Introduction
Osteoarthritis (OA) is a type of degenerative joint
disease that is most common in the elderly (aged ≥60 years),
typically originates from chondrocytes (1,2).
Patients with OA typically present with evidence of chondrocytes
apoptosis, joint inflammation and cartilage sclerosis (3). The main treatment methods, such as
acupuncture, drugs and electromagnetic therapies, are used to
relieve pain or control symptoms; however, they cannot cure OA
(4-8).
Therefore, novel treatment strategies are required to cure OA.
An increasing number of studies have investigated
long non-coding (lnc) RNAs and OA progression, and have revealed
that lncRNAs play important roles in some cellular processes of OA
(9-11).
Tang et al (9) reported that
silencing of lncRNA-p21 significantly increased cell viability and
inhibited apoptosis in human chondrocytes. Both Hu et al
(10) and Luo et al
(11) discovered that in the human
C28/I2 cartilage cell line, cell viability was promoted, whereas
apoptosis and inflammatory cytokines levels were suppressed
following transfection with short inhibiting RNA targeting H19 or
MFI2-AS1. In addition, Li and Zhang (12) reported that LINC01385 may have a
enhancing effect on the proliferation of nasopharyngeal carcinoma.
Xiao et al (13) found that
LINC01385 mRNA expression level was increased in human OA tissues
using microarray and bioinformatics analyses. However, the detailed
regulatory mechanism of LINC01385 in OA progression remains
unclear.
Emerging evidence has confirmed that microRNAs
(miRNAs/miR), such as miR-451(9),
miR-130a-3p (10,11,14),
miR-142-5p (15), miR-137(16), and miR-20b (17), play protective roles against the
development of OA. In addition, an increasing amount of research
has been directed towards understanding the anti-osteoarthritic
role of miR-140 (18-20).
Tardif et al (18) showed
that miR-140 was regulated by NFAT3 and transforming growth
factor-β/SMAD3 to inhibit the progression of OA. Furthermore,
Tardif et al (19)
demonstrated that the suppressive role of miR-140 in human OA was
associated with the protein level of insulin-like growth
factor-binding protein 5 and matrix metalloproteinase 13. Notably,
Wang et al (20) showed that
miR-140 exhibited its anti-osteoarthritic effects by promoting cell
survival and suppressing inflammation; however, the ability of
miR-140 to interact with LINC01385 to modulate OA progression
requires further investigation.
In the present study, the effects of the
LINC01385/miR-140-3p/TLR4 axis on the development and progression
of OA was investigated and the results could identify a potential
therapeutic target for OA.
Materials and methods
Tissues samples
A total of 25 patients with OA (males, 7; females,
18; mean age, 51.34±16.27 years) and 25 age-matched patients
(males, 9; females, 16; mean age, 50.12±14.38 years) with a femoral
fracture without OA or rheumatoid arthritis were selected from
Liaocheng People's Hospital (Shandong, China) between December 2016
and July 2018. The inclusion criteria were: i) Patients who were
diagnosed as OA (21); ii) patients
who were willing to participate; iii) patients who fully understood
the experimental protocol. The exclusion criteria were as follows:
i) Patients with other disease complications, such as chronic
inflammatory diseases; ii) patients who were treated within 3
months before admission. OA cartilage was collected from the
patients with OA, who had undergone total knee replacement surgery,
whereas the normal cartilage tissues were obtained from the knee
joints of patients without OA or rheumatoid arthritis. All the
cartilage samples were obtained in accordance with the diagnostic
criteria of osteoarthritis from the Orthopaedic Society of the
Chinese Medical Association (21).
All participants provided written informed consent and the
protocols of the present study were approved by the ethical
committee of Liaocheng People's Hospital (approval no.
2020037).
Cell culture, transfection, and
induction
The human articular chondrocytes (HC-a) were
purchased from Tongpai Biotech, Co., Ltd., then cultured in DMEM
containing 10% FBS at 37˚C in a humidified incubator with 5%
CO2. To establish OA in vitro model, HC-a cells
were treated with 10 ng/ml of IL-1β (Sigma-Aldrich; Merck KGaA) for
24 h at 37˚C and HC-a cells without IL-1β treatment were served as
the controls. Afterwards, the short hairpin (sh)RNA targeting
LINC01385 (sh-LINC01385), toll-like receptor 4 (sh-TLR4), and the
non-targeting negative control (sh-NC) were purchased from
VectorBuilder Inc. The linear pSIH1-H1-copGFP shRNA Vector was
obtained from System Biosciences, LLC. Overexpression TLR4
(pcDNA-TLR4), overexpression LINC01385 (pcDNA-LINC01385), and their
respective NCs (pcDNA3.1); miR-140-3p mimics
(5'-UACCACAGGGUAGAACCACGG-3') and its NC (NC mimics,
5'-GCAAGAGACAAGCGCUUAGCC-3'); and miR-140-3p inhibitor
(5'-CCGUGGUUCUACCCUGUGGUA-3') and its NC (NC inhibitor,
5'-GGUCCUGAUUCGUGCUACUCG-3') were obtained from Hunan Fenghui
Biotechnology Co., Ltd. The aforementioned agents (20 nM) were
transfected into the IL-1β-induced HC-a (2x106) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C. Subsequently, 48 h after transfection,
the transfected HC-a cells were harvested to perform the following
experiments.
Reverse transcription
(RT)-quantitative PCR (RT-qPCR)
Total RNA was extracted from the OA and normal
cartilage tissues, and the transfected HC-a using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
RNA was then quantified using a NanoDrop® 1000 (Thermo
Fisher Scientific, Inc.) and RT was performed using a FastQuant
cDNA First Chain Synthesis kit (Tiangen Biotech, Co., Ltd.) from 2
µg total RNA, according to the manufacturer's instructions. The
conditions of RT were as follows: 10 min at 25˚C, 35 min at 50˚C
and 15 min at 80˚C. qPCR was performed using Fast SYBR™ Green
Master Mix (Thermo Fisher Scientific, Inc.) and an ABI 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used: Initial
denaturation at 94˚C for 10 min, followed by 40 cycles at 94˚C for
10 sec, 60˚C for 20 sec and 72˚C for 1 min. GADPH and U6 were used
as the internal references. Gene expression was quantified using
the 2-ΔΔCq method (22).
The following primer sequences were used: LINC01385 forward,
5'-TGTTTCTCGAGTGTGGGCAG-3' and reverse, 5'-GGCACTCGCGTTTTCTTCTG-3';
miR-140-3p forward, 5'-CACTCCAGCTGGGAGGCGGGGCGCCGCGGGA-3' and
reverse, 5'-CTCAACTGGTGTCGTGGA-3'; TLR4 forward,
5'-AGTTGATCTACCAAGCCTTGAGT-3' and reverse,
5'-GCTGGTTGTCCCAAAATCACTTT-3'; GAPDH forward,
5'-CCAGGTGGTCTCCTCTGA-3' and reverse, 5'-GCTGTAGCCAAATCGTTGT-3' and
U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
MTT assay
The viability of the HC-a was detected using a MTT
assay. Briefly, the transfected and IL-1β-induced HC-a were seeded
into a 96-well plate (2x105 cells per well) and
incubated for 48 h at 37˚C. Subsequently, 20 µl MTT (Nanjing KeyGen
Biotech Co., Ltd.) was added to each well and the cells were then
incubated for 2 h at 37˚C. Following which, 150 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added to dissolve the
formazan crystal. The viability (measured at 450 nm) was analyzed
using a Multiskan Spectrum microplate reader (Thermo Fisher
Scientific, Inc.).
ELISA
The concentrations of the inflammatory cytokines
[IL-6 (cat. no. 70-EK106/2-96), tumor necrosis factor- α (TNF-α,
cat. no. 70-EK182HS-96), and prostaglandin E2
(PGE2, cat. no. 70-EK8103/2-48; http://www.liankebio.com/gallery.html?scontent=n,70-EK8103%2F2-48)]
in IL-1β-induced HC-a were measured using the corresponding ELISA
kits [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.] according
to the manufacturer's protocols. The absorbance at 450 nm was
measured using a Multiskan Spectrum microplate reader (Thermo
Fisher Scientific, Inc.).
Target prediction
The miRNA targets of LINC01385 were predicted using
the StarBase database version 2.0 (http://starbase.sysu.edu.cn/) and LncBase Predicted
v.2 database (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted).
In addition, the mRNA targets of miR-140-3p were predicted using
the StarBase database. TLR4 was selected for further analysis due
to its important role in OA (23-25)
and unknown relationship with miR-140-3p in OA.
Dual luciferase reporter (DLR)
assay
LINC01385 with wild-type (WT) or mutant (mut)
miR-140-3p-binding sites were ligated into the pGL3 vector (Promega
Corporation). In addition, the 3'-untranslated region from TLR4
containing the predicted miR-140-3p recognition sequence was
amplified by PCR using Platinum™ Direct PCR Universal Master Mix
(cat. no. A44647100; Thermo Fisher Scientific, Inc.). The DNA
template was isolated from the normal cartilage tissues of the
patients without OA or rheumatoid arthritis using DNAzol™ Reagent
(Thermo Fisher Scientific, Inc., cat. no. 10503027). The primer
sequences were: forward, 5'-TGTATAGCAGAGTTCGTAT-3' and reverse
5'-ACTGAATTTTGTTGTCT-3'. The following thermocycling conditions
were used: Initial denaturation at 94˚C for 5 min, followed by 30
cycles at 94˚C for 5 sec, 60˚C for 10 sec and 72˚C for 30 sec. The
amplified product was ligated into the pGL3 vector. HC-a
(2x105) were then co-transfected with
LINC01385-mut/TLR4-mut or LINC01385-WT/TLR4-WT (80 ng) and
miR-140-3p mimics or NC mimics (80 ng) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C. Following transfection for 48 h,
Renilla and firefly luciferase activities were measured
using the dual-luciferase reporter assay system (Promega
Corporation). The activity of firefly luciferase was normalized to
the activity of Renilla luciferase.
Western blot analysis
Following protein extraction from the HC-a using
RIPA buffer containing protease inhibitor, the BCA protein assay
kit (Abcam) was used to detect the protein concentrations. Proteins
(~50 µg) were separated using 10% SDS-PAGE, then transferred onto
PVDF membranes and blocking with 5% bovine serum albumin (Thermo
Fisher Scientific, Inc.) at room temperature for 2 h. Following
which, the membranes were incubated with primary antibodies against
TLR4 (1:1,000; Abcam, cat. no. ab22048), and α-tubulin (1:1,000;
Abcam, cat. no. ab7291) overnight at 4˚C. The membranes were then
washed a minimum of three times using TBS-Tween-20 (TBST; Tween-20,
0.05%) and incubated with the HRP-conjugated anti-mouse IgG
secondary antibody (1:5,000; Abcam, cat. no. ab6728) for 1 h at
room temperature. Tubulin was used as the internal reference. The
membranes were visualized using a ECL kit (Invitrogen; Thermo
Fisher Scientific, Inc.) and quantified using ImageLab software
version 1.46 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS v23.0
(IBM Corp). The differences between two groups or among multiple
groups was analyzed using either a Student's t-test (unpaired) or
one-way ANOVA followed by Tukey's multiple comparison test,
respectively. The data are presented as the mean ± SD. The
correlation was determined using Pearson's correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference. All the experiments were conducted in triplicate, from
at least three independent experiments.
Results
LINC01385 is highly expressed in OA
tissues and in IL-1β-induced HC-a
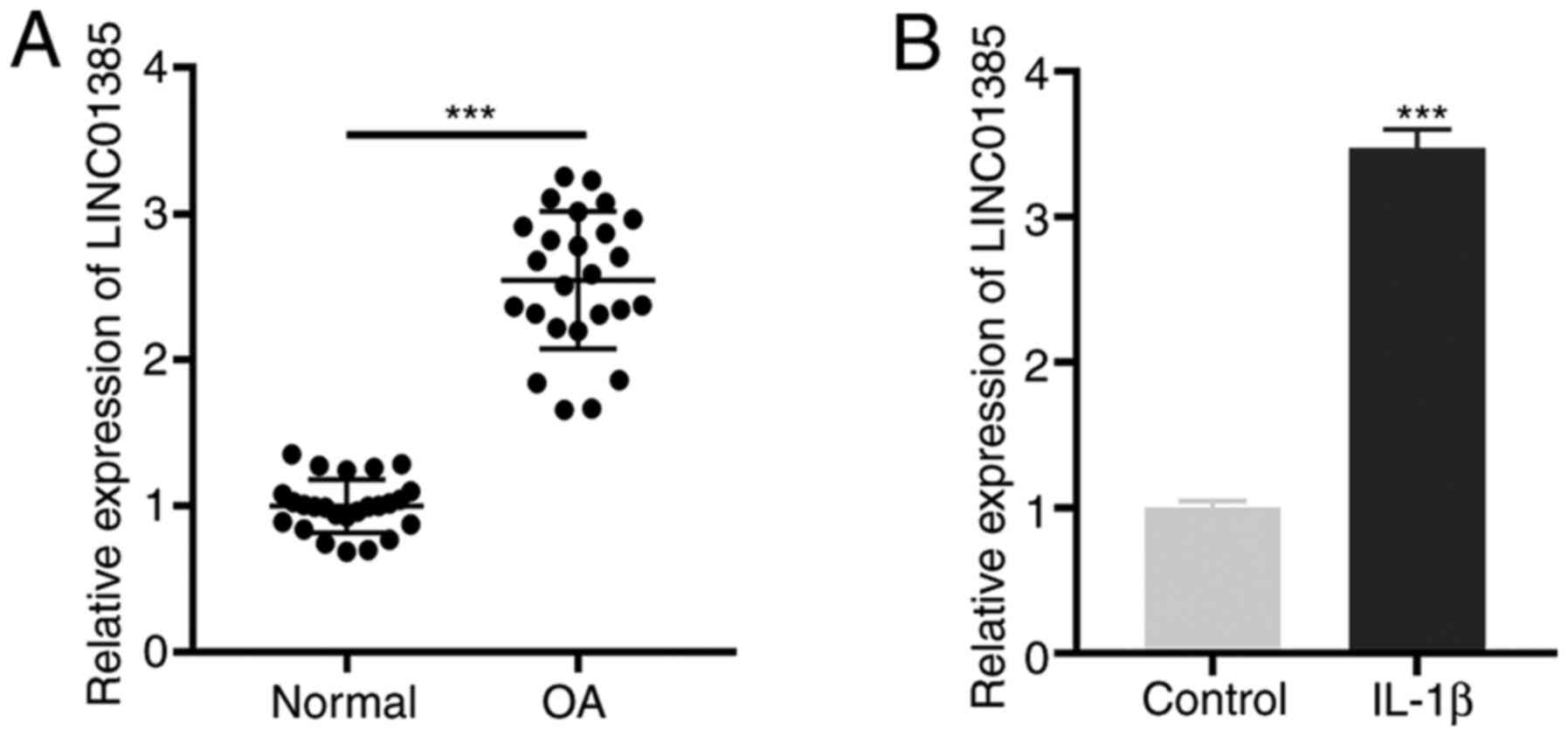
First, the mRNA expression level of LINC01385 in OA
tissues was determined and the results showed that LINC01385 was
significantly expressed in OA tissues compared with that in normal
tissues (P<0.001; Fig. 1A).
Similarly, an increased mRNA expression level of LINC01385 was
observed in IL-1β-induced HC-a compared with that in the controls
(P<0.001; Fig. 1B).
LINC01385 knockdown suppresses
inflammatory protein concentrations and promotes cell viability in
the IL-1β-induced HC-a
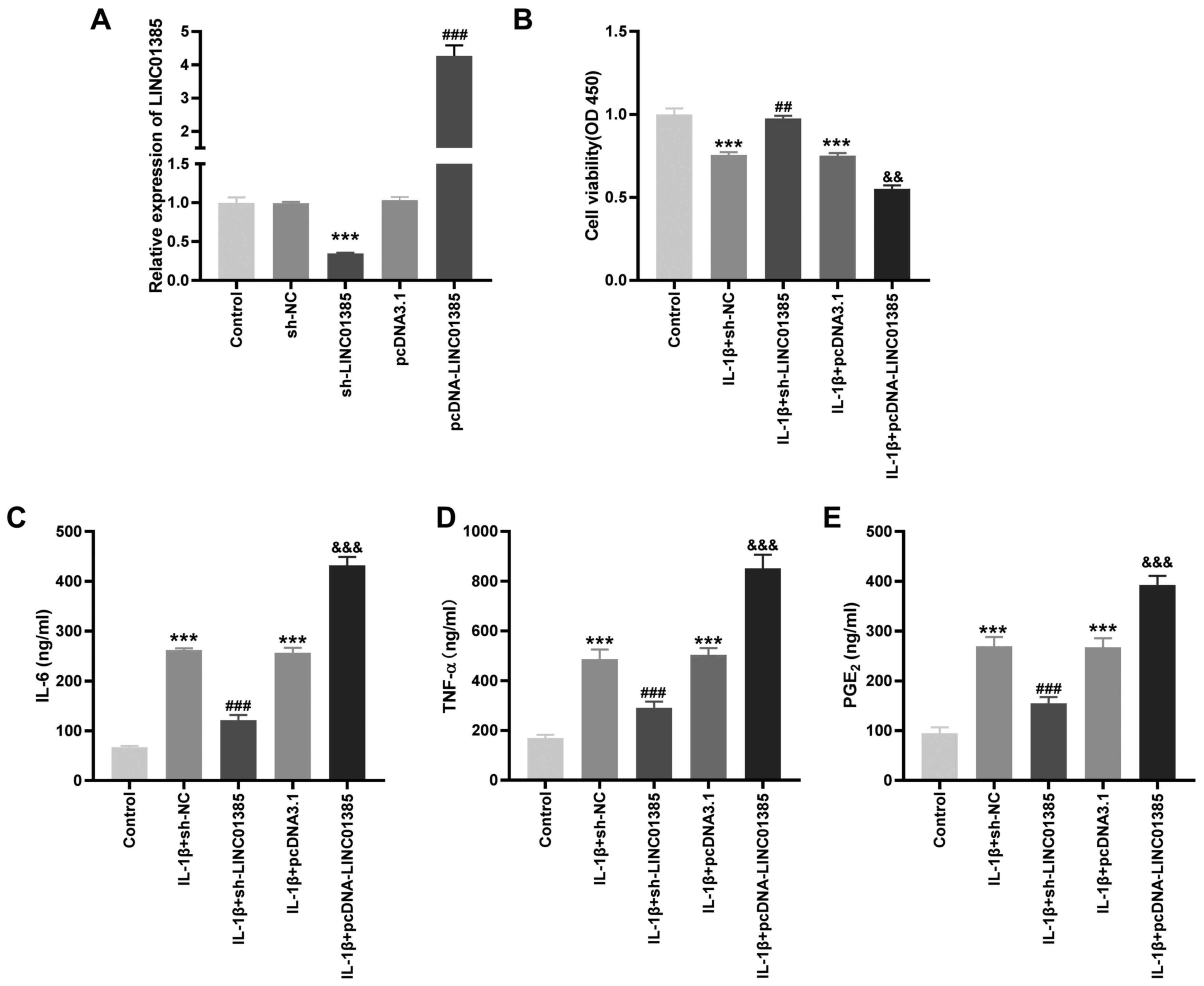
To investigate the potential role of LINC01385 on OA
progression in vitro, the transfection efficiency of
sh-LINC01385 and pcDNA-LINC01385 was evaluated. The mRNA expression
level of LINC01385 was notably decreased following transfection
with sh-LINC01385, whereas it was increased following transfection
with pcDNA-LINC01385 (P<0.001; Fig.
2A). Following which, the viability of the IL-1β-induced HC-a
was measured. Cell viability was inhibited in the IL-1β + sh-NC and
IL-1β + pcDNA3.1 groups compared with that in the control
(P<0.001; Fig. 2B). However, the
viability of the HC-a was increased in the IL-1β + sh-LINC01385
group compared with that in the IL-1β + sh-NC group, whereas it was
suppressed in the IL-1β + pcDNA-LINC01385 group compared with that
in the IL-1β + pcDNA3.1 group (P<0.01; Fig. 2B). The concentrations of the
inflammatory cytokines, which were measured using ELISA, revealed
contrasting results. As shown in Fig.
2C-E, transfection of sh-NC or pcDNA3.1 increased the secretion
of the inflammatory cytokines compared with that in the control.
Compared with that in the sh-NC group, transfection with
sh-LINC01385 significantly reduced the concentration of the
inflammatory cytokines in the IL-1β-induced HC-a, while
transfection with pcDNA-LINC01385 significantly increased the
concentration of inflammatory cytokines compared with that in the
pcDNA3.1 group (P<0.001).
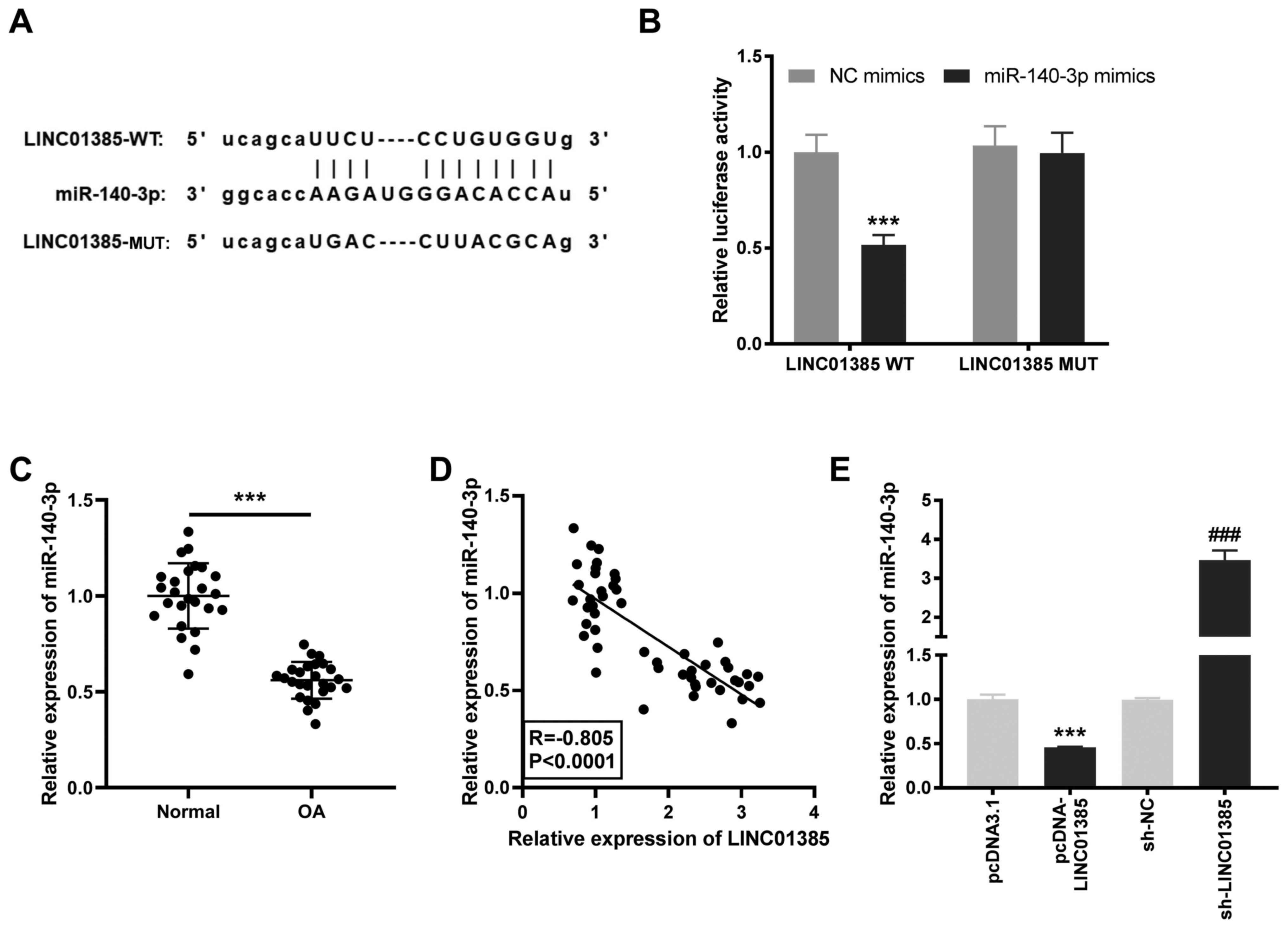
Identification miR-140-3p as a target
of LINC01385
To investigate the downstream target of LINC01385,
the StarBase database was used to predict potential binding sites
between LINC01385 and miRNAs and miR-140-3p was found to be a
target (Fig. 3A). DLR verified that
the luciferase activity was significantly reduced in the presence
of WT LINC01385 and miR-140-3p mimics; but not with miR-NC,
suggesting that miR-140-3p was a target of LINC01385 (P<0.001;
Fig. 3B). As illustrated in
Fig. 3C, decreased mRNA expression
level of miR-140-3p was detected in OA tissues compared with that
in the normal tissues (P<0.001). In addition, there a negative
correlation between the mRNA expression levels of LINC01385 and
miR-140-3p in OA tissues (R=-0.805; P<0.0001; Fig. 3D). To further confirm the target
relationship between LINC01385 and miR-140-3p, miR-140-3p mRNA
expression level was detected following transfection with
pcDNA-LINC01385 or sh-LINC01385 in the HC-a. The results from
RT-qPCR revealed that miR-140-3p mRNA expression level was
significantly decreased following transfection with
pcDNA-LINC01385, while it was increased following transfection with
sh-LINC01385 (P<0.001; Fig. 3E),
which demonstrated that miR-140-3p was negatively regulated by
LINC01385.
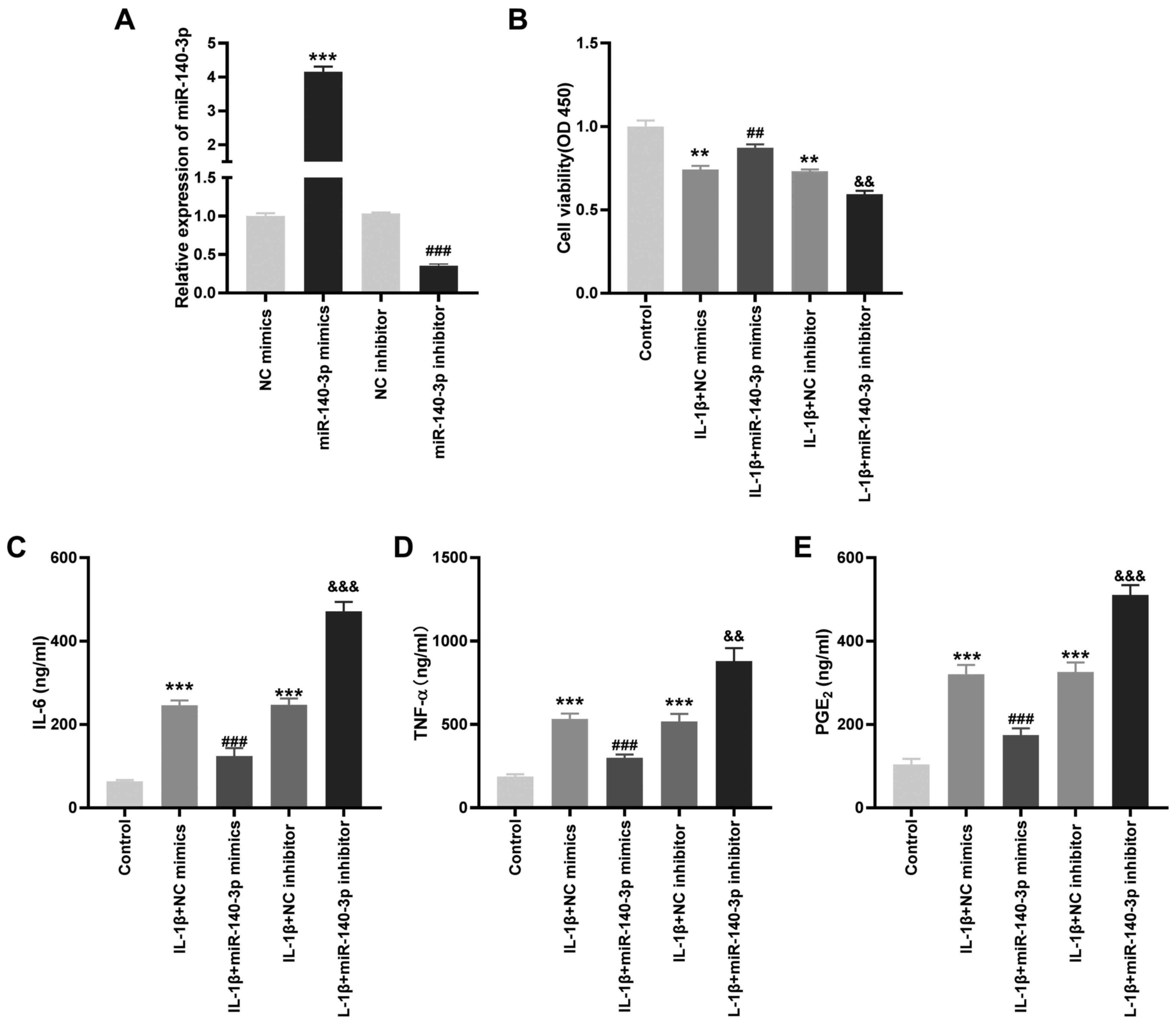
Overexpression of miR-140-3p reduces
the concentration of the inflammatory cytokines in IL-1β-induced
HC-a
To investigate the role of miR-140-3p on the
biological functions of OA in vitro, the transfection
efficiency of miR-140-3p mimics and inhibitors was detected.
miR-140-3p mRNA expression level was significantly increased by
miR-140-3p mimics, whereas it was decreased by the miR-140-3p
inhibitor (P<0.001; Fig. 4A). As
shown in Fig. 4B-E, it was found
that transfection with miR-140-3p mimics significantly increased
cell viability and reduced the concentration of the inflammatory
cytokines compared with that in the control group, whereas
miR-140-3p inhibitor significantly reduced cell viability and
increased the concentration of the inflammatory cytokines
(P<0.01).
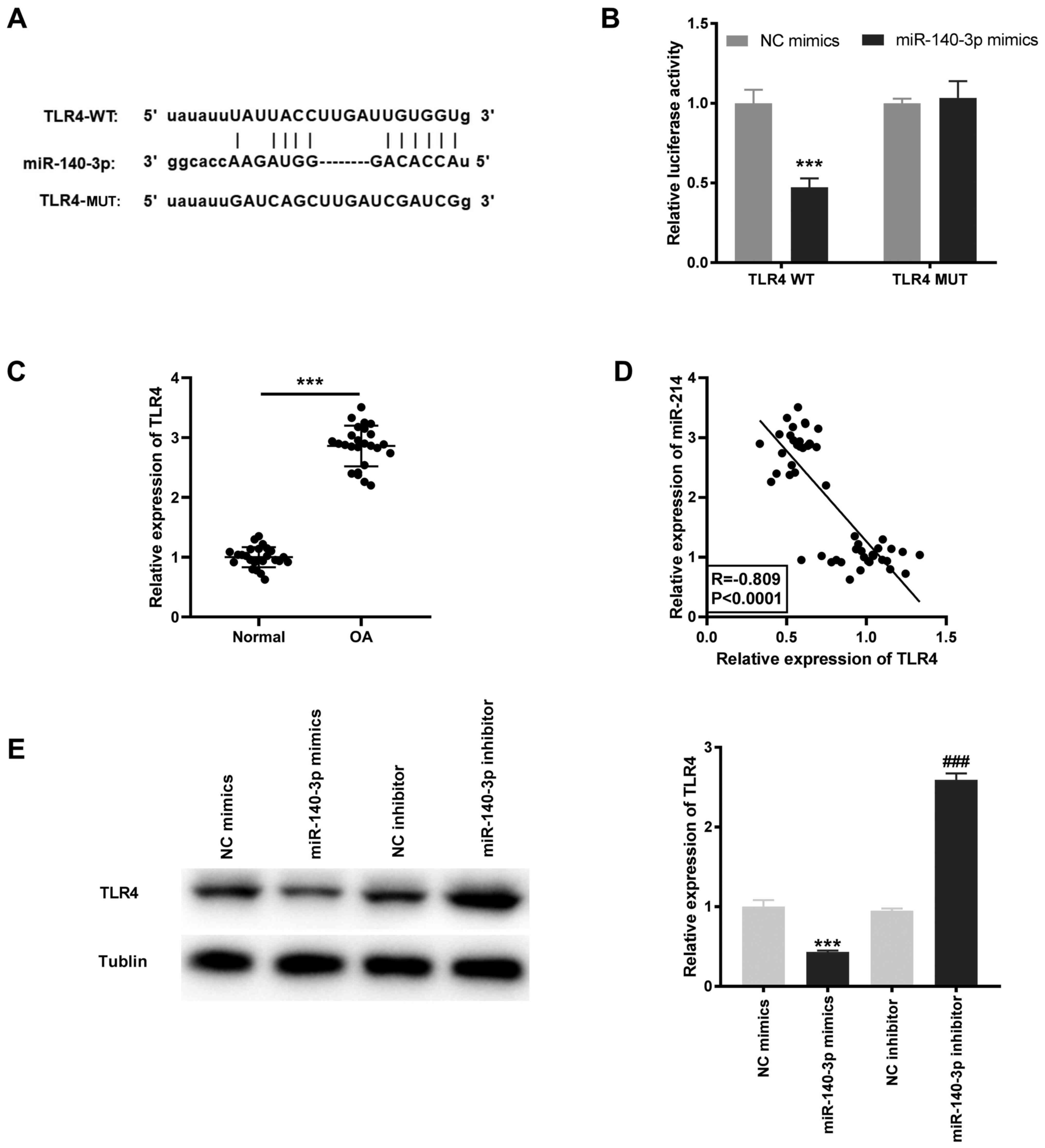
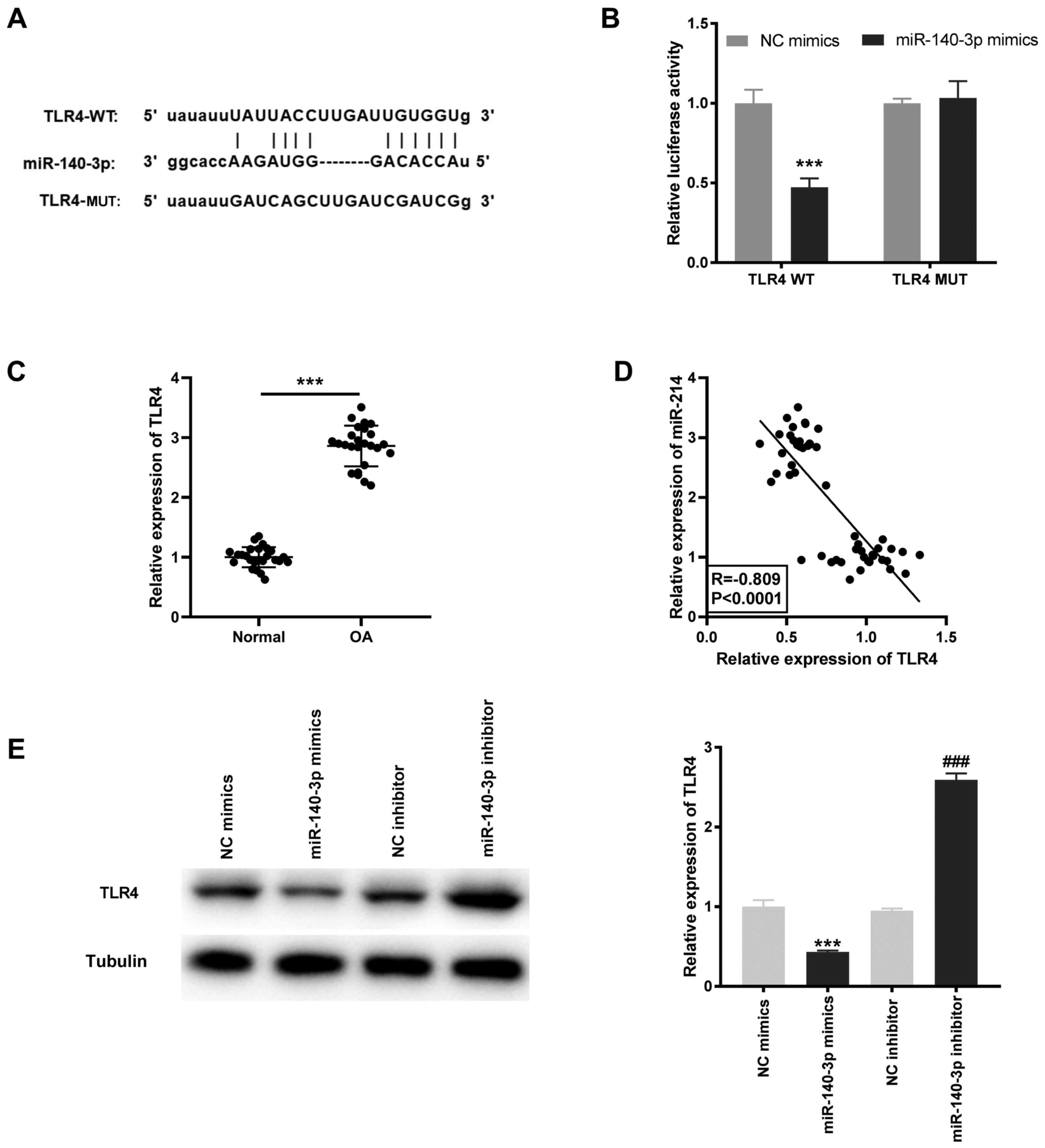
miR-140-3p targets TLR4
Based on the results of the StarBase database, the
potential binding site between miR-140-3p and TLR4 was identified
(Fig. 5A). Using the DLR assay, it
was found that the luciferase activity was significantly decreased
in the TLR4 WT/miR-140-3p mimics group compared with that in the
TLR4 WT/miR-NC group (P<0.001; Fig.
5B). TLR4 mRNA expression level was significantly higher in OA
tissues compared with that in the normal tissues (P<0.001;
Fig. 5C) and was negatively
correlated with miR-140-3p mRNA expression levels (R=-0.809;
P<0.0001; Fig. 5D). Furthermore,
the TLR4 protein expression level was determined following
transfection with miR-140-3p mimics or inhibitor into the HC-a to
further confirm the interaction between miR-140-3p and TLR4. The
results showed that the TLR4 protein expression level was
significantly inhibited by miR-140-3p mimics and increased by
miR-140-3p inhibitor (P<0.001; Fig.
5E).
Decreased TLR4 mRNA expression level
suppresses the concentration of the inflammatory cytokines in OA in
vitro
To investigate the effect of TLR4 on OA inflammation
in vitro, the transfection efficiency of sh-TLR4 and
pcDNA-TLR4 was initially determined. TLR4 mRNA expression level in
the HC-a transfected with sh-TLR4 was decreased, while it was
increased following transfection with pcDNA-TLR4 (P<0.001;
Fig. 6A). Similarly, the protein
expression level of TLR4 was significantly reduced following
transfection with sh-TLR4 and was increased by pcDNA-TLR4
(P<0.001; Fig. 6B). As
illustrated in Fig. 6C-E, the
concentrations of IL-6, TNF-α, and PGE2 were
significantly reduced following TLR knockdown and increased
following overexpression of TLR (P<0.01).
Knockdown of LINC01385 suppresses the
development of OA by regulating the miR-140-3p/TLR4 axis in
vitro
To investigate the regulatory mechanisms of
LINC01385, miR-140-3p and TLR4 on OA in vitro, sh-LINC01385,
sh-LINC01385 + miR-140-3p inhibitor or sh-LINC01385 + pcDNA-TLR4
was transfected into the HC-a. The protein expression level of TLR4
was decreased following transfection with sh-LINC01385, whereas it
was increased following transfection with sh-LINC01385 + miR-140-3p
inhibitor or sh-LINC01385 + pcDNA-TLR4 (P<0.01; Fig. 7A). Following which, rescue
experiments revealed that transfection with miR-140-3p inhibitor
and TLR4 overexpression vector reversed the enhancing effect of
LINC01385 knockdown on cell viability, and the inhibitory effects
on secretion of the inflammatory cytokines in IL-1β-treated HC-a
(P<0.01; Fig. 7B-E).
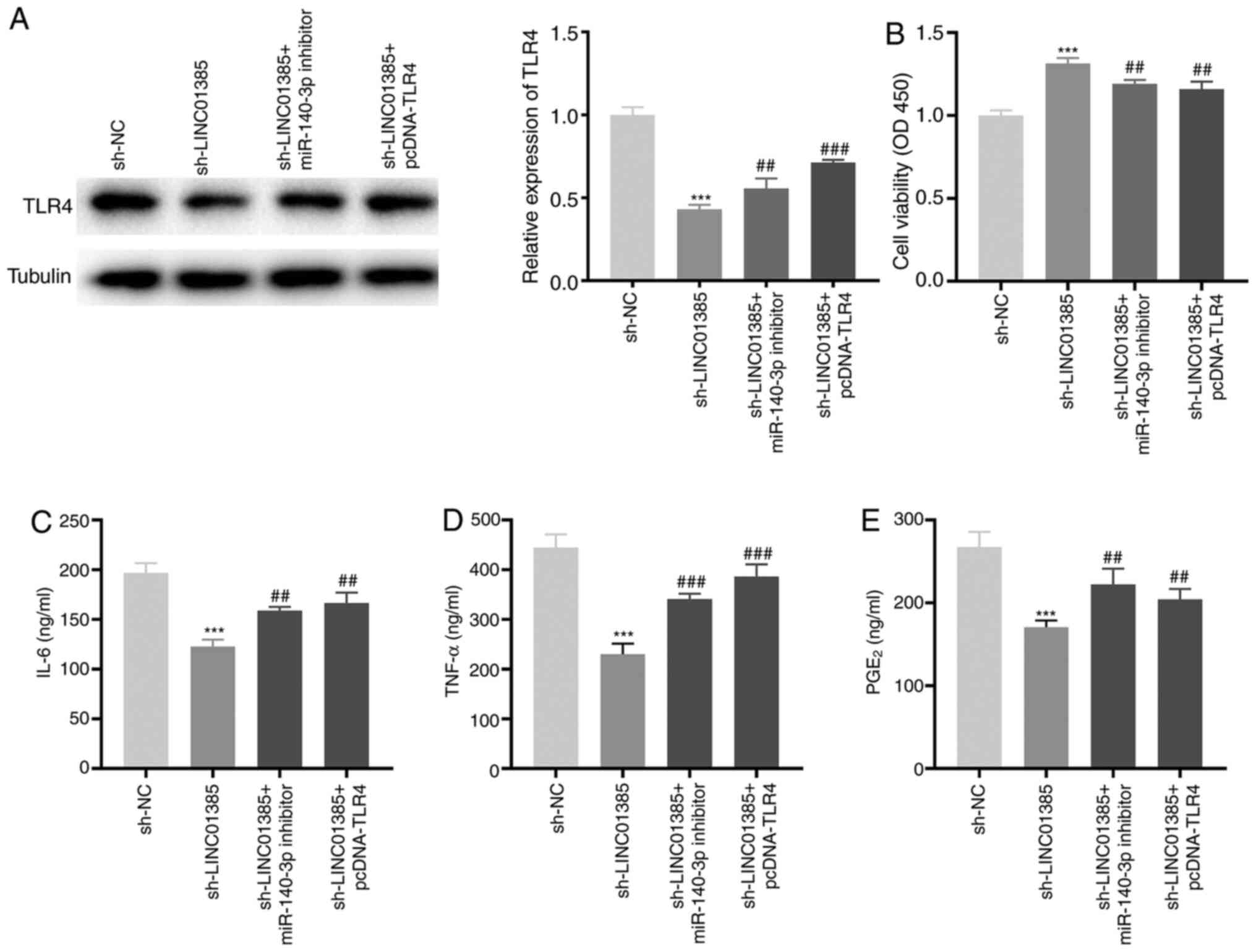 | Figure 7Knockdown of LINC01385 suppresses the
development of OA by regulating the miR-140-3p/TLR4 axis in
vitro. (A) The protein expression level of TLR4 following
transfection with sh-LINC01385, sh-LINC01385 + miR-140-3p inhibitor
or sh-LINC01385 + pcDNA-TLR4 into the HC-a was measured using
western blot analysis. ***P<0.001 vs. the sh-NC
group. ##P<0.01, ###P<0.001 vs. the
sh-LINC01385 group. (B) The viability of the IL-1β-induced HC-a was
measured using a MTT assay. ***P<0.001 vs. the sh-NC
group. ##P<0.01 vs. the sh-LINC01385 group. The
concentration of (C) IL-6, (D) TNF-α and (E) PGE2 in
IL-1β-induced HC-a was measured using ELISA.
***P<0.001 vs. the sh-NC group.
##P<0.01, ###P<0.001 vs. the
sh-LINC01385 group. NC, negative control; miR, microRNA; sh, short
hairpin; TNF, tumor necrosis factor; PGE2, prostaglandin
E2; TLR4, toll-like receptor 4. |
Discussion
OA is a common joint disease, which causes suffering
and inconvenience to the patients, due to joint pain on a daily
basis (26). Furthermore, it is a
leading cause of disability and shortening of an adult working life
globally (26). Numerous lncRNAs
have been associated with the development of OA (27-29).
For example, lncRNA anti-differentiation non-coding RNA expression
was increased in plasma specimens from patients with OA and
CHON-001 human chondrocytes (27).
The mRNA expression level of lncRNA MALAT1 was increased in OA
tissues and in a time-dependent manner in IL-1β-induced
chondrocytes (28). Furthermore,
increased mRNA expression of FOXD2-AS1 was detected in OA cartilage
tissue (29). Similar results were
obtained in the present study, as LINC01385 mRNA expression was
found to be higher in OA tissues and in IL-1β-induced HC-a,
suggesting that LINC01385 may play a pathogenic role in OA.
Over the past decade, lncRNAs have been shown to
play crucial roles in regulating proliferation, apoptosis and
inflammatory reactions in OA progression (10,11,15).
For example, MFI2-AS1 silencing reversed the inhibitory effect of
lipopolysaccharide (LPS)-induced OA on cell viability and the
promoting effect on apoptosis and inflammation in chondrocytes
(11). In addition, knockdown of
XIST played a stimulatory role in the proliferation of human SW1353
chondrocytes (15). The cell
viability of LPS-treated human C28/I2 cartilage cells was
facilitated by H19 knockdown, whereas apoptosis and the
concentrations of inflammatory cytokines (IL-6, IL-1β, and TNF-α)
were suppressed (10). Consistent
with this previous study, in the present study it was found that
transfection with sh-LINC01385 significantly reduced cell viability
in IL-1β-induced HC-a, whilst it decreased the concentration of
IL-6, TNF-α and PGE2. However, contrasting results were obtained
following transfection with pcDNA-LINC01385. Therefore, we
hypothesized that silencing of LINC01385 protected against OA.
Numerous studies have indicated that miRs serve as
suppressors in the progression of OA (11,15,16).
Luo et al (11) demonstrated
that miR-130a-3p was minimally expressed in OA tissues, whereas
overexpression of miR-130a-3p notably promoted cell viability and
inhibited inflammation in LPS-induced C28/I2 cells. Sun et
al (15) showed that the mRNA
expression level of miR-142-5p was decreased in IL-1β-stimulated
SW1353 chondrocytes, while the proliferation of these cells was
increased following transfection with miR-142-5p mimics. A recent
study conducted by Wang et al (16) detected low mRNA expression level of
miR-137 in OA tissues and increase of miR-137 expression in
LPS-treated human chondrocytes significantly elevated cell
viability, suppressed apoptosis and reduced the concentration of
inflammatory cytokines (16). In
the present study, decreased mRNA expression level of miR-140-3p
was found in OA tissues. Overexpression of miR-140-3p significantly
increased cell viability, whereas it reduced the concentration of
inflammatory factors in IL-1β-induced HC-a, suggesting that
miR-140-3p could be an anti-inflammatory miRNA in OA. In accordance
with the results from the present study, Wang et al revealed
that miR-140 expression level was reduced in OA tissues, and
transfection of miR-140 mimics into IL-1β-induced chondrocytes
increased cell viability and reduced the concentration of IL-6 and
TNF-α (20). By contrast, it was
shown in the present study that transfection with miR-140-3p
inhibitor reduced cell viability and increased the concentration of
inflammatory factors. Simultaneously, miR-140-3p was verified as a
target of LINC01385 and was found to be negatively regulated by it.
We hypothesized that silencing of LINC01385 attenuated OA
progression by negatively regulating miR-140-3p. The results from
the present study verified that knockdown of miR-140-3p reversed
the promoting effect of LINC01385 knockdown on cell viability and
the inhibitory effect on inflammation in IL-1β-induced HC-a cells.
Therefore, miR-140-3p was negatively regulated by LINC1385 and
found to be associated with the progression of OA.
TLR4, a member of the TLR family, has been
associated with inflammation, including in OA (30,31).
Liu et al (32) showed that
TLR4 mRNA expression was increased in human OA chondrocytes. In
addition, other studies have demonstrated that TLR4 was
overexpressed in OA tissues (23-25).
In accordance with these studies, it was found that mRNA expression
level of TLR4 was increased in OA tissues compared with that in
normal tissues. These results suggested that TLR4 might be a
pro-inflammatory gene in OA. The ELISA results showed that the
concentration of IL-6, TNF-α and PGE2 in IL-1β-induced
HC-a was inhibited following transfection with sh-TLR4; however, it
was increased following transfection with pcDNA-TLR4, which
supports the aforementioned hypothesis. Furthermore, TLR4
was found to be a target gene of miR-140-3p. As aforementioned, the
results from the present study showed that LINC01385 decreased OA
progression by regulating miR-140-3p. Therefore, we hypothesized
that silencing of LINC01385 ameliorated OA by regulating the
miR-140-3p/TLR4 axis. The results of rescue experiments showed that
TLR4 overexpression reversed the effect of LINC01385 knockdown on
increasing cell viability and the inhibitory effect on the increase
in the concentration of inflammatory factors in IL-1β-induced HC-a,
which confirmed our hypothesis. In conclusion, the results of the
present study suggested that silencing of LINC01385 inhibited OA
progression by modulating the miR-140-3p/TLR4 axis.
However, there are some limitations to the present
study. First, OA progression was investigated at a cellular level
and in vivo experiments should be performed. Second, OA
pathogenesis is complex and the LINC01385/miR-140-3p/TLR4 may not
be the sole regulatory axis involved. Therefore, further
elucidation of alternate pathways and conduction of in vivo
experiments are warranted.
In summary, the present study elucidated that
LINC01385 acted as an endogenous sponge of miR-140-3p to promote
cell viability and suppress inflammation in IL-1β-induced HC-a. In
addition, TLR4 was found to be associated with OA
progression, as a target gene of miR-140-3p. The present study
demonstrated that the LINC01385/miR-140-3p/TLR4 axis could be
important in the development of OA, providing a potential
therapeutic target for OA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ZW and CH were involved in the conception and design
of the study, analyzed the data, and drafted the manuscript. CZ, HZ
and ZZ made substantial contributions to analysis and
interpretation of data and revised the article critically for
important intellectual content. DX made substantial contributions
to conception and design and revised the article. ZW and CH confirm
the authenticity of all the raw data. All the authors performed the
experiments and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was conducted after obtaining ethics
approval from the Liaocheng People's Hospital's Ethics Committee
(no. 2020037).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neogi T and Zhang Y: Epidemiology of
osteoarthritis. Rheum Dis Clin North Am. 39:1–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Markstedt K, Mantas A, Tournier I,
Martinez Avila H, Hagg D and Gatenholm P: 3D bioprinting human
chondrocytes with nanocellulose-alginate bioink for cartilage
tissue engineering applications. Biomacromolecules. 16:1489–1496.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang W, Nuki G, Moskowitz RW, Abramson S,
Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty
M, et al: OARSI recommendations for the management of hip and knee
osteoarthritis: Part III: Changes in evidence following systematic
cumulative update of research published through January 2009.
Osteoarthritis Cartilage. 18:476–499. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Majeed MH, Sherazi SA, Bacon D and Bajwa
ZH: Pharmacological treatment of pain in osteoarthritis: A
descriptive review. Curr Rheumatol Rep. 20(88)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wollheim FA: Current pharmacological
treatment of osteoarthritis. Drugs. 52 (Suppl 3):27–38.
1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chun-Lei Y: Clinical observation on
treatment of knee osteoarthritis with internal and external
therapies in Chinese medicine. Liaoning J Tradit Chin Med.
9:1744–1745. 2010.(In Chinese).
|
|
8
|
Baker CL Jr and Ferguson CM: Future
treatment of osteoarthritis. Orthopedics. 28 (Suppl 2):s227–s234.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang L, Ding J, Zhou G and Liu Z:
LncRNAp21 promotes chondrocyte apoptosis in osteoarthritis by
acting as a sponge for miR451. Mol Med Rep. 18:5295–5301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu Y, Li S and Zou Y: Knockdown of lncRNA
H19 relieves LPS-induced damage by modulating miR-130a in
osteoarthritis. Yonsei Med J. 60:381–388. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luo X, Wang J, Wei X, Wang S and Wang A:
Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced
osteoarthritis progression by miR-130a-3p/TCF4. Life Sci.
240(117019)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li L and Zhang F: Novel long noncoding RNA
LINC01385 promotes nasopharyngeal carcinoma proliferation via the
miR-140-3p/Twist1 signaling pathway. Cell Cycle. 19:1352–1362.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao K, Yang Y, Bian Y, Feng B, Li Z, Wu
Z, Qiu G and Weng X: Identification of differentially expressed
long noncoding RNAs in human knee osteoarthritis. J Cell Biochem.
120:4620–4633. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang H, Li J, Shao W and Shen N: LncRNA
CTBP1-AS2 is upregulated in osteoarthritis and increases the
methylation of miR-130a gene to inhibit chondrocyte proliferation.
Clin Rheumatol. 39:3473–3478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sun P, Wu Y, Li X and Jia Y: miR-142-5p
protects against osteoarthritis through competing with lncRNA XIST.
J Gene Med. 22(e3158)2020.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Wang J, Fang L, Ye L, Ma S, Huang H, Lan X
and Ma J: miR-137 targets the inhibition of TCF4 to reverse the
progression of osteoarthritis through the AMPK/NF-κB signaling
pathway. Biosci Rep. 40(BSR20200466)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Y, Zhang L, Li E, Zhang G, Hou Y,
Yuan W, Qu W and Ding L: Long-chain non-coding RNA HOTAIR promotes
the progression of osteoarthritis via sponging miR-20b/PTEN axis.
Life Sci. 253(117685)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tardif G, Pelletier JP, Fahmi H, Fahmi H,
Hum D, Zhang Y, Kapoor M and Martel-Pelletier J: NFAT3 and
TGF-β/SMAD3 regulate the expression of miR-140 in osteoarthritis.
Arthritis Res Ther. 15(R197)2013.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10(148)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Shen S, Li Z, Li W and Weng X:
miR-140-5p affects chondrocyte proliferation, apoptosis, and
inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res.
69:63–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang MY: Review and prospect: 2009's
annual report of Orthopaedic Trauma Society of Chinese Medical
Association. Chinese Journal of Orthopaedic Trauma. 1(3)2010.(In
Chinese).
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
De Nardo D: Toll-like receptors:
Activation, signalling and transcriptional modulation. Cytokine.
74:181–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang P, Zhu F, Tong Z and Konstantopoulos
K: Response of chondrocytes to shear stress: Antagonistic effects
of the binding partners Toll-like receptor 4 and caveolin-1. FASEB
J. 25:3401–3415. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY,
Oh HJ and Kim HY: The catabolic pathway mediated by toll-like
receptors in human osteoarthritic chondrocytes. Arthritis Rheum.
54:2152–2163. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schiphof D, van den Driest JJ and Runhaar
J: Osteoarthritis year in review 2017: Rehabilitation and outcomes.
Osteoarthritis Cartilage. 26:326–340. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Q, Zhang Z, Guo S, Tang G, Lu W and Qi
X: LncRNA ANCR is positively correlated with transforming growth
factor-β1 in patients with osteoarthritis. J Cell Biochem.
120:14226–14232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Y, Wang F, Chen G, He R and Yang L:
LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3
axis. Cell Biosci. 9(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Cao L, Wang Q, Huang J and Xu S:
LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging
miR-27a-3p in osteoarthritis. Artif Cells Nanomed Biotechnol.
47:1241–1247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Herrero-Beaumont G, Perez-Baos S,
Sanchez-Pernaute O, Roman-Blas JA, Lamuedra A and Largo R:
Targeting chronic innate inflammatory pathways, the main road to
prevention of osteoarthritis progression. Biochem Pharmacol.
165:24–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gómez R, Villalvilla A, Largo R, Gualillo
O and Herrero-Beaumont G: TLR4 signalling in osteoarthritis-finding
targets for candidate DMOADs. Nat Rev Rheumatol. 11:159–170.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu L, Gu H, Liu H, Jiao Y, Li K, Zhao Y,
An L and Yang J: Protective effect of resveratrol against
IL-1β-induced inflammatory response on human osteoarthritic
chondrocytes partly via the TLR4/MyD88/NF-κB signaling pathway: an
‘in vitro study’. Int J Mol Sci. 15:6925–6940. 2014.PubMed/NCBI View Article : Google Scholar
|