Introduction
Pulmonary fibrosis (PF) is a chronic, progressive
and potentially lethal pulmonary interstitial disease that
primarily occur in middle-aged and elderly individuals (1,2). This
disease is characterized by an unusual interstitial pneumonitis,
where fibrosis and honeycomb-like lesions can be observed at the
sub-pleura and basal pleura (3,4). In
addition, deposition of collagen and extracellular matrix (ECM)
occur at the peripheral PF locus, which ultimately results in
structural changes in the pulmonary tissues, loss of pulmonary
ventilation, pulmonary diffusion and even mortality (3,4). PF is
a pulmonary disease, with a median survival time of only 2-4 years
in patients who do not receive lung transplants, making it a severe
lung disease (5). In particular,
the incidence of PF continues to rise with an increased aging
population worldwide (6). However,
the underlying pathogenic mechanism of PF remain poorly understood
(7).
It has been previously hypothesized that PF is
closely associated with alterations in alveolar epithelial cells
and pulmonary fibroblasts (6). In
PF, the initiating factor is typically alveolar epithelial cell
injury (8). After the epithelial
cells are aberrantly activated due to external damage and
stimulation, they secrete large quantities of inflammatory factors,
including IL-6, IL-17 and TGF-β1 (9,10).
This induces the conversion of epithelial cells into fibroblast,
causes the chemotaxis of mononuclear cells in the circulatory
system into the pulmonary space, transformation of pulmonary
fibroblasts into myofibroblasts and the secretion and deposition of
large amounts of ECM into the pulmonary tissues, leading to
morphological and structural changes in the pulmonary tissues
(11-13).
At present, a therapeutic strategy that is fully
effective for the treatment of PF remain unavailable. The
clinically applied pirfenidone and nintedanib can only delay the
deterioration of lung function in patients with slight-to-moderate
PF (14,15). Additionally, N-acetylcysteine can
only improve the survival rate in patients with the recombinant
toll interacting protein TT genotype (16). Over the past decade, with increased
interest in traditional Chinese medicine (TCM) and symptom-based
and disease-based approaches, a growing number of clinical trials
and studies have emerged regarding the treatment of PF using
extracts of Chinese medicine, highlighting the potentially novel
methods and for the treatment of PF (17-21).
Therefore, it may prove to be beneficial to explore the
applicability of Chinese medicine to fully exploit its unique
advantages, whilst at the same time to characterize the bioactive
medicinal compounds contained within the TCM for the treatment of
PF.
In the present study, a single-use bleomycin (BLM)
instillation method was used to establish a PF mouse model, which
was subsequently treated using MWYF by intragastric administration.
In the PF mice, the role of MWYF decoction was investigated, in
addition to the possible mechanism of action.
Materials and methods
Reagents and instruments
The MWYF decoction was obtained from Jiangsu
Province Hospital of Chinese Medicine. BLM was supplied by TCI
(cat. no. B3972-10MG, Shanghai, China). Hydroxyproline (HYP; cat.
no. F10614), pyridinoline (PYD; cat. no. F11442), collagen I
(F5760; all from Shanghai Westang Bio-Tech Co., Ltd.), TNF-α (cat.
no. 70-EK282/3-96), IL-6 (cat. no. 70-EK206/3-96) and IL-7 [cat.
no. 70-EK207/2-96; all from MULTISCIENCES (LIANKE) BIOTECH, CO.,
LTD] were ELISA test kits. α-Smooth muscle actin (α-SMA; cat. no.
14395-1-AP), vimentin (cat. no. 10366-1-AP 14395-1-AP), fibronectin
(cat. no. 15613-1-AP), collagen I (cat. no. 14695-1-AP), collagen
III (cat. no. 22734-1-AP), TNF-α (cat. no. 17590-1-AP), IL-6 (cat.
no. 21865-1-AP), IL-17 (cat. no. 13082-1-AP), TGF-β1 (cat. no.
21898-1-AP), Smad3 (cat. no. 25494-1-AP) and β-actin (cat. no.
20536-1-AP) antibodies were purchased from Wuhan Sanying.
Preparation of MWYF decoction and
quality control standards
The MWYF decoction included 15 g Codonopsis
pilosula, 15 g Michaelmas daisy, 10 g Radix
ophiopogonis, 10 g Schisandra chinensis, 10 g perilla
fruit, 10 g Pinellia ternate, 10 g Scutellaria baicalensis,
10 g Angelica sinensis, 10 g tangerine peel, 6 g fried
Chinese ephedra, 3 g Asarum sieboldi and 3 g fried
glycyrrhiza. The raw drug material was identified and authenticated
by Professor Zhu Yufeng (Department of Pharmacy, Jiangsu Province
Chinese Medicine Hospital, Jiangsu, China). A total of 5 doses of
MWYF raw drug material was immersed in 3 l ddH2O at 20˚C
for 30 min. The mixture of water and raw drug material was then
boiled to 120˚C for 30 min and a decoction was collected. This
procedure was repeated twice and the decoctions were pooled
together. The 5 doses of decoction were centrifuged at 500 x g
before the supernatant was collected, filtered, concentrated and
freeze-dried to obtain a freeze-dried powder, which was used to
prepare different concentrations of MWYF.
The characteristic components in the MWYF decoction
were qualitatively analysed using liquid chromatography-mass
spectrometry (LC-MS). Briefly, 50 mg freeze-dried samples were
extracted using 800 µl 80% methanol and 10 µl internal standard
(2.8 mg/ml, DL-o-chlorophenylalanine) and then vortexed for 30 sec.
Next, the samples were ultrasonicated for 30 min at 40 kHz at 50˚C
and all owed to stand for 1 h at 20˚C. The samples were centrifuged
1,800 x g and 4˚C for 15 min. The supernatant was collected in
small vials and then used for evaluation by LC-MS (UltiMate™ 3000,
Q Exactive™; Thermo Fisher Scientific, Inc.). LC-MS was performed
using a C18 chromatographic column (Hypersil GOLD C18; 100x2.1 mm,
1.9 µm; Thermo Fisher Scientific, Inc.). Chromatographic isolation
was performed at a column temperature of 40˚C with a 0.3 ml/min
flow rate. The composition of the mobile phase A was water + 5%
acetonitrile and 0.1% formic acid, while mobile phase B was
composed of acetonitrile + 0.1% formic acid. The injection volume
was 10 µl. The temperature of the autosampler was 4˚C.
Method for model construction
A single dose intratracheal instillation of BLM was
used to construct the PF mouse model. The mice were first
anesthetized by an intraperitoneal injection of 3% sodium
pentobarbital (40 mg/kg) and fixed on the operating table (22,23).
Along the neck, the skin was cut open lengthways and the trachea
was passively exposed. A blunt no. 7 lumbar spinal needle was
inserted slowly into the trachea along the root of the tongue. The
needle was then inserted with the depth of 2.8-cm and 5 mg/kg BLM
was slowly instilled (24). The
control group was treated in a manner similar to that in the
treatment group, with physiological saline instilled instead of
BLM. To ensure even distribution of BLM into the lung tissues, the
mice were moved back and forth and upside down.
Test animals and grouping
A total of 50 SPF grade C57BL/6 male mice (weight,
20-25 g) were supplied by the Qinglongshan Animal Centre
[Jiangning, Nanjing; licence no. SCXK (Su) 2017-0001; certification
no. 201903602]. The mice were reared at the SPF grade test animal
centre at the Nanjing University of Traditional Chinese Medicine at
22˚C and 46% relative humidity, with a 12-h light/dark cycle. The
mice were provided with ad libitum access to food and water
and were allowed to acclimatise to the new environment for 1 week
prior to experimentation. All experimental studies followed the
Guide for the Care and Use of Laboratory Animals (25). The present study was approved by the
Ethics Commission for Animal Tests of the Nanjing University of
Traditional Chinese Medicine (Nanjing, China). A total of 50 mice
were randomly assigned into five groups (10 mice in each group).
For the control group, saline was administered through oral gavage.
After the PF model was successfully established, mice in the model
group were administered with saline through oral gavage. The
treatment groups also received BLM to establish the model, but then
subsequently received 20, 40 or 60 g/kg/day MWYF soup by gavage
from days 1 to 21. Body weight was measured every 2 days. The unit
used for the MWYF dosage is termed the total raw material. Fur
color, behaviour and other vital signs were observed every day. A
total of 21 days after continuous administration of MWYF, the mice
were intraperitoneally injected with 3% sodium pentobarbital (150
mg/kg) for euthanasia (23). Lung
tissues and bronchoalveolar lavage fluid (BALF) were then
collected. For each mouse, the lower lobe of the right lung was
collected and fixed in 4% paraformaldehyde (4˚C; 24 h). The
remaining lung tissues were maintained in 2 ml tubes and
flash-frozen in liquid nitrogen. The samples were then stored at
-80˚C until required for further analysis.
Pulmonary alveolitis and PF
scoring
Lung tissues were fixed in 4% polyoxymethylene (4˚C)
for 24 h. The tissues were embedded in paraffin and cut into 4-µm
thick sections. The slices were then subjected to H&E and
Masson staining. The levels of alveolar inflammation were scored
using Szapiel's method (26). The
levels of PF were scored using Ashcroft's method (27).
Collagen I, HYP, PYD and inflammatory
cytokines in pulmonary tissues and BALF
The optical density (OD) value was measured after
ELISA was performed according to the manufacturer's protocols,
before the final content of HYP and PYD was calculated using a
standard curve.
TNF-α, IL-6 and IL-17 levels in BALF were determined
using ELISA, according to the manufacturer's protocol. The colour
was developed at room temperature, the OD was recorded and TNF-α,
IL-6 and IL-17 levels in the BALF were calculated using a standard
curve.
Immunohistochemical determination of
collagen I and collagen III
Lung tissue sections (5 µm) were deparaffinized with
xylene and ethanol and washed with PBS. A few drops of 3%
H2O2 were added to block endogenous
peroxidase activity at 25˚C for 25 min in the dark. Antigen
retrieval was performed by boiling in citrate buffer solution at
95˚C for 20 min. The tissues were then blocked with 3% BSA (cat.
no. R22294; ShangHai YuanYe Biotechnology Co., Ltd.) at 25˚C for 30
min. Collagen I and collagen III antibodies were added to the
samples at 4˚C for 12 h, which were placed in a wet box. After
overnight incubation, the secondary antibody (Biotin-conjugated
Affinipure Goat Anti-Rabbit; 1:500; cat. no. SA00004-2; ProteinTech
Group, Inc.) was applied at 25˚C for 50 min, and the signal was
revealed using 3,3'-diaminobenzidine reagent (cat. no. 8059S; Cell
Signalling Technology, Inc.) followed by sealing of the sections
with neutral resin. The expression of collagen I and collagen III
were observed under a light microscope (XSP-C204; Chongqing
Chongguang Industrial Co., Ltd.) at a magnification of x200.
Results were quantified using ImageJ software (v.1.52; National
Institutes of Health).
Determination of vimentin and α-SMA
expression using immunofluorescence analysis
Lung tissue slices (5 µm) were incubated in citrate
buffer (0.01 mol/l; pH 6.0) at 95˚C for 20 min. The sections were
then sealed with 10% goat serum at 37˚C for 30 min. The primary
antibody (α-SMA or vimentin; 1:200) was added and the sections were
incubated overnight at 4˚C. The following day, the sections were
washed with PBS and then incubated with the secondary antibody
(Alexa Fluor 647; 1:500; cat. no. A0468; Beyotime Institute of
Biotechnology) at 25˚C for 50 min in the dark. Finally, the cell
nuclei were stained using DAPI (cat. no. C1002; Beyotime Institute
of Biotechnology) at 37˚C for 10 min in the dark. The slides were
observed under a fluorescence microscope (magnification, x200;
Leica Microsystems GmbH).
Western blotting
Briefly, 20 mg lung tissue was added to RIPA Lysis
Buffer (cat. no. P0013B; Beyotime Institute of Biotechnology). The
tissues were homogenised and total protein was extracted. The
protein content was measured using a bicinchoninic acid assay. The
protein sample was subjected to 10% SDS-PAGE with 30 µg/well and
then transferred onto a PVDF membrane, before and the membrane was
blocked at room temperature for 2 h with 5% skimmed milk and washed
with TBS with 100.1% Tween-20 (TBST) three times, 10 min per wash.
The primary antibody was prepared according to the manufacturer's
protocol and incubated at 4˚C for 12-18 h. The dilution of the
α-SMA antibody used was 1:2,000, whilst the dilution of
fibronectin, vimentin, collagen I, collagen III, TGF-β1 and Smad3
antibodies was 1:1,000. The membranes were washed again with TBST
three times for 10 min. Subsequently, the membrane was incubated
with an HRP-conjugated secondary antibody (cat. no. 3999S; Cell
Signalling Technology, Inc.) at room temperature for 1 h. Next, the
membranes were washed with TBST three times for 10 min. Signals
were visualized using chemiluminescence reagent (cat. no. 170-5061;
Bio-Rad Laboratories, Inc.). Densitometry analysis was performed
using Image Lab software (v.5.1; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Pulmonary tissues were homogenised, and total RNA
was extracted using TRIzol® reagent (cat. no. 15596026;
Thermo Fisher Scientific, Inc.). The concentration of RNA was
calculated, and cDNA was synthesised using Hifair® Ⅲ 1st
Strand cDNA Synthesis SuperMix kit (cat. no. 11141ES60; Shanghai
Yeasen Biotechnology Co., Ltd.) according to the manufacturer's
protocol. Hieff® qPCR SYBR Green Master Mix kit (cat.
no. 11201ES03; Shanghai Yeasen Biotechnology Co., Ltd.) was used to
assay the relative expression levels of TGF-β1 and Smad3 in the
pulmonary tissues. The thermocycling conditions used were as
follows: 95˚C for 5 min; followed by 40 cycles of denaturation at
95˚C for 10 sec, annealing at 60˚C for 20 sec and elongation at
72˚C for 20 sec. The average expression of target genes and GAPDH
was calculated using the Cq values. In each group, the relative
amount of gene expression was calculated using the
2-ΔΔCq value of the model group (28). Primers for TGF-β1 and Smad3 were
synthesised by Sangon Biotech Co., Ltd. The sequences of the
primers were TGF-β1 forward, 5'-CCAGATCCTGTCCAAACTAAGG-3' and
reverse, 5'-CTCTTTAGCATAGTAGTCCGCT-3' Smad3 forward,
5'-ATTCCATTCCCGAGAACACTAA-3' and reverse,
5'-TAGGTCCAAGTTATTGTGTGCT-3'; GAPDH forward,
5'-GGTTGTCTCCTGCGACTTCA-3'; and reverse,
5'-TGGTCCAGGGTTTCTTACTCC-3'.
Statistical analysis
All results are based on triplicate experiments and
presented as the mean ± standard deviation (SD). Statistical
analysis was performed using GraphPad Prism 8 software (GraphPad
Software, Inc.). Inflammation and fibrosis scores were compared
using Kruskal-Wallis test, followed by the Dunn post hoc test. One-
or two-way analysis of variance followed by Tukey's test was used
to determine statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Primary components in MWYF
MWYF consists of 12 Chinese herbal medicines,
indicating a complex composition. Amino acids, flavonoids, phenols,
terpenes and organic acids were identified in the MWYF decoction
and LC-MS was used for the analysis of these chemical ingredients.
Under optimised LC-MS conditions, the primary components of MWYF
were isolated and detected. The typical components are shown in
Table I. The peak signals for the
12 chemicals were identified according to the retention time, mass
of fragment ions (mass/valence), the wavelength of UV absorption.
The total ion flow of these components is shown in Fig. 1. In the chromatograms, succinic
acid, ephedrine, ferulic acid, esperidin, rosmarinic acid,
lobetyolin, baicalin, glycyrrhizic acid, schizandrin, asarinin,
ruscogenin and shionone were detected.
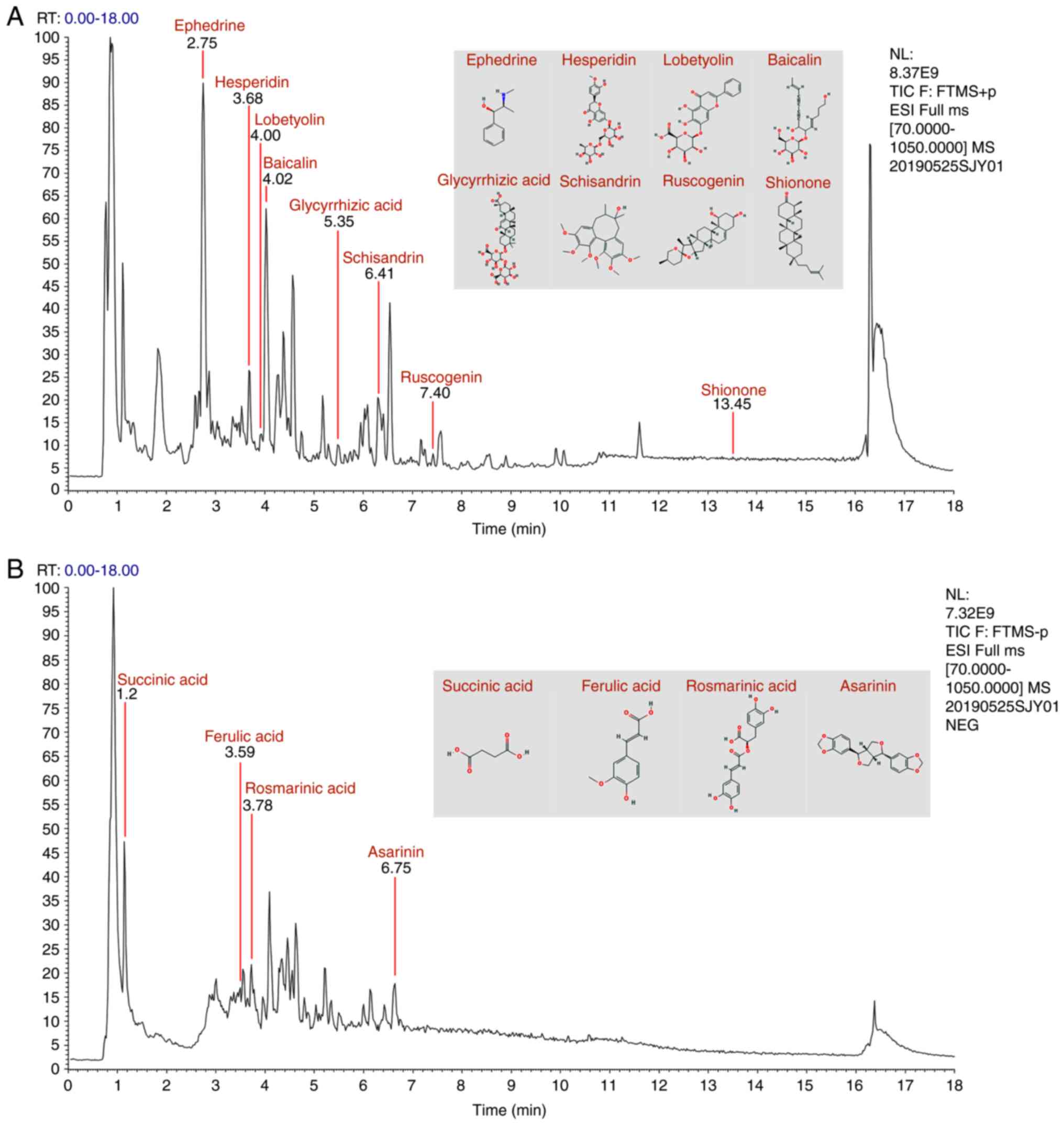 | Figure 1Elementary particle flow graph
chromatogram of the Maiwei Yangfei decoction. Monitoring in (A)
positive ion mode and (B) negative ion mode. In the chromatograms,
succinic acid, ephedrine, ferulic acid, esperidin, rosmarinic acid,
lobetyolin, baicalin, glycyrrhizic acid, schizandrin, asarinin,
ruscogenin and shionone were detected. |
 | Table IComposition of Maiwei Yangfei
decoction. |
Table I
Composition of Maiwei Yangfei
decoction.
| Compound
number | Component | Formula | Adduct | Exact Mass | Retention time
(min) | Mass/charge
ratio | Parts per
million | Chinese name |
|---|
| 1 | Succinic acid |
C4H6O4 |
[M-H]- | 117.01823 | 1.20 | 191.01891 | -3.548 | Ban xia |
| 2 | Ephedrine |
C10H15NO |
[M+H]+ | 166.12264 | 2.75 | 447.09247 | 0.417 | Ma huang |
| 3 | Ferulic acid |
C10H10O4 |
[M-H]- | 193.04953 | 3.59 | 193.04967 | 0.698 | Dang gui |
| 4 | Hesperidin |
C28H34O15 |
[M+H]+ | 611.19704 | 3.70 | 611.19733 | 0.464 | Chen pi |
| 5 | Rosmarinic
acid |
C18H16O8 |
[M-H]- | 359.07614 | 3.78 | 359.07700 | 3.470 | Zi su zi |
| 6 | Lobetyolin |
C20H28O8 |
[M+Na]+ | 419.16763 | 4.00 | 419.16718 | -0.969 | Dang shen |
| 7 | Baicalin |
C21H18O11 |
[M+H]+ | 447.09218 | 4.02 | 447.09247 | 0.631 | Huang qin |
| 8 | Glycyrrhizic
acid |
C42H62O16 |
[M+H]+ | 823.41106 | 5.48 | 823.41187 | 0.981 | Gan cao |
| 9 | Schisandrin |
C24H32O7 |
[M+H]+ | 433.22207 | 6.41 | 433.22208 | 0.000 | Wu wei zi |
| 10 | Asarinin |
C20H18O6 |
[M-H]- | 353.10196 | 6.75 | 353.10315 | 3.357 | Xi xin |
| 11 | Ruscogenin |
C27H42O4 |
[M+H]+ | 431.31558 | 7.40 | 431.20694 | 0.774 | Mai dong |
| 12 | Shionone |
C30H50O |
[M+H]+ | 427.39344 | 13.45 | 427.39380 | 0.836 | Zi wan |
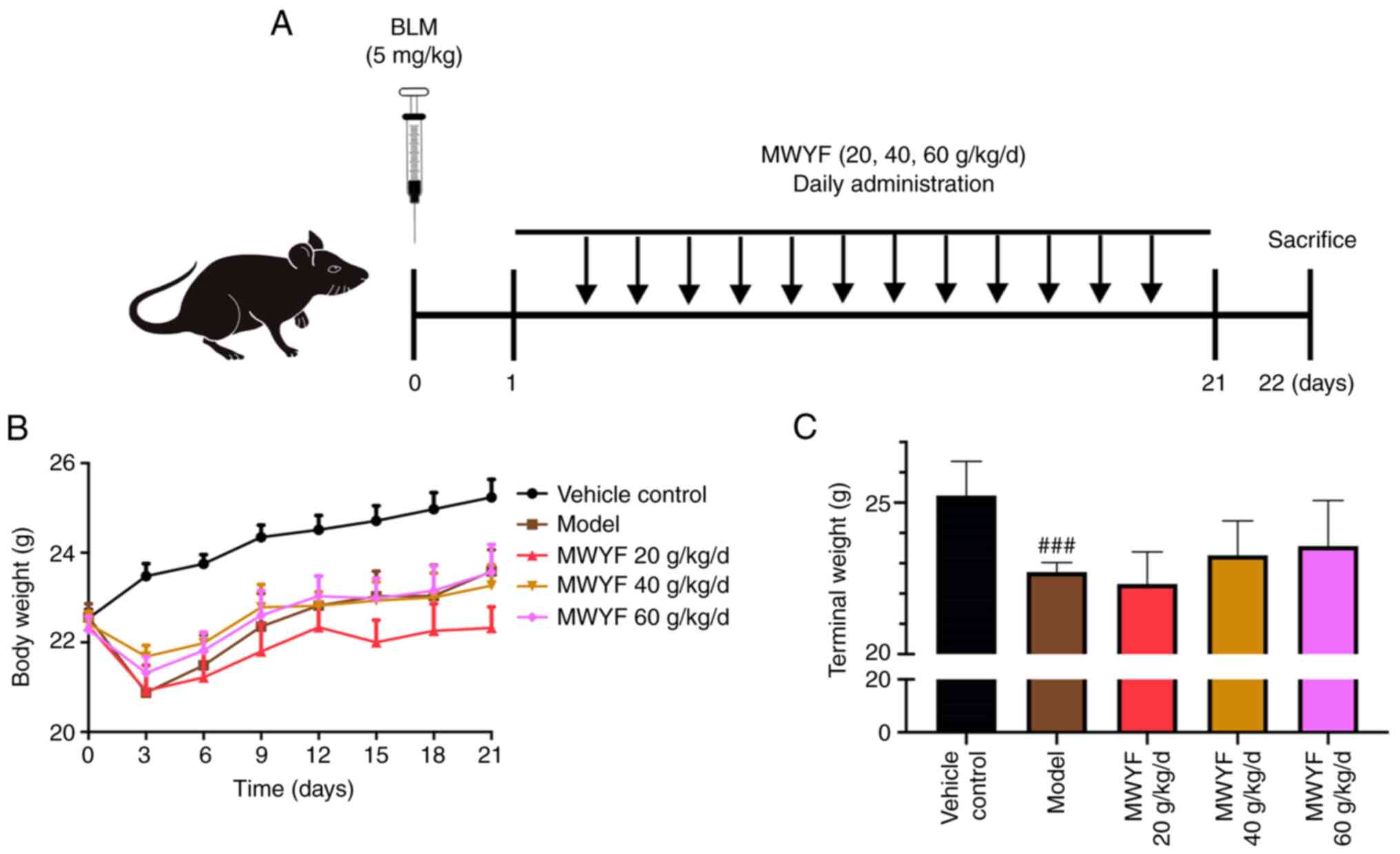
Body weight
To assess the effects of MWYF fibrosis in
vivo, mice were instilled with BLM to induce lung fibrosis.
MWYF at doses of 20, 40 or 60 g/kg/day were orally given to the
mice for 21 days (Fig. 2A). The
weights of the mice were markedly reduced within 3 days of model
construction, with the exception of the control group. However, 1
week after the model was constructed, the weight of mice in all
groups continued to recover (Fig.
2B). Compared with those in the control group, the terminal
mice weights in the model group were significantly reduced.
Although the body weights of mice in the treatment group increased,
no statistical difference could be observed compared with those in
the model group (Fig. 2C).
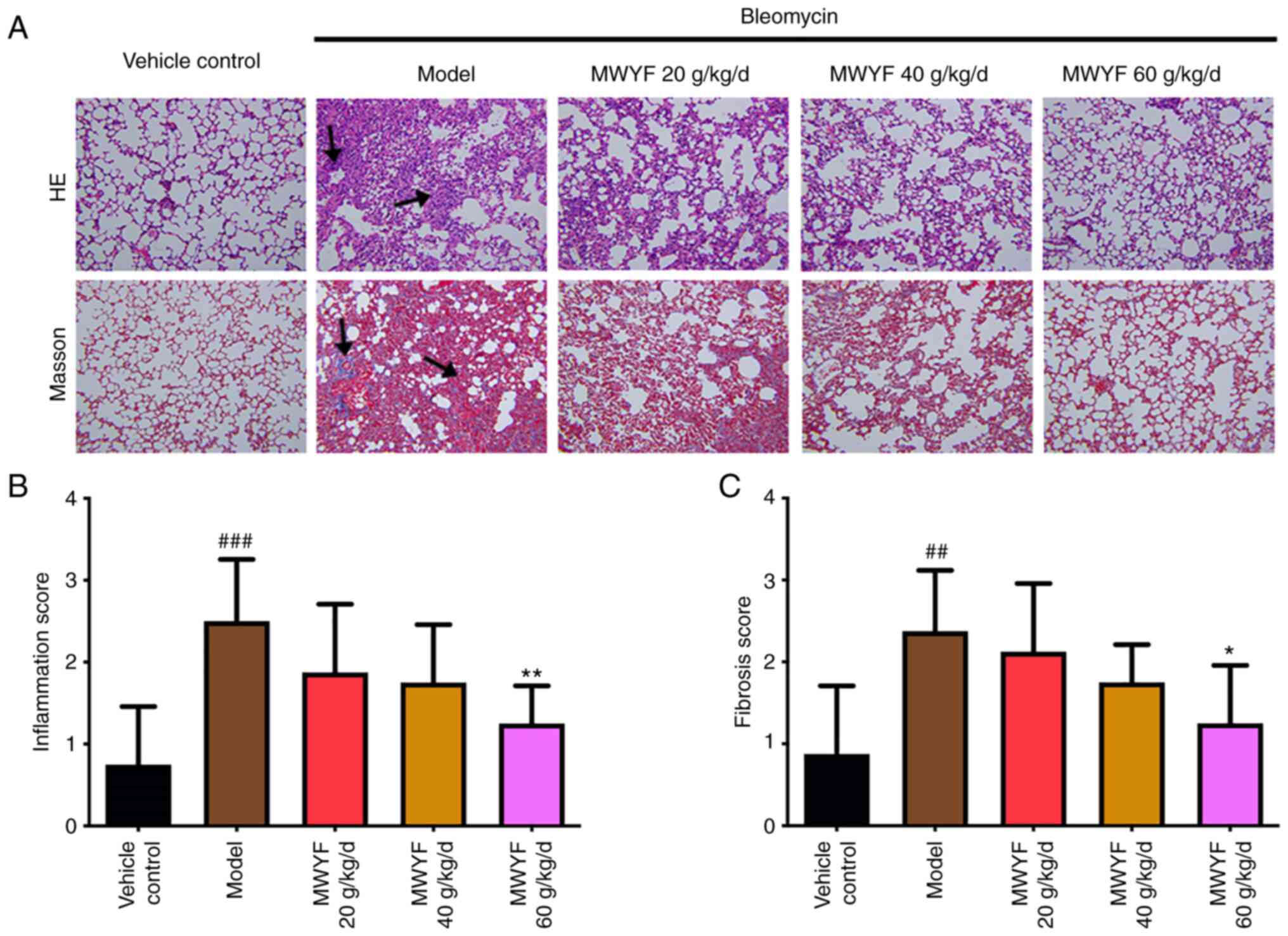
Pulmonary alveolitis and PF
scores
Pulmonary alveolitis and PF were scored based on the
H&E and Masson staining images to evaluate the degree of
inflammation and fibrosis. Using H&E staining, the structure of
the mouse lung tissues in the control group was clear, the
morphology of pulmonary alveoli was normal, the walls of the
pulmonary alveoli were thin and no inflammatory cell infiltration
was observed in the interstitial lung (Fig. 3A). In mice in the model group, the
structure of the pulmonary alveoli was severely damaged. Several
pulmonary alveoli had collapsed, merged and had become necrotic
(Fig. 3A). The walls of the
pulmonary alveoli were thickened and exhibited large degrees of
inflammatory cell infiltration (Fig.
3A). Compared with that in the model group, in the high dose
MWYF group, marked improvements were observed, with the structure
of pulmonary alveoli almost intact, in addition to fewer collapsed
pulmonary alveoli or parenchymal lesions and a reduced degree of
inflammatory cell infiltration in the cavity of pulmonary alveoli
(Fig. 3A). These parameters were
also improved to a certain extent in the low and medium dose MWYF
groups (Fig. 3A).
In the control group, Masson staining demonstrated
that the structure of pulmonary alveoli tissues was regular and
arranged in an orderly manner, with only a small number of fine
light blue fibres observed (Fig.
3A). In the lung tissues of the model group, large quantities
of blue collagen were deposited in the alveolar septum and in the
pulmonary mesenchyme (Fig. 3A).
Compared with that in the model group, significant improvements
were observed in the high dose MWYF group, with blue collagen
deposition markedly alleviated in the lung tissues. A degree of
improvement albeit to a reduced extent was also observed in the low
and medium dose MWYF groups (Fig.
3A).
The inflammation score was calculated to be
significantly higher in the model group compared with that in the
normal control group (Fig. 3B). In
the high dose MWYF group, the pulmonary alveolitis score was
significantly reduced compared with the scores of the model group
(Fig. 3B). Compared with that in
the control group, the PF score was significantly higher in the
model group (Fig. 3C). The mouse PF
scores in the high dose MWYF group were also significantly lower
compared with that in the model group (Fig. 3C).
Collagen content in the pulmonary
tissues
The typical pathological change during PF is
characterised by the deposition of collagen in the pulmonary
fibres, particularly collagen I and collagen III (29). Compared with that in the control
group, the expression of collagen I and collagen III were
significantly higher in the model group (Fig. 4A-C). By contrast, compared with that
in the model group, the expression of collagen I and collagen III
were reduced to a certain degree in the lung tissues of the
MWYF-treated groups, but a significant reduction was only observed
in the high dose MWYF group (Fig.
4A-C).
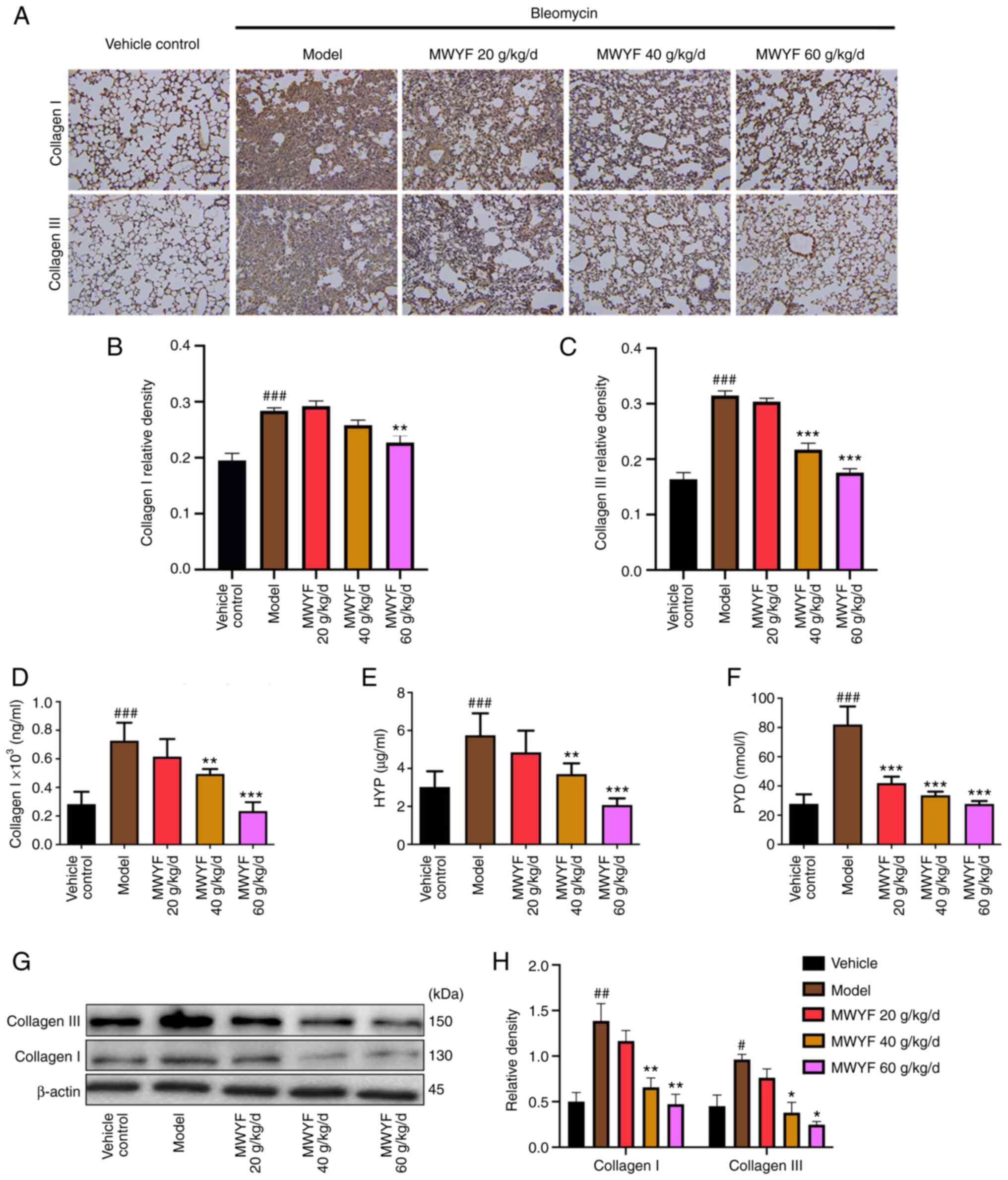 | Figure 4Effects of MWYF on collagen content
in mice with BLM-induced pulmonary fibrosis. (A) Expression of
collagen I and collagen III in the lung tissues was measured by
immunohistochemistry. Magnification, x200. Quantitative results of
immunohistochemical staining for (B) collagen I and (C) collagen
III. Expression of (D) collagen I, (E) HYP and (F) PYD in the lung
tissues was detected by ELISA. (G) Expression of collagen I and
collagen III were measured by western blotting, (H) which was
quantified. All data are expressed as mean ± SD (n=10).
#P<0.05, ##P<0.01 and
###P<0.001 vs. Vehicle; *P<0.05,
**P<0.01 and ***P<0.001 vs. Model.
MWYF, Maiwei Yangfei decoction; Vehicle control, normal group;
Model, model group; HYP, hydroxyproline; PYD, pyridinoline. |
HYP is the primary component of collagen, where its
content is an effective indicator for evaluating collagen
deposition in lung tissues (30).
PYD is a key enzyme involved in collagen synthesis, which can be
used to effectively indicate the state of collagen deposition
(31). Compared with those in the
control group, the levels of collagen I, HYP and PYD were
significantly higher in the lung tissues of the model group, as
indicated by ELISA data. By contrast, in the low, medium and high
dose MWYF groups, the levels of collagen I, HYP and PYD were
reduced to a certain degree compared with those in the model group
(Fig. 4D-F), with the reduction
becoming significant in the high dose MWYF group (Fig. 4D-F). Compared with those in the
control group, western blotting demonstrated that the expression
levels of collagen I and collagen III were significantly increased
in the model group. By contrast, in the low, medium and high dose
MWYF groups, the expression levels of collagen I and collagen III
were reduced to a certain degree compared with those in the model
group, with the reduction becoming significant in the medium and
high dose MWYF groups (Fig. 4G and
H).
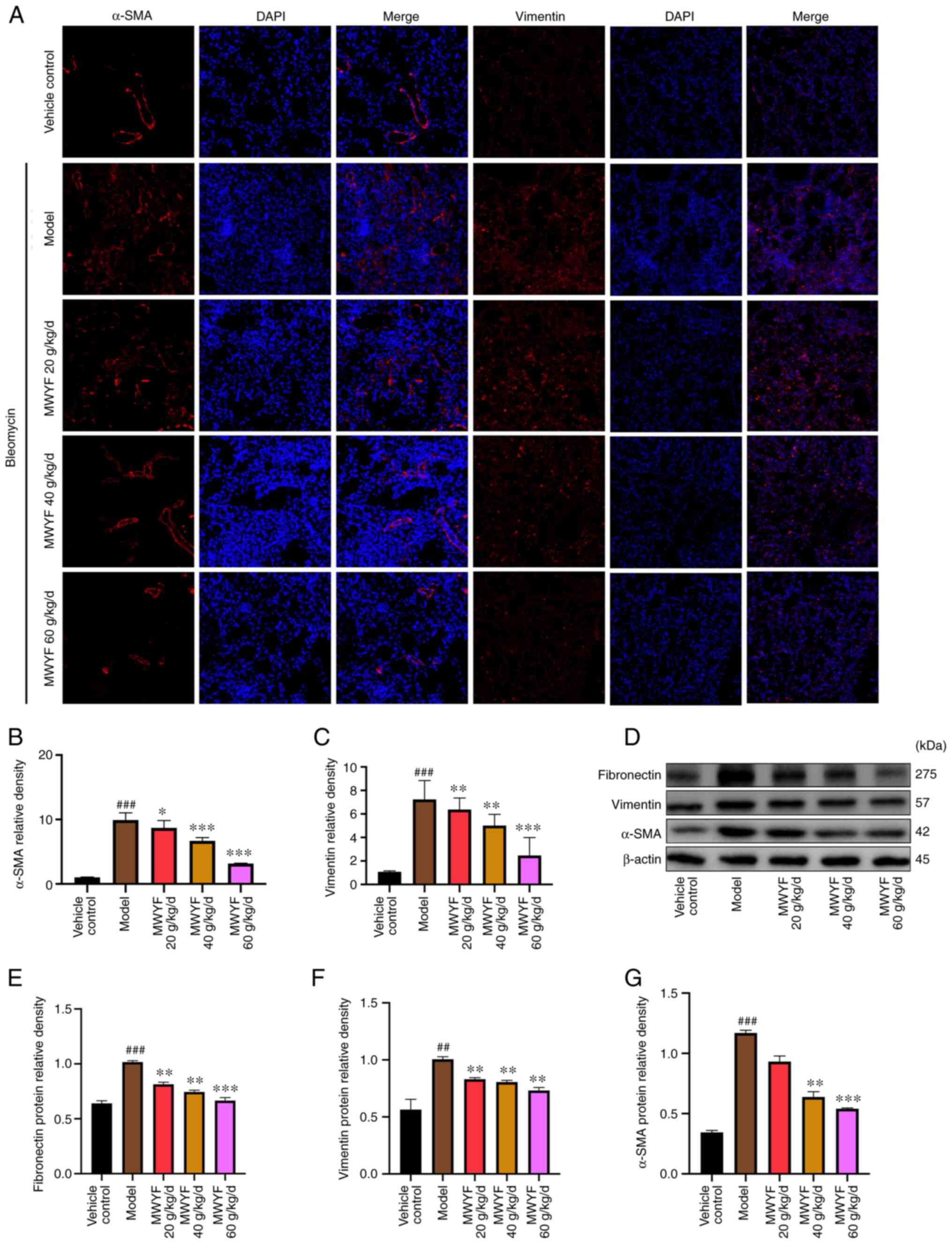
Expression of related marker proteins
in the pulmonary tissues
In PF, the main pathological changes observed are
the proliferation of pulmonary fibroblasts and the differentiation
of fibroblasts into myofibroblasts (32). α-SMA is linked to the activation of
myofibroblasts (33), whilst
vimentin is related to the proliferation of fibroblasts (34) and fibronectin is a component of the
ECM (35). Therefore, the
expression levels of these proteins were assessed to evaluate the
degree of PF using immunofluorescence and western blotting. Based
on the results of the immunofluorescence analysis, in the model
group, the expression levels of α-SMA and vimentin were increased
significantly when compared with those in the control group. By
contrast, treatment with MWYF decoction at all three doses
significantly decreased the expression levels of these two
proteins, with the magnitude particularly high in the medium and
high dose MWYF groups (Fig. 5A-C).
The results of western blotting indicated that the expression
levels of fibronectin, vimentin and α-SMA were significantly
increased in the model group compared with those in the control
group. By contrast, MWYF administration markedly reduced the
expression levels of fibronectin, vimentin and α-SMA, with medium
and high MWYF dose exerting significant reversals (Fig. 5D-G).
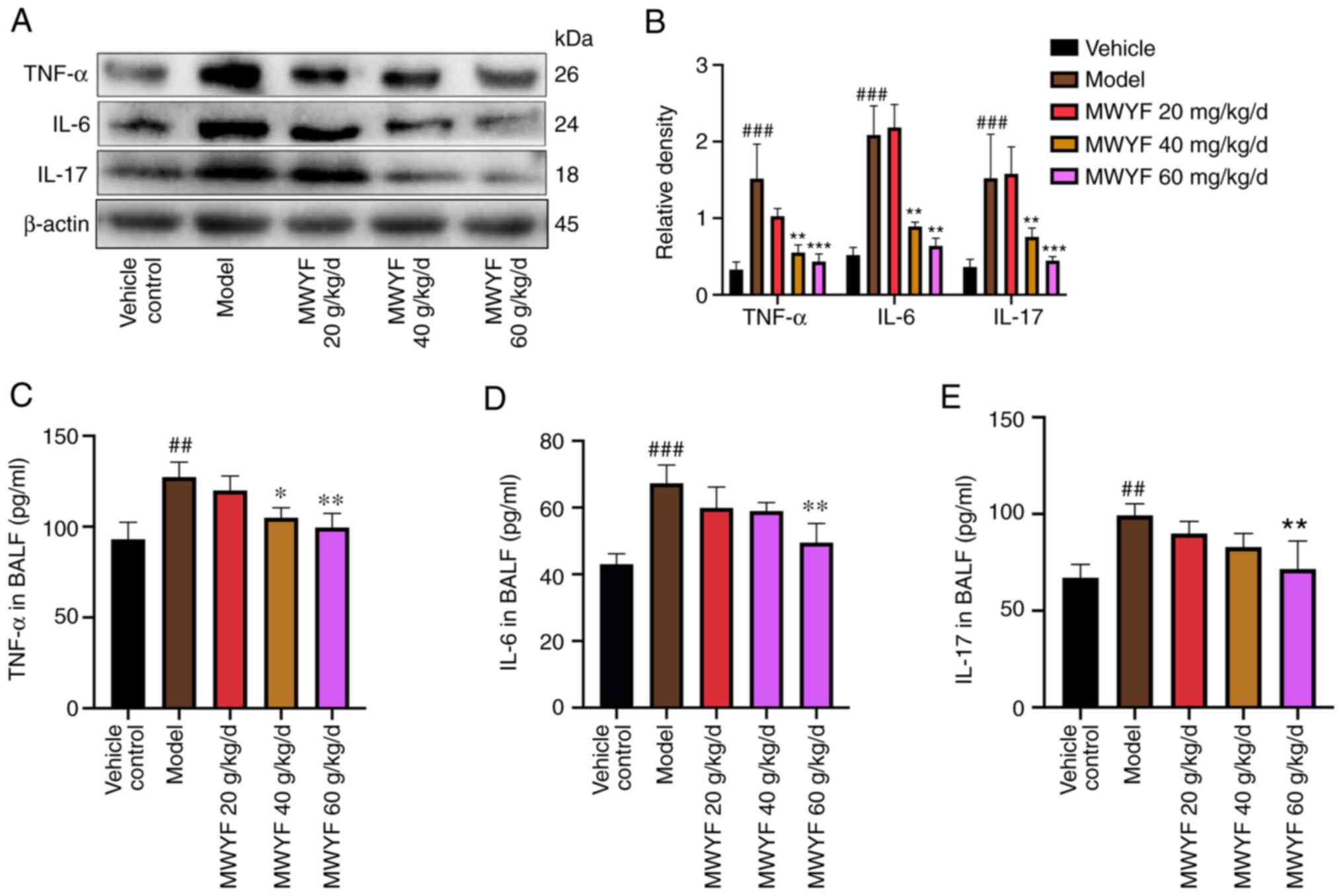
Levels of inflammatory factors in the
pulmonary tissues and BALF
Cytokines serve key roles in the occurrence and
development of PF (36). It has
been previously shown that TNF-α, IL-6 and IL-17 can promote the
progression of PF through inflammatory pathways (37-39).
To assess the role of MWYF in TNF-α, IL-6 and IL-17, the expression
profile of these three cytokines in pulmonary tissues was
determined using western blotting whereas TNF-α, IL-6 and IL-17
levels were detected in BALF using ELISA. Western blotting
indicated that compared with the control group, the expression of
TNF-α, IL-6 and IL-17 proteins in the model group was increased.
Compared with the model group, as the concentration of MWYF
increased, the protein expression decreased (Fig. 6A and B). Compared with those in the control
group, the levels of TNF-α, IL-6 and IL-17 were significantly
increased in the BALF of the model group (Fig. 6C-E). By contrast, in the high dose
MWYF group, the levels of TNF-α, IL-6 and IL-17 were significantly
reduced compared with that in the model group (Fig. 6C-E). This suggests that MWYF exerted
its beneficial effects on PF by reducing the expression of
inflammatory cytokines.
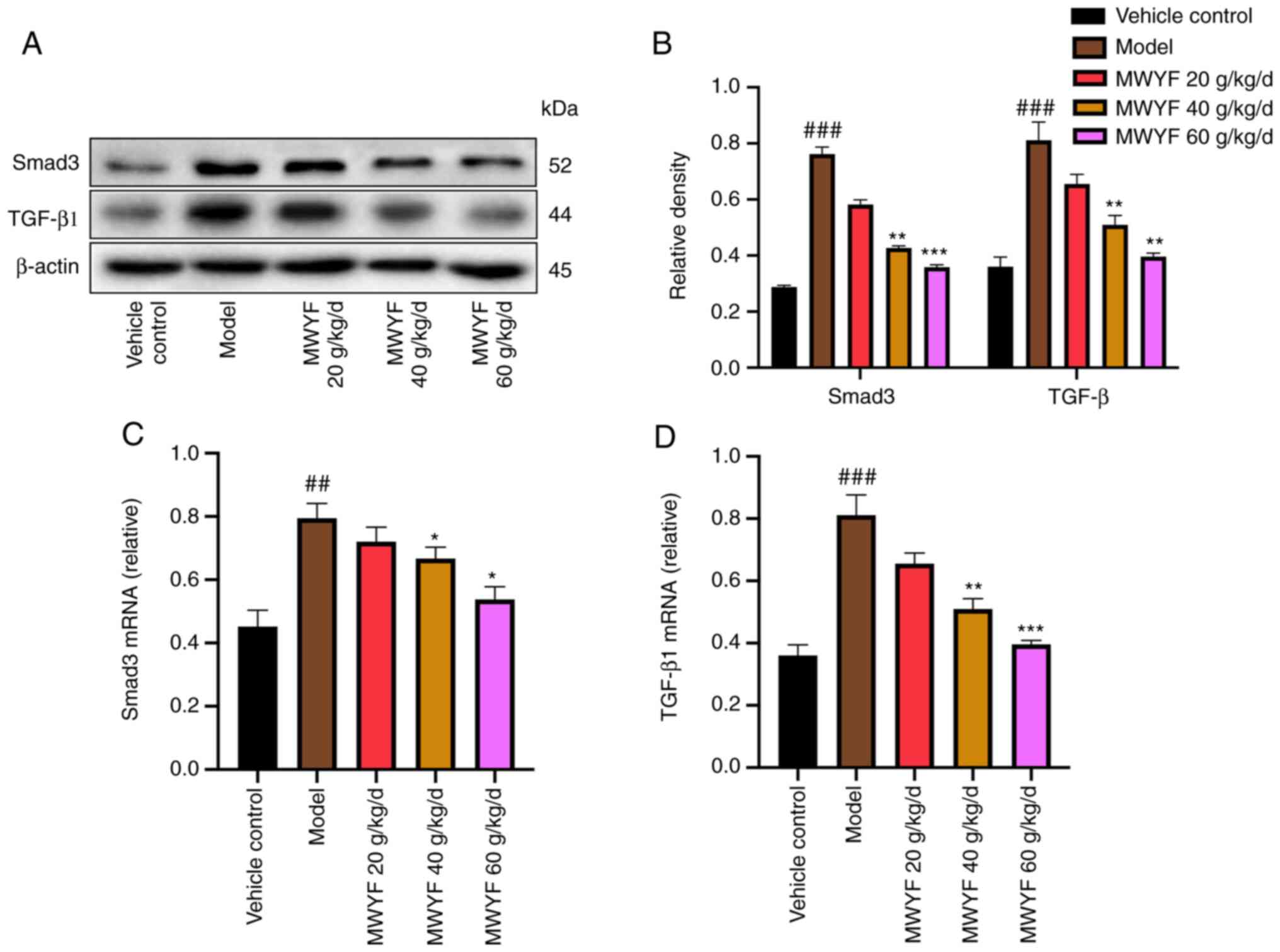
Expression of TGF-β1 and Smad3 in
pulmonary tissues
TGF-β1 serves a key role in the development of PF
(40). TGF-β1 promotes the
deposition of ECM by increasing the transcriptional levels of
collagen-related genes via Smad3, thereby accelerating the
differentiation of pulmonary fibroblasts into myofibroblasts and
exacerbating the degree of PF by promoting the apoptosis of the
type-II epithelial cells (41,42).
In the present study, the expression levels of TGF-β1 and Smad3
proteins and mRNA were measured using western blotting and RT-qPCR,
respectively. High- and medium dose MWYF treatment significantly
decreased the expression of TGF-β1 and Smad3 in mouse lung tissues
at both the transcriptional and translational levels compared with
that in the model group (Fig.
7).
Discussion
Pulmonary interstitial fibrosis is a connective
tissue disease that is primarily characterized by dry cough and
dyspnoea with an unknown pathogenesis (43). The most severe pathological traits
appear to be the aberrant proliferation of fibroblasts,
differentiation of fibroblasts into myofibroblasts and the
excessive deposition of ECM, all of which eventually lead to the
failure of lung function (44,45).
Although nintedanib and pirfenidone can delay the progression of
PF, they are expensive and cause notable adverse reactions,
reducing their efficacy (46).
Therefore, discovery of novel agents and then confirming their
efficacy remain imperative for patients with PF. Therefore, in the
present study, a BLM-induced pulmonary mouse PF model was
established, following which the efficacy of MWYF was assessed with
the underlying mechanism of action investigated.
There are four types of collagens in lung tissues,
where collagen I and collagen III are the most abundant (47). Collagenous fibers are an aggregated
form of collagen secreted by the fibroblasts (48). Collagen primarily contains HYP, an
amino acid present in the body's collagenous fibre (49). Furthermore, HYP is an important
indicator used for estimating the metabolism of collagenous tissues
and the degree of pulmonary interstitial fibrosis (50). Intermolecular cross-linking is an
important post-translational modification during collagen formation
(50). Results from the present
study demonstrated that a high dose MWYF decoction can reduce
collagen I, collagen III, HYP and PYD expression, suggesting a
protective role for the MWYF decoction in BLM-induced PF in
mice.
α-SMA is a marker of myofibroblast activation
(51). The expression of α-SMA in
the cytoplasm allows cellular contractility, which is closely
associated with tissue fibrosis (51). In addition, the expression level of
α-SMA is positively associated with the degree of PF (52). ECM deposition is the primary cause
of PF, where fibronectin is an important component (3). Excessive deposition of fibronectin can
result in the formation of scars and acceleration of fibrosis.
Vimentin is normally expressed in mesenchymal tissues, with little
to no expression in epithelial cells (53). Therefore, vimentin can be used as a
protein marker of fibroblasts. The expression of α-SMA and vimentin
in lung tissues was determined using immunofluorescence analysis in
the present study. The immunofluorescence results indicated that
the proportion of cells exhibiting positive α-SMA and vimentin
expression was significantly higher in the model group compared
with that in the control group. When different concentrations of
the MWYF decoction were administered, the proportion of α-SMA- and
vimentin-positive cells was reduced to a certain degree,
particularly in the high dose MWYF group.
The inflammatory response in the pulmonary alveoli
is one of the primary causes of inflammatory cytokine release
(54). TNF-α is a proinflammatory
cytokine that can stimulate neutrophilic granulocytes and
eosinophils to produce superoxides and release lysosomal enzymes
(55). Furthermore, TNF-α can
accelerate the progression of fibrosis (55). IL-6 is also a proinflammatory
cytokine that can accelerate the early alveolytic response through
the chemotaxis, infiltration and aggregation of inflammatory cells
(56). IL-17 is another
proinflammatory cytokine that can activate various cytokines,
fibroblasts, endothelial cells and epithelial cells to promote the
formation of PF (57). In the
present study, the PF mouse model used appeared to have mimicked
the acute pulmonary injury model, as an inflammatory response was
consistently observed throughout the entire modelling process.
Therefore, although the lung tissues were collected 21 days after
model construction, a compensatory inflammatory response remained.
Results from the present study demonstrated that MWYF reduced the
levels of TNF-α, IL-6 and IL-17 in the BALF of mice with PF,
suggesting that the MWYF decoction could delay the development of
PF by inhibiting the inflammatory response.
TGF-β1/Smad3 is considered to be a classical
signalling pathway associated with PF (58). TGF-β1 has been previously shown to
directly promote fibrosis (59). It
promotes the proliferation, differentiation and secretion of
interstitial cells, induces the differentiation of fibroblasts into
myofibroblasts, thereby increasing the deposition of ECM (60). Therefore, it is considered to be an
inducer and promoter of fibrosis. Smad proteins belong to a family
of unique intracellular signal transduction molecules that can
interact with various transcription factors in cell-specific
patterns (61). This in turn
modulates the transcription of target genes, such as collagen, to
promote the formation of fibrosis (60). TGF-β1 activation results in the
phosphorylation of Smad3 and its subsequent entry into the cell
nuclei, which then activate the expression of a series of genes to
promote the differentiation of fibroblasts into pulmonary
fibroblasts that express contractile proteins, such α-SMA (62). In the present study, the expression
levels of TGF-β1 and Smad3 were increased significantly after model
construction. However, this was reversed after MWYF treatment,
suggesting that MWFY likely alleviated BLM-induced PF in mice via
the TGF-β1/Smad3 signalling pathway.
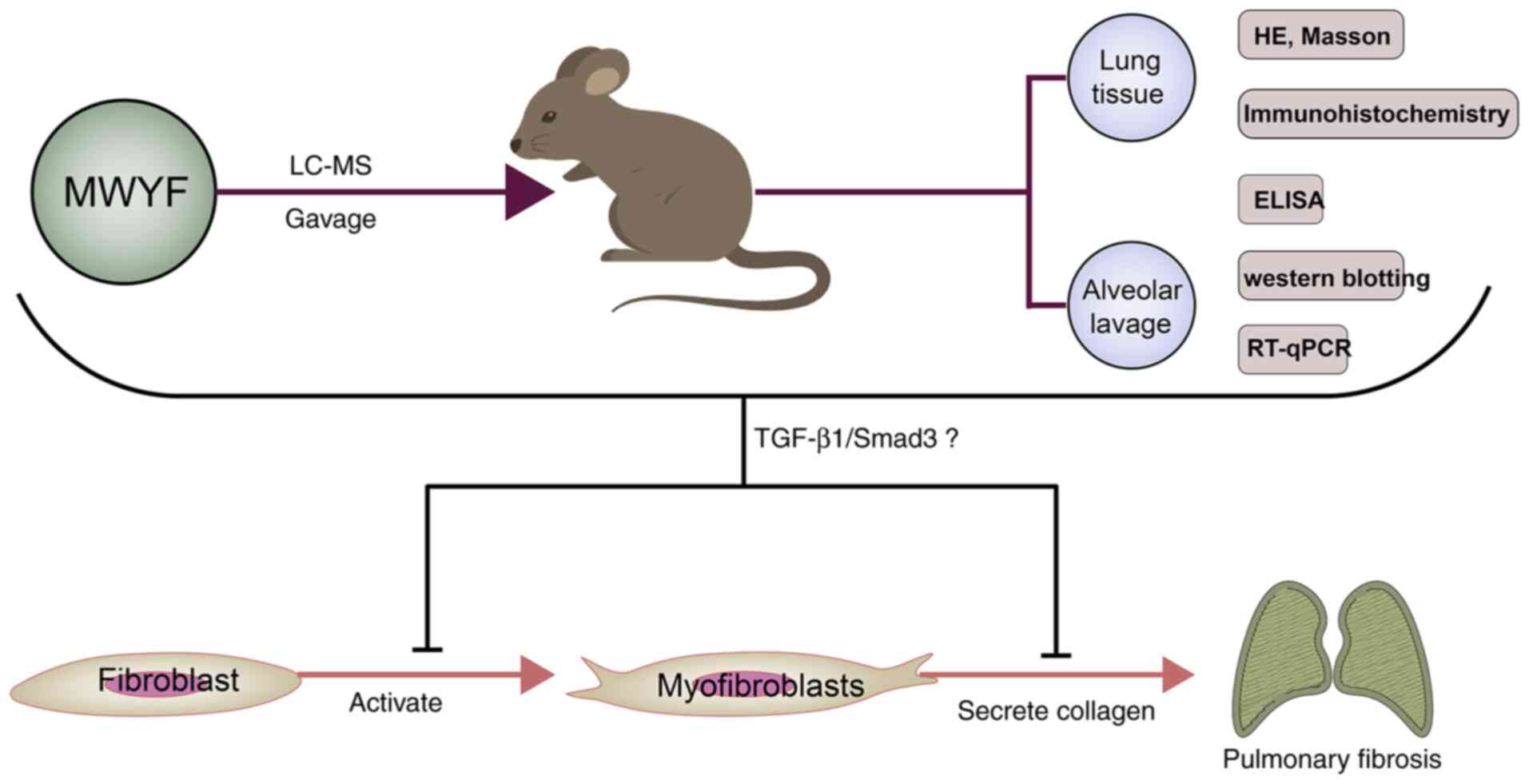
In conclusion, in vivo analysis of the
effects of MWYF treatment was performed, where it was shown to be
effective in alleviating the pathology in mice with BLM-induced PF.
This was mediated by reducing collagen deposition, highlighting its
potentially therapeutic and protective effects. The underlying
mechanism of the effects of the MWYF decoction at least partially
involved inhibition of the TGF-β1/Smad3 signalling pathway
(Fig. 8). However, further study of
the mechanisms underlying the modulation by MWYF treatment in PF is
required. In addition, due to the diversity and variability
contained within MWYF, the results and conclusions observed in the
present study require further verification to confirm the potential
clinical value of MWYF.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially funded by the
National Natural Science Foundation of China (grant no. 82074358)
and Graduate Student Scientific Research Innovation Projects in
Jiangsu Province (grant no. KYCX21_1706).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and QW designed the study. YX, WP and DH
performed the experiments. FF, ZW and CG performed data analysis.
YX and XZ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Commission for Animal Tests of the Nanjing University of Chinese
Medicine (approval no. 012009012015; Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martinez FJ, Collard HR, Pardo A, Raghu G,
Richeldi L, Selman M, Swigris JJ, Taniguchi H and Wells AU:
Idiopathic pulmonary fibrosis. Nat Rev Dis Primers.
3(17074)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lederer DJ and Martinez FJ: Idiopathic
pulmonary fibrosis. N Engl J Med. 378:1811–1823. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Burgstaller G, Oehrle B, Gerckens M, White
ES, Schiller HB and Eickelberg O: The instructive extracellular
matrix of the lung: Basic composition and alterations in chronic
lung disease. Eur Respir J. 50(1601805)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hutchinson J, Fogarty A, Hubbard R and
McKeever T: Global incidence and mortality of idiopathic pulmonary
fibrosis: A systematic review. Eur Respir J. 46:795–806.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chanda D, Otoupalova E, Smith SR,
Volckaert T, De Langhe SP and Thannickal VJ: Developmental pathways
in the pathogenesis of lung fibrosis. Mol Aspects Med. 65:56–69.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Canestaro WJ, Forrester SH, Raghu G, Ho L
and Devine BE: Drug treatment of idiopathic pulmonary fibrosis:
Systematic review and network meta-analysis. Chest. 149:756–766.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Drakopanagiotakis F, Wujak L, Wygrecka M
and Markart P: Biomarkers in idiopathic pulmonary fibrosis. Matrix
Biol. 68-69:404–421. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
da Silva Antunes R, Mehta AK, Madge L,
Tocker J and Croft M: TNFSF14 (LIGHT) exhibits inflammatory
activities in lung fibroblasts complementary to IL-13 and TGF-β.
Front Immunol. 9(576)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heukels P, Moor CC, von der Thüsen JH,
Wijsenbeek MS and Kool M: Inflammation and immunity in IPF
pathogenesis and treatment. Respir Med. 147:79–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang J, Wang D, Wang L, Wang S, Roden AC,
Zhao H, Li X, Prakash YS, Matteson EL, Tschumperlin DJ and Vassallo
R: Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic
pulmonary fibrosis and rheumatoid arthritis-associated lung disease
support a direct role for IL-17A/IL-17RA in human fibrotic
interstitial lung disease. Am J Physiol Lung Cell Mol Physiol.
316:L487–L497. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Penke LR and Peters-Golden M: Molecular
determinants of mesenchymal cell activation in fibroproliferative
diseases. Cell Mol Life Sci. 76:4179–4201. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ballester B, Milara J and Cortijo J:
Idiopathic pulmonary fibrosis and lung cancer: Mechanisms and
molecular targets. Int J Mol Sci. 20(593)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rogliani P, Calzetta L, Cavalli F, Matera
MG and Cazzola M: Pirfenidone, nintedanib and N-acetylcysteine for
the treatment of idiopathic pulmonary fibrosis: A systematic review
and meta-analysis. Pulm Pharmacol Ther. 40:95–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Galli JA, Pandya A, Vega-Olivo M, Dass C,
Zhao H and Criner GJ: Pirfenidone and nintedanib for pulmonary
fibrosis in clinical practice: Tolerability and adverse drug
reactions. Respirology. 22:1171–1178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ,
Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al:
TOLLIP, MUC5B, and the response to N-Acetylcysteine among
individuals with idiopathic pulmonary fibrosis. Am J Respir Crit
Care Med. 192:1475–1482. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li LC and Kan LD: Traditional Chinese
medicine for pulmonary fibrosis therapy: Progress and future
prospects. J Ethnopharmacol. 198:45–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Feng F, Wang Z, Li R, Wu Q, Gu C, Xu Y,
Peng W, Han D, Zhou X, Wu J and He H: Citrus alkaline extracts
prevent fibroblast senescence to ameliorate pulmonary fibrosis via
activation of COX-2. Biomed Pharmacother.
112(108669)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu Y, Sun Z, Shi L, Zhang Y, Zhou Z, Zhang
S and Chao E: Effects of Feiwei granules in the treatment of
idiopathic pulmonary fibrosis: A randomized and placebo-controlled
trial. J Tradit Chin Med. 36:427–433. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen MJ, Yang GL, Ding YX and Tong ZQ:
Efficacy of TCM therapy of tonifying lung-kidney's Qi-deficiency in
a case of idiopathic pulmonary fibrosis: A case report. Medicine
(Baltimore). 98(e15140)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen F, Wang PL, Fan XS, Yu JH, Zhu Y and
Zhu ZH: Effect of Renshen Pingfei Decoction, a traditional Chinese
prescription, on IPF induced by Bleomycin in rats and regulation of
TGF-β1/Smad3. J Ethnopharmacol. 186:289–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giri SN, Hyde DM, Braun RK, Gaarde W,
Harper JR and Pierschbacher MD: Antifibrotic effect of decorin in a
bleomycin hamster model of lung fibrosis. Biochem Pharmacol.
54:1205–1216. 1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Laferriere CA and Pang DS: Review of
intraperitoneal injection of sodium pentobarbital as a method of
Euthanasia in laboratory Rodents. J Am Assoc Lab Anim Sci.
59:254–263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Z, Feng F, Wu Q, Gu C, Xu Y and Zhou
X: Comparison of three methods to establish a mouse model of
pulmonary fibrosis induced by intratracheal instillation of
bleomycin. Chinese Journal of Comparative Medicine. 29:51–57.
2019.(In Chinese).
|
|
25
|
Hotham WE and Henson FMD: The use of large
animals to facilitate the process of MSC going from laboratory to
patient-ʻbench to bedside’. Cell Biol Toxicol. 36:103–114.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hübner RH, Gitter W, El Mokhtari NE,
Mathiak M, Both M, Bolte H, Freitag-Wolf S and Bewig B:
Standardized quantification of pulmonary fibrosis in histological
samples. Biotechniques. 44:507-511:514–517. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Surolia R, Li FJ, Wang Z, Li H, Dsouza K,
Thomas V, Mirov S, Pérez-Sala D, Athar M, Thannickal VJ and Antony
VB: Vimentin intermediate filament assembly regulates fibroblast
invasion in fibrogenic lung injury. JCI Insight.
4(e123253)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Y, Tong X, Ren S, Wang X, Chen J, Mu
Y, Sun M, Chen G, Zhang H and Liu P: Synergistic anti-liver
fibrosis actions of total astragalus saponins and glycyrrhizic acid
via TGF-β1/Smads signaling pathway modulation. J Ethnopharmacol.
190:83–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Q, Hu L, Zhao Z, Ma L, Li J, Xu L and
Wang J: Serum changes in pyridinoline, type II collagen cleavage
neoepitope and osteocalcin in early stage male brucellosis
patients. Sci Rep. 10(17190)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun YB, Qu X, Caruana G and Li J: The
origin of renal fibroblasts/myofibroblasts and the signals that
trigger fibrosis. Differentiation. 92:102–107. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng X, Qi C, Zhang S, Fang Y and Ning W:
TGF-β1 induces Fstl1 via the Smad3-c-Jun pathway in lung
fibroblasts. Am J Physiol Lung Cell Mol Physiol. 313:L240–L251.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Epstein Shochet G, Brook E, Israeli-Shani
L, Edelstein E and Shitrit D: Fibroblast paracrine TNF-α signaling
elevates integrin A5 expression in idiopathic pulmonary fibrosis
(IPF). Respir Res. 18(122)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Epstein Shochet G, Brook E,
Bardenstein-Wald B and Shitrit D: TGF-β pathway activation by
idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble
factors is mediated by IL-6 trans-signaling. Respir Res.
21(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao Y, Yao LF, Zhao Y, Wei LM, Guo P, Yu
M, Cao B, Li T, Chen H and Zou ZM: The Chinese herbal medicine
formula mKG suppresses pulmonary fibrosis of mice induced by
bleomycin. Int J Mol Sci. 17(238)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Malaviya R, Laskin JD and Laskin DL:
Anti-TNFalpha therapy in inflammatory lung diseases. Pharmacol
Ther. 180:90–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang D, Chen X, Wang J, Lou Q, Lou Y, Li
L, Wang H, Chen J, Wu M, Song X and Qian Y: Dysregulated lung
commensal bacteria drive interleukin-17B production to promote
pulmonary fibrosis through their outer membrane vesicles. Immunity.
50:692–706.e7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Papiris SA, Tomos IP, Karakatsani A,
Spathis A, Korbila I, Analitis A, Kolilekas L, Kagouridis K,
Loukides S, Karakitsos P and Manali ED: High levels of IL-6 and
IL-8 characterize early-on idiopathic pulmonary fibrosis acute
exacerbations. Cytokine. 102:168–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barratt SL, Creamer A, Hayton C and
Chaudhuri N: Idiopathic pulmonary fibrosis (IPF): An overview. J
Clin Med. 7(201)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sharif R: Overview of idiopathic pulmonary
fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 23
(Suppl 11):S176–S182. 2017.PubMed/NCBI
|
|
42
|
Saito S, Alkhatib A, Kolls JK, Kondoh Y
and Lasky JA: Pharmacotherapy and adjunctive treatment for
idiopathic pulmonary fibrosis (IPF). J Thorac Dis. 11 (Suppl
14):S1740–S1754. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Herrera J, Forster C, Pengo T, Montero A,
Swift J, Schwartz MA, Henke CA and Bitterman PB: Registration of
the extracellular matrix components constituting the fibroblastic
focus in idiopathic pulmonary fibrosis. JCI Insight.
4(e125185)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shigemura Y, Iwasaki Y, Tateno M, Suzuki
A, Kurokawa M, Sato Y and Sato K: A pilot study for the detection
of cyclic prolyl-hydroxyproline (Pro-Hyp) in human blood after
ingestion of collagen hydrolysate. Nutrients.
10(1356)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu Y, Zheng X, Wang C, Sun X, Sun H, Ma
T, Li Y, Liu K, Chen L and Ma X: Synthesis and biological activity
of thieno[3,2-d]pyrimidines as potent JAK3 inhibitors for the
treatment of idiopathic pulmonary fibrosis. Bioorg Med Chem.
28(115254)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sgalla G, Iovene B, Calvello M, Ori M,
Varone F and Richeldi L: Idiopathic pulmonary fibrosis:
Pathogenesis and management. Respir Res. 19(32)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Beringer A and Miossec P: IL-17 and TNF-α
co-operation contributes to the proinflammatory response of hepatic
stellate cells. Clin Exp Immunol. 198:111–120. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lin J, Shi Y, Men Y, Wang X, Ye J and
Zhang C: Mechanical roles in formation of oriented collagen fibers.
Tissue Eng Part B Rev. 26:116–128. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Oike T, Kanagawa H, Sato Y, Kobayashi T,
Nakatsukasa H, Miyamoto K, Nakamura S, Kaneko Y, Kobayashi S,
Harato K, et al: IL-6, IL-17 and Stat3 are required for
auto-inflammatory syndrome development in mouse. Sci Rep.
8(15783)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lei L, Zhao C, Qin F, He ZY, Wang X and
Zhong XN: Th17 cells and IL-17 promote the skin and lung
inflammation and fibrosis process in a bleomycin-induced murine
model of systemic sclerosis. Clin Exp Rheumatol. 34 (Suppl
100):S14–S22. 2016.PubMed/NCBI
|
|
51
|
Wang YY, Jiang H, Pan J, Huang XR, Wang
YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY and Chen JH:
Macrophage-to-myofibroblast transition contributes to interstitial
fibrosis in chronic renal allograft injury. J Am Soc Nephrol.
28:2053–2067. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhao W, Wang X, Sun KH and Zhou L:
α-smooth muscle actin is not a marker of fibrogenic cell activity
in skeletal muscle fibrosis. PLoS One. 13(e0191031)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ding D, Li C, Zhao T, Li D, Yang L and
Zhang B: LncRNA H19/miR-29b-3p/PGRN axis promoted
epithelial-mesenchymal transition of colorectal cancer cells by
acting on wnt signaling. Mol Cells. 41:423–435. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Schett G, Manger B, Simon D and Caporali
R: COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev
Rheumatol. 16:465–470. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Saha P and Smith A: TNF-α (Tumor Necrosis
Factor-α). Arterioscler Thromb Vasc Biol. 38:2542–2543.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rose-John S: Interleukin-6 family
cytokines. Cold Spring Harb Perspect Biol.
10(a028415)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cipolla E, Fisher AJ, Gu H, Mickler EA,
Agarwal M, Wilke CA, Kim KK, Moore BB and Vittal R: IL-17A
deficiency mitigates bleomycin-induced complement activation during
lung fibrosis. FASEB J. 31:5543–5556. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhou Z, Kandhare AD, Kandhare AA and
Bodhankar SL: Hesperidin ameliorates bleomycin-induced experimental
pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and
IkappaBalpha/NF-kappaB pathways. EXCLI J. 18:723–745.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Guo J, Fang Y, Jiang F, Li L, Zhou H, Xu X
and Ning W: Neohesperidin inhibits TGF-β1/Smad3 signaling and
alleviates bleomycin-induced pulmonary fibrosis in mice. Eur J
Pharmacol. 864(172712)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao
GY, Li M, Zhou H, Lin SQ, Ran JH and Yang BX: Ganoderic acid
hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK
signaling pathways. Acta Pharmacol Sin. 41:670–677. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kurundkar AR, Kurundkar D, Rangarajan S,
Locy ML, Zhou Y, Liu RM, Zmijewski J and Thannickal VJ: The
matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent
profibrotic signaling in fibroblasts and contributes to fibrogenic
responses to lung injury. FASEB J. 30:2135–2150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shimbori C, Bellaye PS, Xia J, Gauldie J,
Ask K, Ramos C, Becerril C, Pardo A, Selman M and Kolb M:
Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis.
J Pathol. 240:197–210. 2016.PubMed/NCBI View Article : Google Scholar
|