Introduction
Intensive care units typically treat numerous
patients with cardiovascular and cerebral vascular diseases
associated with atherosclerosis. In addition to traditional risk
factors, mounting evidence indicates that shear stress is involved
in the initiation and development of atherosclerosis (1). Endothelial cells (ECs) located in the
innermost site of the vascular wall, sense changes in blood flow
shear stress and affect the development of atherosclerotic plaques
via intracellular signal regulatory factors, gene expression and
specific transcription factors (2). Studies show that low-level shear
stress (≤5 dynes/cm2) favours the occurrence of
atherosclerosis and plaque growth (3), whereas high-level shear stress
displays an anti-atherosclerotic effect (4). Therefore, it is important to
demonstrate the underlying mechanism between low shear stress and
the gene regulation network in atherosclerosis.
Melatonin is a notable endocrine hormone secreted by
the pineal gland in a rhythmic manner. Melatonin exhibits diverse
biological functions against the development of atherosclerosis,
including antioxidant and anti-inflammatory functions (5). Multiple melatonin functions are
mediated by membrane or nuclear receptors (6). Among them, retinoid-related orphan
receptor α (RORα) is the nuclear receptor of melatonin (7). RORα regulates the expression of
numerous genes at the transcriptional level and participates in a
number of biological processes, including anti-inflammatory and
anti-apoptotic processes (8). A
previous study also revealed that microRNA (miRNA/miR)-223 inhibits
signal transducer and activator of transcription 3 (STAT-3)
signalling pathway activation and inhibits vascular calcification
of smooth muscle cells (9). In
addition, via bioinformatical analysis, the present study revealed
that melatonin potentially binds to the miR-223 promoter and
promotes miR-223 transcription.
To validate this hypothesis, the present study was
designed to assess the biological effect of melatonin on EC
pyroptosis and dysfunction induced by low shear stress and to
demonstrate the notable role of the RORα-miR-223/STAT-3 signalling
pathway.
Materials and methods
Cell culture and transfection
Human immortalized umbilical vein ECs, purchased
from the China Infrastructure of Cell Line Resource, were cultured
on rectangular glass slides (length, ~4x3-cm2) in
Dulbecco's Modified Eagle's Medium supplemented with 10% foetal
bovine serum (both Invitrogen; Thermo Fisher Scientific, Inc.) and
maintained at 37˚C with 5% CO2. The cell line was
certified by the supplier using the short tandem repeat method.
When 70-80% confluence was achieved, ECs were transfected with
pcDNA-RORα/pGL3-basic-miR-223 promoter plasmid (1 µg/µl), small
interfering (si) RORα, miR-223 inhibitor, miR-223 mimics, siSTAT-3
or the siSTAT-3 scrambled control with each final concentration at
100 nM, which were synthesized by Sangon Biotech Co., Ltd. Empty
plasmids were used as the control for plasmid transfection. For
siRNAs transfection, the control was the siRNA scrambled group. The
negative control with the mutant sequence of miR-223 was the
control group for the miR transfection. All of the transfections
were performed using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. For the siRNAs and miR transfections,
the sequences of all constructs were provided in Table SI and transfection was confirmed
using PCR or western blotting (Fig.
S1, Fig. S2 and Fig. S3).
ECs cultured with low shear
stress
After 24 h transfection, the glass slides were
placed into the plate flow chamber culture system (Shanghai
Naturethink Life & Scientific Co., Ltd.) with or without low
shear stress (5 dynes/cm2) treatment for 24 h. ECs were
then further treated with or without melatonin (2 µmol/l; cat. no.
M5250; Sigma-Aldrich; Merck KGaA) for another 24 h at 37˚C. In
brief, the experimental groups were defined as follows: i) Static
group; ii) low shear stress group; iii) static plus melatonin
group; and iv) low shear stress plus melatonin group.
Bioinformatical analysis
JASPAR is an open-access database of curated,
non-redundant transcription factor (TF) binding profiles stored as
position frequency matrices and TF flexible models for TFs across
multiple species. The candidate transcription factor of the target
miRNA (NCBI gene ID: 407008) was predicted using the JAPAR website
(v8; http://jaspar.genereg.net/) with-3000 to
100 bp of the transcription starting point. The possible binding
sites with a score provided by the website of >0.9 were
selected.
Dual-luciferase reporter assays
A dual-luciferase reporter assay was performed to
further validate the results of the predicted binding site of RORα
with the miR-223 promoter. Luciferase reporter plasmids
(pGL3-basic; Sangon Biotech Co., Ltd.) were constructed with
full-length or truncated promoters of miR-223. In addition, the
full-length RORα gene was cloned into the pcDNA3.0 (Sangon Biotech
Co., Ltd.) plasmid to overexpress RORα. The pcDNA3.0 plasmid cloned
with the RORα gene was co-transfected with the pGL3-basic plasmid
cloned with the miR-223 promoter and Renilla luciferase
reporter plasmids (Promega Corporation) into 293 cells at 37˚C for
24 h using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After another 24 h, firefly and Renilla
luciferase activities were measured in 293 cells using the
Dual-Luciferase Reporter Assay system (Dual-Glo®; cat.
no. E2920; Promega Corporation). The sequences of the miRNA-223
mimics and inhibitor are presented in Table SI.
Western blotting
ECs were lysed in RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.), and the protein
concentration of samples was detected using the BCA method. Protein
samples (20 µg/well) were loaded onto a 10% SDS polyacrylamide gel
and transferred onto a PVDF membrane. After blocking with 5% BSA
for 2 h at room temperature, the membrane was incubated with
primary antibodies overnight at 4˚C. The concentration of primary
antibodies and manufacturers' information are provided as follows:
Cleaved caspase-1 (1:1,000; cat. no. ab207802; Abcam), Cleaved
N-terminal gasdermin D (GSDMD-N; 1:1,000; cat. no. ab215203; Abcam)
and monoclonal STAT-3 (1:1,000; cat. no. #9139; Cell Signaling
Technology, Inc.). Membranes were then washed in TBST (0.1%
Tween-20) routinely and incubated with the horseradish
peroxide-conjugated goat anti-mouse/rabbit IgG secondary antibody
(1:10,000; cat. nos. 21010/21020, respectively; Abbkine Scientific
Co., Ltd.) at room temperature for 1 h. GAPDH (1:10,000; cat. no.
K200057M; Beijing Solarbio Science & Technology Co., Ltd.)
served as the internal control. Western blotting bands were
visualised using the ECL method with a TIANGEN Imaging system
(Tiangen Biotech Co., Ltd.), and quantification analysis was
performed using ImageJ software v1.46 (National Institutes of
Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from ECs using the FastPure
Cell/Tissue Total RNA Isolation kit (cat. no. RC112-01; Vazyme
Biotech Co., Ltd.) according to the manufacturer's instructions.
Total RNA was reverse transcribed using the HiScript II 1st Strand
cDNA Synthesis kit (cat. no. R211-01/02; Vazyme Biotech Co., Ltd.)
and the RT kit was used according to the manufacturer's protocol.
Quantitative real-time PCR was performed using the ChamQ Universal
SYBR® qPCR Master Mix (Q711-02/03; Vazyme Biotech Co.,
Ltd.) and GAPDH served as a housekeeping control. The primers are
as follows: GAPDH Forward, 5'-CATACCAGGAAATGAGCTTG-3', and reverse,
5'-ATGACATCAAGAAGGTGGTG-3'; STAT-3 forward, 5'-CGGA
GAAGCATCGTGAGTGAGC-3' and reverse, 5'-GTTGCCGCCTCTTCCAGTCAG-3';
miR-223 forward, 5'-GGCAGCACCCCATAAACTGTT-3', and reverse
5'-CAGTGCGTGTCGTGTCGTGGAG-3'; RORα forward
5'-GATCGCTCGTGGCTTCAGGAA-3', and reverse,
5'-TGGAGGAAAATGGAGTCGCACA-3'; GSDMD forward
5'-CCATCGGCCTTTGAGAAAGTG-3', and reverse,
5'-ACACATGAATAACGGGGTTTCC-3'; caspase-1 forward,
5'-GGTCCTGAAGGAGAAGAGAA-3' and reverse, 5'-AGGCCTGGATGATGATCACC-3'.
The PCR parameters were 95˚C for 30 sec followed by 40 cycles of
95˚C for 10 sec and 60˚C for 30 sec. The results between different
groups were calculated using the 2-ΔΔCq method (10).
Enzyme-linked immunosorbent assay
(ELISA)
IL-1β and IL-18 expression levels were detected
using ELISA. After treatment, 100 µl of the undiluted supernatants
of HUVECs treated with static or low shear stress supplemented with
or without melatonin were prepared for ELISA measurement. The human
IL-1β (cat. no. 214025) and human IL-18 (cat. no. 215539) ELISA
kits were purchased from Abcam. ELISA was performed according to
the manufacturer's instructions.
Nitric oxide (NO) test
The concentration of NO was detected using a Griess
Reagent assay. Briefly, the supernatants were collected from HUVECs
treated with static or low shear stress supplemented with or
without melatonin, and NO measurement was performed according to
the instructions of the commercial NO kit (Griess Reagent kit; cat.
no. S0021S; Beyotime Institute of Biotechnology). The NO
concentration was calculated based on the optical density value of
the supernatants detected by the microplate reader at a 550-nm
wavelength.
Immunofluorescent analysis
After ECs were treated with low shear stress for 24
h, they were fixed with 4% formaldehyde for 15 min, permeabilized
with 0.1% Triton X-100 for 5 min both at room temperature and then
washed with PBS three times for 3 min each. Subsequently, the cells
were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) for 60 min. The samples were incubated with intercellular
adhesion molecule 1 (ICAM-1; 1:1,000; cat. no. ab109361; Abcam)
overnight at 4˚C. The cells were then washed thrice with PBS for 3
min each and incubated with a goat anti-rabbit IgG (H+L)
Fluor647-conjugated secondary antibody (1:200; cat. no. S0013;
Affinity Biosciences) for 60 min at room temperature. After washing
with PBS three times for 3 min each time, the samples were covered
with DAPI mounting fluid. The images were captured using a laser
confocal microscope (A1R; Nikon Corporation), and ImageJ software
was used to analyse the fluorescent density of the images.
Statistical analysis
Statistical analyses were performed using Excel 2007
(Microsoft Corporation) and GraphPad Prism software v7.0 (GraphPad
Software, Inc.). Error bars are reported as the standard error of
mean. Pairwise comparisons were performed using unpaired two tailed
Student's t-test or one-way ANOVA followed by Tukey's post hoc test
where appropriate. A total of three biologically independent
experiments were performed for each quantified western blotting
experiment.
Results
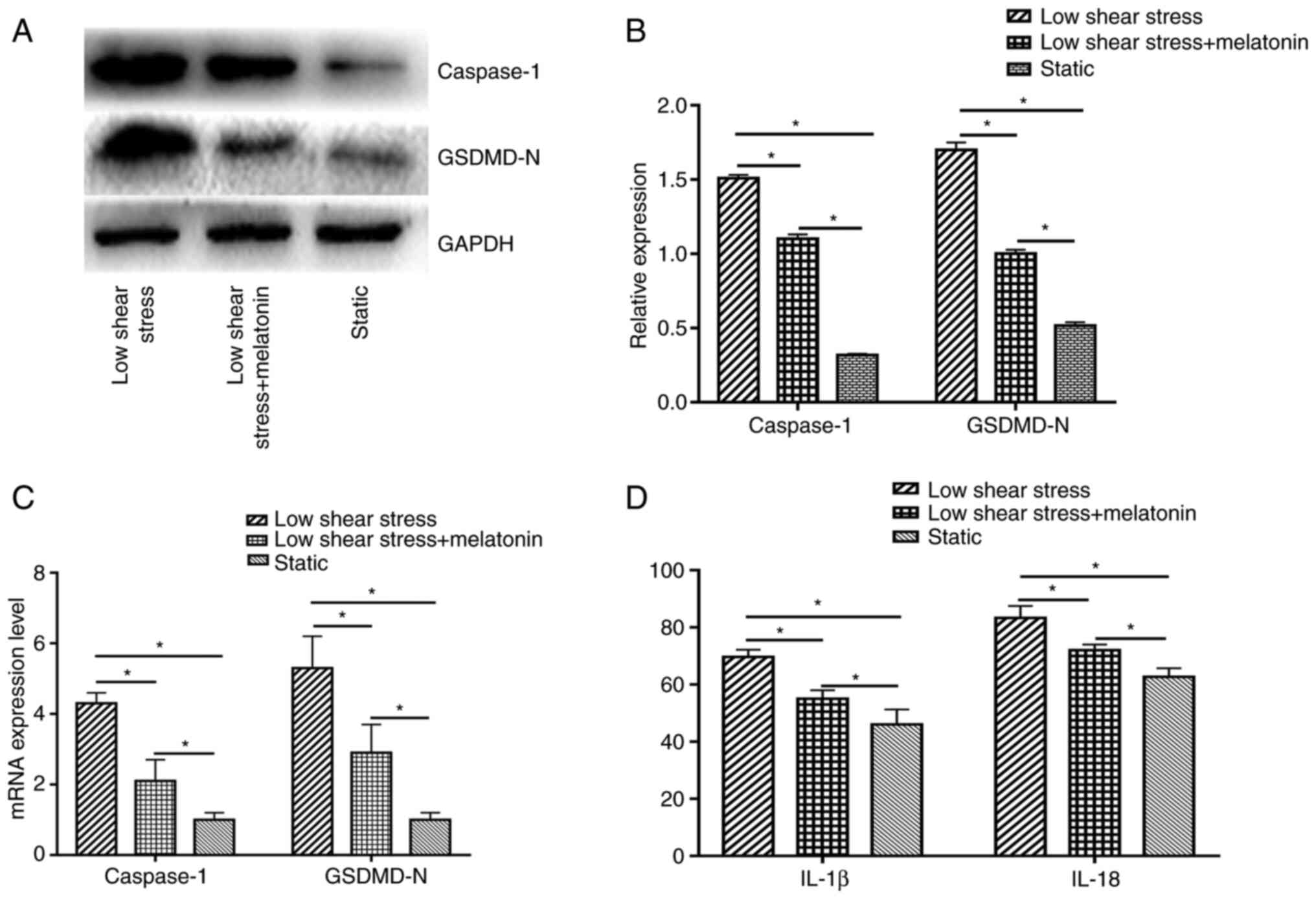
Melatonin suppresses pyroptosis in
ECs
To study the effect of pyroptosis in ECs exposed to
low shear stress and the protective effect of melatonin, the
expression levels of pyroptosis-related proteins were measured,
including caspase-1 and GSDMD-N. The results indicated that low
shear stress significantly induced pyroptosis-related protein
expression compared with the static group; whereas treatment with
melatonin significantly decreased the expression of caspase-1 and
GSDMD-N compared with the low shear stress group (Fig. 1A and B). In addition, Caspase-1 and GSDMD-N
mRNA expression levels were significantly higher in ECs exposed to
low-level shear stress compared with ECs exposed to static stress,
and were significantly suppressed by melatonin in ECs exposed to
low-level shear stress (Fig. 1C).
The expression levels of cytokines were also detected, including
IL-18 and IL-1β. Low-level shear stress significantly increased the
expression levels of IL-18 and IL-1β compared with the static
group, while melatonin suppressed the secretion of IL-18 and IL-1β
compared with the low shear stress group (Fig. 1D).
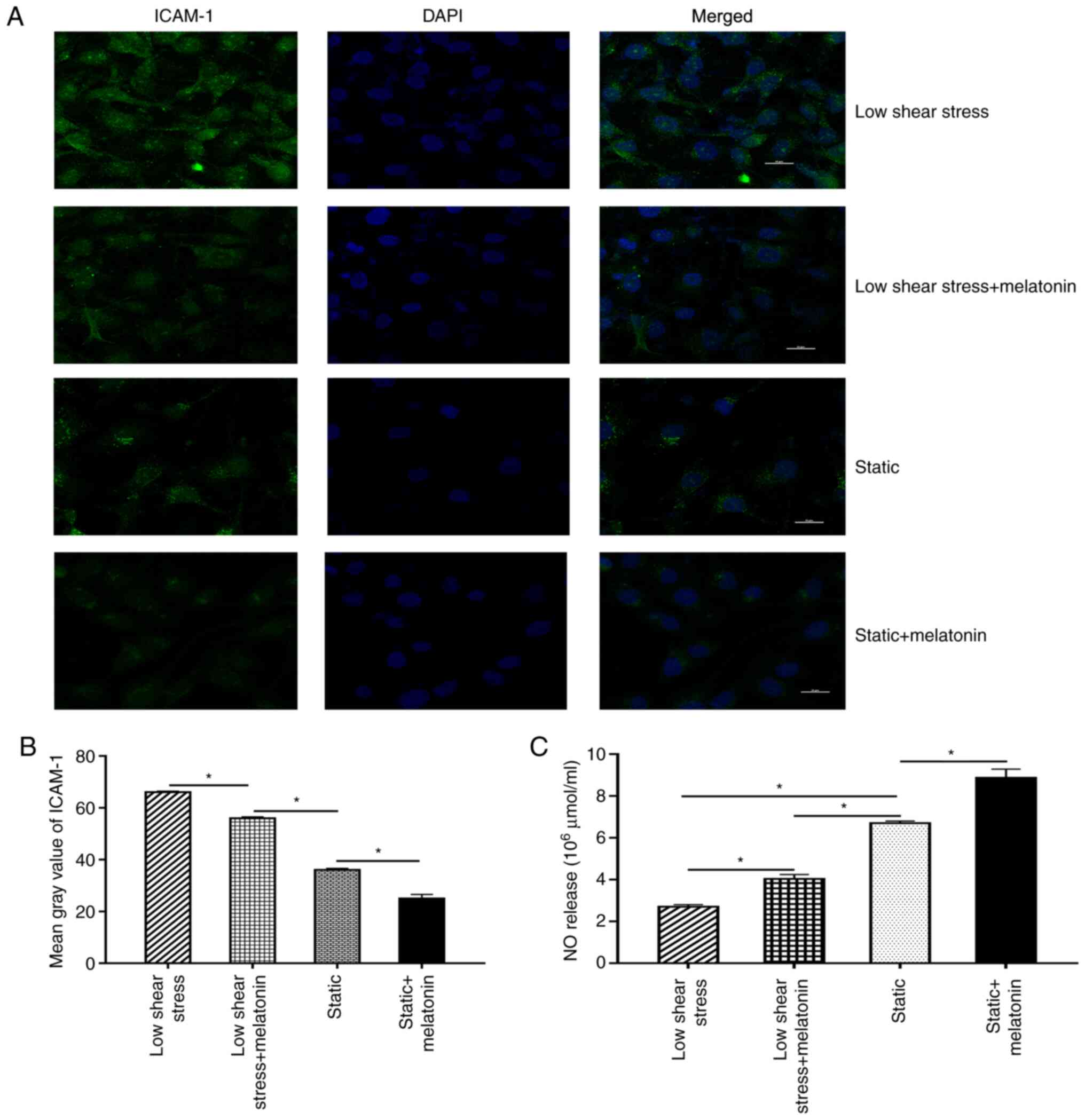
Melatonin ameliorates EC
dysfunction
Next, EC dysfunction was further evaluated by
measuring the expression levels of ICAM-1 and NO. Briefly,
immunofluorescence staining was performed to evaluate the
expression of ICAM-1 in ECs. The results revealed that the mean
grey value of ICAM-1 in the low shear stress treatment group was
significantly increased compared with that in the static group.
After melatonin treatment, the mean grey value of ICAM-1 decreased
significantly compared with that of the low shear stress group
(Fig. 2A and B). In addition, the effect of melatonin
on NO expression in ECs was validated. The results revealed that NO
levels were significantly lower in ECs subject to low-level shear
stress compared with the static group. Moreover, treatment with
melatonin and low shear stress significantly increased NO
expression compared with that in the low shear stress only group
(Fig. 2C).
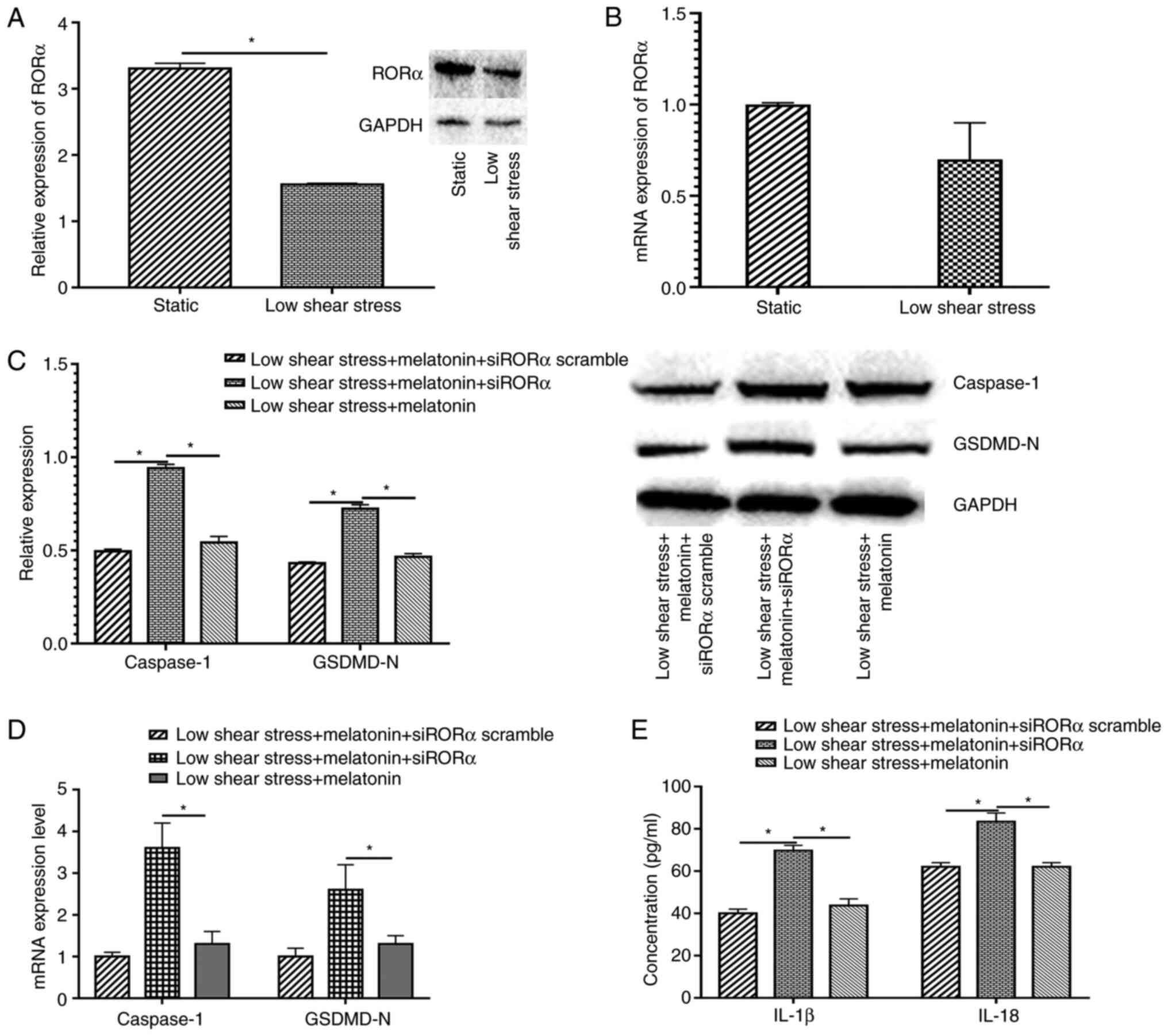
Melatonin suppresses pyroptosis
through RORα
To explore the protective mechanism of melatonin in
ECs exposed to low level shear stress, the relative RORα mRNA and
protein expression levels were measured in ECs with or without low
level shear stress treatment. The results indicated that low shear
stress significantly decreased RORα protein expression and markedly
decreased mRNA expression compared with the static group (Fig. 3A and B). Furthermore, when transfected with
siRORα, the expression levels of pyroptosis-related proteins in
melatonin treated ECs increased compared with those in the siRORα
group (Fig. 3C). The expression of
pyroptosis-related proteins at the mRNA level were also detected in
ECs transfected with siRORα and treated with melatonin. The results
demonstrated that caspase-1 and GSDMD-N expression significantly
increased compared with that of the untransfected low shear stress
and melatonin-treated group (Fig.
3D). The expression levels of IL-18 and IL-1β were detected,
and the data revealed that ECs treated with siRORα exhibited
significantly increased IL-18 and IL-1β secretion compared with the
siRORα group or the scrambled group (Fig. 3E).
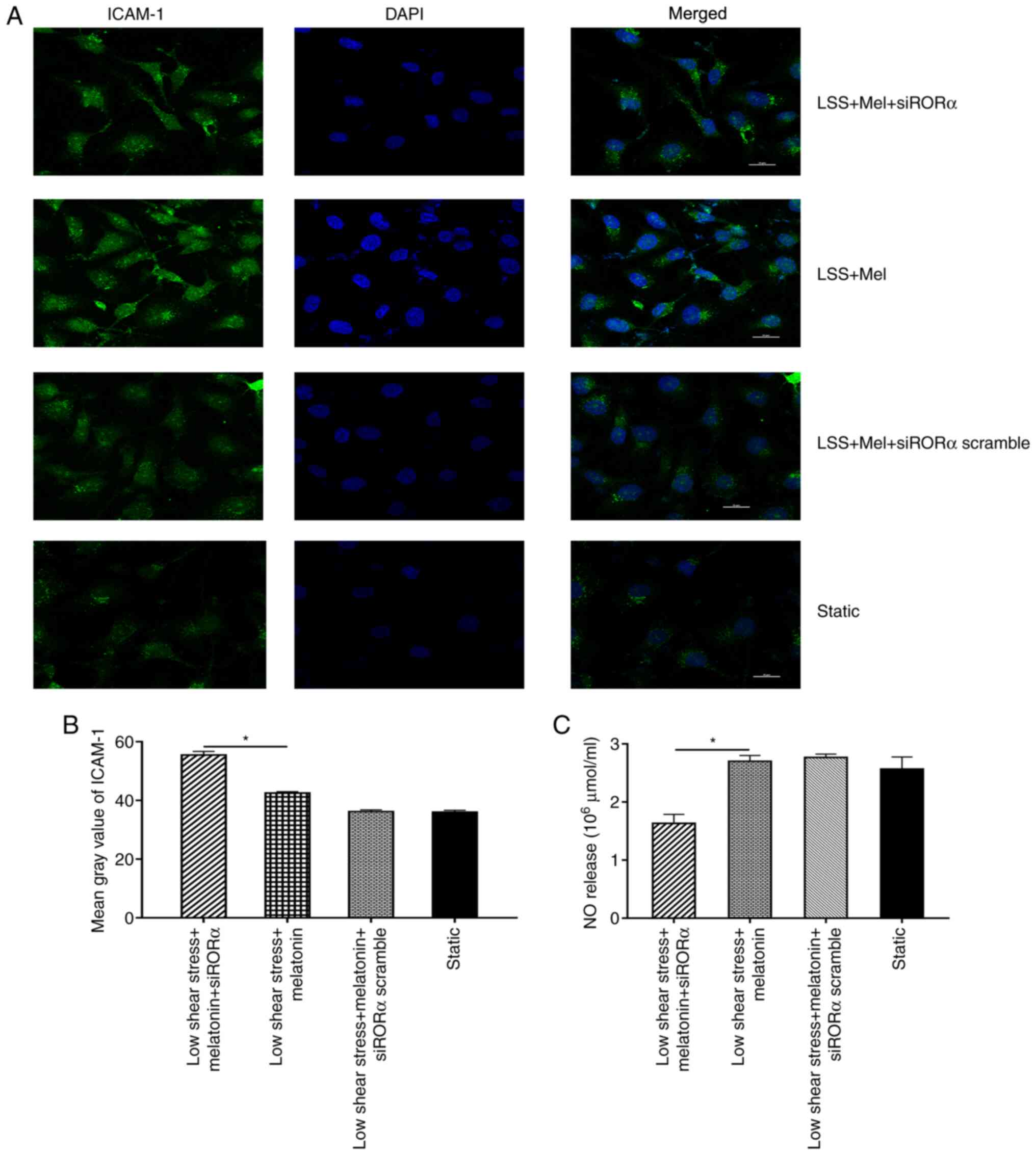
Melatonin ameliorates EC dysfunction
through RORα
ICAM-1 and NO expression levels were analysed in ECs
transfected with siRORα. The results demonstrated that the mean
grey value of ICAM-1 in ECs transfected with siRORα was
significantly increased compared with that in untransfected ECs
treated with melatonin (Fig. 4A
and B). In addition, NO expression
was detected in ECs exposed to low shear stress. The results
revealed that NO expression in ECs transfected with siRORα was
significantly decreased compared with that of untransfected ECs
treated with melatonin (Fig.
4C).
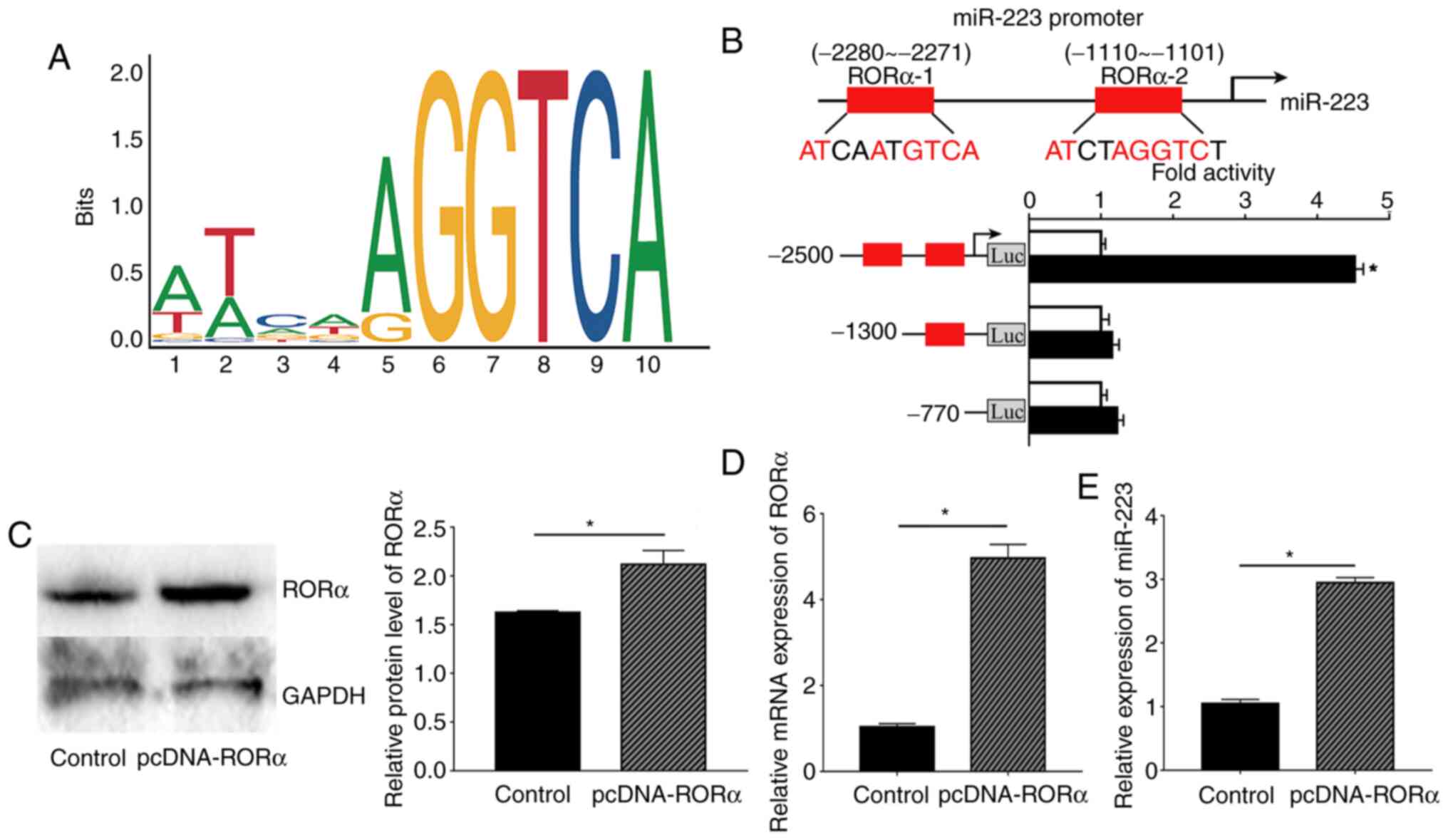
Melatonin regulates miR-223 expression
via RORα
To further study the underlying mechanism by which
melatonin affects miR-223 expression in ECs, the miR-223 promoter
region was analysed and revealed to be a possible putative binding
site of RORα using the JAPAR website. First, the oligonucleotide
sequence of the transcription factor binding site of RORα with the
miR-223 promoter region was predicted (Fig. 5A). Next, the possible binding sites
with a score provided by the website of >0.9 were selected;
therefore, two binding sites were selected as the candidate binding
sites, -2280 to -2271 bp and -1110 to -1101 bp. According to the
dual-luciferase results, the luciferase activity of RORα bound to
the-2280 to -2271 site was considerably increased compared with
that obtained from binding to the -1110 to -1101 site. These
results indicated that RORα could bind to the promoter region of
miR-223 at -2280 to -2271 bp, which also had the highest score
according to the JASPAR website (Fig.
5B).
Based on previous results, RORα was confirmed to
bind to the miR-223 promoter region. Moreover, the present study
further investigated whether RORα could regulate the expression of
miR-223 in ECs. Relative RORα protein and mRNA expression in ECs
were measured to confirm that the pcDNA3.0-RORα plasmid was
successfully transfected into ECs. As expected, both RORα protein
and mRNA expression levels were significantly higher compared with
those in the control group (Fig.
5C and D). Next, ECs
transfected with the RORα plasmid exhibited increased expression of
miR-223 compared with the control group (Fig. 5E).
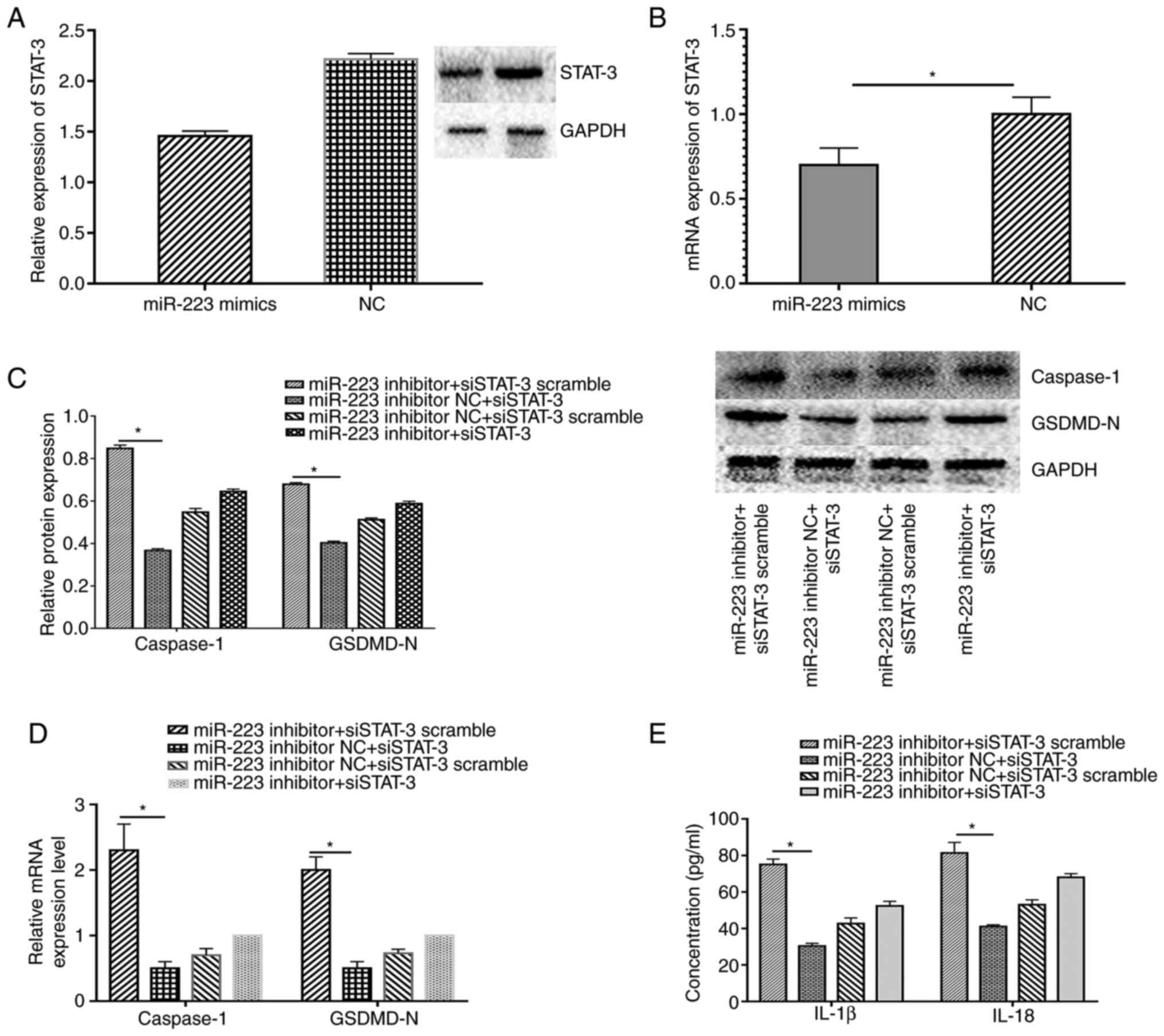
Melatonin prevents pyroptosis and
dysfunction through the RORα/miR-223/STAT-3 signalling pathway
miR-223 regulates STAT-3 expression at the
posttranscriptional level (9). In
the present study, miR-223 and STAT-3 expression levels were
altered to validate whether the RORα-miR-223/STAT-3 signalling
pathway was involved in the regulation of melatonin in ECs exposed
to low shear stress. As expected, miR-223 up-regulation decreased
STAT-3 expression at the protein and mRNA level in ECs treated with
low shear stress compared with the negative control (Fig. 6A and B). In addition, the silencing of STAT-3
down-regulated the expression levels of caspase-1 and GSDMD-N
compared with the control group, whereas the miR-223 inhibitor
partially counteracted the protective effect of silencing STAT-3 in
ECs (Fig. 6C and D). IL-18 and IL-1β secretion displayed
the same trend in ECs (Fig.
6E).
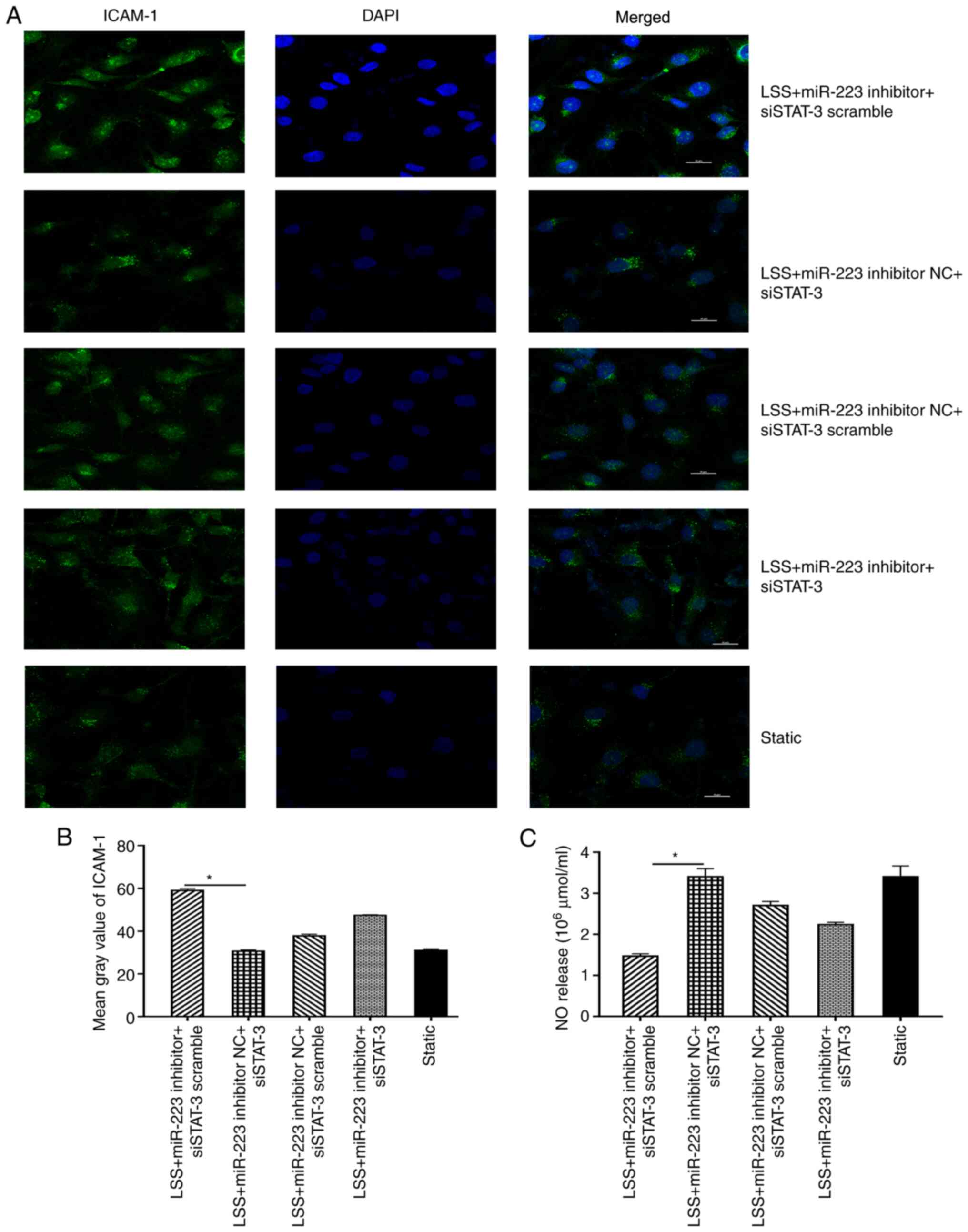
Furthermore, silencing of STAT-3 (miR-223 inhibitor
NC + siSTAT-3) resulted in the decreased expression of ICAM-1
compared with the control (miR-223 inhibitor NC+siSTAT-3 scramble)
group, while introduction of miR-223 inhibitor (miR-223
inhibitor+siSTAT-3) markedly counteracted this trend (Fig. 7A and B). The expression level of NO was also
suppressed when ECs were transfected with miR-223 inhibitor
compared with the inhibitor negative control, and this trend could
be reversed by transfection with siSTAT-3 in ECs subjected to low
shear stress treated with melatonin (Fig. 7C).
Discussion
EC pyroptosis and dysfunction are considered to be
the major causes of the initiation and development of numerous
atherosclerotic cardiovascular diseases (11). The present paper demonstrated that
melatonin could induce miR-223 expression by binding to the
promoter of miR-223. Furthermore, melatonin attenuated low-level
shear stress-induced ECs pyroptosis and dysfunction via the
ROR-α/miR-223/STAT-3 signalling pathway. The present paper
demonstrated that low shear stress-induced EC dysfunction, and that
melatonin prevented ECs pyroptosis and dysfunction. Moreover, the
anti-atherosclerotic effect of melatonin was demonstrated to be
associated with its nuclear receptor, RORα. Overall, the present
study provided a new therapeutic approach for melatonin in
cardiovascular disease.
The Intensive Care Unit Department in Second
Affiliated Hospital of Dalian Medical University (Dalian, China)
normally treats numerous patients with severe cardiovascular
diseases, including acute myocardial infarction, stroke and aortic
dissection. Vascular shear stress plays a notable role in the onset
and development of these diseases (12). High physiological shear stress is
hypothesised to be anti-atherosclerotic, whereas low shear stress
is associated with pro-atherosclerosis effects (13). EC dysfunction is a notable
contributor to the local and systemic manifestations of
atherosclerotic cardiovascular disease (14). Therefore, it is of importance to
inhibit endothelial dysfunction to prevent atherosclerosis induced
by low shear stress. The present study revealed that low shear
stress, which is associated with atherosclerosis, increased the
incidence of pyroptosis and induced the expression of cell adhesion
molecules. These results indicated that low shear stress could
damage the physiological function of ECs and might be associated
with atherosclerosis.
Melatonin regulates numerous biological functions,
including antioxidant effects, anti-inflammatory processes, sleep
regulation and immune regulation (15). Melatonin plays biological functions
mainly via: i) Membrane receptors, such as high-affinity G
protein-coupled receptors (MT)1 and MT2; ii) nuclear receptors,
such as RORα (16); iii)
interactions with cytoplasmic proteins, such as calmodulin and
hydroquinone; and iv) receptor-independent actions, such as
scavenging reactive oxygen species/reactive nitrogen species
(17). A previous study indicated
that melatonin ameliorates intraplaque inflammation in a
rupture-prone vulnerable carotid plaque model induced by low shear
stress in apolipoprotein E-/- mice in a RORα-dependent
manner (18). Another previous
study demonstrated that RORα may modulate pro-inflammatory gene
expression in atherosclerosis (19). Researchers have also reported that
RORα suppresses the expression of cyclooxygenase-2, IL-6 and IL-8
induced by TNF-α in vascular smooth muscle cells (20). In addition, RORα expression levels
in atherosclerotic plaques are suppressed compared with those in
healthy controls (21). Consistent
with the aforementioned evidence, the present paper revealed that
low shear stress could suppress the expression of RORα and that the
STAT-3 signalling pathway may be associated with RORα. Both RORα
and STAT-3 are notable molecules involved in the regulation of
inflammatory processes in atherosclerosis. The present paper
demonstrated that RORα negatively regulated STAT-3 and further
decreased the expression levels of the target molecules of
STAT-3.
The regulation of biological development involves
the regulation of transcription factors, non-coding RNA, DNA
modification and other multi-level regulation mechanisms, in which
transcription factors and miRNAs are closely associated in the
regulatory network (22). The
expression levels of miRNAs are regulated by complex transcription
factors, and the expression of transcription factors themselves are
regulated by miRNAs (23).
Previous research has mainly focused on the expression of miRNAs in
the regulation of melatonin. For instance, Zhang et al
(24) demonstrated that melatonin
could suppress long non-coding RNA maternally expressed-3
expression, and by doing so, increase miR-223 expression to prevent
pyroptosis in atherosclerosis via competing endogenous RNA theory.
This study mainly explores the relationship between melatonin and
non-coding RNA. However, a few associated studies have researched
the effect of melatonin on upstream transcriptional regulation of
miRNAs (25-28).
The present study revealed a novel mechanism by which melatonin
regulated miRNA transcription in cardiovascular disease, and this
regulation was dependent on the RORα pathway. Overexpression of
RORα in ECs induced the expression of miR-223. In previous years,
research has indicated that the abnormal expression of RORα can
cause atherosclerosis (29). Wang
et al (30) demonstrated
that 7-oxysterol, an inverse agonist of RORα, inhibits its
transcriptional activity and reduces the anti-atherosclerotic
effects of RORα. Together with these findings, the present study
provided novel ideas about how to prevent atherosclerosis with
melatonin.
Cell death and inflammation play a pivotal role in
the occurrence and development of atherosclerosis (31). Pyroptosis is an inflammatory form
of cell death and is thought to be associated with multiple
cardiovascular diseases (32). Yin
et al (33) demonstrated
that hyperlipidaemia stimulates caspase-1 activation and pyroptosis
occurrence in ECs; in addition to increasing adhesion molecule
expression and triggering monocyte adhesion to ECs. The present
study demonstrated that low shear stress also stimulated the
development of pyroptosis in ECs, which was consistent with the
hypothesis that low shear stress is pro-atherosclerotic in
cardiovascular disease (34). A
previous study has demonstrated that pyroptosis of ECs induced by
low shear stress plays an important role in the initiation and
progression of atherosclerosis (35). Furthermore, the present study
revealed that down-regulating STAT-3 expression was associated with
a reduction in the expression of pyroptosis-related proteins,
whereas exposure to a miR-223 inhibitor counteracted these effects.
These findings indicated that the miR-223/STAT-3 signalling pathway
plays a notable role in the regulation of pyroptosis in ECs.
ICAM-1 is an important glycoprotein molecule.
Injured ECs exhibit increased ICAM-1 expression compared with
healthy ECs, and atherosclerotic lesion areas also exhibit
increased ICAM-1 expression (36).
Elevated levels of adhesion molecules are associated with the
severity of acute coronary syndrome (37). A number of studies have also
demonstrated that the type, exposure time and magnitude of shear
stress affects ICAM-1 expression (38-40).
Acute exercise may increase cardiac ICAM-1 expression accompanied
by a significant increase in inflammatory mediators (41). Melatonin administration reverses
this effect, suggesting its protective effect against cardiac
damage induced by exercise (42).
A number of molecules can regulate the expression of ICAM-1 at the
transcriptional level (43). The
present study also indicated that ICAM-1 expression was induced by
low shear stress and that melatonin reversed this effect. A
previous study indicated that the STAT-3 signalling pathway plays
an important role in the regulation of ICAM-1(44). The results of the current paper
further validated that silencing of STAT-3 expression suppressed
the expression of ICAM-1, whereas exposure to amiR-223 inhibitor
increased ICAM-1 expression. Therefore, the present study provided
evidence that the miR-223/STAT-3 signalling pathway was involved in
the regulation of ICAM-1 in ECs treated with melatonin.
There are also some limitations in the current
study. First, the present paper did not include a high shear stress
treatment group. According to the relevant references, low shear
stress is pro-atherosclerotic factor, whereas high shear stress
inhibits atherosclerosis (45-48).
Future studies should include a high shear stress group to certify
that melatonin could inhibit pyroptosis in ECs under multiple types
of shear stress via a RORα-dependant manner. Secondly, the present
study did not compare the effect of different concentration of
melatonin on ECs dysfunction. In the future, the effect of
different concentration of melatonin on pyroptosis in ECs treated
with shear stress will be examined.
In conclusion, the results of the current study
demonstrated that melatonin exerted its protective effect via RORα
in ECs exposed to low shear stress. Melatonin decreased pyroptosis
and ICAM-1expression and increased NO bioavailability. The present
study also indicated that the RORα/miR-223/STAT-3 signalling
pathway may represent a promising therapeutic target in
atherosclerotic disease. However, considering that the
pathophysiology of atherosclerosis and the effects of melatonin are
extremely complicated, further studies are needed to demonstrate
the mechanism of melatonin in atherosclerosis. The present paper
provided a novel therapeutic approach and insights into
atherosclerosis.
Supplementary Material
siRORα decreases the expression of
RORα expression in endothelial cells at the protein level.
*P<0.05. si, small interfering; RORα,
retinoid-related orphan receptor α.
siSTAT-3 decreases the expression of
STAT-3 expression in endothelial cells at the protein level.
*P<0.05. si, small interfering; STAT-3, signal
transducer and activator of transcription 3.
miR-223 inhibitor decreases the
expression of miR-223 expression in endothelial cells at the mRNA
level. *P<0.05. miR, microRNA.
Sequences of all constructs usedin
this paper.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and YY confirm the authenticity of all the raw
data. SY conceived the study, analysed the data and participated in
writing, review and editing the manuscript. YY conceived the study,
performed the methodology, data collection and formal analysis and
wrote the original draft. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Collet C, Onuma Y, Sonck J, Asano T,
Vandeloo B, Kornowski R, Tu S, Westra J, Holm NR, Xu B, et al:
Diagnostic performance of angiography-derived fractional flow
reserve: A systematic review and Bayesian meta-analysis. Eur Heart
J. 39:3314–3321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roux E, Bougaran P, Dufourcq P and
Couffinhal T: Fluid shear stress sensing by the endothelial layer.
Front Physiol. 11(861)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Morita T, Kurihara H, Maemura K, Yoshizumi
M, Nagai R and Yazaki Y: Role of Ca2+ and protein kinase C in shear
stress-induced actin depolymerization and endothelin 1 gene
expression. Circ Res. 75:630–636. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zarins CK, Giddens DP, Bharadvaj BK,
Sottiurai VS, Mabon RF and Glagov S: Carotid bifurcation
atherosclerosis. Quantitative correlation of plaque localization
with flow velocity profiles and wall shear stress. Circ Res.
53:502–514. 1983.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun H, Gusdon AM and Qu S: Effects of
melatonin on cardiovascular diseases: Progress in the past year.
Curr Opin Lipidol. 27:408–413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pourhanifeh MH, Dehdashtian E,
Hosseinzadeh A, Sezavar SH and Mehrzadi S: Clinical application of
melatonin in the treatment of cardiovascular diseases: Current
evidence and new insights into the cardioprotective and
cardiotherapeutic properties. Cardiovasc Drugs Ther, Sep 14, 2020
(Epub ahead of print) doi: 10.1007/s10557-020-07052-3.
|
|
7
|
Cook DN, Kang HS and Jetten AM: Retinoic
acid-related orphan receptors (RORs): Regulatory functions in
immunity, development, circadian rhythm, and metabolism. Nucl
Receptor Res. 2(101185)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao CN, Wang P, Mao YM, Dan YL, Wu Q, Li
XM, Wang DG, Davis C, Hu W and Pan HF: Potential role of melatonin
in autoimmune diseases. Cytokine Growth Factor Rev. 48:1–10.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han Y, Zhang J, Huang S, Cheng N, Zhang C,
Li Y, Wang X, Liu J, You B and Du J: MicroRNA-223-3p inhibits
vascular calcification and the osteogenic switch of vascular smooth
muscle cells. J Biol Chem. 296(100483)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang D, Wang Z, Zhang L and Wang Y: Roles
of cells from the arterial vessel wall in atherosclerosis.
Mediators Inflamm. 2017(8135934)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Resnick N, Yahav H, Shay-Salit A, Shushy
M, Schubert S, Zilberman LC and Wofovitz E: Fluid shear stress and
the vascular endothelium: For better and for worse. Prog Biophys
Mol Biol. 81:177–199. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Davies PF, Civelek M, Fang Y and Fleming
I: The atherosusceptible endothelium: endothelial phenotypes in
complex haemodynamic shear stress regions in vivo. Cardiovasc Res.
99:315–327. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baeyens N: Fluid shear stress sensing in
vascular homeostasis and remodeling: Towards the development of
innovative pharmacological approaches to treat vascular
dysfunction. Biochem Pharmacol. 158:185–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Claustrat B and Leston J: Melatonin:
Physiological effects in humans. Neurochirurgie. 61:77–84.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jetten AM, Kurebayashi S and Ueda E: The
ROR nuclear orphan receptor subfamily: Critical regulators of
multiple biological processes. Prog Nucleic Acid Res Mol Biol.
69:205–247. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding S, Lin N, Sheng X, Zhao Y, Su Y, Xu
L, Tong R, Yan Y, Fu Y, He J, et al: Melatonin stabilizes
rupture-prone vulnerable plaques via regulating macrophage
polarization in a nuclear circadian receptor RORα-dependent manner.
J Pineal Res. 67(e12581)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Migita H, Satozawa N, Lin JH, Morser J and
Kawai K: RORalpha1 and RORalpha4 suppress TNF-alpha-induced VCAM-1
and ICAM-1 expression in human endothelial cells. FEBS Lett.
557:269–274. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Delerive P, Monté D, Dubois G, Trottein F,
Fruchart-Najib J, Mariani J, Fruchart JC and Staels B: The orphan
nuclear receptor ROR alpha is a negative regulator of the
inflammatory response. EMBO Rep. 2:42–48. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Besnard S, Heymes C, Merval R, Rodriguez
M, Galizzi JP, Boutin JA, Mariani J and Tedgui A: Expression and
regulation of the nuclear receptor RORalpha in human vascular
cells. FEBS Lett. 511:36–40. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khachigian LM: Transcription factors
targeted by miRNAs regulating smooth muscle cell growth and intimal
thickening after vascular injury. Int J Mol Sci.
20(5445)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao L, Gu C, Ye M, Zhang Z, Li L, Fan W
and Meng Y: Integration analysis of microRNA and mRNA paired
expression profiling identifies deregulated microRNA-transcription
factor-gene regulatory networks in ovarian endometriosis. Reprod
Biol Endocrinol. 16(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu X, Chen S, Jiang Y, Xu Y, Zhao Y, Chen
L, Li C and Zhou X: Analysis of miRNA expression profiles in
melatonin-exposed GC-1 spg cell line. Gene. 642:513–521.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hardeland R: Melatonin, noncoding RNAs,
messenger RNA stability and epigenetics-evidence, hints, gaps and
perspectives. Int J Mol Sci. 15:18221–18252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tian Y, Gong Z, Zhao R and Zhu Y:
Melatonin inhibits RANKL-induced osteoclastogenesis through the
miR-882/Rev-erbα axis in Raw264.7 cells. Int J Mol Med. 47:633–642.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murodumi H, Shigeishi H, Kato H, Yokoyama
S, Sakuma M, Tada M, Ono S, Rahman MZ, Ohta K and Takechi M:
Melatonin-induced miR-181c-5p enhances osteogenic differentiation
and mineralization of human jawbone-derived osteoblastic cells. Mol
Med Rep. 22:3549–3558. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Boukhtouche F, Mariani J and Tedgui A: The
‘CholesteROR’ protective pathway in the vascular system.
Arterioscler Thromb Vasc Biol. 24:637–643. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Solt LA and Burris TP: Regulation
of FGF21 expression and secretion by retinoic acid receptor-related
orphan receptor alpha. J Biol Chem. 285:15668–15673.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang W, Lin J, Dong J and Li D:
Pyroptosis: An inflammatory cell death implicates in
atherosclerosis. Med Hypotheses. 81:484–486. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu YJ, Zheng L, Hu YW and Wang Q:
Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta.
476:28–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yin DL, Zhao XH, Zhou Y, Wang Y, Duan P,
Li QX, Xiong Z, Zhang YY, Chen Y, He H, et al: Association between
the ICAM-1 gene polymorphism and coronary heart disease risk: A
meta-analysis. Biosci Rep. 39(BSR20180923)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen J, Zhang J, Wu J, Zhang S, Liang Y,
Zhou B, Wu P and Wei D: Low shear stress induced vascular
endothelial cell pyroptosis by TET2/SDHB/ROS pathway. Free Radic
Biol Med. 162:582–591. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hoseini Z, Sepahvand F, Rashidi B,
Sahebkar A, Masoudifar A and Mirzaei H: NLRP3 inflammasome: Its
regulation and involvement in atherosclerosis. J Cell Physiol.
233:2116–2132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Raman K, Chong M, Akhtar-Danesh GG,
D'Mello M, Hasso R, Ross S, Xu F and Paré G: Genetic markers of
inflammation and their role in cardiovascular disease. Can J
Cardiol. 29:67–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mulvihill NT, Foley JB, Murphy RT, Pate G,
Crean PA and Walsh M: Enhanced endothelial activation in diabetic
patients with unstable angina and non-Q-wave myocardial infarction.
Diabet Med. 18:979–983. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheng C, Tempel D, van Haperen R, van der
Baan A, Grosveld F, Daemen MJ, Krams R and de Crom R:
Atherosclerotic lesion size and vulnerability are determined by
patterns of fluid shear stress. Circulation. 113:2744–2753.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wei G, Zhu D, Sun Y, Zhang L, Liu X, Li M
and Gu J: The protective effects of azilsartan against oscillatory
shear stress-induced endothelial dysfunction and inflammation are
mediated by KLF6. J Biochem Mol Toxicol. 35:1–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Conway DE, Williams MR, Eskin SG and
McIntire LV: Endothelial cell responses to atheroprone flow are
driven by two separate flow components: Low time-average shear
stress and fluid flow reversal. Am J Physiol Heart Circ Physiol.
298:H367–H374. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li Y, Sun D, Zheng Y and Cheng Y: Swimming
exercise activates aortic autophagy and limits atherosclerosis in
ApoE-/- mice. Obes Res Clin Pract. 14:264–270.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Veneroso C, Tuñón MJ, González-Gallego J
and Collado PS: Melatonin reduces cardiac inflammatory injury
induced by acute exercise. J Pineal Res. 47:184–191.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhong L, Simard MJ and Huot J: Endothelial
microRNAs regulating the NF-κB pathway and cell adhesion molecules
during inflammation. FASEB J. 32:4070–4084. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Han X, Wang Y, Chen H, Zhang J, Xu C, Li J
and Li M: Enhancement of ICAM-1 via the JAK2/STAT3 signaling
pathway in a rat model of severe acute pancreatitis-associated lung
injury. Exp Ther Med. 11:788–796. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Peiffer V, Sherwin SJ and Weinberg PD:
Does low and oscillatory wall shear stress correlate spatially with
early atherosclerosis? A systematic review. Cardiovasc Res.
99:242–250. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Siasos G, Sara JD, Zaromytidou M, Park KH,
Coskun AU, Lerman LO, Oikonomou E, Maynard CC, Fotiadis D, Stefanou
K, et al: Local low shear stress and endothelial dysfunction in
patients with nonobstructive coronary atherosclerosis. J Am Coll
Cardiol. 71:2092–2102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Eshtehardi P and Teng Z: Protective or
destructive: High wall shear stress and atherosclerosis.
Atherosclerosis. 251:501–503. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rashad S, Han X, Saqr K, Tupin S, Ohta M,
Niizuma K and Tominaga T: Epigenetic response of endothelial cells
to different wall shear stress magnitudes: A report of new
mechano-miRNAs. J Cell Physiol. 235:7827–7839. 2020.PubMed/NCBI View Article : Google Scholar
|