Introduction
Sarcoidosis represents a rare condition, affecting
several organs. Its etiology, defined by the development of
non-caseating granulomas in the affected organs, is still unknown
(1). The organ most commonly
affected by sarcoidosis is the lung; however, there is also skin
involvement in 20-35% of the patients with systemic conditions and
it can be the initial presentation as well. This disease affects
both sexes of all ages and races; nonetheless, it occurs more
commonly and severely in women and black individuals. Thus far, it
is still unclear what the cause of sarcoidosis is, even though
possible infectious, immunological, genetic and environmental
factors are to be considered (2,3). The
skin lesions are either specific skin lesions, where histologic
examination indicates the characteristic sarcoid granulomas, or
non-specific skin lesions. Maculopapular eruptions, subcutaneous
nodules, lupus pernio, scars and infiltrated plaques constitute
specific lesions. The main non-specific skin lesion of sarcoidosis
is erythema nodosum. Patients with a chronic course of the disease
(scar infiltration, lupus pernio, skin plaques) present parenchymal
involvement more frequently than those with acute sarcoidosis,
which a better prognosis (maculopapular rash, erythema nodosum)
(1).
Case report
A 35-year-old man presented at the Department of
Dermatology of Ponderas Academic Hospital, Bucharest, Romania for
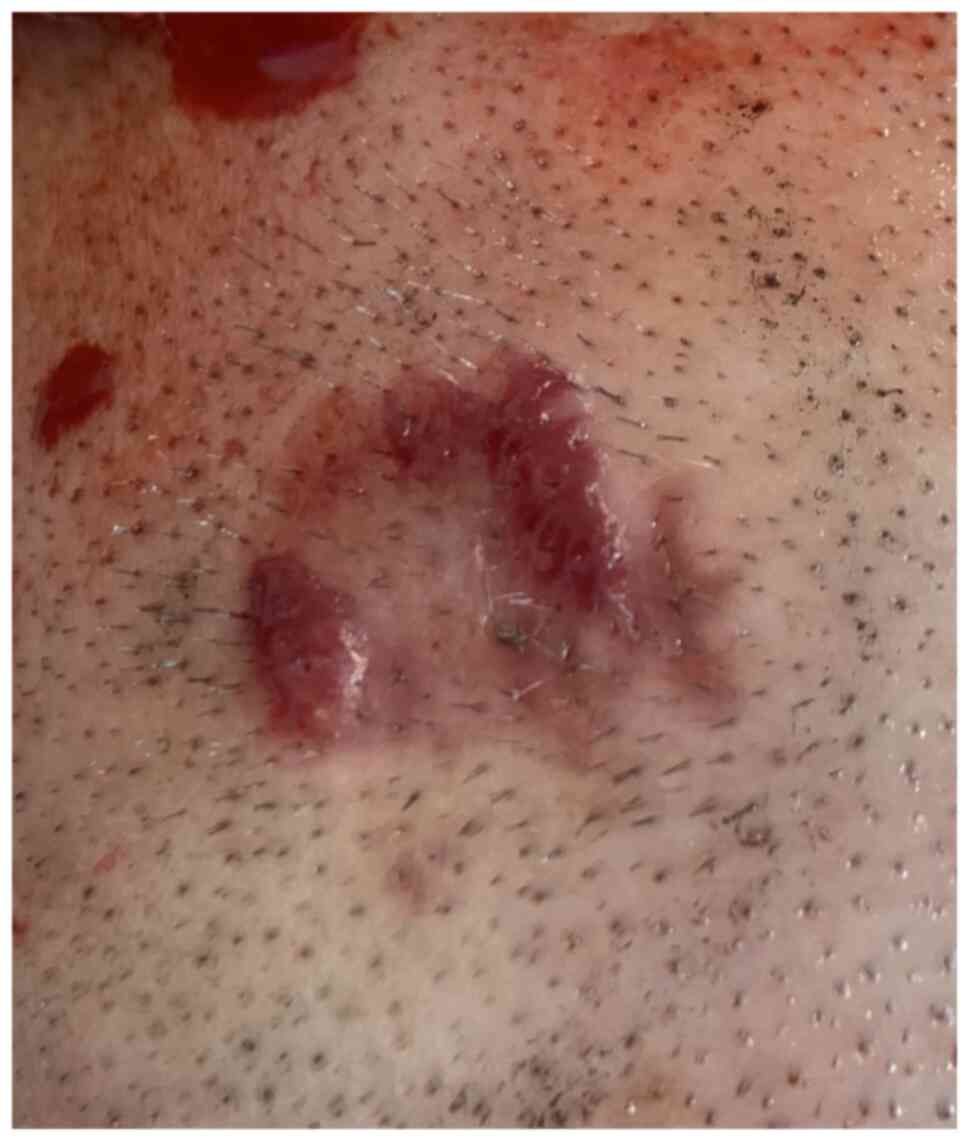
an orange-red indurated plaque with a raised, shiny border on the
scalp, which appeared suddenly during the last 6 months (Fig. 1). The patient did not report any
significant changes, bleeding or itch. However, for the previous 10
days, he had developed a dry cough, along with malaise, night
sweats and shortness of breath on medium exertion. The patient
denied smoking and exposure to any toxic chemicals. Polarized
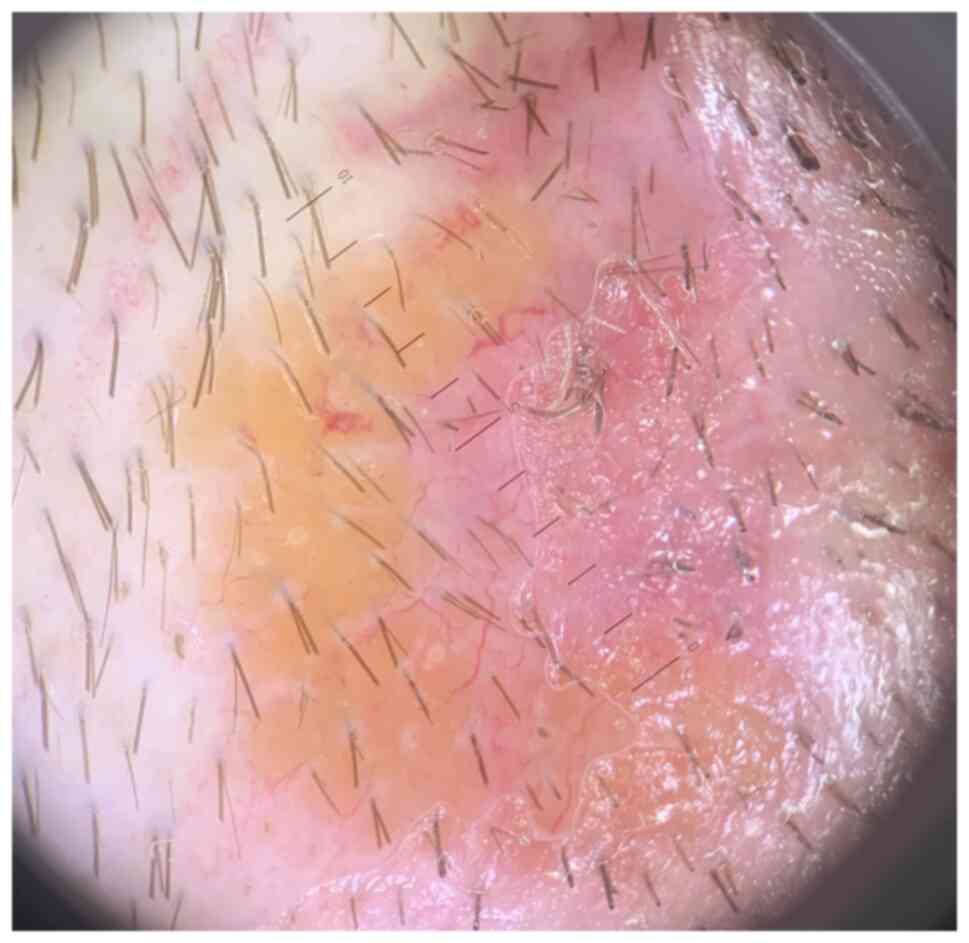
dermoscopy (using DermLite, DL4, x10) with immersion oil showed
diffuse monomorphic linear vessels, as well as yellow-orange and
pink structureless areas (Fig. 2).
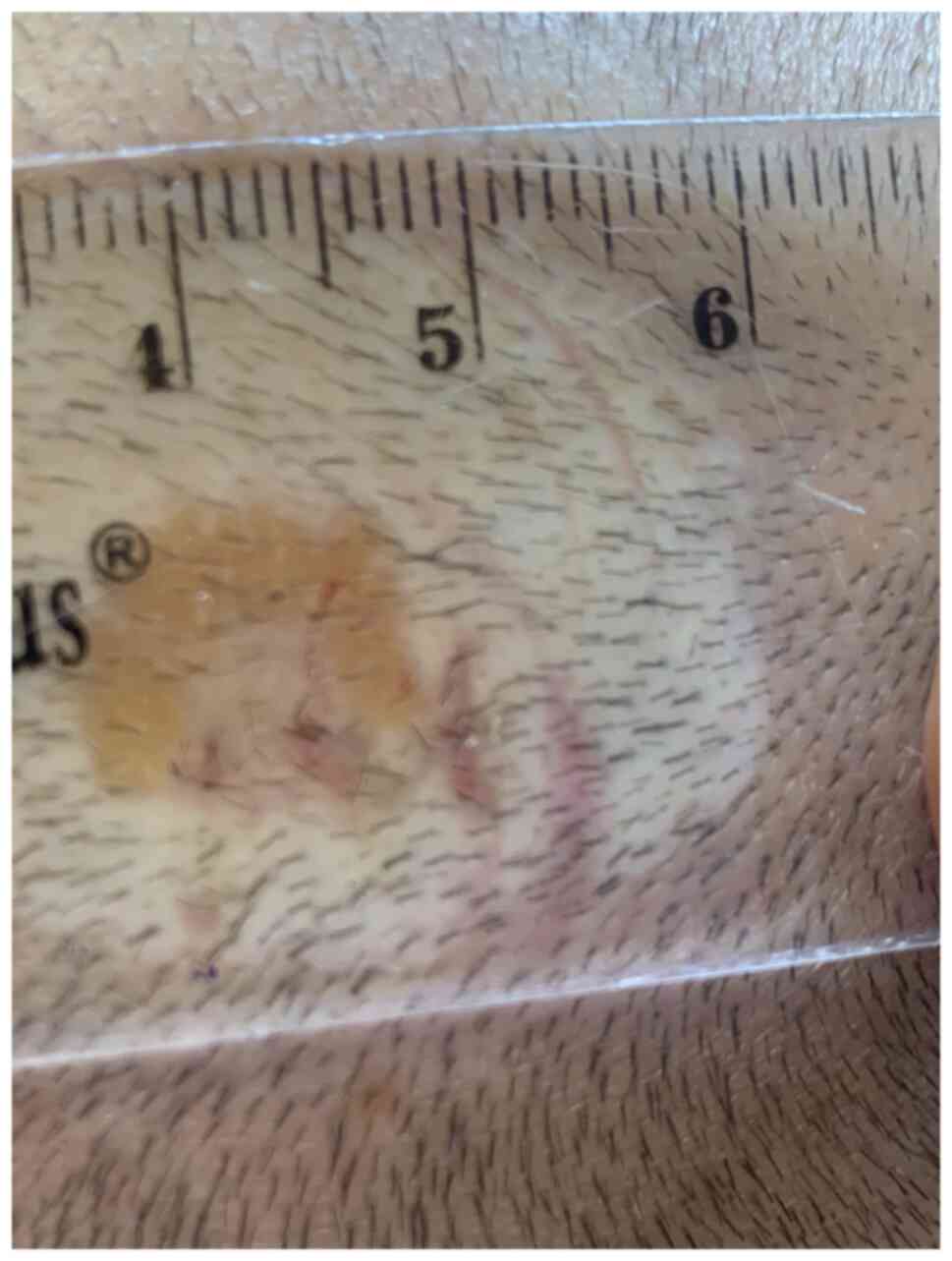
The diascopy revealed the characteristic ‘apple jelly’ nodules
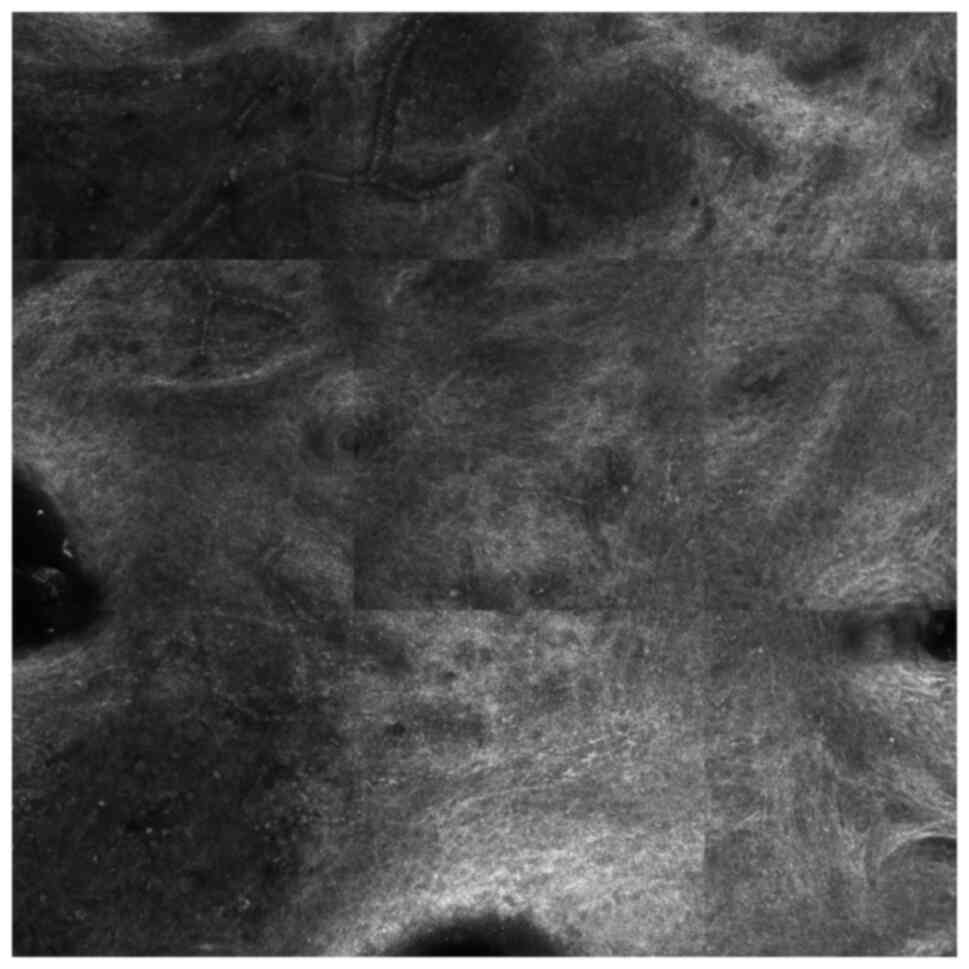
(Fig. 3). The reflectance confocal
microscopy of the lesion showed superficial tortuous vessels, many
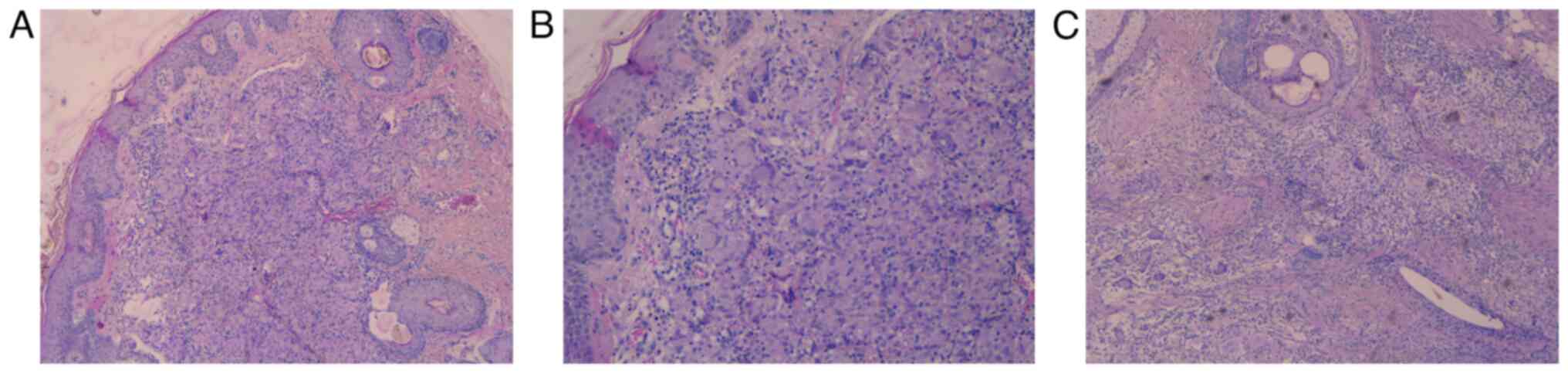
reticulin fibers and inflammatory cells (Fig. 4). A biopsy was performed. Subsequent
histopathology revealed chronic dermal inflammation with multiple
confluent non-caseating granulomas with epithelioid cells,
lymphocytes and multinucleated giant cells (Fig. 5C). The blood tests showed no
abnormalities, except for a high level of angiotensin converting
enzyme (141 U/l) and a slightly elevated C-reactive protein (0.75
mg/dl). In addition, the tests for Mycobacterium
tuberculosis were negative.
After complete excision of the lesion with narrow
margins, the patient was referred to the Pneumology Department for
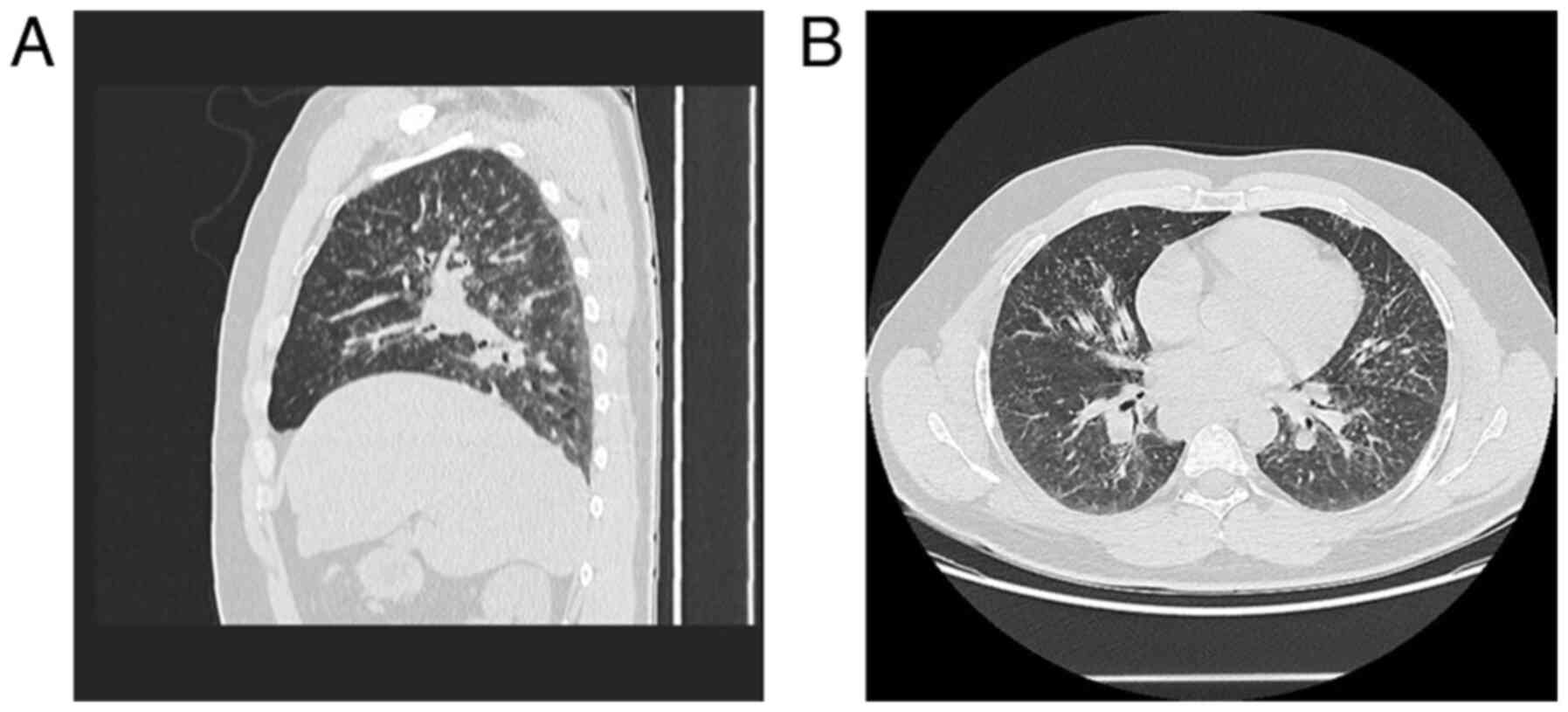
further examination and a CT scan of the thorax, abdomen and pelvis
with contrast. It revealed multiple bilateral mediastinal
lymphadenopathy (23/24 mm, right superior paratracheal lymph node;
34/32 mm, right inferior paratracheal lymph node; 33/24 mm, lateral
aortic lymph node; 28/26 mm, right hilar lymph node; 28/24 mm, left
hilar lymph node) and multiple symmetric pulmonary micronodules
with peribronchovascular distribution (Fig. 6A and B). In addition, the patient was diagnosed
with secondary increased airway hyperreactivity. The ENT
examination showed no pathologic changes. The patient was then
referred to the cardiologist and the ophthalmologist for a thorough
evaluation.
The patient was diagnosed with systemic sarcoidosis
and started systemic treatment with methylprednisolone 32 mg and
inhalation therapy with beclomethasone/formoterol 100/6 mg.
Discussion
Sarcoidosis represents a chronic granulomatous
condition, which is characterized by non-necrotizing granulomas. It
may affect any organ and sometimes constitutes a considerable
diagnostic challenge. The organ most commonly affected by
sarcoidosis is the lung; however, there is also skin involvement in
20-35% of the patients with systemic conditions and it can be the
initial presentation as well. Cutaneous sarcoidosis affects mostly
the face and the limbs (2).
Cutaneous sarcoidosis is also known as the ‘great imitator’ in
dermatology, since it can mimic a great variety of cutaneous
lesions (4).
The skin lesions are either specific skin lesions
where histologic examination indicates the characteristic sarcoid
granulomas, or non-specific skin lesions. Maculopapular eruptions,
subcutaneous nodules, lupus pernio, scars and infiltrated plaques
constitute specific lesions. The main non-specific skin lesion of
sarcoidosis is erythema nodosum, which can be typically seen in
young women as a marker of acute sarcoidosis (1,2).
Conversely, lupus pernio is generally associated with chronic
sarcoidosis, affecting women and older patients. Scar sarcoidosis
may occur on tattoos, surgical scars or vaccination sites. Plaque
sarcoidosis typically involves the limbs, being an indolent form of
the condition (2). Parenchymal
involvement occurs more in patients with a chronic course of the
disease (lupus pernio, scar infiltration and skin plaques) as
opposed to those with acute sarcoidosis (1).
Sarcoidosis can be diagnosed by exclusion and is
backed by recognizing the specific clinical characteristics,
detecting the typical histopathologic findings and excluding other
granulomatous conditions (fungal infection, tuberculosis,
leishmaniasis, foreign body reactions and rheumatoid nodules)
(2,5). From a clinical point of view, diascopy
can prove helpful in displaying the granulomatous inflammation.
This technique is performed by pressing a glass slide against the
skin, which allows the potential ‘apple jelly’ nodules to be seen
(2).
Compulsory standard investigations, accompanied by
physical examination and a complete medical history, should include
pulmonary function tests, chest X-ray, ophthalmologic evaluation,
electrocardiogram, biochemistry, full blood count, serum
immunoglobulins, as well as a 24-h urinary calcium assay (4). The measurement of the serum
angiotensin-converting enzyme (ACE), produced by sarcoidal
granulomas, can prove helpful in monitoring the development of the
disease. However, it is not a very useful diagnostic test,
considering that the levels may be raised in other diseases, such
as alcoholic liver disease and diabetes (2). Specific cutaneous lesions, together
with elevated serum ACE levels, high CD4/CD8
ratio and bronchoalveolar lavage lymphocytosis may act as
predictors of progressive disease in sarcoidosis (6). When there is lung involvement,
physicians should apply the classification of chest X-rays by De
Remee: stage I, bilateral hilar lymphadenopathy (BHL); stage II,
BHL plus pulmonary parenchymal infiltration; stage III, parenchymal
involvement infiltration without BHL (7). In addition, patients with parenchymal
involvement may experience a restrictive pattern of lung
impairment, as well as increased airway hyperreactivity (8).
Dermoscopy represents a non-invasive tool that
enables the visualization of vascular and pigmented structures not
visible to the naked eye. Dermoscopy is conventionally used for
skin tumor diagnosis. However, it has gained an increased interest
during the past years as a useful tool in general dermatology in
the clinical diagnosis of inflammatory and infectious skin
manifestations. Dermoscopy is valuable in differentiating an
extensive range of granulomatous inflammatory and infectious skin
conditions, such as necrobiosis lipoidica, granuloma annulare,
cutaneous leishmaniasis, lupus vulgaris, syphilis, foreign body
reactions, atypical mycobacteriosis, fungal infections or
rheumatoid nodules, as well as skin tumors, such as sebaceous
adenoma or trichoepithelioma, as sarcoidosis can clinically mimic
all of these lesions (9).
Nevertheless, dermoscopy does not seem to be sufficient in order to
accurately establish the diagnosis of cutaneous sarcoidosis, since
a suspicious lesion of cutaneous sarcoidosis displays linear
vessels and yellow-orange structureless areas, but so does any
granulomatous condition (9,10). Hence, conventional methods such as
radiography, laboratory tests or histopathology represent the state
of the art in the diagnosis of sarcoidosis (9).
Reflectance confocal microscopy has been recently
used as support for the diagnosis of cutaneous sarcoidosis. It
represents a non-invasive imaging technique that allows in
vivo visualization of the papillary dermis and epidermis with
cellular level resolution. Granulomatous conditions, such as
sarcoidosis, could be evaluated using this technique. Identifying
bright beaded-like structures that correspond to reticulin fibers
overlying granulomas can prove very helpful, in association with
dermoscopy, in establishing the diagnosis of sarcoidosis (11).
Considering the ease with which to perform a biopsy
for skin lesions, it generally represents the conventional type of
investigation. The biopsy specimens usually show a dermal
infiltrate of non-caseating granulomas, made of epithelioid cells,
multinucleate giant cells and a thin peripheral rim of lymphocytes.
Deep fungal and mycobacterial infections should be excluded by
special stains and culture (2).
Treating cutaneous sarcoidosis can often become
frustrating, since the lesions can either be refractory to
treatment or recur after successful treatment. Intralesional or
topical steroids are used for localized skin involvement.
Intralesional steroids (triamcinolone acetonide 5 mg/ml, with
injections repeated at 2-3 week intervals) are usually more
effective, because even very potent topical steroids do not
penetrate the skin lesion effectively (5,8).
For progressive and multiple lesions and/or systemic
symptoms, systemic treatments are used. Systemic glucocorticoids
are reported to be the most effective agent (used at slow, tapering
dosages, starting at 20-40 mg of oral prednisone daily for four to
six weeks); however, there are many patients that do not respond
well to steroids. In refractory patients, methotrexate,
hydroxychloroquine and thalidomide can prove effective (4). Tofacitinib, adalimumab, etanercept,
pentoxifylline, apremilast, infliximab, and even topical
photodynamic therapy represent several new therapeutic options
(8,12-15).
Nonetheless, the globally acknowledged standard therapies include
the administration of corticosteroids, methotrexate and
antimalarials, considering the need to perform more studies for the
above-mentioned treatments (8).
Most types of cutaneous sarcoidosis present a
chronic course, except maculopapular sarcoidosis and erythema
nodosum. The general prognosis of the disease is linked to the
severity and the extent of the internal involvement (2).
Considering the fact that cutaneous sarcoidosis is
often a precursor of the systemic form, it is of paramount
importance to diagnose correctly as well as any form of cutaneous
sarcoidosis as early as possible. Reflectance confocal microscopy
and dermoscopy represent very useful tools; however, histologic
examination remains the investigation of choice. In addition,
interdisciplinary collaboration between various medical specialties
is required in these cases. In our case, the patient's test results
showed similar aspects with the ones depicted in the
literature.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data and materials supporting the results of the
present study are available in the published article.
Authors' contributions
DB performed the biopsy and the excision of the
lesion and participated in the therapeutic management of the case
study. AC performed the diascopy and the dermoscopy examination and
performed critical review of the literature findings. CC performed
the confocal microscopy examination and performed critical review
of the literature findings. NB performed the histopathologic
examination. All authors read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
There is a general valid ethics approval for this
case presentation that is part of the PATHDERM project no
61PCCDI/2018 (PN-III-P1-1.2-PCCDI-2017-0341).
Patient consent for publication
A signed consent for clinical examination, surgery,
other medical investigations, treatment and capturing images for
publication was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yanardağ H, Pamuk ON and Karayel T:
Cutaneous involvement in sarcoidosis: Analysis of the features in
170 patients. Respir Med. 97:978–982. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilson NJ and King CM: Cutaneous
sarcoidosis. Postgrad Med J. 74:649–652. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ungprasert P, Wetter DA, Crowson CS and
Matteson EL: Epidemiology of cutaneous sarcoidosis, 1976-2013: A
population-based study from Olmsted County, Minnesota. J Eur Acad
Dermatol Venereol. 30:1799–1804. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Katta R: Cutaneous sarcoidosis: A
dermatologic masquerader. Am Fam Physician. 65:1581–1584.
2002.PubMed/NCBI
|
|
5
|
Vasaghi A and Kalafi A: Unusual
Manifestation of cutaneous sarcoidosis: A case report of
morpheaform sarcoidosis. Acta Med Iran. 50:648–651. 2012.PubMed/NCBI
|
|
6
|
Yanardag H, Tetikkurt C, Bilir M, Demirci
S and Iscimen A: Diagnosis of cutaneous sarcoidosis; clinical and
the prognostic significance of skin lesions. Multidiscip Respir
Med. 8(26)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
De Remee RA: The roentgenographic staging
of sarcoidosis. Historic and contemporary perspectives. Chest.
1:128–133. 1983.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi SC, Kim HJ, Kim CR, Byun JI, Lee DY,
Lee JH, Lee ES and Yang JM: A case of morpheaform sarcoidosis. Ann
Dermatol. 22:316–318. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pellicano R, Tiodorovic-Zivkovic R,
Gourhant JY, Catricalà C, Ferrara G, Caldarola G, Argenziano G and
Zalaudek I: Dermoscopy of cutaneous sarcoidosis. Dermatology.
221:51–54. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Conforti C, Giuffrida R, de Barros MH,
Resende FSS, Cerroni L and Zalaudek I: Dermoscopy of a single
plaque on the face: An uncommon presentation of cutaneous
sarcoidosis. Dermatol Pract Concept. 8:174–176. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pasquali P, Gonzalez S, Fortuño A and
Freites-Martinez A: In-vivo assessment of a case of cutaneous
sarcoidosis using reflectance confocal microscopy. An Bras
Dermatol. 94:93–95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heffernan MP and Smith DI: Adalimumab for
treatment of cutaneous sarcoidosis. Arch Dermatol. 142:17–19.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baughman RP, Judson MA, Ingledue R, Craft
NL and Lower EE: Efficacy and safety of apremilast in chronic
cutaneous sarcoidosis. Arch Dermatol. 148:262–264. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Damsky W, Thakral D, Emeagwali N, Galan A
and King B: Tofacitinib treatment and molecular analysis of
cutaneous sarcoidosis. N Engl J Med. 379:2540–2546. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Karrer S, Abels C, Wimmershoff MB,
Landthaler M and Szeimies RM: Successful treatment of cutaneous
sarcoidosis using topical photodynamic therapy. Arch Dermatol.
138:581–584. 2002.PubMed/NCBI View Article : Google Scholar
|