Introduction
Subarachnoid hemorrhage (SAH) is a type of
hemorrhagic stroke defined as hemorrhage in the subarachnoid space,
and it is often caused by brain injury or aneurysm rupture
(1). SAH is classified into five
grades, with grade V being associated with the worst clinical
prognosis (2). The average
mortality of patients with SAH is 40-50% in a population-based
study in the USA (3).
Nevertheless, a previous study has suggested that ~46% of patients
who survive from SAH often suffer from cognitive impairment
(4). Early brain injury (EBI) is
termed the brain injury that occurs within 72 h of SAH (5). The abnormal elevation of the
intracranial pressure, the reduced cerebral blood perfusion and the
development of brain edema are considered to be associated with
SAH-induced EBI (6). However, the
molecular mechanism underlying SAH-induced EBI remains unknown.
Activated protein C (APC) is an activated form of
zymogen that serves an important role in regulating multiple
biological processes (7). APC has
been first recognized for its anticoagulant activity through the
inactivation of coagulation factors and subsequent prevention of
thrombosis (8). In addition, APC
has also been indicated to exert anti-inflammatory activity
(9). In the central nervous
system, APC serves a neuroprotective role in various brain
diseases, such as ischemic stroke and traumatic brain injury
(10,11). APC can be transported through the
blood-brain barrier (BBB) via contact with its receptors and the
stabilized endothelial cells of the BBB (12). APC is antithrombotic and
anti-inflammatory, and has been identified as a neuroprotective
factor that is able to exert antiapoptotic effects (13). A previous report has indicated that
APC causes biased cytoprotective signaling that reduces
ischemia-induced injury by cleaving protease-activated receptor
1(14). Moreover, APC promotes
neurogenesis, and recombinant 3K3A-APC is considered to be a
promising agent for ischemic stroke therapy (11). 3K3A-APC is an artificial protein
made through the replacement of three lysine residues by three
alanine residues, which reduces >90% of the anticoagulant
activity but retains the original biological activity of the
molecule (15). Furthermore, APC
inhibits the activation of the NLR family pyrin domain-containing 3
(NLRP3) inflammasome, which provides a novel framework for the
anti-inflammatory activities of APC (16). However, the role of APC and its
derivative in SAH-induced EBI requires further elucidation.
Excessive inflammation is hypothesized to be a major
cause of SAH-induced EBI (17).
The NLRP3 inflammasome is composed of a NOD-like receptor (NLRP3),
apoptosis-associated speck-like protein containing a C-terminal
caspase recruitment domain and an adaptor protein (18). It is activated in response to
pathogens and induces the secretion of IL-1β and IL-18, which can
lead to an inflammatory cascade and initiate the programmed cell
death pathway known as pyroptosis (19,20).
Previous studies have indicated that the activation of the NLRP3
inflammasome is associated with inflammatory injury and
neurological disorders in SAH (21,22).
Moreover, pyroptosis induced by the NLRP3 inflammasome has been
revealed to be associated with neuroinflammatory injury in the case
of SAH (23). However, to the best
of our knowledge, the role of the NLRP3 inflammasome and pyroptosis
in SAH-induced EBI is rarely reported.
A previous study has indicated that APC can prevent
ischemia-reperfusion injury through the inhibition of the NLRP3
inflammasome (16). However, the
association between APC and the NLRP3 inflammasome in SAH-induced
EBI remains unclear. The present study established SAH cell and rat
models to explore the biological functions of APC and the NLRP3
inflammasome in SAH-induced EBI, aiming to provide novel strategies
for the management of SAH.
Materials and methods
Cell culture and construction of the
SAH model
Rat PC12 cells (The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) were used in the
present study and cultured in RPMI-1640 medium (cat. no. 11875093;
Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated horse serum (cat. no. 04-124-1A; Shanghai Yaoyun
Biological Technology Co., Ltd.) and 5% fetal bovine serum (cat.
no. 16000-044; Gibco; Thermo Fisher Scientific, Inc.). Cells were
treated with 10 mM oxygenated hemoglobin (OxyHb) at 37˚C to
construct the SAH cell model for 24 h, while the control cells were
treated with corresponding PBS solution. BAY11-7082 (5 µmol/l; cat.
no. S2913; Selleck Chemicals), an inhibitor of NLRP3(24), was dissolved in PBS and added to
the culture medium at 37˚C for 48 h, while PBS was used to culture
cells as control. Moreover, 3K3A-APC (FILZB1-03; ZZ Biotech LLC)
was used to treat cells at the concentrations of 5, 10 and 20
ng/ml, respectively.
Reverse transcription-quantitative
PCR
Total RNA from rat PC12 cells that were treated with
3K3A-APC (FILZB1-03; ZZ Biotech LLC) at the concentrations of 5, 10
and 20 ng/ml were extracted using TRIzol® reagent (cat.
no. 1596-026; Invitrogen; Thermo Fisher Scientific, Inc.) after
co-culturing for 24 h. The cDNA synthesis kit (Thermo Fisher
Scientific, Inc.) was used for cDNA synthesis of RNA according to
the manufacturer's protocols. The SYBR® Green
fluorochrome (cat. no. K0223; Thermo Fisher Scientific, Inc.) and
the following thermocycling conditions were used for qPCR: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of
denaturation at 95˚C for 15 sec, annealing at 60˚C for 45 sec and
extension at 72˚C for 30 sec, with a final extension at 72˚C for 3
min. β-actin was used as the reference gene. Gene expression was
calculated using the 2-ΔΔCq method (25). All primers were listed as follows:
APC forward, 5'-CACCCCTTTCAGTTCAAGTAGC-3' and reverse,
5'-AAGACCCAGAATGGCGTTTAG-3'; NLRP3 forward,
5'-ACCTCAACAGACGCTACACCC-3' and reverse, 5'-GCTGCTCCCTGGAACACC-3';
β-actin forward, 5'-CGG TCAGGTCATCACTATC-3' and reverse,
5'-CAGGGCAGT AATCTCCTTC-3'. Each reaction was repeated three
times.
Western blotting
The total protein content of cells or brain tissues
was obtained using RIPA lysis buffer with protease inhibitor
cocktail (Beyotime Institute of Biotechnology). The protein levels
were quantified using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The protein (20 µg/lane) was separated
on 10% SDS-PAGE and transferred to a nitrocellulose membrane. The
membranes were blocked using 5% skimmed milk at room temperature
for 2 h and incubated overnight at 4˚C with the primary antibodies
as follows: APC (1:2,000; cat. no. ab40778), NLRP3 (1:1,000; cat.
no. ab263899), caspase-1 (1:100; cat. no. ab74279) (all from
Abcam), gasdermin D (GSDMD-N; 1:1,000; cat. no. 39754) and β-actin
(1:1,000; cat. no. 3700) (both from Cell Signaling Technology,
Inc.)
After washing with 0.1 M PBS, the membranes were
incubated with the secondary antibodies HRP-labeled goat
anti-rabbit IgG (1:1,000, cat. no. A16104; Thermo Fisher
Scientific, Inc.) and HRP-labeled goat anti-mouse antibody IgG
(1:1,000, cat. no. A0216; Thermo Fisher Scientific, Inc) at 4˚C for
2 h. Enhanced chemiluminescence detection kit (cat. no. WBKLS0100;
MilliporeSigma) was used for signal detection. Each reaction was
replicated three times.
Knockdown of APC
A total of three short hairpin (sh)RNAs were
designed for targeting rat APC (shAPC-1, 5'-GCAUGA
AACUGCCUCCCAUTT-3'; shAPC-2, 5'-GCAGGAAGC CCAUGAACAATT-3'; shAPC-3,
5'-GCCACUGACAUA UCUUCAUTT-3'). A scrambled siRNA negative control
(shNC; 5'-UUCUCCGAACGUGUCACGUTT-3') was used as the control. All
the APC shRNAs were inserted into the pLKO.1 vector (Elibo
Biotechnology, Co., Ltd.). Rat PC12 cells (5x105
cells/well) were transfected with 100 nM APC shRNAs or the
corresponding negative empty using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, and cells were cultured for 48 h before
analysis.
ELISA
The expression levels of IL-1β and IL-18 in cells
that had been treated with 3K3A-APC or BAY11-7082, as indicated
above, were examined using Rat IL-18 ELISA kit (cat. no.
E-EL-R0567c) and Rat IL-1β ELISA kit (cat. no. E-EL-R0012c) (both
from Elabscience, Inc.) according to the manufacturer's protocol.
Briefly, IL-1β and IL-18 antibodies were applied at 37˚C for 2 h.
After rinsing off the washing solution, the secondary antibody was
applied. Subsequently, the stop solution was added and the optical
density at 450 nm was measured. Each reaction was replicated three
times.
Estimation of cell pyroptosis
Activated caspase-1 was estimated to reflect the
level of cell pyroptosis using the Caspase-1 p20 Antibody/FITC
(cat. no. EL900443-100-FITC; Eterlife, Ltd.) according to the
manufacturer's instructions. In brief, PC12 cells were seeded into
6-well plates (5x105 cells/well) and allowed to grow
until reaching 50% confluence. 3K3A-APC or BAY11-7082-treated cells
were incubated with a caspase-1 detection probe for 1 h in the dark
at 37˚C. After rinsing to remove the unconjugated FLICA reagent,
cells were stained with propidium iodide (PI) for 20 min at 37˚C.
Accuri C6 flow cytometer (v1.0.264; BD Biosciences) using CellQuest
Pro software v3.3 (BD Biosciences) was used to identify pyroptotic
cells as being identified by double positive for cleaved caspase-1
and PI. Each reaction was replicated three times.
Construction of the SAH rat model
Male Sprague-Dawley rats (weight, 280-320 g; age, 12
weeks; n=30) were purchased from Elibo Biotechnology, Co., Ltd. The
rats were raised in a 12/12-h dark/light cycle with free access to
food and water at 25±2˚C with humidity (60±5%). The SAH rat model
was constructed through internal carotid artery puncture according
to a method described previously (26). Rats were initially anesthetized
using isoflurane (5%) in an induction chamber and then maintained
on isoflurane (2-2.5%) anesthesia with the aid of a nose cone.
After anesthesia, the external carotid artery was ligated and
sectioned. A nylon thread was inserted through the external carotid
artery into the intracranial part of the internal carotid artery.
When the branch met resistance, the nylon thread was advanced to
puncture the internal carotid artery, which resulted in SAH in
rats. Subsequently, 2 mg/kg of 3K3A-APC and corresponding vehicle
(PBS) were intraperitoneally injected every 12 h. In the
sham-operated group, a similar procedure was performed without
perforation. Animals were euthanized by decapitation under deep
pentobarbital anesthesia at 6, 12, 24, 48 or 72 h after model
constructing (intravenously; 60 mg/kg body weight). Brain tissues
were collected for pathological analysis. There were six rats at
each time point in different groups. All animal procedures involved
in the current study were approved by the Independent Animal Ethics
Committee of Huzhou Central Hospital, Affiliated Hospital of Huzhou
Normal University (approval no. 16470; Huzhou, China) and was
carried out in compliance with the ARRIVE guidelines (27).
Neurological function score
The scoring of neurological functions was performed
using modified Garcia scoring and the balance beam test according
to the method as indicated in a previous report (28). Modified Garcia scoring included
spontaneous activity, the extension of the four limbs, climbing on
a metal mesh wall and touch and whisker response of both sides of
the trunk. The scoring system was as follows: 0 Points, no symptoms
of nerve damage; 1 point, the contralateral forepaws could not be
fully extended; 2 points, when walking, the rat turned to the left
(paralyzed side), indicating moderate neurological deficits; 3
points, when walking, the body of the rat toppled to the left
(paralyzed side), indicating in severe neurological deficits; 4
points, unable to walk spontaneously, loss of consciousness.
Beam walking test
The beam walking test method was performed according
to the methods in Manaenko et al (29). The score of normal rats in the beam
walking test was 4.
SAH score
The scoring of SAH was conducted using modified
Sugawara's scoring (30). The rats
were decapitated under anesthesia and images of the basal cistern
and Willis' circle were captured. The basal cistern was divided
into six parts, and the scores for each part were as follows: i) 0
was defined as no SAH; ii) 1 was defined as a small amount of SAH;
iii) 2 was defined as medium SAH with several distinguishable blood
vessels; and iv) 3 was defined as a large amount of SAH without
distinct blood vessels. The final score was the sum of the scores
of the six parts, among which 0-7, 8-12 and 13-18 points were
defined as mild, moderate and severe hemorrhage, respectively.
Statistical analysis
GraphPad Prism version 7.0 (GraphPad Software, Inc.)
was used for data analyses and visualization. Quantitative data are
presented as the mean ± standard deviation, and each experiment was
repeated three times. Differences between groups were analyzed
using one-way ANOVA followed by Tukey-Kramer multiple comparison
test. Moreover, the ordinal data (all scores) were analyzed using
Kruskal-Wallis test followed by Dunn's post hoc tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
APC and NLRP3 are altered in a
time-dependent manner in the SAH rat model
To preliminarily explore the role of APC and NLRP3
in SAH, their expression levels were detected in the SAH rat model
at different time points. The results demonstrated that the mRNA
expression level of APC was significantly reduced after 48 h
(P<0.05), whereas that of NLRP3 was significantly elevated after
12 h compared with the sham group (P<0.001), and these effects
occurred in a time-dependent manner (Fig. 1A). Furthermore, the protein level
of APC was also revealed to be markedly decreased, whereas NLRP3,
caspase-1 and GSDMD-N were increased in the SAH rat model in a
time-dependent manner (Fig. 1B).
These results indicated that APC and NLRP3 were associated with the
development of SAH and that they exhibited reversed expression
patterns in SAH.
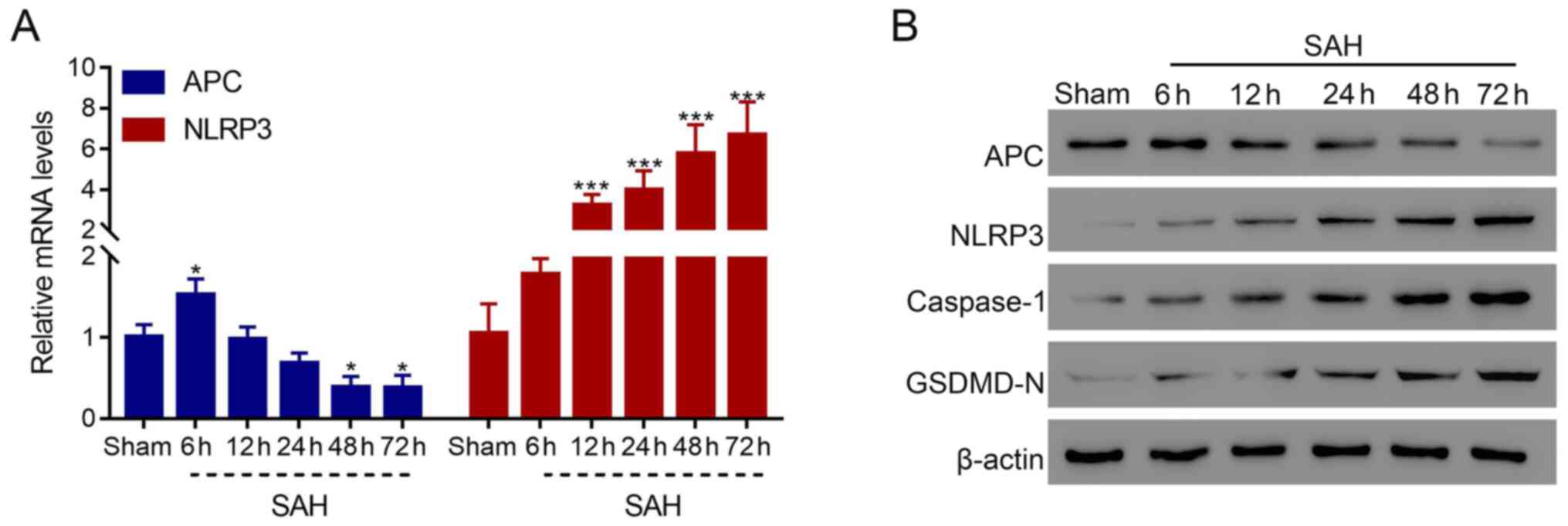 | Figure 1Expression levels of APC and NLRP3 are
altered in a time-dependent manner in the SAH model. (A) mRNA level
of APC and NLRP3 in the brain of the SAH rat model at 6, 12, 24, 48
and 72 h. (B) Protein levels of APC, NLRP3, caspase-1 and GSDMD-N
in the brain of the SAH rat model at 6, 12, 24, 48 and 72 h.
*P<0.05, ***P<0.001 vs. sham. APC,
activated protein C; NLRP3, NLR family pyrin domain-containing 3;
SAH, subarachnoid hemorrhage; GSDMD, gasdermin D. |
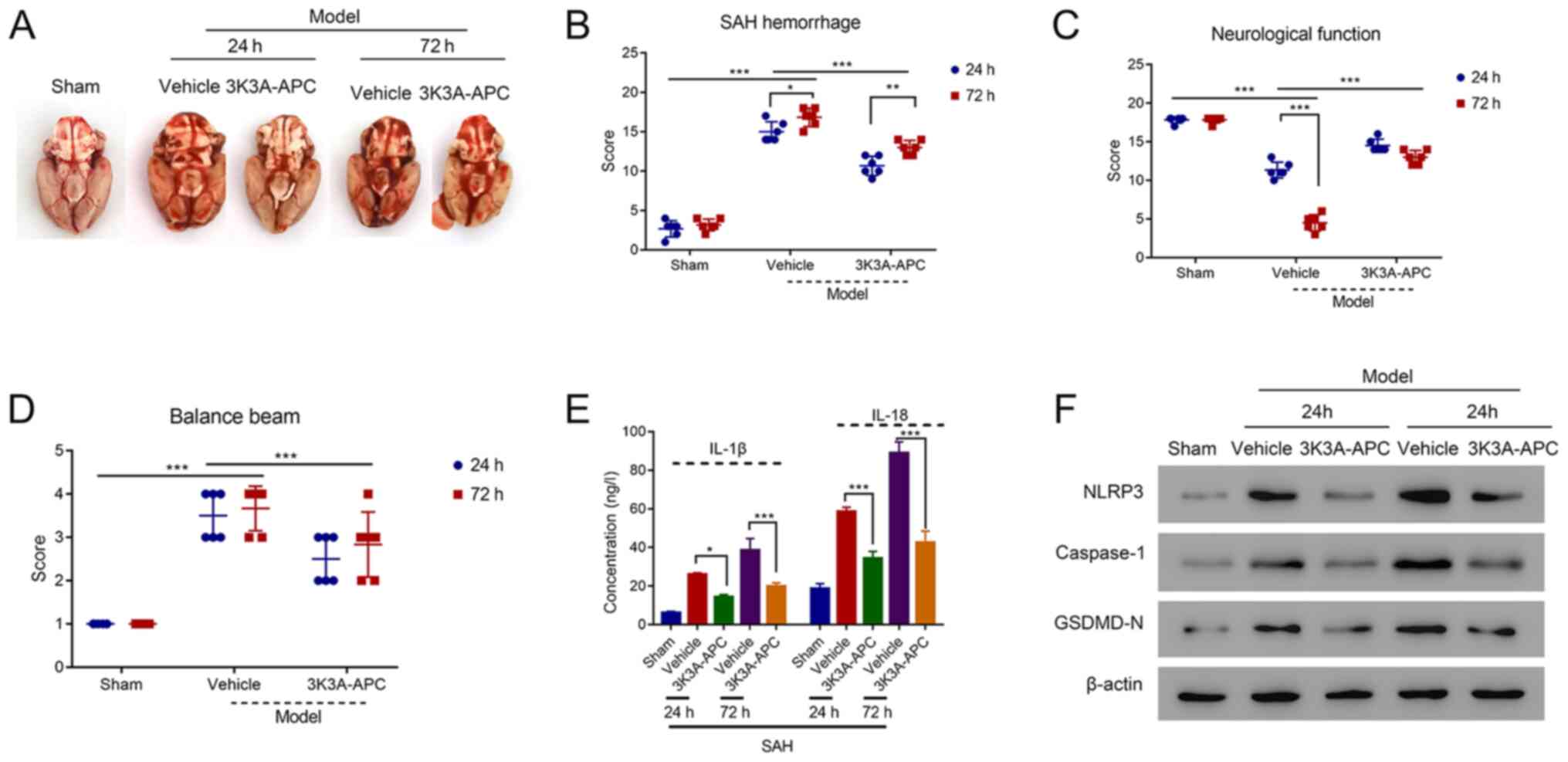
APC recombinant protein 3K3A-APC
ameliorates SAH-induced injury in the rat model
To further explore the function of APC in SAH, a
recombinant APC protein known as 3K3A-APC was applied in the SAH
rat model. After 24 or 72 h of intervention, 3K3A-APC significantly
reduced SAH in rats compared with the vehicle (P<0.001; Fig. 2A and B). In addition, 3K3A-APC significantly
improved the neurological function of SAH rats compared with the
vehicle (P<0.001), which were significantly impaired compared
with the sham group at 24 h (P<0.001; Fig. 2C). In addition, the balance beam
score was significantly reduced in the SAH model after the
application of 3K3A-APC compared with the vehicle (P<0.001;
Fig. 2D). Interestingly, the SAH
hemorrhage, neurological function and balance beam score from 24 to
72 h in the model revealed no significant difference after
treatment with 3K3A-APC.
Subsequently, brain tissues were collected from rats
followed by ELISA, which revealed that the levels of IL-1β and
IL-18 were elevated in the vehicle SAH rats compared with the sham
group; however, 3K3A-APC could significantly reduce their
concentrations (all P<0.05; Fig.
2E). Western blotting revealed that the expression levels of
NLRP3, caspase-1 and GSDMD-N were reduced after the intervention of
3K3A-APC compared with that in vehicle group both at 24 and 48 h
(Fig. 2F). Overall, these results
suggested that recombinant APC protein 3K3A-APC could ameliorate
the hemorrhage and improve neurological functions in SAH.
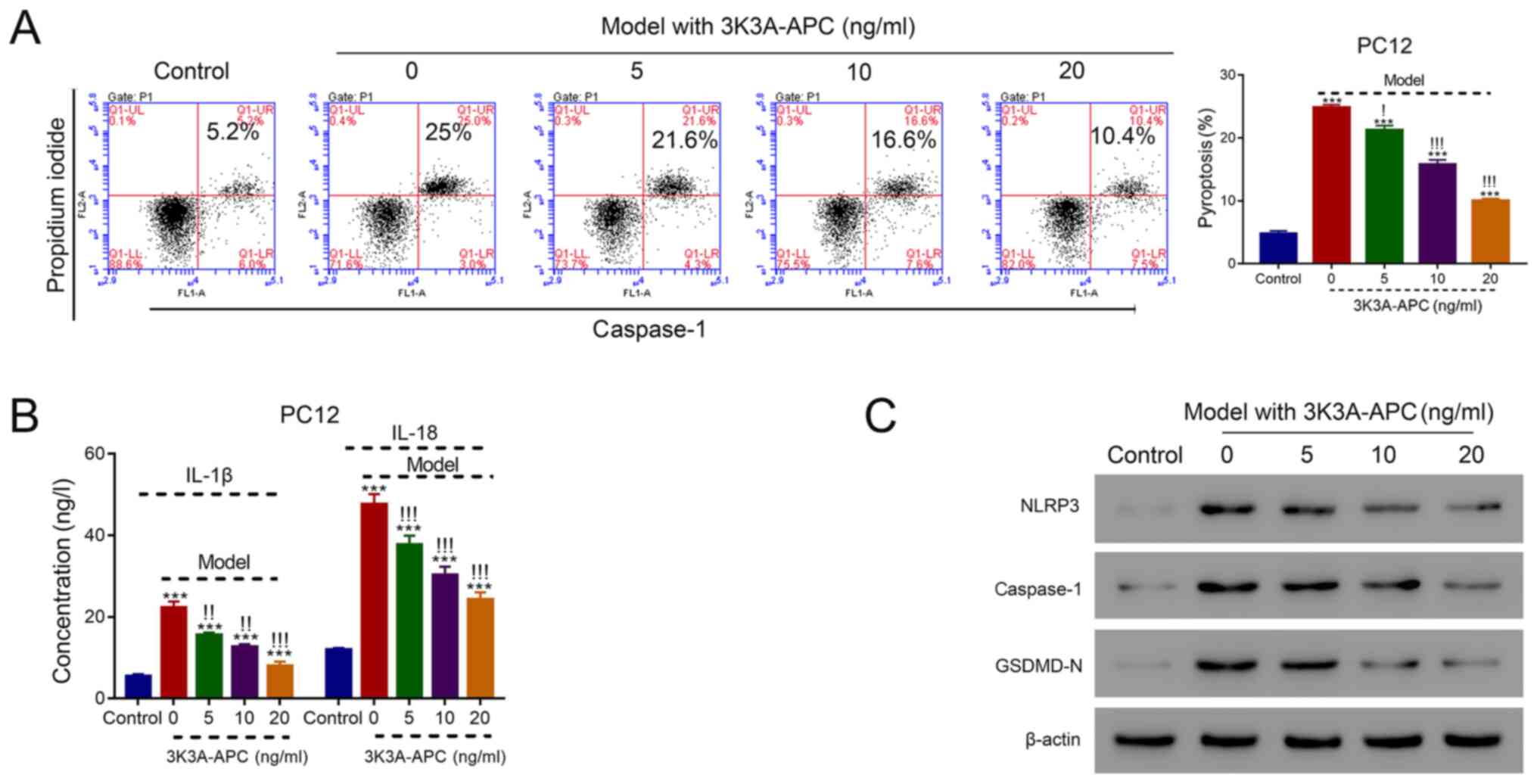
APC recombinant protein 3K3A-APC
inhibits pyroptosis in a dose-dependent manner in the SAH cell
model
The association between 3K3A-APC and pyroptosis in
the SAH cell model was investigated. The construction of the SAH
cell model was performed via application of 10 mM OxyHb, where the
application of 5, 10 and 20 ng/ml 3K3A-APC significantly reduced
the proportion of pyroptotic cells that were PI- and
caspase-1-positive in a dose-dependent manner compared with cells
treated with 0 ng/ml 3K3A-APC (all P<0.05; Fig. 3A). In addition, the levels of IL-1β
and IL-18 were significantly reduced after the application of 5, 10
and 20 ng/ml 3K3A-APC in a dose-dependent manner compared with
cells treated with 0 ng/ml (all P<0.01; Fig. 3B). Moreover, the protein levels of
NLRP3, caspase-1 and GSDMD-N were suppressed in a dose-dependent
manner in cells treated with 3K3A-APC (Fig. 3C). These results indicated that
3K3A-APC could inhibit pyroptosis in the SAH cell model in a
dose-dependent manner.
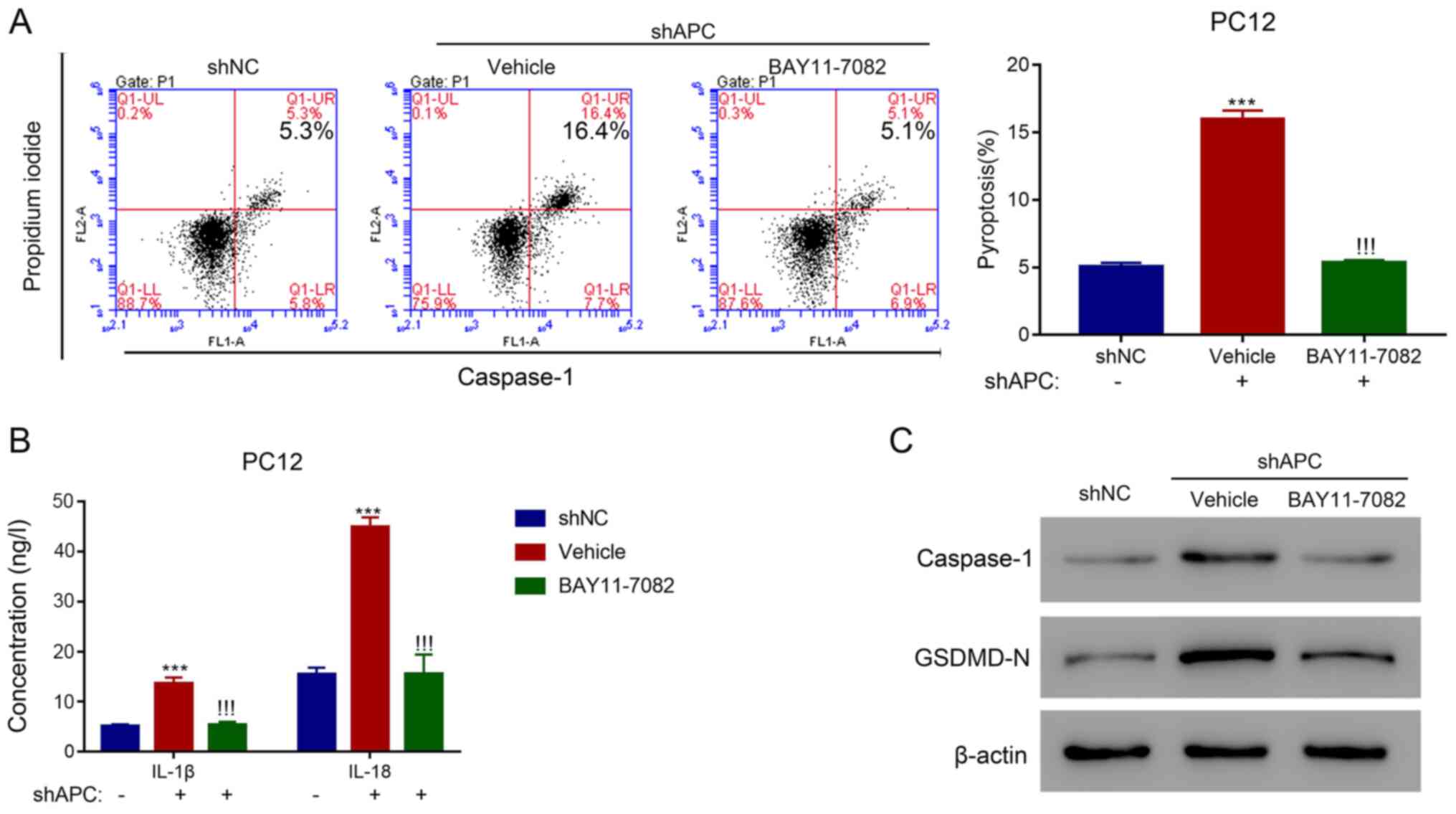
NLRP3 inhibitors reverse the activity
of 3K3A-APC in the SAH cell model
To further verify the association between 3K3A-APC
and pyroptosis in SAH, a specific inhibitor of NLRP3, BAY11-7082,
was applied to explore its effect on 3K3A-APC in PC12 cells. A
total of three specific shRNAs targeting APC were designed to
inhibit the expression of APC at the mRNA and protein levels
(P<0.001; Fig. S1). The
inhibition of APC with shAPC-2 significantly increased the
proportion of pyroptotic cells in the vehicle compared with the
shNC group (P<0.001); however, the application of BAY11-7082
could significantly reverse this effect (P<0.001; Fig. 4A). In addition, the expression
levels of IL-1β and IL-18 were significantly elevated with shAPC in
the vehicle compared with the shNC group (P<0.001); however,
they were significantly suppressed after treatment with BAY11-7082
(P<0.001; Fig. 4B). Moreover,
the expression levels of caspase-1 and GSDMD-N were increased with
shAPC in the vehicle group compared with the shNC group, which were
then decreased after BAY11-7082 treatment (Fig. 4C). These results suggested that
inhibition of APC could promote pyroptosis in the SAH cell model
through activating the NLRP3 inflammasome.
Discussion
Long-term neurocognitive impairment significantly
disrupts the quality of life of patients with SAH (31). A previous report has demonstrated
that microglial pyroptosis is involved in the development of
postcardiac arrest brain injury (32). The results of the present study
indicated that APC was significantly reduced, while NLRP3 was
significantly elevated in the SAH rat model in a time-dependent
manner, and the inhibition of these effects provided a protective
role in SAH-induced EBI. Moreover, 3K3A-APC was identified as a
promising strategy for SAH treatment.
As an engineered product of APC, 3K3A-APC is
synthesized using three alanine residues to replace three lysine
residues, which inhibits its coagulative activity and maintains its
biological activities (15). It
has been demonstrated that 3K3A-APC exerts a promising
neuroprotective activity in the treatment of ischemic stroke
(15,33). In a phase II clinical trial,
patients with acute stroke treated with 3K3A-APC exhibited a
decreased incidence of hemorrhage (34). Moreover, 3K3A-APC can prevent the
deposition of amyloid-β and diminish neuroinflammatory responses in
mice (35). Therefore, 3K3A-APC is
a promising therapy for brain disorders. In the current study,
3K3A-APC significantly ameliorated the hemorrhage and improved
neurological functions in the SAH rat model. Moreover, the present
study indicated that 3K3A-APC may ameliorate SAH-induced injury and
exhibit a protective role in SAH through the inhibition of
pyroptosis.
The NLRP3 inflammasome serves an important role in
SAH-induced EBI. Hu et al (36) reported that the application of a
specific G-protein-coupled bile acid receptor 1 agonist, INT-777,
can significantly reduce neuroinflammation via the inhibition of
the NLRP3 inflammasome. Moreover, the specific blockade of NLRP3
can alleviate SAH-induced EBI by suppressing inflammation (37). In the present study, the expression
of NLRP3 was significantly elevated in the SAH rat model. The
application of an NLRP3 inhibitor, BAY11-7082, could suppress
pyroptosis and reverse the function of APC inhibition in the SAH
cell model. These results indicated that NLRP3 was involved in the
neuroprotective activity of APC in SAH. The current study
demonstrated that the application of 3K3A-APC and the inhibition of
NLRP3 could significantly reduce the levels of IL-1β and IL-18 as
well as the proportion of pyroptotic cells in SAH rat and cell
models. Therefore, the present study indicated that the
neuroprotective role of APC was mediated by the suppression of
pyroptosis via the inhibition of the NLRP3 inflammasome. The
rationale of the current study is presented as a graphical abstract
in Fig. S2.
In the present study, the effects of APC and
3K3A-APC were not examined in a clinical context, and this
limitation discounted the clinical significance of the results. In
the future, it will be necessary to further examine the role of APC
in a clinical context in subsequent analyses. To summarize, the
present study indicated that APC could ameliorate SAH-induced EBI
by suppressing pyroptosis and inhibiting NLRP3 inflammasome, which
could provide a novel strategy for the treatment of SAH.
Supplementary Material
Knockdown of APC in rat PC12 cells.
(A) mRNA and (B) protein levels of APC in PC12 cells transfected
with shNC and three specific shRNAs against APC.
***P<0.001 vs. shNC. APC, activated protein C; sh,
short hairpin; NC, negative control.
Graphical abstract for the present
study. APC, activated protein C; SAH, subarachnoid hemorrhage;
NLRP3, NLR family pyrin domain-containing 3.
Acknowledgements
Not applicable.
Funding
The present research was financially supported by the Medical
Science and Technology Project of Zhejiang Province (grant no.
2020KY302) and Huzhou General Science and Social Development
Project Foundation (grant no. 2019GY39).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AY designed the project and revised the manuscript.
XP performed the experiments and wrote the draft. XW analyzed the
data and edited diagrams. XN and YL performed the analysis and
interpretation of data and confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures of the current study were
approved by the Independent Animal Ethics Committee of Huzhou
Central Hospital, Affiliated Hospital of Huzhou Normal University
(approval no. 16470; Huzhou, China) and were carried out in
compliance with the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dupont SA, Wijdicks EF, Lanzino G and
Rabinstein AA: Aneurysmal subarachnoid hemorrhage: An overview for
the practicing neurologist. Semin Neurol. 30:545–554.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rosen DS and Macdonald RL: Subarachnoid
hemorrhage grading scales: A systematic review. Neurocrit Care.
2:110–118. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Teunissen LL, Rinkel GJ, Algra A and van
Gijn J: Risk factors for subarachnoid hemorrhage: A systematic
review. Stroke. 27:544–549. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Suarez JI, Tarr RW and Selman WR:
Aneurysmal subarachnoid hemorrhage. N Engl J Med. 354:387–396.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cahill J, Calvert JW and Zhang JH:
Mechanisms of early brain injury after subarachnoid hemorrhage. J
Cereb Blood Flow Metab. 26:1341–1353. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sehba FA and Bederson JB: Mechanisms of
acute brain injury after subarachnoid hemorrhage. Neurol Res.
28:381–398. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Griffin JH, Zlokovic BV and Mosnier LO:
Activated protein C, protease activated receptor 1, and
neuroprotection. Blood. 132:159–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ohga S, Ishiguro A, Takahashi Y, Shima M,
Taki M, Kaneko M, Fukushima K, Kang D and Hara T: Japan Childhood
Thrombophilia Study Group. Protein C deficiency as the major cause
of thrombophilias in childhood. Pediatr Int. 55:267–271.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Joyce DE, Gelbert L, Ciaccia A, DeHoff B
and Grinnell BW: Gene expression profile of antithrombotic protein
c defines new mechanisms modulating inflammation and apoptosis. J
Biol Chem. 276:11199–11203. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zlokovic BV and Griffin JH: Cytoprotective
protein C pathways and implications for stroke and neurological
disorders. Trends Neurosci. 34:198–209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Griffin JH, Mosnier LO, Fernández JA and
Zlokovic BV: 2016 Scientific Sessions Sol Sherry Distinguished
Lecturer in Thrombosis: Thrombotic Stroke: Neuroprotective Therapy
by Recombinant-Activated Protein C. Arterioscler Thromb Vasc Biol.
36:2143–2151. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Deane R, LaRue B, Sagare AP, Castellino
FJ, Zhong Z and Zlokovic BV: Endothelial protein C
receptor-assisted transport of activated protein C across the mouse
blood-brain barrier. J Cereb Blood Flow Metab. 29:25–33.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mosnier LO and Griffin JH: Protein C
anticoagulant activity in relation to anti-inflammatory and
anti-apoptotic activities. Front Biosci. 11:2381–2399.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Soh UJ and Trejo J: Activated protein C
promotes protease-activated receptor-1 cytoprotective signaling
through β-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci
USA. 108:E1372–E1380. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mosnier LO, Gale AJ, Yegneswaran S and
Griffin JH: Activated protein C variants with normal cytoprotective
but reduced anticoagulant activity. Blood. 104:1740–1744.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nazir S, Gadi I, Al-Dabet MM, Elwakiel A,
Kohli S, Ghosh S, Manoharan J, Ranjan S, Bock F, Braun-Dullaeus RC,
et al: Cytoprotective activated protein C averts Nlrp3
inflammasome-induced ischemia-reperfusion injury via mTORC1
inhibition. Blood. 130:2664–2677. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu GJ, Tao T, Zhang XS, Lu Y, Wu LY, Gao
YY, Wang H, Dai HB, Zhou Y, Zhuang Z, et al: Resolvin D1 Attenuates
Innate Immune Reactions in Experimental Subarachnoid Hemorrhage Rat
Model. Mol Neurobiol. 58:1963–1977. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu S, Li X, Liu Y, Xia Y, Chang R and
Zhang C: Inflammasome inhibitors: Promising therapeutic approaches
against cancer. J Hematol Oncol. 12(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abbate A, Salloum FN, Vecile E, Das A,
Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby
ED, et al: Anakinra, a recombinant human interleukin-1 receptor
antagonist, inhibits apoptosis in experimental acute myocardial
infarction. Circulation. 117:2670–2683. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kantono M and Guo B: Inflammasomes and
Cancer: The Dynamic Role of the Inflammasome in Tumor Development.
Front Immunol. 8(1132)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cao S, Shrestha S, Li J, Yu X, Chen J, Yan
F, Ying G, Gu C, Wang L and Chen G: Melatonin-mediated mitophagy
protects against early brain injury after subarachnoid hemorrhage
through inhibition of NLRP3 inflammasome activation. Sci Rep.
7(2417)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou K, Shi L, Wang Z, Zhou J, Manaenko A,
Reis C, Chen S and Zhang J: RIP1-RIP3-DRP1 pathway regulates NLRP3
inflammasome activation following subarachnoid hemorrhage. Exp
Neurol. 295:116–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu P, Hong Y, Xie Y, Yuan K, Li J, Sun R,
Zhang X, Shi X, Li R, Wu J, et al: TREM-1 Exacerbates
Neuroinflammatory Injury via NLRP3 Inflammasome-Mediated Pyroptosis
in Experimental Subarachnoid Hemorrhage. Transl Stroke Res.
12:643–659. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng XY, Lv YD, Jin FY, Wu XJ, Zhu J and
Ruan Y: Kainic acid hyperphosphorylates tau via inflammasome
activation in MAPT transgenic mice. Aging (Albany NY).
11:10923–10938. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kenneth JL and Thomas DS: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan Y, Yan G, Liu F, Rong J, Ma W, Yang D
and Yu Y: Potential role of poly (ADP-ribose) polymerase in delayed
cerebral vasospasm following subarachnoid hemorrhage in rats. Exp
Ther Med. 17:1290–1299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gulin JE, Rocco DM and García-Bournissen
F: Quality of Reporting and Adherence to ARRIVE Guidelines in
Animal Studies for Chagas Disease Preclinical Drug Research: A
Systematic Review. PLoS Negl Trop Dis. 9(e0004194)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yue X, Liu L, Yan H, Gui Y, Zhao J and
Zhang P: Intracerebral Hemorrhage Induced Brain Injury Is Mediated
by the Interleukin-12 Receptor in Rats. Neuropsychiatr Dis Treat.
16:891–900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Manaenko A, Lekic T, Ma Q, Zhang JH and
Tang J: Hydrogen inhalation ameliorated mast cell-mediated brain
injury after intracerebral hemorrhage in mice. Crit Care Med.
41:1266–1275. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Naval NS, Kowalski RG, Chang TR, Caserta
F, Carhuapoma JR and Tamargo RJ: The SAH Score: A comprehensive
communication tool. J Stroke Cerebrovasc Dis. 23:902–909.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Al-Khindi T, Macdonald RL and Schweizer
TA: Cognitive and functional outcome after aneurysmal subarachnoid
hemorrhage. Stroke. 41:e519–e536. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chang Y, Zhu J, Wang D, Li H, He Y, Liu K,
Wang X, Peng Y, Pan S and Huang K: NLRP3 inflammasome-mediated
microglial pyroptosis is critically involved in the development of
post-cardiac arrest brain injury. J Neuroinflammation.
17(219)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mosnier LO, Yang XV and Griffin JH:
Activated protein C mutant with minimal anticoagulant activity,
normal cytoprotective activity, and preservation of thrombin
activable fibrinolysis inhibitor-dependent cytoprotective
functions. J Biol Chem. 282:33022–33033. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lyden P, Pryor KE, Coffey CS, Cudkowicz M,
Conwit R, Jadhav A, Sawyer RN Jr, Claassen J, Adeoye O, Song S, et
al: NeuroNEXT Clinical Trials Network NN104 Investigators: Final
Results of the RHAPSODY Trial: A Multi-Center, Phase 2 Trial Using
a Continual Reassessment Method to Determine the Safety and
Tolerability of 3K3A-APC, A Recombinant Variant of Human Activated
Protein C, in Combination with Tissue Plasminogen Activator,
Mechanical Thrombectomy or both in Moderate to Severe Acute
Ischemic Stroke. Ann Neurol. 85:125–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lazic D, Sagare AP, Nikolakopoulou AM,
Griffin JH, Vassar R and Zlokovic BV: 3K3A-activated protein C
blocks amyloidogenic BACE1 pathway and improves functional outcome
in mice. J Exp Med. 216:279–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu X, Yan J, Huang L, Araujo C, Peng J,
Gao L, Liu S, Tang J, Zuo G and Zhang JH: INT-777 attenuates
NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA
signaling pathway after subarachnoid hemorrhage in rats. Brain
Behav Immun. 91:587–600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Luo Y, Lu J, Ruan W, Guo X and Chen S:
MCC950 attenuated early brain injury by suppressing NLRPs3
inflammasome after experimental SAH in rats. Brain Res Bull.
146:320–326. 2019.PubMed/NCBI View Article : Google Scholar
|