Introduction
Endometriosis (EM) is characterized by the presence
of endometrial foci outside the uterus; it is considered a
significant health issue and is commonly observed in women of
reproductive age, affecting ~15% of women in Asia (1). EM is often accompanied by menstrual
disorders, a pelvic mass, dysmenorrhea, chronic pelvic pain,
infertility and a significant decline in quality of life (2). The standard methods of treatment
include drug therapies that inhibit the ovarian activity or
conservative surgical resection of EM. However, some of the
challenges accompanying EM include the severe side effects
resulting from the long-term use of painkillers and hormone
therapies, and the high rate of recurrence, which is expected after
the treatment is discontinued or even after undergoing a surgical
procedure (3). Due to the chronic
and recurrent features, the current treatment strategies of EM
still focus on pain relief. As a primary and continuous symptom,
EM-associated pain has a significant impact on the physical and
mental health of patients, and is considered the primary reason for
individuals to seek medical care. There has been a continuous
debate regarding the development of strategies to optimize the
management of EM-associated pain (4). The underlying mechanism related to
the onset of EM-associated pain is unclear. Therefore, it is
necessary to explore the pain pathways involved in EM to design
more effective treatment strategies that will target the underlying
causes of this disease. Although the pathogenesis of EM remains
unclear, the role of prostaglandin E2 (PGE2)
and prostaglandin F2α (PGF2α) in various female reproductive
processes, particularly in EM-associated infertility and pain, is
well documented (5,6), indicating the direct association
between prostaglandins and the persistent pain of EM.
Furthermore, abnormal expression of PGE2
and PG2α receptors has been observed in patients with EM (7). Although it is clear that
PGE2 and PGF2α play an essential role in EM, the precise
mechanism is still unclear. Prostaglandins are lipid molecules
obtained from arachidonic acid via enzymatic hydrolysis.
PGE2 and PGF2α are mainly produced by the reproductive
system. Being a key mediator of inflammation, PGE2
contributes to the development of hypersensitivity to pain by
overexciting sensory neurons. PGF2α is a type of vasoconstrictor;
therefore, an aberrant increase in its expression can cause the
contraction of uterine smooth muscles and blood vessels, enhancing
the algogenic effect of bradykinin (BK) and ultimately causing
spastic pain (8). BK is known as
one of the most algogenic substances (9); when the tissue is impaired by noxious
stimuli, BK is released from the tissue and binds to one of the
nociceptive receptors at the nerve terminal (10). As a nonapeptide, BK has attracted a
lot of attention due to its potent pain-inducing effect within the
human body (11,12). Several studies have reported the
interaction between prostaglandins and BK in the pathological
process of pain induction; BK is also known to induce the synthesis
of PGE2 in dermal fibroblasts (13). In our previous studies (14,15),
non-clinical and clinical experiments were performed to explore the
pathogenesis of EM. Abnormally high levels of PGF2α and BK
were found in an EM model, indicating that PGF2α and BK had a
crucial role in the pathology of EM. Thus, an evaluation of whether
BK/BKB1 receptor (BKB1R), PGE2 and PGF2α promoted the
onset of EM in a synergistic manner and an assessment of their
mutual associations was required to understand the mechanism of
EM-associated pain. Thus, in the present study, the role of
PGE2, PGF2α, BK and BKB1R in the development and
maintenance of EM-associated pain was investigated.
Materials and methods
Study subjects
General conditions. Serum and eutopic and
ectopic samples were collected during the proliferative phase of
the menstrual cycle from 50 women with EM (EM group), aged
38.34±5.32 years, whose diagnosis was confirmed based on
pathological examinations after laparoscopic surgery at the Fourth
Hospital of Shijiazhuang (Shijiazhuang, China) between January 2019
and December 2020. All participants provided written informed
consent for participation. The degree of dysmenorrhea was assessed
in the EM group based on the Visual Analog Scale (VAS) (16). A scale of 0 (without any pain) to
10 (most severe pain) was used for VAS scoring using a 10-cm ruler;
the patients placed a mark on the line according to their degree of
dysmenorrhea before the operation and this was then measured by the
physician. Based on the VAS scores of the 50 patients, 14 patients
(aged 39.51±6.02 years) had a VAS score of 0 (non-pain group),
while 36 patients (aged 36.93±7.28 years) had different levels of
VAS scores (pain group). EM was staged according to the American
Society for Reproductive Medicine classification (17). Of the patients with EM, 11 had
stage 1 disease, 14 had stage 2, 13 had stage 3 and 12 had stage 4.
During the same time period in the Fourth Hospital of Shijiazhuang,
control samples were also collected from 20 women (control group),
aged 37.83±4.72 years, who had undergone laparoscopic surgery due
to benign gynecological disorders, such as uterine myoma without
period pain. We exhibited no visible evidence of EM upon
laparoscopy. All the patients in the control group provided written
informed consent for participation. The study protocol was approved
by the Ethics Committee of the Hebei University of Chinese Medicine
(approval no. YXLL2015001).
Inclusion criteria
Patients were included in the study based on the
following parameters: i) Premenopausal women aged 20-50 years; ii)
no medical complications; iii) no hormonal medication for at least
1 month before sample collection; and iv) confirmed EM diagnosis
via pathological examination.
Exclusion criteria
Patients were excluded from the study if they had
any of the following: i) Pelvic infection or malignant pelvic
tumor; ii) cardiovascular complications, liver/kidney/blood
diseases or rectal cancer; iii) consumption of hormonal medication
within the month before the operation; and iv) any neurological
disorders.
Tissue collection
Before the operation, venous blood (3 ml) was
collected from the elbow via venous puncture in each patient,
followed by centrifugation at 1,800 x g at 4˚C for 10 min to
separate the serum and storage at -80˚C for ELISA. On the day of
the operation, eutopic and ectopic endometrial tissues were
collected from the patients in the EM group and normal endometrium
samples were collected from the patients in the control group. Some
of the samples were immediately frozen in liquid nitrogen and then
transferred to a -80˚C cryogenic refrigerator for western blotting
and reverse transcription-quantitative PCR (RT-qPCR) analysis. The
remaining samples were fixed in 4% paraformaldehyde for 1 week at
4˚C, then embedded in paraffin for hematoxylin and eosin (H&E)
and immunohistochemical (IHC) staining.
Serum BK, PGE2, PGF2α
measurement
Serum levels of BK, PGE2 and PGF2α were
measured using commercially available ELISA kits (BK ELISA kit:
Enzo Life Science, Inc.; cat. no. ADI-900-206; PGF2α ELISA kit:
Enzo Life Science, Inc.; cat. number, ADI-900-069; and
PGE2 ELISA kit: Cayman Chemical Company; cat. no.
514010), following the manufacturer's instructions.
H&E staining
Paraffin tissues were sliced into 4-µm sections,
which were successively treated with xylene, anhydrous ethanol, 90%
ethanol and 80% ethanol, then rinsed in distilled water for 5 min,
followed by hematoxylin staining for 5 min and eosin staining for
3-5 min at room temperature. Staining was observed under an
electron microscope at x400 magnification.
IHC staining for the detection of
BKB1R
Serial tissue sections (4-µm thick) were washed with
phosphate-buffered saline (PBS) and treated with 3% hydrogen
peroxide to block endogenous peroxidase activity at room
temperature for 15 min. The sections were incubated with primary
antibody for BKB1R (Abcam; cat. no. ab75148; dilution, 1:200)
overnight at 4˚C, and then with HRP-conjugated Affinipure goat
anti-rabbit IgG (ProteinTech Group, Inc.; cat. no. SA00001-2;
dilution, 1:2,000) at room temperature for 1 h, followed by washing
with PBS. Finally, the sections were counterstained with
hematoxylin at room temperature for 5 min, and mounted in resinene
(cat. no. 10004160; Sinopharm Chemical Reagent Co., Ltd.).
The expression of BKB1R-positive cells was
characterized via IHC by pale brown staining of the cytoplasm. A
total of 10 high-power fields were randomly selected to count the
BKB1R-positive cells in each sample under a light microscope,
analyzed the data using the HMIAS-2000 pathology picture analysis
system (Qianping Audiovisual Company) and evaluated it based on
optical density.
Western blot analysis for measuring
BKB1R
Radioimmunoprecipitation assay (RIPA) lysis buffer
containing protease inhibitor (cat. no. BB3201; BestBio) was used
to lyse the tissues, which where then homogenized in the lysis
buffer. Following this, the lysis buffer was kept on ice for 30
min, centrifuged at 8,000 x g at 4˚C for 15 min and diluted in 5X
sample loading buffer (cat. no. G2013-100ML; Servicebio). Next, the
supernatant was collected and the concentration of protein was
determined using a Bradford assay. Protein samples were separated
on 10% gels via SDS-PAGE with 5 µl per lane, followed by transfer
onto a polyvinylidene fluoride membrane. The non-specific sites on
the membrane were blocked by incubation in 5% skimmed milk for 2 h
at room temperature, followed by overnight incubation at 4˚C with
the primary antibodies for BKB1R (Abcam; cat. no. ab75148;
dilution, 1:200) and β-actin (Hangzhou HuaAn Biology Technology
Ltd., Co.; cat. no. R130605; dilution, 1:2,000). Next, the
membranes were washed and incubated with the secondary antibody
(anti-rabbit IgG antibody; Protein Tech Group, Inc.; cat. no.
SA00001-2; dilution, 1:5,000) for 1 h at room temperature. Protein
bands were visualized using an electrochemiluminescence (ECL) kit
(cat. no. P10300; New Cell & Molecular Biotech Co., Ltd.),
prior to being analyzed using Quantity One® 1-D Analysis
Software v4.6 (Bio-Rad Laboratories, Inc.). Data are presented as a
ratio of BKB1R/β-actin.
RT-qPCR for measuring the mRNA
expression of BKB1R
RNA from tissues was extracted using an
E.Z.N.A.® Total RNA kit II (cat. no. R6934-01; Omega
Bio-Tek, Inc.) according to the manufacturer's instructions. Total
RNA was reverse transcribed to cDNA using an Mon Script™ RT III
All-in-one mix with dsDNA (cat. no. MR05101; Monad Biotech Co.,
Ltd.) for incubation at 55˚C for 10 min following the
manufacturer's instructions. The primers were purchased from
Shanghai Sangon Biotechnology Co., Ltd. The synthetic sequences of
the primers were as follows: BKB1R, forward,
5'-AACAACTAGTCACCTAAGGTCC-3' and reverse
5'-TCTCAAGGTTGCTGGCAGAG-3'; GAPDH, forward,
5'-TCCAAAATCAAGTGGGGCGA-3' and reverse, 5'-AAATGAGCCCCAGCCTTCTC-3'.
qPCR was performed using Mon Amp™ Chemo HS qPCR Mix (cat. no.
MQ00401; Monad Biotech Co., Ltd.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
10 min, followed by 40 cycles at 95˚C for 15 sec and 60˚C for 60
sec. The quantitation cycle (Cq) method was used, and the relative
transcript number of the target gene was normalized to that of
GAPDH using the 2-ΔΔCq method (18). The reactions were run in triplicate
using the Applied Biosystems™ 7500 Real-Time PCR system and results
were analyzed with the associated SDS 2.0 software (Thermo Fisher
Scientific, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
software v22.0 (IBM Corp.). Statistical comparisons were performed
using one-way analysis of variance (ANOVA) for >2 groups,
followed by LSD post hoc test. Student's unpaired t-tests were
performed to test the significance between two independent samples.
The correlation between two normally distributed variables was
evaluated via linear correlation analysis with Pearson's
correlation coefficient, and the correlation between VAS score and
other variables was assessed with Spearman's correlation analysis.
All data are presented as the mean ± SD, and P<0.05 was used to
indicate a statistically significant difference.
Results
Serum concentrations of BK,
PGE2 and PGF2α
There was a significant increase in the
concentration of BK (P<0.01), PGE2 (P<0.05) and
PGF2α (P<0.01) in the EM group compared with that in the control
group (Table I).
 | Table IComparison of serum BK,
PGE2 and PGF2α levels between two groups (mean ±
SD). |
Table I
Comparison of serum BK,
PGE2 and PGF2α levels between two groups (mean ±
SD).
| Group | n | BK, ng/ml | PGE2,
pg/ml | PGF2α, ng/ml |
|---|
| Control | 20 | 4.46±1.19 | 548.94±165.22 | 6.44±1.97 |
| EM | 50 |
5.70±1.60b |
684.12±228.10a |
13.64±5.88b |
Serum concentrations of BK,
PGE2 and PGF2α in the three groups
The concentration of BK in the pain group was
significantly higher than that in the control (P<0.01) and
non-pain (P<0.05) groups; however, there was no statistical
significance between the control and non-pain group (P>0.05).
The concentration of PGE2 in the pain group was
significantly higher than that in the control (P<0.01) and
non-pain (P<0.05) groups; however, there was no statistical
significance between the control and non-pain groups (P>0.05).
The concentration of PGF2α in the pain group was also significantly
higher than that in the control (P<0.01) and non-pain
(P<0.05) groups, and there was no statistical significance
between the control and non-pain groups (P>0.05) (Table II).
 | Table IIComparison of serum BK,
PGE2 and PGF2α levels among three groups (mean ±
SD). |
Table II
Comparison of serum BK,
PGE2 and PGF2α levels among three groups (mean ±
SD).
| Group | n | BK, ng/ml | PGF2α, ng/ml | PGE2,
pg/ml |
|---|
| Control | 20 | 4.46±1.19 | 6.44±1.97 | 548.94±165.22 |
| Non-pain | 14 |
5.02±1.59b |
9.68±3.28b |
584.23±173.10b |
| Pain | 36 |
5.97±1.57a |
15.18±5.98a |
722.96±237.00a |
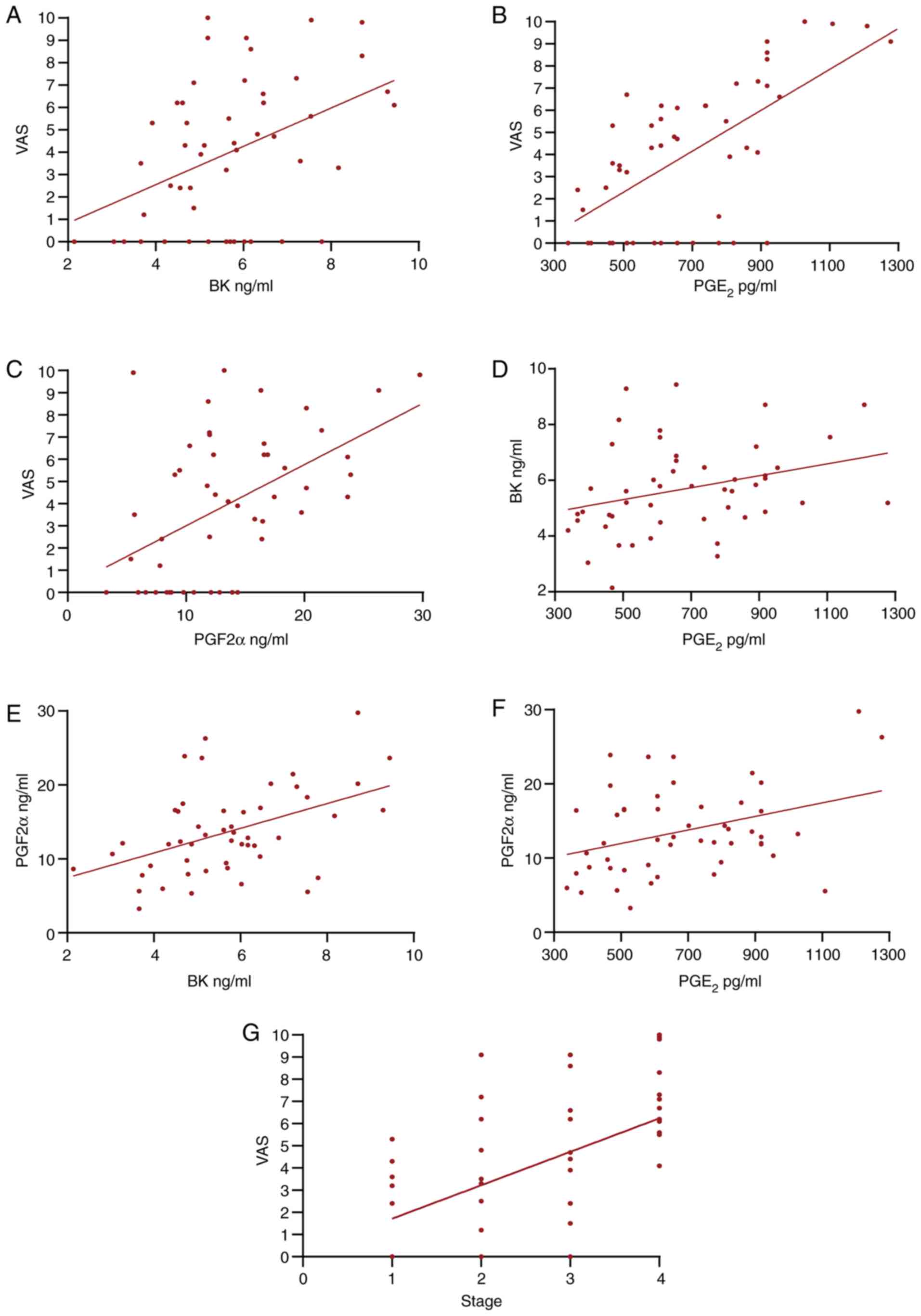
Correlation analysis in EM
To understand the associations between VAS and the
serum molecules in EM, a correlation analysis was performed for BK,
PGE2, PGF2α and VAS. VAS was positively correlated with
BK, PGE2 and PGF2α (P≤0.01), PGF2α was positively
correlated with BK and PGE2 (P≤0.01 and P<0.05), and
PGE2 was correlated with BK (P<0.05). In order to
explore the association between disease stage and the severity of
pain symptoms, a correlation analysis was performed, which found
that VAS score was correlated with stage (P≤0.01). Table III shows the results of the
correlation analysis. Fig. 1 shows
the scatter plots between the two variables.
 | Table IIICorrelation analysis in
endometriosis. |
Table III
Correlation analysis in
endometriosis.
| Value | VAS vs. BK | VAS vs.
PGE2 | VAS vs. PGF2α | PGE2 vs.
BK | BK vs. PGF2α | PGE2 vs.
PGF2α | Stage vs. VAS |
|---|
| r value | 0.401 | 0.611 | 0.457 | 0.303 | 0.458 | 0.354 | 0.518 |
| P-value | 0.004 | ≤0.001 | ≤0.001 | 0.033 | ≤0.001 | 0.012 | ≤0.001 |
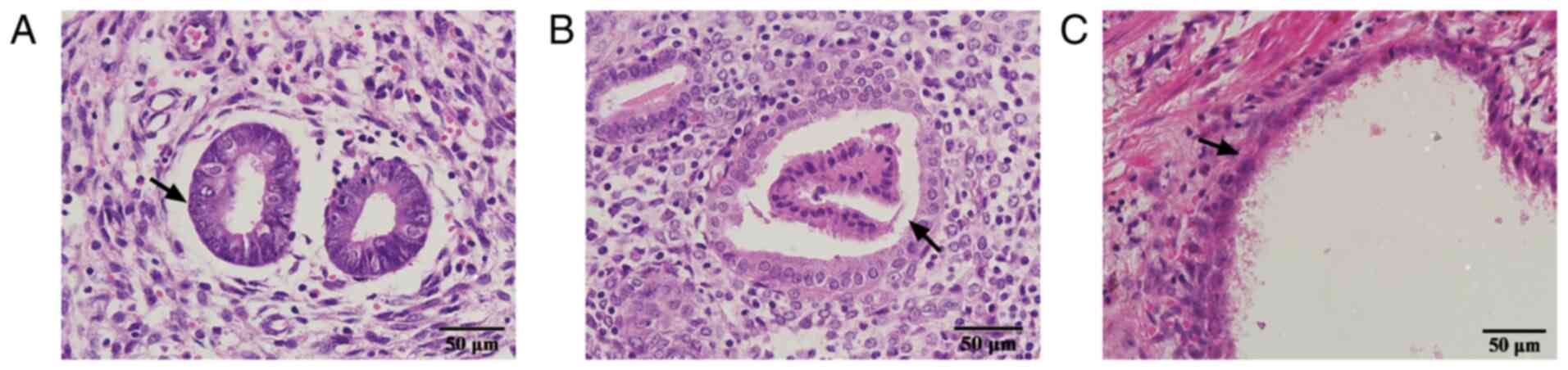
H&E staining analysis
In the control group, endometrial tissue morphology
was regular, cells were not damaged or missing, and no pericellular
inflammatory factors were observed. The endometrial tissue
morphology in the EM group was irregular in shape, with most cells
incomplete, damaged or missing. Complete glands or stroma were
observed in the ectopic foci and the simple columnar epithelium of
the glands exhibited mild disorder (Fig. 2).
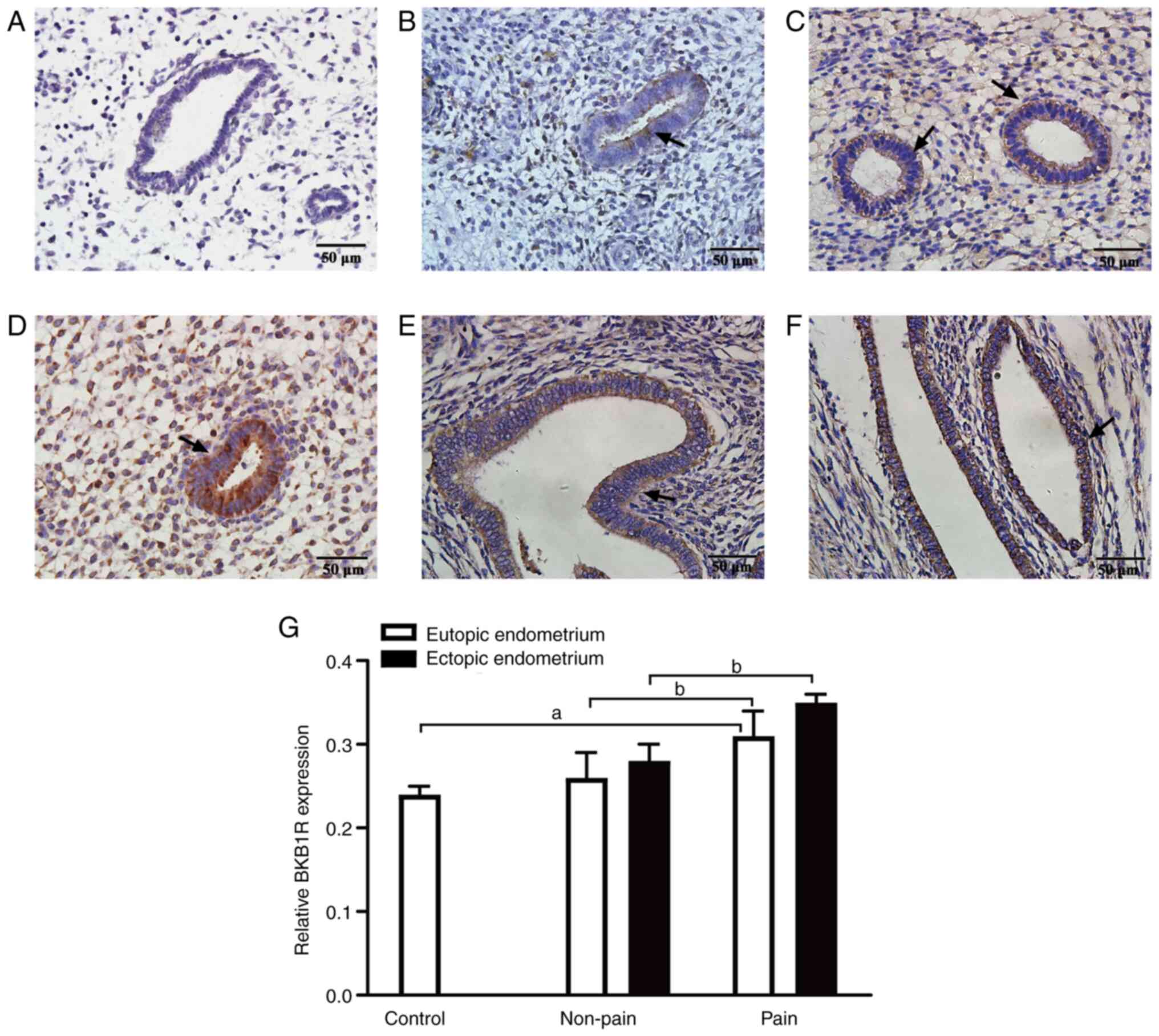
Expression of BKB1R by
immunohistochemistry
Next, an IHC analysis of the endometrium in the
different groups was performed to investigate the expression and
localization of BKB1R protein. The BKB1R protein was mainly
localized on the cytoplasm of endometrial stroma or glands with
brown granular staining. The expression level of BKB1R in the
eutopic endometrium of the pain group was significantly higher than
that in the control and non-pain groups (both P<0.01), and there
was no significant difference between the control and non-pain
groups (P>0.05). Additionally, a significantly higher expression
level of BKB1R was observed in the ectopic endometrium in the pain
group compared with the non-pain group (P<0.01) (Fig. 3). These results suggested
excessively high secretion of BKB1R protein in the pain group.
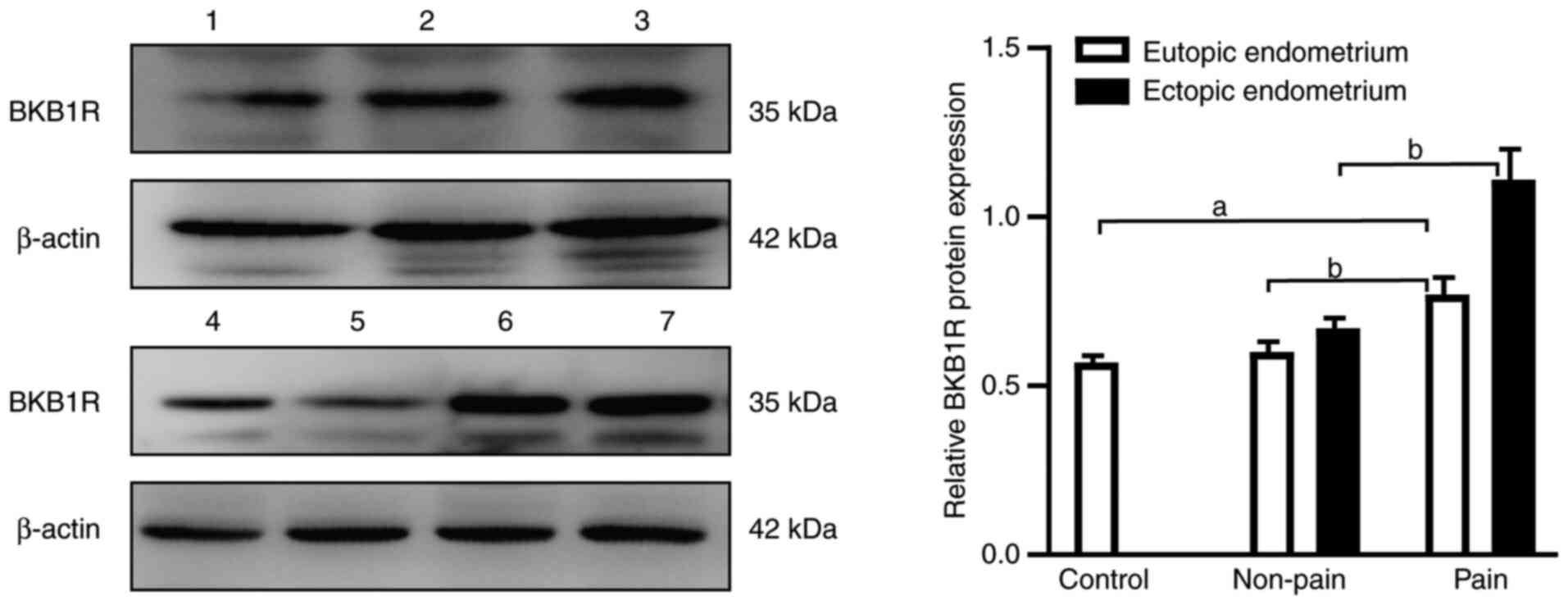
Expression of BKB1R as assessed by
western blotting
The results of western blotting analysis showed a
significantly higher expression level of BKB1R protein in the
eutopic endometrium in the pain group compared with the control and
non-pain groups (all P<0.01), and there was no significant
difference between the control and non-pain groups (P>0.05).
Additionally, a significantly higher expression level of BKB1R
protein was observed in the ectopic endometrium of the pain group
compared with that in the non-pain group (P<0.01) (Fig. 4).
Expression of BKB1R mRNA as assessed
by RT-qPCR
RT-qPCR was performed to investigate the mRNA
expression of BKB1R in EM. The expression level of BKB1R mRNA in
the eutopic endometrium from the pain group was significantly
higher compared with that in the control and non-pain groups (all
P<0.01), and there was no difference between control and
non-pain groups (P>0.05). Furthermore, the expression level of
BKB1R mRNA in the ectopic endometrium from the pain group was
significantly higher than that in the non-pain group (P<0.01).
These results showed that BKB1R mRNA was significantly upregulated
in the lesions in EM, especially in the pain group (Table IV).
 | Table IVComparison of BKB1 receptor mRNA
expression assessed by reverse transcription-quantitative PCR (mean
± SD). |
Table IV
Comparison of BKB1 receptor mRNA
expression assessed by reverse transcription-quantitative PCR (mean
± SD).
| Group | n | Eutopic
endometrium | Ectopic
endometrium |
|---|
| Control | 20 | 0.90±0.05 | - |
| Non-pain | 14 |
1.39±0.12b |
1.76±0.33b |
| Pain | 36 |
3.50±1.12a | 3.78±0.86 |
Discussion
EM is a complex inflammatory disease of the pelvis,
characterized by dysmenorrhea, dyspareunia and chronic pelvic pain.
Pain is one of the typical symptoms that severely affect the
quality of life of a patient. The pathophysiology of EM involves
biological mechanisms that induce pain. Although there are numerous
studies (19-21)
on the related biological processes, the pathological processes
related to the onset of EM-associated pain have remained unclear.
Prostaglandins, such as PGE2 and PGF2α, are mainly
derived from the reproductive system (7). Relevant studies have revealed that
PGE2 and PGF2α are involved in various diseases,
particularly EM, by regulating the cyclic changes in the
endometrium. PGE2 and PGF2α are known to be abnormally
expressed in the peritoneal fluid of patients with EM, which might
be associated with the pathogenesis of EM (22). Furthermore, prostaglandins are also
considered to play multiple roles in the etiology of EM-associated
infertility and pain (6,23-25).
To date, several studies have confirmed that there is a specific
association between prostaglandins and EM-associated pain (26,27).
We previously showed that the serum levels of PGF2α
were abnormally elevated in an EM-associated dysmenorrhea model of
mice (15), indicating that PGF2α
might be involved in the pathogenesis of EM-associated pain. High
concentrations of PGF2α can cause uterine smooth muscle
contraction, and the consequent reduction of blood flow may cause
uterine ischemia and anoxia, which may lead to the accumulation of
acidic metabolites, causing dysmenorrhea (28). Also, abnormally high levels of
prostaglandins can cause pain by inducing aseptic inflammation,
increasing vascular permeability and enhancing the pain effect of
pain molecules such as BK (29).
BK is considered a mediator of inflammatory pain; its elevated
levels after tissue injury and inflammation occur via the
activation of two types of GPCRs, termed BKB1R and BKB2R (30). The B2 receptor is constitutively
expressed in several tissues, while the B1 receptor is minimally
expressed in physiological conditions, but its expression might be
induced under different pathological conditions, such as
inflammation. BKB1R is an inducible GPCR; it is induced or
upregulated at the site of injury or inflammation. In particular,
BKB1R has been shown to be involved in the pathogenesis of numerous
inflammatory diseases (31).
Recent reports (32-34)
have suggested a potential role of the B1 receptor in various
pathophysiological processes. For example, it may be implicated in
inflammation, hyperalgesia, hyperthermia and experimental diabetes.
In these conditions, the B1 receptor has been found to be
significantly upregulated; it plays a critical role in chronic
pain, and it is one of the major causes of prolonged pain (35), playing an important role in the
regulation of hyperalgesia and pain. The combination of BK and B1
receptor results in the activation of phospholipase A2
and stimulates arachidonic acid to release prostaglandins, causing
pain (36). The present study
showed that serum levels of BK, PGE2 and PGF2α were
upregulated in patients with EM compared with those in the control
group, indicating that BK, PGE2 and PGF2α were involved
in the development of EM. Inflammation is one of the causative
factors of pain in EM and previous studies have shown that
prostaglandins and BK are involved in inflammation and pain
(37-39).
In the present study, a correlation analysis was performed to
determine whether these molecules were correlated with the clinical
characteristics of EM and a positive correlation was found between
the VAS score and BK, PGE2 and PGF2α expression. Further
correlation analysis indicated that PGE2, PGF2α and BK
levels were significantly correlated with each other, as well as
with the pain intensity of EM. These results suggested that BK,
PGE2 and PGF2α played crucial roles in the onset of
EM-associated pain. Additionally, the results of the present study
showed that the expression levels of BKB1R protein and mRNA in the
patients with EM were significantly upregulated compared with those
of the control group. These results were consistent with those of a
previous study (40). The present
study investigated apparent changes in the protein and gene
expression levels of BKB1R, and the serum levels of BK,
PGE2 and PGF2α, as well as their association with pain
symptoms. Based on the results, we hypothesized that, along with
the cyclical changes in the ectopic endometrium in EM, surrounding
tissues were injured and released various types of pain mediators,
such as BK, which is a potent algogenic substance and directly
participates in the occurrence of EM-associated pain. Also, after
binding to the BKB1R, it induced the upregulation of
prostaglandins, such as PGE2 and PGF2α, causing pain.
Therefore, based on present study results, it was speculated that
BK, BKB1R, PGE2 and PGF2α were involved in the
development of EM and served a synergistic effect in the pathology
of EM-associated pain.
In our previous study (15), a mouse EM model was used to
demonstrate that the control of BK by herbal medicine could inhibit
the development of EM and relieve dysmenorrhea, suggesting that it
could significantly help treat EM-associated pain by downregulating
BK and BKB1R. Furthermore, certain previous studies demonstrated
that BK regulated autophagic and apoptotic responses (41-43).
Recent studies have shown that BKB1R is a positive regulator of
autophagy, and its overexpression may be involved in the autophagy
of microglia, which causes neuropathic pain (44). BK/BKB1R is known to be involved in
the development of pain in various ways. Therefore, one potential
way to treat pain might include blocking BK. In the present study,
with regard to the disease stage, the severity of pain symptoms was
correlated significantly with the stage of the disease, and this
finding is in agreement with the results of a previous study
(45). The present study results
provided a potential molecular mechanism by which PGE2,
PGF2α and BK/BKB1R are involved in EM-associated pain, suggesting a
possible target for the treatment of EM-associated pain. A
correlation was found between the VAS score of menstrual pain and
the concentration of molecules, as well as the stage of the
disease, so the results may be helpful in the development of
biomarkers to determine the severity of EM-associated pain.
The present study does have limitations to be
considered. First of all, samples were collected from only 50
patients with EM, and it would be necessary for the sample to be
enlarged in order to confirm the conclusions. Secondly, the present
study is a primary analysis of the mechanism of EM-associated pain
focusing on PGE2, PGF2α, BK and BKB1R in EM tissues.
Further studies are required to investigate the expression and
cellular and subcellular location of BKB1R in primary cells and
cell lines to further confirm any conclusions, as well as to
further explore the molecular regulatory mechanism of
pain-producing substances.
In conclusion, the present study shows that the
onset of EM-associated pain is related to the release of several
inflammatory mediators, including BK, BKB1R, PGE2 and
PGF2α. However, the regulatory effects of these molecules on the
onset of EM-associated pain in humans are still unclear. Thus,
further studies are required to elucidate the possible role and
specific mechanisms of these molecules in the pathogenesis of EM
and to improve the quality of life of patients with EM. Since the
overexpression of the BKB1R can result in elevated levels of pain
after a longer stimulation interval, it is anticipated that BKB1R
antagonists could act as potential therapeutic agents for the
treatment of pain in EM. In the future, further meaningful studies
should be conducted in order to improve therapy options and patient
management.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Fund of China (grant nos. 82074483 and 81503608) and the
S&T Program of Hebei (grant nos. 21377725D and 213777116D).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM and YL performed the experiments. XF and MA
collected and analyzed the data. JC and SZ designed the study,
drafted and reviewed the manuscript, and supervised the entire
study. QL collected data and interpreted the results, and JY
analyzed the data and drew the figures. JC and SZ confirm the
authenticity of all the raw data. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Study protocols involving human subjects were
approved by the Ethics Committee of the Hebei University of Chinese
Medicine (Shijiazhuang, China; approval no. YXLL2015001), and
written informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamamoto A, Johnstone EB, Bloom MS,
Huddleston HG and Fujimoto VY: A higher prevalence of endometriosis
among Asian women does not contribute to poorer IVF outcomes. J
Assist Reprod Genet. 34:765–774. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alimi Y, Iwanaga J, Loukas M and Tubbs RS:
The clinical anatomy of endometriosis: A review. Cureus.
10(e3361)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim JH and Han E: Endometriosis and female
pelvic pain. Semin Reprod Med. 36:143–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nezhat C, Vang N, Tanaka PP and Nezhat C:
Optimal management of endometriosis and pain. Obstet Gynecol.
134:834–839. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ahmad SF, Akoum A and Horne AW: Selective
modulation of the prostaglandin F2α pathway markedly impacts on
endometriosis progression in a xenograft mouse model. Mol Hum
Reprod. 21:905–916. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sacco K, Portelli M, Pollacco J,
Schembri-Wismayer P and Calleja-Agius J: The role of prostaglandin
E2 in endometriosis. Gynecol Endocrinol. 28:134–138.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rakhila H, Bourcier N, Akoum A and Pouliot
M: Abnormal expression of prostaglandins E2 and F2α
receptors and transporters in patients with endometriosis. Biomed
Res Int. 2015(808146)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bulun SE, Imir G, Utsunomiya H, Thung S,
Gurates B, Tamura M and Lin Z: Aromatase in endometriosis and
uterine leiomyomata. J Steroid Biochem Mol Biol. 95:57–62.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Linhart O, Obreja O and Kress M: The
inflammatory mediators serotonin, prostaglandin E2 and bradykinin
evoke calcium influx in rat sensory neurons. Neuroscience.
118:69–74. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mizumura K, Sugiura T, Katanosaka K, Banik
RK and Kozaki Y: Excitation and sensitization of nociceptors by
bradykinin: What do we know? Exp Brain Res. 196:53–65.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Petho G and Reeh PW: Sensory and signaling
mechanisms of bradykinin, eicosanoids, platelet-activating factor,
and nitric oxide in peripheral nociceptors. Physiol Rev.
92:1699–1775. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Meotti FC, Campos R, da Silva K, Paszcuk
AF, Costa R and Calixto JB: Inflammatory muscle pain is dependent
on the activation of kinin B1 and B2
receptors and intracellular kinase pathways. Br J Pharmacol.
166:1127–1139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakano R, Kitanaka T, Namba S, Kitanaka N
and Sugiya H: Protein kinase Cε regulates nuclear translocation of
extracellular signal-regulated kinase, which contributes to
bradykinin-induced cyclooxygenase-2 expression. Sci Rep.
8(8535)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang Z, Yuan Y, He L, Yao X and Chen J:
Involvement of angiotensin II receptor type 1/NF-κB signaling in
the development of endometriosis. Exp Ther Med. 20:3269–3277.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jingwei C, Huilan D, Ruixiao T, Hua Y and
Huirong M: Effect of Bushenwenyanghuayu decoction on nerve growth
factor and bradykinin/bradykinin B1 receptor in a endometriosis
dysmenorrhea mouse model. J Tradit Chin Med. 35:184–191.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Osuga Y, Seki Y, Tanimoto M, Kusumoto T,
Kudou K and Terakawa N: Relugolix, an oral gonadotropin-releasing
hormone receptor antagonist, reduces endometriosis-associated pain
in a dose-response manner: A randomized, double-blind,
placebo-controlled study. Fertil Steril. 115:397–405.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee SY, Koo YJ and Lee DH: Classification
of endometriosis. Yeungnam Univ J Med. 38:10–18. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29(e45)2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu X, Zhang P, Li Y, Zhao N and Han H:
The AMPK-mTOR axis requires increased MALAT1 expression for
promoting granulosa cell proliferation in endometriosis. Exp Ther
Med. 21(21)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang J, Chen X and Lv Y: HMGB1 mediated
inflammation and autophagy contribute to endometriosis. Front
Endocrinol (Lausanne). 12(616696)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Machairiotis N, Vasilakaki S and Thomakos
N: Inflammatory mediators and pain in endometriosis: A systematic
review. Biomedicines. 9(54)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ulug U, Goldman S, Ben-Shlomo I and Shalev
E: Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor,
TIMP-1, in human term decidua and fetal membranes: The effect of
prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod.
7:1187–1193. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Banu SK, Lee J, Speights VO Jr,
Starzinski-Powitz A and Arosh JA: Cyclooxygenase-2 regulates
survival, migration, and invasion of human endometriotic cells
through multiple mechanisms. Endocrinology. 149:1180–1189.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ylikorkala O, Koskimies A, Laatkainen T,
Tenhunen A and Viinikka L: Peritoneal fluid prostaglandins in
endometriosis, tubal disorders, and unexplained infertility. Obstet
Gynecol. 63:616–620. 1984.PubMed/NCBI
|
|
25
|
Schenken RS, Asch RH, Williams RF and
Hodgen GD: Etiology of infertility in monkeys with endometriosis:
Measurement of peritoneal fluid prostaglandins. Am J Obstet
Gynecol. 150:349–353. 1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
McAllister SL, Giourgas BK, Faircloth EK,
Leishman E, Bradshaw HB and Gross ER: Prostaglandin levels, vaginal
innervation, and Cyst innervation as peripheral contributors to
endometriosis-associated vaginal hyperalgesia in rodents. Mol Cell
Endocrinol. 437:120–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
French L: Dysmenorrhea. Am Fam Physician.
71:285–291. 2005.PubMed/NCBI
|
|
29
|
Liclican EL, Nguyen V, Sullivan AB and
Gronert K: Selective activation of the prostaglandin E2 circuit in
chronic injury-induced pathologic angiogenesis. Invest Ophthalmol
Vis Sci. 51:6311–6320. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hamza M, Wang XM, Adam A, Brahim JS, Rowan
JS, Carmona GN and Dionne RA: Kinin B1 receptors contributes to
acute pain following minor surgery in humans. Mol Pain.
6(12)2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qadri F and Bader M: Kinin B1 receptors as
a therapeutic target for inflammation. Expert Opin Ther Targets.
22:31–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lau J, Rousseau J, Kwon D, Bénard F and
Lin KS: A systematic review of molecular imaging agents targeting
bradykinin B1 and B2 receptors. Pharmaceuticals (Basel).
13(199)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tang M, He F, Ma L, Liu P, Wang J and Zhu
X: Bradykinin receptors in ischemic injury. Curr Neurovasc Res.
15:359–366. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bertolini F, Carriero V, Bullone M, Sprio
AE, Defilippi I, Sorbello V, Gani F, Di Stefano A and Ricciardolo
FLM: Correlation of matrix-related airway remodeling and bradykinin
B1 receptor expression with fixed airflow obstruction in severe
asthma. Allergy. 76:1886–1890. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Moreau ME, Garbacki N, Molinaro G, Brown
NJ, Marceau F and Adam A: The kallikrein-kinin system: Current and
future pharmacological targets. J Pharmacol Sci. 99:6–38.
2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Okuse K: Pain signalling pathways: From
cytokines to ion channels. Int J Biochem Cell Biol. 39:490–496.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Muscella A, Cossa LG, Vetrugno C and
Marsigliante S: Bradykinin stimulates prostaglandin E2
release in human skeletal muscular fibroblasts. Mol Cell
Endocrinol. 507(110771)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bouhadfane M, Kaszás A, Rózsa B,
Harris-Warrick RM, Vinay L and Brocard F: Sensitization of neonatal
rat lumbar motoneuron by the inflammatory pain mediator bradykinin.
Elife. 4(e06195)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Baek SB, Shin MS, Han JH, Moon SW, Chang
B, Jeon JW, Yi JW and Chung JY: Rocuronium bromide inhibits
inflammation and pain by suppressing nitric oxide production and
enhancing prostaglandin E2 synthesis in endothelial
cells. Int Neurourol J. 20:296–303. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoshino O, Yamada-Nomoto K, Kobayashi M,
Andoh T, Hongo M, Ono Y, Hasegawa-Idemitsu A, Sakai A, Osuga Y and
Saito S: Bradykinin system is involved in endometriosis-related
pain through endothelin-1 production. Eur J Pain. 22:501–510.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hammerschmidt S, Kuhn H, Gessner C,
Seyfarth HJ and Wirtz H: Stretch-induced alveolar type II cell
apoptosis: Role of endogenous bradykinin and PI3K-Akt signaling. Am
J Res Cell Mol Biol. 37:699–705. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kaminskyy VO and Zhivotovsky B: Free
radicals in cross talk between autophagy and apoptosis. Antioxid
Redox Signal. 21:86–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Xu X, Tu L, Jiang W, Feng W, Zhao CX and
Wang DW: Bradykinin prevents the apoptosis of NIT-1 cells induced
by TNF-α via the PI3K/Akt and MAPK signaling pathways. Int J Mol
Med. 29:891–898. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xie H, Lu F, Liu W, Wang E, Wang L and
Zhong M: Remimazolam alleviates neuropathic pain via regulating
bradykinin receptor B1 and autophagy. J Pharm Pharmacol: Jun 1,
2021 (Epub ahead of print).
|
|
45
|
Al-Jefout M, Alnawaiseh N, Yaghi S and
Alqaisi A: Prevalence of endometriosis and its symptoms among young
jordanian women with chronic pelvic pain refractory to conventional
therapy. J Obstet Gynaecol Can. 40:165–170. 2018.PubMed/NCBI View Article : Google Scholar
|