Introduction
Cardiovascular disease (CVD) is an important health
concern and has been the focus of considerable research. In China,
there are an estimated 330 million patients with CVD, and CVD
accounts for >40% of all disease-related resident deaths and has
been identified as the leading cause of mortality (1). The mortality, incidence and prevalence
rates of CVD continue to increase globally (1). Atherosclerosis (AS) is a major cause
of CVD and the result of several factors, among which lipid
metabolism disorders are the leading contributor. In modern
society, lipid metabolism disorders are caused by
hypercholesterolaemia and induce an inflammatory response that is
involved in all processes of AS (2,3). The
nuclear transcription factor controlling their release is
phosphorylated (p)-nuclear factor κB (NF-κB), which has been
revealed to induce an increase in the production of inflammatory
and adhesion factors (4-6).
Usually, NF-κB and the inhibitory protein inhibitor of NF-κB-α
(IκBα) exist as a complex and are inactive. However, when cells are
stimulated or activated, the inhibitor of NF-κB kinase β (IKKβ)
phosphorylates and degrades IκBα, thus activating NF-κB. p-NF-κB
translocates into the nucleus, binds to related genes and regulates
their transcription (7-10).
In addition, numerous studies have reported that oxidative stress
is an important cause of AS and is closely associated with NF-κB
(11-13).
Therefore, the occurrence of AS is closely linked to the
IKK/IκB/NF-κB pathway.
Gentianella acuta (Michx.) Hulten (G.
acuta) belongs to the Gentianella genus of the
Gentianaceae family, also known as the bitter gentian (14). The Elunchun people have been using
G. acuta to treat arrhythmias and other heart diseases for
thousands of years (15,16). Previous studies on G. acuta
mainly have focused on traditional efficacy, such as liver
protection, and anti-arrhythmic, antioxidant and hypoglycaemic
effects; however, further discoveries have been made in other
fields (17); for example, the
bioactive substances of G. acuta have been revealed to exert
a beneficial effect on aberrant intestinal motility (18-20).
Li et al (18) reported
treatment with water extract of G. acuta could ameliorate
cardiac structural disorders, excessive collagenous fiber
accumulation in the heart and cardiac malfunction by regulating the
NF-κB pathway in a model of myocardial fibrosis. Wang et al
(21) indicated that xanthones from
G. acuta exerted cardioprotective effects on myocardial
ischemia/reperfusion (I/R) injury through its antioxidant and
anti-apoptosis properties. Yang et al (22) indicated that the aqueous extract of
G. acuta may improve isoproterenol-induced myocardial
fibrosis through the inhibition of the tumour growth factor
(TGF)-β1/Smads signalling pathway. Numerous studies (16,18,23-25)
have reported that G. acuta exerted a protective effect
against injury of the heart and the aorta of rats under various
conditions, such as I/R. However, the effect and specific mechanism
of action of G. acuta in cardiovascular damage and
inflammation under hypercholesterolaemic conditions remain unclear.
The aim of the present study was to explore the potential role of
G. acuta in mitigating cardiovascular damage and
inflammation in diet-induced hypercholesterolaemic rats.
Materials and methods
Collection and preparation of plant
materials
G. acuta was purchased from The Darhan
Muminggan Joint Banner mongolian medicine plantation, Hulunbeier
district of Inner Mongolia and was identified and authenticated by
Professor Yu-Ping Yan in the field of medicinal plants (College of
Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang,
China). The plants were air-dried and then chopped. G. acuta
(64.51 g) was soaked in 1,400 ml 25˚C distilled water for 30 min.
The mixture was boiled in two batches and combined twice with the
filtrate. The mixture was used at a quantity of 537 ml to obtain a
suspension of G. acuta with a concentration of 0.12
g/ml.
Animals and experimental design
The Ethics Committee of Hebei University of Chinese
Medicine (Shijiazhuang, China) approved and supervised the present
study (approval no. DWLL2018016). A total of 32 specific-pathogen
free male Sprague-Dawley (SD) rats, aged 6-7 weeks, weighing
160-180 g, were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (license no. SCXK 2016-0006) and all
rats had free access to food and water. They were kept at room
temperature with 60% humidity and 12-h light/dark cycle. After
1-week adaptive feeding, the rats were randomized into four groups
(8) as follows: i) Control group
(Control); ii) control administration group (Control-G), iii) model
group (Model); and iv) model administration group (Model-G). While
animals in the Model and Model-G groups received a high-fat diet
(high-fat feed ratio, 80.4% basic feed + 2% cholesterol + 10% lard
+ 0.5% sodium cholate + 0.1% propylthiouracil + 5% sugar + 2% yolk
powder) to induce preliminary hypercholesterolaemia, Control and
Control-G animals received normal feed (100% basic feed: 248.48
g/kg crude protein + 65.18 g/kg crude fat). Normal and high-fat
feed were provided and prepared by Hebei Medical University
(Shijiazhuang, China). On the basis of the previous study, a 1.2
g/kg G. acuta dosage solution was designed (26). After the third week of modelling,
the rats of the Control-G and Model-G groups were administered
water extract of G. acuta and the other groups were treated
with the same 10 ml/kg of physiological saline for 2 weeks.
At the end of the experiment, all rats were only
administered water for the final 12 h. All rats were anesthetized
with 50 mg/kg pentobarbital sodium (Merck KGaA) and euthanized
using cervical dislocation. Following anaesthesia, blood was
collected from the inferior vena cava for analysis of blood
indicators. The serum was separated by centrifugation at 12,000 x g
for 15 min at 4˚C and stored in a refrigerator at -80˚C for further
analysis. The heart was weighed and fixed with the thoracic aorta
in 10% (v/v) formalin 24 h at room temperature for
histopathological studies, and the rest of heart and thoracic aorta
were stored at -80˚C.
Blood biochemical index test
The serum levels of total cholesterol (TC; cat. no.
OSR6216; Beckman Coulter, Inc.), triglycerides (TG; cat. no.
OSR61118; Beckman Coulter, Inc.), low-density lipoprotein (LDL;
cat. no. A113-1-1; Nanjing Jiancheng Bioengineering Institute),
high-density lipoprotein (HDL; cat. no. A112-1-1; Nanjing Jiancheng
Bioengineering Institute), lactate dehydrogenase (LDH; cat. no.
A020-1-2; Nanjing Jiancheng Bioengineering Institute), creatine
kinase (CK; cat. no. A032-1-1; Nanjing Jiancheng Bioengineering
Institute), tumour necrosis factor-α (TNF-α; cat. no. SXR063;
Shanghai Senxiong Biotech Industry, Co., Ltd.) and interleukin-10
(IL-10; cat. no. SXR035; Shanghai Senxiong Biotech Industry, Co.,
Ltd.) were assessed strictly according to the manufacturer's
instructions.
Histopathological examination of the
heart and thoracic aorta
The heart and thoracic aortas isolated from each
group were fixed in 10% (v/v) formalin in 50 mm potassium phosphate
buffer (pH 7.0) for 24 h at 4˚C. The tissues were subsequently
embedded in paraffin, cut into 4-µm sections, and stained 5 min at
room temperature with hematoxylin and then 1 min with eosin at room
temperature. The sections were observed and images were captured
using a light microscope with a Leica DFC 320 digital camera
(magnification, x400; Leica Microsystems, Inc.).
Immunohistochemical analysis of IKKβ,
p-IKKβ, IκBα and p-IκBα in the heart and thoracic aorta
Each section was dewaxed with a dimethylbenzene
gradient and dehydrated using an alcohol gradient. The sections
were then incubated with 3% H2O2 for 15 min
in the dark, blocked with 100% goat serum for 20 min at room
temperature (cat. no. ZLI-9056; ZSGB-BIO; OriGene Technologies,
Inc.), and then rinsed three times with PBS. The primary antibodies
[IKKβ (1:100; cat. no. A2087; ABclonal Biotech Co., Ltd.), p-IKKβ
(1:400; cat. no. bs-5398R), IκBα (1:800; cat. no. bsm-33441M) and
p-IκBα (1:200; cat. no. bs-5515R; all from BIOSS)] were incubated
with the sections at 4˚C overnight. Next, rabbit two-step
HRP-secondary antibody polymers (cat. no. PV-6001; ZSGB-BIO;
OriGene Technologies, Inc.) were added for 60 min at room
temperature and then the avidin-biotin-peroxidase complex (cat. no.
PK-6200; Vector Laboratories, Inc.; Maravai LifeSciences) was added
for 120 min at room temperature. The sections were stained with
diaminobenzidine (DAB) reagent 10 min at room temperature,
dehydrated with alcohol gradient and DAB and finally mounted using
neutral balsam. The sections were viewed under a light microscope
(magnification, x400) and analyzed using ImageJ software (v. d1.47;
National Institutes of Health).
Western blot analysis for p-IKKβ,
p-IκBα and p-NF-κB in the heart and p-NF-κB in the thoracic
aorta
The protein extract from the frozen tissues of the
heart and thoracic aorta were determined using a BCA Protein Assay
Kit (cat. no. P0010; Beyotime Institute of Biotechnology) to ensure
20 µg protein per lane and were separated by 10% SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes. Membranes
were blocked 4˚C for 5 h with 5% non-fat dry milk in Tris-buffered
saline with 0.05% Tween-20 and left overnight. The blots were
incubated with primary antibodies for GAPDH (1:1,000; cat. no.
bs-0755R; BIOSS), IKKβ (1:100; cat. no. AF6013; Affinity
Biosciences), p-IKKβ (1:400; cat. no. bs-5398R), IκBα (1:800; cat.
no. bsm-33441M), p-IκBα (1:200; cat. no. bs-5515R; all from BIOSS),
NF-κB (1:1,000; product no. 8242; Cell Signaling Technology, Inc.),
p-NF-κB (1:250; cat. no. ab247871; Abcam) overnight at 4˚C and then
incubated with a secondary antibody (1:10,000; cat. no. ZB2301;
ZSGB-BIO; OriGene Technologies, Inc.) conjugated to horseradish
peroxidase (1:6,500; Biosharp Life Sciences) for 2 h at room
temperature. After the treatment of Super ECL Detection Reagent
(cat. no. 36208ES60; Shanghai Yeasen Biotechnology Co., Ltd.), the
protein bands were quantified by transmittance densitometry using
ImageJ software (v. d1.47; National Institutes of Health). The
relative protein band intensity was expressed as the ratio of each
protein to the reference GAPDH.
Statistical analysis
All statistical analyses were completed using SPSS
22.0 software (IBM Corp). The data are presented as the mean ± SD.
Differences among the four groups were assessed using one-way
analysis of variance followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of G. acuta on serum
lipids
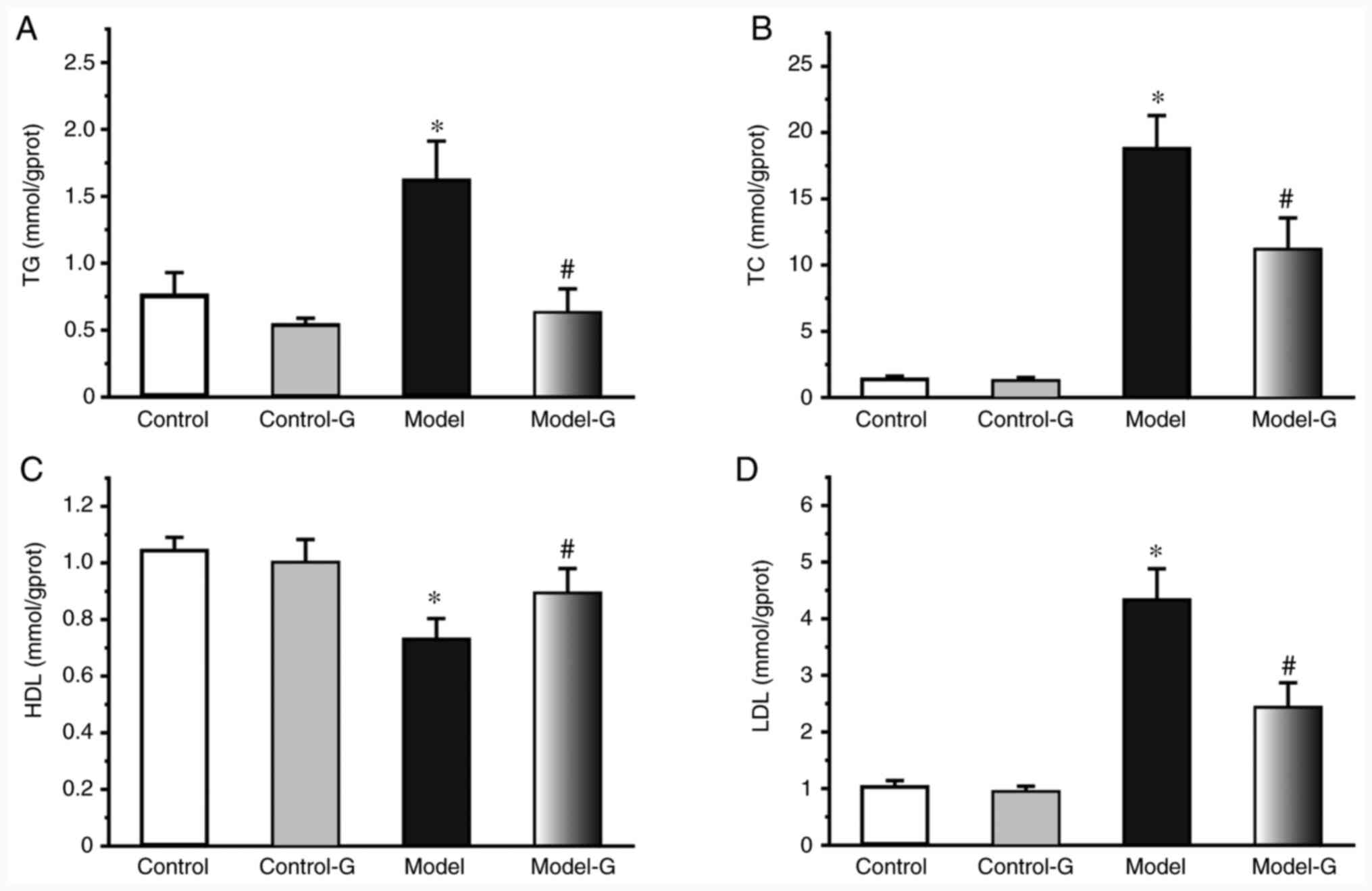
Compared with the Control group, the TG, TC and LDL
levels of the Model and Model-G groups were significantly
increased, while the HDL level was significantly decreased
(P<0.05). In addition, the levels of TG, TC and LDL were
significantly decreased, and those of HDL were significantly
increased in the Model-G group compared with those in the Model
group (Fig. 1; P<0.05).
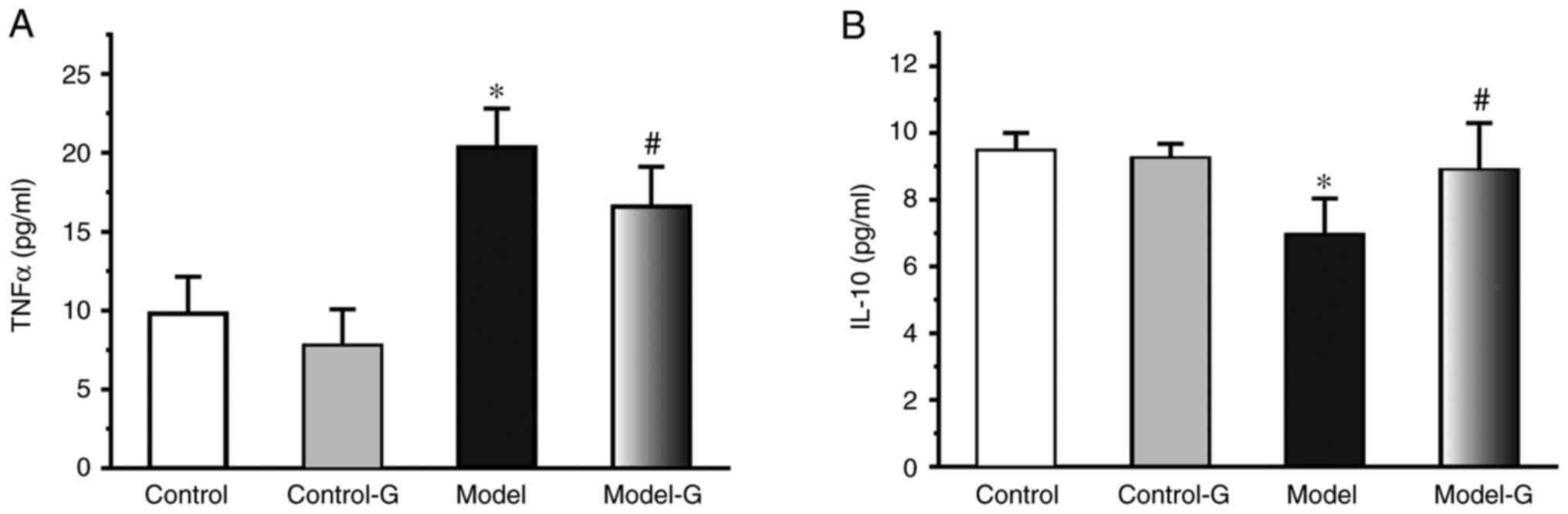
Effects of G. acuta on IL-10 and TNF-α
in the serum
Compared with the Control group, the TNF-α levels in
the Model group were increased >2-fold (P<0.05). Compared
with the Model group, TNF-α was significantly decreased in the
Model-G group (P<0.05). The IL-10 levels in the Model group were
significantly decreased compared with those in the Control and
Model-G groups (Fig. 2;
P>0.05).
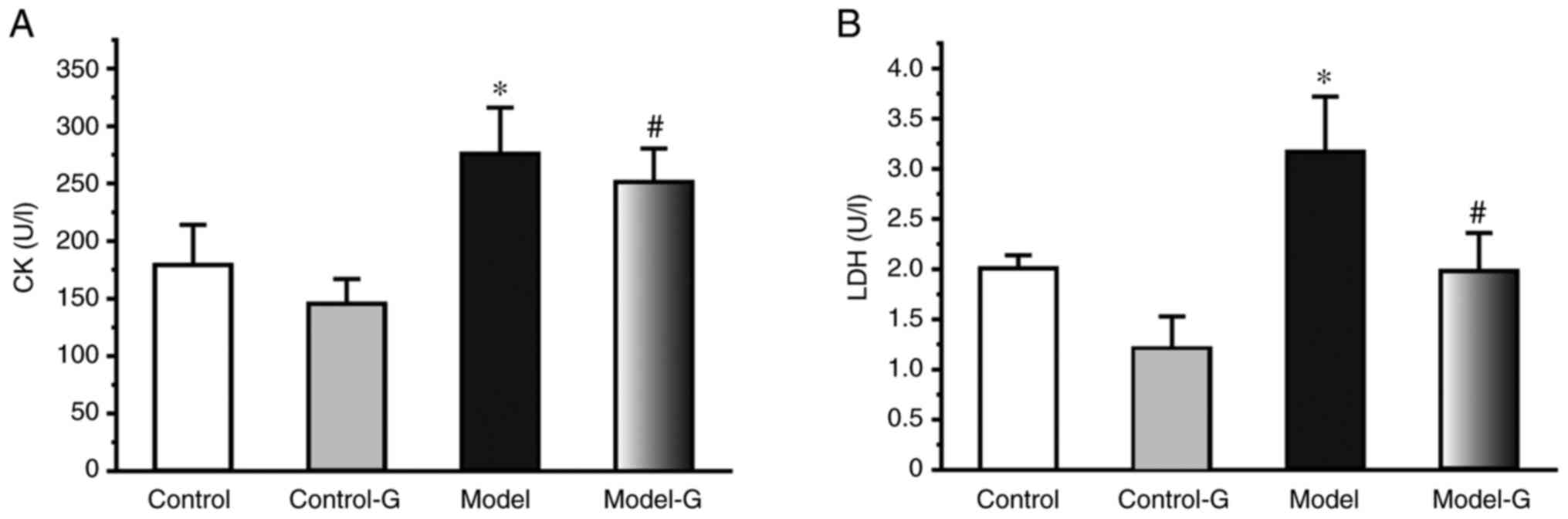
Effects of G. acuta on CK and LDH in
the serum
The CK and LDH levels of the Model group were
significantly higher compared with those of the Control and Model-G
groups (P<0.05). Compared with the Model group, the level of CK
and LDH were significantly decreased in the Model-G group
(P<0.05; Fig. 3).
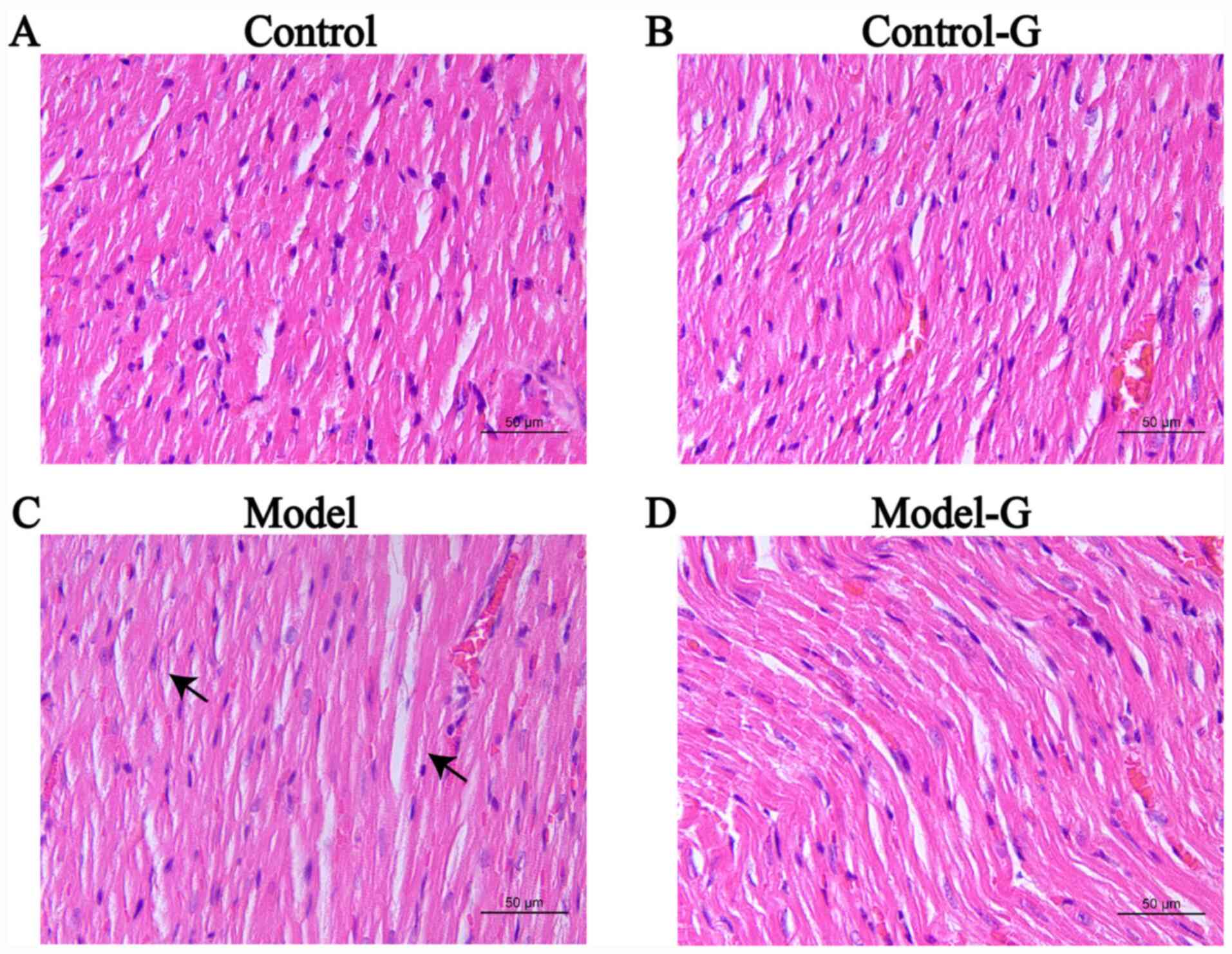
Effects of G. acuta on morphological
and histological changes in the heart
The cardiomyocytes in the Control group were
arranged in an orderly manner with uniform nuclei, uniform H&E
staining of the cytoplasm and obvious striations. The arrangement
of cardiomyocytes in the Model group was disordered, with burrs,
slightly blurry striations, and certain sections were revealed to
have lipid droplets. The arrangement of cardiomyocytes in the
Model-G group was improved and appeared orderly (Fig. 4).
Effect of G. acuta on IKK/IκB/NF-κB in
the heart
The IKKβ, p-IKKβ and p-IκBα protein expression
levels of the Model group were markedly higher compared with those
in the other three groups. While changes in the expression of IκBα
were not significant among the four groups, the levels of
p-IKKβ/IKKβ and p-IκBα/IκBα in the Model group were significantly
higher than the other groups (Fig.
5A-C). Compared with the Control and Model-G groups, the
protein levels of p-IKKβ/IKKβ, p-IκBα/IκBα and p-NF-κB/NF-κB in the
Model group were significantly increased (Fig. 5D-G).
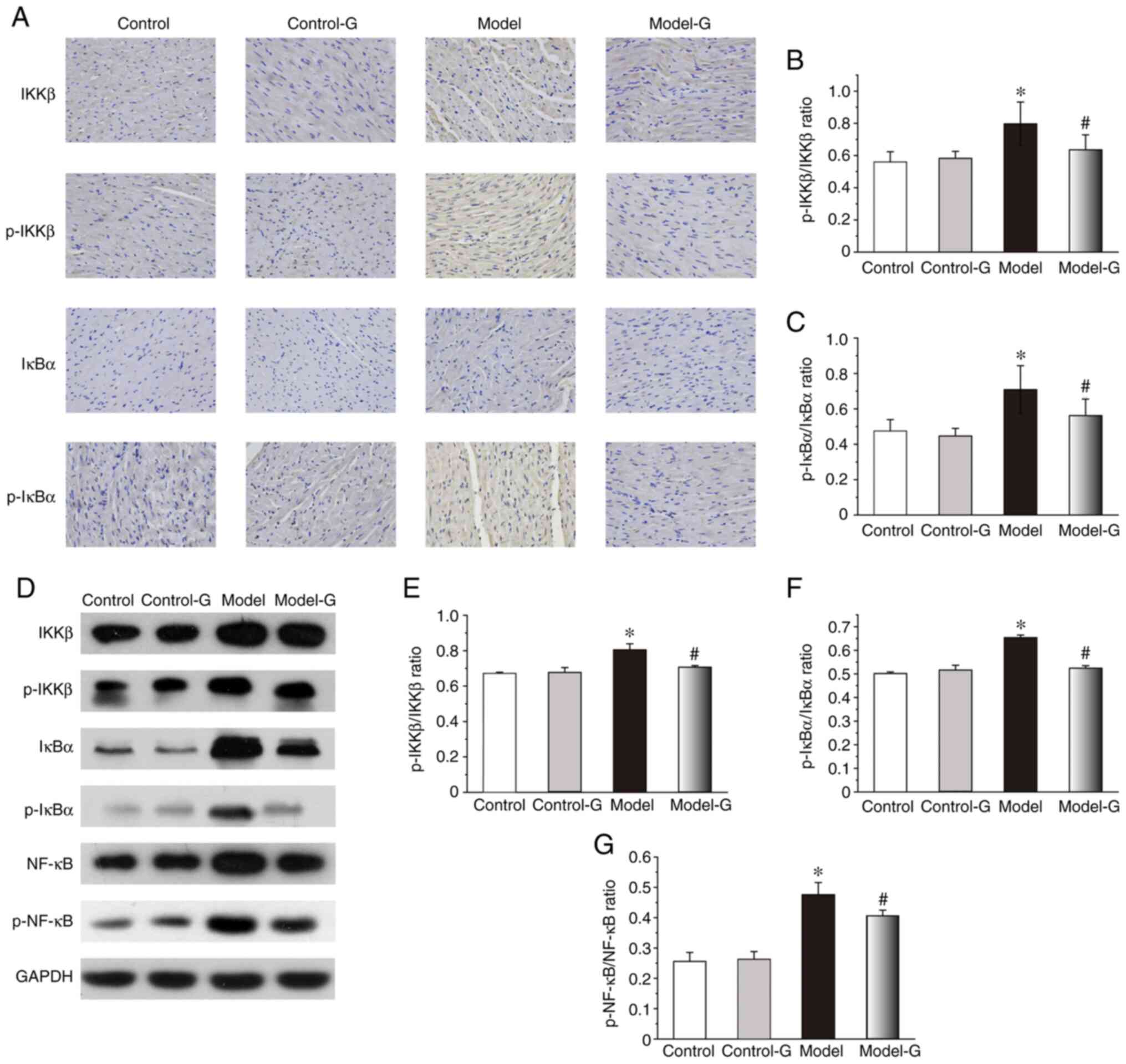 | Figure 5Effects of G. acuta on p-IKKβ,
IKKβ, p-IκBα, IκBα and p-NF-κB expression levels in the heart. (A)
Immunohistochemical staining for p-IKKβ, IKKβ, p-IκBα and IκBα in
the heart (magnification, x400). Heart tissues were obtained from
the Control, Control-G, Model and Model-G groups. (B) Expression of
p-IKKβ/IKKβ in the heart. (C) Expression of p-IκBα/IκBα in the
heart. (D) Typical western blot bands. (E) Expression of
p-IKKβ/IKKβ in the heart was quantified by densitometry. (F)
Expression of p-IκBα/IκBα in the heart was quantified by
densitometry. (G) Expression of p-NF-κB/NF-κB in the heart was
quantified by densitometry. Scale bar, 200 µm. Data are presented
as the mean ± SD. *P<0.05 vs. the Control group and
#P<0.05 vs. the Model group (Immunohistochemistry:
n=8 per group; western blot: n=3 per group). G. acuta,
Gentianella acuta; p-, phosphorylated; IKKβ, inhibitor of
NF-κB kinase β; IκBα, inhibitor of NF-κB α; NF-κB, nuclear factor
κB. |
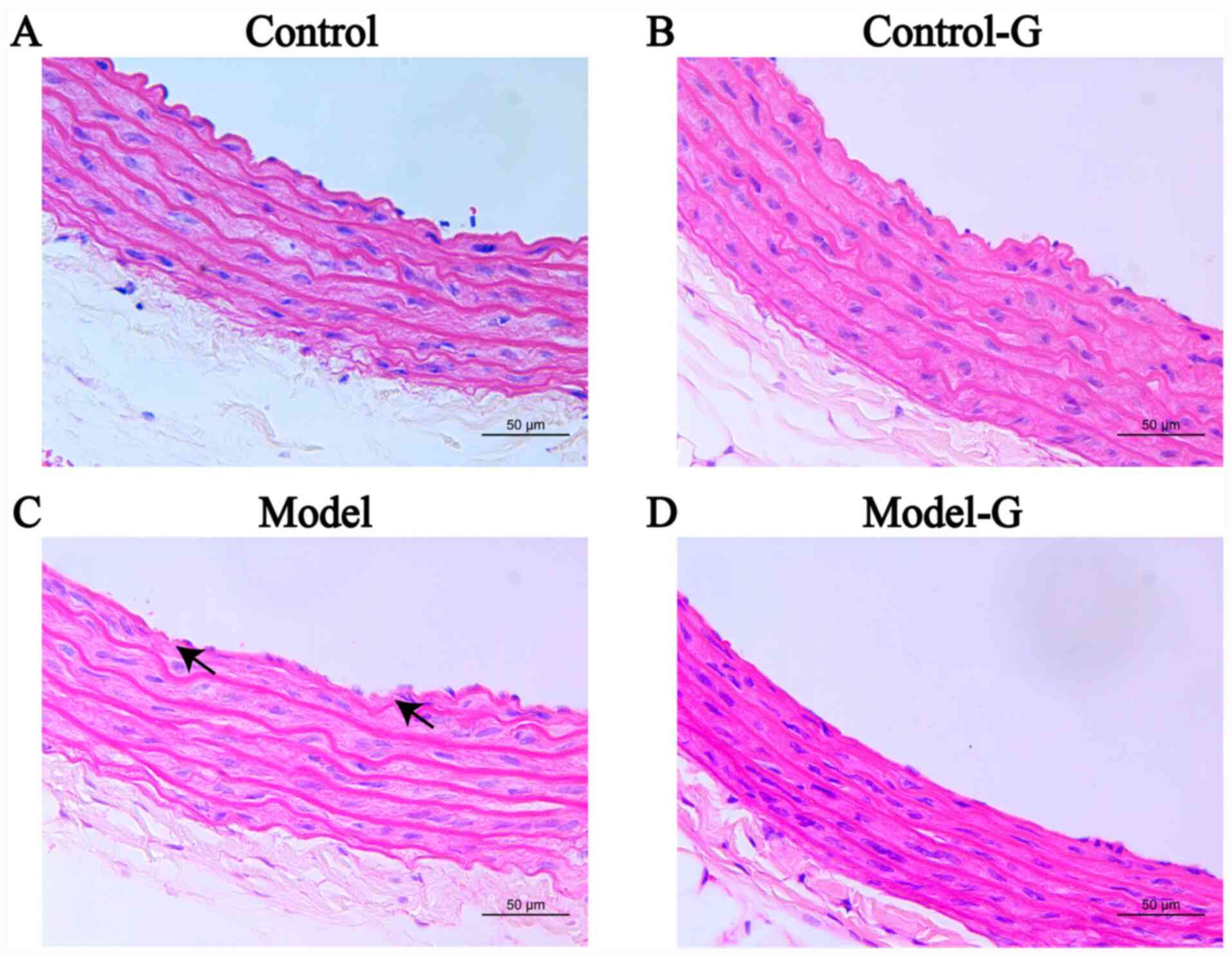
Effects of G. acuta on morphological
and histological changes in the thoracic aorta
The arterial intima in the Control group was
relatively smooth, with clear boundaries between the intima, media
and adventitia, and endothelial cell layer continuity. The intima
of the arteries of the Model group was uneven, and some endothelial
cells had lost their continuity. Intimal concavity was improved in
the Model-G group. The intima of the Control-G group was damaged
(Fig. 6).
Effect of G. acuta on IKK/IκB/NF-κB in
the thoracic aorta
The IKKβ, p-IKKβ and p-IκBα protein expression
levels of the Model group were significantly higher compared with
those in the other three groups. While the changes in the
expression of IκBα were not significant among the four groups, the
levels of p-IKKβ/IKKβ and p-IκBα/IκBα in the Model group were
higher than the other three groups (Fig. 7A-C). Compared with the Model group,
the levels of p-NF-κB/NF-κB in the Control and Model-G groups were
significantly decreased (Fig. 7D
and E).
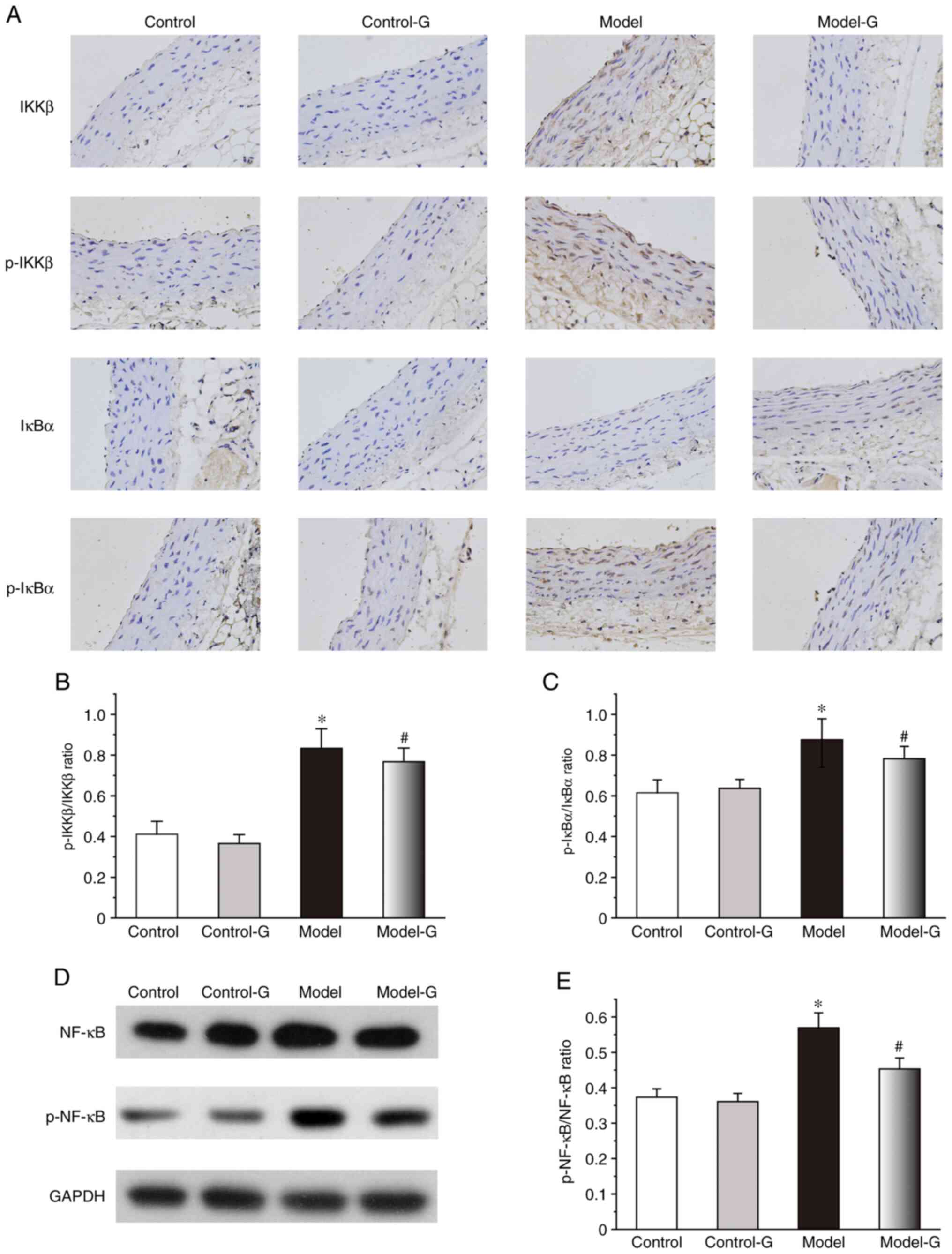 | Figure 7Effects of G. acuta treatment
on p-IKKβ, IKKβ, p-IκBα, IκBα and p-NF-κB expression levels in the
thoracic aorta. (A) Immunohistochemical staining for p-IKKβ, IKKβ,
p-IκBα and IκBα in the thoracic aorta (magnification, x400).
Thoracic aorta samples were obtained from the Control, Control-G,
Model and Model-G groups. (B) Expression of p-IKKβ/IKKβ in the
thoracic aorta. (C) Expression of p-IκBα/IκBα in the thoracic
aorta. (D) Typical western blot bands. (E) Expression of
p-NF-κB/NF-κB in the thoracic aorta was quantified by densitometry.
Scale bar, 200 µm. Data are presented as the mean ± SD.
*P<0.05 vs. the Control group; #P<0.05
vs. the Model group (Immunohistochemical: n=8 per group; western
blot: n=3 per group). G. acuta, Gentianella acuta;
p-, phosphorylated; IKKβ, inhibitor of NF-κB kinase β; IκBα,
inhibitor of NF-κB α; NF-κB, nuclear factor kappa-B. |
Discussion
Several studies have reported that
hypercholesterolaemia is not only a risk factor for AS development,
but also an important cause of the exacerbation of AS (27,28).
It has been revealed that G. acuta exerts a protective
effect against myocardial ischemia (19). On this basis, its anti-AS effects
and mechanism were studied herein. In the present study, a rat
model of hypercholesterolaemia was established using a high-fat
diet (29) to explore the effect
and mechanisms of G. acuta in mitigating cardiovascular
damage and inflammation.
Hypercholesterolaemia model rats exhibited increases
in serum lipids and inflammatory factors, aortic muscular layer
thickening and widespread myocardial structural disruption. These
histopathological changes in the body were important formative
indices of hypercholesterolaemia, with some beneficial changes
appearing in the Model-G group, such as improved aortic wall
structure and neatly arranged myocardial cells. Lipid deposition
has been identified as an important cause of AS, which can lead to
the increase of free radicals, thereby damaging endothelial cell
function (30-32).
Thus, the release of protective factors is reduced, leading to a
reduction in the tightness of endothelial cells and increased
permeability, which in turn results in increased lipid deposition,
forming a vicious circle (33). As
a consequence of a continuous high-fat diet, LDL is elevated and
deposited in the endothelial cells of the arteries in which it is
oxidized to ox-LDL, which can cause necrosis and disintegration of
macrophages, release of lipids from atheromatous necrosis and
plaque formation (34). When
comparing the Model-G and the Model groups, it was revealed that
G. acuta could effectively reduce the serum lipid level with
further increasing the level of HDL.
A change in TNF-α and IL-10 levels in the serum of
hypercholesterolaemic rats was also observed. TNF-α and IL-10 are
important inflammatory factors leading to AS. TNF-α has been
reported to promote the production of various inflammatory
cytokines through T cells and has been identified as an important
indicator of inflammation (35).
Conversely, IL-10 has been reported to inhibit mononuclear
macrophages from performing specific immune functions, such as the
release of inflammatory mediators (36). Compared with the Control group, the
levels of TNF-α were significantly increased, and those of IL-10
were decreased in the Model group. Following treatment with G.
acuta, TNF-α and IL-10 levels were significantly altered in the
Model group. These results demonstrated that the inflammatory
response induced by the high-fat diet was inhibited by G.
acuta.
CK and LDH have been revealed to be important
indices reflecting functional heart status (37). Compared with the control group, the
levels of CK and LDH in the blood vessels of the Model group were
significantly decreased, indicating the protective effect of G.
acuta in the heart. It was also revealed by H&E staining
that the arrangement of cardiomyocytes in the Model-G group was
improved and appeared orderly. These results further demonstrated
that G. acuta effectively alleviated cardiovascular damage
and inflammation in diet-induced hypercholesterolaemic rats.
NF-κB has been identified as an important nuclear
factor that controls inflammatory cytokines and is normally bound
to IκB in the cytoplasm. After NF-κB has been activated and
translocated to the nucleus, several downstream
inflammation-related factors, such as TNF-α and IL-6, promote its
synthesis and release. Such factors are important causes of the
occurrence and deterioration of AS (38-40).
As a pattern recognition receptor, activated IKK is a major
upstream target for NF-κB regulation. Multiple members of the IKK
family, such as IKKα and IKKβ, have important regulatory effects on
the activity of NF-κB. Both of these have been revealed to
phosphorylate the IκB protein at different serine residues, while
the main function has been assumed by IKKβ (41). Thus, IKKβ is an important indicator
of NF-κB activation. When G. acuta was administered, the
expression of p-IKKβ/IKKβ and p-IκBα/IκBα both in the heart and
thoracic aorta in the Model-G group were significantly decreased.
Compared with the Model group, the phosphorylation ratio of IKKβ,
IκBα, NF-κB in the heart and NF-κB in thoracic aorta was decreased
in the Model-G group. In addition, G. acuta significantly
decreased the expression levels of IKKβ, p-IKKβ and p-IκBα in
endothelial cells of the thoracic aorta, indicating its protective
role. These results demonstrated that the anti-inflammatory effect
of G. acuta may be mediated by inhibiting the
IKKβ/IκBα/NF-κB pathway in the heart as well as the thoracic
aorta.
In conclusion, G. acuta mitigated
cardiovascular damage and inflammation in diet-induced
hypercholesterolaemic rats, possibly through the inhibition of the
IKK-β/IκB/NF-κB pathway. Thus, G. acuta may prove useful in
the treatment of hypercholesterolaemia.
A limitation of the present study was that it lacked
direct assessment of physiological parameters and
immunohistochemical analysis could reveal the expression levels but
was weaker in protein comparison than western blotting in thoracic
aorta. In addition, G. acuta water extract was selected, but
water extract is comprised of numerous components and these were
not fractionated and explored individually.
Acknowledgements
The authors would like to thank Professor Gang Cao
and Professor Huazhou Xu (School of Basic Medicine, Hebei
University of Chinese Medicine, Shijiazhuang, China) for technical
assistance.
Funding
Funding: The present was supported by the National Natural
Science Foundation of China (grant no. 81573698), the innovative
research program for postgraduate of Hebei University of Chinese
Medicine (grant no. XCXZZSS2021003) and the 333 Talent Project of
Hebei (grant no. 2020-7).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DM and ZM conceived the project and designed the
experiments. AL and SG carried out the experiments. MS, MW and YH
established the rat model of hypercholesterolaemia and carried out
the experiments. YH conducted the statistical analysis. MS and MW
wrote the manuscript and confirmed the authenticity of the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Hebei University of Chinese
Medicine (Shijiazhuang, China) approved and supervised the present
study (approval no. DWLL2018016). The animal treatments in this
study were in compliance with the Laboratory Animal Management of
National Animal Science and Technology Commission's Regulations
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Annual report on cardiovascular health and
diseases in China 2019. Journal of Cardiovascular & Pulmonary
Diseases. 39:1145–1156. 2020.(in Chinese).
|
|
2
|
Libby P: The molecular mechanisms of the
thrombotic complications of atherosclerosis. J Intern Med.
263:517–527. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao X, Zhu J, Wang L, Li Y, Zhao T, Chen
X, Sun Y, Dai Y, Wei G, Altamirano A, et al: U. diffracta extract
mitigates high-fat diet and VD3-induced atherosclerosis and
biochemical changes in the serum liver and aorta of rats. Biomed
Pharmacother. 120(109446)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ding L, Gu H, Lan Z, Lei Q, Wang W, Ruan
J, Yu M, Lin J and Cui Q: Downregulation of cyclooxygenase1
stimulates mitochondrial apoptosis through the NF-κB signaling
pathway in colorectal cancer cells. Oncol Rep. 41:559–569.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Akhtar M, Guo S, Guo YF, Zahoor A, Shaukat
A, Chen Y, Umar T, Deng PG and Guo M: Upregulated-gene expression
of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs
following NF-κB and MAPKs in bovine mastitis. Acta Trop.
207(105458)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rezagholizadeh L, Pourfarjam Y, Nowrouzi
A, Nakhjavani M, Meysamie A, Ziamajidi N and Nowrouzi PS: Effect of
Cichorium intybus L. on the expression of hepatic NF-κB and IKKβ
and serum TNF-α in STZ- and STZ+
niacinamide-induced diabetes in rats. Diabetol Metab Syndr.
8(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moallemian R, Rehman AU, Zhao N, Wang H,
Chen H, Lin G, Ma X and Yu J: Immunoproteasome inhibitor DPLG3
attenuates experimental colitis by restraining NF-κB activation.
Biochem Pharmacol. 177(113964)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang CH, Liu XM, Si JJ, Shi HS, Xue YX,
Liu JF, Luo YX, Chen C, Li P, Yang JL, et al: Role of IKK/NF-κB
signaling in extinction of conditioned place aversion memory in
rats. PLoS One. 7(e39696)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu C, Yang Y, Ou J, Zhu L, Zhao W and Cui
J: LRRC14 attenuates toll-like receptor-mediated NF-κB signaling
through disruption of IKK complex. Exp Cell Res. 347:65–73.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leibowitz SM and Yan J: NF-κB pathways in
the pathogenesis of multiple sclerosis and the therapeutic
implications. Front Mol Neurosci. 9(84)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Y, Song F, Li Y, Li J, Cui Y, Hong Y,
Han W, Wu W, Lakhani I, Li G and Wang Y: Acacetin exerts
antioxidant potential against atherosclerosis through Nrf2 pathway
in apoE(-/-) mice. J Cell Mol Med. 25:521–534. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang F, Feng J, Zhang J, Kang X and Qian
D: Quercetin modulates AMPK/SIRT1/NF-kappaB signaling to inhibit
inflammatory/oxidative stress responses in diabetic high fat
diet-induced atherosclerosis in the rat carotid artery. Exp Ther
Med. 20(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu S, Zhang J and Lv Y: Glaucocalyxin A
inhibits hydrogen peroxide-induced oxidative stress and
inflammatory response in coronary artery smooth muscle cells. Clin
Exp Pharmacol Physiol. 47:765–770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Ni Y, Ruan J, Qu L, Yu H, Han L,
Zhang Y and Wang T: Bioactive gentixanthone and gentichromone from
the whole plants of Gentianella acuta (Michx.) Hulten. Fitoterapia.
113:164–169. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wunir Chunliang Khasbagan: Ewenki folk
medicinal plants and its comparison with mongolian medicine. Chin J
Ethnomed Ethnopharm. 17:156–158. 2009.(in Chinese).
|
|
16
|
Ding Z, Liu Y, Ruan J, Yang S, Yu H, Chen
M, Zhang Y and Wang T: Bioactive constituents from the whole plants
of Gentianella acuta (Michx.) Hulten. Molecules.
22(1309)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Z, Wu Q, Yu Y, Yang C, Jiang H, Wang
Q, Yang B and Kuang H: Determination and pharmacokinetic study of
four xanthones in rat plasma after oral administration of
Gentianella acuta extract by UHPLC-ESI-MS/MS. J Ethnopharmacol.
174:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li AY, Wang JJ, Yang SC, Zhao YS, Li JR,
Liu Y, Sun JH, An LP, Guan P and Ji ES: Protective role of
Gentianella acuta on isoprenaline induced myocardial fibrosis in
rats via inhibition of N-κB pathway. Biomed Pharmacother.
110:733–741. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jindarat S: Xanthones from mangosteen
(Garcinia mangostana): Multi-targeting pharmacological
properties. J Med Assoc Thai. 97 (Suppl 2):S196–S201.
2014.PubMed/NCBI
|
|
20
|
Tantapakul C, Maneerat W, Sripisut T,
Ritthiwigrom T, Andersen RJ, Cheng P, Cheenpracha S, Raksat A and
Laphookhieo S: New benzophenones and xanthones from Cratoxylum
sumatranum ssp. neriifolium and their antibacterial and antioxidant
activities. J Agric Food Chem. 64:8755–8762. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Z, Wu G, Yu Y, Liu H, Yang B, Kuang H
and Wang Q: Xanthones isolated from Gentianella acuta and their
protective effects against H2O2-induced myocardial cell injury. Nat
Prod Res. 32:2171–2177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang HX, Xu GR, Zhang C, Sun JH, Zhang Y,
Song JN, Li YF, Liu Y and Li AY: The aqueous extract of Gentianella
acuta improves isoproterenol-induced myocardial fibrosis via
inhibition of the TGF-β1/Smads signaling pathway. Int J Mol Med.
45:223–233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ren K, Su H, Lv LJ, Yi LT, Gong X, Dang
LS, Zhang RF and Li MH: Effects of four compounds from Gentianella
acuta (Michx.) Hulten on hydrogen peroxide-induced injury in H9c2
Cells. Biomed Res Int. 20(2692970)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koo YE, Song J and Bae S: Use of plant and
herb derived medicine for therapeutic usage in cardiology.
Medicines (Basel). 22(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guedes L, Reis P, Machuqueiro M, Ressaissi
A, Pacheco R and Serralheiro ML: Bioactivities of centaurium
erythraea (Gentianaceae) decoctions: Antioxidant activity, enzyme
inhibition and docking studies. Molecules. 24(3795)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Z, Wu G, Liu H, Xing N, Sun Y, Zhai
Y, Yang B, Kong AT, Kuang H and Wang Q: Cardioprotective effect of
the xanthones from Gentianella acuta against myocardial
ischemia/reperfusion injury in isolated rat heart. Biomed
Pharmacother. 93:626–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y and Zhang Y: Pterostilbene, a
novel natural plant conduct, inhibits high-fat-induced
atherosclerosis inflammation via NF-κB signaling pathway in
toll-like receptor 5 (TLR5) deficient mice. Biomed Pharmacother.
81:345–355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kumar SU, Kumar DT, Bithia R, Sankar S,
Magesh R, Sidenna M, Doss CG and Zayed H: Analysis of
differentially expressed genes and molecular pathways in familial
hypercholesterolemia involved in atherosclerosis: A systematic and
bioinformatics approach. Front Genet. 11(734)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tan CX, Chong GH, Hamzah H and Ghazali HM:
Effect of virgin avocado oil on diet-induced hypercholesterolemia
in rats via1 H NMR-based metabolomics approach.
Phytother Res. 32:2264–2274. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Dettlaff-Pokora A, Sucajtys-Szulc E and
Sledzinski T: Up-regulation of PCSK9 gene expression and diminished
level of LDL-receptor in rat liver as a potential cause of
post-lipectomy hypercholesterolemia. Mol Cell Biochem. 455:207–217.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Adedayo MR, Akintunde JK, Sani A and
Boligon AA: Effect of dietary supplement from mono-culture
fermentation of Moringa oleifera seeds by Rhizopus stolonifer on
hematology and markers linked to hypercholesterolemia in rat model.
Food Sci Nutr. 6:1826–1838. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Wang X, Hasegawa J, Kitamura Y, Wang Z,
Matsuda A, Shinoda W, Miura N and Kimura K: Effects of hesperidin
on the progression of hypercholesterolemia and fatty liver induced
by high-cholesterol diet in rats. J Pharmacol Sci. 117:129–138.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu Y, Gao J, Gong Y, Chen M, Chen J, Zhao
W and Tan S: Hsa-miR-140-5p down-regulates LDL receptor and
attenuates LDL-C uptake in human hepatocytes. Atherosclerosis.
297:111–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Klein-Szanto AJP and Bassi DE: Keep
recycling going: New approaches to reduce LDL-C. Biochem Pharmacol.
164:336–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Leko MB, Perkovic MN, Klepac N, Strac DS,
Borovecki F, Pivac N, Hof PR and Simic G: IL-1β, IL-6, IL-10, and
TNF-α single nucleotide polymorphisms in human influence the
susceptibility to Alzheimer's disease pathology. J Alzheimers Dis.
75:1029–1047. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qin J, Zhao N, Wang S, Liu S, Liu Y, Cui
X, Wang S, Xiang Y, Fan C, Li Y, et al: Roles of endogenous IL-10
and IL-10-competent and CD5+ B cells in autoimmune
thyroiditis in NOD.H-2h4 mice. Endocrinology. 161(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saliu JA, Oyeleye SI, Olasehinde TA and
Oboh G: Modulatory effects of stonebreaker (Phyllanthus
amarus) and bitter gourd (Momordica charantia) on
enzymes linked with cardiac function in heart tissue of
doxorubicin-stressed rats. Drug Chem Toxicol. 11:1–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xian H, Feng W and Zhang J: Schizandrin A
enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB
signaling in non-small cell lung cancer. Eur J Pharmacol.
855:10–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li T, Chen RR, Gong HP, Wang BF, Wu XX,
Chen YQ and Huang ZM: FGL2 regulates IKK/NF-κB signaling in
intestinal epithelial cells and lamina propria dendritic cells to
attenuate dextran sulfate sodium-induced colitis. Mol Immunol.
117:84–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yan C, Li B, Liu X, Deng C, Cai R, Shen Y
and Tang H: Involvement of multiple transcription factors in
regulation of IL-β-induced MCP-1 expression in alveolar type II
epithelial cells. Mol Immunol. 111:95–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shin JS, Im HT and Lee KT: Saikosaponin B2
suppresses inflammatory responses through IKK/I-κBα/NF-κB signaling
inactivation in lps-induced RAW 264.7 macrophages. Inflammation.
42:342–353. 2019.PubMed/NCBI View Article : Google Scholar
|