Introduction
Asthma is a heterogeneous disease, usually
characterized by chronic airway inflammation. Severe asthma is
defined as asthma that remains uncontrolled despite adherence with
maximal optimized therapy (with high dose ICS-LABA) or that that
requires high dose ICS-LABA to prevent it from becoming
uncontrolled (1). Asthma is a
common, chronic respiratory disease affecting 1-18% of the
population worldwide. However, severe asthma remains underdiagnosed
and poorly managed despite the emergence of new effective
biological treatments (2).
Clinical recommendations regarding the treatment of
severe asthma, established by ERS (European Respiratory
Society)/ATS (American Thoracic Society) aimed to reduce the
exacerbation frequency and to improve the quality of life of these
patients. Specific attention has been made in individualized and
phenotypic-driven management (3).
It is argued that national and local asthma programs are more
effective than conventional treatment guidelines in improving
asthma care and reducing costs (4).
Omalizumab, the first novel biologic approved for
severe asthma changed the landscape in the management of the
disease. Omalizumab is a recombinant monoclonal anti-IgE antibody
that binds to free IgE and down-regulates high-affinity IgE
receptors on mast cells as well as basophils, eosinophils and
dendritic cells. It was the first and for a long time the only
biological drug available in clinical practice for the add-on
therapy of uncontrolled asthma. Its long-term effectiveness has
been widely demonstrated by various studies (5,6). The
second biologic therapy approved was Mepolizumab a humanized
monoclonal antibody against interleukin-5. IL-5 involves in the
maturation, recruitment and activation of eosinophils thus
anti-IL-5 treatment such as mepolizumab proved to be an effective
add-on treatment for severe eosinophilic asthma (7-9).
Although mepolizumab binds circulating IL-5, benralizumab is
another approved biologic agent for severe eosinophilic asthma and
binds to IL-5 receptor α subunit, leading to apoptosis of
eosinophils (1).
The prevalence of severe asthma varies widely among
the different countries (ranging from 3.6% in the Netherlands to
8.1% in Denmark) (10,11). The International Severe Asthma
Registry (ISAR) is the first global adult severe asthma registry
which aims to improve our understanding of severe asthma through
the implementation of existing and the generation of new knowledge
in this field (12). In Greece the
self-reported prevalence of physician diagnosed asthma was 9% in a
nation-wide survey (13) which was
in accordance with other recent surveys of self-reported prevalence
of asthma (9.1%) (14). The aim of
the present study was to provide real life data of referrals of
patients with mainly (but not exclusively) uncontrolled or
difficult to treat asthma in the community to an asthma expert
centre. An additional aim was the establishment of a severe asthma
registry in Crete since 2008 with characterization of patients at
their baseline assessment.
Materials and methods
Patients
Patients with asthma uncontrolled or difficult to
treat in the community, were mainly referred to the Heraklion
University Hospital's asthma expert center between 2008 and 2019.
The study was conducted in a retrospective manner.
All patients were evaluated systematically during a
1-day visit and according to the medical decisions made they were
distributed into the following 3 groups: Group 1, patients with
mild-moderate asthma; group 2, patients who were prescribed a step
4 or 5 asthma therapy according to Global Initiative for Asthma
(GINA) (1), such as medium/high
dose inhaled corticosteroids with or without LABA, oral
corticosteroid, tiotropium or had a change in a step 4 or 5
treatment during the first visit and group 3, patients under
treatment with a biologic agent such as omalizumab or
mepolizumab.
Patients' demographics were documented, including
age, gender and nationality as well as certain clinical parameters
such as the body mass index, smoking status (non-smoker, ex-smoker,
current smoker), history of comorbidities, allergies and asthma age
of onset (late-onset if 18 years old or older), history of
exacerbations in the last 12 months and use of inhaled medication
and oral steroids were recorded. Data on spirometry especially
pre-bronchodilation forced expiratory volume in 1 sec
(FEV1), and blood testing for blood eosinophil count,
total and specific serum IgE were provided where available.
The presence of self-reported comorbidities was
included in the evaluation such as: eczema, allergic rhinitis,
nasal polyps, chronic rhinosinusitis, atopic disease, hypertension,
diabetes, hyperlipidemia, chronic heart or other cardiovascular
disease, anxiety, depression, obstructive sleep apnea,
gastroesophageal reflux disease, medication intake such as
nonsteroidal anti-inflammatory drugs and angiotensin-converting
enzyme inhibitors.
Data on the patients' medication regarding asthma
and the Asthma Control Questionnaire 7 (ACQ7) were obtained. A
lower score corresponds to better asthma control, using a minimal
clinically important difference of 0.5, which indicates the minimal
difference in mean scores that is regarded as important (15,16).
Data presented for patients currently on biologics are from the
baseline assessment prior to initiation of biologics including
omalizumab, mepolizumab or combination.
Statistical analysis
Current analysis included data until December 2019.
Distribution of continuous variables was assessed. Data were
expressed as median (interquartile range), and for categorical
variables as counts (percentage). Statistical analysis was
performed using R studio [R version 3.6.2 (2019-12-12)].
Kruskal-Wallis test was used for comparison of non-parametric
continuous variables and Chi-square or Fisher's exact for the
comparison of categorical variables. P<0.05 was considered to
indicate a statistically significant difference. As anticipated in
a real life study, the data set contains a substantial number of
missing data.
Results
Patients' baseline
characteristics
Patients' baseline data are displayed in Table I. The total number of patients
included in the study was 198. A total of 109 patients (55.1%) were
characterized as difficult to treat asthma at the time of
presentation, whereas 63 patients (31.8%) were categorized as
having mild to moderate asthma and 26 (13.1%) out of the total were
already on biologic agent, either omalizumab (46.2%) or mepolizumab
(53.8%). The median age of asthma patients referred to our center
was 55 years old and 183 (68.5%) of the subjects were females.
Patients presenting with mild to moderate asthma were younger than
those with severe asthma and those already on biologic agent at the
time of presentation, in a statistically significant way (median
age of 45 vs. 57 and 60.5, respectively P<0.001). Most of the
patients included in the study were never smokers (154 patients,
58.3%) while the median BMI of the participants was 28.7.
 | Table IBaseline demographic characteristics
of patients with asthma. |
Table I
Baseline demographic characteristics
of patients with asthma.
| | Groups included in
present study (n=198) | |
|---|
| Characteristics | Group 1 (n=63) | Group 2 (n=109) | Group 3 (n=26) | Total registry
patients (n=267) | P-value |
|---|
| Age, years, median
(interquartile range) | 45 (32-59) | 57 (47-68) | 61 (44-67) | 55 (43-65) | <0.001 |
| Sex | | | | | 0.307 |
|
Male, n
(%) | 21 (33.3) | 26 (23.9) | 9 (34.6) | 84 (31.5) | |
|
Female, n
(%) | 42 (66.7) | 83 (76.1) | 17 (65.4) | 183 (68.5) | |
| Smoking status | | | | | 0.081 |
|
Non-smoker,
n (%) | 32 (51.6) | 63 (57.8) | 15 (57.7) | 154 (58.3) | |
|
Ex-smoker, n
(%) | 11 (17.7) | 27 (24.8) | 9 (34.6) | 59 (22.3) | |
|
Current
smoker, n (%) | 19 (30.6) | 19 (17.4) | 2 (7.7) | 51 (19.3) | |
|
Pack-yearsa, median (interquartile
range) | 0.0 (0.0-12.0) | 0.0 (0.0-20.0) | 2.5 (0.0-23.8) | 0.0 (0.0-17.0) | 0.498 |
| BMI, median
(interquartile range) | 26.7
(22.4-30.7) | 29.7
(24.8-33.2) | 30.4
(25.9-35.0) | 28.7
(24.6-33.1) | 0.025 |
Asthma parameters
The asthma related variables of the study population
are shown in Table II. Most
patients had late onset asthma (137 subjects, 75.7%). 8 patients
(3%) were on oral corticosteroids. The vast majority of patients
were classified in group 2. The median number of exacerbations
during the last 12 months was 1 (0-3).
 | Table IIAsthma parameters. |
Table II
Asthma parameters.
| | Groups included in
present study (n=198) | |
|---|
|
Characteristics | Group 1 (n=63) | Group 2
(n=109) | Group 3 (n=26) | Total registry
patients (n=267) | P-value |
|---|
| Asthma onset | | | | | 0.006 |
|
Early, n
(%) | 18 (40.9) | 13 (16.3) | 4 (17.4) | 44 (24.3) | |
|
Late, n
(%) | 26 (59.1) | 67 (83.8) | 19 (82.6) | 137 (75.7) | |
| Maintenance OCS, n
(%) | 0 (0.0) | 5 (4.6) | 1 (3.8) | 8 (3.0) | <0.001 |
| Exacerbations in
prior year, median (interquartile range) | 0 (0-1) | 1 (1-3) | 1 (1-3) | 1(2) | 0.001 |
| Predicted % FEV1,
median (interquartile range) | 93.4
(80.0-110.5) | 79.9
(63.9-94.5) | 79.4
(60.0-98.5) | 85.5
(70.0-100.8) | <0.001 |
| Serum positive
allergy test, n (%) | 11 (47.8) | 16 (53.3) | 7 (50.0) | 34 (50.7) | 0.922 |
| Current blood
eosinophil count, cells/µl, median (interquartile range) | 200
(100.0-308.0) | 342
(231.5-602.5) | 500
(400.0-800.0) | 300
(188.0-508.5) | 0.007 |
| Total serum IgE,
median (interquartile range) | 145.0
(49.0-400.0) | 94.3
(26.0-256.3) | 109.0
(28.0-473.0) | 117.5
(29.4-360.5) | 0.327 |
| ACQ7 score, median
(interquartile range) | 0.7 (0.4-1.3) | 1.1 (0.7-2.0) | 2.1 (1.4-3.1) | 1.1 (0.7-2.1) | <0.001 |
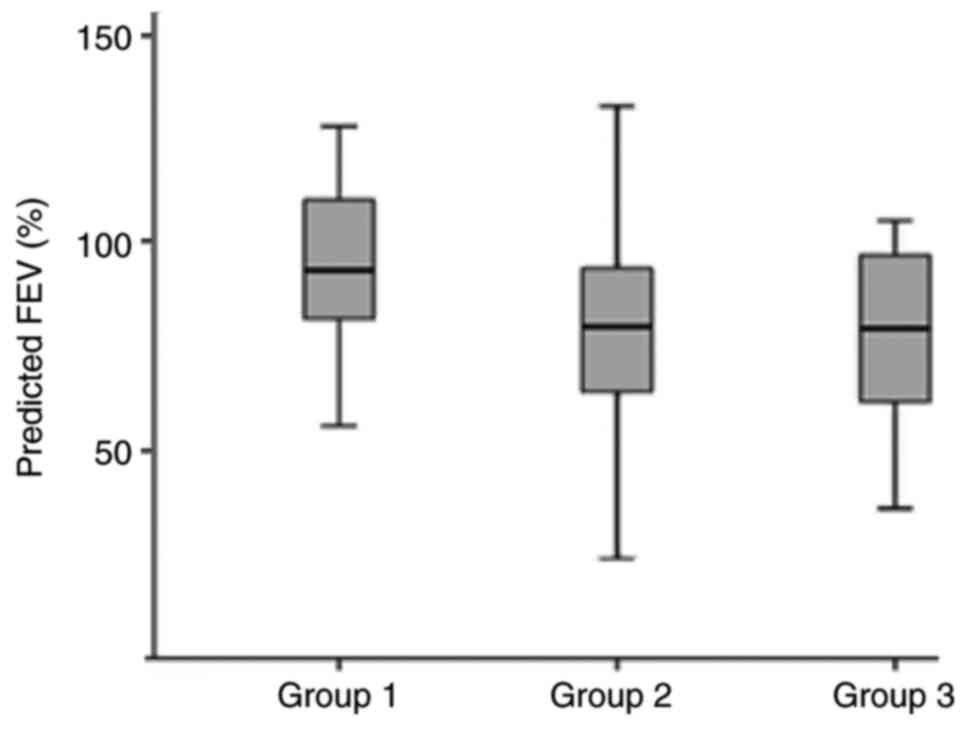
Patients with mild to moderate asthma had
significantly higher FEV1% of predicted compared to patients with
difficult to treat asthma and those on biologic agent (93.4% vs.
79.9% and 79.4%, respectively, P<0.001, Fig. 1). Approximately half of the
patients had positive serum allergy test (50.7%), median eosinophil
count was 300 (108-508.5) cells/mcl and median total IgE level was
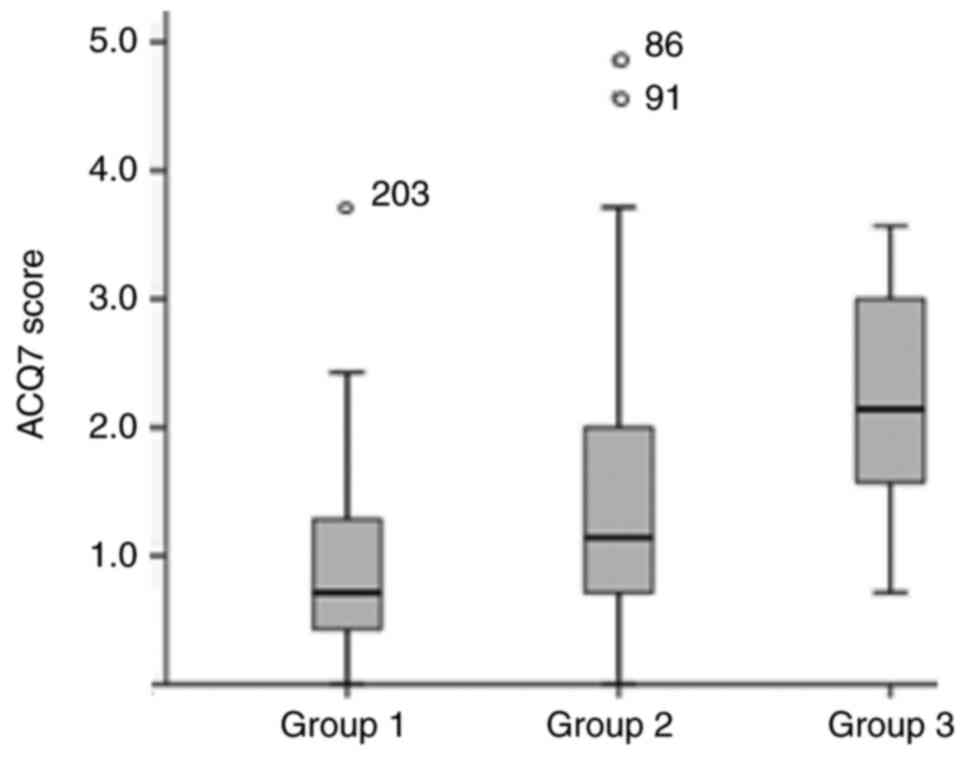
117.5 (29.4-360.5) IU/ml. Finally, median ACQ7 score was 1.14
(0.7-2.1), with patients from group 2 and group 3 presenting higher
ACQ7 scores compared to group 1 patients as expected 1.1 (0.7-2)
and 2.1 (1.4-3.1) vs. 0.7 (0.4-1.3) respectively, P<0.001,
Fig. 2).
Patient treated with biologic
agent
Both Tables III
and IV show the characteristics
of patients who were treated with a biologic agent after their
initial assessment any time during their follow up (totally 86
patients, 32%). A total of 32 asthma patients were given
omalizumab, whereas 54 were treated with mepolizumab and 13 of the
latter were previously on omalizumab and had switched due to poor
response. 48% of the patients were already on high dose inhaled
corticosteroids and LABA and 52% were prescribed add on treatment
with LAMA. 10% of patients on biologic agents received maintenance
treatment with oral steroids. As demonstrated, there are no
significant differences between the groups treated with a different
biologic agent.
 | Table IIICharacteristics of patients with
asthma on biologic agent. |
Table III
Characteristics of patients with
asthma on biologic agent.
| | Biologic agent | |
|---|
|
Characteristics | Omalizumab
(n=32) | Mepolizumab
(n=41) | Omalizumab to
mepolizumab (n=13) | Total (n=86) | P-value |
|---|
| Age, years, median
(interquartile range) | 56 (47.5-64.0) | 59 (48.5-67.5) | 56 (48.5-62.0) | 57 (48.8-60.0) | 0.636 |
| Sex | | | | | 0.712 |
|
Male, n
(%) | 11 (34.4) | 14 (34.1) | 6 (46.2) | 31 (36.0) | |
|
Female, n
(%) | 21 (65.6) | 27 (65.9) | 7 (53.8) | 55 (64.0) | |
| Smoking status | | | | | 0.076 |
|
Non-smoker,
n (%) | 21 (70.0) | 22 (53.7) | 6 (46.2) | 49 (58.3) | |
|
Ex-smoker, n
(%) | 4 (13.3) | 17 (41.5) | 5 (38.5) | 26 (31.0) | |
|
Current
smoker, n (%) | 5 (16.7) | 2 (4.9) | 2 (15.4) | 9 (10.7) | |
|
Pack-yearsa, median (interquartile
range) | 0.0 (0.0-15.3) | 7.0 (1.5-30.0) | 37.5
(22.5-58.8) | 5.0 (0.0-30.0) | 0.001 |
| BMI, median
(interquartile range) | 30.1
(25.7-35.1) | 29.7
(25.0-33.9) | 28.1
(24.8-33.2) | 29.3
(25.4-34.7) | 0.820 |
 | Table IVAsthma parameters of patients on
biologic agent. |
Table IV
Asthma parameters of patients on
biologic agent.
| | Biologic agent | |
|---|
| Parameters | Omalizumab
(n=32) | Mepolizumab
(n=41) | Omalizumab to
mepolizumab (n=13) | Total (n=86) | P-value |
|---|
| Asthma onset | | | | | 0.151 |
|
Early, n
(%) | 5 (26.3) | 5 (14.3) | 0 (0.0) | 10 (15.4) | |
|
Late, n
(%) | 14 (73.7) | 30 (85.7) | 11 (100.0) | 55 (84.6) | |
| Maintenance OCS, n
(%) | 0 (0.0) | 5 (14.7) | 2 (22.2) | 7 (10.0) | 0.149 |
| Exacerbations in
prior year, median (interquartile range) | 2.5 (0.8-6.3) | 1.5 (1.0-3.0) | 4 (3.0-6.5) | 2.5 (1.0-4.3) | 0.162 |
| Predicted % FEV1,
median (interquartile range) | 67.2
(53.8-92.5) | 76.6
(62.1-86.3) | 65.6
(43.1-78.2) | 75.6
(56.6-85.4) | 0.384 |
| Serum positive
allergy test, n (%) | 8 (88.9) | 6 (42.9) | 4 (66.7) | 18 (62.1) | 0.082 |
| Current blood
eosinophil count, cells/µl, median (interquartile range) | 300 (150-550) | 375 (125-636) | 100 (65-550) | 321 (100-563) | 0.532 |
| Total serum IgE,
median (interquartile range) | 365.5
(115.8-571.3) | 99.5
(20.1-429.3) | 337.0
(46.1-1,705.5) | 277.0
(31.2-538.5) | 0.150 |
| ACQ7 score, median
(interquartile range) | 2.3 (1.1-3.4) | 2.1 (1.3-2.8) | 2.9 (2.0-3.4) | 2.2 (1.3-3.2) | 0.438 |
Discussion
In the present study we presented a Greek difficult
to treat asthma registry. The main findings of this study were the
differences observed among the 3 groups in certain clinical,
physiological and immunological factors such as late onset asthma,
lung function, IgE, blood eosinophils and ACQ7 scale. Severe
uncontrolled asthma treatment in daily clinical practice poses a
great challenge to the pulmonologists. New strategies have been
approved the recent years, implemented within a stepwise approach,
taking into consideration relevant phenotypic characteristics of
individuals and specific biomarkers (17,18).
Asthma prevalence is high in Greece and it imposes a
high economic burden on society and the healthcare system with both
direct and indirect costs (19,20).
As such asthma should be recognized as a priority disease in health
care policies and detailed asthma registries should exist. It is
the respiratory physicians' responsibility to make ‘visible’ a
disease that has until now been mostly ‘invisible’ (21) and this is something we may have
achieved through our study.
Severe asthma in Europe is heterogeneous in both
clinical characteristics and treatment. The European Respiratory
Society Severe Heterogeneous Asthma Research collaboration,
Patient-centered (SHARP) Clinical Research Collaboration was the
first study to compare characteristics of patients in European
severe asthma registries and treatments before starting biological
therapies. It is very important to achieve harmonization of severe
asthma databases across Europe (22). For example, the Portuguese Severe
Asthma Registry is a national web-based disease registry of adult
and pediatric severe asthma patients. It collects evidence on
severe asthma in Portugal and aims at improving the healthcare
delivery of severe asthma and supporting collaborative research
projects (23).
As we showed in our registry, patients with severe
asthma were on high dose inhaled corticosteroids and/or oral
steroids. A recent German study showed that 33.6% of asthma
patients treated with high dose inhaled corticosteroids/long-acting
β agonists received additional oral corticosteroids. In addition,
those patients had higher prevalence of other underlying disorders
and more steroid side effects (24). In our registry patients on
biologics received maintenance oral corticosteroids in a lesser
degree (10%).
Patients with severe asthma in our cohort generally
benefit from visits to our asthma clinic because of the
optimization of their treatment, which may include the initiation
of biologic agents after the necessary step by step
characterization approach (25).
We emphasized on avoidable risk factors, such as lack of education,
that lead commonly to uncontrolled asthma and frequent Emergency
Department visits (26). The
precise asthma diagnosis in our asthma outpatient clinic is
essential as it is widely accepted that asthma diagnosis is
confirmed only in two third of asthma cases referred to tertiary
specialists (27).
Approved biologics targeting IL-5/IL-5R, IL-4/IL-13,
and IgE have shown significant reduction in exacerbations rate and
other asthma outcomes such as lung function, oral corticosteroid
use and quality of life in appropriately selected patients
(28-32).
15% of patients in our cohort with an indication to receive a
biologic agent switched from omalizumab to mepolizumab. However,
further understanding is needed on how and when to switch from one
biological therapy to another (32). A simple algorithm on switching
possibilities in case that the physicians' initial choice is proven
not to be the best has been recently suggested by a Greek expert
group (33). Moreover, the
clinical approach for choosing an initial biologic, the assessment
of response to biologics, and the process of troubleshooting and
adjusting biologic treatment for those patients with suboptimal
responses are discussed in the recent literature (34).
Super-responders (upper 25% of ACQ5 responders) were
found to have a higher T2 disease burden and fewer comorbidities at
baseline in The Australian Mepolizumab Registry (8). We recently participated, as one of
the specialized asthma clinics in Greece, that in patients with
severe eosinophilic asthma, 1 year of treatment with mepolizumab
was safe and resulted in significant reduction of the annual
exacerbation rate, reduction (or even discontinuation) of the
needed dose of OCS, and improvements of asthma control and lung
function (35).
Our study has both strengths and limitations. Αn
important limitation is the retrospective nature of the study
making the lack of a large amount of information and missing data,
a common phenomenon in real-life studies. Additionally, it was a
single-center study, however, the only one in the island of Crete,
which also could carry bias. The strengths are that it provides
real life data regarding a lot of aspects of asthma management in a
large group of Cretan patients for a period of several years. As
such it yields more precisely the reality of the daily management
of these patients. Furthermore, it analyses a large group of severe
asthma patients on biologic treatment for asthma. Such studies are
unfortunately limited in our country, so, we hope that we add
important information about the best clinical and holistic approach
for our patients, in the era of the biologics in the severe asthma
patients.
The majority of the patients included had severe
asthma. Particularly those on biologic treatment were on high dose
ICS or/and oral steroids while commonly presented with allergic
rhinitis and nasal polyposis. Our study demonstrates the importance
of a detailed assessment in expert asthma centers for uncontrolled
asthmatic patients for precise identification of severe disease and
timely initiation of targeted therapy.
Acknowledgements
The abstract was presented at the virtual 2020 ERS
International Congress September 06-09 2020, in session ‘Clinical
parameters in severe asthma’ and published as abstract no. 2239 in
European Respiratory Journal 2020; 56: Suppl. 64. On behalf of ‘The
Cretan Registry of the use of Biologics in Severe Asthma’, the
authors would like to acknowledge the following collaborators: Dr
Argiana Evi, Dr Chronaki Emmanouela, Dr Politis Ioannis, Dr Vakouti
Eleutheria (Private respiratory physician, Heraklion, Greece), Dr
Damianaki Aggeliki, Dr Krietsepi Vasiliki (Chania General Hospital,
Chania, Greece), Dr Georgoudakis Grigoriοs, Dr Markatos Miltiadis,
Dr Michelaki Eleni, Dr Solakis Athanasios, Dr Vittorakis Stylianos,
Dr Voulgaraki Olga (Private respiratory physician, Chania, Greece),
Dr Ieronimakis Astrinos, Dr Toupaki-Kalitsounaki Anna Maria
(Private respiratory physician, Moires, Heraklion, Greece), Dr
Kallergis Kostantinos (Rethimno General Hospital, Rethimno,
Greece), Dr Kostaki Aggeliki and Dr Varoucha Maria (Private
respiratory physician, Rethimno, Greece). These individuals
contributed by referring patients to the specialized asthma clinic
at the University Hospital of Heraklion for further investigation
and management.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KMA, DAS and NT were responsible for designing the
study, writing the manuscript, approving the data analysis and
reviewing the manuscript. KMA, KK and NT confirm the authenticity
of all the raw data. MB, KK, AT and DI were responsible for
collecting the data, performing data analysis and writing the
manuscript. VS, CC and SM acquired and analyzed the daya. GP, IL
and IM were involved in the study design, conception and
interpretation of data. ES acquired and analyzed the data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of University General Hospital Heraklion (Heraklion,
Greece; approval no. 404A-9751). This study was conducted in a
retrospective manner, and thus the need for consent was waivered by
the ethics committee of University General hospital Heraklion.
Patient consent for publication
All identifying information has been removed, public
interest considerations outweigh the potential harm.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Global Initiative for Asthma: 2021 GINA
Report, Global Strategy for Asthma Management and Prevention. GINA,
Fontana, 2021. https://ginasthma.org/gina-reports.
|
|
2
|
Chung KF, Wenzel SE, Brozek JL, Bush A,
Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et
al: International ERS/ATS guidelines on definition, evaluation and
treatment of severe asthma. Eur Respir J. 43:343–373.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Holguin F, Cardet JC, Chung KF, Diver S,
Ferreira DS, Fitzpatrick A, Gaga M, Kellermeyer L, Khurana S,
Knight S, et al: Management of severe asthma: A European
respiratory society/American thoracic society guideline. Eur Respir
J. 55(1900588)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Selroos O, Kupczyk M, Kuna P, Łacwik P,
Bousquet J, Brennan D, Palkonen S, Contreras J, FitzGerald M,
Hedlin G, et al: National and regional asthma programmes in Europe.
Eur Respir Rev. 24:474–483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
MacDonald KM, Kavati A, Ortiz B, Alhossan
A, Lee CS and Abraham I: Short- and long-term real-world
effectiveness of omalizumab in severe allergic asthma: Systematic
review of 42 studies published 2008-2018. Expert Rev Clin Immunol.
15:553–569. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pelaia C, Calabrese C, Terracciano R, de
Blasio F, Vatrella A and Pelaia G: Omalizumab, the first available
antibody for biological treatment of severe asthma: More than a
decade of real-life effectiveness. Ther Adv Respir Dis.
12(1753466618810192)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deeks ED: Mepolizumab: A review in
eosinophilic asthma. BioDrugs. 30:361–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harvey ES, Langton D, Katelaris C, Stevens
S, Farah CS, Gillman A, Harrington J, Hew M, Kritikos V,
Radhakrishna N, et al: Mepolizumab effectiveness and identification
of super-responders in severe asthma. Eur Respir J.
55(1902420)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khatri S, Moore W, Gibson PG, Leigh R,
Bourdin A, Maspero J, Baros M, Buhl R, Howarth P, Alberts F, et al:
Assessment of the long-term safety of mepolizumab and durability of
clinical response in patients with severe eosinophilic asthma. J
Allergy Clin Immunol. 143:1742–1751.e7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hekking PW, Wener RR, Amelink M,
Zwinderman AH, Bouvy ML and Bel EH: The prevalence of severe
refractory asthma. J Allergy Clin Immunol. 135:896–902.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
von Bülow A, Kriegbaum M, Backer V and
Porsbjerg C: The prevalence of severe asthma and low asthma control
among Danish adults. J Allergy Clin Immunol Pract. 2:759–767.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
ISAR Study Group. International severe
asthma registry: Mission statement. Chest. 157:805–814.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zervas E, Loukides S, Kostikas K, Bakakaos
P, Tzortzaki E and Gaga M: On Behalf of Asthma Working Group of
Hellenic Thoracic Society. Asthma and asthma-like symptoms in
Greece. The Greece asthma national prevalence survey. Eur Respir J.
40 (Suppl 56)(P3936)2012.
|
|
14
|
Kourlaba G, Bakakos P, Loukides S,
Vellopoulou K, Solakidi A and Maniadakis N: The self-reported
prevalence and disease burden of asthma in Greece. J Asthma.
56:478–497. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Juniper EF, O'byrne PM, Guyatt GH, Ferrie
PJ and King DR: Development and validation of a questionnaire to
measure asthma control. Eur Respir J. 14:902–907. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khusial RJ, Honkoop PJ, van der Meer V,
Snoeck-Stroband JB and Sont JK: Validation of online asthma control
questionnaire and asthma quality of life questionnaire. ERJ Open
Res. 6:00289–2019. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zervas E, Samitas K, Papaioannou AI,
Bakakos P, Loukides S and Gaga M: An algorithmic approach for the
treatment of severe uncontrolled asthma. ERJ Open Res.
4:00125–2017. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Papi A, Saetta M and Fabbri L: Severe
asthma: Phenotyping to endotyping or vice versa? Eur Respir J.
49(1700053)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vellopoulou K, Bakakos P, Loukides S,
Maniadakis N and Kourlaba G: The Economic burden of asthma in
Greece: A cross-sectional study. Appl Health Econ Health Policy.
17:629–640. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Souliotis K, Kousoulakou H, Hillas G,
Bakakos P, Toumbis M, Loukides S and Vassilakopoulos T: Direct and
indirect costs of asthma management in Greece: An expert panel
approach. Front Public Health. 5(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wenzel SE, Brillhart S and Nowack K: An
invisible disease: Severe asthma is more than just ‘bad asthma’.
Eur Respir J. 50(1701109)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Bragt JJMH, Adcock IM, Bel EHD,
Braunstahl GJ, ten Brinke A, Busby J, Canonica GW, Cao H, Chung KF,
Csoma Z, et al: Characteristics and treatment regimens across ERS
SHARP severe asthma registries. Eur Respir J.
55(1901163)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sá-Sousa A, Fonseca JA, Pereira AM,
Ferreira A, Arrobas A, Mendes A, Drummond M, Videira W, Costa T,
Farinha P, et al: The portuguese severe asthma registry:
Development, features, and data sharing policies. Biomed Res Int.
2018(1495039)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taube C, Bramlage P, Hofer A and Anderson
D: Prevalence of oral corticosteroid use in the German severe
asthma population. ERJ Open Res. 5:00092–2019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bousquet J, Brusselle G, Buhl R, Busse WW,
Cruz AA, Djukanovic R, Domingo C, Hanania NA, Humbert M, Menzies
Gow A, et al: Care pathways for the selection of a biologic in
severe asthma. Eur Respir J. 50(1701782)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
AL-Jahdali H, Anwar A, AL-Harbi A,
Baharoon S, Halwani R, Al Shimemeri A and Al-Muhsen S: Factors
associated with patient visits to the emergency department for
asthma therapy. BMC Pulm Med. 12(80)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stensen L, Porsbjerg C, Sverrild A, Nybo
Jensen B and Vibeke B: Managing asthma in the outpatient clinic: Is
the diagnosis of asthma confirmed objectively according to
guidelines? Eur Respir J. 40 (Suppl 56)(P2263)2012.
|
|
28
|
Assaf SM and Hanania NA: Biological
treatments for severe asthma. Curr Opin Allergy Clin Immunol.
19:379–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
McGregor MC, Krings JG, Nair P and Castro
M: Role of biologics in asthma. Am J Respir Crit Care Med.
199:433–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rabe KF: New biologics for severe asthma:
What patients, what agents, what results, at what cost? Am J Respir
Crit Care Med. 199:406–408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pavord I, Bahmer T, Braido F, Cosío BG,
Humbert M, Idzko M and Adamek L: Severe T2-high asthma in the
biologics era: European experts' opinion. Eur Respir Rev.
28(190054)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Doroudchi A, Pathria M and Modena BD:
Asthma biologics: Comparing trial designs, patient cohorts and
study results. Ann Allergy Asthma Immunol. 124:44–56.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Papaioannou AI, Fouka E, Papakosta D,
Papiris S and Loukides S: Switching between biologics in severe
asthma patients. When the first choice is not proven to be the
best. Clin Exp Allergy. 51:221–227. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pepper AN, Hanania NA, Humbert M and
Casale TB: How to assess effectiveness of biologics for asthma and
what steps to take when there is not benefit. J Allergy Clin
Immunol Pract. 9:1081–1088. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kallieri M, Zervas E, Katsoulis K, Fouka
E, Porpodis K, Samitas K, Papaioannou AI, Kipourou M, Gaki E,
Vittorakis S, et al: Mepolizumab in severe eosinophilic asthma: A
2-year follow-up in specialized asthma clinics in Greece: An
interim analysis. Int Arch Allergy Immunol. 181:613–617.
2020.PubMed/NCBI View Article : Google Scholar
|