Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection. Essentially, sepsis is the body's response to
infectious factors (1). The
incidence of sepsis is high, with >30 million cases of severe
sepsis annually worldwide and a continually increasing number every
year (2). Despite the significant
progress achieved in anti-infection treatment and organ function
support technology in recent years (3), the fatality rate of sepsis remains
high at ~35%. The high cost of sepsis treatment and the high demand
for medical resources severely affect the quality of life of the
patients, and sepsis poses a significant threat to human health.
Sepsis can be caused by infection in any part of the human body,
particularly in the gastrointestinal tract. The gastrointestinal
tract is the first organ affected by sepsis, resulting in
functional damage (4). Moreover,
intestinal disease itself can also induce sepsis and lead to
multiple organ dysfunction (5).
Therefore, it is crucial to study the association between sepsis
and the intestine, and to develop methods for alleviating the
damage in intestinal function.
Phosphodiesterases (PDEs) can hydrolyze
intracellular cyclic adenosine monophosphate (cAMP) or cyclic
guanosine monophosphate (cGMP) (6), thereby blocking the biochemical
effects mediated by these second messengers (7). cAMP and cGMP serve important roles in
regulating cell activities. PDEs are widely distributed in the
human body, and their physiological effects have been determined in
numerous research fields (8). In
recent years, PDEs have been attracting increased attention as new
therapeutic targets and have become a new research hotspot. The
clinical research of selective PDE4 and PDE5 inhibitors has also
received significant attention (9,10).
PDE4 has multiple isozymes, which are divided into four subtypes:
PDE4A, B, C and D (11).
Furthermore, PDE4 has been found to be associated with cAMP
hydrolysis in numerous types of inflammatory cells (12). Therefore, inhibiting PDE4 may help
inhibit the physiological functions of inflammatory cells. More
importantly, it was found that PDE4B knockdown could relieve
intestinal dysregulation, bacterial overgrowth and endotoxemia
(13).
Roflumilast is a highly selective PDE4 inhibitor
used for the treatment of severe chronic obstructive pulmonary
disease (COPD), and has been found to reduce the expression levels
of pro-inflammatory factors in patients with COPD (14). Roflumilast has important
anti-inflammatory properties, and the latest research has shown
that roflumilast can reduce sepsis-induced lung (15), liver (14) and renal (16) tissue injury. However, whether
roflumilast can reduce intestinal tissue damage induced by sepsis
remains unknown.
Currently available research suggests that
roflumilast can reduce the release of inflammatory factors in
ulcerative colitis, decrease inducible nitric oxide synthase levels
and enhance the expression of cAMP (17). Therefore, the aim of the present
study was to investigate whether roflumilast could reduce
sepsis-induced intestinal injury.
Materials and methods
Cell culture
IEC-6 cells (a rat small intestinal epithelial cell
line) were purchased from the American Type Culture Collection. The
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
10 U/ml insulin and 100 U/ml penicillin and streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.), and maintained at
37˚C in an incubator with 5% CO2. The cells were treated
with lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) for 24 h
to construct the intestinal injury cellular model. Roflumilast
(AdooQ Bioscience) was initially diluted to four concentrations,
namely 1, 5, 10 and 50 µg/ml. In subsequent experiments, 5 µg/ml
represented the low dose and 10 µg/ml the high dose.
Model establishment
A total of 15 male Sprague-Dawley rats (weight,
250-300 g; age, 8 weeks) were purchased from Jiangsu ALF
Biotechnology Co., Ltd. and kept at ~25˚C under a 12-h light/dark
cycle and ~55% relative humidity, with free access to food and
water. The rats were anesthetized using isoflurane inhalation
(induction dose, 4%; maintenance dose, 1.5%) before cecal ligation
and puncture (CLP), and their normal body temperature was
maintained using heating pads. Briefly, the procedure was as
follows: Laparotomy was performed, followed by ligation of the
bowel below the ileocecal valve. The cecum was pierced twice using
a needle, and then the abdominal cavity was closed and the rats
were resuscitated. Prior to collecting the intestinal tissues, the
rats were euthanized with intraperitoneal injection of 200 mg/kg
pentobarbital sodium followed by cervical dislocation. All the
experiments were conducted according to the institutional criteria
for the care and use of laboratory research animals and all efforts
were made to minimize animal suffering.
H&E staining
The small intestinal tissues of rats were rinsed,
fixed in 4% paraformaldehyde at room temperature overnight,
dehydrated and embedded in paraffin. The sections were then stained
with hematoxylin solution for 10 min at room temperature followed
by eosin solution for 1 min at room temperature. After rinsing with
70% alcohol, an ascending alcohol gradient was used for dehydration
and xylene was used to transparentize the sections. The sections
were then sealed using neutral gum. The physiological changes of
the tissues were observed under a light microscope (magnification,
x200; Olympus Corporation) and an appropriate field of view was
selected to capture the image.
Intestinal function index
detection
Small intestinal tissues were lysed using RIPA lysis
buffer, and tissue samples were used to detect the activity of
lactate dehydrogenase (LDH), D-amino acid oxidase (DAO) and
intestinal fatty acid-binding protein (iFABP). The assays were
conducted using an LDH Assay kit (cat. no. ab197004; Abcam), DAO
Activity Assay kit (cat. no. ab273325; Abcam) and iFABP ELISA kit
[cat. no. JK-(a)-5035; Jingkang Biotechnology Co. Ltd.], according
to the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from small intestinal tissue
and IEC-6 cells using the RNAsimple total RNA kit (Tiangen Biotech
Co., Ltd.), and cDNA was obtained using a Reverse Transcriptase kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. qPCR was performed using the
QuantiTect SYBR-Green PCR kit (Qiagen, Inc.). The thermocycling
condition was as follows: 95˚C For 2 min, followed by 40 cycles at
95˚C for 10 sec, 60˚C for 35 sec and 72˚C for 10 sec. The
2-∆∆Cq method (18) was
applied for data analysis, with normalization to GAPDH. The primer
sequences are listed in Table
I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR analysis. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR analysis.
| Gene | Sequence (5'-3') |
|---|
| PDE4A sense |
TTCTGCAAGAGGGAGACAGGA |
| PDE4A antisense |
GAGTGTCCTCCTCGCTGAAG |
| PDE4B sense |
GGCCAGGCTTTGCTTACTGT |
| PDE4B antisense |
ATTTGGGAAGCCGTGATGGT |
| PDE4D sense |
TCAAAGCCCCCAAGCATCTCTG |
| PDE4D antisense |
GTCTGAGTCCCTGGAAAGACG |
| TNF-α sense |
TTCCCAAATGGGCTCCCTCT |
| TNF-α antisense |
GTGGGCTACGGGCTTGTCAC |
| IL-1β sense |
TGCCACCTTTTGACAGTGATG |
| IL-1β antisense |
ATGTGCTGCTGCGAGATTTG |
| IL-6 sense |
TCTGGGAAATCGTGGAAATGAG |
| IL-6 antisense |
TCTCTGAAGGACTCTGGCTTTGTC |
| GAPDH sense |
GCATCTTCTTGTGCAGTGCC |
| GAPDH
antisense |
GATGGTGATGGGTTTCCCGT |
Western blotting
Total protein was extracted from small intestinal
tissue and IEC-6 cells and homogenized in RIPA lysis buffer
(Beyotime Institute of Biotechnology). After protein quantification
was conducted using a BCA protein assay kit (Glpbio Technology,
Inc.), the same amount of 30 mg protein was used for
electrophoresis, and the protein separated by 10 or 12%
SDS-polyacrylamide gel electrophoresis was transferred to PVDF
membranes via electroporation. The membranes were first incubated
in 5% skimmed milk for 2 h at room temperature, and then incubated
with primary antibodies against PDE4A (cat. no. ab14628; 1:1,000;
Abcam), PDE4B (cat. no. ab170939; 1:1,000; Abcam), PDE4D (cat. no.
ab114613; 1:1,000; Abcam), p65 (cat. no. ab32536; 1:1,000; Abcam),
histone H3 (cat. no. ab1791; 1:1,000; Abcam), β-actin (cat. no.
ab8226; 1:1,000; Abcam), Bax (cat. no. ab32503; 1:1,000; Abcam),
cleaved caspase 3 (cat. no. ab32042; 1:1,000; Abcam), cleaved
poly(ADP-ribose) polymerase 1 (PARP; cat. no. ab32064; 1:1,000;
Abcam) and Bcl-2 (cat. no. ab32124; 1:1,000; Abcam) at 4˚C
overnight. Then, the membranes were washed with 0.05% TBS-Tween 20,
and were incubated with HRP-conjugated anti-rabbit antibody (cat.
no. 65-6120; 1: 10,000; Thermo Fisher Scientific, Inc.). The strip
was developed using an ECL reagent (Thermo Fisher Scientific,
Inc.), and ImageJ software (v1.8; National Institutes of Health)
was used to analyze the gray value.
When detecting the nuclear and cytoplasmic protein
levels, the cells were centrifuged at 12,000 x g for 5 min at 4˚C.
The cytoplasmic protein extract was added to the pellet, which was
then centrifuged at 12,000 x g at 4˚C, and this supernatant was
considered as the cytoplasmic protein lysis solution. The cells
were centrifuged at 12,000 x g for 1 min at 4˚C, the nuclear lysate
was added and sample was centrifuged at 12,000 x g for 5 min at
4˚C, after being vortexed several times. This supernatant was
considered as the nuclear protein lysate.
Cell Counting Kit-8 (CCK-8) assay
The cells (5x103/well) were added to a
96-well plate and cultured in an incubator with 5% CO2
at 37˚C. The CCK-8 solution (Beyotime Institute of Biotechnology)
was added after cells were treated with different concentrations of
LPS or additional roflumilast for 24 h. The cells were cultured for
1 h and then the optical density was measured at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
TUNEL assay
Cells (5x105/well) were seeded into a
24-well plate and cultured until they reached ~80% confluence. The
cells were fixed with 4% paraformaldehyde for 30 min at room
temperature and permeated with PBS containing 0.3% Triton X-100 for
another 5 min at room temperature. Following blocking with 3%
H2O2 for 5 min at room temperature, TUNEL
staining was performed according to the procedure provided in the
TUNEL assay kit (Beyotime Institute of Biotechnology). DAPI was
used to counterstain the nuclei for 10 min at room temperature. The
results were observed at five random fields of view under a
fluorescence microscope (magnification, x200; Olympus
Corporation).
Statistical analysis
The data are presented as the mean ± SD and were
analyzed using GraphPad Prism software, version 8.0 (GraphPad
Software, Inc.). Statistical significance was determined using
one-way ANOVA followed by Tukey's post hoc test for multiple
groups, and unpaired Student's t-test for two groups. Each
experiment was conducted ≥3 times. P<0.05 was considered to
indicate a statistically significant difference.
Results
PDE4 expression in septic small
intestinal tissue
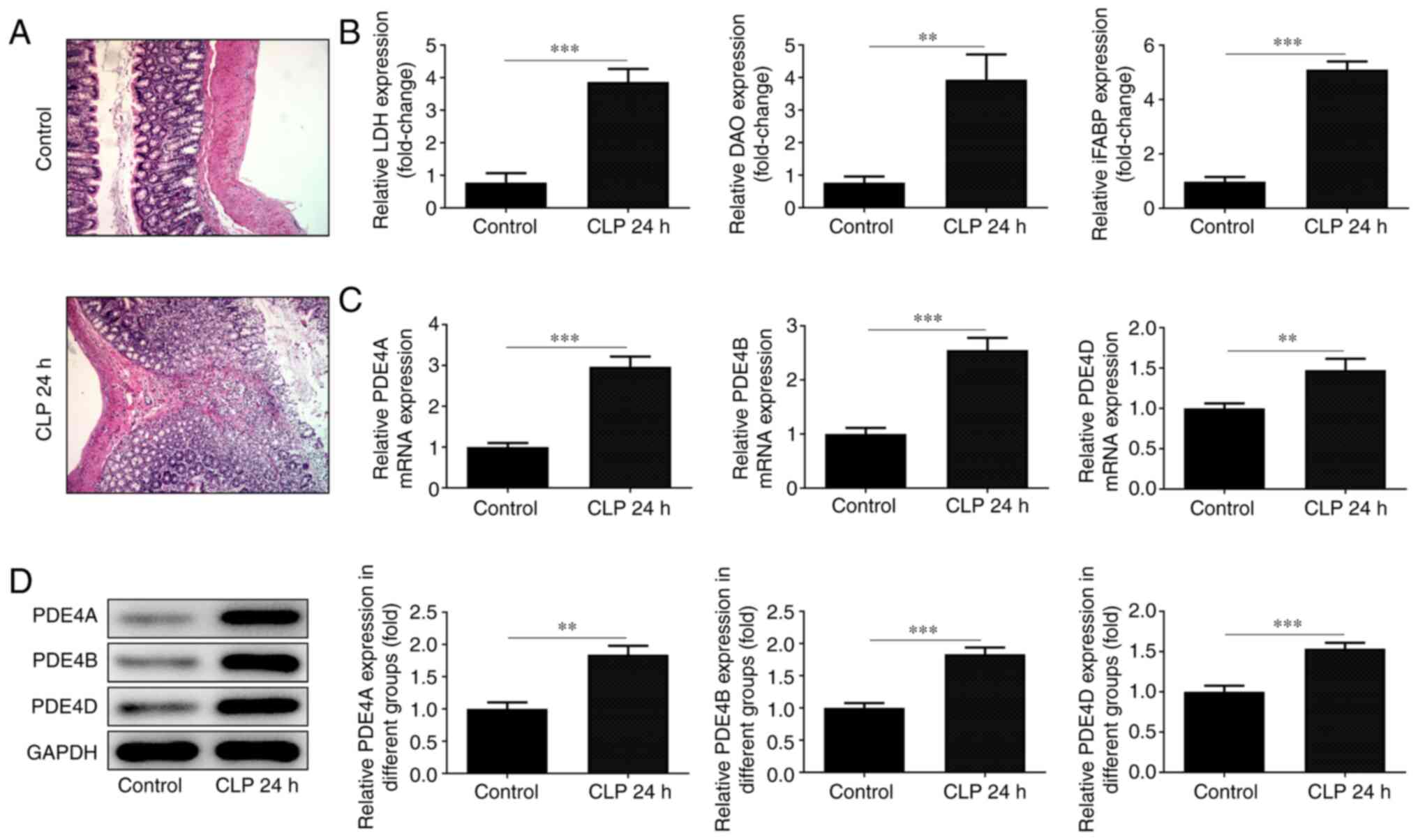
The pathological changes in small intestinal tissue
were observed following H&E staining. The cells in the normal
group were arranged in an orderly manner, and obvious intestinal
villi and columnar cells could be observed at x200 magnification.
However, the integrity of the intestinal tissue and the structure
of villi were disrupted in the CLP group, and the arrangement of
the cells became disordered. The number of vacuolar goblet cells
increased significantly, accompanied by inflammatory cell
infiltration (Fig. 1A). The levels
of the intestinal indicators LDH, DAO and iFABP were detected using
corresponding kits and were found to be low in normal tissues and
high in damaged intestinal tissues (Fig. 1B). RT-qPCR analysis (Fig. 1C) and western blotting (Fig. 1D) were employed to detect the
expression level of PDE4. All three detected subtypes of PDE4 were
elevated in the damaged tissue, although the difference in the
expression of PDE4D was not as significant as that of the other two
subtypes.
PDE4 expression in small intestinal
cells
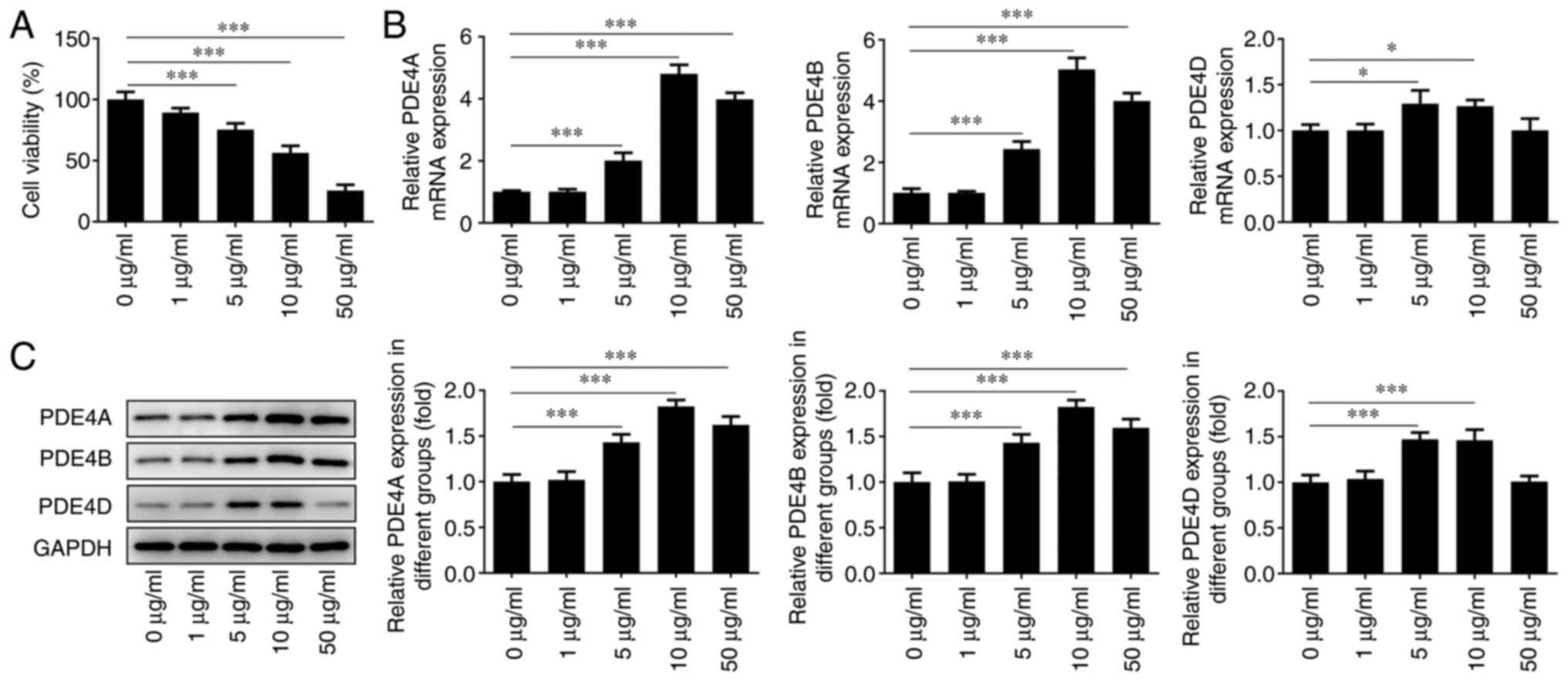
IEC-6 cells were treated with different
concentrations of LPS for 24 h to detect its effect on cell
viability. It was found that the cell survival rate decreased
significantly with increasing LPS concentrations (Fig. 2A). Moreover, RT-qPCR analysis
(Fig. 2B) and western blotting
(Fig. 2C) were conducted to detect
the expression level of PDE4 in cells treated with increasing doses
of LPS. After treatment with different concentrations, it was
observed that the expression level of PDE4 reached a peak at an LPS
concentration of 10 µg/ml. However, when the concentration was
increased to 50 µg/ml, PDE4 expression was decreased. This may be
due to the fact that the majority of cells were necrotic. In the
subsequent experiments, the cells were treated with LPS at a
concentration of 10 µg/ml, and the cell survival rate was
maintained at ~50%.
Intestinal indicators reflect the
effect of roflumilast on LPS-induced cells
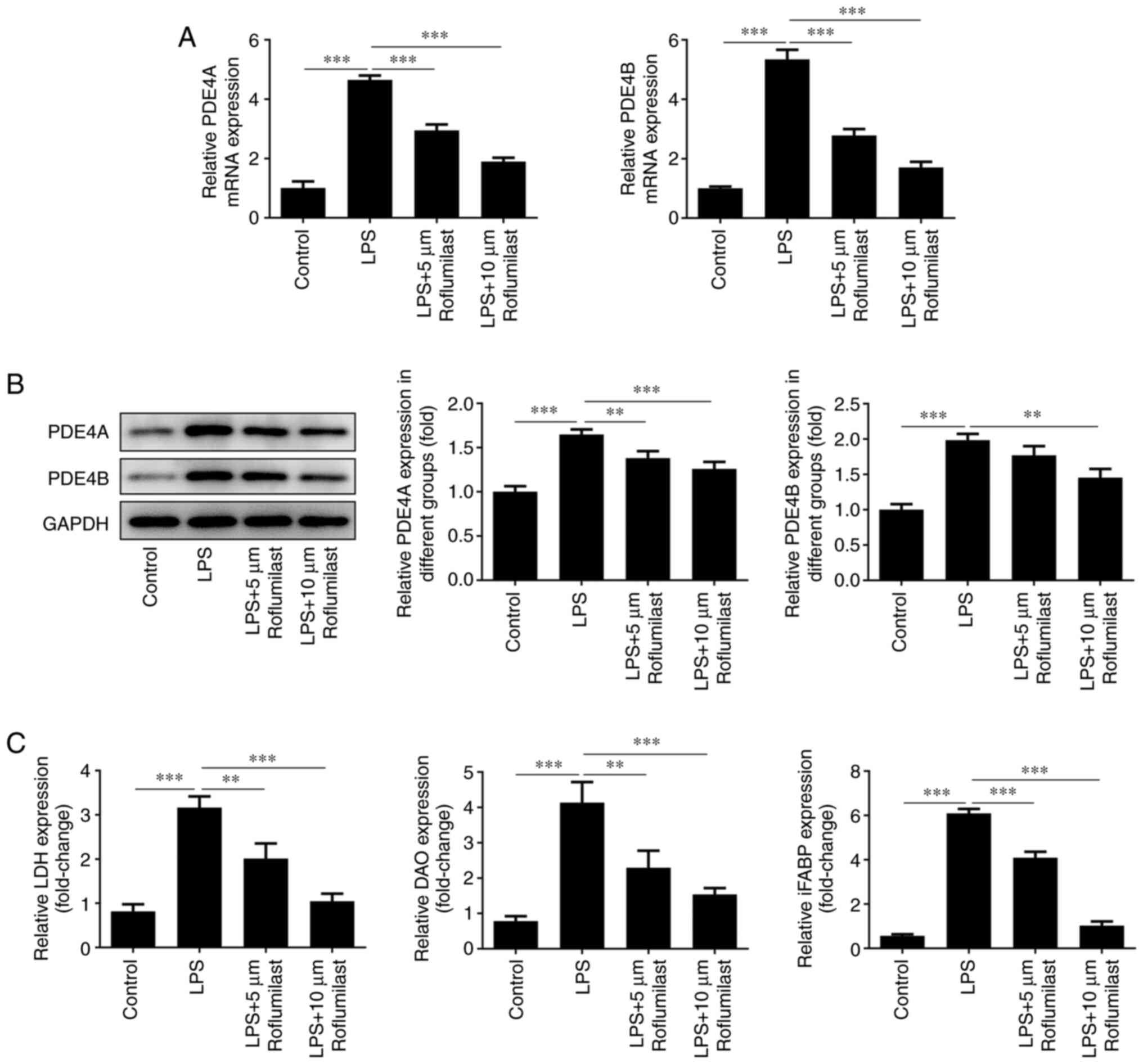
RT-qPCR analysis (Fig.
3A) and western blotting (Fig.
3B) were conducted to detect the expression levels of PDE4A and
PDE4B in cells treated with LPS or LPS + roflumilast. The
expression levels of these factors were decreased in the LPS +
roflumilast group compared with the LPS alone group. Furthermore,
LDH, DAO and iFABP levels were detected using respective assay
kits. In the LPS-induced group, the levels of these three factors
were significantly increased. However, the addition of roflumilast
suppressed their levels, and this inhibitory effect was more
notable with a high concentration of roflumilast (Fig. 3C).
Roflumilast suppresses LPS-induced
intestinal cell injury and release of inflammatory factors
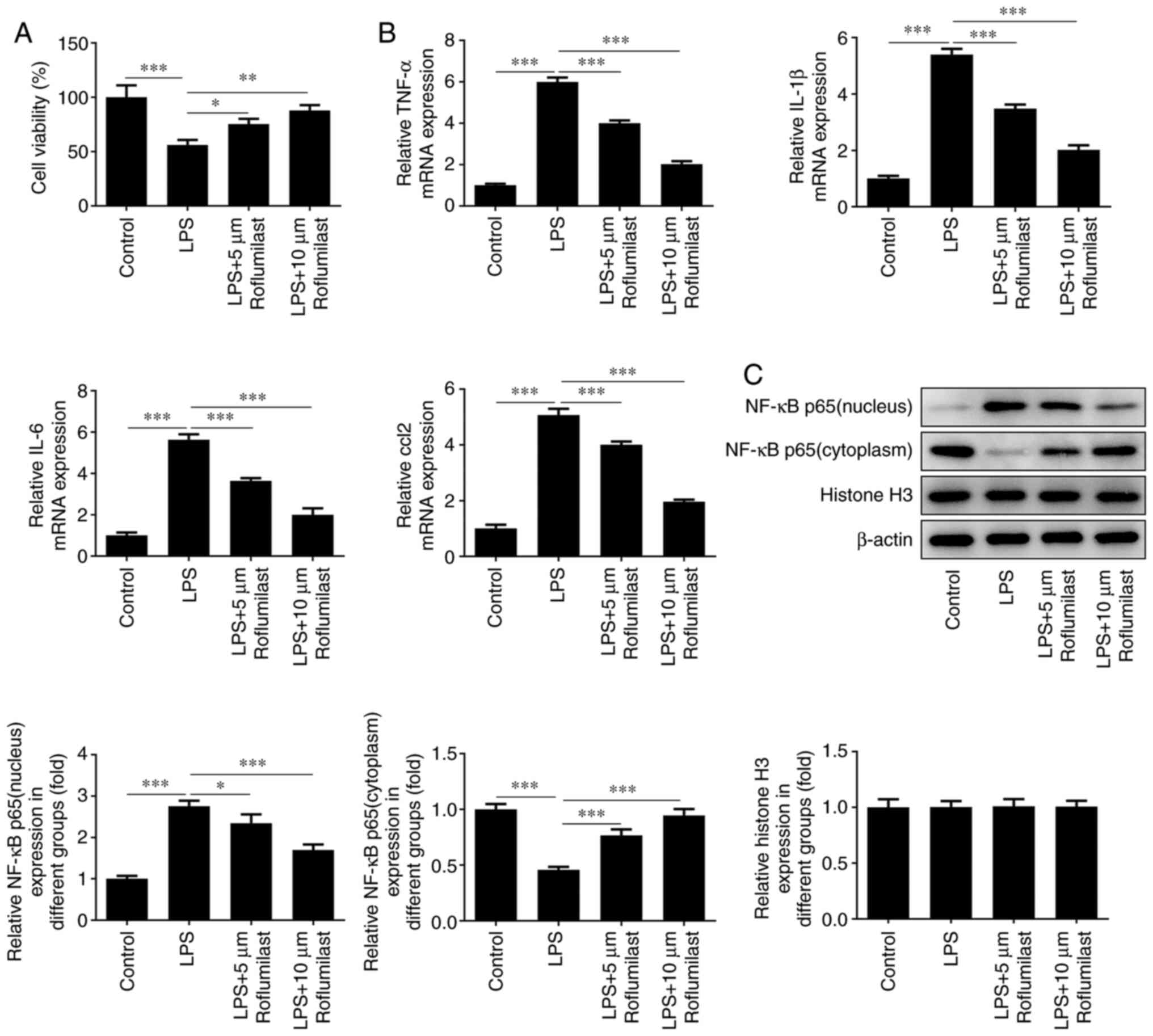
The survival rate of the cells in the control group,
the LPS induction group and the roflumilast groups was examined
using a CCK-8 assay. The cell survival rate of the roflumilast
groups was higher compared with that of the induction group
(Fig. 4A). In addition, RT-qPCR
analysis was used to detect the expression levels of the
inflammation-related factors TNF-α, IL-1β, IL-6 and C-C motif
chemokine ligand 2, and it was found that the expression levels of
these inflammatory factors were increased in the LPS induction
group, while they were inhibited after treatment with roflumilast
(Fig. 4B). Western blotting was
used to detect the expression level of NF-κB p65 in the nucleus and
cytoplasm. It was observed that NF-κB p65 was mainly expressed in
the cytoplasm in normal cells, and was mainly localized to the
nucleus after cells were exposed to LPS. Following treatment with
roflumilast, the expression of NF-κB p65 in the nucleus was
decreased and its expression in the cytoplasm was increased, which
suggested that roflumilast relieved the activation of NF-κB p65
(Fig. 4C).
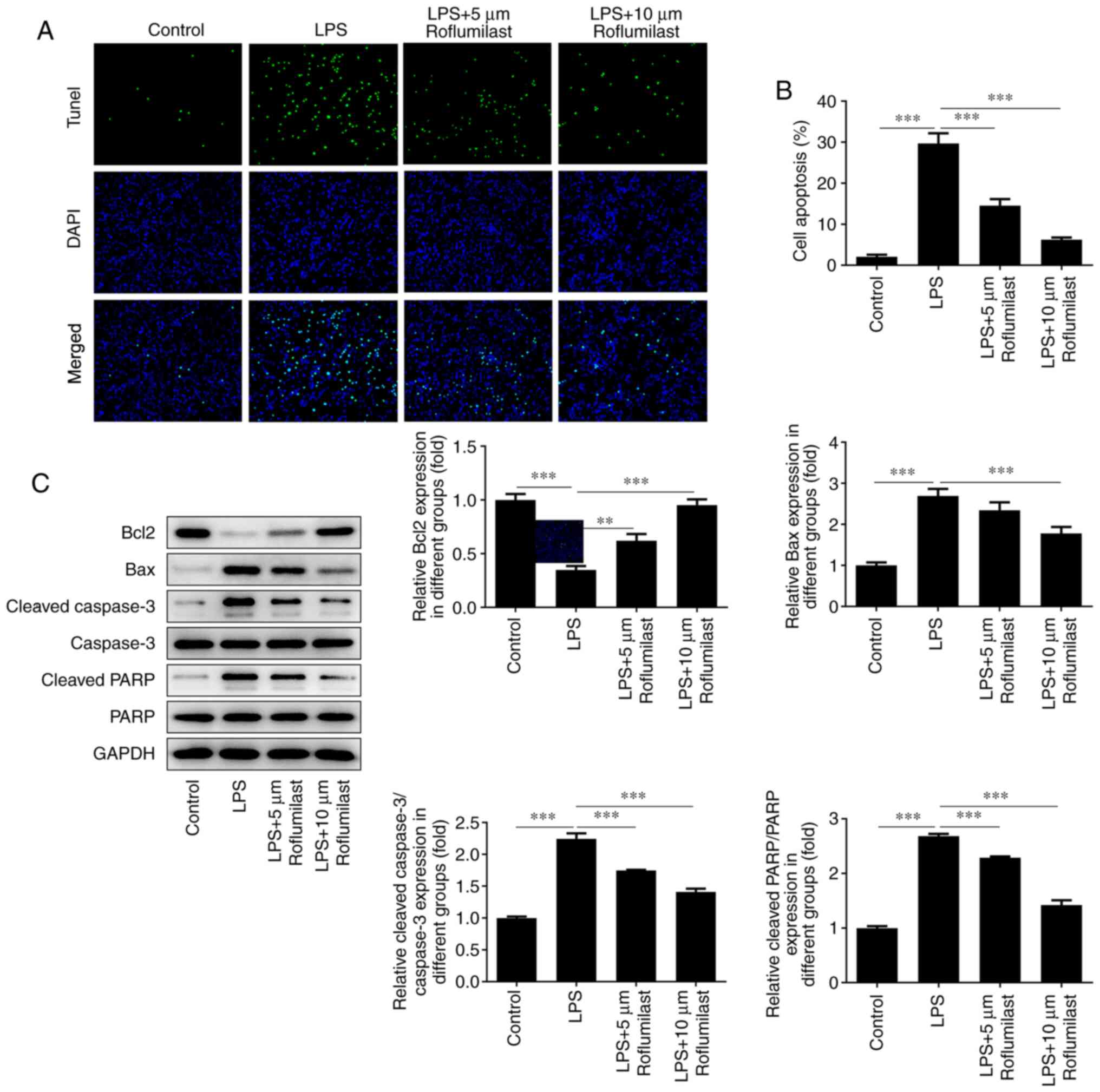
Roflumilast suppresses LPS-induced
intestinal cell apoptosis
TUNEL staining was used to detect cell apoptosis.
The fluorescence of the cells in the LPS group was significantly
decreased compared with that of the control group, and the
fluorescence of the roflumilast-treated groups was restored to
varying degrees (Fig. 5A and
B). Western blotting was performed
to detect the expression levels of the apoptosis-related proteins
Bax, cleaved caspase 3, cleaved PAPR and Bcl-2. The expression of
the anti-apoptotic Bcl-2 protein was decreased in the LPS induction
group, but was increased in the roflumilast groups. The changing
trend in the expression levels of other genes that promote
apoptosis was the opposite, indicating that roflumilast reduces the
apoptosis of LPS-induced cells (Fig.
5C).
Discussion
The mortality rate of sepsis remains the leading
cause of disease-related mortality in intensive care medicine
(19). Two main mechanisms
underlying sepsis-induced gastrointestinal functional damage are
currently known. The first mechanism is direct damage. For example,
inflammation directly acts on the gastrointestinal mucosa, causing
congestion and damage to the tight junctions between cells,
resulting in intestinal mucosal damage (20). The second mechanism is indirect
damage, involving insufficient blood supply to the intestines and
malnutrition (21). There are
numerous hypotheses regarding the mechanism of sepsis caused by
gastrointestinal injury (22,23),
some of which have been confirmed (23); however, the exact mechanism remains
to be determined. More importantly, treatment strategies targeting
PDE4 have been attracting increased attention, and the expression
levels of PDE4A, PDE4B and PDE4D have all been found to be
increased in intestinal tissues (24). In view of the anti-inflammatory
effects of the PDE inhibitor roflumilast, the current study was
undertaken to examine whether roflumilast can relieve
sepsis-induced intestinal damage.
In order to confirm the construction of the rat
model of intestinal tissue injury and cell damage, certain
indicators were evaluated. LDH is mainly found in cardiomyocytes,
liver cells and skeletal muscle cells. Damage to these cells will
lead to excessive LDH release, which is an indicator of tissue
damage (25). The results of the
present study demonstrated that the LDH levels were increased in
the injury group, while roflumilast reduced LDH levels. DAO is an
intracellular enzyme found in the small intestinal mucosa or
ciliated epithelial cells, which can promote cell repair and
protect the intestinal mucosa, and its levels increase when the
mucosal barrier of the small intestine is damaged (26). Therefore, the activity of DAO may
reflect the extent of intestinal damage. iFABP is specifically
present in large quantities in the epithelial cells of the mucosal
layer of small intestinal tissue. Following mucosal injury, iFABP
is quickly released into the circulation, serving as an indicator
of intestinal epithelial cell damage (27). The results of the present study
revealed that roflumilast reduced the levels of these two
indicators, suggesting that it may alleviate intestinal tissue
damage and epithelial cell damage caused by sepsis. In addition,
the expression level of PDE4 in septic intestinal tissue was
upregulated, suggesting that there may be an association between
PDE4 and sepsis-induced intestinal injury. Furthermore, the effect
of dobutamine, which is used to treat septic shock, was reduced in
septic mice suffering from CLP due to the upregulation of
PDE4(28), which appears to
confirm this hypothesis.
Roflumilast has been used in the clinical treatment
of COPD, and there are multiple preclinical studies being conducted
(14-16).
In the present study, roflumilast was found to alleviate the
intestinal damage caused by sepsis. Moreover, medical researchers
observed that the combination of roflumilast with cisplatin could
inhibit the proliferation of ovarian cancer cells, induce their
apoptosis and arrest the cell cycle in the
G0/G1 phase (29). Takano et al (30) reported that PDE4 inhibition can
improve the cognitive function of rhesus monkeys via the
intravenous injection of roflumilast, while Peng et al
(31) revealed that roflumilast
can increase the viability of sevoflurane-treated rat neurons and
reduce the level of apoptosis. In addition, El-Ashmawy et al
(17) observed that roflumilast
can increase the length of healthy colon in a rat model of
ulcerative colitis, improve the histological score of the colon and
thereby alleviate the severity of colitis. The present study
provided a novel research direction for the clinical application of
roflumilast based on the discovery of its effects in alleviating
intestinal damage caused by sepsis.
In conclusion, cell injury is closely associated
with inflammation, ultimately resulting in cell apoptosis. To the
best of our knowledge, the present study was the first to
demonstrate that roflumilast can reduce inflammatory factor release
and cell apoptosis during intestinal damage resulting from sepsis.
Moreover, roflumilast was found to downregulate PDE4 expression in
LPS-induced intestinal cells, indicating that it may target PDE4 to
reduce the intestinal damage caused by sepsis. The results of the
present study uncovered the role of roflumilast in septic
intestinal injury, and may help expand the scope of the clinical
use of roflumilast; however, whether roflumilast inhibits
intestinal injury by targeting PDE4 requires further in-depth
investigation, and will be the focus of research in future studies.
Furthermore, its application in the clinical treatment of
intestinal injury requires numerous additional preclinical studies
to verify its effects and elucidate the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ conceived the study and wrote the manuscript. ML
participated in the experiments and revised the manuscript. XW
participated in the experiments and data processing. All authors
have read and approved the final manuscript. ZZ and ML confirm the
authenticity of the raw data.
Ethics approval and consent to
participate
All procedures performed followed the ethical
guidelines of Tianyou Hospital Affiliated to Wuhan University of
Science & Technology (Wuhan, China). All efforts were made to
minimize animal suffering. The Institutional Animal Care and Use
Committee of Tianyou Hospital Affiliated to Wuhan University of
Science & Technology has approved this study (June 2018-June
2021; approval no. 20180602).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Faix JD: Biomarkers of sepsis. Crit Rev
Clin Lab Sci. 50:23–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20(5376)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rossaint J and Zarbock A: Pathogenesis of
multiple organ failure in sepsis. Crit Rev Immunol. 35:277–291.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang CT, Tsai YJ, Tsai PR, Yu CJ and Ko
WJ: Epidemiology and outcome of severe sepsis and septic shock in
surgical intensive care units in Northern Taiwan. Medicine
(Baltimore). 94(e2136)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martinez A and Gil C: cAMP-specific
phosphodiesterase inhibitors: Promising drugs for inflammatory and
neurological diseases. Expert Opin Ther Pat. 24:1311–1321.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lynch MJ, Hill EV and Houslay MD:
Intracellular targeting of phosphodiesterase-4 underpins
compartmentalized cAMP signaling. Curr Top Dev Biol. 75:225–259.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ioakeimidis N and Kostis JB: Pharmacologic
therapy for erectile dysfunction and its interaction with the
cardiovascular system. J Cardiovasc Pharmacol Ther. 19:53–64.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peng T, Qi B, He J, Ke H and Shi J:
Advances in the development of phosphodiesterase-4 inhibitors. J
Med Chem. 63:10594–10617. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Di Luigi L, Sansone M, Sansone A, Ceci R,
Duranti G, Borrione P, Crescioli C, Sgrò P and Sabatini S:
Phosphodiesterase type 5 inhibitors, sport and doping. Curr Sports
Med Rep. 16:443–447. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu M, Yu X, Meng X, Huang S, Zhang Y,
Zhang A and Jia Z: Inhibition of PDE4/PDE4B improves renal function
and ameliorates inflammation in cisplatin-induced acute kidney
injury. Am J Physiol Renal Physiol. 318:F576–F588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sakkas LI, Mavropoulos A and Bogdanos DP:
Phosphodiesterase 4 inhibitors in immune-mediated diseases: Mode of
action, clinical applications, current and future perspectives.
Curr Med Chem. 24:3054–3067. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Myers SA, Gobejishvili L, Saraswat Ohri S,
Garrett Wilson C, Andres KR, Riegler AS, Donde H, Joshi-Barve S,
Barve S and Whittemore SR: Following spinal cord injury, PDE4B
drives an acute, local inflammatory response and a chronic,
systemic response exacerbated by gut dysbiosis and endotoxemia.
Neurobiol Dis. 124:353–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feng H, Chen J, Wang H, Cheng Y, Zou Z,
Zhong Q and Xu J: Roflumilast reverses polymicrobial sepsis-induced
liver damage by inhibiting inflammation in mice. Lab Invest.
97:1008–1019. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chang X, Hu LF, Ma XJ, Yin J, Liu XY and
Li JB: Influence of roflumilast on sepsis mice through the JAK/STAT
signaling pathway. Eur Rev Med Pharmacol Sci. 23:1335–1341.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu X, Liao L, Hu B, Jiang H and Tan M:
Roflumilast, a phosphodiesterases-4 (PDE4) inhibitor, alleviates
sepsis-induced acute kidney injury. Med Sci Monit.
26(e921319)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
El-Ashmawy NE, Khedr NF, El-Bahrawy HA and
El-Adawy SA: Roflumilast, type 4 phosphodiesterase inhibitor,
attenuates inflammation in rats with ulcerative colitis via
down-regulation of iNOS and elevation of cAMP. Int Immunopharmacol.
56:36–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stoller J, Halpin L, Weis M, Aplin B, Qu
W, Georgescu C and Nazzal M: Epidemiology of severe sepsis:
2008-2012. J Crit Care. 31:58–62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sertaridou E, Papaioannou V, Kolios G and
Pneumatikos I: Gut failure in critical care: Old school versus new
school. Ann Gastroenterol. 28:309–322. 2015.PubMed/NCBI
|
|
21
|
Yang H, Song Z, Jin H, Cui Y, Hou M and
Gao Y: Protective effect of rhBNP on intestinal injury in the
canine models of sepsis. Int Immunopharmacol. 19:262–266.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Deitch EA: Bacterial translocation or
lymphatic drainage of toxic products from the gut: What is
important in human beings? Surgery. 131:241–244. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Deitch EA, Xu D and Kaise VL: Role of the
gut in the development of injury- and shock induced SIRS and MODS:
The gut-lymph hypothesis, a review. Front Biosci. 11:520–528.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Li H, Fan C, Feng C, Wu Y, Lu H, He P,
Yang X, Zhu F, Qi Q, Gao Y, et al: Inhibition of
phosphodiesterase-4 attenuates murine ulcerative colitis through
interference with mucosal immunity. Br J Pharmacol. 176:2209–2226.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the lactate dehydrogenase assay. Cold
Spring Harb Protoc. 2018:2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wolvekamp MC and de Bruin RW: Diamine
oxidase: An overview of historical, biochemical and functional
aspects. Dig Dis. 12:2–14. 1994.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Derikx JP, Schellekens DH and Acosta S:
Serological markers for human intestinal ischemia: A systematic
review. Best Pract Res Clin Gastroenterol. 31:69–74.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sakai M, Suzuki T, Tomita K, Yamashita S,
Palikhe S, Hattori K, Yoshimura N, Matsuda N and Hattori Y:
Diminished responsiveness to dobutamine as an inotrope in mice with
cecal ligation and puncture-induced sepsis: Attribution to
phosphodiesterase 4 upregulation. Am J Physiol Heart Circ Physiol.
312:H1224–H1237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong S, Chen Y, Meng F, Zhang Y, Li C,
Zhang G, Huan W and Wu F: Roflumilast enhances
cisplatin-sensitivity and reverses cisplatin-resistance of ovarian
cancer cells via cAMP/PKA/CREB-FtMt signalling axis. Cell Prolif.
51(e12474)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takano A, Uz T, Garcia-Segovia J, Tsai M,
Lahu G, Amini N, Nakao R, Jia Z and Halldin C: A nonhuman primate
PET study: Measurement of brain PDE4 occupancy by roflumilast using
(R)-[11C]Rolipram. Mol Imaging Biol. 20:615–622.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peng S, Yan HZ, Liu PR, Shi XW, Liu CL,
Liu Q and Zhang Y: Phosphodiesterase 4 inhibitor roflumilast
protects rat hippocampal neurons from sevoflurane induced injury
via modulation of MEK/ERK signaling pathway. Cell Physiol Biochem.
45:2329–2337. 2018.PubMed/NCBI View Article : Google Scholar
|