Introduction
Inhalation drug products have been developed for the
treatment of respiratory diseases, such as asthma, chronic
obstructive pulmonary disease (COPD) and pulmonary infections.
Inhalation drug products are also used to treat systemic diseases,
including diabetes (1,2), and their application for pulmonary
vaccination (3) is expected. Three
types of inhalation drug products are currently on the market:
nebulizers, pressurized metered dose inhalers (pMDIs) and dry
powder inhalers (DPIs). In contrast to nebulizers and pMDIs, DPIs
deliver a powder formulation without synchronizing the timing of
nebulization and inhalation. Furthermore, a nebulizer and
propellant are not necessary for DPI and the device is small.
However, drug release from DPIs is affected by the inhalation
patterns of patients (4-7),
inhalation devices (5), inhalation
formulations (8) and the
physiology of the airways (9).
There are two types of DPIs, the multi- and single-unit-dose
inhalers (10-12).
A typical example of a single-unit-dose inhaler is capsule-based
DPIs, including Intal® Spinhaler®,
Spiriva® Handihaler®, Onbrez®,
Ultibro® and Seebri® Breezhaler®.
These inhalers are widely distributed as commercial inhalers. The
advantages of capsule-based DPIs are the accurate and uniform drug
delivery as well as the simplicity of use for patients (13). Patients can also visually confirm
whether a dose has been administered (14). Drug retention in capsules affects
inhalation performances and attenuates therapeutic efficacy for
pulmonary diseases (15,16). Therefore, the development of a
formulation with controlled drug release from capsules is needed
for capsule-based DPIs. To improve drug release from capsules,
previous studies focused on inhalation particles. For example, drug
release from capsules is improved by preparing drug-carrier
particles and drug-drug granulate particles or adding dispersive
agents, including L-leucine, L-phenylalanine, DPPC and DOTAP. These
inhalation particles preparation techniques attenuate drug-drug and
drug-capsule adhesive-cohesive forces (17-21).
The capsule body has also been previously investigated. Wauthoz
et al (22) and Saleem
et al (23) have reported
that inhalation characteristics depend on capsule compositions
[e.g., gelatin and hydroxypropyl methylcellulose (HPMC)], capsule
loss on drying, capsule piercing pattern, the manufacturing method
and the lubricant content. Most of these findings were related to
the capsule outer properties; however, there are few studies on the
effect of capsule inner properties. In addition, most of the
previous studies have been performed using a low resistance device
instead of a high resistance device.
The present study aimed to evaluate the effects of
capsule inner surface physical properties on drug release from
capsules and lung drug delivery using a high resistance device.
Materials and methods
Materials
As a model capsule-based DPIs, Spiriva®
Handihaler® was purchased from Boehringer Ingelheim
Japan Ltd. Spiriva® Handihaler® is a typical
capsule-based DPIs, used for the treatment of COPD and for reducing
COPD exacerbations. A dry powder in Spiriva® inhalation
capsules containing tiotropium bromide (TIO) as the main component
was used as a model drug. The capsule contained 5.5 mg of a powder
formulation consisting of 18 µg micronized tiotropium (as bromide
hydrate, 22.5 µg) with coarse lactose monohydrate (24). The Handihaler®
(Boehringer Ingelheim Japan Ltd.) was used as a model high
resistance device for capsule-based DPIs (device specific
resistance 3.03 Pa* min2/l2) (25). Size 3 capsules with different
compositions were supplied by Qualicaps, Co., Ltd. (G-Cap, gelatin
capsule; PEG/G-Cap, gelatin capsule containing 5% PEG4000 as
plasticizer; and HPMC-Cap, HPMC capsule). PEG/G-Cap is a gelatin
capsule that is resistant to cracking due to the presence of PEG.
HPMC-Cap is resistant to cracking under dry conditions and may be
filled with highly hygroscopic drugs. Each capsule was stored at
15-25˚C and under 40-50% relative humidity (RH) in a glass
desiccator. The characteristics of each capsule are presented in
Table I. All other reagents and
solvents were of analytical or HPLC grade.
 | Table ICharacteristics of each capsule. |
Table I
Characteristics of each capsule.
| | G-Cap | PEG/G-Cap | HPMC-Cap |
|---|
| Main material | Gelatin | Gelatin,
PEG4000 | Hydroxypropyl
methyl cellulose |
|---|
| Length diameter,
mm | 12.7±1.53 | 13.9±0.79 | 14.1±0.56 |
| Width diameter,
mm | 4.37±0.53 | 4.67±0.14 | 4.52±0.27 |
| Surface area,
mm2 | 289±6.14 | 245±20.5 | 268±24.6 |
| Volume,
mm3 | 304±1.03 | 301±2.04 | 313±17.9 |
| Loss of drying,
% | 14.3 | 13.2 | 5.0 |
| Lubricant
content | NR | NR | NR |
Characterization of three type of
capsule with different compositions
Each capsule image was analyzed using the image
analysis software ImageJ, and the length diameter, width diameter,
surface area and volume were calculated (Table I). The loss on drying (LOD) and
lubricant content of each capsule are shown as stated in the
analysis certificate. The numbers shown in Table I correspond to the average of three
capsules of each capsule type except for LOD and lubricant content.
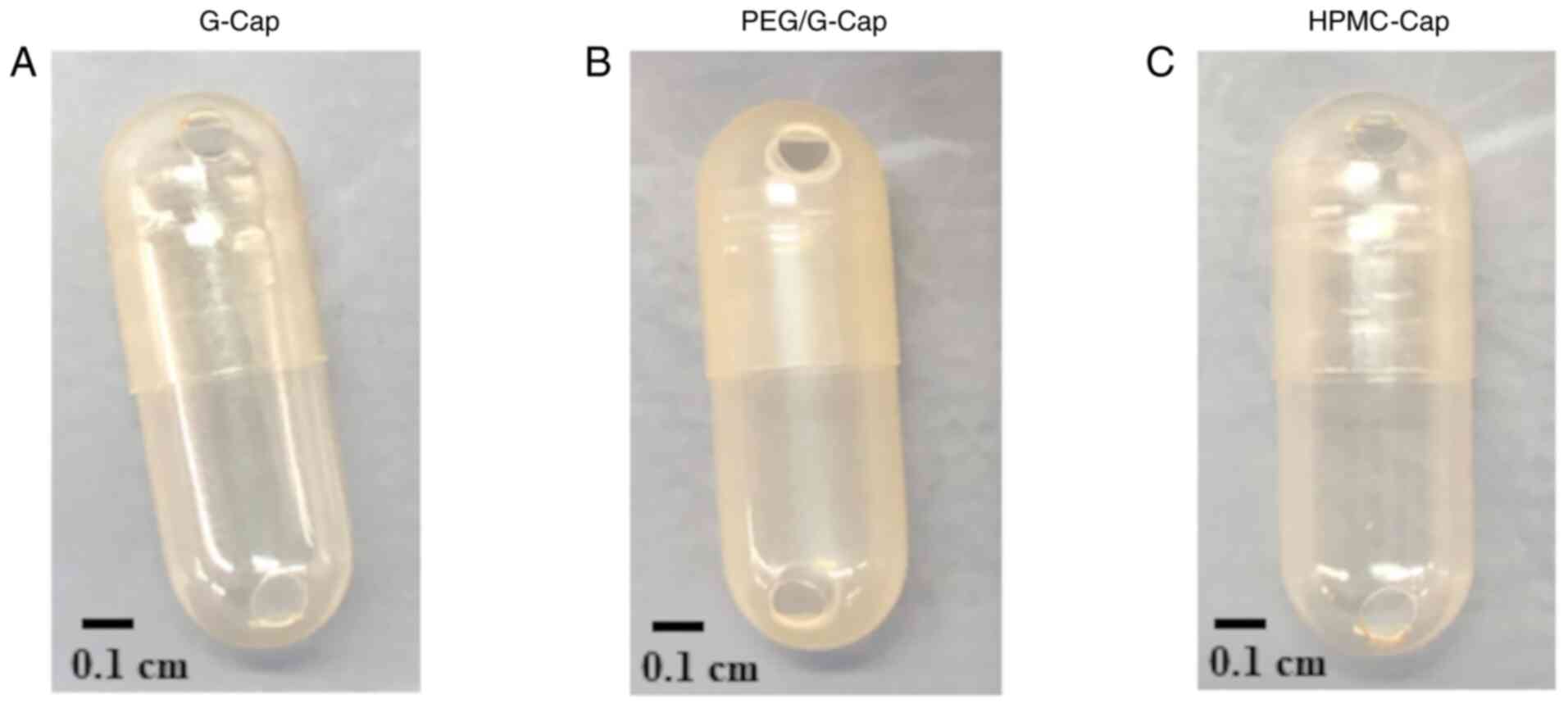
Fig. 1A-C display the appearance
of each capsule.
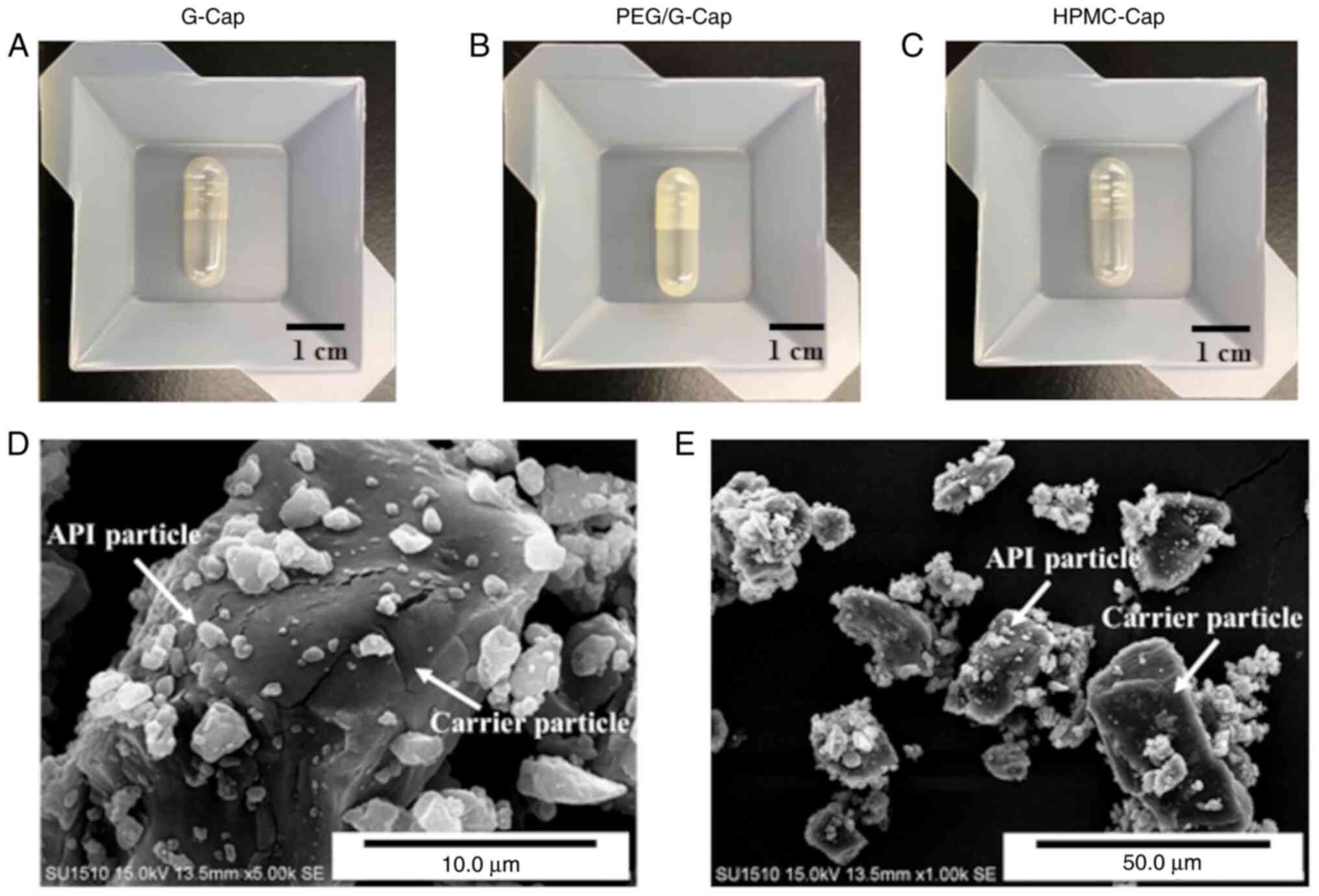 | Figure 1Images of the (A-C) three capsule
types and (D and E) SEM images of TIO. (A) G-Cap, (B) PEG/G-Cap,
and (C) HPMC-Cap. (D) High magnification, x5000 image of TIO. (E)
Low magnification, x1,000 image of TIO. G-Cap, gelatin capsule;
PEG/G-Cap, gelatin capsule containing 5% PEG4000 as plasticizer;
HPMC-Cap, hydroxypropyl methylcellulose capsule; API, active
pharmaceutical ingredient; TIO, tiotropium bromide. |
Morphological analysis of TIO by
scanning electron microscopy
The morphology of TIO was examined using scanning
electron microscopy (SEM; SU1510; Hitachi High-Technologies Corp.).
Prior to observations, free falling powders from a spatula were
manually dispersed on a specimen mount with double-side tape and
were then coated with platinum using an ion sputter coater (E-1013;
Hitachi High-Technologies Corp.).
Capsule hole examination after
piercing in Handihaler®
Ten capsules of each type were successively placed
in the Handihaler® to be pierced. Capsule hole images
were taken of the most representative hole type of each capsule. In
addition, each capsule hole image was analyzed using the image
analysis software ImageJ (version 1.8.0_172; National Institutes of
Health), and the hole diameter and hole area were calculated.
Topographical analysis of capsule
inner surface by AFM
AFM is widely used for micro- and nanoscale material
conditions to create a 3D image of a physical surface (26). In the present study, the AFM
topography of each capsule inner surface was obtained by scanning
probe microscopy (SPM-9700HT; Shimadzu Corp.). Prior to
observations, the capsule was cut off and placed on a specimen
mount with double-side tape. AFM topography was performed in air
using an EFM cantilever with a force constant 42 N/m in the phase
mode. All experiments were performed in triplicate with an area of
20.0x20.0 µm in each capsule. The obtained AFM images were
evaluated and quantified using ImageJ software. The capsule inner
surface of each capsule using AFM was characterized by calculating
the valley diameter, the valley area and the valley ratio. The
valley diameter corresponds to the average diameter of capsule
valleys in the total observed area. The valley area is the average
area of capsule valleys in the total observed area. The valley
ratio was expressed as the ratio of valley areas in the total
observed area. The valley diameter, valley area and valley ratio
were evaluated for 40 valleys in one observation area, and the
average value was calculated.
Measurement of capsule inner surface
potential distribution with the KFM mode
The potential distribution of each capsule inner
surface was obtained using the KFM mode from SPM-9700HT. The KFM
mode is a method used to measure the potential distribution of the
sample surface. This distribution is evaluated by detecting the
electrostatic force acting between the sample surface and the probe
tip by applying an alternating current signal to the electric
conductive cantilever. A KFM image is obtained in air using the EFM
cantilever with a force constant of 2.8 N/m in KFM mode. Prior to
observation, the capsule was cut off and placed on a specimen mount
with double-side tape. All experiments were performed in triplicate
with an area of 5.0x5.0 µm in each capsule. Image analysis was
performed on a representative image of each capsule. Images were
analyzed using SPM-9700HT manager software (Shimadzu Corp.).
In vitro inhalation performance
evaluation
Inhalation performance was evaluated using a
twin-stage liquid impinger (TSLI; European Pharmacopoeia Apparatus;
Copley Scientific Ltd.) equipped with a suction pump. Stages 1 and
2 in TSLI contained 7 and 30 ml of purified water and methanol
(75:25, volume ratio), respectively. Each capsule (G-Cap, PEG/G-Cap
and HPMC-Cap) was manually filled with 5.5±0.1 mg of TIO while the
weight was measured using sensitive digital balance (±0.1 mg;
Balance XS64; Mettler-Toledo GmbH). Once Handihaler® was
connected to the mouthpiece of TSLI, the TIO-loaded capsule was
placed in the holder of Handihaler® with a pin to pierce
it. The Handihaler® pierced one hole in the side wall of
the cap and the body. The suction pump was operated to disperse the
powder in the capsule at a flow rate 30 l/min. Since the lower
limit of peak inhalation flow rates for Handihaler® is
>20 l/min (25), the flow rate
of the suction pump was set to 30 l/min in the present study. Dry
powder remaining in the capsule and device at each stage after
dispersion by the suction pump was collected by rinsing with
purified water and methanol (75:25, volume ratio). The collected
samples were diluted into 50 ml purified water and methanol (75:25,
volume ratio), and the concentration of TIO in each sample was
measured by high performance liquid chromatography (HPLC). All
experiments were performed in triplicate. The inhalation
performance of TIO by TSLI was characterized using the following
parameters: i) Recovered dose (RD) is the mass of TIO recovered
from all parts of the apparatus (capsule, device and TSLI); ii)
mass balance (MB) is the ratio between RD to the amount of TIO to
be loaded into capsule; iii) drug retention for capsule is the
ratio of the amount of capsule drug deposition of the RD, while
Emitted dose (ED) is the amount of drug particles emitted from a
capsule to that from an inhalation device; iv) fine particle dose
(FPD) is the mass of drug particles from Stage 2 within the RD; and
v) fine particle fraction (FPF) is the ratio between FPD to RD.
Conditions for measuring TIO by
HPLC
The concentration of TIO in in vitro
inhalation characteristics samples was determined using Shimadzu
LC-20AT system (Shimadzu Corp.), which consists of a UV-vis
detector (SPD-20A), column oven (CTO-20A), degassing unite
(DGU-20A3R) and an auto sampler (SIL-10AF). The mobile
phase was composed of 100 mM potassium dihydrogenphosphate solution
(pH 4.0) and acetonitrile (80:20, volume ratio). The flow rate was
set to 0.25 ml/min. The column (Inertsil® ODS-3, 3.0 µm,
2.1x50 mm; GL Science Co., Inc.) was heated at 35˚C. The injection
volume was 100 µl. The UV absorbance of each sample was measured at
238 nm. Under these HPLC condition, the limit of detection for TIO
was 0.03 µg/ml, and a calibration curve was linear at a
concentration range of 0.1-20 µg/ml. The correlation coefficients
(R2) was 0.999 (y=326.12x+2684.8). All samples were
properly diluted to fit within this calibration range and peak area
was used for quantification of TIO.
Statistical analysis
All data were presented as the means ± standard
errors of measurements. The ‘BellCurve for Excel’ (Social Survey
Research Information Co., Ltd.) was used for statistical data
analysis. One-way ANOVA followed by Dunnett's post hoc test for
multiple comparison and an unpaired Student's t-test were used for
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Capsule characterization
The characterization of the three type of capsules
(G-Cap, PEG/G-Cap and HPMC-Cap) are presented in Table I. The appearance of each capsule is
shown in Fig. 1A-C. G-Cap and
HPMC-Cap were clear capsule bodies, whereas PEG/G-Cap had a
translucent capsule body. No significant differences were observed
in the length diameter, width diameter, surface area and volume of
each capsule between G-Cap, PEG/G-Cap and HPMC-Cap [non-significant
(NS), ANOVA]. The LOD of HPMC-Cap was much lower than the LOD of
G-Cap and PEG/G-Cap, with no difference between G-Cap to PEG/G-Cap
(Table I).
Morphology of a model dry powder
In the present study, TIO was used as a dry powder
model for capsule-based DPIs. TIO consists of micronized tiotropium
particles and coarse lactose monohydrate particles as carriers. The
morphology of TIO is presented in Fig.
1D and E. The SEM images
clearly demonstrated that micronized tiotropium particles [active
pharmaceutical ingredient (API) particles] adhered to coarse
lactose monohydrate particles (carrier particles).
Pierced capsule hole
characterization
The image of capsule hole of each capsule is
presented in Fig. 2. The
characteristics of pierced capsule holes of each capsule are
presented in Table II. In terms
of capsule hole after piercing, no cracked holes between G-Cap,
PEG/G-Cap and HPMC-Cap were seen (Fig.
2). The capsule hole diameter and hole area of G-Cap tended to
be larger than that of PEG/G-Cap and HPMC-Cap (Table II; NS, ANOVA).
 | Table IICharacteristics of pierced capsule
holes of each capsule. |
Table II
Characteristics of pierced capsule
holes of each capsule.
| | Hole diameter,
mm | Hole area,
mm2 |
|---|
| G-Cap | 1.28±0.02 | 1.56±0.08 |
| PEG/G-Cap | 1.14±0.98 | 1.26±0.06 |
| HPMC-Cap | 1.17±0.15 | 1.35±0.19 |
Comparison of inner surface structure
of each capsule
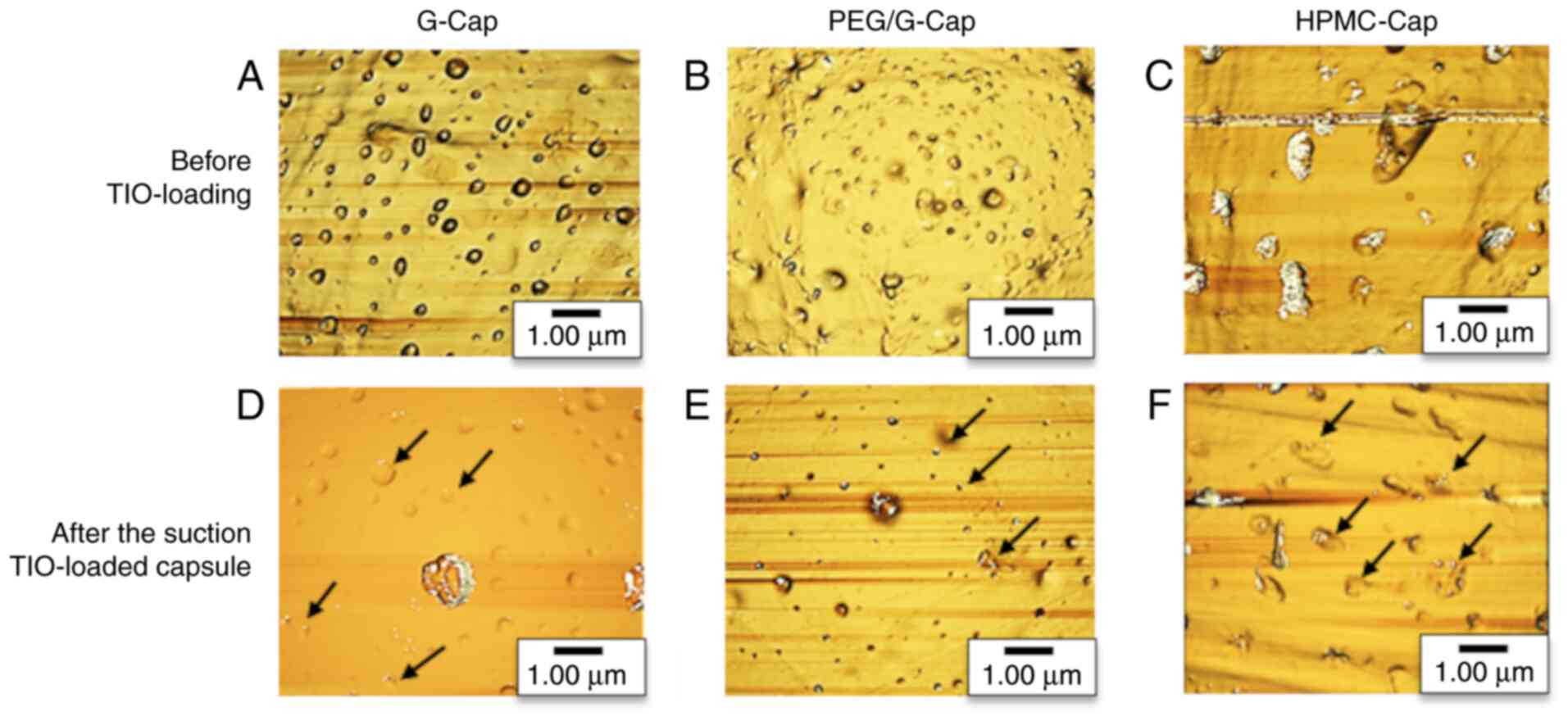
Fig. 3 presents the
inner surface structure of each capsule before TIO loading and
after suction of TIO-loaded capsules. The valley diameter, valley
area and valley ratio of capsule inner surface before TIO loading
and after the suction of TIO-loaded capsules are shown in Table III. In Fig. 3A-C, the areas darker than
background indicated the valleys while the lighter areas indicated
the mountains. Numerous valleys and mountains were observed on the
capsule inner surface regardless of the capsule composition. After
the suction of TIO-loaded capsules, TIO was observed in valleys on
the capsule inner surface (Fig.
3D-F). The black arrows in Fig.
3D-F indicated the area where particles were placed in the
valley. As presented in Table
III, the valley diameter, valley area and valley ratio were
decreased following suction, regardless of the type of capsule.
Furthermore, valley diameter, valley area and valley ratio of
HPMC-Cap were significantly difference before TIO loading and after
the suction of TIO-loaded capsules (P<0.05, unpaired Student's
t-test).
 | Table IIIValley diameter, valley area and
valley ratio of each capsule type determined by atomic force
microscopy images analysis. |
Table III
Valley diameter, valley area and
valley ratio of each capsule type determined by atomic force
microscopy images analysis.
| | Valley diameter,
µm | Valley area,
µm2 | Valley ratio,
% |
|---|
| | Before | After | Before | After | Before | After |
|---|
| G-Cap | 0.59±0.26 | 0.54±0.28 | 0.28±0.22 | 0.24±0.16 | 4.60±0.75 | 3.68±0.09 |
| PEG/G-Cap | 0.68±0.39 | 0.64±0.36 | 0.64±0.58 | 0.46±0.32 | 5.24±3.65 | 4.51±0.54 |
| HPMC-Cap | 1.97±0.98 |
1.06±0.40a | 2.05±1.89 |
1.02±0.67a | 10.6±6.24 |
6.39±0.16a |
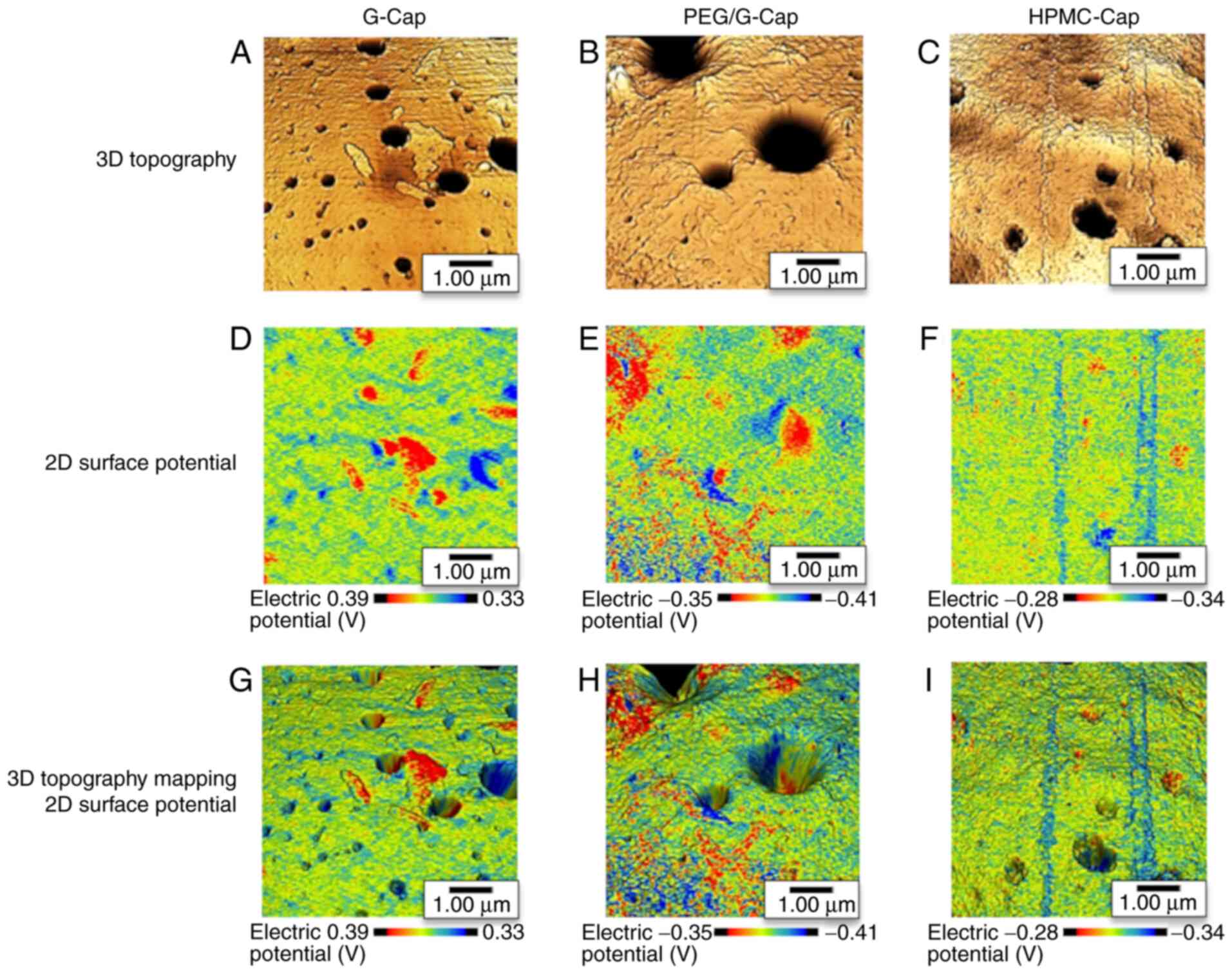
3D mapping analysis of the capsule
inner surface structure and surface potential distribution
The 3D topography of the capsule inner surface and
capsule inner surface potential distribution were evaluated using
SPM-9700HT. The 3D topographies of the three capsule inner surfaces
are presented in Fig. 4A-C. In
Fig. 4A-C, the areas darker than
background indicated the valleys, and the lighter areas indicated
the mountains. These topographies revealed the distribution of
valleys and mountains on the inner surface of each capsule. KFM
images of the three capsule inner surfaces are shown in Fig. 4D-F. In these images, the red
fraction indicates a higher potential and the blue fraction a lower
potential. KFM images showed mixed high and low potential fractions
in all three capsules. In addition, 3D topography and KFM image
mapping were performed to confirm the relationship between the
capsule inner surface structure and surface potential. The results
of image mapping demonstrated that the distribution of valleys and
mountains on the capsule inner surface corresponded to high and low
potential fractions, respectively (Fig. 4G-I).
Comparison of inhalation
performance
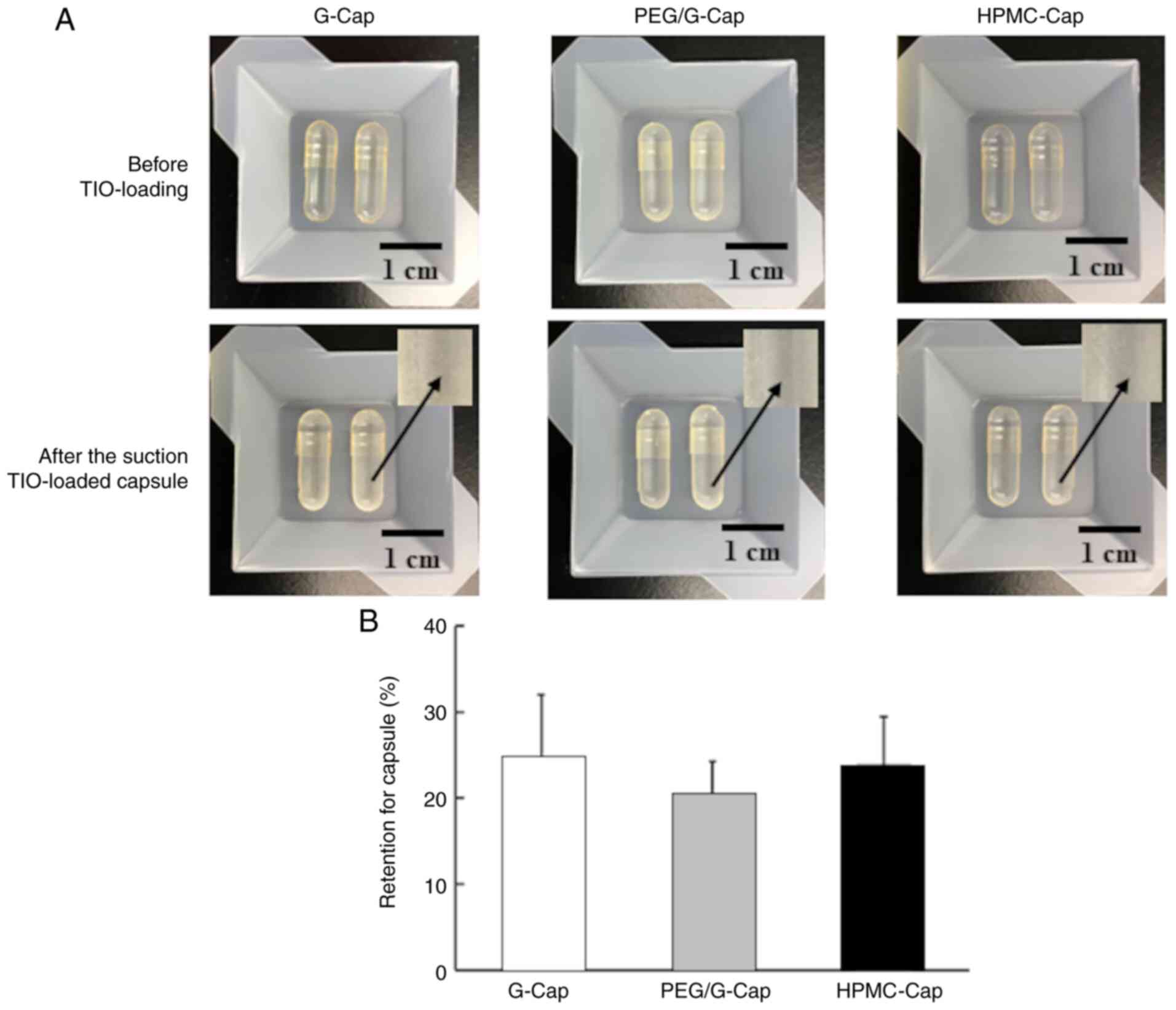
We evaluated drug release from capsule and lung drug
delivery with different compositions using TSLI. Fig. 5A shows an image of the inner
surface of each capsule before TIO loading and after the suction of
TIO-loaded capsules. Images of TIO-loaded capsules after the
suction demonstrated the adherence of TIO to the surface of capsule
regardless of the capsule type. The results for inhalation
performance parameters are presented in Fig. 5B and Table IV. The drug retention for capsule,
RD, MB, ED, FPD and FPF were not significantly different between
G-Cap, PEG/G-Cap and HPMC-Cap (NS, ANOVA).
 | Table IVInhalation performance of tiotropium
bromide loaded in three capsules with different compositions using
twin-stage liquid impinger. |
Table IV
Inhalation performance of tiotropium
bromide loaded in three capsules with different compositions using
twin-stage liquid impinger.
| | G-Cap | PEG/G-Cap | HPMC-Cap |
|---|
| Recovery dose,
µg | 17.4±0.18 | 16.8±0.42 | 17.5±0.6 |
| Mass balance,
% | 96.9±0.98 | 93.2±2.36 | 97.5±3.54 |
| Emitted dose,
µg | 4.37±0.53 | 4.67±0.14 | 4.52±0.27 |
| Fine particle dose,
µg | 7.94±0.85 | 7.54±0.45 | 8.27±0.74 |
| Fine particle
fraction, % | 25.7±4.85 | 28.3±4.50 | 26.7±1.25 |
Discussion
The advantages of capsule-based DPIs over other DPIs
are as follows: Accurate and uniform drug delivery, ease to use for
patients and the visual confirmation of whether a dose has been
administered (13,14). The inhalation performance of
capsule-based DPIs is related to numerous factors, such as the
inhalation patterns of patients (4-7),
the inhalation devices (5) and the
inhalation formulations (8). The
inhalation performance of capsule-based DPIs was recently reported
to be affected by the capsule properties. In a previous study,
inhalation performance was evaluated by filling five capsules with
binary or ternary particles of different compositions and
manufacturing methods, and the findings demonstrated that
inhalation performance was affected by capsule compositions and
manufacturing methods (22).
However, few studies have investigated the effects of the physical
properties of the capsule inner surface on inhalation performance.
The present study aimed therefore to determine the capsule inner
surface structures and surface potential distributions using the 3D
mapping technologies, AFM topography and KFM imaging. Because the
previous studies of capsule-based DPIs mainly focused on low
resistance devices, not high resistance devices (22,23),
the present study investigated the effects of capsule physical
properties on inhalation performance using a high resistance device
of capsule-based DPIs.
To characterize the properties of the capsules, each
capsule was evaluated by image analysis using ImageJ software. No
significant difference was observed in the length diameter, width
diameter, surface area and volume of each capsule, while the LOD of
HPLC-Cap (5.0%) was much lower than of G-Cap (14.3%) and PEG/G-Cap
(13.2%; Table II). As the result
of pierced capsule hole characteristics, there were no cracked
holes in G-Cap, PEG/G-Cap and HPMC-Cap (Fig. 2). The capsule hole diameter and
hole area of G-Cap tended to be larger than that of PEG/G-Cap and
HPMC-Cap (Table II). The results
of a previous study reported that different capsule compositions
exhibit different levels of water content. The gelatin capsule and
HPMC capsule demonstrated 13-16% and 3-7% water content,
respectively (27). Under low RH
conditions, a decreased water content increases brittleness, with
gelatin capsules being more sensitive to this effect (27). The capsule shell may therefore
crack or fracture when the capsule is pierced, thereby affecting
inhalation performance (28). In
the present study, the capsule compositions were different, and it
was expected that the capsules would crack during capsule piercing
due to the difference in LOD. Although the hole diameter and hole
area in each capsule was slightly different, no cracks occurred
during capsule piercing. We considered that the capsule storage
condition (15-25˚C, 40-50% RH) and the piercing condition using two
needles in the Handihaler® had a role in the lack of
cracking.
To confirm the capsule inner surface structure, each
capsule was observed using SPM. The inner surface structure on the
center, top, bottom, left and right parts were observed in a
previous experiment, and the results confirmed that there was no
significant difference among these regions. The images from
Fig. 3 were obtained by SPM of the
central part of the capsule body. Numerous valleys and mountains
were observed on each capsule inner surface before TIO loading,
irrespective of the capsule composition (Fig. 3A-C). Following the suction
TIO-loaded capsules, the inner surface of the capsule became smooth
and TIO was placed in the valleys (Fig. 3D-F). To confirm whether the
smoothness of the capsule inner surface was due to the TIO in the
valleys, the valley diameter, valley area and valley ratio were
compared before TIO loading and after the suction of TIO-loaded
capsules (Table III). The valley
diameter, valley area and valley ratio were slightly decreased
after suction, regardless of the type of capsule. In particular,
the valley diameter, area and ratio of the HPMC-Cap following the
suction of TIO-loading were significantly different compared with
these characteristics prior to TIO loading. The results suggested
that, although the capsule had valley and mountain structure on the
inner surface regardless of the capsule composition, the
characteristics of valleys were affected by the capsule
composition. Furthermore, drug particles may be trapped in the
valleys on the capsule inner surface, which would affect the
inhalation performance.
Subsequently, to confirm the electric properties of
capsule inner surface, we evaluated the surface potential
distribution using the KFM mode of SPM. KFM images demonstrated
that regions with high and low potential fractions were mixed in
each capsule (Fig. 4D-F). The 3D
topography and KFM images mapping of capsules showed that the
distribution valleys and mountains on the capsule inner surface
corresponded to high and low potential fractions, respectively
(Fig. 4G-I). Therefore, a local
potential difference may have occurred due to the distribution of
valleys and mountains on the capsule inner surface. These results
suggested that the effect of the inner surface structure and the
surface potential difference between the drug particles and the
valleys may both contribute to the increase in TIO amount in the
valleys.
To clarify the effects of the physical properties of
the capsule inner surface on inhalation performance, the inhalation
performance of each capsule type were evaluated using TSLI. The
results of drug retention for capsule and lung drug delivery were
not significantly different between G-Cap, PEG/G-Cap and HPMC-Cap
(Fig. 5B and Table IV). These findings may be due to
the fact that no significant difference in hole diameter and hole
area between capsules was observed. In addition, drug particles
were present in the inner surface valleys of each capsule, although
their impact on drug retention for capsule and lung drug delivery
was expected to be minimal. In the present study, we evaluated the
inhalation performance using the high resistance device at an
appropriate flow rate of 30 l/min. The influence of inhalation
device and flow rate on the drug release from capsule was therefore
considered to be greater than that of the capsule compositions and
physical properties of the capsule inner surface. In other words,
at an appropriate flow rate using high resistance device, the
physical properties of the capsule inner surface due to the capsule
composition did not significantly affect the drug release from
capsule and lung drug delivery. In this study, we particularly
focused on the drug release from capsule, and inhalation
performance was evaluated using TSLI. The TSLI is a simple
inhalation characteristic apparatus that contains Throat, Stage 1
and Stage 2. The TSLI was set up so that drug particles reaching
Stage 2 at a flow rate of 60 l/min showed a cut-off of less than
6.4 µm. Unfortunately, in the present study, the TSLI was examined
at a flow rate of 30 l/min, and the exact cut-off diameter could
not be measured. Therefore, the stage grouping in the inhalation
performance evaluation could not be performed, and the mass median
aerodynamic diameter (MMAD) ± geometric standard deviation (GSD)
could not be calculated. To understand the exact aerodynamic
differences in inhalation characterization, representative stage
grouping data and MMAD ± GSD are therefore needed (29,30).
Future investigation will evaluate the inhalation performance by
calculating MMAD ± GSD using Andersen cascade impactor, Next
generation impactor and Multistage liquid impinger.
In summary, the present study investigated the
effects of the physical properties of the capsule inner surface on
drug release form capsules and lung drug delivery using AFM and a
high resistance device. The results demonstrated that the capsule
inner surface had many valleys and mountains regardless of the type
of capsule. In addition, the valley and mountain areas on the
capsule inner surface presented with potential fluctuations where
there were significant differences in the surface structure.
Following inhalation of capsule-based DPIs, the drug remained in
valleys on the capsule inner surface; however, no significant
difference was observed in drug retention for capsule and lung drug
delivery. Therefore, drug release from capsule and lung drug
delivery in capsule-based DPIs when a high resistance device, such
as Handihaler®, is used at an appropriately flow rate
may have not significantly been affected by the physical properties
of the capsule inner surface of the capsule due to capsule
composition. However, since the present study only evaluated one
DPI formulation and inhalation device, the effect of the physical
properties of capsule inner surface on drug release and lung drug
delivery remain currently unclear. Further studies using various
DPI formulations, including indacaterol, glycopyrronium and
andrographolide, and inhalation devices, such as
Breezhaler®, are needed to elucidate the relationship
between the physical properties of capsule inner surface and
inhalation performance.
Acknowledgements
We would like to thank Qualicaps, Co., Ltd., for
supplying G-Cap, PEG/G-Cap and HPMC-Cap, and Shimadzu Corp. for
providing AFM topography and KFM images of each capsule surface
using SPM-9700HT. This study was partly supported by the Hosokawa
Powder Technology Foundation.
Funding
The present study was supported partly by The Mochida Memorial
Foundation for Medical and Pharmaceutical Research (grant no.
14).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
HO and MM performed the experiments and were the
major contributors to writing the manuscript. MY, SA and KI
analyzed and interpreted the data. HO and NN made substantial
contributions to the conception and design of the study. NN
designed the experiments and gave final approval for the version of
the manuscript to be published. MM and MY confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethic approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Claus S, Weiler C, Schiewe J and Friess W:
How can we bring high drug doses to the lung? J Pharm Biopharm.
86:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang MY, Chan JG and Chan HK: Pulmonary
drug delivery by powder aerosols. J Control Rel. 193:228–240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tonnis WF, Lexmond AJ, Frijlink HW, de
Bore AH and Hinrichs WL: Devices and formulations for pulmonary
vaccination. Expert Opin Drug Deliv. 10:1383–1397. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouwmeester C, Kraft J and Bungay KM:
Optimizing inhaler use by pharmacist-provided education to
community-dwelling elderly. Respir Med. 109:1363–1368.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Broeders ME, Vincken W, Corbetta L and
Group AW: The ADMIT series-Issues in inhalation therapy. 7. Ways to
improve pharmacological management of COPD: The importance of
inhaler choice and inhalation technique. Prime Care Respir J.
20:338–343. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cochrane MG, Bala MV, Downs KE, Mauskopf J
and Ben-Joseph RH: Inhaled corticosteroids for asthma therapy:
Patient compliance, devices, and inhalation technique. Chest.
117:542–550. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baily MM, Gorman EM, Munson EJ and
Berkland C: Pure insulin nanoparticle agglomerates for pulmonary
delivery. Lungmuir. 24:13614–13620. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weer JG and Miller DP: Formulation design
of dry powders for inhalation. J Pharm Sci. 104:3259–3288.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stocks J and Hislop AA: Structure and
function of the respiratory system: Developmental aspects and their
relevance to aerosol therapy. In: Drug Delivery to the Lung:
Clinical Aspects. Bisgaard H, O'Callaghan C, Smaldone CG (eds.)
Marcel Dekker, Inc., New York, NY pp47-104, 2002.
|
|
10
|
Lavorini F, Pistolesi M and Usmani OS:
Recent advances in capsule-based dry powder inhaler technology.
Multidiscip Respir Med. 12(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Berkenfeld K, Lamprecht A and McConville
JT: Devices for dry powder drug delivery to the lung. AAPS
PharmSciTech. 16:479–490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Faulhammer E, Flink M, Liusa M, Lawrence
SM, Biserni S, Calzolari V and Khinast JG: Low-dose capsule filling
of inhalation products: Critical material attributes and process
parameters. Int J Pharm. 473:617–626. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wauthoz N, Hennia I, Dejaeger B, Ecenarro
S and Amighi K: Proposed algorithm for healthcare professionals
based on product characteristics and in vitro performances in
different use conditions using formoterol-based marketed products
for inhalation. Int J Pharm. 530:415–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Smith IJ, Bell J, Bowman N, Everard M,
Stein S and Weer JG: Inhaler devices: What remains to be done? J
Aerol Med Pulm Drug Deliv. (Suppl 2):S25–S37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Steckel H and Muller BW: In-vitro
evaluation of dry powder inhalers 1: Drug deposition of commonly
used devices. Int J Phar. 154:19–29. 1997.
|
|
16
|
Vidgren M, Kärkkäinen A, Karjalainen P,
Paronen P and Nuutinen J: Effect of powder inhaler design on drug
deposition in the respiratory tract. Int J Pharm. 42:211–216.
1988.
|
|
17
|
Chew NY, Shekunov BY, Tong HH, Chow AH,
Savage C, Wu J and Chan H: Effect of amino acids on the dispersion
of disodium cromoglycate powders. J Pharm Sci. 94:2289–2300.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Otake H, Okuda T and Okamoto H:
Development of spray-freeze dried powders for inhalation with high
inhalation performance and antihygroscopic property. Chem Pharm
Bull (Tokyo). 64:239–245. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li HY, Seville PC, Williamson IJ and
Birchall JC: The use of amino acids to enhance the aerosolisation
of spray-dried powders for pulmonary gene therapy. J Gene Med.
7:343–353. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Ungaro F, Giovino C, Coletta C, Sorrentino
R, Miro A and Quaglia F: Engineering gas-foamed large porous
particles for efficient local delivery of macromolecules to the
lung. Eur J Pharm Sci. 41:60–70. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Duan J, Vogt FG, Li X, Hayes Jr D and
Mansour HM: Design, characterization, and aerolization of organic
solution advanced spray-dried moxifloxacin and ofloxacin
dipalmitoylphosphatidylcholine (DPPC)
microparticulate/nanoparticulate powders for pulmonary inhalation
aerosol delivery. Int J Nanomedicine. 8:3489–3505. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wauthoz N, Hennia I, Ecenarro S and Amighi
K: Impact of capsule type on aerodynamic performance of inhalation
products: A case study using a formoterol-lactose binary or ternary
blend. Int J Pharm. 553:47–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saleem IY, Diez F, Jones BE and Polo L:
Investigation on the aerosol performance of dry powder inhalation
hypromellose capsules with different lubricant levels. Int J Pharm.
492:258–263. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Horhota ST, van Noord JA, Verkleij CB,
Bour LJ, Sharma A, Trunk M and Cornelissen PJ: In vitro,
pharmacokinetic, pharmacodynamic, and safety comparisons of single
and combined administration of Tiotropium and Salmeterol in COPD
patients using different dry powder inhalers. AAPS J. 17:871–880.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hira D, Koide H, Nakamura S, Okada T,
Ishizeki K, Yamaguchi M, Koshiyama S, Oguma T, Ito K, Funayama S,
et al: Assessment of inhalation flow patterns of soft mist inhaler
co-prescribed with dry powder inhaler using inspiratory flow meter
for multi inhalation devices. PLoS One. 13(e0193082)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
García R and Pérez R: Dynamic atomic force
microscopy methods. Surf Sci Rep. 47:197–301. 2002.
|
|
27
|
Nagata S: Gellulose capsules-an
alternative to gelatin. In: Biolodical Polymers and Polymer
Therapeutics. Chiellini E, Sunamoto J, Migliaresi C, Ottenbrite RM,
Cohn D (eds). Kluwer Academics/Plenum Publishers, New York, NY, pp
53-62, 2001.
|
|
28
|
Coate MS, Fletcher DF, Chan HK and Raper
JA: The role of capsule on the performance of a dry powder inhaler
using computational and experimental analysis. Pharm Res.
22:923–932. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mehta PP, Kadam SS and Pawar AP: Effect of
USP induction ports and modified glass sampling apparatus on
aerosolization performance of lactose carrier-based fluticasone
propionate dry powder inhaler. J Drug Deliv Sci Technol.
58(101794)2020.
|
|
30
|
Timsina MP, Martin GP, Marriott C,
Ganderton D and Yianneskis M: Drug delivery to the respiratory
tract using dry powder inhalers. Int J Pharm. 101:1–13. 1994.
|