8-12).
It has been reported that the early use of moxibustion is able to
generate a favorable stress response to resist or reduce the
subsequent disease and delay the degradation of normal tissues
(13). To date, studies on the
mechanisms underlying the improvement in ovarian function by
moxibustion have mainly focused on the
hypothalamic-pituitary-ovarian axis (HPOA) (14,15).
There is a lack of studies focusing on androgen balance and
AR-mediated signaling pathways. It remains elusive whether the
protective roles and mechanisms underlying moxibustion intervention
on ovarian function prior to the onset or at early stages of DOR
are different.
In the present study, moxibustion was performed on
the BL23 and RN4 acupoints of female rats daily for a total of 20
times. For this, two moxibustion groups were established with
different intervention times: One group was subjected to
pre-disease intervention and the other group to early-disease
intervention. The ovarian function was evaluated based on the
levels of several hormones related to ovarian function,
particularly androgens. To further investigate the downstream
regulatory factors of AR after moxibustion intervention prior to
the onset or at early stages of POF, FSHR and miR-125b expression
in ovaries were also analyzed. The present study focused on rat
physiology, providing a strong foundation and a complementary
understanding of moxibustion application as both physiological and
pathological research.
Materials and methods
Animal experiment
A total of 32 female Sprague-Dawley rats weighing
220-280 g and aged 8-12 weeks were provided by Shanghai
Super-B&K Laboratory (Animal production license no. SYXK,
Shanghai 2017-0002). The rats were maintained under a normal 12-h
light/dark cycle at 22±2˚C with 50-70% relative humidity. The rats
were allowed to adapt to the surrounding environment for two weeks.
Animals with a regular estrus cycle and the same estrus stage were
detected through vaginal smears. Rats were housed with four animals
per cage and food pellets and water provided ad libitum.
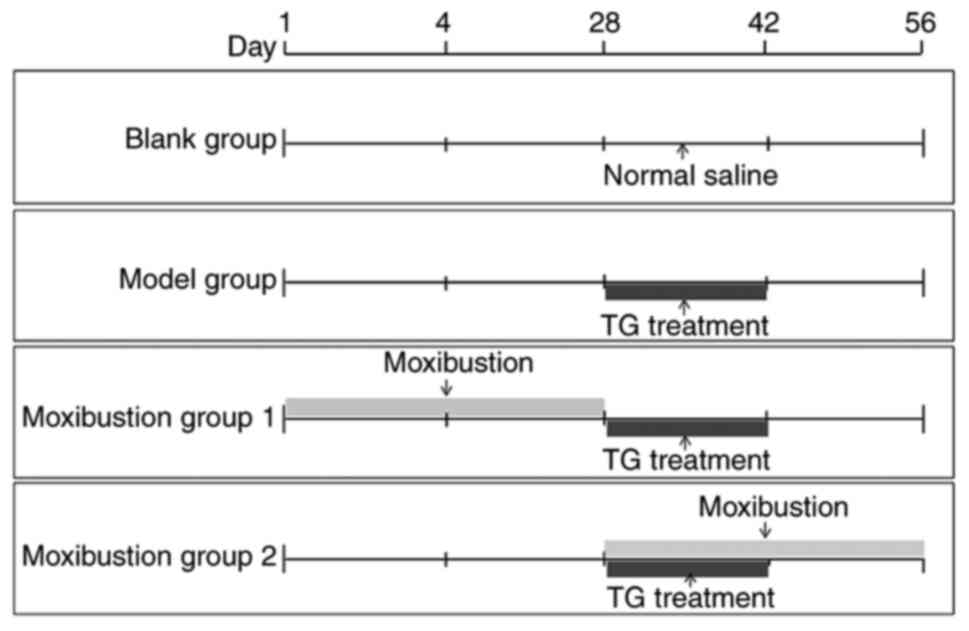
A total of 32 rats were randomly divided into four
groups (Fig. 1): Blank group
(normal saline was administered), Model group (rat model was
established via intragastric administration of tripterygium
glycosides), Moxibustion group 1 (rat model was established after 4
weeks of moxibustion treatment, making this the pre-disease
intervention group) and Moxibustion group 2 (rats were treated with
tripterygium glycosides and moxibustion for 2 weeks, followed by
another 2 weeks of moxibustion treatment, making this the
early-disease intervention group). The day of grouping was recorded
as day 1. All experimental procedures were approved by the Animal
Care and Use Committee of Nanjing University of Chinese Medicine
(Nanjing, China; no. ACU170709).
Observation of estrus stages
A thin cotton swab dipped in saline was gently
inserted into the vagina of the rats and used to scrape around the
cervix of the rats. The vaginal secretions were evenly smeared onto
slides. After 15 min of fixation, the vaginal secretions were
subjected to Pap staining and cytological examination was performed
under the microscope. The observation of estrus stages began on the
43rd day of the experiment and was continually performed once a day
for 14 consecutive days and ended on the 56th day. A summary of the
regularity of the estrus stages in the rats was generated at the
end of the study. The estrus stages of normal female rats lasted
for 4-5 days, including the proestrus, estrus, metestrus and
diestrus stages (16). ‘Extension
of estrus stages’ is defined as estrus stages that lasted >5
days and ‘disorder of estrous stages’ was defined as estrous cycles
that could not be observed or continued to be diestrus.
Model construction methods
The DOR model was established by referring to the
methods reported in the literature (17,18)
and began on the 29th day of the experiment (Fig. 1). Normal saline (2 ml) was
administered once a day by gavage in the blank group, while 2 ml of
tripterygium glycosides (Shanghai Fudan Fuhua Pharmaceutical Co.,
Ltd.; no. Z31020415) was administered at 75 mg/kg/day once a day IG
for 14 consecutive days in the other groups. The whole modeling
process was uneventful and no death occurred. If the vaginal smear
of the rats exhibited no change of the sexual cycle in >2 sexual
cycles or prolonged estrus stage of >6 days, the model was
considered to be successfully established.
Moxibustion application
The acupoint location was determined referring to
‘Experimental Acupuncture’ (19).
Moxibustion was applied to the bilateral ‘Shenshu’ acupoint (BL 23,
7 mm lateral to the spinous process of the second lumbar spine) in
the prone position and the ‘Guanyuan’ acupoint (RN 4, 25 mm below
the navel) in the supine position daily. The specific moxibustion
methods were performed as follows: Two assistants performed the
procedure. One researcher was responsible for holding the animals
with its hand, and the other researcher performed the moxibustion.
The rat was blindfolded. The neck of the rats were first touched to
quieten them and then the rats gently held in the hand without
anesthetization (20,21). Hair on the treatment area was shaved
and Vaseline was applied. A grain-sized moxa cone (5 mg of pure
moxa cone with a base of 3.0-3.5 mm and a height of 4-5 mm) was
placed on the acupoints using tweezers and ignited with a match
(the burning time of each cone was 8-10 sec, with a temperature of
48-52˚C at the acupoints). A new moxa cone was applied when the
prior one was completely burned. Seven grain-sized moxa cones were
applied at each acupoint per treatment for a total of 20 days (once
a day, five times a week for a total of 4 weeks). The duration of
one treatment session for each rat was ~3 min. The moxibustion in
moxibustion group 1 and moxibustion group 2 began on the 1st day
and the 29th day of the experiment, respectively (Fig. 1). The rats in the non-moxibustion
group were also fixed with the assistant's hand and blindfolded
synchronously. During and after the procedure, the wellbeing of the
rats was assessed by observing their behavior and attempting to
detect any moxibustion ulcer formation. It was observed that the
rats were quiet when treated with moxibustion: No foot lifting and
licking, no back arching, no tremor or spasm and regular breathing.
Following moxibustion, there were no moxibustion ulcers in any of
the rats with regular food and water consumption (22).
Hormone assays
Hormones were detected at the end of the experiment
to ensure that the blood samples were collected at the same stage
of the menstrual cycle. Whole blood samples (2 ml) were harvested
by a retro-orbital puncture after animals were anesthetized with a
single intraperitoneal injection of 7% chloral hydrate (350 mg/kg)
from eight rats in each group. The animals were sacrificed 10 min
after injection of chloral hydrate by cervical dislocation. Then,
the animals were sacrificed by cervical dislocation. The samples
were centrifuged at 1,500 x g for 20 min at room temperature and
the supernatants were collected. Serum anti-mullerian hormone
(AMH), FSH, estradiol (E2), T, DHEA and DHT were detected using the
rat AMH ELISA kit (cat. no. ml060605), FSH ELISA kit (cat. no.
ml002872), E2 ELISA kit (cat. no. ml002871), T ELISA kit (cat. no.
ml003368), DHEA ELISA kit (cat. no. ml003097) and DHT ELISA kit
(cat. no. ml002998), respectively (Shanghai Mlbio Co., Ltd.). The
optical density of the ELISA plates was measured at 450 nm by a
microplate reader.
Reverse transcription-quantitative
(q)PCR
The fresh ovarian tissue was separated from six
sacrificed rats in each group and they were used for RNA analysis.
Total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and was reverse
transcribed using a First-strand cDNA Synthesis kit (Invitrogen;
Thermo Fisher Scientific, Inc.). miRNA was harvested using the
miRcute miRNA isolation kit (Tiangen Biotech Co., Ltd.) and was
reverse transcribed using stem-loop primers and the Thermo
First-strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
The following primers were used: AR forward,
5'-GAGACGACACGATGGACAATT-3' and reverse,
5'-GCGGAAGGGAAACAGAAGTAT-3'; FSHR forward,
5'-TGAATGATTAAGAGGGACAAGC-3' and reverse,
5'-AAGCCCAGATTTACAGGACAG-3'; Rat 18S rRNA forward,
5'-GAATTCCCAGTAAGTGCGGGTCATA-3' and reverse,
5'-CGAGGGCCTCACTAAACCATC-3'. miR-125b forward,
5'-CGGGCTCCCTGAGACCCTAA-3' and reverse, 5'-CAGCCACAAAAGAGCACAAT-3',
miRNA/U6 forward, 5'-CCTGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'. The qPCR Fluorescence Quantitation kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the CFX384
Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.)
were used for detecting AR and FSHR expression. The
TaqMan® Fast Advanced Master Mix (Thermo Fisher
Scientific, Inc.) and the CFX384 Touch™ Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.) were used for detecting
miRNA-125b expression. The reaction for detecting AR and FSHR
expression was performed in a 10-µl system (5, 0.2, 0.2, 1, 0.2 and
3.4 µl of 2X ChamQ SYBR qPCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.), forward primer 10 µM, reverse primer 10
µM, template DNA, 50X ROX Reference Dye 1, nuclease-free
H2O, respectively). The program was set to two steps for
real-time quantitation: Initial denaturation was performed at 95˚C
for 10 min. Subsequently, each denaturation was 95˚C for 15 sec,
followed by an annealing elongation at 63˚C for 30 sec. The above
steps comprised one cycle and there were 40 cycles in total. The
reaction for detecting miR-125b expression was performed in a 20-µl
system [SDW 7.5 µl, TaqMan® Fast Advanced Master Mix
(2X) 10.0 µl, miRNA-125b forward 0.5 µl, universal miRNA-125b
reverse 0.5 µl, universal TaqMan probe 0.5 µl and template DNA 1.0
µl]. The program was set to two steps for real-time quantitation:
Initial denaturation was performed at 50˚C for 2 min. Subsequently,
each denaturation was 95˚C for 5 sec, followed by an annealing
elongation at 60˚C for 25 sec. The above steps comprised one cycle
and there were 40 cycles in total. The fluorescence value was read
during each extension stage and the dissolution curve was prepared
after the end of the cycle. Each sample was analyzed in triplicate
and ultimately, the relative expression levels of each gene were
calculated with the 2-∆∆Cq method (23).
Histopathology
The right ovaries from eight sacrificed rats in each
group were fixed in 4% paraformaldehyde for histopathological
examination. After fixation, each tissue sample was routinely
processed and embedded in paraffin. Subsequently, they were
sectioned at 4 µm thickness and stained with H&E for
observation. Ovarian follicles were classified into the primordial
follicle (an oocyte surrounded by one layer of flattened graunlosa
cells), the primary follicle (an oocyte surrounded by one layer of
cuboidal graunlosa cells), the secondary follicle (two or three
layers of cuboidal granulose cells with no antral space) and antral
follicle (more than four layers of granulosa cells with one or more
independent antral spaces) (16).
Atretic follicles contained ≥20 apoptotic granulosa cells,
disorganized granulosa cells, fragmentation of the oocyte nucleus
or a degenerating oocyte. The number of primary follicles,
secondary follicles, antral follicles and atretic follicles was
calculated according to the morphological characteristics of
follicles in the different groups.
Statistical analysis
Values are expressed as the mean ± standard
deviation. The statistical analysis was performed by SPSS 22.0
statistical software (IBM Corporation). Statistical significance
was determined by one-way ANOVA with Tukey's post-hoc test. The
difference in estrus stages among the different groups was analyzed
by using the χ2 test. P<0.05 was considered to
indicate statistical significance.
Results
Moxibustion stimulation improves
tripterygium glycoside-induced histopathological changes in
rats
As presented in Fig.
2 and Table I, compared with
the blank group, the number of atretic follicles was significant
increased in the model group (5.00±1.15 vs. 9.12±2.45, P<0.001),
while the number of mature follicular cells was decreased and the
ovarian granuloma cells were observed to be undergoing significant
apoptosis, with rare corpus luteum. Compared with the model group,
the number of atretic follicles in both moxibustion group 1 and
moxibustion group 2 were significantly reduced (9.12±2.45 vs.
7.00±3.15, P<0.05 and 9.12±2.45 vs. 5.44±1.69, P<0.05,
respectively). The number of mature follicular cells was increased
with enhanced corpus luteum and decreased apoptotic ovarian
granuloma cells as compared with the model group.
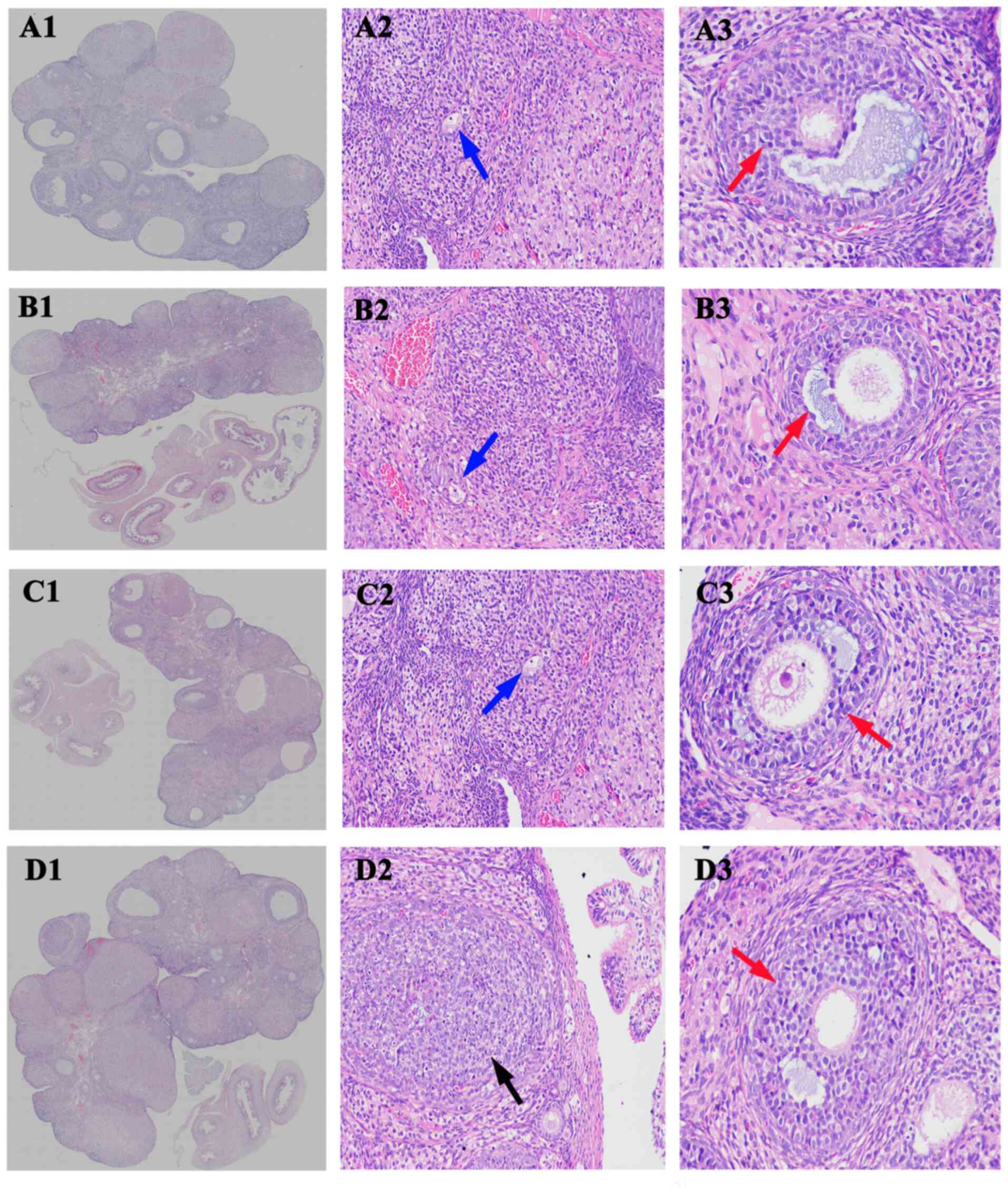 | Figure 2Moxibustion stimulation improves
tripterygium glycoside-induced histopathological changes in rats.
Histopathology images of ovaries from (A) the blank group, (B)
model group, (C) moxibustion group 1 and (D) moxibustion group 2
(H&E staining; original magnification, x20, x200 and x400, in
column 1, 2 and 3, respectively). The blue, red and black arrows
indicate atretic follicles, mature follicular cells and the ovarian
granuloma cells, respectively. |
 | Table INumber of various types of follicle
per ovary in the different groups. |
Table I
Number of various types of follicle
per ovary in the different groups.
| Group | Primary
follicles | Secondary
follicles | Antral
follicles | Atretic
follicles |
|---|
| Blank | 7.00±3.09 | 5.38±2.06 | 3.37±0.89 | 5.00±1.15 |
| Model | 4.50±2.97 |
3.50±1.26a | 2.71±1.44 |
9.12±2.45b |
| Moxibustion 1 | 4.90±2.02 |
5.57±1.91c | 3.50±1.96 |
7.00±3.15c |
| Moxibustion 2 |
7.33±3.25c,d | 4.67±1.94 | 4.89±5.14 |
5.44±1.69c |
| χ2 | 4.370 | 4.099 | 1.577 | 10.813 |
| P-value | 0.007 | 0.010 | 0.204 | 0.000 |
Changes in the estrus stages in
rats
As presented in Table
II, the estrus stage of rats in the blank group was regular,
while the stages were expanded to different degrees in the model
group, moxibustion group 1 and moxibustion group 2 in comparison to
the blank group, particularly in the model group (P<0.05). A
portion of the rats in the model group continued to exhibit
diestrus. No significant difference was observed between the
moxibustion group and the blank group. No significant difference
was observed between the moxibustion group and the model group.
 | Table IIChanges in estrus stages in the
rats. |
Table II
Changes in estrus stages in the
rats.
| Group | Total number | Normal | Extension | Disorder |
|---|
| Blank | 8 | 7 | 1 | 0 |
| Modela | 8 | 1 | 4 | 3 |
| Moxibustion 1 | 8 | 3 | 4 | 1 |
| Moxibustion 2 | 8 | 4 | 4 | 0 |
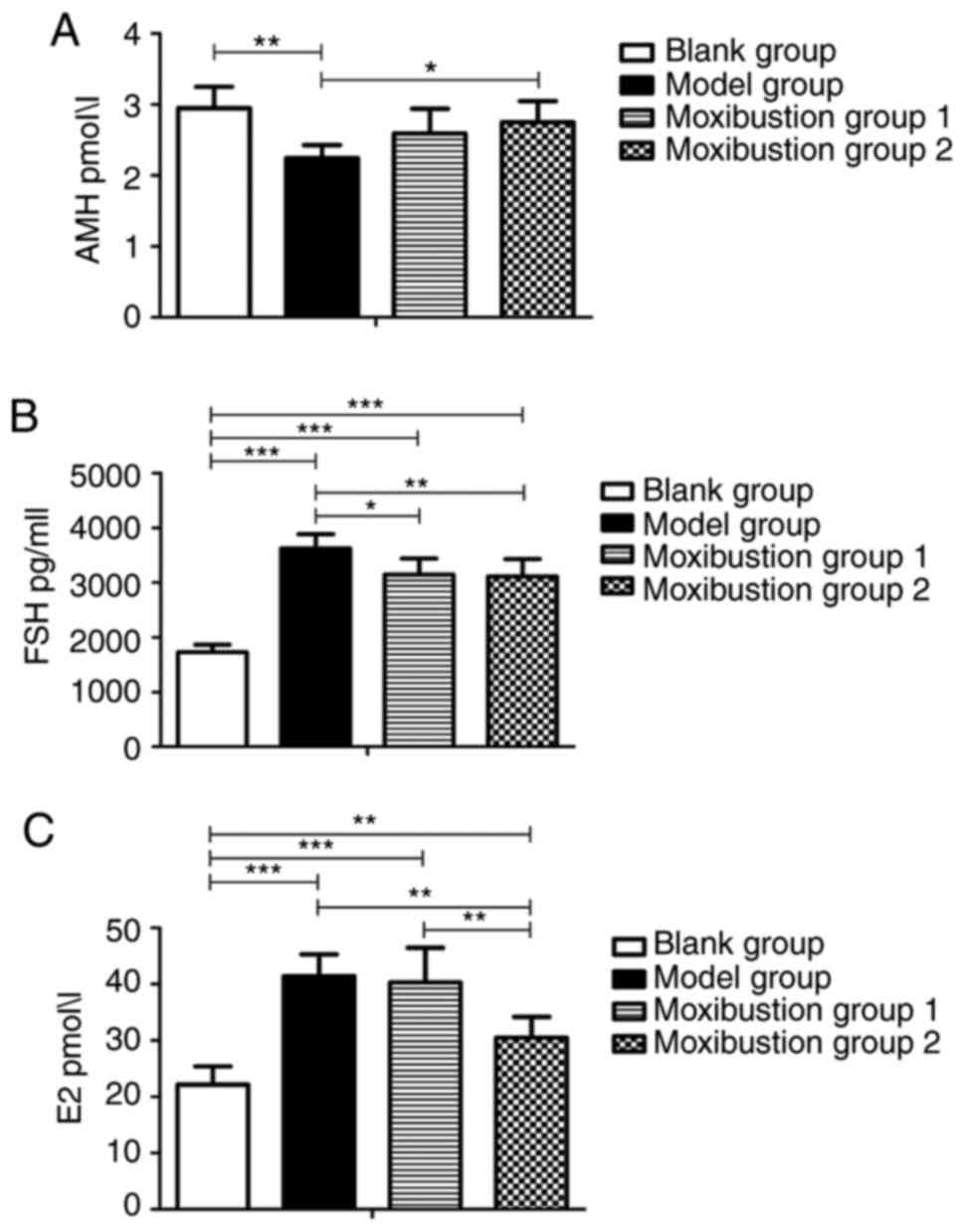
Moxibustion attenuates DOR-associated
changes in AMH, FSH and E2 in serum
The serum concentrations of AMH, FSH and E2 were
analyzed to evaluate ovarian function. As presented in Fig. 3A, AMH levels were significantly
reduced after modeling compared with the blank group (2.24±0.18 vs.
2.94±0.30 pmol/l, P<0.01), while they were increased in
Moxibustion group 1 and Moxibustion group 2 compared with the model
group (2.59±0.35 vs. 2.24±0.18 pmol/l, P=0.153 and 2.74±0.30 vs.
2.24±0.18 pmol/l, P<0.05, respectively). There was no difference
in AMH levels between the 2 moxibustion groups.
As presented in Fig.
3B, FSH levels significantly increased after establishing the
model (1,725.32±138.96 vs. 3,616.87±262.46 pg/ml, P<0.001). FSH
levels were reduced in Moxibustion group 1 and Moxibustion group 2
compared with the model group (3,142.52±290.95 vs. 3,616.87±262.46
pg/ml, P<0.05 and 3,109.37±317.38 vs. 3,616.87±262.46 pg/ml,
P<0.01, respectively). There was no difference in FSH levels
between the 2 moxibustion groups. As indicated in Fig. 3C, serum E2 levels were significantly
increased in the model group compared with the blank group
(41.45±3.88 vs. 22.14±3.24 pmol/l, P<0.001); they decreased
significantly in Moxibustion group 2 compared with the model group
(30.53±3.64 vs. 41.45±3.88 pmol/l, P<0.01). Significant
differences in E2 levels were observed between Moxibustion group 1
and Moxibustion group 2 (40.30±6.16 vs. 30.53±3.64 pmol/l,
P<0.01).
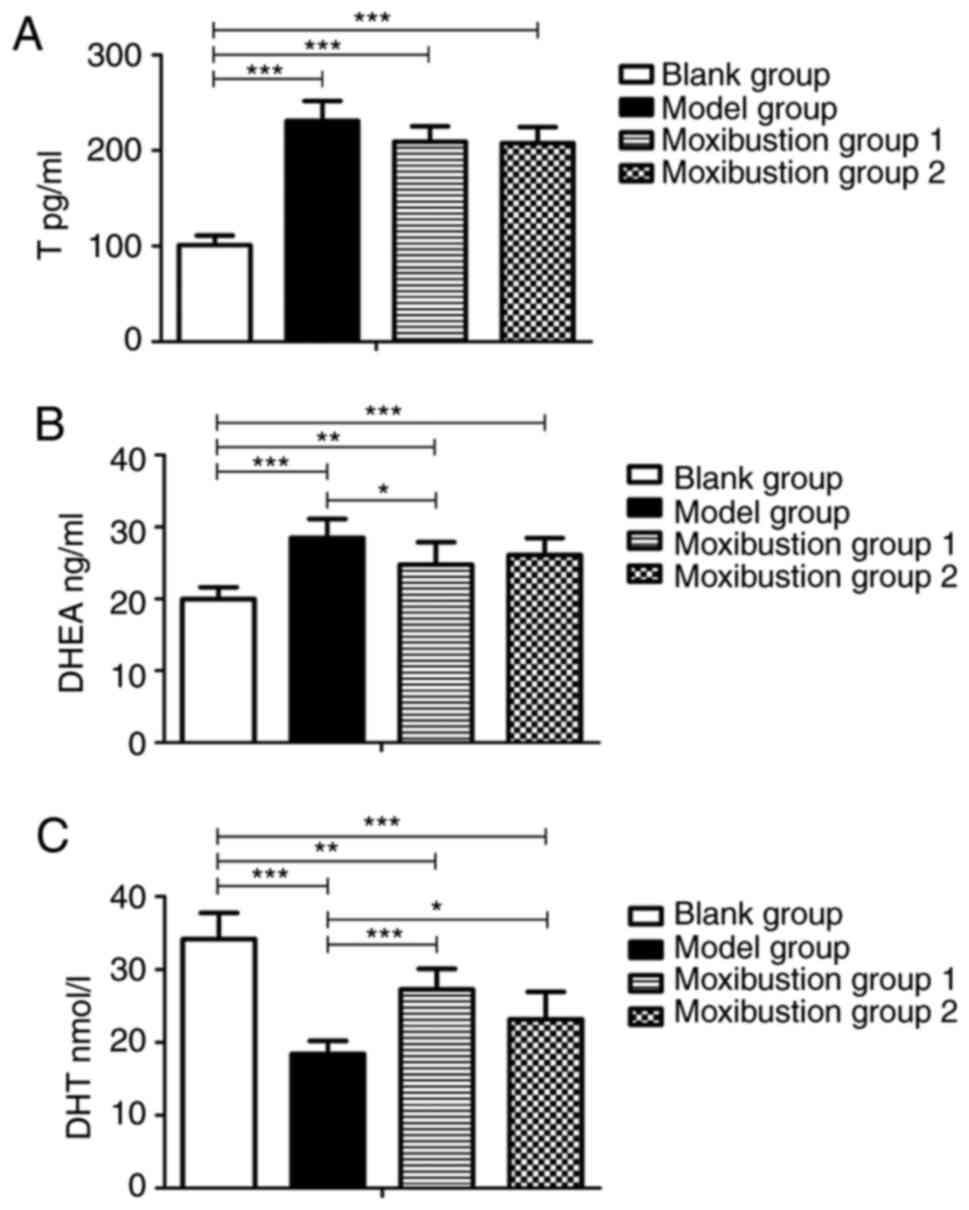
Moxibustion stimulation attenuates
DOR-associated changes in T, DHEA and DHT levels in serum
As T, DHEA and DHT are the three major androgens and
changes in their levels affect ovarian function, their serum levels
were analyzed to investigate whether there is an association
between androgen balance and ovarian function after moxibustion
treatment. As illustrated in Fig.
4A and B, T and DHEA levels
significantly increased after establishing the model (101.24±9.76
vs. 230.80±21.03 pg/l, P<0.001 and 19.98±1.63 vs. 28.49±2.63
ng/l, P<0.001, respectively). T levels were lower in Moxibustion
group 1 and Moxibustion group 2 compared with the model group
(209.10±16.22 vs. 230.80±21.03 pg/l, P=0.078 and 207.75±16.77 vs.
230.80±21.03 pg/l, P=0.064, respectively). No significant
difference in T levels was observed between moxibustion group and
model group. No difference in T levels was observed between the 2
moxibustion groups. Serum DHEA levels decreased in Moxibustion
group 1 as compared with the model group (24.77±3.11 vs. 28.49±2.63
ng/ml, P<0.05), but no significant difference was observed in
Moxibustion group 2 compared with the model group (26.10±2.36 vs.
28.49±2.63 ng/ml, P=0.324).
As presented in Fig.
4C, DHT levels significantly decreased after establishing the
model (34.20±3.60 vs. 18.41±1.81 nmol/l, P<0.001), while they
increased in Moxibustion group 1 and Moxibustion group 2 compared
with the model group (27.28±2.83 vs. 18.41±1.81 nmol/l, P<0.001
and 23.19±3.77 vs. 18.41±1.81 nmol/l, P<0.05, respectively). DHT
levels were higher in Moxibustion group 1 than in Moxibustion group
2 (27.28±2.83 vs. 23.19±3.77 nmol/l, P=0.058). No significant
difference in DHT levels was observed between Moxibustion group 1
and Moxibustion group 2.
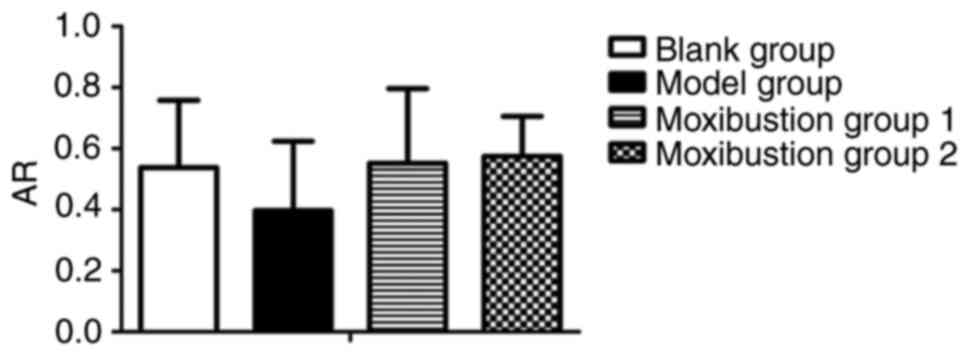
Effects of moxibustion stimulation on
AR expression in the ovary with DOR
AR is an essential factor required for the androgen
during follicular development. Thus, AR expression in the ovary was
determined to assess whether there was any change after moxibustion
treatment. As presented in Fig. 5,
the relative mRNA expression of AR was decreased after establishing
the model (0.54±0.22 vs. 0.40±0.23, P=0.193). Compared to the model
group, the AR content of the ovary was increased in Moxibustion
group 1 and Moxibustion group 2 (0.40±0.23 vs. 0.55±0.24, P=0.130
and 0.40±0.23 vs. 0.58±0.13, P=0.062, respectively). There was no
significant difference in AR mRNA expression between each
group.
Moxibustion stimulation induces
different changes in FSHR and miR-125b expression in the ovary
Since FSHR and miRNA-125b are two different
regulators of the AR-mediated signaling pathway, their expression
in the ovary was analyzed to investigate whether their expression
is affected by DOR and different methods of moxibustion treatment.
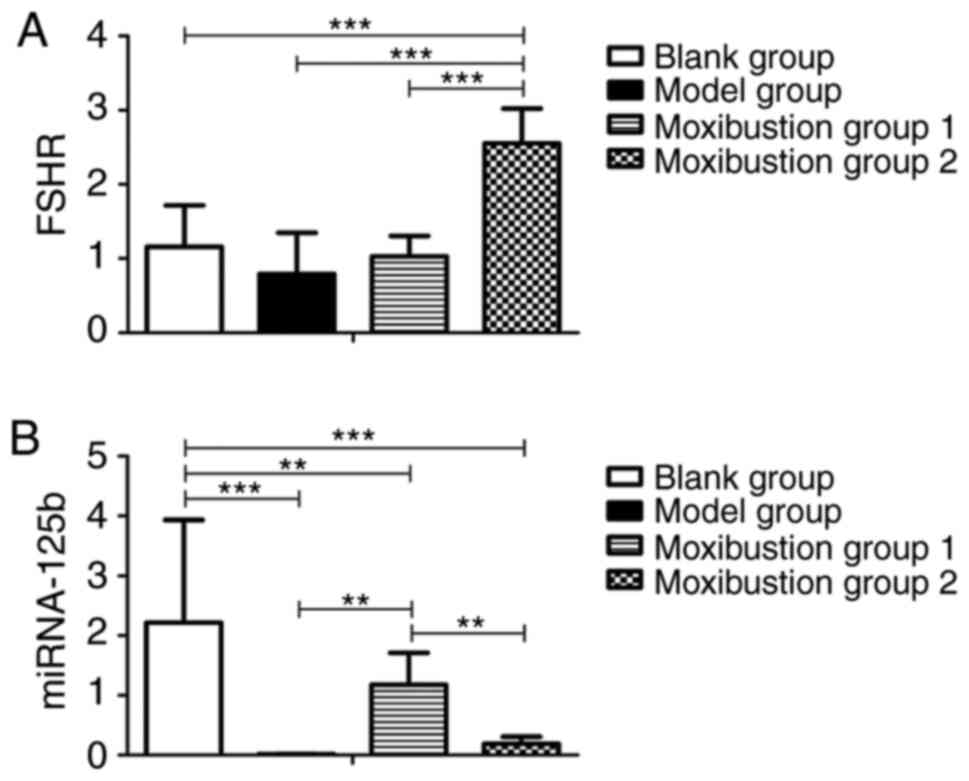
As presented in Fig. 6A and
B, the relative expression of FSHR
mRNA and miR-125b decreased after establishing the model (1.16±0.56
vs. 0.79±0.55, P=0.109 and 2.22±1.72 vs. 0.01±0.01, P<0.001,
respectively). There was no significant difference in FSHR mRNA
expression between blank group and model group. Compared to the
blank and model groups, the FSHR content in the rat ovary of
Moxibustion group 2 was significantly increased (1.16±0.56 vs.
2.55±0.47, P<0.001 and 0.79±0.55 vs. 2.55±0.47, P<0.001,
respectively). However, there was no difference in FSHR mRNA in the
ovaries between Moxibustion group 1 and the model group (1.03±0.28
vs. 0.79±0.55, P=0.457). miR-125b was not changed in Moxibustion
group 2 compared with the model group (0.19±0.12 vs. 0.01±0.01,
P=0.934), but a significant increase was observed in Moxibustion
group 1 (1.18±0.53 vs. 0.01±0.01, P<0.01). Significant
differences in FSHR and miR-125b mRNA expression were observed
between Moxibustion group 1 and Moxibustion group 2 (1.03±0.28 vs.
2.55±0.47, P<0.001 and 1.18±0.53 vs. 0.19±0.12, P<0.01,
respectively).
Discussion
Previous studies have indicated that moxibustion is
effective in improving ovarian function, but most of them focused
on treatments of disease states and there is a lack of studies on
‘prevention’. Wang et al (24) found that moxibustion could reduce
the rate of estrus cycle disorder, improve the level of serum sex
hormones and antioxidant stress in DOR rats, and the mechanism may
be related to the regulation of Nrf2/HO-1 signaling pathway. Yang
et al (13) found that
moxibustion could improve ovary function by suppressing apoptosis
events and upregulating antioxidant defenses in the natural aging
ovary.
Combined with the variation rule of reproductive
aging, the average fertility of females reaches a peak at the age
of 25 and then begins to decrease, declining rapidly after 35 years
of age (25). The reduced fertility
is manifested as a reduction in the number and quality of follicles
in the ovary. In addition to the natural aging of the ovary,
genetic, iatrogenic, environmental and psychological factors may
also cause a premature decline in ovarian function and eventually
DOR in certain females (26,27).
DOR is a precursor of POF. The transition from DOR to POF is a
gradual dynamic evolution process. If DOR cannot be treated in a
timely manner and prevented from progressing, POF will develop
(28-30).
Therefore, certain scholars have proposed a ‘peri-premature ovarian
failure stage’ (31) and the
gradual physiological decline in the ovary and DOR occur at this
stage. According to the TCM theories of ‘preventing disease in
healthy states’ and ‘preventing progression in disease states’
(32), if prevention measures are
applied prior to or early in the ‘peri-premature ovarian failure
stage’, they may effectively protect and improve ovarian function.
The experiments of the present study aimed to verify this
hypothesis.
The present study indicated that moxibustion prior
to establishing DOR in a rat model or during the early stage of
model establishment may protect ovarian function and alleviate
ovarian injury caused by Tripterygium wilfordii. The present
experiments demonstrated that after 2 weeks of tripterygium
glycosides administration, the serum levels of AMH, FSH and E2 in
rats were significantly different from those in the blank group.
The rats' estrus cycle in the model group was disordered,
indicating that the model was established successfully and stable.
Both pre-disease and early intervention with moxibustion rescued
the increase in FSH levels and the decrease in AMH levels induced
by tripterygium glycosides. The E2 level in moxibustion group 1 was
not different from that in the model group, while the E2 level in
moxibustion group 2 was significantly lower than that in the model
group. AMH, FSH and E2 are common indicators to evaluate ovarian
reserve and are frequently used jointly (33,34).
An increase in FSH levels is related to a decrease in ovarian
reserve. Elevated FSH levels promote the early recruitment and
growth of follicles and thus produce higher E2 levels. However, the
increase in FSH in the early stage of DOR is not stable. Due to the
negative feedback regulation of E2 on the secretion of FSH, the
change in E2 is earlier than that of FSH (35). Previous studies indicated that the
sensitivity and specificity of AMH in predicting ovarian response
are better than those of FSH and E2 (36,37).
The present results suggested that moxibustion intervention prior
to disease onset and during the early disease stage may improve
ovarian function. Of note, the effect of moxibustion treatment at
the early disease stage was better than that prior to disease onset
under the same treatment conditions.
The present study focused on the effect of
moxibustion on the androgen balance in a rat model of DOR. The
experimental results indicated that the T levels were reduced in
both moxibustion groups. T and E2 are positively correlated. When T
increases in vivo, the level of estradiol converted from T
by aromatase increases, thus causing a positive correlation between
the two, and the present results are consistent with those of a
previously published study (38).
However, the increase in serum T and E2 levels in rats with DOR
were compensatory. If ovarian function was to be further reduced
and decompensated, the levels of T and E2 may decrease (38). After model establishment, the serum
DHT levels in rats of the model group were lower than those in the
blank group, the DHT levels in the two moxibustion groups were
significantly higher than those in the model group and the serum
DHT levels in moxibustion group 1 were higher than those in
moxibustion group 2. A total of 20% of DHT is converted from T,
which is an indicator of androgen levels in peripheral tissues. DHT
does not convert to E2 without aromatase and T is a major indicator
reflecting the secretion of ovarian androgen (39,40).
Therefore, DHT and T may have different trends. The present study
also indicated that compared with the serum DHEA level in the blank
group, the serum DHEA level in the model group was higher. The
serum DHEA level in moxibustion group 1 was significantly lower but
the serum DHEA level in moxibustion group 2 was the same as that in
the model group. DHEA cannot directly exert its role and its
conversion depends on its own production and the expression levels
of metabolic enzymes in tissue. Furthermore, DHEA secretion has no
negative feedback regulatory mechanism; it is an unstable androgen
that acts as a ‘hormone buffer’ (41-44).
Therefore, after model establishment, higher DHEA levels may be
associated with inhibition of DHEA conversion by T.
wilfordii. However, prior to model establishment, moxibustion
treatment may significantly reduce DHEA, indicating that DHEA may
be one of the targets, revealing a mechanism underlying the
prevention of POF by moxibustion.
The present study also indicated that AR expression
in the model group rats was lower than that prior to model
establishment, while AR expression in the two moxibustion groups
was higher than that in the model group. AR is an important
essential in androgen-mediated signaling pathways. Studies have
indicated that the female model of overall AR knockout exhibited
reproductive defects, follicular development disorders and POF
(45,46). AR may promote follicular development
through two pathways (6). First, it
induces miR-125b expression, which inhibits granulose cell
apoptosis, avoiding follicular atresia. Furthermore, it enhances
FSHR expression, increases FSH sensitivity and promotes granulosa
cell proliferation and FSH-mediated follicular growth and
development. The present study also investigated the effect of
moxibustion on the expression levels of FSHR and miR-125b. It was
observed that the expression levels of FSHR and miR-125b in the
model group were lower than those prior to model establishment, but
the results for the two moxibustion groups were different. The
content of miR-125b in moxibustion group 1 was significantly higher
than that in the model group, while the miR-125b level in
moxibustion group 2 was the same as that in the model group.
However, the changes in the FSHR content were the opposite. In
brief, the FSHR levels in moxibustion group 1 were the same as
those in the model group, while the FSHR levels in moxibustion
group 2 were significantly higher than those in the model group and
even significantly higher than those in the blank group. Therefore,
it may be speculated that moxibustion intervention prior to model
establishment and at the early stage of model establishment may
improve ovarian function via modulating different AR signaling
pathways. Moxibustion treatment prior to model establishment is a
preventive measure before disease onset and its function may be
related to the delayed follicular atresia; moxibustion treatment at
the early stage of model establishment is a treatment method for
the early disease stage and its function may be related to an
increase of FSH sensitivity. Therefore, it may explain why the
outcomes of moxibustion group 1 and moxibustion group 2 were
different even though the moxibustion regimens, sites and degree of
stimulation were the same for the two moxibustion groups. Changes
occurred in the acupoints' functional status after disease onset
and action pathways change accordingly, which manifested as
‘enhanced’ acupoint functions, resulting in ‘large responses to
small stimulations’ (47,48). Studies have indicated that
acupuncture also has a regulatory function on the rat HPOA under
physiological conditions (49),
which may explain why the FSHR levels in the moxibustion group 2
were significantly higher than those in the blank group of the
present study.
A limitation of the study was that no placebo
moxibustion was performed in a designated sham group. This problem
will be addressed in in future studies. A higher concentration of
chloral hydrate was more likely to cause abdominal inflammation,
which could affect the ovaries that were harvested. Therefore,
chloral hydrate with concentration of <5% may be used for
anesthesia in future studies.
In summary, the present study suggested that
moxibustion intervention both prior to disease onset and at the
early disease stage improved ovarian function and their protective
roles are associated with AR-mediated androgen balance. However,
the effects and mechanisms of the two moxibustion interventions are
different. It may be speculated that to achieve the same ovarian
protection effect, the treatment course of moxibustion prior to
disease onset should be longer than that of moxibustion at the
early disease stage; however, the appropriate duration of treatment
and the time-effect relationship require to be studied further.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81603674, 81873371 and
81804179), the Natural Science Foundation of Jiangsu Province of
China (grant no. BK20161049), and Nanjing University of Chinese
Medicine ‘Construction Project of Superior Nursing Discipline in
Jiangsu Universities’ (grant no. 2019YSHL057).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XJ was responsible for experimental design, and was
a major contributor in writing the manuscript. JC, JS and YM were
responsible for collecting and analyzing data. XL, QL, HB and YL
were responsible for moxibustion and index detection. YX was
responsible for experimental design and paper revision. XJ and JS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of Nanjing University of Chinese
Medicine (Nanjing, China; no. ACU170709).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Devine K, Mumford SL, Wu M, DeCherney AH,
Hill MJ and Propst A: Diminished ovarian reserve in the United
States assisted reproductive technology population: Diagnostic
trends among 181,536 cycles from the Society for assisted
reproductive technology clinic outcomes reporting system. Fertil
Steril. 104:612–619.e3. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Greene AD, Patounakis G and Segars JH:
Genetic associations with diminished ovarian reserve: A systematic
review of the literature. J Assist Reprod Genet. 31:935–946.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao M, Feng F, Chu C, Yue W and Li L: A
novel EIF4ENIF1 mutation associated with a diminished ovarian
reserve and premature ovarian insufficiency identified by
whole-exome sequencing. J Ovarian Res. 12(119)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gleicher N, Kim A, Weghofer A, Kushnir VA,
Shohat-Tal A, Lazzaroni E, Lee HJ and Barad DH: Hypoandrogenism in
association with diminished functional ovarian reserve. Hum Reprod.
28:1084–1091. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vodo S, Bechi N, Petroni A, Muscoli C and
Aloisi AM: Testosterone-induced effects on lipids and inflammation.
Mediators Inflamm. 2013(183041)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sen A, Prizant H, Light A, Biswas A, Hayes
E, Lee HJ, Barad D, Gleicher N and Hammes SR: Androgens regulate
ovarian follicular development by increasing follicle stimulating
hormone receptor and microRNA-125b expression. Proc Natl Acad Sci
USA. 111:3008–3013. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prizant H, Gleicher N and Sen A: Androgen
actions in the ovary: Balance is key. J Endocrinol. 222:R141–R151.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shi XL, Zhao C, Yang S, Hu XY and Liu SM:
Moxibustion reduces ovarian granulosa cell apoptosis associated
with perimenopause in a natural aging rat model. Evid Based
Complement Alternat Med. 2015(742914)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang CR, Deng JL, Zhu WN, Miao MX, Shen
WW, Cao SJ and Tang Y: Involvement of PI 3 K/Akt/mTOR signaling in
protective effects of moxibustion for premature ovarian failure in
rats. Zhen Ci Yan Jiu. 43:75–79. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
10
|
Wu S and Yan J: Clinical observation on
premature ovarian failure by warming acupuncture at Zusanli (ST 36)
and Guanyuan (CV 4) combined with ginger moxibustion at Baliao
points. Zhongguo Zhen Jiu. 38:1267–1271. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Zhu S, Wang Y, Chang X, Chen H and Jin X:
The protective effect of pre-moxibustion on reproductive hormones
profile of rats with tripterygium glycosides-induced ovarian
damage. Complement Med Res. 27:401–409. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen J, Shen M, Li Z, Zhang R, Li X and Ai
B: Effects of moxibustion at Shenshu (BL 23) on level of sex
hormones and AMH in sub-health peri-menopausal women. Zhongguo Zhen
Jiu. 37:381–385. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Yang X, Wang W, Zhang Y, Wang J and Huang
F: Moxibustion improves ovary function by suppressing apoptosis
events and upregulating antioxidant defenses in natural aging
ovary. Life Sci. 229:166–172. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Y, Yu B, Chen J, Zhao Z, Wang Jiali,
Huang F, Lin Y, Wang M, Zhang Y and Wei B: Effects of acupuncture
on PI3K/Akt/mTOR signaling pathway in rats with premature ovarian
failure. Zhongguo Zhen Jiu. 35:53–58. 2015.PubMed/NCBI(In Chinese).
|
|
15
|
Xia L and Xia Y: Clinical research and the
effect mechanism on premature ovarian failure treated with
acupuncture in recent 20 years. Zhongguo Zhen Jiu. 38:5653–5670.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX,
Zhang XM, Liu WJ, Luo LL and Fu YC: SIRT1 activator (SRT1720)
improves the follicle reserve and prolongs the ovarian lifespan of
diet-induced obesity in female mice via activating SIRT1 and
suppressing mTOR signaling. J Ovarian Res. 7(97)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma M, Chen XY, Gu C, Xiao XR, Guo T and Li
B: Biochemical changes of oxidative stress in premature ovarian
insufficiency induced by tripterygium glycosides. Int J Clin Exp
Pathol. 7:8855–8861. 2014.PubMed/NCBI
|
|
18
|
Zhang T, Yan D, Yang Y, Ma A, Li L, Wang
Z, Pan Q and Sun Z: The comparison of animal models for premature
ovarian failure established by several different source of
inducers. Regul Toxicol Pharmacol. 81:223–232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo Y and Fang JQ: Experimental
acupuncture. Beijing: China Traditional Medicine Press, pp402-407,
2012.
|
|
20
|
Machholz E, Mulder G, Ruiz C, Corning BF
and Pritchett-Corning KR: Manual restraint and common compound
administration routes in mice and rats. J Vis Exp.
(2771)2012.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Buerge T and Weiss T: The laboratory
mouse: Handling and restraint. Elsevier, pp517-526, 2004.
|
|
22
|
Morton DB and Griffiths PH: Guidelines on
the recognition of pain, distress and discomfort in experimental
animals and an hypothesis for assessment. Vet Rec. 116:431–436.
1985.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Q, Lu G, Xie Z, Xie ZJ, Li HX and
Shen MH: Effect of moxibustion on Nrf2/HO-1 signaling pathway in
rats with diminished ovarian reserve. Zhongguo Zhen Jiu. 41:53–58.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
25
|
Li X and Xu CJ: Diseases of the female
reproductive system 1nd edition. Beijing: People's Medical
Publishing House, pp1-4, 2015.
|
|
26
|
Galey-Fontaine J, Cédrin-Durnerin I,
Chaïbi R, Massin N and Hugues JN: Age and ovarian reserve are
distinct predictive factors of cycle outcome in low responders.
Reprod Biomed Online. 10:94–99. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shatavi SV, Llanes B and Luborsky JL:
Association of unexplained infertility with gonadotropin and
ovarian antibodies. Am J Reprod Immunol. 56:286–291.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cohen J, Chabbert-Buffet N and Darai E:
Diminished ovarian reserve, premature ovarian failure, poor ovarian
responder-a plea for universal definitions. J Assist Reprod Genet.
32:1709–1712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li HF and Tan Y: Discussion on the
prevention and treatment of ovarian reserve function decline from
the idea of treating pre-disease. J Tradit Chin Med. 31:155–157.
2016.
|
|
30
|
Huang XC, Cao XC and Wang XY: Evaluation
and revision of TCM guidelines on premature ovarian failure. Henan
Tradit Chin Med. 39:82–86. 2019.
|
|
31
|
Liao HH, Zhao Y and Shi Y: ZHANG Yu-zhen's
ideas and approaches concerning treatment of premature ovarian
failure. Glob Tradit Chin Med. 8:780–782. 2015.
|
|
32
|
Meng JC and Wang XH: Internal classic.
Plain questions 1nd edition. Shanghai: Shanghai Science and
Technology Press, pp12-20, 2019.
|
|
33
|
Xu H, Zeng L, Yang R, Feng Y, Li R and
Qiao J: Retrospective cohort study: AMH is the best ovarian reserve
markers in predicting ovarian response but has unfavorable value in
predicting clinical pregnancy in GnRH antagonist protocol. Arch
Gynecol Obstet. 295:763–770. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zebitay AG, Cetin O, Verit FF, Keskin S,
Sakar MN, Karahuseyinoglu S, Ilhan G and Sahmay S: The role of
ovarian reserve markers in prediction of clinical pregnancy. J
Obstet Gynaecol. 37:492–497. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Du EQ, Gao X and Shang L: Assessments of
anti-mullerian hormone combining with FSH and FSH/LH in the
advanced maternal women during the reproductive year with ovarian
reserve. Med J West China. 30:701–703. 2018.
|
|
36
|
Zhang H, Luo Q, Lu X, Yin N, Zhou D, Zhang
L, Zhao W, Wang D, Du P, Hou Y, et al: Effects of hPMSCs on
granulosa cell apoptosis and AMH expression and their role in the
restoration of ovary function in premature ovarian failure mice.
Stem Cell Res Ther. 9(20)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lunding SA, Aksglaede L, Anderson RA, Main
KM, Juul A, Hagen CP and Pedersen AT: AMH as predictor of premature
ovarian insufficiency: A longitudinal study of 120 turner syndrome
patients. J Clin Endocrinol Metab. 100:E1030–E1038. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Song JM: Women testosterone levels and
ovarian reserve evaluation index and the correlation of IVF outcome
studies. Kunming: Kunming Medical University, pp26-27, 2016.
|
|
39
|
Walters KA and Handelsman DJ: Role of
androgens in the ovary. Mol Cell Endocrinol. 465:36–47.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gleicher N, Weghofer A, Lee IH and Barad
DH: FMR1 genotype with autoimmunity-associated polycystic
ovary-like phenotype and decreased pregnancy chance. PLoS One.
5(e15303)2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qin JC, Fan L and Qin AP: The effect of
dehydroepiandrosterone (DHEA) supplementation on women with
diminished ovarian reserve (DOR) in IVF cycle: Evidence from a
meta-analysis. J Gynecol Obstet Hum Reprod. 46:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Burger HG: Androgen production in women.
Fertil Steril. 77 (Suppl 4):S3–S5. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Labrie F and Labrie C: DHEA and
intracrinology at menopause, a positive choice for evolution of the
human species. Climacteric. 16:205–213. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gleicher N and Barad DH:
Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian
reserve (DOR). Reprod Biol Endocrinol. 9(67)2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Walters KA, Allan CM, Jimenez M, Lim PR,
Davey RA, Zajac JD, Illingworth P and Handelsman DJ: Female mice
haploinsufficient for an inactivated androgen receptor (AR) exhibit
age-dependent defects that resemble the AR null phenotype of
dysfunctional late follicle development, ovulation, and fertility.
Endocrinology. 148:3674–3684. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Walters KA, Middleton LJ, Joseph SR, Hazra
R, Jimenez M, Simanainen U, Allan CM and Handelsman DJ: Targeted
loss of androgen receptor signaling in murine granulosa cells of
preantral and antral follicles causes female subfertility. Biol
Reprod. 87(151)2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhu B: The plasticity of acupoint.
Zhongguo Zhen Jiu. 35:1203–1208. 2015.PubMed/NCBI(In Chinese).
|
|
48
|
Xu B and Han X: Discussion on primordial
and sensitized states of acupuncture points. Zhen Ci Yan Jiu.
43:273–276. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
49
|
Zhu H, Nan S, Suo C, Zhang Q, Hu M, Chen
R, Wan J, Li M, Chen J and Ding M: Electro-acupuncture affects the
activity of the hypothalamic-pituitary-ovary axis in female rats.
Front Physiol. 10(466)2019.PubMed/NCBI View Article : Google Scholar
|