Introduction
Rheumatoid arthritis (RA) is an immune-mediated
systemic inflammatory disease of unknown etiology, characterized by
polyarticular synovial inflammation and bone erosion (1). Besides arthritis symptoms and other
complications, 17-60% of patients with RA experience rheumatoid
cachexia (2). Cachexia is a serious
complication of RA and other chronic illnesses, such as diabetes,
cancer and heart failure (3).
However, RA-related musculoskeletal alterations are frequently
overlooked, despite their contributions to the physical disability
and reduced mobility and quality of life of patients (4). Arthritis and rheumatoid cachexia may
be modeled in collagen-induced arthritis (CIA) mouse models, which
provides the possibility of studying the disease (5).
Although the outcomes of numerous patients with RA
have been significantly improved by the wide use of conventional
synthetic disease-modifying antirheumatic drugs (DMARDs),
biological DMARDs and targeted synthetic DMARDs, a considerable
proportion of patients with RA remain in non-remission or
experience side effects from the drugs, which is mainly due to the
incomplete understanding of the etiology and drug intolerance
(6). Thus, advancing the
understanding of RA to identify novel therapeutic strategies is
critical for improving the efficacy of current treatments.
Stem cell-based therapy has emerged as a promising
approach to treat arthritic diseases. It was previously reported
that transplantation of adult stem cells, such as bone marrow stem
cells, adipose mesenchymal stem cells (MSCs) or umbilical
cord-derived MSCs (UC-MSCs) into the blood was able to ameliorate
synovitis and bone erosion in animal models of arthritis (7). Accumulating evidence has indicated the
therapeutic potential of UC-MSCs in animal models of arthritis and
clinical trials (8). These effects
are systemic and integrated, suggesting that UC-MSCs may be a
preferred resource to systematically improve synovitis and bone
erosion. Compared with other types of stem cell, UC-MSCs are able
to secrete a wide range of multifunctional factors and have been
demonstrated to possess systemic immunoregulatory properties
(9). Therefore, UC-MSCs may be
considered more promising for clinical application compared with
other types of stem cell.
However, there are certain emerging limitations that
cannot be ignored. For instance, the therapeutic doses and
frequencies remain critical issues that require to be determined
(10,11). Furthermore, whether frequent
injections of high-dose UC-MSCs cause enhanced efficacy or
aggravated adverse effects has remained elusive. In addition,
muscle atrophy in patients with RA has received little attention in
previous studies. Whether doses and frequencies of UC-MSC
injections that differ from those applied previously are a more
favorable choice for treating arthritis and cachexia also requires
further research. Therefore, the present study aimed to investigate
the effects of UC-MSCs on the joints and muscles of mice with CIA
when administered frequently at high doses, hoping that the results
provide novel insight into the therapeutic efficacy of UC-MSCs in
RA-related muscle cachexia.
Materials and methods
Preparation of UC-MSCs
The UC was obtained from a 25-year-old healthy
mother from March 28, 2019 at Henan Provincial People's Hospital
(Zhengzhou, China) after cesarean deliveries. Written informed
consent was obtained from the donor several weeks prior to
delivery. The UC-MSCs were cultured, purified and identified as
previously described (12). The
viability of UC-MSCs digested by 0.2% Type I collagenase (cat. no.
17100017; Gibco; Thermo Fisher Scientific, Inc.) was >95% (as
assessed via trypan blue staining) and all cultures were negative
for pathogenic microorganisms, including bacteria (on blood agar
plates), fungi (in Sabouraud dextrose medium), endotoxins (as
detected using a horseshoe crab agent) and mycoplasma [as
determined via reverse transcription-PCR (RT-PCR)] (13). Flow cytometric analysis identified
the presence of CD73, CD90 and CD105 (>95% of cells) and the
absence of CD34, CD11b, CD19, CD45 and human leukocyte antigen-DR
using a Human MSC Analysis kit (BD Biosciences; cat. no. 562245)
(<2% of cells) on the cell surface (14) (Fig.
S1). All procedures for isolating, culturing and harvesting
UC-MSCs were performed under good manufacturing practice
conditions. All UC-MSCs used for transfusion were from passage
3.
Mice and CIA model
The experiments were performed on 34 male DBA/1 mice
(weight, 15-18 g; age, 10 weeks; Beijing Vital River Laboratory
Animal Technology). All mice, with 4 mice housed per cage, were
maintained in a specific pathogen-free environment on a standard
chow and water with a 12 h dark-light cycles at 20±2˚C and 55±10%
humidity. The mice were randomly divided into three groups: Normal
group (n=11), CIA group (n=12) and a CIA group treated with UC-MSCs
(n=11; dose, 5x106 per mouse every week for 3 weeks).
CIA mice were established as described previously (15,16).
In brief, equal volumes of bovine type II collagen (Chondrex, Inc.)
and complete Freund's adjuvant (4 mg/ml; Chondrex, Inc.) were mixed
to form an emulsion. On day 0, 0.1 ml emulsion was injected
intradermally at a site 1.5 cm distal to the base of the tail. On
day 21, the mice received an identical dose of type II collagen
mixed with an equal volume of normal saline injected
intraperitoneally. Normal mice were injected with 0.1 ml normal
saline on both above-mentioned occasions. The study protocol was
approved by the Animal Ethics Committee of Peking University
(Beijing, China; approval no. J201985). All experiments were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Experimental procedures
Locomotor function was assessed via an open field
test (OFT) in mice with CIA when the arthritis clinical score
reached 3(15) at days 28. Then,
mice were assigned to the UC-MSC group received 5x106
UC-MSCs on days 28, 35 and 42(11),
which were injected via the tail vein. No animals died unexpectedly
prior to the end of this experiment. Body weight, food intake
(measured as the weight of the remaining food pellets subtracted
from the initial food weight), arthritis clinical score and hind
ankle diameters measured using a caliper were evaluated each week
(15). On day 84(11), the mice were anesthetized via
intraperitoneal injection of pentobarbital sodium at a
concentration of 50 mg/kg and were sacrificed by cervical
dislocation. The left gastrocnemius muscle and the knee joints of
each mouse were dissected for histopathological analyses and the
gastrocnemius muscle on the right side was stored at -196˚C in
liquid nitrogen for RT-quantitative (q)PCR.
Open Field Test (OFT)
The mice were first familiarized with the square
testing arena (50x50x35 cm) in a quiet, dimly lit room for 6 min (1
min for adapting to the environment and 5 min for the OFT). On days
0 and 28, the mice were placed in the open field arena for 5 min
and spontaneous locomotor activities were recorded using a video
analysis system of OFT (BW-OF302, Shanghai Ranlong Technology
Development Co., Ltd.) mounted on top of the arena for measurements
of the total time spent active and immobile, the total movement
distance and the mean velocity of motion (17).
Histology and scoring of
arthritis
The knee joints from the hind limbs were excised and
fixed in 10% buffered formalin for 24 h at room temperature.
Subsequently, the samples were decalcified in 10% EDTA for 21 days
at room temperature and embedded in paraffin. Slides with a
thickness of 4 µm were dewaxed with 100% xylene and then rehydrated
with anhydrous, 95 and 70% ethanol and H2O in sequence.
The slices were then stained with haematoxylin for 5 min and eosin
for 1 min and then dehydrated at room temperature. All HE-stained
slides were captured and analyzed using an Olympus BX53 light
microscope (Olympus Corporation) at 20x magnification. Synovial
inflammation was scored by two independent observers (LA and LW)
according to the percentage area exhibiting synovitis in five
high-power magnification fields per animal: 0, 0% (absent); 1,
1-10% (mild); 2, 11-50% (moderate); and 3, 51-100% (severe).
Cartilage erosion was scored according to the percentage of the
cartilage surface that was eroded: 0, 0% (absent); 1, 1-10% (mild);
2, 11-50% (moderate); and 3, 51-100% (severe). The absence of bone
erosion was scored as 0, minor erosion(s) observed only at high
magnification was scored as 1, moderate erosion(s) observed at low
magnification was scored as 2 and severe transcortical erosion(s)
was scored as 3(18).
The gastrocnemius muscles from the hind limbs were
excised and placed in a fixative solution (Wuhan Servicebio
Technology Co., Ltd.) for 24 h before paraffin embedding, cut into
5 µm sections and stained with HE at room temperature.
Myofiber cross-sectional area
Myofiber cross-sectional areas were calculated as
described previously (18). In
brief, fibers were measured in two randomly selected images of the
gastrocnemius muscle from a series of 10 images per mouse, totaling
100-200 measured myofibers. The cross-sectional area of the
gastrocnemius fibers was calculated using ImageJ software (version
1.8.0; National Institutes of Health) (19).
Toluidine blue staining
Toluidine Blue O Cartilage Stain solution containing
an amine group and a quinone type benzene ring as chromogenic
agents (cat. no. G2543; Beijing Solarbio Science & Technology
Co., Ltd.) was added to cartilage samples for 15 min at room
temperature. Acetone (1,000 ml) was then applied to differentiate
chondrocytes for 5 min at room temperature.
Immunohistochemical staining
Gastrocnemius muscle tissues were fixed in muscle
fixation fluid (cat. no. G1111; Wuhan Servicebio Technology Co.,
Ltd.) at room temperature for 24 h, subsequently dehydrated and
thereafter embedded in paraffin. Tissue sections (4 µm) were
deparaffinized according to the above-mentioned methods and
incubated with 3% H2O2 at 37˚C for 10 min.
Antigen retrieval was performed by boiling in 0.01 M sodium citrate
at 95˚C for 15 min. After cooling for 2 h, the sections were
blocked at room temperature for 1 h using 10% goat serum (z
sbio.com, SAP-9102). The slides were then incubated at
4˚C overnight with antibodies against CD3 (cat. no. GB13014; 1:100
dilution), CD4 (cat. no. GB11064; 1:500 dilution), CD19 (cat. no.
GB11061-1; 1:300 dilution), F4/80 (cat. nos. GB11061-1 and GB11027;
1:1,500 dilution) and inducible nitric oxide synthase (iNOS; cat.
no. GB11119; 1:2,000 dilution). Subsequently, the sections were
incubated with biotinylated goat anti-rabbit horseradish peroxidase
IgG secondary antibody (cat. no. G1213-100UL; 1:1,000 dilution) at
37˚C for 30 min. All antibodies were from Wuhan Servicebio
Technology Co., Ltd. Diaminobenzidine was used as chromogenic
agent. Following washing, the sections were counterstained with
hematoxylin, dehydrated with ethanol (70, 85, 95 and 100%) and
xylene and then sealed with neutral resin. Images were obtained
using a light microscope (magnification, x400; Olympus
Corporation).
RT-qPCR
Total RNA was isolated from gastrocnemius muscle
using TRIzol® (Takara Bio, Inc.) and cDNA was
synthesized at 37˚C for 15 min and 85˚C for 5 sec using reverse
transcriptase (PrimeScript RTase, RNase Inhibitor, Random 6 mers,
Oligo dT Primer, dNTP Mixture, Buffer; cat. no. RR036A; Takara Bio,
Inc.). The expression levels of genes encoding IL-6, IL-1β, TNF-α,
iNOS, IL-10, α-smooth muscle actin (α-SMA), fibronectin 1 (FN1),
collagen type I α 1 chain (COLIa1), COLIa2, COLIIIa1 and TGF-β1
were assessed via RT-qPCR using SYBR green mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the primers are listed in
Table SI. The thermocycling
conditions were as follows: Initial denaturation at 50˚C for 2 min
and 95˚C for 2 min, followed by 40 cycles of 95˚C for 15 sec, 60˚C
for 15 sec and 72˚C for 1 min. Data were collected and
quantitatively analysed on an ABI Prism 7900 sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Finally, relative gene expression values were calculated using the
2-∆∆Cq method (20). All
quantities are expressed as fold changes relative to the expression
of β-actin. The RT-qPCR reactions were performed in triplicate.
Statistical analysis
Values are expressed as the mean ± standard
deviation. The data were compared using the Mann-Whitney U-test,
unpaired Student's t-test or one-way and mixed ANOVA followed by
Bonferroni's post-hoc test. Data were analysed using GraphPad Prism
version 6.0 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Locomotor deficits at day 28 of
CIA
Baseline locomotion, including total active time,
total immobility time, total movement distance and mean motion
velocity, did not significantly differ between mice assigned to the
normal group and those assigned to the model group (Fig. S2A). A total of 28 days after the
first induction of CIA, locomotion was again assessed via the OFT.
As expected, mice with CIA traveled shorter distances in the 5-min
assessment but had a significantly higher total active time,
resulting in a lower mean motion velocity compared with that of
mice in the normal group (Fig.
S2B). However, there were no locomotor differences between mice
in the CIA group and those assigned to the UC-MSC treatment group
(Fig. S2C-E).
Clinical arthritis and ankle thickness
are unaffected by UC-MSC treatment
UC-MSCs are a potent treatment modality with
beneficial effects in arthritis models and human patients with RA
(7,8). However, in the CIA model of the
present study (Fig. S3A),
treatment with a total of 15x106 UC-MSCs did not
significantly affect the ankle thickness or arthritis scores
compared with those of untreated mice with CIA (Fig. S3B-D).
Knee joint arthritis histopathology is
unaffected by UC-MSC treatment
Joint inflammation and cartilage damage in the CIA
model were examined via HE and toluidine blue staining,
respectively. It was indicated that CIA resulted in synovitis and
bone erosion (as detected by HE staining), as well as articular
cartilage and proteoglycan loss (as indicated by toluidine blue
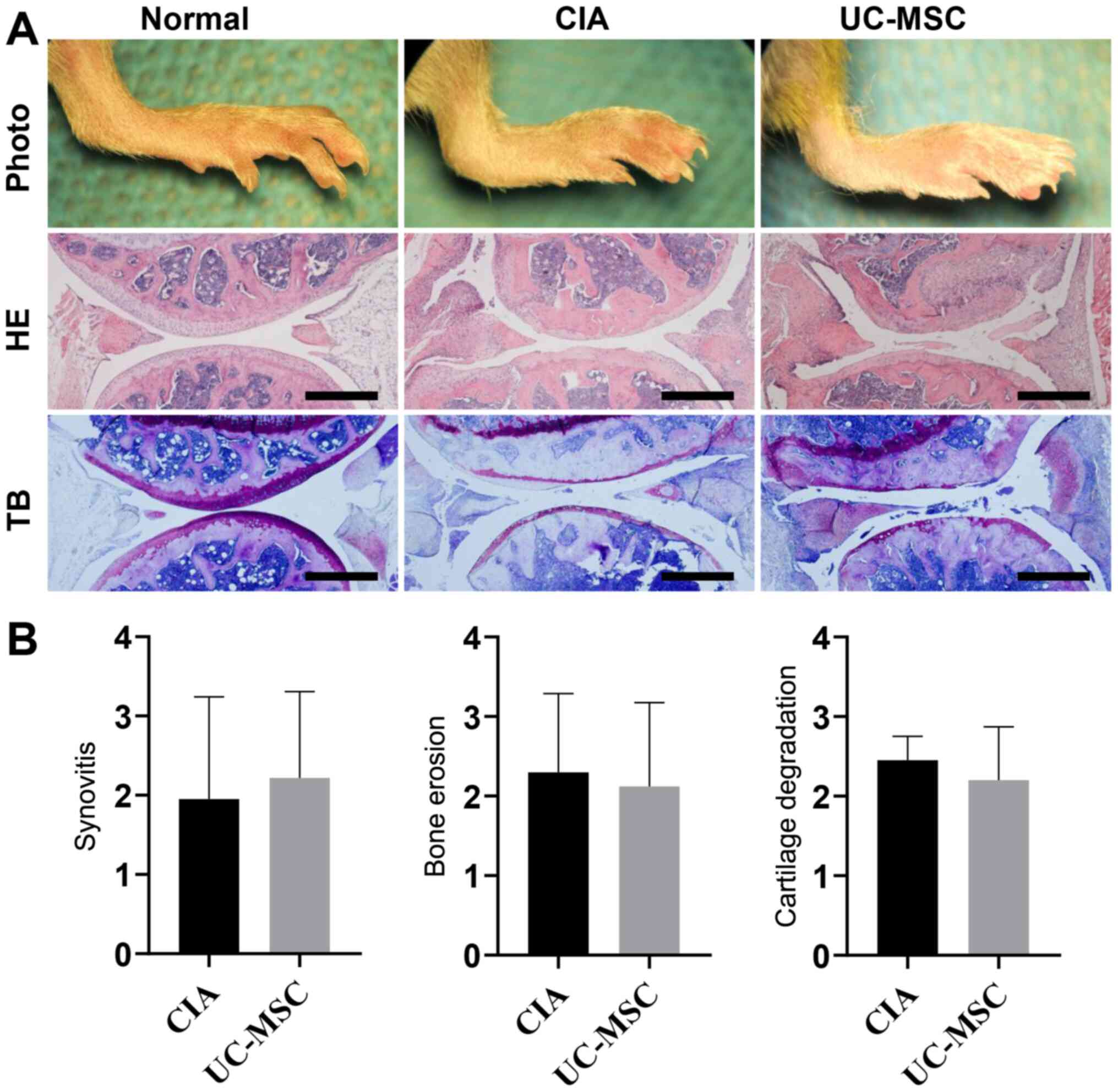
staining) in comparison with the normal group (Fig. 1A). However, the results in the group
receiving UC-MSC treatment did not differ from the untreated CIA
group. Furthermore, the histology was consistent with the observed
increase in clinical arthritis scores and left/right paw swelling
in mice with CIA, regardless of UC-MSC treatment. In addition,
UC-MSC treatment did not decrease synovitis, bone erosion or
cartilage degradation in mice with CIA (Fig. 1B).
Rheumatoid cachexia is unaffected by
UC-MSC treatment
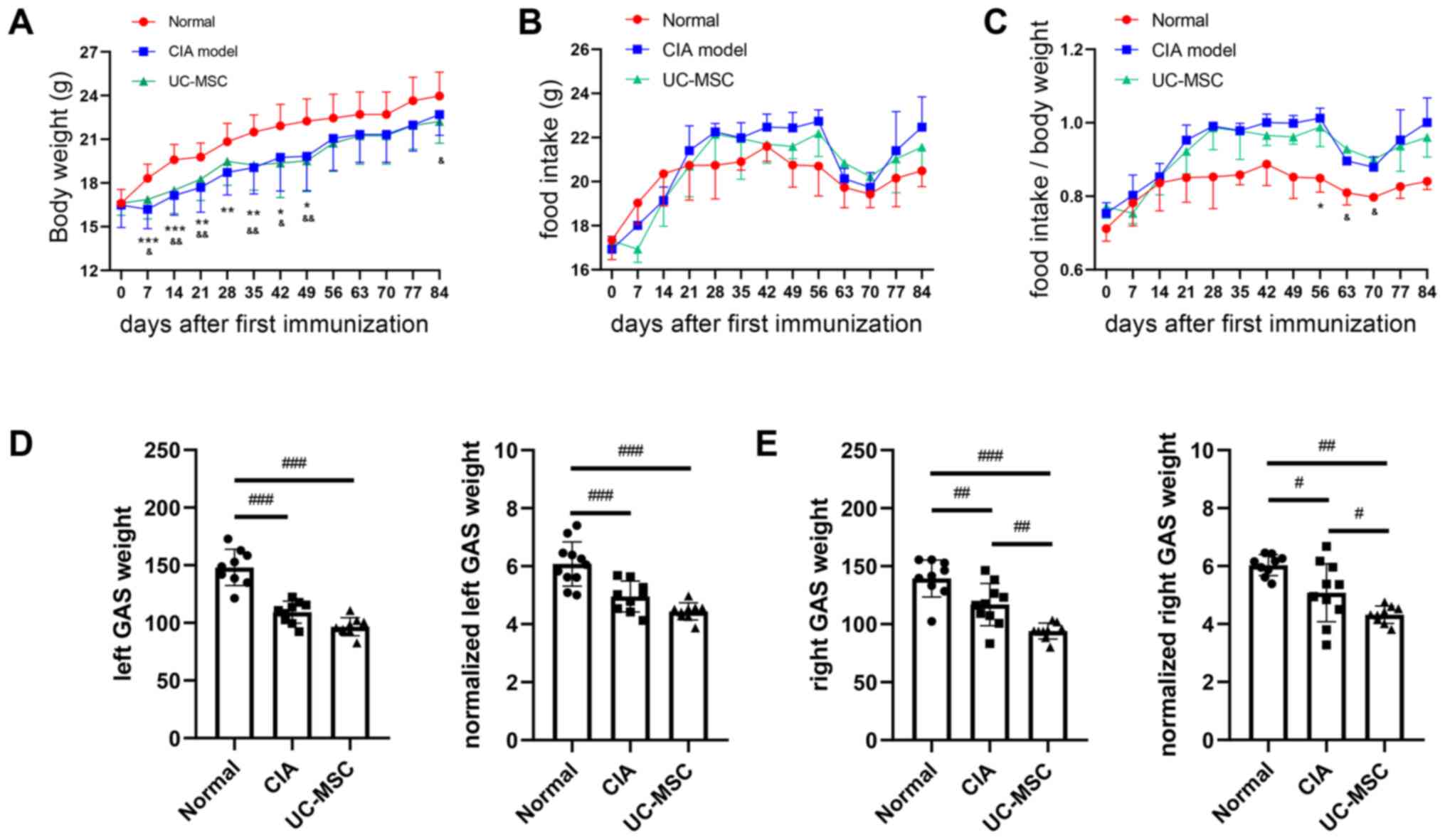
Mice with CIA exhibited a significant decrease in
body weight at various points after the first immunization.
However, there was no significant difference between the CIA group
and the UC-MSC group (Fig. 2A).
Although food intake did not differ among the groups (Fig. 2B), the normalized food intake (food
intake/body weight) was higher in mice with CIA from day 42 onwards
(there was no significance on day 77) (Fig. 2C). After the mice were sacrificed on
day 84, the gastrocnemius muscle weight and normalized
gastrocnemius muscle weight (ratio of muscle weight to body weight)
in the UC-MSC group were significantly lower compared with those in
the CIA group, and a similar if insignificant trend was observed on
the left (Fig. 2D and E).
Muscle fiber cross-sectional area is
unaffected by UC-MSC treatment
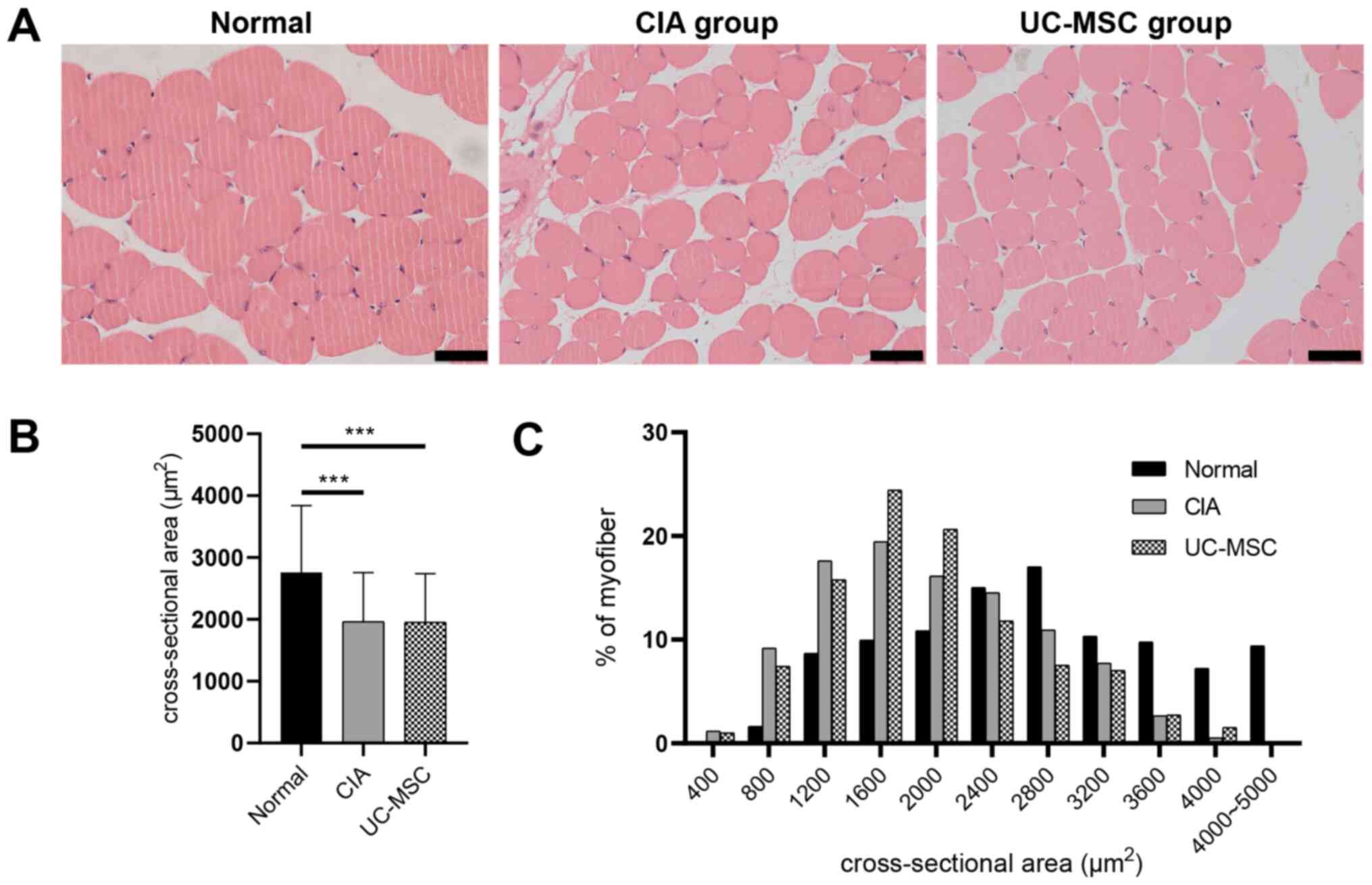
Histological analysis of cross-sections of
gastrocnemius muscles with HE staining revealed additional
pathological features of CIA (Fig.
3A). The cross-sectional area of gastrocnemius muscle fibers
was significantly decreased in mice with CIA, as well as in those
treated with UC-MSCs (Fig. 3B). Of
note, mice with CIA that were treated with frequent high doses of
UC-MSCs did not exhibit any improved muscle fiber areas (Fig. 3C).
Proinflammatory and fibrosis cytokines
in CIA
Inflammation and fibrosis may lead to changes in
muscle weight (17,18). As the gastrocnemius muscle weight
was further decreased in CIA mice treated with UC-MSCs, the present
study investigated whether UC-MSCs induce an inflammatory response.
Immunostaining of tissue collected on day 84 was negative for
markers of B cells (CD19), T cells (CD3 and CD4) and macrophages
(iNOS and F4/80), indicating that there was no inflammatory-cell
infiltration. However, RT-qPCR analyses identified significant
increases in the expression levels of the inflammatory cytokines
TNF-α, IL-6 and IL-1β in mice with CIA as compared with those in
normal mice (Fig. 4). Furthermore,
mice treated with UC-MSCs exhibited similar increases, except for
the expression of TNF-α, which was significantly enhanced compared
with that in untreated mice with CIA. There were neither any
significant differences among the groups in the expression levels
of iNOS, TGF-β, FN1 and α-SMA, nor in the expression levels of
fibrosis genes (COLIa1, COLIa2 and COLIIIa1) (Fig. S4).
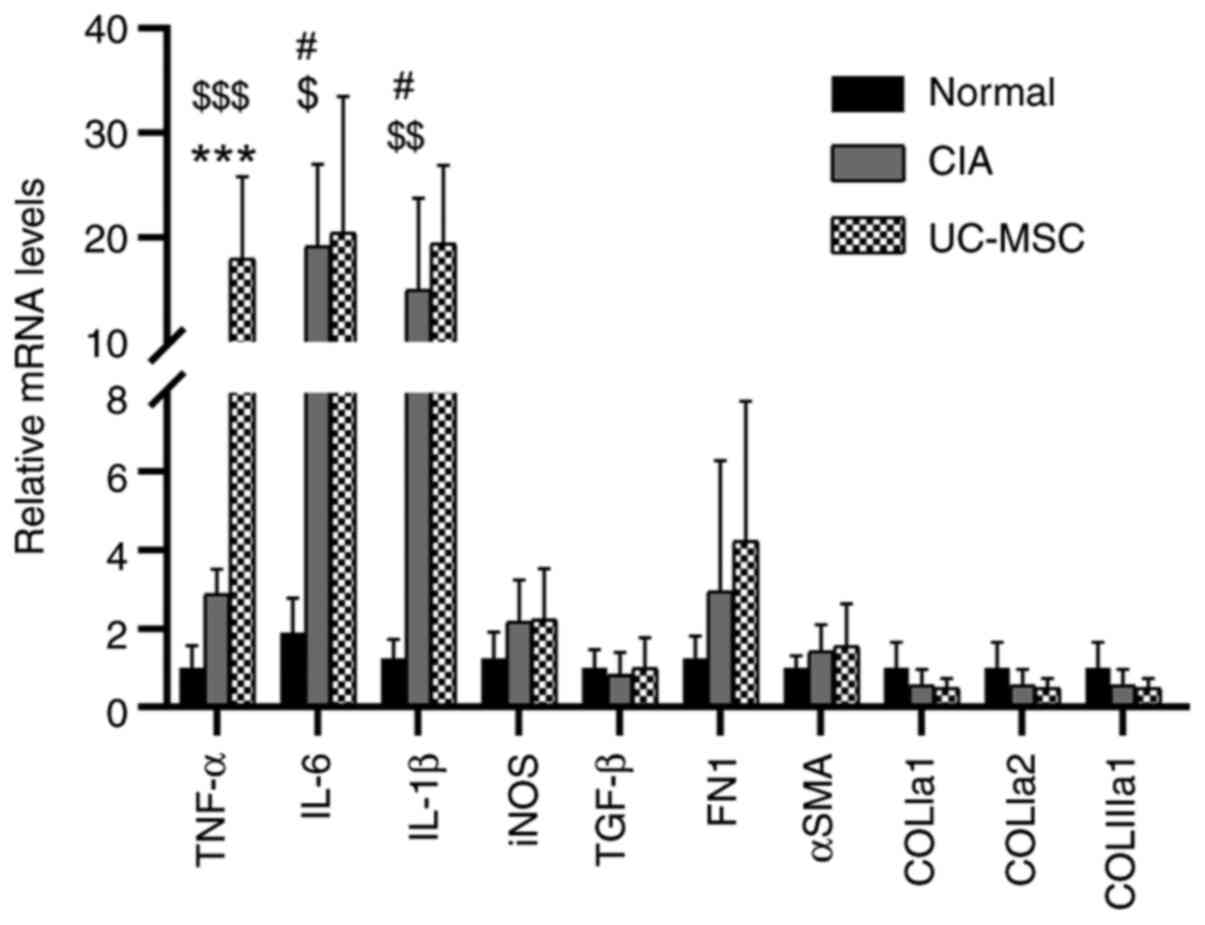 | Figure 4UC-MSC treatment does not decrease
the immune response in the muscles of mice with CIA. Levels of
relative mRNA expression were normalized to those of β-actin.
Values are expressed as the mean ± standard deviation (n=6-9).
#P<0.05, normal vs. CIA; $P<0.05,
$$P<0.01, $$$P<0.001, normal vs.
UC-MSC; ***P<0.001, CIA vs. UC-MSC. CIA,
collagen-induced arthritis; UC-MSC, umbilical cord mesenchymal stem
cell; iNOS, inducible nitric oxide synthase; SMA, smooth muscle
actin; COLIa1, collagen type I a1 chain; COLIIIa1, collagen type
III a1 chain; FN, fibronectin. |
Discussion
MSCs, known for their immunomodulatory properties,
have been reported to improve clinical symptoms and joint pathology
(21-24).
However, MSCs do not always exert immunoregulatory functions and
may only promote immune responses under certain conditions
(25). Specifically, the present
study examined a novel cell-based therapeutic strategy for animal
arthritis using high-frequency and -dose injection of UC-MSCs,
which has so far remained largely elusive. The present study first
addressed whether a high-frequency and -dose injection of UC-MSCs
possessed anti-arthritis properties and the results demonstrated
the inability of this treatment to exert a beneficial effect on
clinical symptoms and joint protection in the experimental
arthritis model. Next, the effect of high-frequency and -dose
injection of UC-MSCs on muscle atrophy was assessed and the results
indicated that this treatment decreased gastrocnemius muscle weight
and increased the levels of TNF-α in this muscle. Therefore, the
present UC-MSC therapy aggravated muscle wasting and had no
significant effect on arthritis symptoms and body weight in an
experimental model of RA.
RA is a systemic disease mainly affecting small
joints. The treatment of RA usually comprises systemic
administration of agents such as methotrexate and leflunomide,
which may control disease activity of inflamed joints via
immunoregulation (1). Local
intraarticular injection is generally suitable for large joints and
has the advantage of controlling the symptoms of the injected
joint, with relatively fewer effects on the non-injected joints
(26). Thus, due to the small joint
cavity and poor maneuverability, local intraarticular injection is
rarely used to treat RA. Based on the above considerations,
intravenous administration of UC-MSCs was used in the present
study. It was previously reported that the injected UC-MSCs
preferentially migrated to the inflammatory joint sites of the rats
as proven by UC-MSCs being labeled with superparamagnetic iron
oxide nanoparticles and bromodeoxyuridine (27). In the present study, no staining for
UC-MSC markers in the inflamed joints was performed after animal
sacrifice, so it cannot be decisively proven whether all of the
effects observed were induced by UC-MSCs directly. However, it was
demonstrated that UC-MSCs are able to secrete a wide range of
functional factors, including cytokines, chemokines and
metabolites, which mostly have beneficial effects on RA due to
their anti-inflammatory activity (7) and systemic immunomodulatory effects
(9), suggesting that the
therapeutic effect is not only entirely dependent on local effects
of articular administration but also on systemic effects. It was
previously reported that a single dose of 5x106 UC-MSCs
was effective in relieving clinical symptoms in arthritis models
(10). Furthermore, Liu et
al (11) revealed that
injections of 5x106 UC-MSC cells on days 28 and 56
improved joint damage and served a beneficial role in
arthritis-related osteoporosis. However, there is an important
balance between the pro-inflammatory and anti-inflammatory effects
of the functional factors. It is well documented that MSCs fail to
exhibit a therapeutic effect on RA (28,29),
and that intravenously infused MSCs induce inflammatory responses
in vivo (30). The present
study suggested that three weekly injections of 5x106
UC-MSCs did not relieve arthritis and muscle atrophy. The
inconsistencies among previous studies and the present results may
be associated with the stimulatory effects of the microenvironment
on MSCs, which may hamper their immunosuppressive properties or
even enhance the inflammatory process. Thus, it was suggested that
the lack of therapeutic benefit may be due to the dose and
frequency of UC-MSCs administered, which may have led to UC-MSCs
losing their immunomodulatory properties and becoming
pro-inflammatory.
Similar to RA, the CIA model is a chronic and
progressive disease manifested by arthritis and muscle cachexia
prior to day 45(31) or day
65(5). The molecular phenotype of
muscle mass loss involves increases in inflammatory cytokines,
including IL-1β, IL-8, IL-6 and Toll-like receptor-4(32). A study from another field of
research reported that MSCs promoted muscle regeneration by
enhancing angiogenesis (33).
However, the effects of MSCs on RA muscle atrophy have remained
largely elusive. The present study focused on the effects MSC
interventions on muscle atrophy in a CIA model. The results
demonstrated that body and muscle weight were reduced, while the
levels of pro-inflammatory cytokines, including TNF-α, were
increased in gastrocnemius muscles after UC-MSC therapy, as
detected via RT-qPCR. Although inflammation is an important driver
of RA skeletal muscle disease, immune cells did not infiltrate into
the muscle, which suggests that the skeletal cachexia of CIA was
not due to systemic inflammation but rather the muscle cells
themselves. A possible reason for this is that the UC-MSC group
demonstrated more severe arthritis than the CIA group, meaning that
animals moved less and the muscles wasted more. Furthermore, muscle
fibrosis has been detected in skeletal cachexia in animal models of
RA (19). The present study
examined the effects of CIA up to 84 days after immunization but
did not observe any fibrotic manifestations.
The present study had certain limitations that are
worth mentioning. It was previously suggested that syngeneic or
allogeneic MSCs provided a clinical benefit when administered
intravenously prior to the onset of arthritis (infused MSCs at days
1 and 5 or 4 and 9 post-Freund's complete adjuvant injection) in an
adjuvant-induced arthritis rat model (29); thus, a study with earlier injection
of UC-MSCs prior to arthritis onset should be designed.
Furthermore, only one therapeutic dosage regimen was tested and
further studies using different dose gradients in CIA models are
required to investigate the optimal dose and frequency. Of note,
the effectiveness of UC-MSC injections at a low frequency and dose
have been demonstrated in previous studies. However, the present
results indicated that frequent high-dose injections of UC-MSCs
failed to exert anti-arthritis and anti-muscle cachexia
effects.
In conclusion, skeletal muscle cachexia persisted
even in the CIA mice at day 84. However, frequent injection of
high-dose of UC-MSCs slightly aggravated arthritis and skeletal
muscle cachexia in CIA mice.
Supplementary Material
Characterization of hUC-MSCs. (A and
B) hUC-MSCs exhibit a phenotype typical for mesenchymal stem cells
at (A) day 5 and (B) day 14. Arrows indicate the cell colonies. (C
and D) Pluripotent differentiation abilities of hUC-MSCs. (C)
Alizarin red S staining of calcium deposits indicated that UC-MSCs
were able to differentiate into osteoblasts, while (D) oil red O
staining demonstrated that they differentiated into adipocytes
after culture in the respective induction medium for 14 days (scale
bars, 2 mm). (E) Flow cytometric analysis of UC-MSC makers at
passage 1 when the cells were undifferentiated. The cells were
positive for CD73, CD90 and CD105 and negative for CD34, CD11b,
CD19, CD45 and HLA-DR antibody cocktail. Blue indicates the
negative calibration control and red indicates cells. HLA, human
leukocyte antigen; hUC-MSC, human umbilical cord mesenchymal stem
cell.
Locomotor behavior in the open field
test. (A and B) Total immobility time, total active time, total
movement distance and the mean motion velocity were recorded (A) at
baseline and (B) 28 days after induction for CIA.
*P<0.05, **P<0.01. (C) Locomotion was
also assessed at day 28 in mice with CIA. Representative movement
tracking patterns of mice in (D) normal mice and (E) CIA mice
without treatment. CIA, collagen-induced arthritis; UC-MSC,
umbilical cord mesenchymal stem cell; D, day; s, seconds.
Clinical arthritis assessments. (A)
Experimental timeline of treatments for the CIA model. Caliper
measurements of (B) left and (C) right ankle swelling during the
experimental period in normal mice, mice with CIA and mice with CIA
receiving UC-MSCs (5x106 per mouse; weekly; three
times). (D) Clinical arthritis scores. Values are expressed as the
mean ± standard deviation (n=11 mice per group). CIA,
collagen-induced arthritis; UC-MSC, umbilical cord mesenchymal stem
cell; iv, intravenous.
Immunohistochemical staining images
for CD3, CD4, CD19, F4/80 and iNOS in gastrocnemius muscles from
each group (scale bars, 2 mm). Positive results are indicated by
brown staining. iNOS, inducible nitric oxide synthase.
Primers used for quantitative
PCR.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Department of Henan Province (grant no. 212102310200)
and the National Natural Science Foundation of China (grant no.
81970312).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LA, HY and ZZ contributed to the study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. LA, TC, LW, SA, YL and HH performed the
experiments and data curation. LA, HH, ZZ and HY performed
manuscript editing and review. LA, ZZ and HY confirmed the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval for the human experiments was
obtained from the Ethical Board of the Stem Cell Clinical Research
Center of Henan Provincial People's Hospital [Zhengzhou, China;
approval no. 1 (2019)]. The donor provided written informed
consent. Ethics approval for the animal experiments was obtained
from the Animal Ethics Committee of Peking University (Beijing,
China; approval no. J201985).
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
References
|
1
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A review. JAMA. 320:1360–1372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Santo RCE, Fernandes KZ, Lora PS, Filippin
LI and Xavier RM: Prevalence of rheumatoid cachexia in rheumatoid
arthritis: A systematic review and meta-analysis. J Cachexia
Sarcopenia Muscle. 9:816–825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cohen S, Nathan JA and Goldberg AL: Muscle
wasting in disease: Molecular mechanisms and promising therapies.
Nat Rev Drug Discov. 14:58–74. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Lin JZ, Liang JJ, Ma JD, Li QH, Mo YQ,
Cheng WM, He XL, Li N, Cao MH, Xu D and Dai L: Myopenia is
associated with joint damage in rheumatoid arthritis: A
cross-sectional study. J Cachexia Sarcopenia Muscle. 10:355–367.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alabarse PVG, Lora PS, Silva JMS, Santo
RCE, Freitas EC, de Oliveira MS, Almeida AS, Immig M, Teixeira VON,
Filippin LI and Xavier RM: Collagen-induced arthritis as an animal
model of rheumatoid cachexia. J Cachexia Sarcopenia Muscle.
9:603–612. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Burmester GR and Pope JE: Novel treatment
strategies in rheumatoid arthritis. Lancet. 389:2338–2348.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leyendecker A Jr, Pinheiro CCG, Amano MT
and Bueno DF: The use of human mesenchymal stem cells as
therapeutic agents for the in vivo treatment of immune-related
diseases: A systematic review. Front Immunol.
9(2056)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu L, Farhoodi HP, Han M, Liu G, Yu J,
Nguyen L, Nguyen B, Nguyen A, Liao W and Zhao W: Preclinical
evaluation of a single intravenous infusion of hUC-MSC (BX-U001) in
rheumatoid arthritis. Cell Transplant.
29(963689720965896)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi Y and Wang Y, Li Q, Liu K, Hou J, Shao
C and Wang Y: Immunoregulatory mechanisms of mesenchymal stem and
stromal cells in inflammatory diseases. Nat Rev Nephrol.
14:493–507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Y, Kong W, Huang S, Shi B, Zhang H,
Chen W, Zhang H, Zhao C, Tang X, Yao G, et al: Comparable
therapeutic potential of umbilical cord mesenchymal stem cells in
collagen-induced arthritis to TNF inhibitor or anti-CD20 treatment.
Clin Exp Rheumatol. 35:288–295. 2017.PubMed/NCBI
|
|
11
|
Liu C, Zhang H, Tang X, Feng R, Yao G,
Chen W, Li W, Liang J, Feng X and Sun L: Mesenchymal stem cells
promote the osteogenesis in collagen-induced arthritic mice through
the inhibition of TNF-α. Stem Cells Int.
2018(4069032)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
13
|
Mushahary D, Spittler A, Kasper C, Weber V
and Charwat V: Isolation, cultivation, and characterization of
human mesenchymal stem cells. Cytometry A. 93:19–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini Fc, Krause Ds, Deans Rj, Keating A,
Prockop Dj and Horwitz Em: Minimal criteria for defining
multipotent mesenchymal stromal cells. The international society
for cellular therapy position statement. Cytotherapy. 8:315–317.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Miyoshi M and Liu S: Collagen-induced
arthritis models. Methods Mol Biol. 1868:3–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kong X, Zhang Z, Fu T, Ji J, Yang J and Gu
Z: TNF-α regulates microglial activation via the NF-κB signaling
pathway in systemic lupus erythematosus with depression. Int J Biol
Macromol. 125:892–900. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brenner M, Meng HC, Yarlett NC, Griffiths
MM, Remmers EF, Wilder RL and Gulko PS: The non-major
histocompatibility complex quantitative trait locus Cia10 contains
a major arthritis gene and regulates disease severity, pannus
formation, and joint damage. Arthritis Rheum. 52:322–332.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oyenihi AB, Ollewagen T, Myburgh KH,
Powrie YSL and Smith C: Redox status and muscle pathology in
rheumatoid arthritis: Insights from various rat hindlimb muscles.
Oxid Med Cell Longev. 2019(2484678)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
An L, Li Z, Shi L, Wang L, Wang Y, Jin L,
Shuai X and Li J: Inflammation-targeted celastrol nanodrug
attenuates collagen-induced arthritis through NF-κB and Notch1
pathways. Nano Lett. 20:7728–7736. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Park KH, Mun CH, Kang MI, Lee SW, Lee SK
and Park YB: Treatment of collagen-induced arthritis using immune
modulatory properties of human mesenchymal stem cells. Cell
Transplant. 25:1057–1072. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park MJ, Lee SH, Moon SJ, Lee JA, Lee EJ,
Kim EK, Park JS, Lee J, Min JK, Kim SJ, et al: Overexpression of
soluble RAGE in mesenchymal stem cells enhances their
immunoregulatory potential for cellular therapy in autoimmune
arthritis. Sci Rep. 6(35933)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu CC, Liu FL, Sytwu HK, Tsai CY and Chang
DM: CD146+ mesenchymal stem cells display greater therapeutic
potential than CD146-cells for treating collagen-induced arthritis
in mice. Stem Cell Res Ther. 7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shu J, Pan L, Huang X, Wang P, Li H, He X
and Cai Z: Transplantation of human amnion mesenchymal cells
attenuates the disease development in rats with collagen-induced
arthritis. Clin Exp Rheumatol. 33:484–490. 2015.PubMed/NCBI
|
|
25
|
Inoue S, Popp FC, Koehl GE, Piso P,
Schlitt HJ, Geissler EK and Dahlke MH: Immunomodulatory effects of
mesenchymal stem cells in a rat organ transplant model.
Transplantation. 81:1589–1595. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Santos JM, Bárcia RN, Simões SI, Gaspar
MM, Calado S, Agua-Doce A, Almeida SC, Almeida J, Filipe M,
Teixeira M, et al: The role of human umbilical cord tissue-derived
mesenchymal stromal cells (UCX®) in the treatment of inflammatory
arthritis. J Transl Med. 11(18)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gu J, Gu W, Lin C, Gu H, Wu W, Yin J, Ni
J, Pei X, Sun M, Wang F, et al: Human umbilical cord mesenchymal
stem cells improve the immune-associated inflammatory and
prothrombotic state in collagen type-II-induced arthritic rats. Mol
Med Rep. 12:7463–7470. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luz-Crawford P, Torres MJ, Noël D,
Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C,
Illanes SE, Figueroa FE, et al: The immunosuppressive signature of
menstrual blood mesenchymal stem cells entails opposite effects on
experimental arthritis and graft versus host diseases. Stem Cells.
34:456–469. 2016.
|
|
29
|
Papadopoulou A, Yiangou M, Athanasiou E,
Zogas N, Kaloyannidis P, Batsis I, Fassas A, Anagnostopoulos A and
Yannaki E: Mesenchymal stem cells are conditionally therapeutic in
preclinical models of rheumatoid arthritis. Ann Rheum Dis.
71:1733–1740. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoogduijn MJ, Roemeling-van Rhijn M,
Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes
MG, Weimar W and Baan CC: Mesenchymal stem cells induce an
inflammatory response after intravenous infusion. Stem Cells Dev.
22:2825–2835. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Filippin LI, Teixeira VN, Viacava PR, Lora
PS, Xavier LL and Xavier RM: Temporal development of muscle atrophy
in murine model of arthritis is related to disease severity. J
Cachexia Sarcopenia Muscle. 4:231–238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huffman KM, Jessee R, Andonian B, Davis
BN, Narowski R, Huebner JL, Kraus VB, McCracken J, Gilmore BF, Tune
KN, et al: Molecular alterations in skeletal muscle in rheumatoid
arthritis are related to disease activity, physical inactivity, and
disability. Arthritis Res Ther. 19(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arutyunyan I, Fatkhudinov T, Kananykhina
E, Usman N, Elchaninov A, Makarov A, Bolshakova G, Goldshtein D and
Sukhikh G: Role of VEGF-A in angiogenesis promoted by umbilical
cord-derived mesenchymal stromal/stem cells: In vitro study. Stem
Cell Res Ther. 7(46)2016.PubMed/NCBI View Article : Google Scholar
|