Introduction
Chronic myeloid leukemia (CML) is a clonal malignant
tumor resulting from hematopoietic disorders, with an incidence of
1-2/10,000 adults per year (1).
Currently, the most effective treatment for CML is bone marrow
transplantation. However, high risk of mortality and recurrence
rates and the difficulty in obtaining a matched donor limit its
application (2). Although many
patients with CML have a response to hematopoietic stem cell
implantation, refractory disease is common, and relapse represents
the major cause of treatment failure (3). Therefore, it is urgent to understand
the pathogenesis of CML and discover novel effective treatments for
CML.
In most cases, CML arises owing to the aberrant
formation of a chimeric gene for a constitutively active tyrosine
kinase. Thus, tyrosine kinase inhibitors (TKIs) are approved for
the first-line treatment of chronic phase CML (4). However, second-generation TKIs have
cardiovascular risks that are greater than with imatinib treatment
(5). To the best of our knowledge,
traditional Chinese medicines or natural products exhibit
advantages in tumor prevention and treatment (6,7).
Several lines of evidence suggest that salidroside, a major active
component of Rhodiola rosea which has been widely used as a
tonic herb exhibiting antitumor properties in various cancers, may
be a promising novel drug candidate for cancer therapy. For
instance, salidroside reduced cell proliferation and induced G1
phase cell cycle arrest and apoptosis of renal cell carcinoma cells
(8). In human colorectal cancer
HT29 cells, salidroside enhanced autophagic effects by inducing
apoptosis via the inactivation of PI3K/Akt/mTOR signaling pathway
(9). Salidroside suppressed
migration and invasion and induced apoptosis of poorly
differentiated thyroid cancer cells via the inhibition of
JAK2/STAT3 pathway (10).
Furthermore, it has been shown that salidroside inhibited the
malignant behaviors of gastric cancer cells (11). Kang et al (12) reported that salidroside induced the
angiogenesis, migration and metastasis of breast cancer cells
through the STAT3 signaling pathway. Yu et al (13) reported that salidroside was a potent
inducer of apoptosis in human ovarian cancer cells through the p53
signaling pathway. Ren et al (14) reported that salidroside had
anticarcinogenic activities in human lung cancer cells by
suppressing proliferation, migration and invasion through AKT and
MEK/ERK signaling. However, the potential antitumor function of
salidroside in CML has not been extensively investigated.
The present study aimed to elucidate whether
salidroside could be used as a therapeutic agent to modulate the
growth of CML cell lines and to further evaluate the antitumor
potential of salidroside against CML. Considering the anticancer
mechanisms of Chinese medicinal herbs targeting microRNAs (miRNAs),
we herein examined the potential mechanisms underlying
salidroside's antitumor properties by focusing on miRNAs (15,16).
miR-140-5p is one of the most studied miRNAs in the field of human
cancer (17,18). In regard of CML, miR-140-5p was
observed to be downregulated in CML patients and CML cell lines,
and the overexpression of miR-140-5p promoted CML cell apoptosis
(19). Based on these previous
observations, miR-140-5p was selected for further investigation.
The findings of the present study may provide evidence for novel
therapeutic strategies for CML.
Materials and methods
Cell lines
CML cell lines, K562 and KCL22, purchased from
American Type Culture Collection, were cultured in RPMI-1640 media
with fetal bovine serum (FBS), penicillin and streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a normoxic
environment (5% CO2 and 95% air). Cells were incubated
with 0, 20, 40, 60, 80 and 100 µM salidroside (Sigma-Aldrich; Merck
KGaA; cat. no. 43866; analytical grade; purity ≥98.0%) for 12, 24,
48 and 72 h. When indicated, cells were transfected with miR-140-5p
mimic (5'-GACUACGAUAUCGAGCCAUA-3'; 50 nM), inhibitor
(5'-UGUACACACGAUUGACGUG-3'; 100 nM) or negative controls (NC;
miR-NC, 5'-AGUCUAACGCGAGCGUAUAA-3'; 50 nM; inhibitor-NC,
5'-GGUAUUACAAAGGUCGCACAA-3'; 100 nM), which were obtained from
Shanghai GenePharma Co., Ltd., by using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. After 48 h, the cells were harvested for further
experiments.
Cell viability assay
Cell viability was determined by CCK-8 assay
(Beyotime Institute of Biotechnology). Briefly, CCK-8 solution (10
µl) was added to each well at 12, 24, 48 and 72 h. After incubation
at 37˚C for 4 h, the absorbance was detected at 450 nm.
Cell apoptosis assay
K562 and KCL22 cells were inoculated in a six-well
plate for 24 h. Next, the culture medium was replaced with a medium
containing 10% FBS. After 24 h, the cells were harvested and
stained with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) using a double-staining apoptosis detection
kit (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols. Cell apoptosis was determined by flow
cytometry analysis using a FACSCanto II flow cytometer
(Becton-Dickinson and Company) and calculated using Cell Quest
acquisition software (version 2.9; BD Biosciences).
RNA extraction and reverse
transcription-quantitative PCR analysis
Total RNA was extracted from K562 and KCL22 cells by
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and was reverse transcribed to cDNA by using MiRcute miRNA
first-strand cDNA synthesis kit (Tiangen Biotech Co., Ltd.),
according to the manufacturer's protocol. Relative expression
levels of miR-140-5p were determined using MiRcute miRNA qPCR
detection kit (Tiangen Biotech Co., Ltd.) on an ABI 7500 real-time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions used for qPCR were as follows: Initial
denaturation at 95˚C for 3 min; followed by 40 cycles of 12 sec at
95˚C and 40 sec at 62˚C; the fluorescence signal was collected
after 40 cycles. Relative fold changes in mRNA expression were
calculated using the formula 2-ΔΔCq method (20). U6 was used as an internal control.
The primer sequences were as follows: miR-140-5p, forward,
5'-TGCGGCAGTGGTTTTACCCTATG-3' and reverse,
5'-CCAGTGCAGGGTCCGAGGT-3'; U6, forward, 5'-CTCGCTTCGGCAGCACATA-3'
and reverse, 5'-AACGATTCACGAATTTGCGT-3'.
Western blotting
RIPA lysis buffer (Beyotime Institute of
Biotechnology) was used to extract the protein from cultured cells.
Total protein concentration was determined using a bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Equal amounts (40
µg/lane) of protein were loaded onto a 12% SDS-PAGE gel and then
electroblotted onto polyvinylidene fluoride membranes. Following
blocking with 5% skimmed milk for 2 h at room temperature, these
membranes were incubated with primary antibodies (1:1,000 dilution)
overnight at 4˚C. The primary antibodies were (Cell Signaling
Technology, Inc.): Cyclin D1 (cat. no. 55506), p21 (cat. no. 2947),
Bcl-2 (cat. no. 3498), Bax (cat. no. 5023), pro-caspase-3 (cat. no.
14220), cleaved caspase-3 (cat. no. 9579) and β-actin (cat. no.
4970). The next day, membranes were incubated at room temperature
for 2 h with FITC-labeled IgG secondary antibodies (cat. no. 7074;
1:2,000 dilution; Cell Signaling Technology, Inc.). The bands were
visualized using Novex ECL HRP chemiluminescent substrate reagent
kit (Invitrogen; Thermo Fisher Scientific, Inc.) and the protein
expression levels were quantified using ImageJ software (version
1.6.0; National Institutes of Health).
Luciferase activity assay
Binding sites for miR-140-5p on the 3'untranslated
region (UTR) of wnt5a were identified using the StarBase database
(http://starbase.sysu.edu.cn/) (21) and were confirmed by performing a
dual luciferase reporter assay. The 3'-UTR region of wnt5a
encompassing the putative miR-140-5p binding site was amplified
using PCR and was subsequently sub-cloned into the psiCHECK-2 dual
luciferase vector (Promega Corporation). K562 and KCL22 cells were
co-transfected with wnt5a recombinant plasmids [wild-type
(wnt5a-WT) or mutant (wnt5a-Mut)] and miR-140-5p mimic or miR-NC
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.). After 48 h transfection, luciferase activity was determined
using the dual luciferase assay kit after adding firefly or
Renilla luciferase reagents (Promega Corporation), according
to the manufacturer's instructions.
Statistical analysis
Data were expressed as mean ± standard deviation.
Comparisons were assessed using SPSS 19.0 (IBM Corp.). Differences
between two groups were analyzed by Student's t-test. Comparisons
between multiple groups were performed by one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Salidroside inhibits proliferation and
downregulates cell cycle-related protein expression in CML
cells
The effects of salidroside on the proliferation and
cell cycle-related protein expression of CML cells were first
determined. In the present study, human CML cell lines K562 and
KCL22 were treated with different concentrations (0, 20, 40, 60, 80
and 100 µM) of salidroside for 12, 24, 48 and 72 h. Then, changes
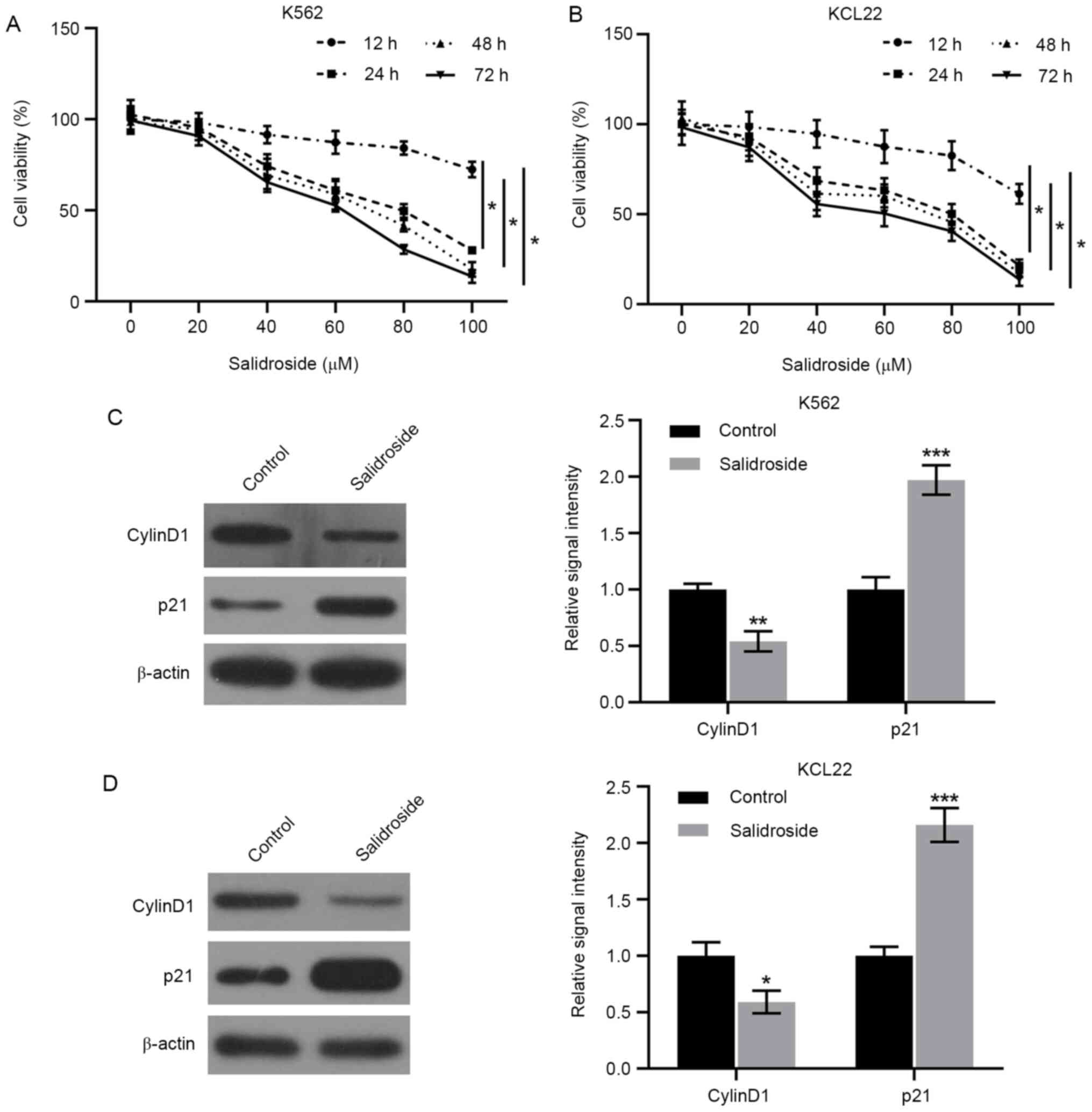
in cell viability were monitored by CCK-8 assay. The results
revealed that, compared with the untreated cells, salidroside
decreased the numbers of viable K562 (Fig. 1A) and KCL22 (Fig. 1B) cells in a dose and time-dependent
manner. Since the IC50 of viability was presented at the
concentration of 80 µM following 24 h treatment, the 80 µM dose and
the 24 h timepoint were selected for further experimental
treatments with salidroside in the present study. Next, changes in
expression levels of cell cycle regulatory proteins were assessed.
Western blotting revealed that salidroside treatment resulted in
the upregulation of p21 and the downregulation of Cyclin D1
expression levels in K562 and KCL22 cells (Fig. 1C and D). These data suggested that salidroside
suppressed the proliferation of human CML cells and regulated cell
cycle-related proteins.
Salidroside promotes the apoptosis of
CML cells
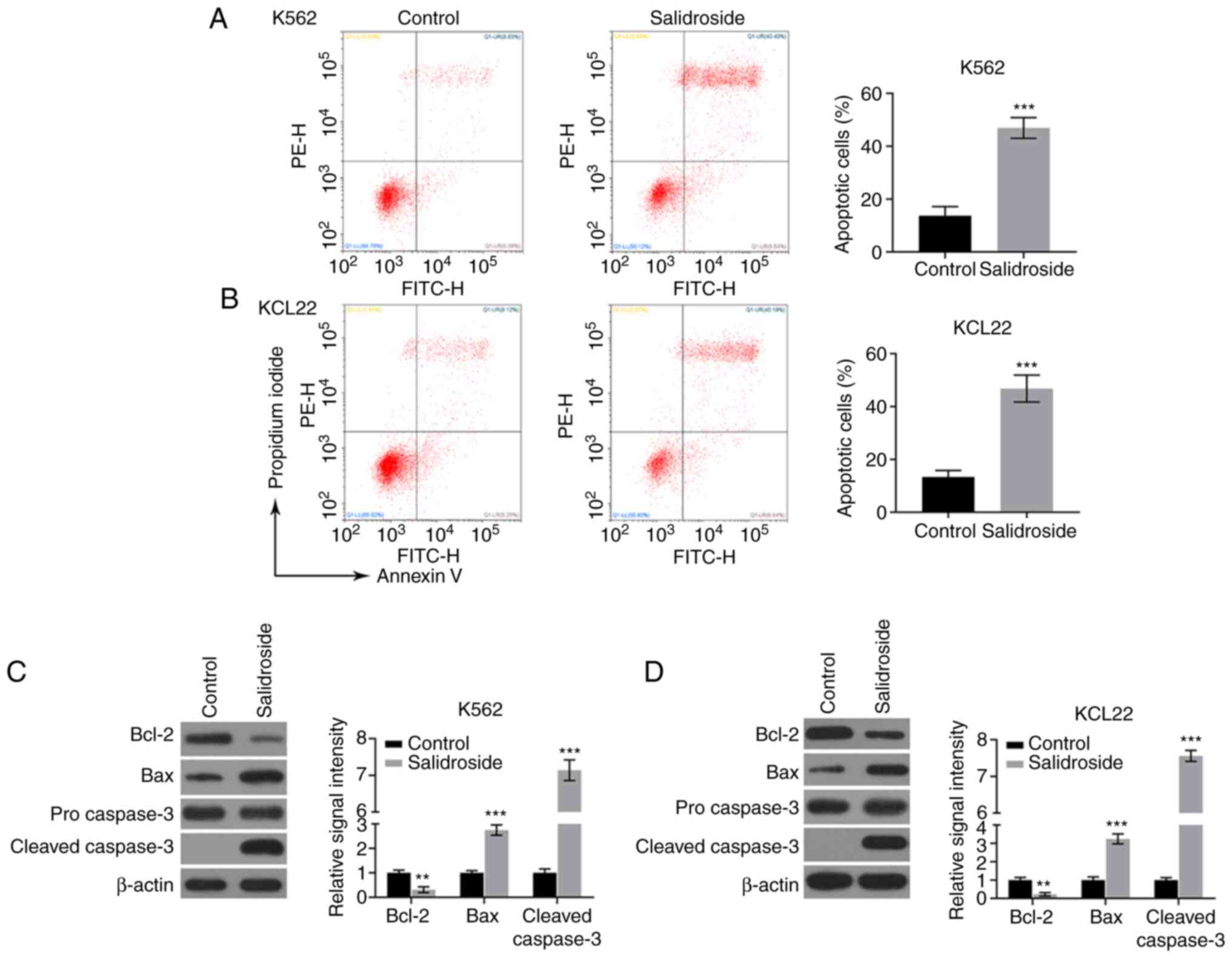
Furthermore, the effects of salidroside on CML cell
apoptosis were assessed. The results showed that after treatment
with 80 µM salidroside for 24 h, K562 and KCL22 cell apoptosis was
induced, as demonstrated by flow cytometry analysis with Annexin
V-FITC/PI double staining (Fig. 2A
and B). In addition, the
proapoptotic protein Bax and apoptosis marker protein cleaved
caspase-3 were significantly upregulated, while the antiapoptotic
protein Bcl-2 expression was decreased, following treatment of K562
and KCL22 cells with salidroside, as evidenced by western blotting
(Fig. 2C and D). These data indicated that salidroside
had proapoptotic effects on cultured human CML cells.
Salidroside affects CML cell
proliferation, apoptosis and cell cycle-related protein expression
by upregulating miR-140-5p
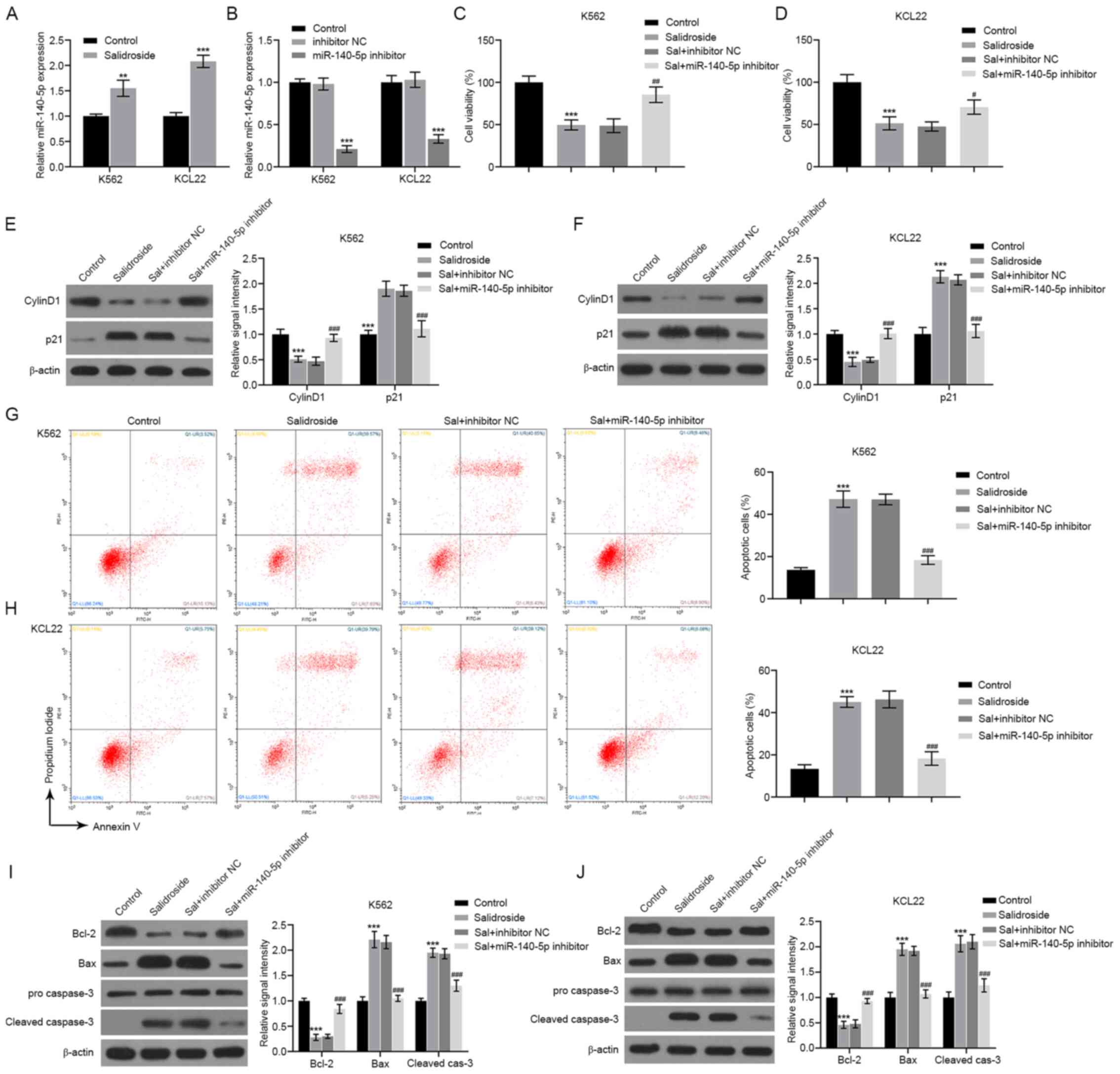
The results of qPCR analysis demonstrated that, in
both CML cell lines, treatment with salidroside resulted in the
upregulation of miR-140-5p expression (Fig. 3A). Furthermore, silencing of
miR-140-5p expression by using a miR-140-5p-specific inhibitor
(Fig. 3B) reversed the effects of
salidroside on cell proliferation (Fig.
3C and D), on the Cyclin D1 and
p21 protein expression (Fig. 3E and
F), on cell apoptosis (Fig. 3G and H), and on the the protein levels of Bcl-2,
Bax and cleaved caspase-3 (Fig. 3I
and J). Additionally, when
miR-140-5p was overexpressed by transfection with a miR-140-5p
mimic (Fig. S1A), the cell
viability was significantly inhibited (Fig. S1B), while cell apoptosis was
significantly increased (Fig. S1C
and D) for both K562 and KCL22
cell lines. The present results indicated that salidroside might
influence CML cells through the regulation of miR-140-5p.
Salidroside regulates the
miR-140-5p/wnt5a/β-catenin axis in CML cells
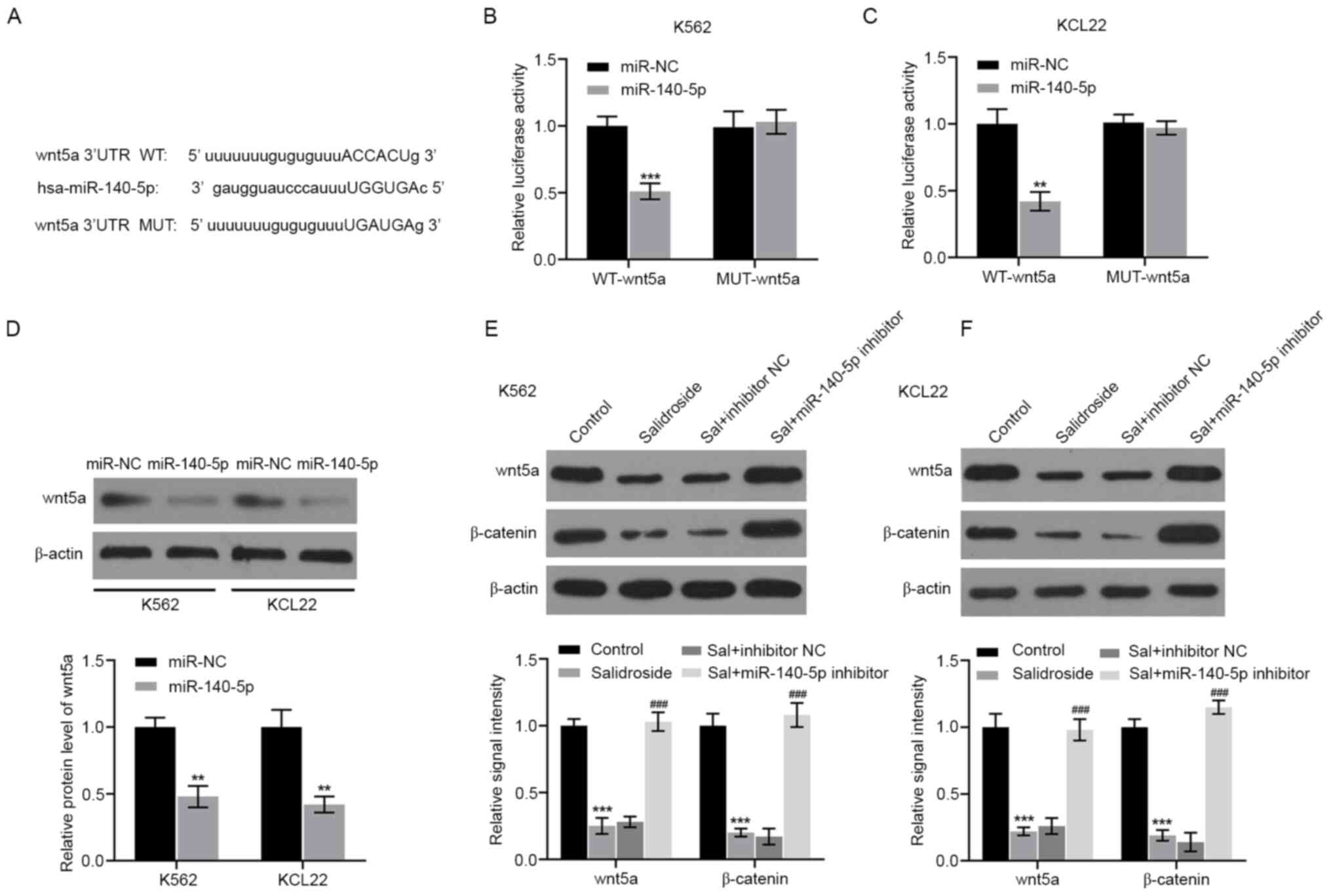
Finally, the potential involvement of miR-140-5p in
salidroside-modulated signaling pathways was investigated. The
online bioinformatic database StarBase was used to identify
potential target genes of miR-140-5p. According to the analysis,
wnt5a was predicted to be a potential target gene of miR-140-5p.
The predicted binding sites between miR-140-5p and wnt5a are
presented in Fig. 4A. In order to
investigate whether miR-140-5p regulated wnt5a expression by
directly binding to its 3'UTR, a luciferase reporter assay was
performed. The results showed that miR-140-5p overexpression
significantly decreased the luciferase activity of wnt5a-WT,
whereas K562 and KCL22 cells co-transfected with Wnt5a-Mut and
miR-140-5p mimic showed no obvious changes in their luciferase
activity (Fig. 4B and C). In addition, ectopic expression of
miR-140-5p in K562 and KCL22 cells resulted in a decrease of Wnt5a
protein expression levels (Fig.
4D). These findings indicated that wnt5a was a direct target of
miR-140-5p in CML cells. The present study then explored whether
miR-140-5p could mediate the effects of salidroside on the
wnt5a/β-catenin pathway. As shown in Fig. 4E and F, the protein expression levels of Wnt5a
and β-catenin were significantly decreased by salidroside.
Conversely, miR-140-5p inhibition reversed this effect.
Discussion
The present study provided the first demonstration
of the antitumor mechanism of salidroside in CML. The current
findings revealed that salidroside accelerated CML cell apoptosis
and inhibited proliferation through downregulation of miR-140-5p
and the wnt5a/β-catenin signaling pathway.
A previous study has shown that salidroside
inhibited the growth of K562 cells in a dose and time-dependent
manner, and induced apoptosis (22). Similarly, the present results
demonstrated that salidroside significantly reduced the cell
viability of two CML cell lines (K562 and KCL22) in a
concentration-dependent manner. In addition, salidroside at 80 µM
significantly reduced CML cell proliferation, and induced
apoptosis. In the cell cycle progression, Cyclin D1, a regulatory
subunit of cyclin-dependent kinase (CDK)4 and CDK6, is required for
G1/S transition of the cell cycle to entry into the S-phase
(23). p21 is a potent CDK
inhibitor by abrogating all cyclin-CDK complexes and thus functions
as a regulator of cell cycle progression at G1 and S phases
(24). Qie et al (23) and Ren et al (14) found that salidroside could cause
G1-phase arrest, a decrease of cyclin D1 expression and an increase
of p21 expression in breast cancer cells and lung cancer cells. The
current study revealed that salidroside treatment resulted in a
decrease in the expression of Cyclin D1, but an increase in the
expression of p21, in K562 and KCL22 cells.
Extensive research on miRNAs has confirmed that they
serve important roles in the occurrence and development of CML by
acting as oncogenes or tumor suppressors (25). Among them, the antitumor roles of
miR-140-5p have been described in multiple types of human cancer by
regulating tumor cell proliferation, apoptosis and cell cycle
progression (26). A previous study
has demonstrated that miR-140-5p was downregulated in CML and its
overexpression was associated with the proliferation and apoptosis
of CML cells by targeting SIX homeobox 1(19). Based on this previous literature,
the present study speculated that targeting miR-140-5p might be a
promising strategy for CML treatment. The present study further
revealed that the addition of salidroside upregulated the
expression of miR-140-5p in CML cells, implying that miR-140-5p
might be implicated in the antitumor effects of salidroside.
Accumulating studies have highlighted the
involvement of several signaling pathways in the initiation and
development of CML, including NF-κB (27), JAK/STAT (28), and PI3K/AKT/mTOR (29). In recent years, several reports have
proposed that salidroside displays its antitumor activities via the
modulation of p53(13),
JAK2/STAT3(30) and Wnt/β-catenin
(31) signaling pathways. Cha et
al (32) uncovered that
miR-140-5p suppressed gastric cancer cell proliferation and
invasion by regulating WNT1 expression. Han et al (33) showed that miR-140-5p promoted the
cerebral protective effects of dexmedetomidine against
hypoxic-ischemic brain damage in neonatal rats by targeting Wnt1
through the negative regulation of the Wnt/β-catenin signaling
pathway. Zhao et al (34)
reported that miR-140-5p hindered cell proliferation, invasion and
tumorigenesis by targeting SRY-box transcription factor 4 via
inactivation of the Wnt/β-catenin and NF-κB signaling pathways in
malignant melanoma. The present study further investigated whether
Wnt/β-catenin signaling was involved in the antitumor action of
salidroside in CML cells, because Wnt5a was identified as a target
of miR-140-5p. Additionally, the present results demonstrated that
salidroside treatment blocked the activation of the Wnt5a/β-catenin
signaling pathway. Notably, the effects of salidroside on the
Wnt5a/β-catenin signaling pathway were partially reversed by
miR-140-5p inhibition, suggesting that salidroside blocked the
Wnt5a/β-catenin signaling pathway possibly through the upregulation
of miR-140-5p. The present study has nonetheless several
limitations. Firstly, clinical evaluations and in vivo
experiments were not performed at this time. Furthermore, a
detailed cell cycle analysis is needed to further evaluate the
effects of salidroside on K562 and KCL22 cyclin expressions. In
addition, other molecules and signaling pathways may be involved in
the antitumor effects of salidroside and future studies will be
required to elucidate these.
In conclusion, the present findings confirmed the
antitumor properties of salidroside in CML via the inhibition of
Wnt5a/β-catenin signaling pathway by upregulating miR-140-5p
expression. Further studies are needed to confirm the potential of
salidroside as a therapeutic agent in CML.
Supplementary Material
Overexpression of miR-140-5p affects
cell viability, apoptosis and apoptosis-related protein expression
in CML cells. K562 and KCL22 cells were transfected with miR-140-5p
mimic or mimic-NC. (A) The transfection efficiency was confirmed by
reverse transcription-quantitative PCR. (B) CCK-8 assay was
performed to assess cell viability. (C) Apoptosis was measured by
flow cytometry. (D) The protein expression levels of Bcl-2, Bax,
pro-caspase-3 and cleaved caspase-3 were determined by western blot
analysis. Resultsfor Bcl-2 and Bax were plotted as fold-ratios
relative to β-actin. Results for cleaved caspase-3 were plotted as
fold-ratios relative topro-caspase-3. ***P<0.001 vs.
mimic-NC. CML, chronic myeloid leukemia; NC, negative control; OD,
optical density.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by a 2019 Guiding project of
Hengyang Science and Technology Bureau (no grant number available),
a 2019 Hunan Provincial Natural Science Youth Fund (grant no.
2019JJ50536) and a 2018 National Natural Science Youth Fund (grant
no. 81803473).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL designed the study. CL and DC performed the
experiments, analyzed the data and prepared the manuscript. CL
reviewed the manuscript. All authors read and approved the final
manuscript. CL and DC confirm the authenticity of all the raw data.
All authors agree to be accountable for all aspects of the research
in ensuring that the accuracy or integrity of any part of the work
are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Flis S and Chojnacki T: Chronic
myelogenous leukemia, a still unsolved problem: Pitfalls and new
therapeutic possibilities. Drug Des Devel Ther. 13:825–843.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gupta A and Khattry N: Current status of
hematopoietic stem cell transplant in chronic myeloid leukemia.
Indian J Med Paediatr Oncol. 35:207–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hino Y, Doki N, Yamamoto K, Senoo Y,
Sasajima S, Sakaguchi M, Hattori K, Kaito S, Kurosawa S, Harada K,
et al: Chronic myeloid leukemia relapsing ten years after allogenic
bone marrow transplantation. Rinsho Ketsueki. 57:608–612.
2016.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
4
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2016 update on diagnosis, therapy, and
monitoring. Am J Hematol. 91:252–265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ross DM, Arthur C, Burbury K, Ko BS, Mills
AK, Shortt J and Kostner K: Chronic myeloid leukaemia and tyrosine
kinase inhibitor therapy: Assessment and management of
cardiovascular risk factors. Intern Med J. 48 (Suppl 2):S5–S13.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li H, Liu L, Liu C, Zhuang J, Zhou C, Yang
J, Gao C, Liu G, Lv Q and Sun C: Deciphering key pharmacological
pathways of qingdai acting on chronic myeloid leukemia using a
network pharmacology-based strategy. Med Sci Monit. 24:5668–5688.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Y, Xiao Y, Dong Q, Ouyang W and Qin
Q: Neferine in the lotus plumule potentiates the antitumor effect
of imatinib in primary chronic myeloid leukemia cells in vitro. J
Food Sci. 84:904–910. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lv C, Huang Y, Liu ZX, Yu D and Bai ZM:
Salidroside reduces renal cell carcinoma proliferation by
inhibiting JAK2/STAT3 signaling. Cancer Biomark. 17:41–47.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shang H, Wang S, Yao J, Guo C, Dong J and
Liao L: Salidroside inhibits migration and invasion of poorly
differentiated thyroid cancer cells. Thorac Cancer. 10:1469–1478.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qi Z, Tang T, Sheng L, Ma Y, Liu Y, Yan L,
Qi S, Ling L and Zhang Y: Salidroside inhibits the proliferation
and migration of gastric cancer cells via suppression of
Src-associated signaling pathway activation and heat shock protein
70 expression. Mol Med Rep. 18:147–156. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kang DY, Sp N, Kim DH, Joung YH, Lee HG,
Park YM and Yang YM: Salidroside inhibits migration, invasion and
angiogenesis of MDAMB 231 TNBC cells by regulating EGFR/Jak2/STAT3
signaling via MMP2. Int J Oncol. 53:877–885. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu G, Li N, Zhao Y, Wang W and Feng XL:
Salidroside induces apoptosis in human ovarian cancer SKOV3 and
A2780 cells through the p53 signaling pathway. Oncol Lett.
15:6513–6518. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ren M, Xu W and Xu T: Salidroside
represses proliferation, migration and invasion of human lung
cancer cells through AKT and MEK/ERK signal pathway. Artif Cells
Nanomed Biotechnol. 47:1014–1021. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang L, Yu Y, Zhang Q, Li X, Zhang C, Mao
T, Liu S and Tian Z: Anti-gastric cancer effect of Salidroside
through elevating miR-99a expression. Artif Cells Nanomed
Biotechnol. 47:3500–3510. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li H, Huang D and Hang S: Salidroside
inhibits the growth, migration and invasion of Wilms' tumor cells
through down-regulation of miR-891b. Life Sci. 222:60–68.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu Y, Li J, Chen S and Yu Z: The effects
of miR-140-5p on the biological characteristics of ovarian cancer
cells through the Wnt signaling pathway. Adv Clin Exp Med.
29:777–784. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16(139)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nie ZY, Liu XJ, Zhan Y, Liu MH, Zhang XY,
Li ZY, Lu YQ, Luo JM and Yang L: miR-140-5p induces cell apoptosis
and decreases Warburg effect in chronic myeloid leukemia by
targeting SIX1. Biosci Rep. 39(BSR20190150)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin YL, Zhang XM, Chen X, Liu J, Chang YY,
Gao XY, Xue YM, Dong XS, Liu Y, Tian YY, et al: Salidroside induces
apoptosis via ERK1/2 in human leukemia. K562 cells. 9:17588–17595.
2016.
|
|
23
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
El-Deiry WS: p21(WAF1) mediates cell-cycle
inhibition, relevant to cancer suppression and therapy. Cancer Res.
76:5189–5191. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Litwinska Z and Machalinski B: miRNAs in
chronic myeloid leukemia: Small molecules, essential function. Leuk
Lymphoma. 58:1297–1305. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liao Y, Yin X, Deng Y and Peng X:
MiR-140-5p suppresses retinoblastoma cell growth via inhibiting
c-Met/AKT/mTOR pathway. Biosci Rep: Nov 30, 2018 (Epub ahead of
print). doi: 10.1042/BSR20180776.
|
|
27
|
Jia Q, Sun H, Xiao F, Sai Y, Li Q, Zhang
X, Yang S, Wang H, Wang H, Yang Y, et al: miR-17-92 promotes
leukemogenesis in chronic myeloid leukemia via targeting A20 and
activation of NF-κB signaling. Biochem Biophys Res Commun.
487:868–874. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai H, Qin X and Yang C: Dehydrocostus
lactone suppresses proliferation of human chronic myeloid leukemia
cells through Bcr/Abl-JAK/STAT signaling pathways. J Cell Biochem.
118:3381–3390. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li L, Qi Y, Ma X, Xiong G, Wang L and Bao
C: TRIM22 knockdown suppresses chronic myeloid leukemia via
inhibiting PI3K/Akt/mTOR signaling pathway. Cell Biol Int.
42:1192–1199. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang L, Huang Z, Lin W, Wang L, Zhu X,
Chen X, Yang S and Lv C: Salidroside suppresses the growth and
invasion of human osteosarcoma cell lines MG63 and U2OS in vitro by
inhibiting the JAK2/STAT3 signaling pathway. Int J Oncol.
54:1969–1980. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao J, Du X, Wang M, Yang P and Zhang J:
Salidroside mitigates hydrogen peroxide-induced injury by
enhancement of microRNA-27a in human trabecular meshwork cells.
Artif Cells Nanomed Biotechnol. 47:1758–1765. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cha Y, He Y, Ouyang K, Xiong H, Li J and
Yuan X: MicroRNA-140-5p suppresses cell proliferation and invasion
in gastric cancer by targeting WNT1 in the WNT/β-catenin signaling
pathway. Oncol Lett. 16:6369–6376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han XR, Wen X, Wang YJ, Wang S, Shen M,
Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, et al: MicroRNA-140-5p
elevates cerebral protection of dexmedetomidine against
hypoxic-ischaemic brain damage via the Wnt/β-catenin signalling
pathway. J Cell Mol Med. 22:3167–3182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao G, Yin Y and Zhao B: miR-140-5p is
negatively correlated with proliferation, invasion, and
tumorigenesis in malignant melanoma by targeting SOX4 via the
Wnt/β-catenin and NF-κB cascades. J Cell Physiol. 235:2161–2170.
2020.PubMed/NCBI View Article : Google Scholar
|