Introduction
Alzheimer's disease (AD) is defined as progressive
memory loss and cognitive dysfunction and its pathological features
are the accumulation of amyloid-β (Aβ) to form senile plaques (SPs)
and intracellular hyperphosphorylated Tau protein to form
neurofibrillary tangles (NFTs), leading to neuronal death (1,2). With
global aging, the incidence of AD has been increasing year by year;
the number of affected patients has doubled in the past two decades
and has been estimated to reach 131.5 million by 2050, bringing a
serious economic burden to families, as well as social disruption
(3). To date, the intricate
etiology of the development of AD has remained to be fully
elucidated, but there is evidence that both the
microtubule-associated protein tau and amyloid-β (Aβ) exert complex
and synergistic effects contributing to the hypometabolism and
symptomatology of AD (4,5). Although several therapeutic strategies
have achieved results based on these hypotheses, no satisfactory
treatment is available to date. Given the numerous synchronic
pathways involved in the development of AD, it is thought that
therapies acting on multiple targets may be beneficial for the
prevention and treatment of AD and will be superior to current
single-target therapies against AD (6).
Traditional Chinese Medicine (TCM), which is well
known for working in synergy on multiple targets and pathways, may
provide novel potentially effective treatments for diseases such as
AD (7,8). It is recorded in the ‘Inner Canon of
the Yellow Emperor’, the earliest medical classic in China, that in
TCM, the occurrence of AD is thought to be closely associated with
kidney deficiency (9,10). Certain clinical studies have
demonstrated that TCM syndrome in AD mainly manifests as kidney
deficiency (11). Erjing pill,
which is composed of Polygonatum sibiricum and Lycium
chinense, was first recorded in the ‘Sheng Ji Zong Lu’
(12), a medical book that recorded
numerous famous prescriptions and was compiled in the Song Dynasty.
The Erjing pill was also recorded in numerous classic works of TCM,
such as ‘Pu Ji Fang’ (13), ‘Tai Yi
Yuan Qi Xiao Liang Fang Da Quan’ (14) and ‘Zun Sheng Ba Jian’ (13). In the clinic, Erjing pill is mainly
used to protect the kidneys, delay aging and enhance immunity and
memory (13). It has been used for
the prevention and treatment of AD for numerous years. Its
ingredients Polygonatum sibiricum and Lycium chinense
are both included in the ‘List of Items that Are Both Food and
Drugs’ issued by the Ministry of Health of China in 2002 [Ministry
of Health Legal Supervision Department issued (2002) no. 51]
(15,16). Polygonatum sibiricum may be
used to treat the reproductive system, regulate immunity and
enhance brain function (17,18).
Lycium chinense is used as an adjuvant therapy for various
clinical diseases. It is frequently used to protect the eyes and
liver, treat impotence and improve memory (19,20).
Animal experiments have reported that Erjing pill may improve the
learning and memory ability of rats induced by long-term irritation
stress (21). It also significantly
improved the learning and memory ability of mice with
D-galactose-induced aging (22) and
aluminum trichloride-induced dementia (23). Erjing pill may improve the learning
and memory abilities of rats induced by Aβ1-40 upon
injection into the hippocampus, reduce the deposition of Aβ and
inhibit the overphosphorylation of tau protein (24). However, the clinical application of
Erjing pill for AD has been limited and remains to be further
explored due to the unknown mechanism of action.
Given the characteristics of multicomponent and
multitargeting of TCMs (25), it is
usually a time- and cost-consuming process to pinpoint its active
components and then test the experimental effects of these active
components individually, as the results are easily affected by
experimental animals and external factors (26). Exploring the mechanism of action of
a single component commonly commences using a simplified single
target, which sets substantial limitations on studies. Network
pharmacology is an emerging method and strategy for drug design
based on systems biology and multidirectional pharmacology
(27). By constructing a complex
network among drugs, targets and signal pathways, including active
molecular screening, target prediction, molecular docking and
network analysis, network pharmacology combines a drug-target
network with a biological system network to provide novel
approaches and strategies for the development of new drugs
(28). The present study sought to
unravel the effectiveness of Erjing pill through networks
pharmacology to explore the major active components and the
multi-target groups of Erjing pill in the treatment of AD. The
present study also provided a systematic approach for the
exploration of the molecular mechanisms of Erjing pill to provide a
foundation for the application of Erjing pill as a potential TCM
treatment for AD.
Materials and methods
Databases, tools and software
The following databases, tools and software were
used in the present study: The TCM (http://tcm.cmu.edu.tw/) and TCM Systems Pharmacology
(TCMSP; http://lsp.nwu.edu.cn/index.php) are the networks
pharmacology platforms and databases of TCM (29). UniProt (https://www.UniProt.org/) is a protein database
(30). PubChem (https://pubchem.ncbi.nlm.nih.gov/) is a
cheminformatics database (31).
Gene Ontology (GO) is a bioinformatics platform for analyzing the
biological functions of genes (32). GeneCards (https://www.genecards.org/) is an integrative website
used for predicting human genes (33). The Therapeutic Target Database (TTD;
https://db.idrblab.org/ttd/) (34), and the Online Mendelian Inheritance
in Man (OMIM; http://www.omim.org/) (35) and DiGSeE (https://digsee.com/en/) are databases of known and
exploited therapeutic proteins, as well as targeted diseases and
pathways (36). Discovery Studio
4.5 (DS) is a software for structure construction (37). SwissTargetPrediction (https://www.swisstargetprediction.ch/)
is a chemical target prediction platform (38). Metascape (http://metascape.org/) is used for gene function
analysis (39). Cytoscape 3.2.1 is
a platform-independent open-source software for visualizing
networks and combining them with various types of attribute data
(40).
Screening of active ingredients in
Erjing pill
The active ingredients in Erjing pill were screened
from the literature (search terms: ‘Erjing pill’, ‘Polygonatum
sibiricum’ and ‘Lycium chinense’), TCMSP, Chinese chemistry
database, TCM-Database@Taiwan (41) and experimental separated
systematically in the present laboratory, and the collected
ingredients were screened and analyzed for drug-likeness using DS
software. Pharmacokinetic properties, including absorption,
distribution, metabolism, excretion and toxicity (ADMET), are the
major factors influencing biological activity. In the present
study, five ADMET-related parameters, including aqueous solubility,
blood-brain-barrier (BBB) penetration, human intestinal absorption
(HIA), plasma protein binding (PPB) and hepatotoxicity, were
employed to identify the potential bioactive components of Erjing
pill. The screening criteria for active components in Erjing pill
were as follows: Water-solubility parameter, 3-4; BBB penetration,
1-2; HIA, 0; PPB, FALSE; and toxicity parameter, FALSE.
Prediction of compound targets and
AD-associated targets
To predict the potential targets of screened
compounds in Erjing pill, two public databases, PubChem and
SwissTargetPrediction, were used for identification of the
compound-related targets depending on the canonical Simplified
Molecular Input Line Entry System formula by limiting the species
to ‘Homo sapiens’.
The TTD, OMIM and DiGSeE databases were employed to
identify AD-associated targets. The standard gene names and UniProt
ID of AD-related targets were obtained from the UniProtKB database
as well.
GO and pathway enrichment
analyses
The related targets were inputted into the Metascape
platform, which is an online open source for gene annotation and
analysis, for GO and pathway annotation. The amount of genes was
used as the analysis standard.
Protein-protein interaction network
construction
Cytoscape (version 3.2.1) software, which is an
open-source bioinformatics platform for visualizing molecular
interaction networks, was used to construct a
‘compound-pathway-disease’ network for obtaining the interacting
genes between Erjing pill and AD.
Molecular docking
N-methyl-D-aspartic acid (NMDA) receptor antagonist
memantine, cholinesterase inhibitor donepezil, tacrine,
galanthamine and rivastigmine, are approved by the Food and Drug
Administration (USA) for the treatment of AD. The English names of
the drugs were used as key words, which were searched using the
GeneCards platform. To obtain the highly correlative targets of
drugs and compare them with the potential targets of Erjing pill
for preventing and treating AD, all processes of molecular docking
were performed using DS software. The docking active pocket was
defined by the original ligand molecule. Subsequently, the docking
parameters were set as follows: SDS site sphere radius, 9; top hit,
3; post cluster radius, 0.5; and the other parameters were set to
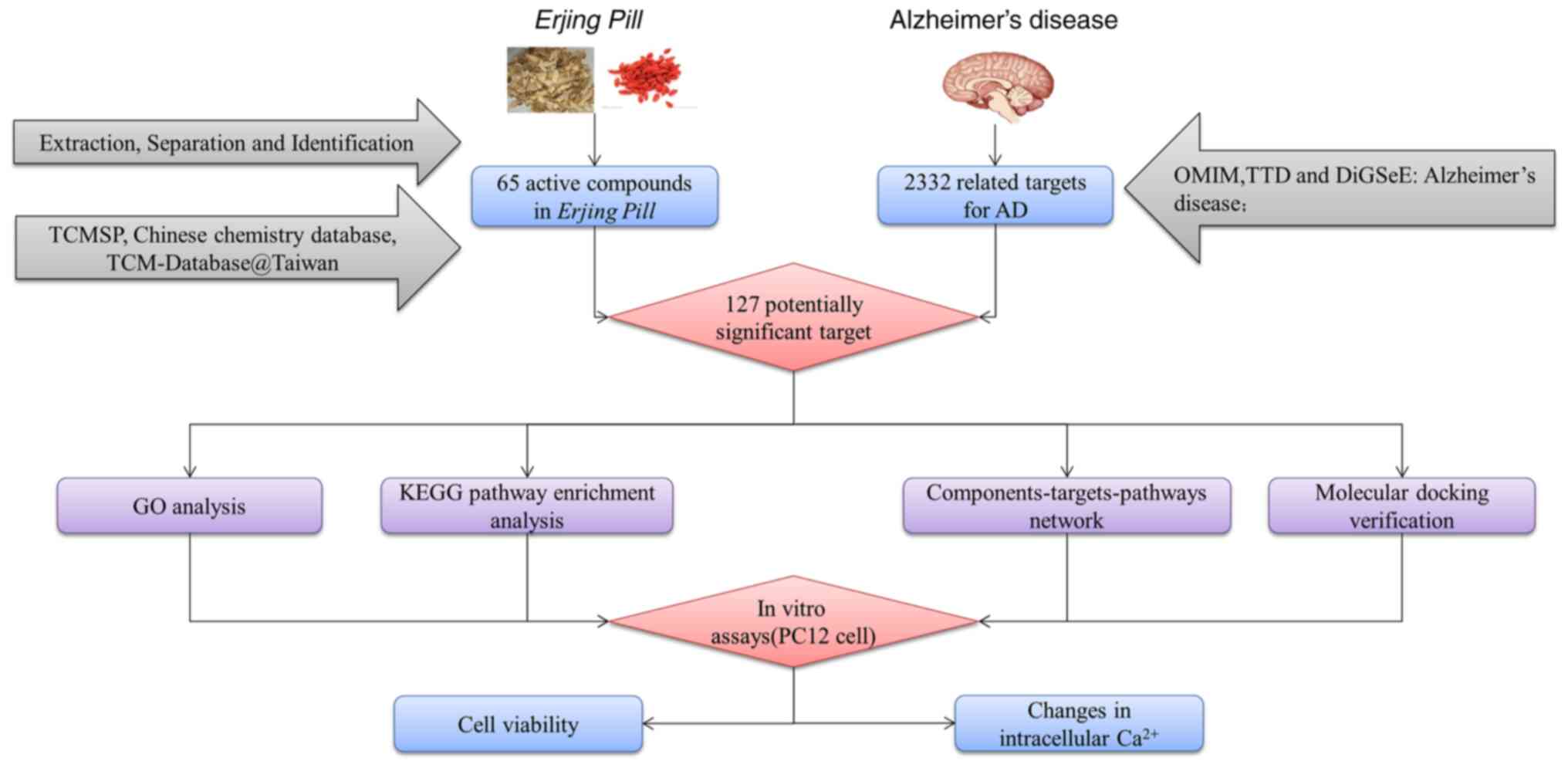
default values. The technology roadmap of the present study is
presented in Fig. 1.
Validation of compounds by in vitro
assays Cell culture
The rat pheochromocytoma cell line PC12 is an
internationally recognized ideal model for in vitro studies
of neurobiology, neurochemistry and nervous system diseases. These
cells exhibit the characteristics of neuroendocrine cells, such as
exhibiting obvious synapse formation. These cells may produce
neuron-associated proteins and may be stably cultured (42).
The PC12 cell line was purchased from the Shanghai
Cell Bank of the Chinese Academy of Sciences. PC12 cells were
cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) with 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 5% (v/v) horse serum (MRC, Jiangsu, China).
Cells were maintained at 37˚C in an atmosphere containing 5%
CO2, and saturated humidity of 95%.
Polygonatine A and polygonatine C
extraction
According to literature (43), the air-dried Rhizomes of P.
sibiricum [30 kg; purchased from Jiangxi Province Zhengyi
Chinese Medicinal Materials Co., Ltd.; Identified as the dried
rhizome of P. sibiricum, a Liliaceae plant, by professor
Xiaomei FU (Jiangxi University of Traditional Chinese Medicine,
China); Stored in the Herbal Medicine Laboratory of Jiangxi
Provincial Institute of Drug Testing and Research] were Processed
in accordance with the Regulations for processing Chinese herbal
medicines in Jiangxi Province of the Polygonati Rhizoma (44) for 24 h. Briefly, the air-dried
Rhizomes of processed P. sibiricum were extracted using 70%
ethanol, two repeats for 120 min both times. The ethanol extract
was evaporated under reduced pressure to yield a brown residue
(2,300 g). The residue was suspended in water and separated using
dichloromethane, ethyl acetate and n-butanol in sequence at room
temperature. The dichloromethane extract (210 g) was fractionated
and subjected to a silica gel column (100-200 mesh; Petroleum
Ether-Ethyl Acetate, 8:1) to produce 6 fractions (Fr.1-Fr.6). Fr.6
was further fractionated and subjected to a silica gel column
(100-200 mesh; petroleum ether-ethyl acetate; 20:1, 10:1, 5:1, 2:1
and 1:1) to produce 22 fractions (Fr.6-a-Fr.6-v), Fr.6-t was
further separated using semi-preparative HPLC (Semipreparative
RP-HPLC column; Agilent Eclipse; XDB-C18 Semi-Prep 5 µm, 9.4x250
mm; Agilent Technologies Inc.), with a flow rate of 3.0 ml/min and
column temperature, 30˚C, using MeOH-H2O (37:63) to give
compound polygonatine C (28.2 mg). Repeated purification of Fr.6-v
was performed using semi-preparative HPLC using MeOH-H2O
(11:89) to give compound Polygonatine A (50.2 mg).
Cell viability
The effects of 4',5-dihydroxyflavone (Yuanye;
purity, ≥95%), polygonatine A and polygonatine C on the PC12 cell
line were determined by MTT assays. First, 100 µl cells
(5x104 cells/ml) were seeded into a 96-well plate coated
with poly-L-lysine (Boster Biotechnology, Inc.). After the cells
attached, treatments with various concentrations of
4',5-dihydroxyflavone, polygonatine A and polygonatine C (5, 10,
20, 40, 80 or 160 µM) were performed for 24 h. In the MTT assay,
cells were incubated with 10 µl MTT (Beijing Solarbio Science &
Technology Co., Ltd.; 5 mg/ml) for 4 h at 37˚C, the supernatant was
discarded and formazan was then dissolved in DMSO. Subsequently,
optical density values were measured at 490 nm using a microplate
reader (ELx800; Biotek). The effect of the three compounds on the
survival rate of PC12 cells was thereby determined. In another
experiment, 100 µl cells (5x104 cells/ml) were seeded
into a 96-well plate coated with poly-L-lysine. After the cells
attached, they were stimulated with 60 µM Aβ25-35
(Sigma-Aldrich; Merck KGaA) and then treated with different
concentrations of 4',5-dihydroxyflavone, polygonatine A or
polygonatine C (10, 20 or 40 µM) for 24 h prior to the MTT
assay.
Changes in intracellular
Ca2+
PC12 cells were seeded in 24-well plates at a
density of 5x104 cells/ml and treated with different
concentrations of 4',5-dihydroxyflavone (20 µM), polygonatine A (40
µM) or polygonatine C (20 µM) for 24 h, followed by washing thrice
with 10 mM HEPES buffer (Sigma-Aldrich; Merck KGaA). Subsequently,
300 µl of Fluo 3-AM working solution (Sigma-Aldrich; Merck KGaA)
was added to each well and samples were incubated for 30 min in the
dark at 37˚C and 5% CO2. The cells were washed thrice
with 10 mM HEPES solution to ensure that any unbound nonspecific
dye on the cell surface was washed away. Subsequently, 300 µl of 10
mM HEPES buffer was added to each well, followed by incubation for
30 min at 37˚C in a cell incubator, with 5% CO2, and
subsequently, new HEPES solution was added. Finally, the
fluorescence intensity was detected with a fluorescence microscope
(excitation wavelength, 488 nm) and the fluorescence intensity of
calcium ions was analyzed using ImageJ v1.8.0 software (National
Institutes of Health).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc.). Significant differences were determined
by one-way ANOVA with Dunnett's post-hoc test. Values are expressed
as the mean ± standard deviation. P<0.05 was considered to
indicate statistical significance.
Results
Identification of the main active
components of Erjing pill
The active components of Erjing pill were retrieved
from TCMSP, TCM-Database@Taiwan and the
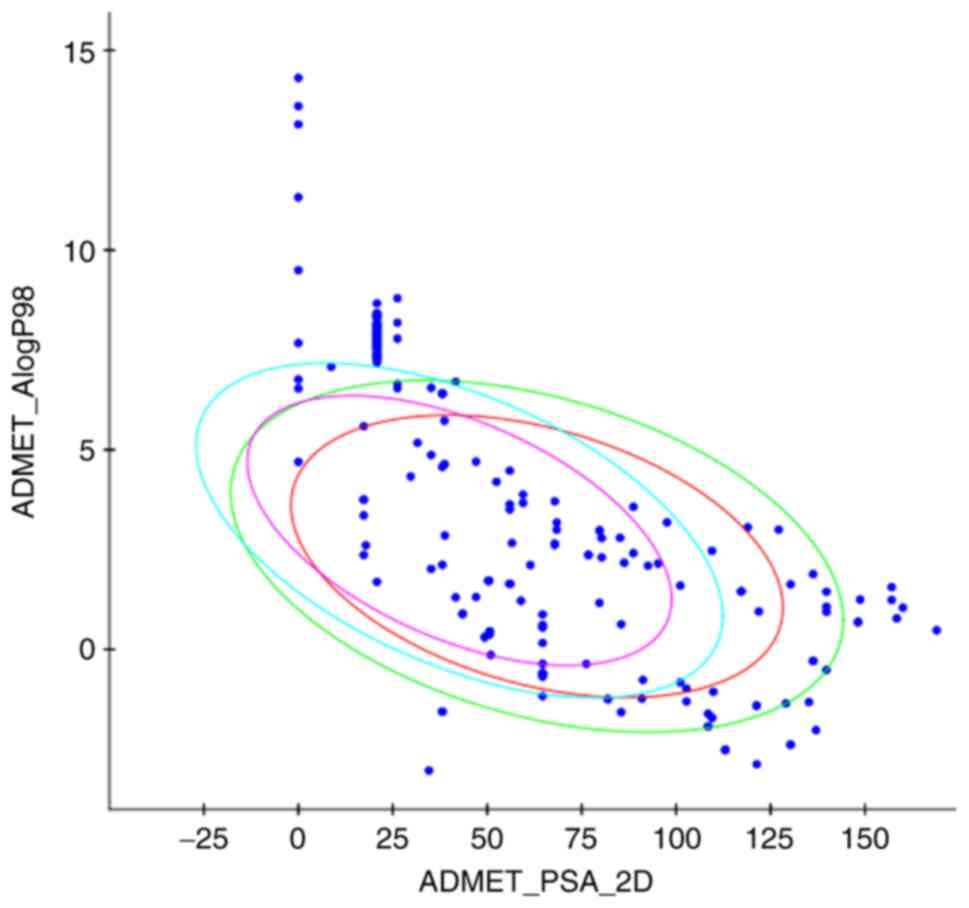
Chinese chemistry database. The absorption, distribution,
metabolism and excretion (ADME)-related properties of the selected
ingredient are presented in Fig. 2.
A total of 65 active components for AD treatment were identified
using DS [Table SI (22,23,45-52)].
Prediction of drug target
By using the Swiss Target Prediction platform, the
potential protein targets of these 65 active components of Erjing
pill were analyzed. In total, 265 potential targets were identified
after eliminating duplicates. In total, 2,332 potential targets of
AD-related genes were obtained using the OMIM, TTD and DiGSeE
diseases and 127 potentially significant targets for the treatment
of AD were annotated, as presented in Table SII.
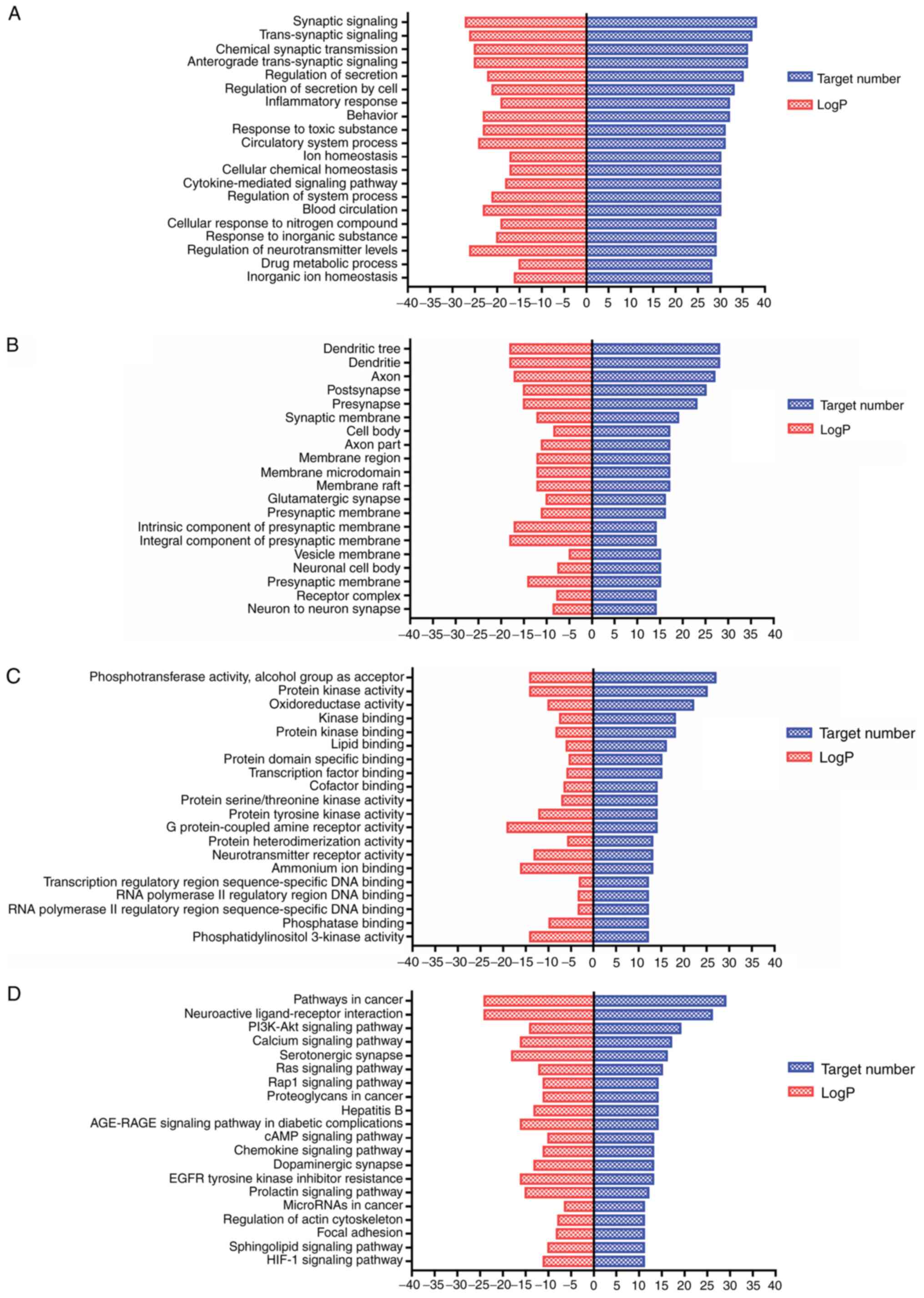
Signaling pathway enrichment analyses
with GO and Kyoto Encyclopedia of Genes and Genomes (KEGG)
To further establish the effect of the active
components of Erjing pill, 127 significant target proteins of AD
were analyzed. GO functional enrichment and determination of KEGG
signaling pathways for both active components and target proteins
were performed using the Metascape database, as presented in
Fig. 3. The results of the GO
analysis demonstrated that in the category biological process,
enrichment was mainly in the synaptic signaling pathway in response
to synaptic signal transmission, transsynaptic signal transmission,
anterograde transsynaptic signal transmission, chemical synaptic
transmission and secretory regulation (Fig. 3A). Furthermore, in the category
cellular component, cellular response to dendrites, axons,
posterior synapses, presynapses and synaptic membranes were
relevant enriched terms (Fig. 3B).
In the category molecular function, obvious enrichment in
phosphotransferase activity, protein kinase activity,
oxidoreductase activity, lipid binding and transcription factor
binding was obtained (Fig. 3C).
KEGG pathway enrichment analysis revealed 145
pathways as targets of Erjing pill for the treatment of AD,
including neuroactive ligand-receptor interactions, the PI3K-Akt
signaling pathway, serotoninergic synapses, the calcium ion
signaling pathway and dopaminergic synapses. The pathways closely
associated with the pathogenesis of AD and those with high
significance are presented in Fig.
3D.
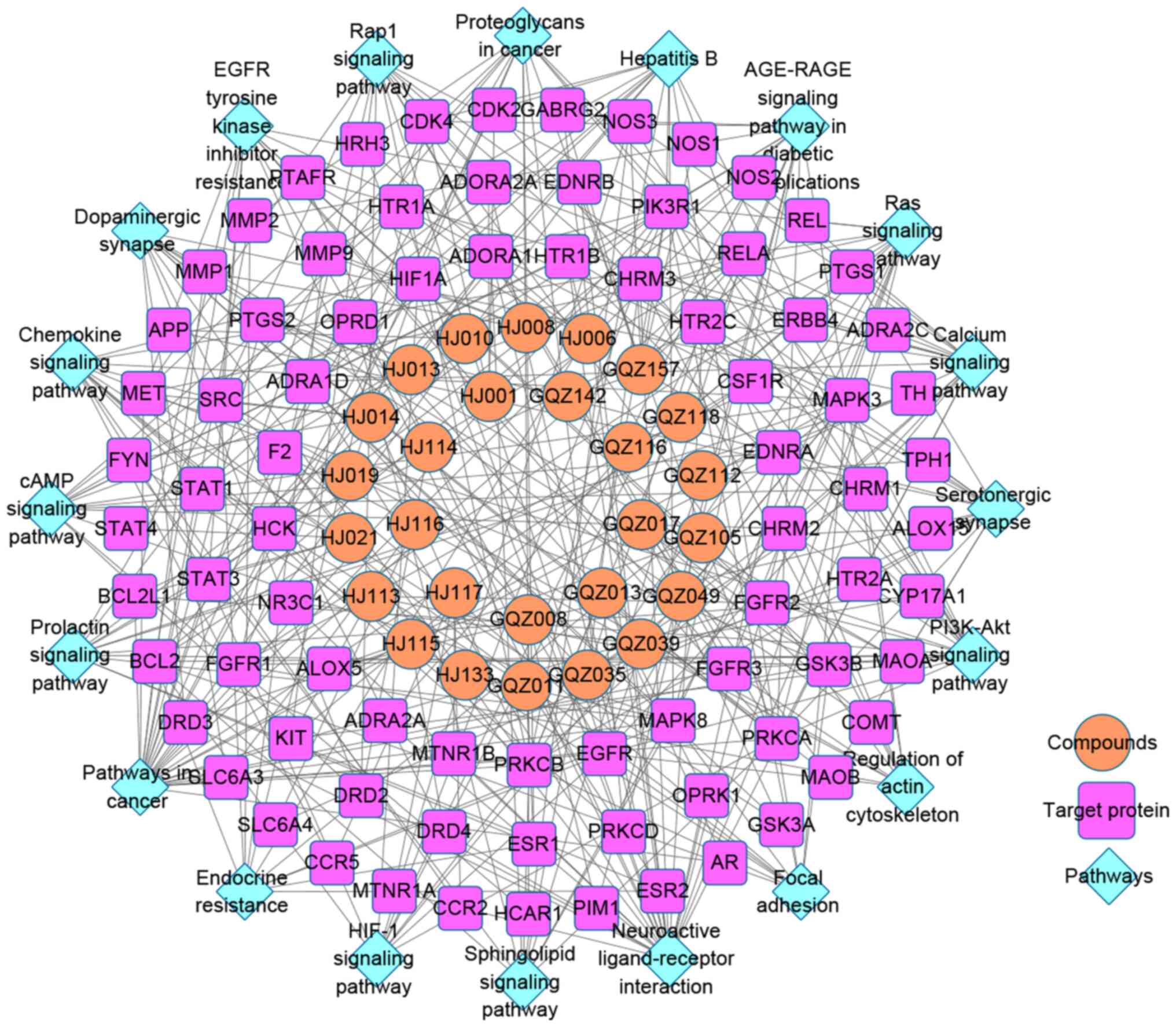
Active components-targets-pathways
network of Erjing pill for the prevention and treatment of AD
The network map of significant components and
potential AD targets of Erjing pill was constructed using the
Cytoscape database (Fig. 4). The
network contained 128 nodes and 378 edges in this interaction
network. Central network evaluation was performed to identify the
main targets. The results further demonstrated that in the
prevention and treatment of AD, 27 nodes are directly related to
the target of Erjing pill, 81 nodes are indirectly related to that
target and 20 nodes are the signaling pathways involved in the
targeting of AD. In the network, the components with the highest
degree of interaction were GQZ157 (zederone, 9), HJ021
(sibiricoside B_qt, 6), GQZ013 [dibutylphthalate (DBP), 6], GQZ035
(atropine, 6), HJ113 (2',7-dihydroxy-3',4'-dimethoxyisoflavone, 5)
and GQZ017 (paeonol, 5), and the network suggested that the effect
of Erjing pill on AD may be the result of multicomponent and
multitargeting effects.
Molecular docking
Using the five control drugs memantine, tacrine,
donepezil, galantamine and rivastigmine as keywords, the top 10
target genes were obtained using the GeneCards platform and are
listed in Table SIII. Upon
comparison with 127 potential targets of Erjing pill for AD, the
results suggested that acetylcholinesterase (ACHE),
butyrylcholinesterase (BCHE), amyloid protein precursor (APP),
5-hydroxytryptamine receptor 2A (HTR2A), muscarinic acetylcholine
receptor M2 (CHRM2) and microtubule-associated protein tau (MAPT)
were shared targets. The Root mean square deviation (RMSD) was
<0.2 nm and the results revealed that the docking method was
reliable (Table I). The 2D
structure of 65 active components of Erjing Pill and 5 control
drugs were inputted into the DS platform using the CDOCKER module
and -CDOCKER ENERGY was obtained. The results highlighted 20
compounds with better anti-AD properties compared with 4 control
drugs (Table SIV).
 | Table ITarget protein function and root mean
square deviation values. |
Table I
Target protein function and root mean
square deviation values.
| Gene symbol | Protein
function | PDB ID | RMSD |
|---|
| ACHE | Terminates signal
transduction at the neuromuscular junction by hydrolysis of the
acetylcholine released into the synaptic cleft. | 4EY5 | 0.19 |
| BCHE | Esterase with broad
substrate specificity. Contributes to the inactivation of the
neurotransmitter acetylcholine. | 4B0O | 1.13 |
| APP | Performs
physiological functions on the surface of neurons relevant to
neurite growth, neuronal adhesion and axonogenesis. | 3IVH | 1.76 |
| HTR2A | G-protein coupled
receptor for 5-hydroxytryptamine (serotonin) | 4IB4 | 0.75 |
| CHRM2 | The muscarinic
acetylcholine receptor mediates various cellular responses. | 5ZKC | 1.11 |
| MAPT | Promotes
microtubule assembly and stability and may be involved in the
establishment and maintenance of neuronal polarity. | 4Y5I | 1.23 |
Validation of compounds in vitro
From the aforementioned results and analysis, three
compounds were selected for the assays. The results of the MTT
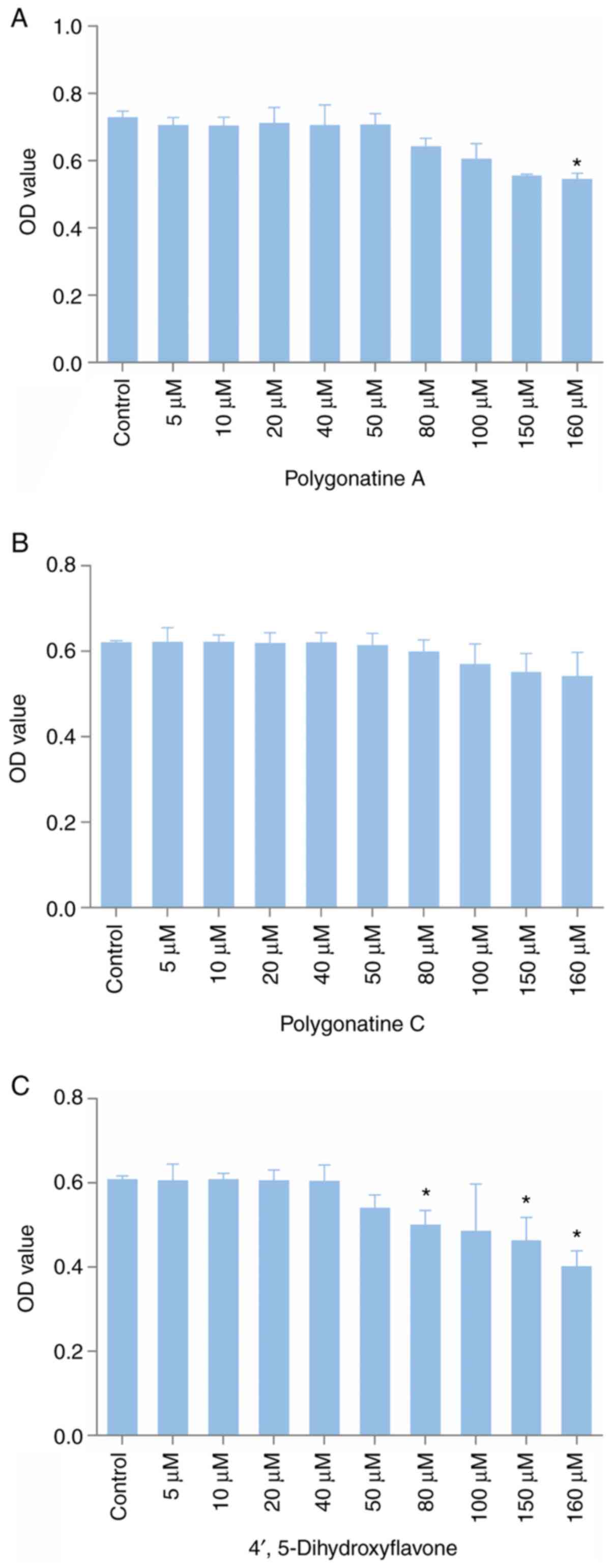
assays are presented in Fig. 5. At
low concentrations, the three compounds had no significant effect
on the survival rate of PC12 cells. However, when the
concentrations of 4',5-dihydroxyflavone, polygonatine A and
polygonatine C were >50, 80 and 100 µM, respectively, the
survival rate of PC12 cells exhibited a decreasing trend. To
administer a gradient of doses, 10, 20 and 40 µM were
selected as the doses of the three compounds.
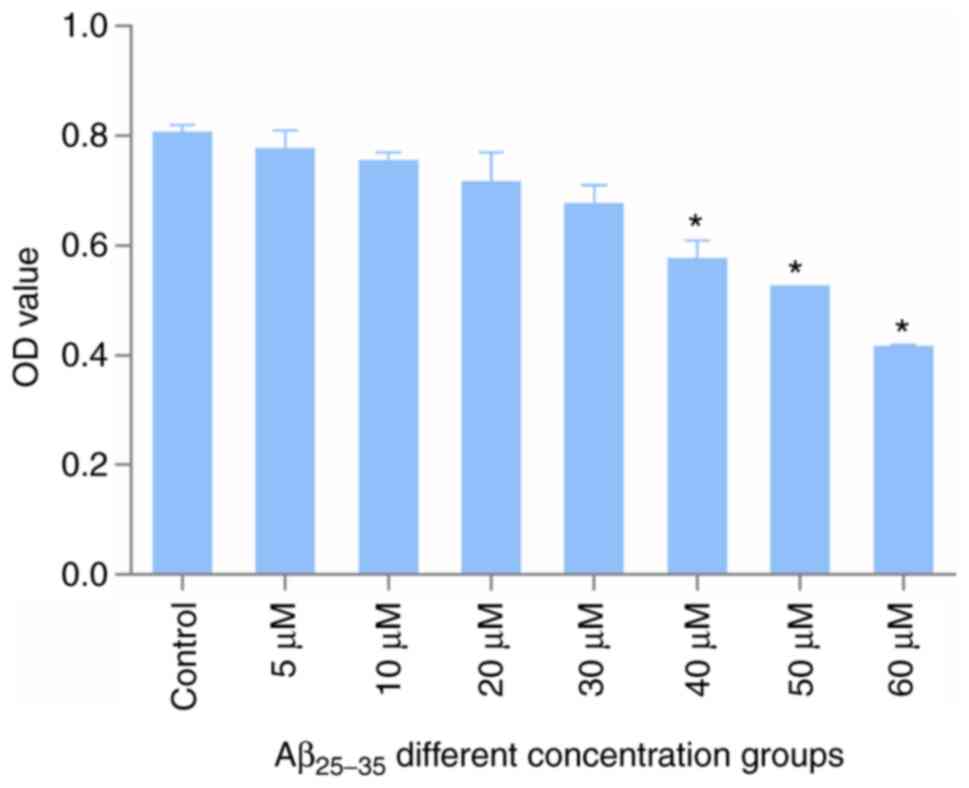
The results of establishing the AD cell model are
presented in Fig. 6. Compared with
that in the control group, the 24-h survival rate of cells in the
Aβ25-35 groups decreased with increasing drug
concentrations. When the concentration of the modeling drug was 60
µM, the cell inhibition rate was 42%. Therefore, the optimal
modeling concentration was selected as 60 µM.
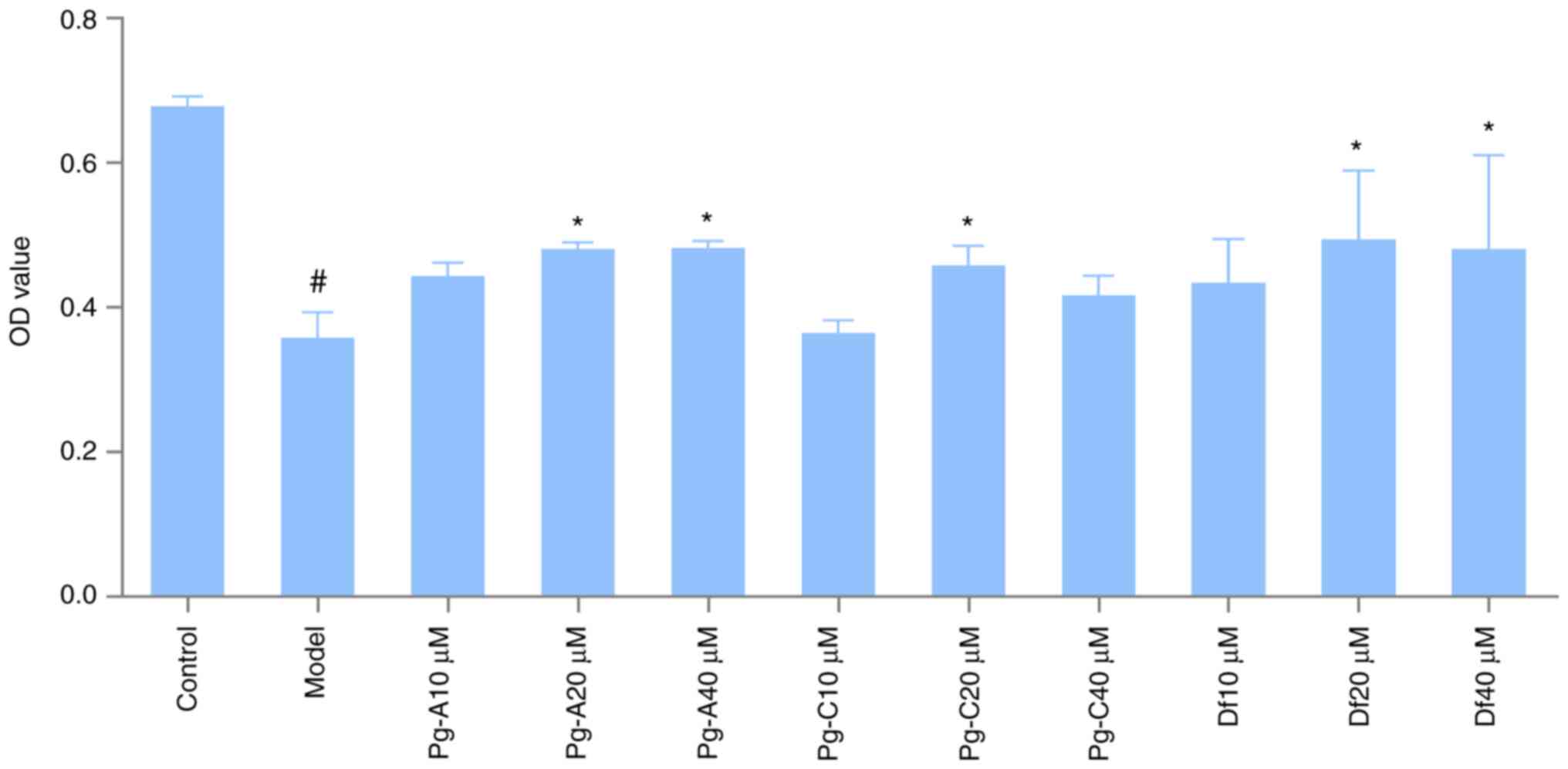
The effects of the three compounds on the survival
rate of the PC12 cell model of AD induced by Aβ25-35 are
presented in Fig. 7. Compared with
that in the control group, the cell survival rate in the model
group was significantly reduced. Under the microscope, the cells in
the model group appeared more shrunken, the cell refractive index
was increased, and certain cells floated, indicating that the
modeling was successful (data not shown). Compared with that in the
model group, the survival rate of PC12 cells in the polygonatine A
20 and 40 µM groups was significantly increased (P<0.05). The
survival rate of the polygonatine C 20 µM dose group was also
significantly increased (P<0.05) and the survival rates of the
4'5-dihydroxyflavone 20 and 40 µM dose groups were significantly
increased compared with that of the model group (P<0.05).
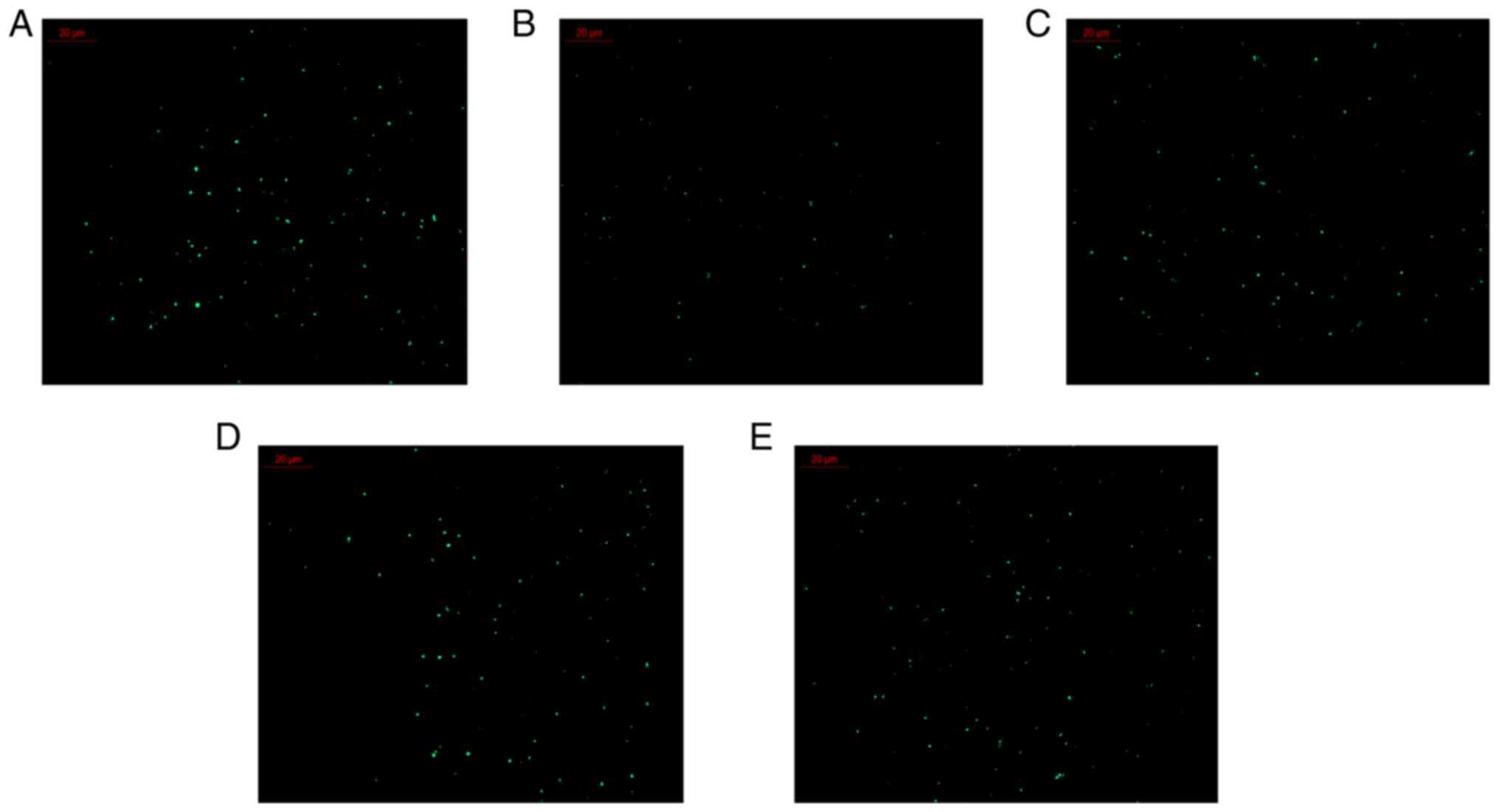
Changes in intracellular Ca2+ are
presented in Fig. 8 and Table II. Compared with that in the
control group, the intracellular Ca2+ level in the model
group was significantly reduced (P<0.01). Compared with those in
the model group, Ca2+ levels in the polygonatine A 40 µM
group, polygonatine C 20 µM group and 4',5-dihydroxyflavone 20 µM
group were significantly increased (P<0.05).
 | Table IIEffects of Erjing pill monomer
compounds on Ca2+ fluorescence in the Alzheimer's
disease model of PC12 cells induced by
amyloid-β25-35. |
Table II
Effects of Erjing pill monomer
compounds on Ca2+ fluorescence in the Alzheimer's
disease model of PC12 cells induced by
amyloid-β25-35.
| Group | Concentration
(µM) | Fluorescence
intensity |
|---|
| Control | - |
3,473.00±358.86 |
| Model | - |
1,172.25±30.74a |
| Polygonatine A | 40 |
2,883.00±32.14b |
| Polygonatine C | 20 |
2,972.33±230.91b |
|
4',5-Dihydroxyflavone | 20 |
2,542.75±309.52b |
Discussion
As a neurodegenerative disease, AD is the most
common cause of senile dementia. With the rapid global aging
process, the incidence of AD has been exhibiting annual increases,
which has provided challenges in the pharmacology, nursing and
elderly care sectors (53). Current
single-target drugs may relieve the symptoms of AD, but the effect
is unsatisfactory and requires to be improved further. The
therapeutic advantages of TCM with its multicomponent and
multitarget properties have begun to receive widespread attention
(54). Erjing pill, a famous
classic prescription composed of Polygonatum sibiricum and
Lycium chinense, may be traced back to the ‘Sheng Ji Zong
Lu’ in the Northern Song Dynasty (A.D. 1117) and has the effect
of nourishing the kidneys and promoting intellectual performance.
Studies have demonstrated that Erjing pill is able to remedy
impairments in the learning and memory ability of rats induced by
long-term irritation stress (55)
and significantly improve cognitive impairment of mice induced by
aluminum trichloride (56), as well
as thyroxine combined with reserpine (57). However, the mechanisms of action of
Erjing pill on AD have remained elusive and further research is
necessary. In the present study, with the application of network
pharmacological technology, the targets were identified and
analyzed via active components, and an interaction network was
constructed. In addition, functional enrichment analysis provided
terms and pathways that are of importance in the prevention and
treatment of AD by Erjing pill. Furthermore, molecular docking
techniques were adopted to validate key nodes of the network. The
results of previous studies clearly indicated a correlation between
multiple active components and multiple targets of diseases. Thus,
unraveling such a pharmacological network may provide a scientific
basis for the development of effective strategies to treat AD.
In the present study, a combination of compound
target prediction and disease target databases/tools was used to
compare the 265 potential targets screened by the SwissTarget
prediction platform with 2,332 targets obtained by screening
disease databases, such as OMIM and TTD. As a result, 127 common
targets, including APP and phosphoinositide-3-kinase regulatory
subunit 1 (PIK3R1), were obtained, all of which may be correlated
with the prevention and treatment of AD. GO analysis results from
the Metascape database revealed that these 127 target proteins are
mainly related to synaptic signaling, transsynaptic signaling,
dendrites, axons and other signaling pathways. By comparing the
control drug targets retrieved by the GeneCards platform with the
potential targets of Erjing pill, common targets, including ACHE,
BCHE, APP, HTR2A, CHRM2 and MAPT, were identified. Previous studies
have demonstrated the following: i) In the brains of patients with
AD, ACHE activity in SP and NFTs are significantly increased,
affecting the release of choline neurotransmitters and leading to
the formation of Aβ deposition and NFTs, resulting in a vicious
cycle. Inhibition of ACHE activity to improve the ACH content in
the brain may relieve the symptoms of patients with AD (58); ii) the development of BCHE
inhibitors may increase the hydrolysis of neurotransmitter
acetylcholine by acetylcholinesterase to lower synaptic levels and
induce BCHE overexpression to improve the symptoms of AD (59); iii) APP has a fundamental
relationship with the pathogenesis of AD. APP is a precursor
protein of Aβ, which produces Aβ through continuous hydrolysis of
β-secretase and γ-secretase (60).
Reducing the expression of APP and changing the activity of
secretase may decrease Aβ production, thereby helping to prevent
the excessive deposition of Aβ to form SP; iv) The 5HT-2A receptor
is a serotonin receptor subtype. As a type of G-protein-coupled
receptor, these receptors are located in the central nervous system
and exhibit functions related to neurotransmitter transmission,
such as cognition, learning and memory. The change in the 5HT-2A
receptor exhibits a correlation with mental symptoms in patients
with AD (61); v) mediating
muscarinic ACH receptors that inhibit a variety of cellular
responses, such as adenylate cyclase (AC) and phosphoinositide,
affecting phospholipase C (PLC) activity and increasing the calcium
ion concentration; thus, increasing synaptic signal transmission
improves the cognitive, learning and memory functions of patients
with AD (62). vi) Tau protein is a
protein with numerous functions, such as connecting microtubules in
axons and maintaining the stability of the microtubule assembly
process. Under pathological conditions, abnormally phosphorylated
Tau proteins lose the ability to transport microtubules, causing a
large amount of intracellular deposition of Tau proteins to form
NFTs, which leads to cognitive impairment in patients with AD
(63). Thus, inhibiting the
hyperphosphorylation of Tau protein represents another treatment
for improving the clinical manifestations of AD. The active
components in Erjing pill may increase the contents of ACH, BCH and
serotonin among neurons by six proteins, including ACHE, BCHE and
APP, to reduce the production of Aβ and the hyperphosphorylation of
Tau proteins and thus prevent AD.
Using the Metascape database for KEGG pathway
analysis, 127 target proteins of Erjing pill were identified as
being involved in the prevention and treatment of AD, and these
proteins mainly participate in signaling pathways involved in
neuroactive ligand-receptor interactions and dopaminergic synapses,
including the PI3K-Akt signaling pathway and other existing
pathogenesis pathways of AD (64).
The anastomotic pathway also contains signaling pathways involved
in the pathogenesis of neuropathic diseases, such as serotonin
synapse (65) and dopamine synapse
formation. Studies have demonstrated that 5-HT receptor blockade
induces ACH release, regulates the balance between glutamate and
gamma-aminobutyric acid, and has a wide-ranging effect on
neurotransmission and neuronal activity (66,67). A
study using the early AD genetic mouse model (Tg2576) (68) discovered that the degeneration of
dopamine neurons in the ventral tegmental area was closely
correlated with AD progression, thus confirming the relevance of
dopamine levels for AD (69).
Erjing pill may increase the amount of dopamine receptors and the
content of dopamine in the brain, promote synapse formation,
improve synaptic excitatory transmission and thus prevent AD. In
previous research by our group, an AD model was established by
D-galactose (i.p.) combined with Aβ1-40 (i.c.v.) to
observe the pharmacodynamic effects of Erjing pill on AD.
Comparison of data from a proteomics analysis (70), with the network pharmacology
screening pathways indicated that dopaminergic synapses were the
most involved proteins. Existing research indicates that in the
dopaminergic synapse pathway, the D1 receptor is able bind to the
glutamine synthetase protein, activate AC and increase cAMP, which
subsequently increases the level of proteinase A, and the increase
of protein kinase A (PKA) may accelerate the physiological and
biochemical processes. On the other hand, dopamine may activate D1
receptor-mediated PLC-dependent calcium burst calcium flow, thereby
participating in phosphoinositide metabolism and Ca2+
signal transduction process and thus having a role in promoting
synapse production (71). The
expression of D1 receptor mRNA in 8-month-old transgenic female
mice with AD was increased compared with that of wild-type female
mice of the same month of birth. Immunohistochemical staining
revealed that the amount of D1 receptor in the CA1 area of the
mouse exhibited an increasing trend, but there was no significant
difference in the expression of D2 receptor, which indicates that
the expression of D1 receptor is increased in AD model mice. The
mechanism of action is related to the activation of AC, cAMP and
PKA (72). Given that Erjing pill
has the effect of increasing the expression of Gq-protein (Gq),
PLC, inositol 1,4,5-triphate receptor (IP3R) and
calmodulin-dependent protein kinase 2d (Camk2d), Erjing pill may
act through the TH-DA-D1-Gq-PLC-IP3R-CaM-Camk2d pathway in the
dopaminergic synapse pathway, increasing the content of
Ca2+ between cells, and has the effect of promoting the
transmission of synapses in the brain.
Using the ADMET descriptors module in DS software,
302 chemical constituents collected from three databases, including
TCMSP, were screened for 65 potential active components, including
lauric acid, zederone, DBP, polysaccharides, flavonoids and
alkaloids. Using molecular docking technology, it was indicated
that 20 active ingredients, such as lauric acid and paeono,
exhibited improved binding to disease-related proteins compared
with control drugs. Studies suggested that lauric acid (73), paeonol (74), isolecin (75), phenylalanine tocopherin (76), tryptophane (77) and DBP (78) exhibit correlations with AD. There
are 7 components that both act on the dopaminergic synapse pathway
and are better than the four control drug docking results. The 7
components include DBP, paeonol, tyrosine, apigenin,
4',5-dihydroxyflavone, polygonatine A and polygonatine C.
Literature verification indicated that in addition to tyrosine,
which is involved in the dopamine synaptic pathway, DBP, paeonol
(74) and apigenin (79) act on DA receptors and promote DA
production, whereas 4',5-dihydroxyflavone, polygonatine A and
polygonatine C have not been previously reported. Therefore, the
activity was were verified by detecting the effects of
4',5-dihydroxyflavone, polygonatine A and polygonatine C on the
intracellular Ca2+ levels in an AD model generated from
PC12 cells. The results indicated that 4',5-dihydroxyflavone,
polygonatine A and polygonatine C significantly improved the
survival rate of PC12 cells challenged with Aβ25-35 and
increased the intracellular Ca2+ concentration in the
PC12 cells of the AD model. Thus, the extract of Erjing pill may
exert a neuroprotective effect through the dopaminergic synapse
pathway.
In conclusion, in the present study, the
biologically active components of Erjing pill on AD were analyzed
by networks pharmacology and molecular docking. The results
provided 65 active components of Erjing pill, including lauric
acid, zederone and DBP, which may act on 127 protein targets, such
as APP and PIK3R1, and through multiple protein functions, such as
synaptic signal transmission and chemical synaptic transmission. In
addition, multiple pathways, such as neuroactive ligand-receptor
interactions and dopaminergic synapses, appear to be involved,
further suggesting that Erjing pill is a multiple
component-multiple target-multiple pathway to prevent and treat
Alzheimer's disease. Subsequently, 4',5-dihydroxyflavone,
polygonatine A and polygonatine C were selected to verify the
results of the networks pharmacology analysis through in
vitro tests. The results indicated that the tested compounds
are able to significantly increase the survival rate of PC12 cells
injured by Aβ25-35 and increase the Ca2+
concentration in PC12 cells in the AD model. The present study
preliminarily elucidated the active components and mechanism of
Erjing pill in preventing and treating AD and provided a direction
for follow-up research on the scientific mechanisms of action of
Erjing pill in the treatment of AD.
Supplementary Material
Significant active components of the
Erjing pill that meet the absorption, distribution, metabolism,
excretion and toxicity conditions (n=65).
Potential targets of Erjing pill in
the treatment of Alzheimer's disease.
Top 10 genes related to five drugs
from Gene Cards.
Docking score of 20 active components
of Erjing pill and five control drugs.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Academic and Technical
Leader Program of Jiangxi Province (grant nos. 20182BCB22005 and
20165BCB18006), the Natural Science Foundation of Jiangxi Province
(grant nos. 20171BAB205083 and 20172BCB22010), the National Natural
Science Foundation of China (grant nos. 82060759, 31660275 and
31960193), the Jiangxi Provincial Health and Family Planning
Commission for Chinese Medicine Research Project (grant no.
2016A023), Jiangxi Provincial Traditional Chinese Medicine
First-class Discipline Special Research Fund (grant nos.
JXSYLXK-ZHYAO119 and JXSYLXK-ZHYAO137), Project of Science and
Technology Department of Jiangxi Province (grant nos. 20165BCB18006
and 20172BCB22010), Postgraduate Innovation Fund Project of Jiangxi
University of Traditional Chinese Medicine (grant no. JZYC20S03)
and Funds from China Scholarship Council (grant no. 201808360274).
The funders had no role in the design of the study or the
collection, analysis, interpretation of data or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and YG designed the research and wrote the
original draft. BY and YX performed the software analysis. MZ, YW
and FY performed the formal analysis. LY and XQ performed the cell
experiments and date curation. YC and LH performed the data
analysis and reviewed the manuscript. YC and LH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blennow K, Hampel H, Weiner M and
Zetterberg H: Cerebrospinal fluid and plasma biomarkers in
Alzheimer disease. Nat Rev Neurol. 6:131–144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kozlov S, Afonin A, Evsyukov I and
Bondarenko A: Alzheimer's disease: As it was in the beginning. Rev
Neurosci. 28:825–843. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bonet-Costa V, Pomatto LC and Davies KJ:
The proteasome and oxidative stress in Alzheimer's Disease.
Antioxid Redox Signal. 25:886–901. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nisbet RM, Polanco JC, Ittner LM and Götz
J: Tau aggregation and its interplay with amyloid-β. Acta
Neuropathol. 129:207–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Myeku N, Clelland CL, Emrani S, Kukushkin
NV, Yu WH, Goldberg AL and Duff KE: Tau-driven 26S proteasome
impairment and cognitive dysfunction can be prevented early in
disease by activating cAMP-PKA signaling. Nat Med. 22:46–53.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Zeng Q, Li L, Siu W, Jin Y, Cao M, Li W,
Chen J, Cong W, Ma M, Chen K and Wu Z: A combined molecular biology
and network pharmacology approach to investigate the multi-target
mechanisms of Chaihu Shugan San on Alzheimer's disease. Biomed
Pharmacother. 120(109370)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li C, Zhang WY, Yu Y, Cheng CS, Han JY,
Yao XS and Zhou H: Discovery of the mechanisms and major bioactive
compounds responsible for the protective effects of gualou xiebai
decoction on coronary heart disease by network pharmacology
analysis. Phytomedicine. 56:261–268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Q, Li R, Peng W, Zhang M, Liu J, Wei
S, Wang J, Wu C, Gao Y and Pu X: Identification of the active
constituents and significant pathways of guizhi-shaoyao-zhimu
decoction for the treatment of diabetes mellitus based on molecular
docking and network pharmacology. Comb Chem High Throughput Screen.
22:584–598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Du YJ, Tao YM, Tian Q, Wang Y, Wang L and
Wu WH: Based on the ERK/CREB signal pathway to explore the
mechanism of moxibustion on Shenshu points to improve neuron loss
in ovariectomized AD rats with D-galactose. Chin J Trad Chin Med.
1–18. 2020.(In Chinese).
|
|
10
|
Gao YY and Wang X: The effect of ‘Kidney
and Brain Xiangji’ electroacupuncture on the behavior and
expression of IL-1b and TNF-¦Á in Alzheimer's disease model mice.
Shanghai J Acupuncture. 39:359–364. 2020.
|
|
11
|
Hao da C and Xiao PG: Network
pharmacology: A rosetta stone for traditional Chinese medicine.
Drug Dev Res. 75:299–312. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Q, Bai SF, Lai XJ, Wang HF, Liang J
and Lai WY: Comparison of two methods for determination of
polysaccharides in Erjing pill. Chin J Exp Trad Chin Med. 16:32–34.
2010.
|
|
13
|
Ding C, Luo J, Shu H, Zhang XY, He GX and
Zhou Y: A summary of the health effects of Shengji Erjing Recipe
and analysis of its industrialization direction. Hubei J Trad Chin
Med. 34:73–74. 2012.
|
|
14
|
Bai SF, Lai XJ, Liu Q and Lai WY: Study on
the effect of Erjing pill polysaccharide on reducing blood sugar in
diabetic rats. China Mod Appl Pharm. 27:577–580. 2010.
|
|
15
|
Li SX: Understanding and suggestions on
the variety list of ‘Food and Medicine’. Chin J Nat Med. 4:232–242.
2001.
|
|
16
|
Xie GZ, Liu H, Wang Z, Tang XY and Zhang
SH: Comparison of Polygonatum odoratum and Polygonatum odoratum, a
traditional Chinese medicine for food and medicine. Mod Chin Med.
22:1447–1152. 2020.
|
|
17
|
Han C, Zhu Y, Yang Z, Fu S, Zhang W and
Liu C: Protective effect of Polygonatum sibiricum against
cadmium-induced testicular injury in mice through inhibiting
oxidative stress and mitochondria-mediated apoptosis. J
Ethnopharmacol. 261(113060)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tu MF and Ye WF: Research progress of
pharmacological effects and clinical application of Polygonatum
sibiricum. J Yichun Univ. 40:27–31. 2018.(In Chinese).
|
|
19
|
Gao Y, Wei Y, Wang Y, Gao F and Chen Z:
Lycium barbarum: A traditional Chinese herb and a promising
anti-aging agent. Aging Dis. 8:778–791. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen LG: Analysis of pharmacological
effect and clinical application value of wolfberry. The World's
Latest Medical Information Digest. 15(92)2015.(In Chinese).
|
|
21
|
Zhang R, Zhu X, Bai H and Ning K: Network
pharmacology databases for traditional Chinese medicine: Review and
assessment. Front Pharmacol. 10(123)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahn MJ, Kim CY, Yoon KD, Ryu MY, Cheong
JH, Chin YW and Kim J: Steroidal saponins from the rhizomes of
Polygonatum sibiricum. J Nat Prod. 69:360–364.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu HS, Zhang J, Kang LP, Han LF, Zou P,
Zhao Y, Xiong CQ, Tan DW, Song XB, Yu K and Ma BP: Three new
saponins from the fresh rhizomes of Polygonatum kingianum. Chem
Pharm Bull (Tokyo). 57:1–4. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen YH, Yan B, Guan Y and Liu YQ: Effect
of Erjing Pills on learning and memory abilities of AD rats with
kidney yin deficiency induced by ovariectomy+D-galactose combined
with Aβ1-40. Trad Chin Drug Res Clin Pharmacol. 30:1421–1427.
2019.
|
|
25
|
Leng EL, Cao ZW, Jiang ZH, Zhou H and Liu
L: Network-based drug discovery by integrating systems biology and
computational technologies. Brief Bioinform. 14:491–505.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Che CT, Wang ZJ, Chow MS and Lam CW:
Herb-herb combination for therapeutic enhancement and advancement:
Theory, practice and future perspectives. Molecules. 18:5125–5141.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao S and Iyengar R: Systems
pharmacology: Network analysis to identify multiscale mechanisms of
drug action. Annu Rev Pharmacol Toxicol. 52:505–521.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zeng Q, Li L, Jin Y, Chen Z, Duan L, Cao
M, Ma M and Wu Z: A network pharmacology approach to reveal the
underlying mechanisms of Paeonia lactiflora pall. On the treatment
of Alzheimer's Disease. Evid Based Complement Alternat Med.
2019(8706589)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6(13)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
UniProt Consortium: UniProt: A hub for
protein information. Nucleic Acids Res. 43 (Database
Issue):D204–D212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hähnke VD, Kim S and Bolton EE: PubChem
chemical structure standardization. J Cheminform.
10(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gene Ontology Consortium. Gene ontology
consortium: Going forward. Nucleic Acids Res. 43 (Database
Issue):D1049–D1056. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Safran M, Dalah I, Alexander J, Rosen N,
Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, et al:
GeneCards version 3: The human gene integrator. Database (Oxford).
2010(baq020)2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li YH, Yu CY, Li XX, Zhang P, Tang J, Yang
Q, Fu T, Zhang X, Cui X, Tu G, et al: Therapeutic target database
update 2018: Enriched resource for facilitating bench-to-clinic
research of targeted therapeutics. Nucleic Acids Res. 46
(D1):D1121–D1127. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Amberger JS and Hamosh A: Searching online
mendelian inheritance in man (OMIM): A knowledgebase of human genes
and genetic phenotypes. Curr Protoc Bioinformatics.
58:1.2.1–1.2.12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim J, So S, Lee HJ, Park JC, Kim JJ and
Lee H: DigSee: Disease gene search engine with evidence sentences
(version cancer). Nucleic Acids Res. 41 (Web Server
Issue):W510–W517. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu X, Shi Y, Deng Y and Dai R: Using
molecular docking analysis to discovery Dregea sinensis Hemsl.
Potential mechanism of anticancer, antidepression, and
immunoregulation. Pharmacogn Mag. 13:358–362. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Daina A and Zoete V: Application of the
SwissDrugDesign online resources in virtual screening. Int J Mol
Sci. 20(4612)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qiao B, Wu Y, Li X, Xu Z, Duan W, Hu Y,
Jia W, Fan Q and Xing H: A network pharmacology approach to explore
the potential mechanisms of Yifei Sanjie formula in treating
pulmonary fibrosis. Evid Based Complement Alternat Med.
2020(8887017)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lotia S, Montojo J, Dong Y, Bader GD and
Pico AR: Cytoscape app store. Bioinformatics. 29:1350–1351.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen CY: TCM Database@Taiwan. The world's
largest traditional Chinese medicine database for drug screening in
silico. PLoS One. 6(e15939)2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Su WT and Shi YA: Nanofiber containing
carbon nanotubes enhanced PC12 cell proliferation and
neuritogenesis by electrical stimulation. Biomed Mater Eng. 26
(Suppl 1):S189–S195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ning HH, Yuan MM, Wu QP, Ping YH, Zhou ZQ,
Xu Y, Wu Y and Yin HX: Separation and identification of chemical
constituents of Polygonatum multiflorum. Chin J Exp Formulas.
24:77–82. 2018.
|
|
44
|
Yi BX, Zhong LY and Gong QF: Jiangxi
Jianchang's special Wen method and its modern research ideas.
Lishizhen Trad Chin Med. 23:1755–1756. 2012.
|
|
45
|
Chen H, Feng SS and Sun YJ: Research
progress on chemical constituents and pharmacological activities of
three kinds of medicinal Huangjing. Chin Herbal Med. 46:2329–2338.
2015.
|
|
46
|
Ma K, Huang XF and Kong LY: Steroidal
saponins from Polygonatum cyrtonema. Chem Nat Compd. 49:888–891.
2013.
|
|
47
|
Yu HS, Ma BP, Kang LP, Zhang T, Jiang FJ,
Zhang J, Zou P, Zhao Y, Xiong CQ, Tan DW, et al: Saponins from the
processed rhizomes of Polygonatum kingianum. Chem Pharm Bull
(Tokyo). 57:1011–1014. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yu HS, Ma BP and Song XB: Two new
steroidal saponins from the processed Polygonatum kingianum. Helv
Chim Acta. 93:1086–1092. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nakanishi T, Inada A, Kambayashi K and
Yoneda K: Flavonoid glycosides of the roots of Glycyrrhiza
uralensis. Phytochemistry. 24:339–341. 1985.
|
|
50
|
Aida K, Tawata M, Shindo H, Onaya T,
Sasaki H, Yamaguchi T, Chin M and Mitsuhashi H: Isoliquirigenin: A
new aldose reductase inhibitor from glycyrrhizae radix. Planta Med.
56:254–258. 1990.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sun LR, Li X and Wang SX: Two new
alkaloids from the rhizome of Polygonatum sibiricum. J Asian
Nat Prod Res. 7:127–130. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang YF, Lu CH, Lai GF, Cao JX and Luo SD:
A new indolizinone from Polygonatum kingianum. Planta Med.
69:1066–1068. 2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chiquita S, Rodrigues-Neves AC, Baptista
FI, Carecho R, Moreira PI, Castelo-Branco M and Ambrósio AF: The
retina as a window or mirror of the brain changes detected in
Alzheimer's disease: Critical aspects to unravel. Mol Neurobiol.
56:5416–5435. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ma J, Chen X, Bian YQ, Chen ZJ, Qiao YJ
and Zhang YL: Study on efficacy markers of Salviae Miltiorrhizae
Radix et Rhizoma for promoting blood circulation and remving blood
stasis based on systematic traditional Chinese medicine. Zhongguo
Zhong Yao Za Zhi. 45:3259–3265. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
55
|
Xu XQ, Huang JY, Zhao T, Tu XY, Huang BB,
Gao SL, Xiao BQ, Luo L and Zhao XF: Mechanism of total
polysaccharides from Erjing Pill against learning and memory
impairment in rats with long-term stress. J Jiangxi College Trad
Chin Med. 19:54–57. 2007.
|
|
56
|
Huang JY, Zhou J, Wei H, Tu XY and Lou LY:
Pharmacological experimental study of compound Erigo against
Alzheimer's Disease. J Jiangxi Med College. 41:53–56. 2001.
|
|
57
|
Xu XQ, Huang JY, Luo R, Xiao BQ, Zhao T
and Tu XY: Effect of the effective fraction of Erjing Pill on
learning and memory impairment in rats with kidney-yin deficiency
model and its molecular mechanism. Chin Trad Herbal Drugs.
38:564–569. 2007.
|
|
58
|
Capatina L, Todirascu-Ciornea E, Napoli
EM, Ruberto G, Hritcu L and Dumitru G: Thymus vulgaris essential
oil protects zebrafish against cognitive dysfunction by regulating
cholinergic and antioxidants systems. Antioxidants (Basel).
9(1083)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Baumann K, Kordić L, Močibob M, Šinko G
and Tomić S: Synthesis and in vitro screening of novel heterocyclic
β-d-Gluco- and β-d-galactoconjugates as butyrylcholinesterase
inhibitors. Molecules. 24(2833)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Galvão F Jr, Grokoski KC, Silva BB, Lamers
ML and Siqueira IR: The amyloid precursor protein (APP) processing
as a biological link between Alzheimer's disease and cancer. Ageing
Res Rev. 49:83–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Craig D, Donnelly C, Hart D, Carson R and
Passmore P: Analysis of the 5HT-2A T102C receptor polymorphism and
psychotic symptoms in Alzheimer's disease. Am J Med Genet B
Neuropsychiatr Genet. 144B:126–128. 2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ma LQ, Liu C, Wang F, Xie N, Gu J, Fu H,
Wang JH, Cai F, Liu J and Chen JG: Activation of
phosphatidylinositol-linked novel D1 dopamine receptors inhibits
high-voltage-activated Ca2+ currents in primary cultured striatal
neurons. J Neurophysiol. 101:2230–2238. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
DeTure MA and Dickson DW: The
neuropathological diagnosis of Alzheimer's disease. Mol
Neurodegener. 14(32)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Park HJ, Kwon H, Lee JH, Cho E, Lee YC,
Moon M, Jun M, Kim DH and Jung JW: β-Amyrin ameliorates Alzheimer's
disease-like aberrant synaptic plasticity in the mouse hippocampus.
Biomol Ther (Seoul). 28:74–82. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ledo JH, Azevedo EP, Beckman D, Ribeiro
FC, Santos LE, Razolli DS, Kincheski GC, Melo HM, Bellio M,
Teixeira AL, et al: Cross talk between brain innate immunity and
serotonin signaling underlies depressive-like behavior induced by
Alzheimer's Amyloid-β oligomers in mice. J Neurosci.
36:12106–12116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
de Jong IEM and Mørk A: Antagonism of the
5-HT(6) receptor-Preclinical rationale for the treatment of
Alzheimer's disease. Neuropharmacology. 125:50–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ferrero H, Solas M, Francis PT and Ramirez
MJ: Serotonin 5-HT6 receptor antagonists in Alzheimer's
disease: Therapeutic rationale and current development status. CNS
Drugs. 31:19–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Krashia P, Nobili A and D'Amelio M:
Unifying hypothesis of dopamine neuron loss in neurodegenerative
diseases: Focusing on Alzheimer's disease. Front Mol Neurosci.
12(123)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nardone R, Höller Y, Thomschewski A, Kunz
AB, Lochner P, Golaszewski S, Trinka E and Brigo F: Dopamine
differently modulates central cholinergic circuits in patients with
Alzheimer disease and CADASIL. J Neural Transm (Vienna).
121:1313–1320. 2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Huang LP, Yang XY, Yan B and Qiu XP:
Effect of Erjing Pill on hippocampal proteomics of AD rats with
kidney yin deficiency induced by ovariectomy + D-galactose combined
with Aβ_ (1-40). Chin J Exp Pharmacol. 26:15–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sun BJ and Ling H: Research progress on
the relationship between dopamine receptors and Alzheimer's
disease. J Clin Ration Use. 8:177–180. 2015.
|
|
72
|
Lian YX and He L: Therapeutic effects of
dopamine D1 receptor agonists on central nervous system diseases
and their target pathways. J of Clin Rational Use. 6:102–103.
2013.(In Chinese).
|
|
73
|
Nafar F, Clarke JP and Mearow KM: Coconut
oil protects cortical neurons from amyloid beta toxicity by
enhancing signaling of cell survival pathways. Neurochem Int.
105:64–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Han F, Xu H, Shen JX, Pan C, Yu ZH, Chen
JJ, Zhu XL, Cai YF and Lu YP: RhoA/Rock2/Limk1/cofilin1 pathway is
involved in attenuation of neuronal dendritic spine loss by paeonol
in the frontal cortex of D -galactose and aluminum-induced
Alzheimer’s disease-like rat model. Acta Neurobiologiae
Experimentalis. 80:225–244. 2020.PubMed/NCBI
|
|
75
|
Li BL, Li X and Li JC: Metabolomics study
on serum of Tg2576 mice transgenic model of Alzheimer's disease.
Chin J Exp Anim. 25:218–224. 2017.
|
|
76
|
Sethiya NK, Nahata A, Singh PK and Mishra
SH: Neuropharmacological evaluation on four traditional herbs used
as nervine tonic and commonly available as Shankhpushpi in India. J
Ayurveda Integr Med. 10:25–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Liu X: Study on the limiting rate enzyme
of tryptophan metabolism IDO1 and its inhibitors. Chem Life.
6:774–782. 2016.
|
|
78
|
Preciados M, Yoo C and Roy D: Estrogenic
endocrine disrupting chemicals influencing NRF1 regulated gene
networks in the development of complex human brain diseases. Int J
Mol Sci. 17(2086)2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Nakazawa T, Yasuda T, Ueda J and Ohsawa K:
Antidepressant-like effects of apigenin and
2,4,5-trimethoxycinnamic acid from perilla frutescens in the forced
swimming test. Biol Pharm Bull. 26:474–480. 2003.PubMed/NCBI View Article : Google Scholar
|