Introduction
Lung cancer is the leading cause of
cancer-associated mortality, accounting for 18.4% of total cancer
deaths according to a 2018 Global Cancer Statistics report on the
global burden of cancer (1).
Non-small cell lung cancer (NSCLC) accounts for ~85% of all lung
cancer cases (2,3). Aside from surgical resection, which is
the gold standard for NSCLC treatment, chemoradiotherapy,
EGFR-tyrosine kinase inhibitors and anaplastic lymphoma kinase
inhibitors are common NSCLC treatment strategies (4). However, the prognosis of patients with
NSCLC remains poor. Therefore, the molecular mechanisms of NSCLC
should be investigated to develop new treatment and therapeutic
targets for NSCLC.
MicroRNAs (miRNAs/miRs) are endogenous small
non-coding RNAs with a length of 20-24 nucleotides that play
important roles in numerous biological processes (2). The regulatory roles of miRNAs involve
the negative regulation of gene expression by binding to the 3'
untranslated region (UTR) of target mRNAs (5). miRNAs may be key mediators of tumor
development in various types of cancer, such as NSCLC (6,7),
prostate cancer (8), colorectal
cancer (9) and ovarian cancer
(10). According to the miRbase
database, the let-7f-1 family includes two types, namely, let-7f-5p
and let-7f-1-3p. Let-7f-5p is involved in regulating all types of
biological disease processes (11-18),
including cancer (17,18). Moreover, according to the tumor
suppressor gene, tumor-associated gene and TRANSFAC databases,
let-7f-1-3p is downregulated in colorectal cancer tissues (19). However, the involvement of
let-7f-1-3p in NSCLC development is poorly understood. Thus, the
function and potential application of let-7f-1-3p in NSCLC biology
should be extensively investigated.
Integrin β1 (ITGB1) is considered a potential
oncoprotein that promotes the malignant progression of cancer
(20), particularly in cancer cell
invasion and migration (21). ITGB1
is activated during the tumorigenesis and migration of gastric
cancer via lumican (22). The more
ITGB1 is aggregated by C-X-C motif chemokine 12, the more invasion
is promoted (23). In addition,
ITGB1 is stabilized to promote small-cell lung cancer migration by
cullin-5(21), and cellular
retinoic acid binding protein 1 requires Hu antigen R to promote
the ITGB1 expression necessary for lung cancer metastasis (24).
The present study described the role of let-7f-1-3p
in suppressing the cancer progression of NSCLC by regulating ITGB1.
Moreover, let-7f-1-3p-overexpression combined with doxorubicin
(DOX)-induced apoptosis of A549 cells provided new insights into
the mechanism of miRNA-mediated tumor suppression and a new target
for NSCLC treatment .
Materials and methods
Patients and tissue samples
Tumorous lung tissue and matched paratumor tissues
(5 cm away from the tumor tissue) were collected from 15 patients
(8 female and 7 male) who underwent curative surgery for NSCLC at
Yantaishan Hospital, The Teaching Hospital of Binzhou Medical
University (Yantai, China). None of the patients were subjected to
radiotherapy and chemotherapy before the operation. Informed
consent was obtained from all the participants before the study.
The samples were collected from May to August in 2019. This study
was approved by The Ethics Committee of Binzhou Medical University
(Yantai, China; approval number: 2018-07-06). The
clinicopathological data of the patients is presented in Table I. The data was assessed by a
pathologist at Yantaishan Hospital (Yantai, China).
 | Table IClinicopathological data of patients
with non-small cell lung cancer. |
Table I
Clinicopathological data of patients
with non-small cell lung cancer.
| Sample no. | Sex | Age, years | Tumor stage | TNM stage | Tumor grade |
|---|
| 1 | Female | 62 | ⅡA | T2aN1M0 | G1 |
| 2 | Female | 50 | ⅠB | T2aN0M0 | G1 |
| 3 | Female | 57 | ⅠA | T1aN0M0 | G1 |
| 4 | Female | 56 | ⅠB | T2aN0M0 | G1 |
| 5 | Female | 65 | ⅡA | T2aN1M0 | G1 |
| 6 | Female | 67 | ⅠA | T1aN0M0 | G1 |
| 7 | Female | 57 | ⅠA | T1aN0M0 | G1 |
| 8 | Female | 76 | ⅠB | T2aN0M0 | G1 |
| 9 | Male | 61 | ⅠA | T1aN0M0 | G1 |
| 10 | Male | 39 | ⅠA | T2aN0M0 | G1 |
| 11 | Male | 64 | ⅠA | T1aN0M0 | G1 |
| 12 | Male | 62 | ⅠA | T1aN0M0 | G1 |
| 13 | Male | 66 | ⅠA | T1aN0M0 | G1 |
| 14 | Male | 61 | ⅠB | T2aN0M0 | G1 |
| 15 | Male | 64 | ⅡA | T1bN0M0 | G1 |
Cell culture
The NSCLC cell line A549 and 293T cells were
obtained from the Shanghai Institute of Biochemistry and Cell
Biology. NCI-H1975 was purchased from Procell Life Science &
Technology Co., Ltd. Human bronchial epithelial (HBE)135-E6E7 cells
were obtained from the American Type Culture Collection (cat. no.
ATCC® CRL-2741™). The NSCLC cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific Inc.) and anti-mycoplasma reagent (cat. no. HB-SV1000;
Hanbio Biotechnology Co., Ltd.). All the cells were maintained in a
humidified incubator at 37˚C under 5% CO2 condition.
let-7f-1-3p mimics, let-7f-1-3p inhibitors and negative controls
(NC) were synthesized by Shanghai GenePharma Co., Ltd. The
corresponding sequences were as follows: let-7f-1-3p mimic, sense,
5'-CUAUACAAUCUAUUGCCUUCCC-3', and antisense,
5'-GAAGGCAAUAGAUUGUAUAGUU; let-7f-3p inhibitor,
5'-GGGAAGGCAAUAGAUUGUAUAG-3'; mimic NC (miRNA mimic negative
control), sense, 5'-UUCUUCGAACGUGUCACGUTT-3', and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'; inhibitor NC (scrambled),
CAGUACUUUUGUGUAGUACAA. The final concentration of transfected miRNA
mimics/inhibitors was 30 nM. pcDNA3.1-ITGB1-3*Flag and negative
controls (pcDNA3.1-3*Flag) were purchased by Shanghai GeneChem Co.,
Ltd. Plasmid (2 µg) was used for transfection. Transfection was
performed in triplicate at ~60% confluence using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
The transfection mixture was added dropwise to the experimental
wells and mixed and incubated at 37˚C, 5% CO2 for 6-10
h. The transfection solution was aspirated and 2 ml of RPMI-1640
culture solution containing 10% fetal bovine serum was added to the
wells and incubated at 37˚C, 5% CO2 for 24-48 h. The
cells were observed under a light microscope (BX43; Olympus, Inc.)
(magnification, x100). Following this the cells were used for
subsequent experimentation.
Reverse transcription-quantitative
(RT-q)PCR
NSCLC cells were incubated at 37˚C 5% CO2
with let-7f-1-3p mimics or let-7f-1-3p inhibitor and used in
subsequent experiments after 48 h of transfection. Small RNA was
purified using RNAiso for small RNA reagent (Takara Biotechnology
Co., Ltd.). The primers used to amplify let-7f-1-3p (Shanghai
GenePharma Co., Ltd.) were as follows: Forward,
5'-CTATACAATCTATTGCCTTCCC-3', and reverse,
5'-AACATGTACAGTCCATGGATG-3'. Human 5S ribosomal (r)RNA served as
the positive control. The primers used to amplify 5S rRNA (Shanghai
GenePharma Co., Ltd.) were as follows: Forward,
5'-GCCATACCACCCTGAACG-3', and reverse, 5'-AACATGTACAGTCCATGGATG-3'.
According to the Poly(A) Polymerase (cloned) 2 U/ul kit manual
(cat. no. AM2030; Invirtogen; Thermo Fisher Scientific Inc.), a
poly A tail was added to the 3' end of the miRNA. The subsequent
reverse transcription (PrimeScript™ RT reagent Kit with gDNA
Eraser, cat. no. RR047A; Takara Biotechnology Inc.) was the same as
the reverse transcription of total RNA and was performed according
to the manufacturer's protocol. Total RNA was purified using
TRIzol® reagent (Takara Biotechnology Co., Ltd.). The
primers used to magnify ITGB1 (Shanghai GenePharma Co., Ltd.) were
as follows: Forward, 5'-AAAATGTAACCAACCGTAGCAAAG-3', and reverse,
5'-GACAGGTCCATAAGGTAGTAGA-3'. Human GAPDH mRNA served as the
positive control. The primers used to amplify GAPDH mRNA (Shanghai
GenePharma Co., Ltd.) were as follows: Forward,
5'-GTCTTCACCACCATGGAGAAGG-3', and reverse,
5'-GCCTGCTTCACCACCTTCTTGA-3. TB Green (cat. no. RR420A; Takara Bio
Inc.) was used for the RT-qPCR reaction. qPCR was performed on a
StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Inc.)
under the following reaction conditions: Initial denaturation at
95˚C for 10 min, followed by 40 cycles of 95˚C for 15 sec and
fluorescence signal acquisition at 60˚C for 1 min. The relative
expression levels of the gene of interest were calculated using the
2-∆∆Ct method (2).
MTT assay
A549 and NCI-H1975 cells were seeded onto 96-well
plates at 4,000 cells/well at 24 h after transfection. MTT assay
was used to confirm cell viability at 48 h after the cells were
plated. Purple formazan was dissolved with dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck KGaA). The absorbance at 491 nm was
measured using a Multiskan FC microplate reader (Thermo Fisher
Scientific, Inc.).
Colony formation assay
For the colony formation assay, the A549 and
NCI-H1975 cells were plated onto a 10-cm cell culture dish at a
density of 1,500 cells/plate at 24 h after transfection. The
RPMI-1640 culture solution containing 10% fetal bovine serum was
replaced every 4 days. After ~12 days, the majority of the cell
clones contained >40 cells. The plate was gently washed with 1X
PBS and 4% paraformaldehyde was used to fix the clones for 1 h at
room temperature, which were subsequently stained with crystal
violet for ~30 min at room temperature. Images were captured and
the clones were quantified by eye.
Western blotting
A549 and NCI-H1975 cells were lysed using RIPA lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.) with a
complete protease inhibitor cocktail tablet (cat. no. CORO;
MilliporeSigma) on ice. BCA protein concentration determination kit
(cat. no. P0012; Beyotime Institute of Biotechnology) to detect
protein concentration. The absorbance at 560 nm was measured using
a Multiskan FC microplate reader (Thermo Fisher Scientific, Inc.).
Total protein (50 µg/lane) was separated on a 10% SDS-PAGE gel,
transferred to a PVDF membrane and blocked with 5% skim milk powder
blocking solution for 2-3 h at room temperature. The primary
antibodies were incubated with the membrane at 4˚C overnight.
Rabbit antibodies against GAPDH (1:5,000; cat. no. AP0063), ITGB1
(1:2,000; cat. no. BS9835M), MYC (1:800; cat. no. BS2462), Bax
(1:800; cat. no. BS2538; all Bioworld Technology, Inc.), BCL2
(1:2,000; cat. no. 12789-1-AP; ProteinTech Group, Inc.), E-cadherin
and vimentin (both 1:1,000; cat. no. 9782; Cell Signaling
Technology, Inc.) were used. The membranes were then incubated with
goat anti-rabbit HRP-conjugated secondary antibody (1:2,000; cat.
no. SA00001-2; ProteinTech Group, Inc.) for 2 h at 4˚C. GAPDH was
used as an internal reference. The chemiluminescence substrate a
Chemistar™ High-sig ECL Western Blotting substrate (cat. no.
180-5001; Tanon Science and Technology Co., Ltd.) with the Tanon
4600 series automatic chemiluminescence/fluorescence image analysis
system (Tanon Science and Technology Co., Ltd.) was used for
detection and analysis.
Cell migration and invasion
assays
The cells were transfected with oligonucleotides and
treated with DOX (200 ng/ml) for 36 h. Subsequently, 50,000 cells
were resuspended in a serum-free medium and plated in the upper
chamber of a modified two-compartment Corning Transwell plate
(24-well; cat. no. 3428; Corning, Inc.) with a pore size of 8.0 µm.
The lower chamber medium contained 20% fetal bovine serum (Gibco;
Thermo Fisher Scientific Inc.). For the invasion experiment,
Matrigel was diluted with RPMI-1640 at a ratio of 1:3 on ice and
pre-coated onto the chamber on the Transwell plate at 37˚C, 5%
CO2 for 2 h for subsequent experiments. Unlike the
invasion experiment, the migration experiment did not require
Matrigel. After incubation at 37˚C 5% CO2 for 24 h, the
migration test setup was removed from the culture plate, and the
invasion test setup was removed after 48 h of incubation at 37˚C
and 5% CO2. The cells that were transferred to the lower
chamber were washed with 1X PBS, fixed in 4% paraformaldehyde for
30 min at room temperature and stained with crystal violet for 30
min at room temperature. The cells were rinsed gently with
ultrapure water and dried and a fully automatic upright fluorescent
microscope (DM6000 B; Leica Microsystems Inc.) (magnification, x20)
was used with randomly switching 5 fields of views to take images
that were then visually counted.
Luciferase reporter assay
293T cells were cultured in a 10-cm culture dish to
90% confluence, trypsin (cat. no. 25200056; Gibco; Thermo Fisher
Scientific Inc.) digested for 30 sec and counted with a beef
abalone counter. A total of ~5x104 cells were added to
each well of a six-well plate, and transfection was performed after
16 h of culture at 37˚C and 5% CO2. let-7f-1-3p (sense,
5'-CUAUACAAUCUAUUGCCUUCCC-3' and antisense,
5'-GAAGGCAAUAGAUUGUAUAGUU) or NC (sense,
5'-UUCUUCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3') were co-transfected with
GP-miRGLO-ITGB1 wild-type (WT) or GP-miRGLO-ITGB1 mutant (MUT)
(Shanghai GeneChem Co., Ltd.). Plasmid (2 µg), miRNA mimic/NC (30
nM) were transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific Inc.) and replaced with a
whole medium after 8 h, further cultured for 36 h, lysed and
collected using a Dual-Luciferase® Reporter Assay System
(cat. no. E1910; Promega Corporation) in accordance with the
manufacturer's instructions. Renilla luciferase activity was
used for normalization.
Tumor experiment involving nude
mice
A total of 12 SPF BALB/c nude mice with an average
weight of 14 g (6 weeks old; male) were purchased from
GemPharmatech Co., Ltd. (animal certificate no. 201904776). Mice
were raised in SPF conditions with a 12/12 h light/dark cycle and
randomly divided into cages, given SPF immunodeficiency mouse feed
(Beijing Keao Xieli Feed Co., Ltd.) and the drinking water was
autoclaved. All animal experiments in the present study were
approved by The Committee on the Ethics of Animal Experiments of
Binzhou Medical University. Mice were randomly divided into 2
groups, NC injection group and let-7f-1-3p injection group, with 6
animals in each group. A549 cells that were transiently transfected
with let-7f-1-3p or NC were thoroughly digested and counted through
trypsinization. The cell concentration was adjusted to
5x107/ml in 1X PBS, and 100 µl of cells was injected
into the shoulder of each mouse. Nude mice were anesthetized via
intraperitoneal injection of 1% sodium pentobarbital (50 mg/kg) for
~10 min. A Vernier caliper was used to measure tumor width, and
tumor volume was calculated using the following formula: Tumor
volume=(length x width2)/2. After 9 weeks, the mouse
tumor had grown to ~40 mm3. The transfection solution
prepared by let-7f-1-3p mimics or NC and Lipofectamine 2000 was
diluted to 100 µl with PBS to make the final concentration of
mimics 1.0 µM, and then injected at multiple sites of the tumor
twice a week (25). At the same
time, the mice in the NC injection group/let-7f-1-3p injection
group were randomly divided into a DOX-administered group or a
saline-administered group with 3 mice in each group and treated
intraperitoneally at a dose of 1.5 mg/kg body weight. The control
group was given the same volume of physiological saline every 3
days. The treatments were continuously administered for 2 weeks,
and every 3 days, use a vernier caliper to measure the width of the
tumor. After 12 weeks, the mice were sacrificed through cervical
dislocation, and tumor tissues were excised and weighed.
miRNA target genes
TargetScanHuman Release v.7.2 (http://www.targetscan.org/vert_72/) was used to
predict the biological targets of miRNAs. The target genes and
their miRNA binding sites were predicted using this online
tool.
Statistical analyses
SPSS 22.0 software (IBM Corp.) was used in this
study. Measured data are presented as mean ± standard deviation
(unless otherwise shown). One-way analysis of variance (ANOVA) was
used to analyze the differences among ≥3 groups, followed by
Tukey's post hoc test. Group means were compared using an unpaired,
two-sided Student's t-test. Wilcoxon signed-rank test was used to
compare the expression of let-7f-1-3p in paracarcinoma and
carcinoma tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
let-7f-1-3p is downregulated in NSCLC
tissues
Although let-7f-1-3p is downregulated in human small
airway epithelial cells (HSAEC) and human pulmonary alveolar
epithelial cells (HPAEpiC) (26),
let-7f-1-3p expression in NSCLC remains unclear. To identify the
role of let-7f-1-3p in lung cancer, RT-qPCR was performed to detect
the expression profile of let-7f-1-3p in NSCLC tissues. let-7f-1-3p
expression levels were significantly decreased in NSCLC tissues
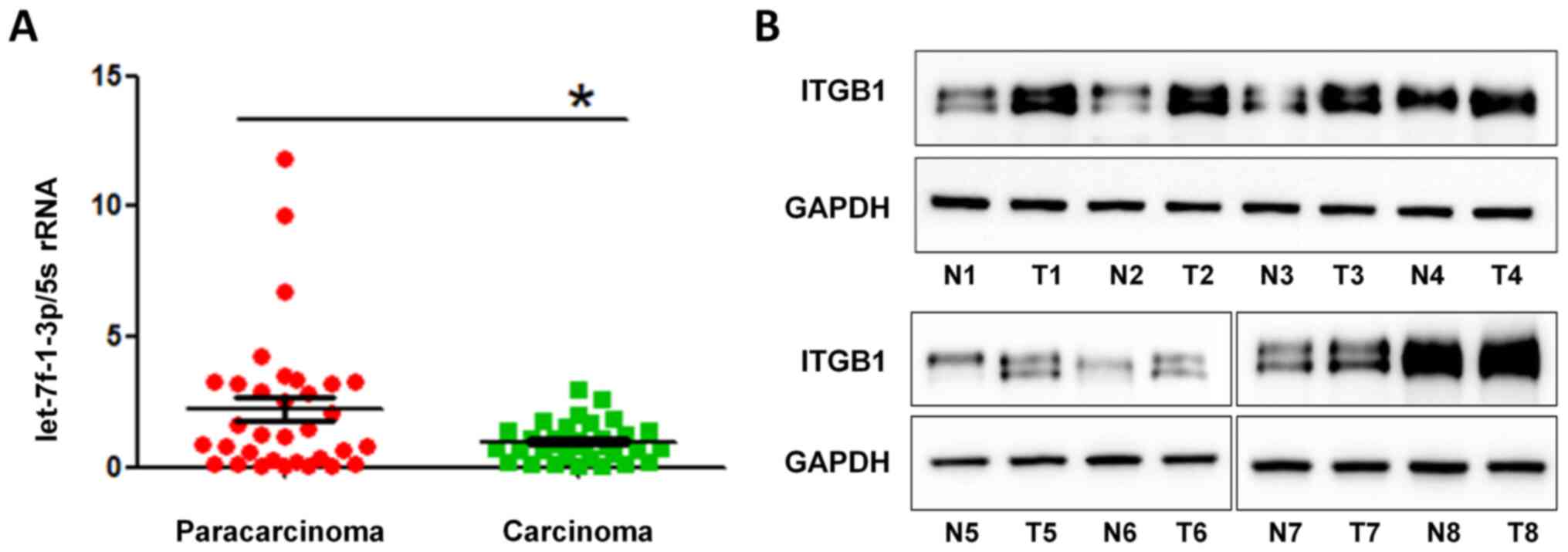
compared with those in adjacent paracarcinoma tissues (Fig. 1A), suggesting the suppressive role
of let-7f-1-3p in tumorigenesis of lung cancer. Thus, lower levels
of let-7f-1-3p may be associated with NSCLC development.
Next, the expression levels of ITGB1 protein in
patients with NSCLC were examined, which revealed that ITGB1
expression in NSCLC tissues was higher compared with in adjacent
tissues (Fig. 1B). These results
suggested that increased expression levels of ITGB1 may be
associated with NSCLC development.
let-7f-1-3p expression decreases in
NSCLC cell lines
To further investigate whether let-7f-1-3p inhibited
the proliferation of NSCLC, let-7f-1-3p expression levels were
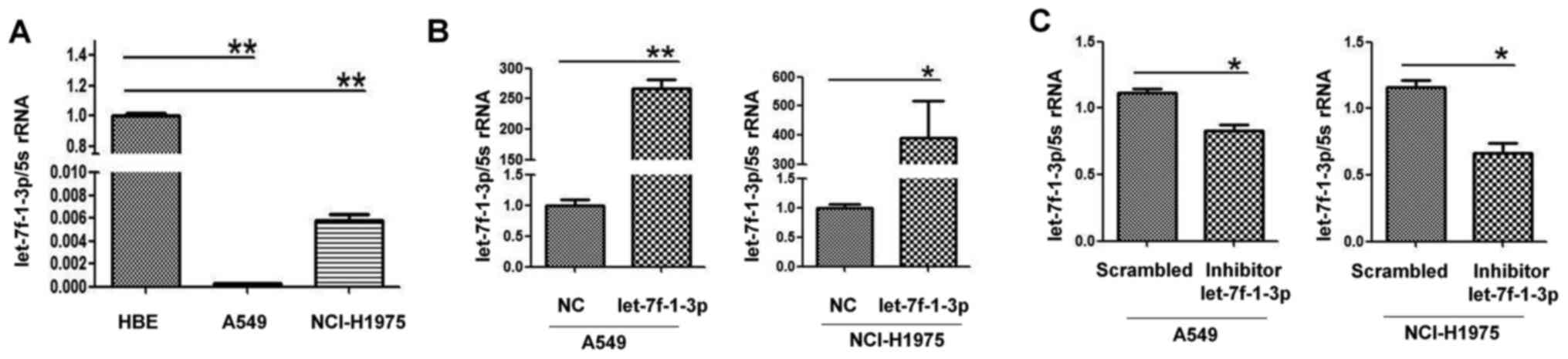
detected in HBE, A549 and NCI-H1975 cells using RT-qPCR.
let-7f-1-3p expression was significantly decreased in A549 and
NCI-H1975 cells compared with HBE cells as a control group
(Fig. 2A). Therefore, the A549 and
NCI-H1975 cell lines were selected to be transfected with the
let-7f-1-3p oligonucleotide. As presented in Fig. 2B, the expression levels of
let-7f-1-3p in the two cell lines transfected with let-7f-1-3p
mimics were upregulated compared with the cells transfected with
NC. Conversely, let-7f-1-3p expression levels were significantly
downregulated by transfection with the let-7f-1-3p inhibitor
compared with the NC (Fig. 2C).
let-7f-1-3p directly regulates the
expression of ITGB1
To gain insight into the molecular mechanism of
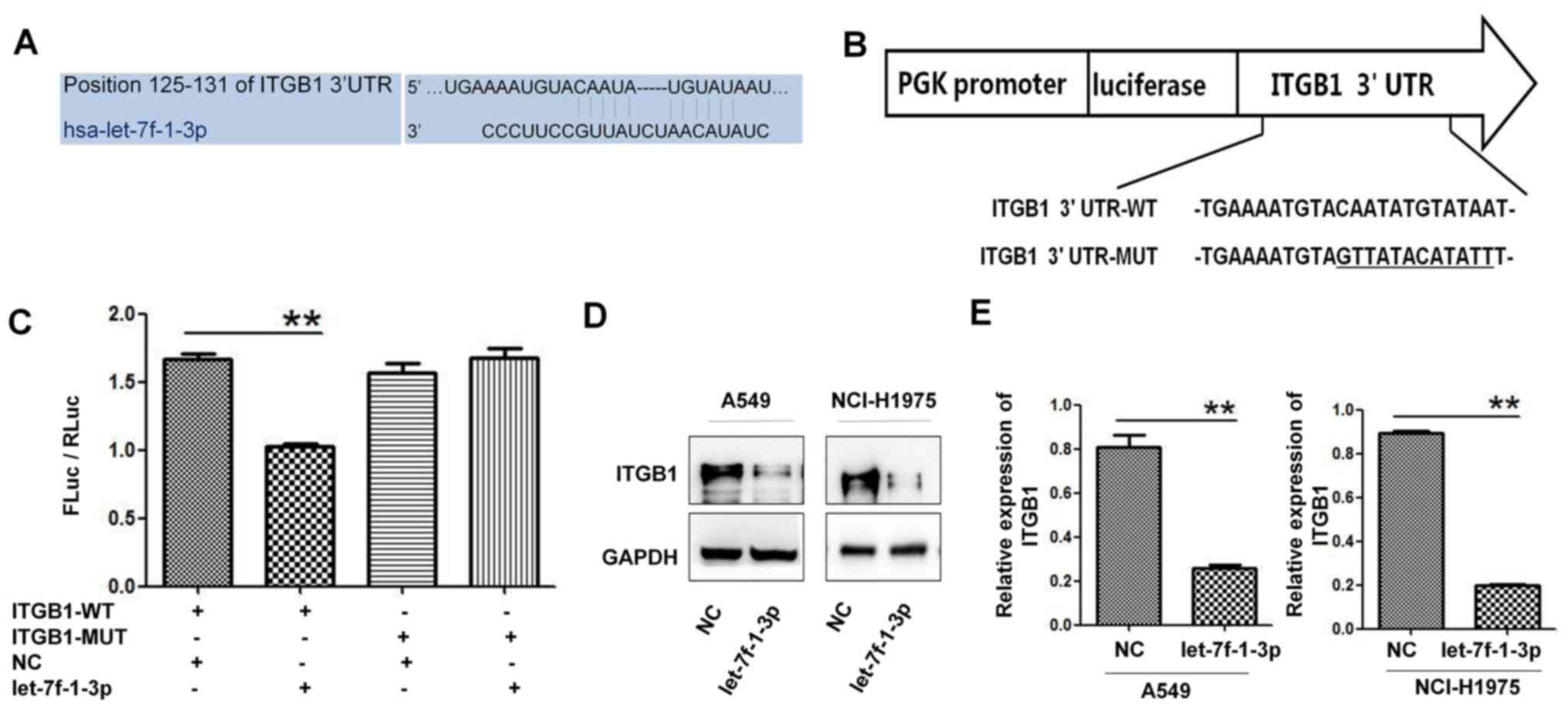
let-7f-1-3p in suppressing lung cancer, ITGB1 was predicted to be a
target gene of let-7f-1-3p using the TargetScan database (Fig. 3A). Subsequently, the ITGB1-3' UTR-WT
vector and its mutant vector were constructed for transfection of
let-7f-1-3p or NC into 293T cells (Fig.
3B). After 48 h, double-luciferase reporter gene assays
indicated that the luciferase activity of the ITGB1-3' UTR-WT
vector was significantly inhibited when the cells were
co-transfected with let-7f-1-3p compared with the NC. However,
there was no significant change in luciferase activity in cells
co-transfected with ITGB1-3' UTR-MUT and let-7f-1-3p compared with
those co-transfected with ITGB1-3' UTR-MUT and NC (Fig. 3C). Subsequently, western blotting
suggested that the expression levels of ITGB1 were significantly
downregulated in let-7f-1-3p-treated A549 and NCI-H1975 cells
compared with the NC (Fig. 3D and
E). In summary, these observations
suggested that let-7f-1-3p targeted ITGB1 inhibition.
let-7f-1-3p inhibits the viability of
NSCLC cells
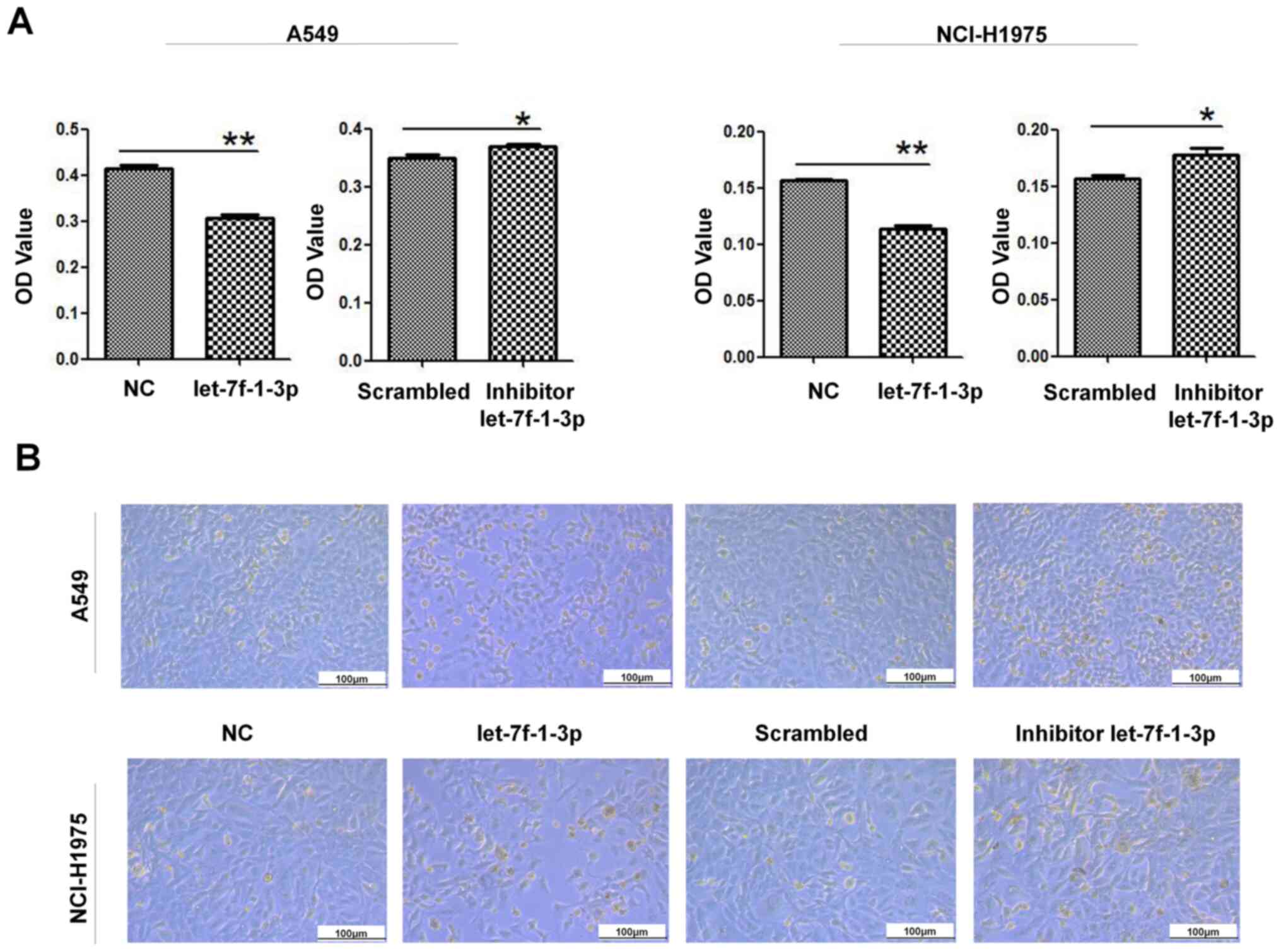
To explore the effect of let-7f-1-3p on the
viability of NSCLC cells, an MTT assay was used to detect the
effect of cell viability after overexpression or inhibition of
let-7f-1-3p. Cell viability was inhibited after
let-7f-1-3p-overexpression compared with the NC in both A549 and
NCI-H1975 cells, whereas the let-7f-1-3p-inhibitor significantly
increased cell viability in the cells compared with that of the
control group (Fig. 4A).
Subsequently, the effect of the oligonucleotides on A549/NCI-H1975
cells was analyzed morphologically. After 48 h of treatment,
overexpression of let-7f-1-3p decreased the number of cells and
their adhesion compared with the NC; by contrast, inhibition of
let-7f-1-3p increased the number of cells (Fig. 4B).
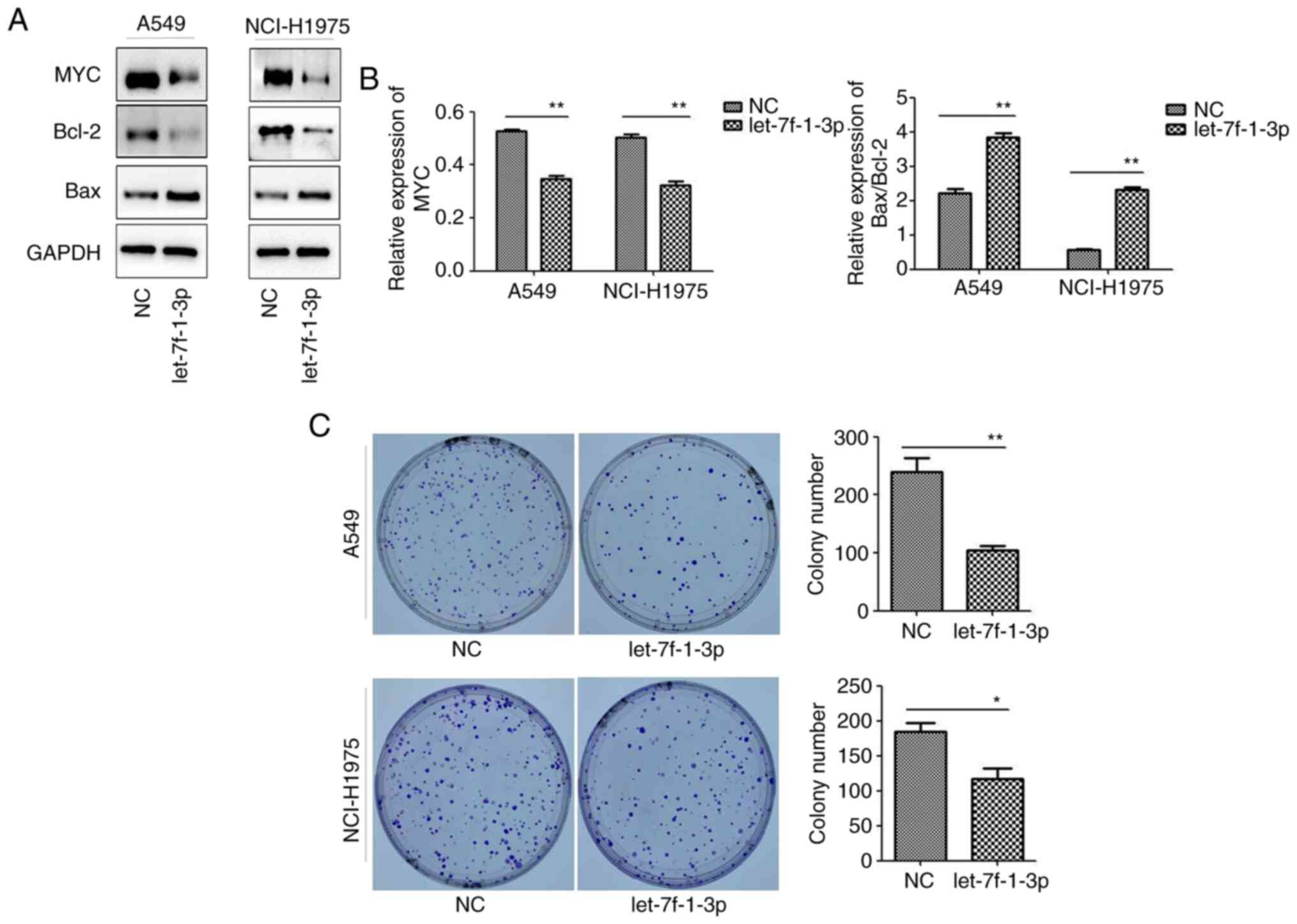
let-7f-1-3p affects apoptosis and
decreases the colony number of NSCLC cells
To explore the molecular mechanism of the
let-7f-1-3p-induced effects on NSCLC cells, the expression levels
of apoptosis-related proteins were analyzed. After let-7f-1-3p was
overexpressed for 48 h, the Bax/Bcl-2 ratio significantly
increased, whereas the expression of MYC significantly decreased,
as determined via western blotting (Fig. 5A and B). Cell clone formation experiments
supported these results, as let-7f-1-3p overexpression
significantly decreased the colony numbers compared with the NC
(Fig. 5C).
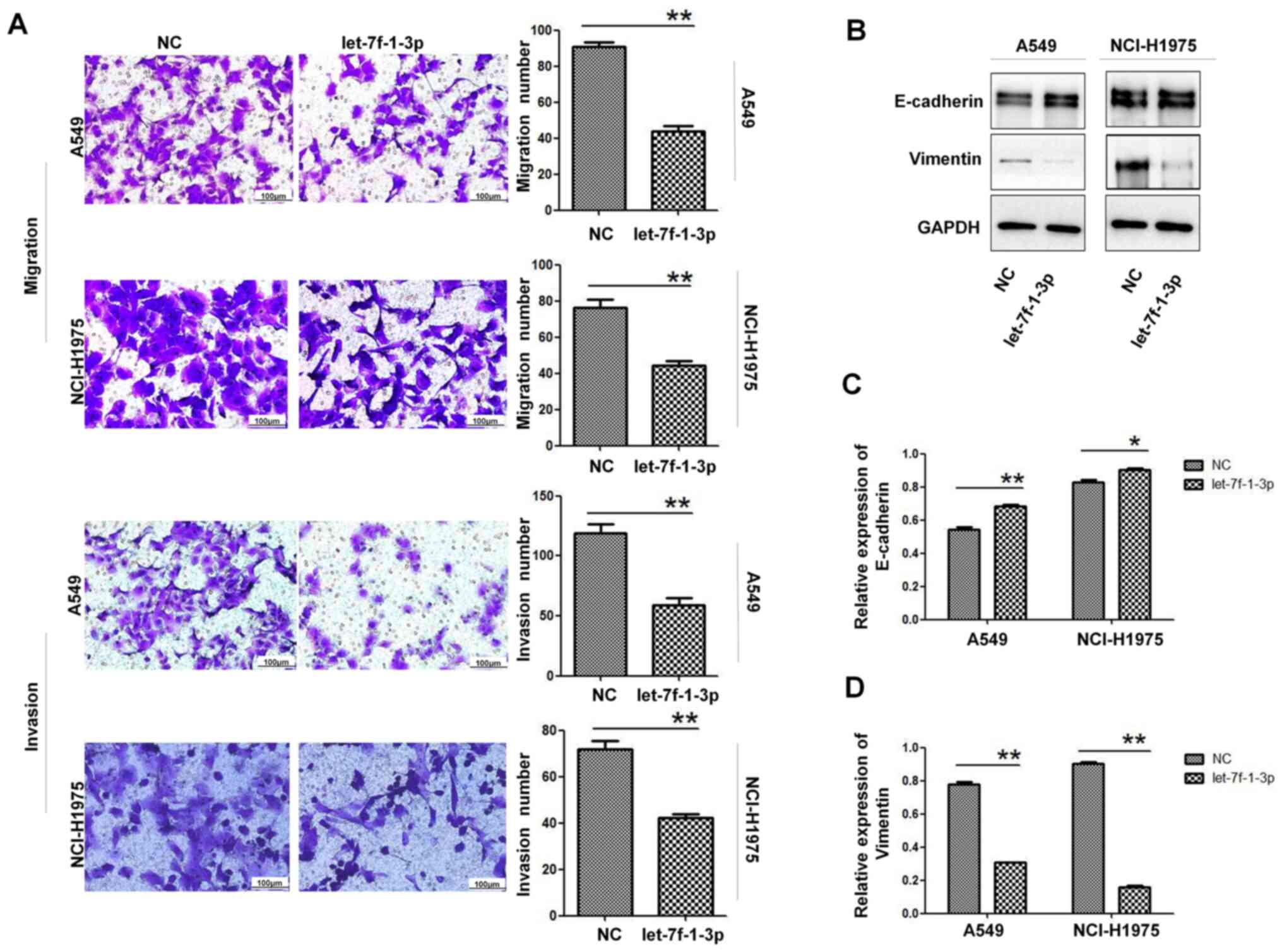
let-7f-1-3p inhibits cell migration
and invasion
Given that the malignancy of tumor cells is closely
associated with their local infiltration and distant migratory
ability, the effect of let-7f-1-3p on the migration and invasion of
NSCLC cells was investigated. The results revealed that the
migratory ability of A549 and NCI-H1975 cells overexpressing
let-7f-1-3p was significantly decreased compared with the NC group
(Fig. 6A). Moreover,
let-7f-1-3p-overexpression inhibited the invasive ability of A549
and NCI-H1975 cells compared with the NC cells (Fig. 6A). Furthermore,
let-7f-1-3p-overexpression increased the expression levels of
E-cadherin (an epithelial cell marker) and reduced the expression
levels of vimentin (a mesenchymal cell marker; Fig. 6B-D). These results suggested that
let-7f-1-3p suppressed the migration and invasion ability of
NSCLC.
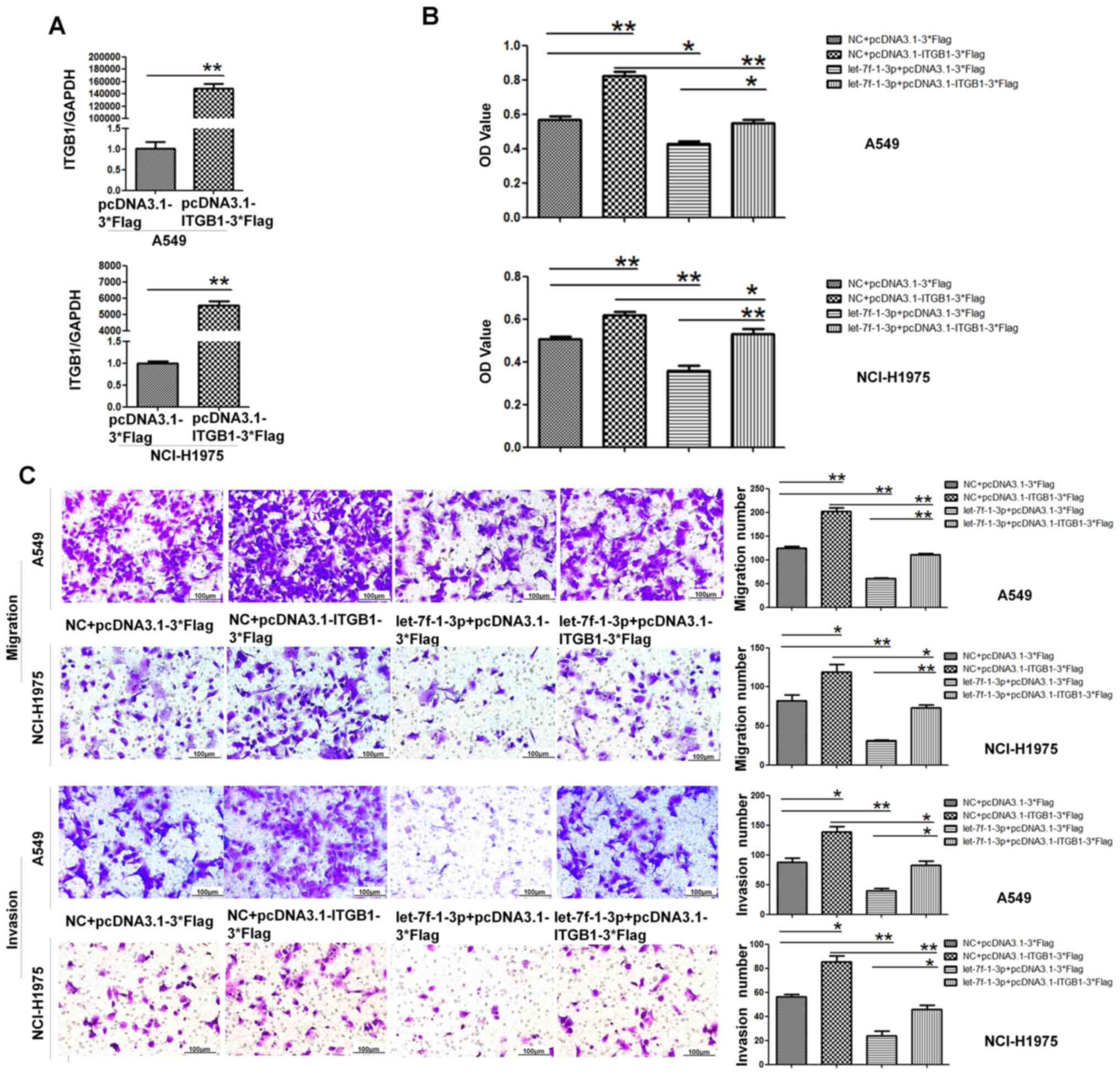
Suppressive role of let-7f-1-3p is
attenuated by the overexpression of ITGB1
Given that let-7f-1-3p suppressed the migration and
invasion of A549 and NCI-H1975 cells, whether overexpression of
ITGB1 could attenuate the effects of let-7f-1-3p was investigated.
First, the transfection efficiency was detected via RT-qPCR. As
presented in Fig. 7A, the
expression level of ITGB1 in two cell lines after overexpression
was significantly increased compared with the control. A549 and
NCI-H1975 cells were treated with NC + pcDNA3.1-3*Flag, NC +
pcDNA3.1-ITGB1-3*Flag, let-7f-1-3p mimics + pcDNA3.1-3*Flag and
let-7f-1-3p mimics + pcDNA3.1-ITGB1-3*Flag. The results of the MTT
assays suggested that the viability potential of A549 and NCI-H1975
cells transfected with let-7f-1-3p and ITGB1 was significantly
increased compared with that of let-7f-1-3p and pcDNA3.1-3*Flag
(Fig. 7B). Moreover, the Transwell
assay results demonstrated that the migration and invasion ability
of the let-7f-1-3p + ITGB1 overexpression group was significantly
increased compared with the let-7f-1-3p mimics + pcDNA3.1-3*Flag
group (Fig. 7C). In conclusion,
these data revealed that let-7f-1-3p inhibited cell viability,
migration and invasion by targeting ITGB1.
let-7f-1-3p promotes the role of DOX
in apoptosis and in inhibiting cell viability, migration and
invasion in vitro
To evaluate the biological consequences of DOX on
the development of NSCLC cells, viability assays and western
blotting were performed. The MTT assay revealed that the viability
of A549 cells treated with 0, 200, 400, 600, and 800 ng/ml DOX
gradually but significantly decreased (Fig. 8A). In addition, DOX treatment
markedly inhibited A549 cell proliferation (Fig. 8B). Next, DOX was revealed to
significantly inhibit ITGB1 expression in A549 cells (Fig. 8C).
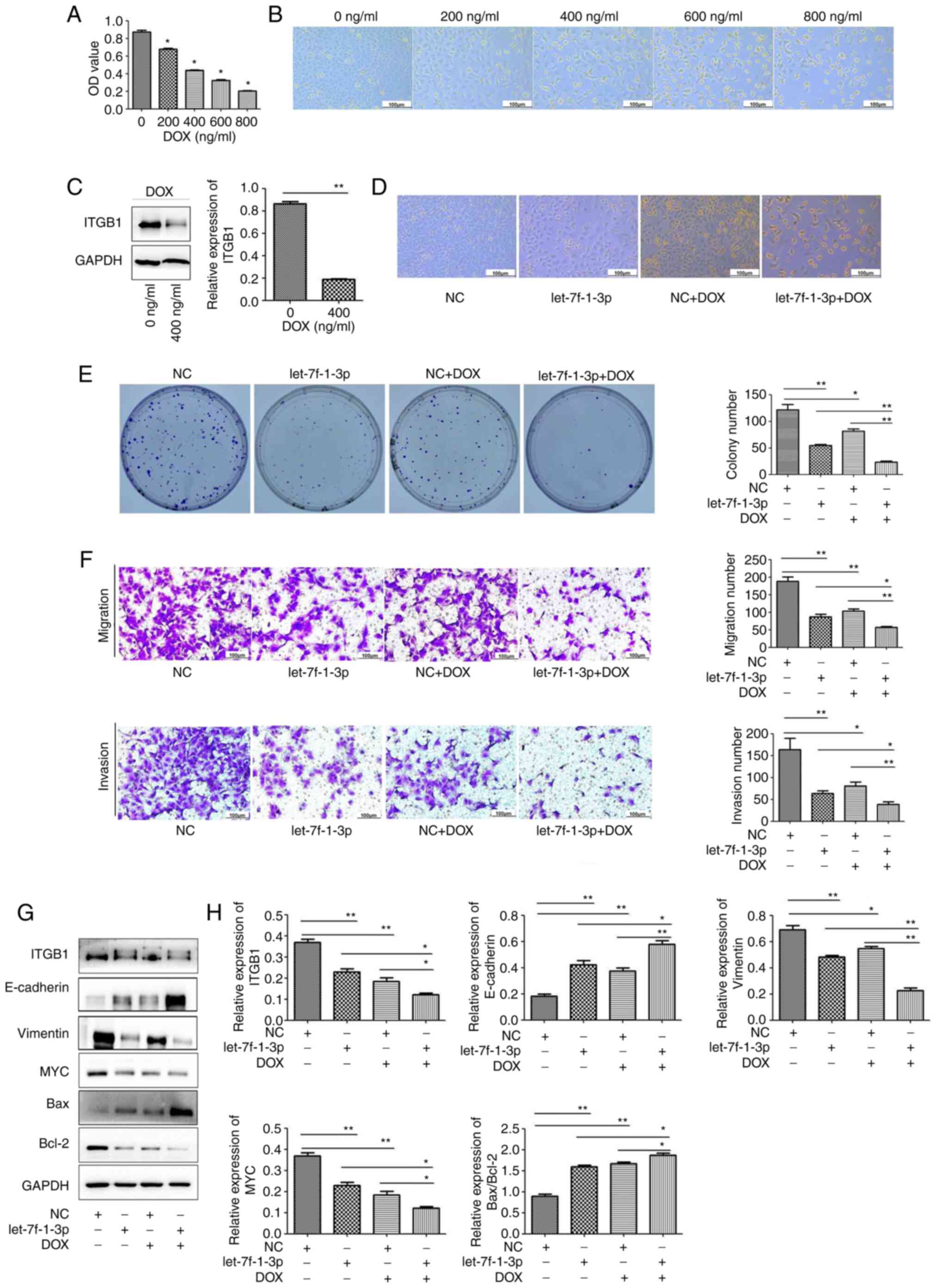 | Figure 8let-7f-1-3p promotes the roles of DOX
in cell apoptosis, viability, migration, and invasion in
vitro. (A) DOX significantly inhibited A549 cell viability in a
dose-dependent manner in the MTT assay. *P<0.05 vs. 0
ng/ml. (B) Fewer living A549 cells were identified in the
DOX-treated lung adenocarcinoma cultures compared with the control
cells (scale bar, 100 µm). (C) DOX (400 ng/ml) suppressed ITGB1
expression in A549 cells, as shown via western blotting on the left
and densitometry analysis on the right. (D) Changes in the number
of cells were detected via microscopy after NC, let-7f-1-3p mimics,
NC + DOX and let-7f-1-3p mimics + DOX treatment. (E) Colonies from
the cell treatment groups are indicated on the left, and colony
formation ability is shown on the right (scale bar, 100 µm). (F)
Images of cell migration and invasion are shown on the left and
quantified on the right. (G) Western blotting and (H) densitometry
analysis results of ITGB1, E-cadherin, vimentin, MYC, Bax and
Bcl-2. *P<0.05, **P<0.01. DOX,
doxorubicin; NC, negative control; OD, optical density; ITGB1,
integrin β1. |
Given that DOX suppressed the proliferation of A549
cells, whether combining DOX with let-7f-1-3p hindered cell
proliferation was investigated. A549 cells were treated with NC +
DOX (200 ng/ml) and let-7f-1-3p mimics + DOX (200 ng/ml). The
number of surviving A549 cells after treatment with let-7f-1-3p
mimics and DOX was decreased compared with that in the control
group and the let-7f-1-3p group (Fig.
8D). Moreover, a significantly decreased number of clones was
observed in the co-treated let-7f-1-3p mimics and DOX group
compared with the let-7f-1-3p mimics group (Fig. 8E). Consistent with the
aforementioned experiments, the results demonstrated that the
number of let-7f-1-3p-overexpressing + DOX clones that passed
through the Transwell chambers were significantly decreased
compared with the number of let-7f-1-3p group that passed through
the chambers (Fig. 8F). The
treatment with let-7f-1-3p + DOX significantly suppressed the
expression of ITGB1, vimentin and Bcl-2 compared with single miRNA
or DOX treatment (Fig. 8G and
H). These data suggested that the
promotive effect of let-7f-1-3p on the anticancer roles of DOX was
associated with the regulation of ITGB1.
let-7f-1-3p and DOX suppress cancer
progression in vivo
To further address the tumor-suppressive role of
let-7f-1-3p in promoting the effect of DOX on NSCLC, A549 cells
transfected with NC and let-7f-1-3p mimics were injected into
BALB/c nude mice to establish A549 lung cancer xenografts, then the
mice with xenografts were treated with DOX. When let-7f-1-3p was
overexpressed, the xenograft growth measured in tumor size, volume
and weight was decreased compared with that of the corresponding
control group (Fig. 9A-C).
Moreover, the tumor growth curves indicated that the tumor volume
in let-7f-1-3p + DOX mice decreased compared with that in
let-7f-1-3p or DOX-treated mice (Fig.
9A and B). Compared with the
NC+NS group, let-7f-1-3p in the let-7f-1-3p+NS group was
significantly increased. Compared with the NC+DOX group,
let-7f-1-3p was significantly upregulated in the let-7f-1-3p+DOX
group (Fig. 9D). ITGB1 expression
significantly decreased in both let-7f-1-3p and DOX-treated mice
compared with that in let-7f-1-3p or DOX-treated xenografts
(Fig. 9E and F). In summary, these results demonstrated
that let-7f-1-3p and DOX co-treatment significantly suppressed
cancer progression via ITGB1 in vivo.
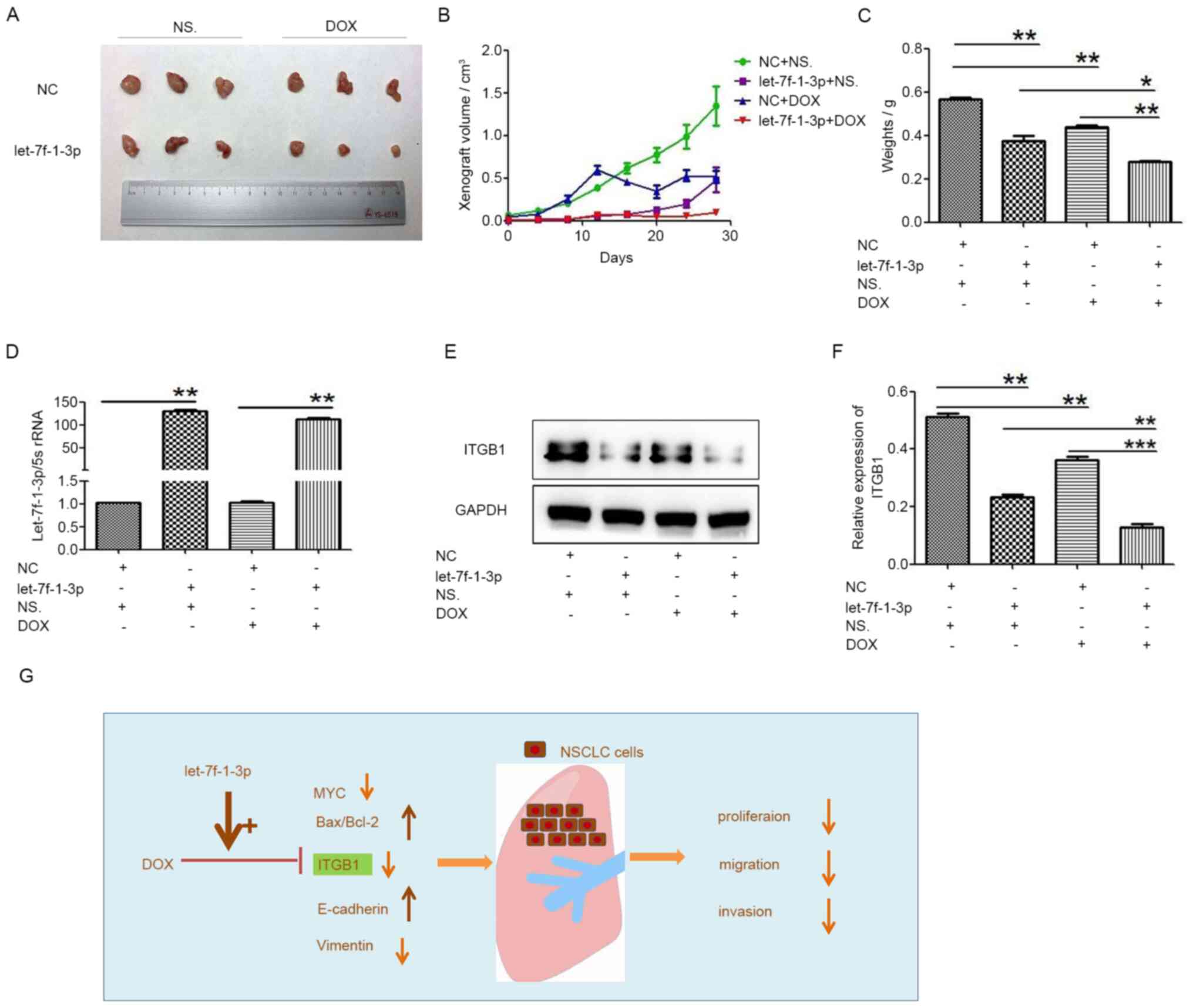 | Figure 9DOX and let-7f-1-3p suppress cancer
progression in vivo. (A) Volumes of A549 cancer xenografts
were detected after NC + saline, let-7f-1-3p mimics + saline, NC +
DOX, and let-7f-1-3p mimics + DOX treatments. (B) Tumor volumes and
(C) weights were the smallest when treated with let-7f-1-3p mimics
+ DOX treatment. (D) Expression of let-7f-1-3p in nude mouse tumors
was analyzed using reverse transcription-quantitative PCR. (E)
Western blotting and (F) quantification of ITGB1 expression in
tumors treated with let-7f-1-3p mimics + DOX compared with control
tumors (G) Compared with let-7f-1-3p group, Bax/Bcl-2 ratio and
E-cadherin were significantly increased, while the expression of
MYC and vimentin were significantly decreased, indicating that
let-7f-1-3p overexpression and DOX inhibited NSCLC cell
proliferation and migration. *P<0.05,
**P<0.01, ***P<0.001. DOX, doxorubicin;
NC, negative control; ITGB1, integrin β1; rRNA, ribosomal RNA;
NSCLC, non-small cell lung cancer; NS, Normal saline. |
Discussion
As a small non-coding RNA, miRNA can promote or
inhibit tumor development and play a regulatory role in cancer
metastasis. For example, miR-200 inhibits epithelial-to-mesenchymal
transition and tumorigenic effects by targeting quaking (27). In triple-negative breast cancer,
miR-200c promotes apoptosis by directly targeting phosphodiesterase
7B (28). miR-532-3p is negatively
associated with the kinesin family member C11 expression, leading
to metastasis via the activation of the gankyrin/AKT signaling
pathway in hepatocellular carcinoma (29). Han et al (26) demonstrated that let-7f attenuates
smoke-induced apoptosis in HSAEC and HPAEpiC. In the present study,
let-7f-1-3p suppressed the proliferation of NSCLC cells in
vitro and in vivo by directly decreasing ITGB1
expression. Furthermore, let-7f-1-3p overexpression reduced MYC
expression and upregulated the Bax/Bcl-2 ratio. let-7f-1-3p also
inhibited the migration and invasion of NSCLC by increasing the
expression of E-cadherin and decreasing the expression of vimentin.
Moreover, let-7f-1-3p promoted the ability of DOX to inhibit cell
viability, migration and invasion both in vitro and in
vitro (Fig. 9G).
Integrins directly bind to the components of the
extracellular matrix and provide the traction necessary for cell
motility and invasion (30). ITGB1,
a potential oncoprotein, contributes to tumor growth and migration
(31). In the present study,
let-7f-1-3p was negatively associated with ITGB1, which was
consistent with the results of DOX treatment. Moreover, the
expression levels of ITGB1 increased in NSCLC tissues compared with
paracancerous lung tissues. ITGB1 plays a notable role in mediating
metastatic dissemination and preventing tumor cell senescence
(32). In addition to migration and
invasion, integrins can regulate proliferation (33). In particular, let-7f-1-3p prevents
tumor viability, migration and invasion by regulating ITGB1.
The co-treatment of DOX and miRNA has been used in
the treatment process of multiple animal cancer models (34,35).
miR-101/DOX-liposome suppresses the malignant progression of liver
cancer cells in vitro and in vivo through the
combinatory effect on miR-101 and DOX (34). Zhang et al (36) revealed that combining DOX and miR-21
inhibitor significantly increases the expression of tumor
suppressor genes to reduce tumor cell proliferation, invasion and
migration in glioblastoma cells compared with the effect of DOX or
miR-21 inhibitor treatment alone. Moreover, co-delivering miR-159
and DOX contributed to the treatment of triple-negative breast
cancer (35). The viability of
breast cancer cells treated with DOX and miR-154 mimic considerably
decreased compared with that of cells treated with DOX alone
(37). Furthermore, miR-608
enhances the efficacy of DOX in the treatment of NSCLC by
suppressing the expression of transcription factor-activated
enhancer-binding protein 4(38).
The present study demonstrated that let-7f-1-3p and DOX suppressed
not only cancer progression by ITGB1 but also viability, migration
and invasion.
The present study did not further explore the ways
in which DOX's inhibitory effect on ITGB1 inhibits the development
of NSCLC. It can be speculated that DOX may affect the expression
of ITGB1 by regulating the expression of an intermediate molecule.
Follow-up studies should use co-immunoprecipitation to identify
molecules that interact with ITGB1, to construct the molecular
regulatory network of ITGB1 and provide new clinical treatment
strategies for NSCLC.
In summary, the current study provided novel
evidence that let-7f-1-3p acted as a tumor suppressor in inhibiting
the viability of NSCLC by targeting ITGB1. let-7f-1-3p can also
promote the anticancer ability of DOX by inhibiting the development
of NSCLC. Future studies will continue to investigate the specific
mechanism by which DOX regulates the development of NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by National Natural
Science Foundation of China (grant nos. 81800169, 81772281 and
81702296), Shandong Science and Technology Committee (grant nos.
2018GSF118056 and ZR2019MH022), Foundation of Shandong Educational
Committee (grant nos. J17KA121 and 2019KJK014), Yantai Science and
Technology Committee (grant no. 2018XSCC051) and Shandong Province
Taishan Scholar Project (grant no. ts201712067).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YaY, YuL, NX, YS, LS, HS, YW and YuY performed the
experiment. YaY, YuL and YoL drafted the manuscript. YuY, PW and YS
analyzed the data. YS, YoL and SX contributed in experimental
design and manuscript revision. SX, YuL and YaY conceived the
study, designed the experiment, and finalized the manuscript. YaY
and YuL confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments with human specimens were performed
in accordance with the relevant guidelines and were approved by The
Medical Ethics Committee of Binzhou Medical University (Yantai,
China; approval number: 2018-07-06). Prior to study inclusion,
written informed consent was obtained from all patients. All animal
experiments in the present study were approved by The Committee on
the Ethics of Animal Experiments of Binzhou Medical University
(approval number: 2018-07-06).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feng H, Ge F, Du L, Zhang Z and Liu D:
MiR-34b-3p represses cell proliferation, cell cycle progression and
cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting
CDK4. J Cell Mol Med. 23:5282–5291. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lang N, Wang C, Zhao J, Shi F, Wu T and
Cao H: Long noncoding RNA BCYRN1 promotes glycolysis and tumor
progression by regulating the miR149/PKM2 axis in nonsmallcell lung
cancer. Mol Med Rep. 21:1509–1516. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zou A, Liu X, Mai Z, Zhang J, Liu Z, Huang
Q, Wu A and Zhou C: LINC00472 acts as a tumor suppressor in NSCLC
through KLLN-mediated p53-signaling pathway via MicroRNA-149-3p and
MicroRNA-4270. Mol Ther Nucleic Acids. 17:563–577. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai FQ, Li CR, Fan XQ, Tan L, Wang RT and
Jin H: miR-150-5p inhibits non-small-cell lung cancer metastasis
and recurrence by targeting HMGA2 and β-catenin signaling. Mol Ther
Nucleic Acids. 16:675–685. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang W, Shen XB, Jia W, Huang DB, Wang Y
and Pan YY: The p53/miR-193a/EGFR feedback loop function as a
driving force for non-small cell lung carcinoma tumorigenesis. Ther
Adv Med Oncol. 11(1758835919850665)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu X, Min S, Wu N, Liu H, Wang T, Li W,
Shen Y, Zhao C, Wang H, Qian Z, et al: miR-193a-3p inhibition of
the Slug activator PAK4 suppresses non-small cell lung cancer
aggressiveness via the p53/Slug/L1CAM pathway. Cancer Lett.
447:56–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y,
Gong F and Jiang W: Exosomes derived from miR-143-overexpressing
MSCs inhibit cell migration and invasion in human prostate cancer
by downregulating TFF3. Mol Ther Nucleic Acids. 18:232–244.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gu C, Cai J, Xu Z, Zhou S, Ye L, Yan Q,
Zhang Y, Fang Y, Liu Y, Tu C, et al: MiR-532-3p suppresses
colorectal cancer progression by disrupting the ETS1/TGM2
axis-mediated Wnt/beta-catenin signaling. Cell Death Dis.
10(739)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tung CH, Kuo LW, Huang MF, Wu YY, Tsai YT,
Wu JE, Hsu KF, Chen YL and Hong TM: MicroRNA-150-5p promotes cell
motility by inhibiting c-Myb-mediated Slug suppression and is a
prognostic biomarker for recurrent ovarian cancer. Oncogene.
39:862–876. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shen GY, Ren H, Shang Q, Zhao WH, Zhang
ZD, Yu X, Huang JJ, Tang JJ, Yang ZD, Liang D and Jiang XB:
Let-7f-5p regulates TGFBR1 in glucocorticoid-inhibited osteoblast
differentiation and ameliorates glucocorticoid-induced bone loss.
Int J Biol Sci. 15:2182–2197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tan W, Gu Z, Leng J, Zou X, Chen H, Min F,
Zhou W, Zhang L and Li G: Let-7f-5p ameliorates inflammation by
targeting NLRP3 in bone marrow-derived mesenchymal stem cells in
patients with systemic lupus erythematosus. Biomed Pharmacother.
118(109313)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li ZH, Wang YF, He DD, Zhang XM, Zhou YL,
Yue H, Huang S, Fu Z, Zhang LY, Mao ZQ, et al: Let-7f-5p suppresses
Th17 differentiation via targeting STAT3 in multiple sclerosis.
Aging (Albany NY). 11:4463–4477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao XR, Ge J, Li WY, Zhou WC, Xu L and
Geng DQ: NF-kappaB/let-7f-5p/IL-10 pathway involves in wear
particle-induced osteolysis by inducing M1 macrophage polarization.
Cell Cycle. 17:2134–2145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shen GY, Ren H, Huang JJ, Zhang ZD, Zhao
WH, Yu X, Shang Q, Qiu T, Zhang YZ, Tang JJ, et al: Plastrum
testudinis extracts promote BMSC proliferation and osteogenic
differentiation by regulating Let-7f-5p and the TNFR2/PI3K/AKT
signaling pathway. Cell Physiol Biochem. 47:2307–2318.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han L, Zhou Y, Zhang R, Wu K, Lu Y, Li Y,
Duan R, Yao Y, Zhu D and Jia Y: MicroRNA Let-7f-5p promotes bone
marrow mesenchymal stem cells survival by targeting caspase-3 in
Alzheimer disease model. Front Neurosci. 12(333)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tie Y, Chen C, Yang Y, Qian Z, Yuan H,
Wang H, Tang H, Peng Y, Du X and Liu B: Upregulation of let-7f-5p
promotes chemotherapeutic resistance in colorectal cancer by
directly repressing several pro-apoptotic proteins. Oncol Lett.
15:8695–8702. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Di Fazio P, Maass M, Roth S, Meyer C,
Grups J, Rexin P, Bartsch DK and Kirschbaum A: Expression of
hsa-let-7b-5p, hsa-let-7f-5p, and hsa-miR-222-3p and their putative
targets HMGA2 and CDKN1B in typical and atypical carcinoid tumors
of the lung. Tumour Biol. 39(1010428317728417)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chang J, Huang L, Cao Q and Liu F:
Identification of colorectal cancer-restricted microRNAs and their
target genes based on high-throughput sequencing data. Onco Targets
Ther. 9:1787–1794. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cui J, Huang W, Wu B, Jin J, Jing L, Shi
WP, Liu ZY, Yuan L, Luo D, Li L, et al: N-glycosylation by
N-acetylglucosaminyltransferase V enhances the interaction of
CD147/basigin with integrin beta1 and promotes HCC metastasis. J
Pathol. 245:41–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao G, Gong L, Su D, Jin Y, Guo C, Yue M,
Yao S, Qin Z, Ye Y, Tang Y, et al: Cullin5 deficiency promotes
small-cell lung cancer metastasis by stabilizing integrin β1. J
Clin Invest. 129:972–987. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang X, Zhou Q, Yu Z, Wu X, Chen X, Li J,
Li C, Yan M, Zhu Z, Liu B and Su L: Cancer-associated
fibroblast-derived Lumican promotes gastric cancer progression via
the integrin beta1-FAK signaling pathway. Int J Cancer.
141:998–1010. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Izumi D, Ishimoto T, Miyake K, Sugihara H,
Eto K, Sawayama H, Yasuda T, Kiyozumi Y, Kaida T, Kurashige J, et
al: CXCL12/CXCR4 activation by cancer-associated fibroblasts
promotes integrin beta1 clustering and invasiveness in gastric
cancer. Int J Cancer. 138:1207–1219. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu JI, Lin YP, Tseng CW, Chen HJ and Wang
LH: Crabp2 promotes metastasis of lung cancer cells via HuR and
integrin β1/FAK/ERK signaling. Sci Rep. 9(845)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han Z, Zhu Y, Cui Z, Guo P, Wei A and Meng
Q: MicroRNA Let-7f-1-3p attenuates smoke-induced apoptosis in
bronchial and alveolar epithelial cells in vitro by targeting
FOXO1. Eur J Pharmacol. 862(172531)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim EJ, Kim JS, Lee S, Lee H, Yoon JS,
Hong JH, Chun SH, Sun S, Won HS, Hong SA, et al: QKI, a miR-200
target gene, suppresses epithelial-to-mesenchymal transition and
tumor growth. Int J Cancer. 145:1585–1595. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang DD, Li Y, Xu Y, Kim J and Huang S:
Phosphodiesterase 7B/microRNA-200c relationship regulates
triple-negative breast cancer cell growth. Oncogene. 38:1106–1120.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Han J, Wang F, Lan Y, Wang J, Nie C, Liang
Y, Song R, Zheng T, Pan S, Pei T, et al: KIFC1 regulated by
miR-532-3p promotes epithelial-to-mesenchymal transition and
metastasis of hepatocellular carcinoma via gankyrin/AKT signaling.
Oncogene. 38:406–420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen MB, Lamar JM, Li R, Hynes RO and Kamm
RD: Elucidation of the roles of tumor integrin β1 in the
extravasation stage of the metastasis cascade. Cancer Res.
76:2513–2524. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kren A, Baeriswyl V, Lehembre F, Wunderlin
C, Strittmatter K, Antoniadis H, Fässler R, Cavallaro U and
Christofori G: Increased tumor cell dissemination and cellular
senescence in the absence of beta1-integrin function. EMBO J.
26:2832–2842. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Assoian RK and Klein EA: Growth control by
intracellular tension and extracellular stiffness. Trends Cell
Biol. 18:347–352. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu F, Liao JZ, Xiang GY, Zhao PX, Ye F,
Zhao Q and He XX: MiR-101 and doxorubicin codelivered by liposomes
suppressing malignant properties of hepatocellular carcinoma.
Cancer Med. 6:651–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding
X, Qiang L, Li G, Han Z, Yuan Y and Gao S: Functional
exosome-mediated co-delivery of doxorubicin and hydrophobically
modified microRNA 159 for triple-negative breast cancer therapy. J
Nanobiotechnology. 17(93)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang S, Han L, Wei J, Shi Z, Pu P, Zhang
J, Yuan X and Kang C: Combination treatment with doxorubicin and
microRNA-21 inhibitor synergistically augments anticancer activity
through upregulation of tumor suppressing genes. Int J Oncol.
46:1589–1600. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bolandghamat Pour Z, Nourbakhsh M,
Mousavizadeh K, Madjd Z, Ghorbanhosseini SS, Abdolvahabi Z, Hesari
Z and Ezzati Mobasser S: Suppression of nicotinamide
phosphoribosyltransferase expression by miR-154 reduces the
viability of breast cancer cells and increases their susceptibility
to doxorubicin. BMC Cancer. 19(1027)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang YF, Ao X, Liu Y, Ding D, Jiao WJ, Yu
Z, Zhai WX, Dong SH, He YQ, Guo H and Wang JX: MicroRNA-608
promotes apoptosis in non-small cell lung cancer cells treated with
doxorubicin through the inhibition of TFAP4. Front Genet.
10(809)2019.PubMed/NCBI View Article : Google Scholar
|