Introduction
Diminished ovarian reserve (DOR) is a condition that
reduces the quantity and/or quality of ovarian follicles before the
age of 40 as a result of ovarian dysfunction. Ovarian dysfunction
is a result of various factors, such as endocrine disorders and
ovarian injury, which lead to a decline in fertility (1). Due to DOR, an increasing number of
young women experience premature oligomenorrhea, amenorrhea,
infertility or miscarriage, which greatly reduces the success rate
of assisted reproductive technology used for infertility (2). If not treated in a timely manner,
this condition can develop into premature ovarian failure (3). The most commonly used treatment
method in modern medicine is hormone replacement therapy (HRT),
which involves the supplement of estrogen (4) and the use of ovulation stimulators.
Medications used to stimulate ovarian function in clinical practice
include letrozole, clomiphene citrate, gonadotropins and pulsatile
gonadotropin releasing hormone (5). Despite this therapeutic option being
available, not only is the morbidity as a result of DOR increasing
annually and the efficacy of HRT not improving, potential risks
associated with the development of breast cancer, endometrial
cancer and heart disease are also increased (6-9).
Pueratin (Pue) is a flavonoid that can be extracted
from the perennial vine Pueraria lobata with various reported
pharmacological activities, including antioxidant,
anti-inflammatory, anti-apoptotic, neuroprotective and
cardioprotective properties (10).
It has been previously demonstrated to exert effective protection
in several types of cell injury, including neurons, epithelial
cells, vascular endothelial cells and cardiomyocytes (11-14).
However, the potential effect of Pue on DOR remains unclear.
Previous studies have suggested that Bcl-2 and Bax are associated
with apoptosis in mammalian cells (15,16).
A previous study (7) found that
Pue exerted therapeutic effects against ovarian failure via
regulation of the Wnt/β-catenin signaling pathway and oxidative
stress. However, since the concentration of Pue was low and the
effects of Pue on follicular stimulating hormone (FSH) levels in
the rats with DOR remain unknown (4).
Therefore, in the present study, a physiological DOR
rat model was established by vinyl-cyclohexene-dioxide (VCD)
injection to assess the effects of Pue on the expression levels of
the apoptosis-related proteins, Bcl-2 and Bax. The aim was to
investigate the effects of Pue on the pathophysiology of DOR on a
cellular level. It is hoped that findings from the present study
may lay a foundation for its potential application in the clinical
treatment of DOR.
Materials and methods
Animals
A total of 75 female specific-pathogen-free
Sprague-Dawley rats (age, 4 weeks; weight, 60-70 g) were supplied
by Beijing Vital River Laboratory Animal Technology Co., Ltd.
[certificate no. SCXK (Beijing) 2017-0001]. The rats were housed in
a temperature-controlled environment at 25˚C and 50±10% humidity,
with a 12-h light/dark cycle and free access to a standard diet and
water. The experiments and operations related to the animals
involved in this study were performed with the approval of the
Animal Ethics Committee of Guangdong Women and Children Hospital
(Guangzhou, China).
Drugs, antibodies and reagents
The following drugs, reagents, antibodies and kits
were used in the present study: Pue (Merck KGaA), VCD, sesame oil
(Sigma-Aldrich; Merck KGaA), FSH ELISA kit (cat. no. E-EL-R0391c;
Elabscience Biotechnology, Inc.), luteinizing hormone (LH) ELISA
kit (cat.E-EL-H6019; Elabscience Biotechnology, Inc.), estradiol
(E2) kit (cat. no. KGE014; R&D Systems, Inc.), Bcl-2
antibodies (cat. no. ab182858; Abcam), Bax antibodies (cat. no.
ab216985; Abcam), caspase-3 antibodies (cat. no. ab32150; Abcam)
and GAPDH antibodies (cat. no. ab8245; Abcam).
Instruments
The following instruments were used: CX21 light
microscope (Olympus Corporation), EnSpire® 2300 Enzyme
Multimode Plate Reader (PerkinElmer, Inc.), MiniPROTEAN Tetra Cell
and Trans-Blot SD Cell (Bio-Rad Laboratories, Inc.), PowerPac HC
Electrophoresis system (Bio-Rad Laboratories, Inc.), TS-12F
Automatic Biological Tissue Dehydrator (Xiaogan Hongye Medical
Instrument Co., Ltd.) and a BMJ-M Pathological Tissue Embedding
Machine and Embedding Freezer (Tianjin Tianli Aeronautical
Mechanical and Electrical Co., Ltd.).
Establishment of the DOR animal
model
After conducting a standard 7-day feeding period
with the 4-week-old female Sprague Dawley rats, the rats were
screened using smears of the vaginal exfoliated cells after opening
the vulva, where the estrous cycle was found to be normal
(Proestrus, a large number of oval nucleated epithelial cells and a
small number of leukocytes; during estrus, a large number of
a-nucleated keratinized epithelial cells; late estrus,
non-nucleated keratinocytes, oval nucleated cells and leukocytes at
the same time; estrous interval, a large number of white blood
cells and a small number of nuclear epithelial cells). A total of
12 of the 60 rats (15 rats died due to the modeling process) were
randomly selected for the normal group, whilst the remaining 48
rats were used in the model, Pue-L, Pue-M and Pue-H groups, with 12
rats in each group. Model, Pue-L, Pue-M and Pue-H groups were
modeled by injection with VCD.
The feasibility of model establishment was mainly
evaluated based on the following criteria: i) Observation of
vaginal smears showed that the rats had a prolonged estrous cycle;
ii) microscopic analysis revealing ovarian atrophy; iii) a
decreased number of preantral and antral follicles; iv) intensified
atresia; and v) significant hyperplasia of the ovarian stroma.
VCD was dissolved to a concentration of 80 mg/kg in
sesame oil in preparation for injection, where an intraperitoneal
injection was conducted once daily (starting at 10:00 a.m.) for 45
days, as described previously (15). Rats in the normal group were
injected with an equal volume of sesame oil once a day. At 24 h
after the last administration, all rats were anesthetized via an
intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg),
following which blood from the inferior vena cava was collected
(8-10 ml). Subsequently, the anaesthetized rats were sacrificed by
cervical dislocation before ovarian and uterus tissues were
extracted after confirmation of cardiac arrest.
Grouping and administration
A total of 60 rats were randomly divided into the
model, Pue-low dose (L) group (50 mg/kg), Pue-medium dose (M) group
(100 mg/kg), Pue-high dose (H) group (300 mg/kg) and normal groups
(17). The rats in each group were
administered with Pue dissolved in saline by intragastric
administration starting on the first day of modeling, then once a
day for 45 days. The model and normal groups were administered with
the same volume of saline once a day for the same period.
Sampling
The weight of the rats was measured and recorded 1
day before modeling, 1 week after modeling and at the end of
modeling. Vena cava blood was drawn before the animals were
sacrificed. The ovarian and uterus tissue samples were then
obtained using the rapid aseptic method, where the wet weight was
measured and the organ indices was calculated as follows: i) Ovary
index = ovary mass / rat body weight; and ii) uterus index = uterus
mass / rat body weight. In total, 50% ovarian tissues from each rat
was stored at -70˚C, whilst the other 50% was fixed in 4%
paraformaldehyde at room temperature for 48 h for subsequent
use.
Ovarian tissue observation using
H&E staining
Ovarian tissues were fixed in paraformaldehyde,
progressively dehydrated with increasing concentrations of ethanol,
embedded in paraffin, sectioned (4 µm) and deparaffinized with
ascending series of alcohol (70% alcohol for 45 min, 75% alcohol
for 45 min). The sections were then stained with hematoxylin for 3
min and eosin for 20 sec at room temperature, and visualized using
an optical light microscope (magnification, x100).
ELISAs
After resting for 30 min, the blood was centrifuged
at 8000 x g and 4˚C for 30 sec before the serum was collected.
Serum FSH, LH and E2 levels were measured using the
respective FSH, LH and E2 kits according to the
manufacturers' protocols.
TUNEL staining of ovarian tissues
Paraffin-embedded tissue was cut into sections,
placed in water and treated with a protease K (Sigma-Aldrich; Merck
KGaA) solution at 37˚C for 15 min. After washing three times with
PBS for 3 min, the TUNEL reaction mixture (including the TdT enzyme
and dUTP marker solution) was added for 1 h in a humidified
incubator at 37˚C, before being washed five times with PBS for 5
min. A confining liquid (cat. no. 4112APG; Richard Allan
Scientific™; Thermo Fisher Scientific, Inc.) was then added and the
samples were placed in a humidified incubator at 37˚C for 30 min.
Positive and negative controls were included by adding
deoxyribonuclease I reaction mixture and omitting the TdT enzyme
reaction mixture, respectively. TUNEL positive cells were observed
in >4 randomly selected fields under a fluorescent microscope
(magnification, x200).
Immunohistochemical (IHC) staining of
ovarian tissue
The 4-µm paraffin-embedded ovarian tissue sections
after boiling in 0.1 mol/l citric acid buffer solution (pH 6.0),
antigen retrieval was performed at room temperature for 10 min,
followed by inactivation of endogenous catalase activity with 3%
H2O2 at room temperature for 5 min. A Dako
pen was used to mark the tissue site. Next, ~50 µl 10% normal
donkey serum (cat. no. 017-000-001; Jackson ImmunoResearch
Laboratories, Inc.) was added to the slides, which were incubated
for 20 min at 37˚C. The serum on the slide was then removed and
primary antibodies against Bcl-2 (1:200; cat. no. ab182858; Abcam),
Bax (1:200; cat. no. ab216985; Abcam), caspase-3 (1:200; cat. no.
ab32150; Abcam) and GAPDH (1:200; cat. no. ab8245; Abcam) were
added dropwise. The diluent solution (PBS) without primary antibody
was used as the negative control. The samples were then placed and
incubated at 4˚C overnight. Incubation with HRP-conjugated
secondary antibodies (cat. no. BA1054; 1:2,000; Wuhan Boster
Biological Technology, Ltd.) was performed at room temperature for
2 h. DAB color development was then performed, including staining
by hematoxylin at room temperature for 1 h and differentiation by
hydrochloric acid ethanol. Progressive dehydration with increasing
ethanol alcohol concentrations was then performed, followed by
mounting in neutral balsam. The cells were observed and
photographed under an optical light microscope (magnification,
x200). Protein expression was analyzed using ImageJ v1.8.0.112
(National Institutes of Health).
Detection of Bcl-2 and Bax protein
expression in the ovary using western blotting
The protein was extracted from rat ovarian tissues
by RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.). Protein concentration was determined by a BCA protein
assay kit according to the manufacturer's protocol and proteins (45
µg/lane) were separated via 10% SDS-PAGE and transferred onto PVDF
membranes. The membranes were subsequently blocked with 5% BSA
(Nanjing KeyGen Biotech Co., Ltd.) for 2 h at 4˚C, then incubated
with Bcl-2 (1:1,000; cat. no. ab182858; Abcam), Bax (1:1000; cat.
no. ab216985; Abcam), caspase-3 (1:1,000; cat. no. ab32150; Abcam)
and GAPDH (1:1000; cat. no. ab8245; Abcam) rabbit anti-rat
polyclonal antibodies overnight at 4˚C. Following the primary
antibody incubation, the membrane was washed and incubated with a
HRP-conjugated goat anti-rabbit secondary antibody (cat. no.
BA1054; 1:2,000; Wuhan Boster Biological Technology, Ltd.) at 37˚C
for 2 h. The blots were then incubated with WesternBright ECL (APG
Bio, Ltd.). GAPDH was used as the internal reference. The results
were analyzed using ImageJ version 1.47 (National Institutes of
Health).
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. The results are presented as the mean ± SD from 12 rats
per group. One way ANOVA was used for comparison between multiple
groups, followed by Tukey's tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Pue on rat ovarian and
uterine indices in the DOR model
Compared with those in the normal group, the ovarian
and uterine indices in the model group were significantly decreased
(two rats died during the experiment due to injection injury;
P<0.001; Fig. 1), suggesting
that the ovaries and uteri of rats in the DOR model were atrophied.
Compared with those in the model group, the ovarian and uterine
indices in the Pue intervention group were significantly increased
(one rat died during the experiment due to injection injury;
P<0.05; Fig. 1) in a
dose-dependent manner (P<0.05; Fig.
1). This suggests that the extent of ovary and uterus atrophy
was at least partially reduced in rats following Pue treatment.
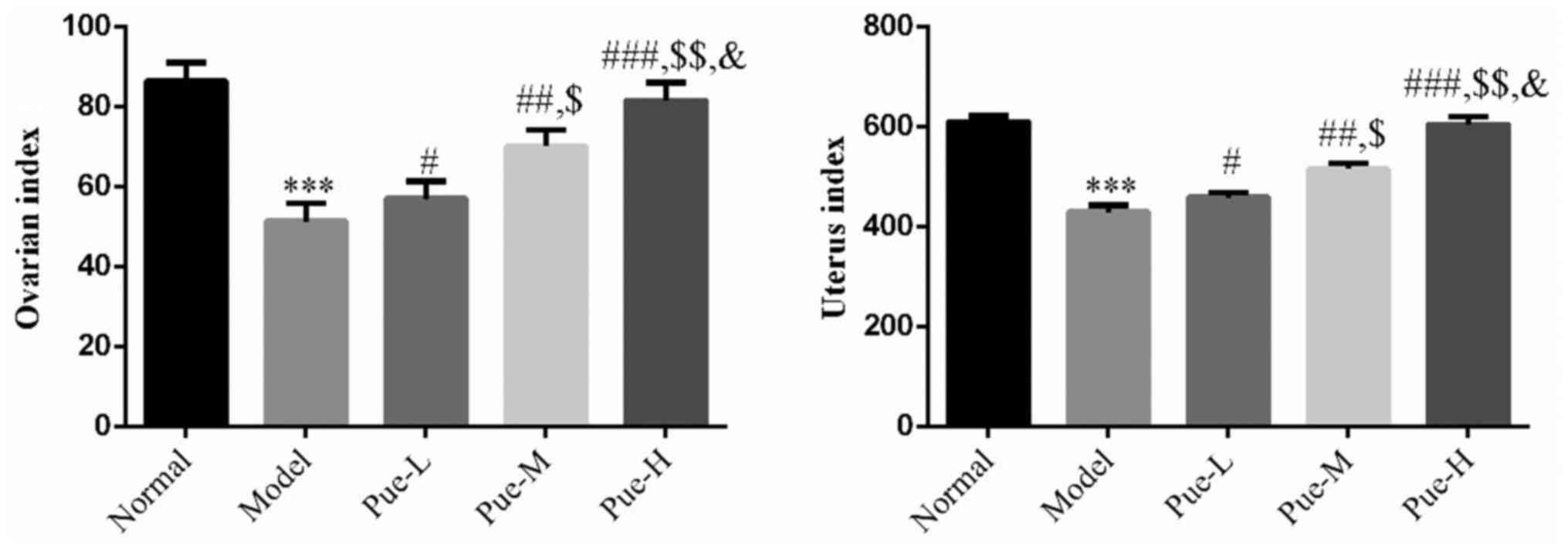 | Figure 1Effects of Pue on the ovarian and
uterine indices of DOR model rats. Rats were divided into normal,
model, Pue-L, Pue-M and Pue-H groups. ***P<0.001 vs.
normal; #P<0.05, ##P<0.01,
###P<0.001 vs. Model; $P<0.05,
$$P<0.01 vs. Pue-L; &P<0.05 vs.
Pue-M. DOR, diminished ovarian reserve; Pue, pueratin; L, low dose;
M, medium dose; H, high dose. |
Effect of Pue on the morphology of
ovaries of rats in the DOR model Macroscopic observations
Compared with that in the normal group, the volume
of the ovary was observed to be markedly decreased after modeling,
where there were different degrees of adhesion to the surrounding
tissues (Fig. 2; black arrow).
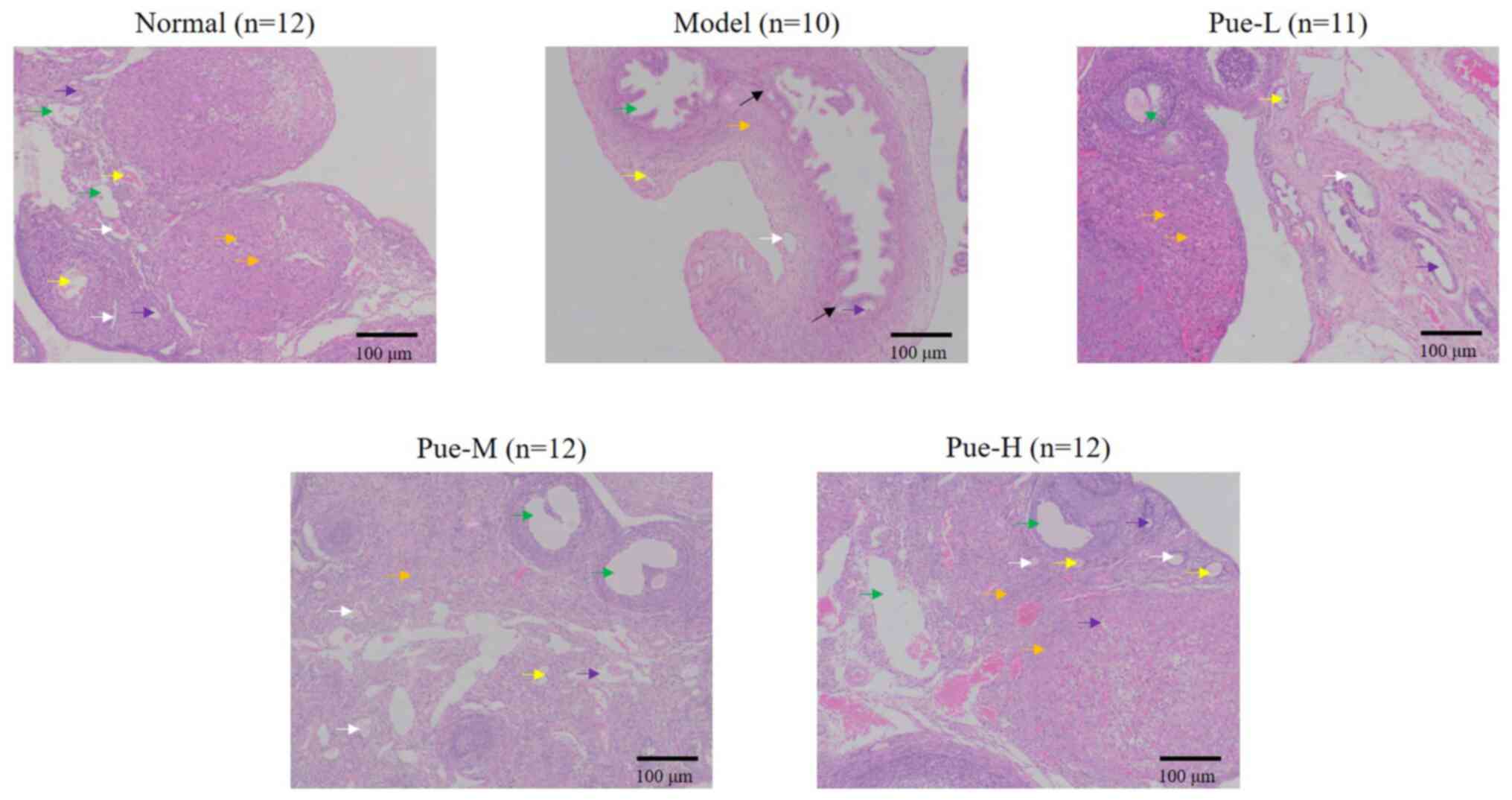 | Figure 2Effects of Pue on the morphology of
ovaries in DOR model rats. Rats were divided into normal, model,
Pue-L, Pue-M and Pue-H groups. Scale bars, 100 µm. White arrow,
primordial follicles; yellow arrow, primary follicles; green arrow,
mature follicles; violet arrow, oocytes; orange arrow, granulosa
cells. DOR, diminished ovarian reserve; Pue, pueratin; L, low dose;
M, medium dose; H, high dose. |
Microscopic findings
Compared with that in the normal group, the number
of rat ovarian primordial and primary follicles in the model group
was markedly decreased. It was also found indirectly that the
number of mature follicles was also decreased. The size of oocytes
was reduced, the number of granulosa cells was decreased and the
arrangement became disordered. However, after intragastric Pue
administration, the pathology of the ovary was markedly improved,
for example, the number of follicles were higher in the Pue groups
compared with the model group, suggesting a curative relationship
(Fig. 2).
Effects of Pue on the apoptosis of rat
ovarian cells in the DOR model
TUNEL assay results showed that, compared with that
in the normal group, the number of apoptotic cells in the ovaries
of the model group was significantly increased (P<0.001;
Fig. 3). After intervention with
Pue, compared with that in the model group, the number of apoptotic
cells in the ovarian tissue of the Pue groups was significantly
decreased (P<0.05; Fig. 3).
These significant reductions in the number of apoptotic cells in
the ovarian tissue among Pue groups were also found to be
dose-dependent (P<0.05; Fig.
3).
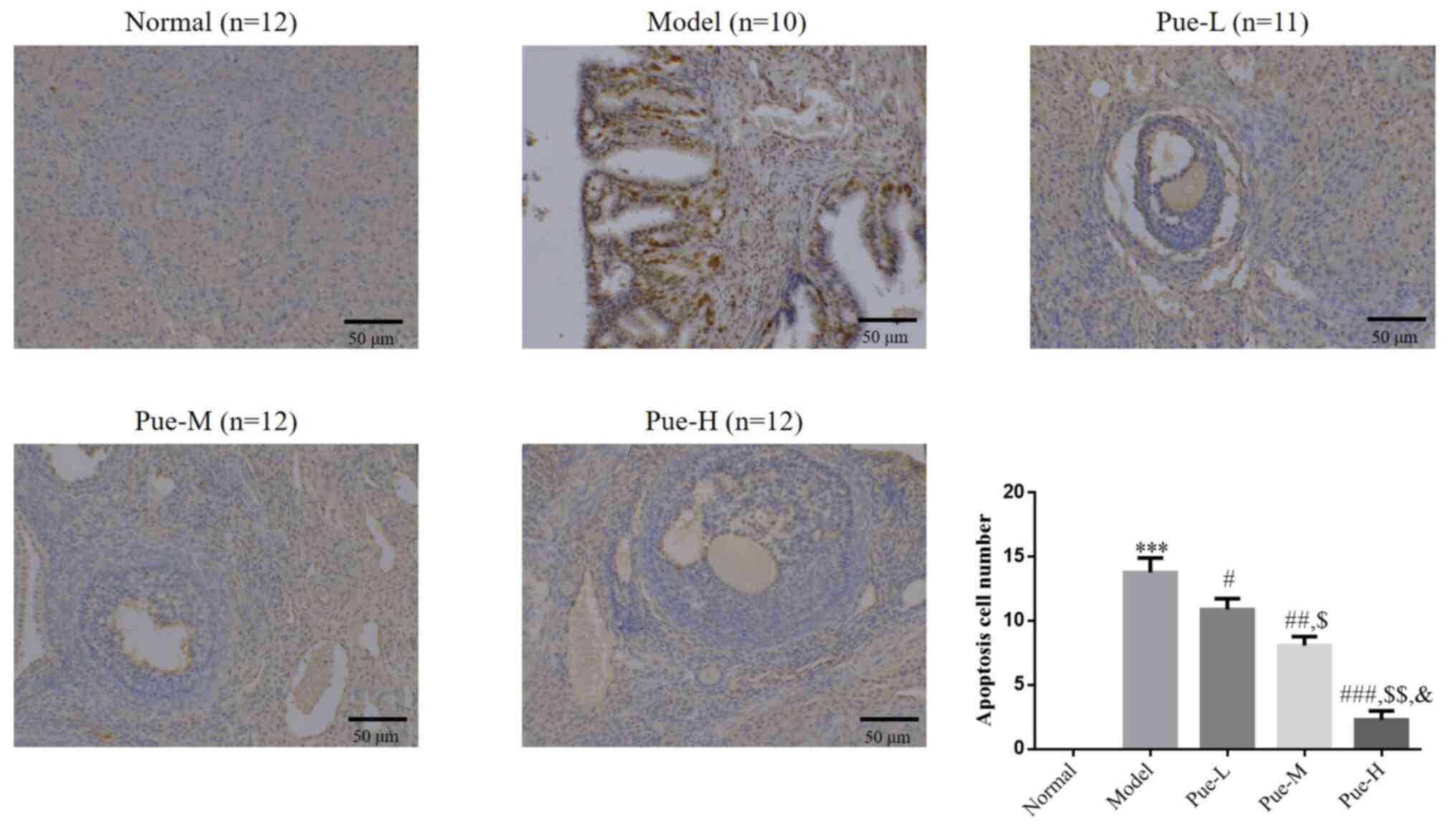 | Figure 3Apoptotic cell number in the
different treatment groups. Rats were divided into normal, model,
Pue-L, Pue-M and Pue-H groups. Scale bars, 50 µm.
***P<0.001 vs. normal; #P<0.05,
##P<0.01, ###P<0.001 vs. model;
$P<0.05, $$P<0.01 vs. Pue-L;
&P<0.05 vs. Pue-M. DOR, diminished ovarian
reserve; Pue, pueratin; L, low dose; M, medium dose; H, high
dose. |
Effects of Pue on rat serum FSH, LH
and E2 levels in the DOR model
Compared with those in the normal group, FSH, LH and
E2 levels in the model group were significantly changed
(P<0.001; Fig. 4).
Specifically, FSH and LH levels were significantly increased,
whilst E2 levels were significantly decreased. After
intervention with Pue, compared with those in the model group, the
levels of FSH and LH in each Pue group were significantly
decreased, whereas and the level of E2 was significantly
increased (P<0.05; Fig. 4). In
addition, there was a significant dose-effect relationship among
the Pue groups (P<0.05; Fig.
4).
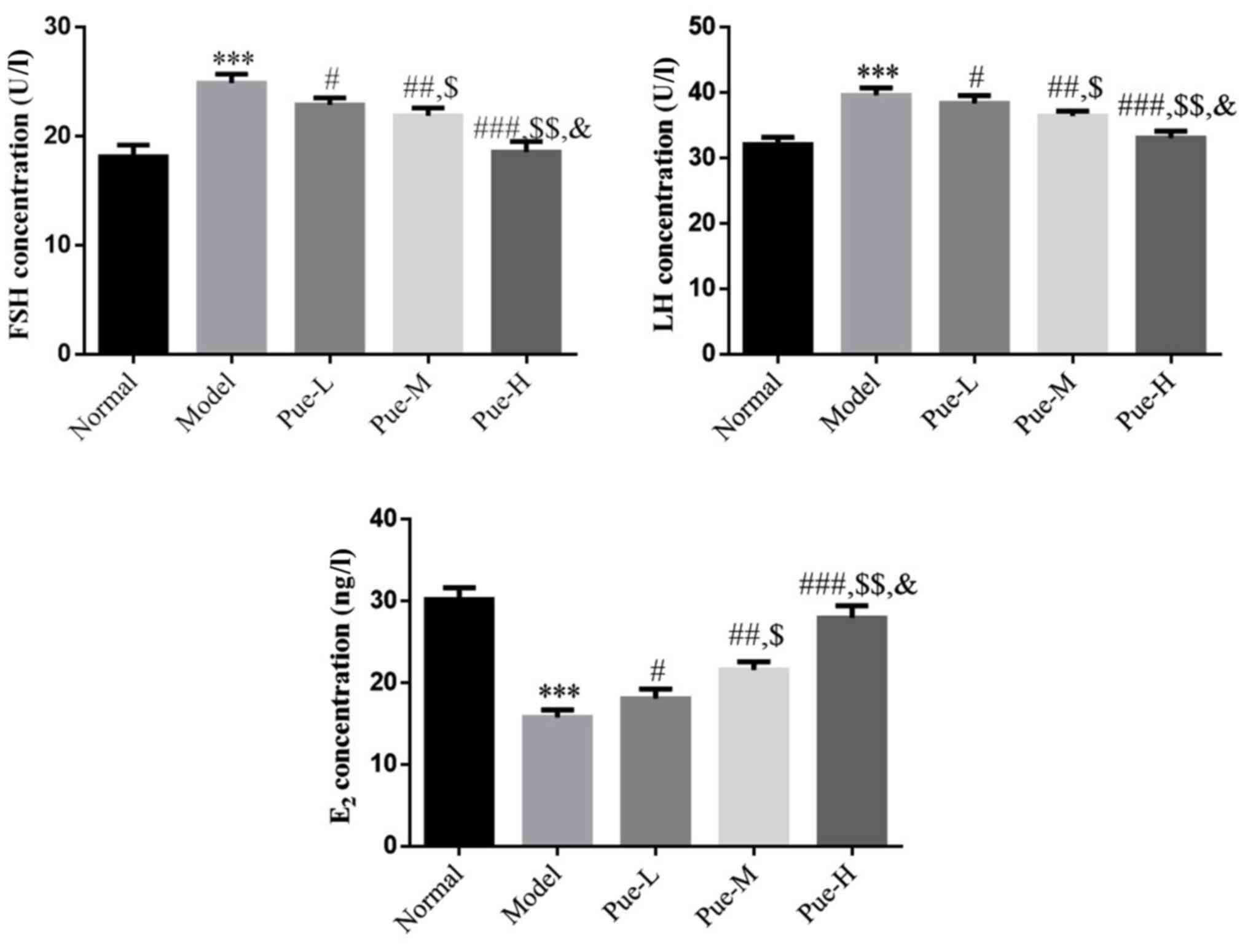 | Figure 4Effects of Pue on FSH, LH and
E2 levels in the serum of DOR model rats. Rats were
divided into normal, model, Pue-L, Pue-M and Pue-H groups.
***P<0.001 vs. normal; #P<0.05,
##P<0.01 and ###P<0.001 vs. model;
$P<0.05 and $$P<0.01 vs. Pue-L;
&P<0.05 vs. Pue-M. DOR, diminished ovarian
reserve; Pue, pueratin; L, low dose; M, medium dose; H, high dose;
FSH, follicle-stimulating hormone; LH, luteinizing hormone;
E2, estradiol. |
Apoptotic caspase-3 protein expression
analysis using IHC and WB
Compared with that in the normal group, the
expression of caspase-3 protein in the model group was
significantly upregulated (P<0.001; Fig. 5A and B). However, following Pue administration,
the protein expression levels of caspase-3 were significantly
downregulated compared with that the model group in a
dose-dependent manner (P<0.05; Fig.
5A and B).
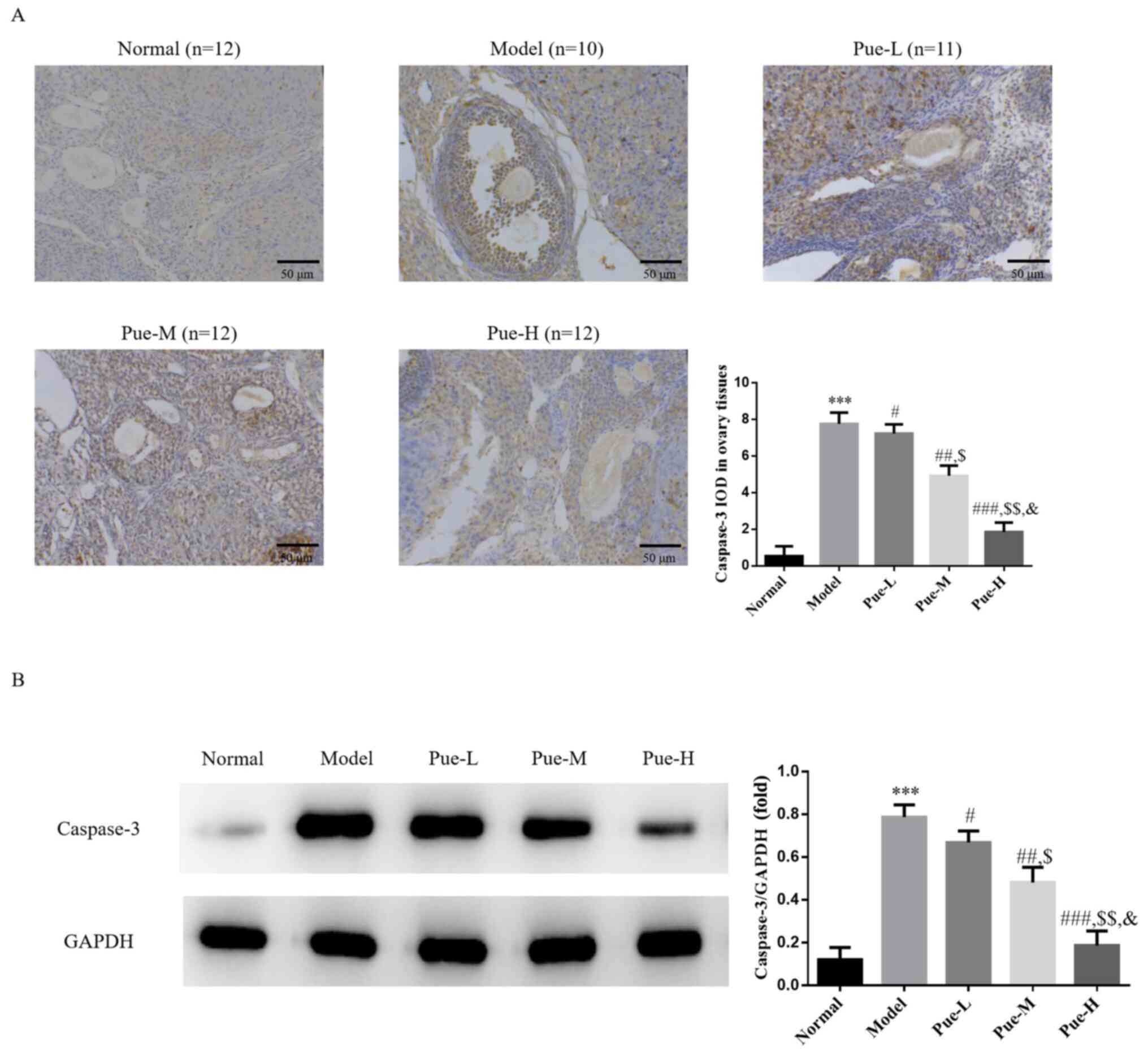 | Figure 5Caspase-3 protein expression in
ovaries as determined using IHC and WB. Rats were divided into
normal, model, Pue-L, Pue-M and Pue-H groups. (A) Caspase-3 protein
expression was analyzed using IHC assays. Scale bars, 50 µm. (B)
Caspase-3 protein expression was measured using WB assays.
***P<0.001 vs. normal; #P<0.05,
##P<0.01, ###P<0.001 vs. model;
$P<0.05, $$P<0.01 vs. Pue-L;
&P<0.05 vs. Pue-M. DOR, diminished ovarian
reserve; Pue, pueratin; L, low dose; M, medium dose; H, high dose;
IHC, immunohistochemistry; WB, western blotting; IOD, integrated
optical density. |
Detection of the expression levels of
Bcl-2 and Bax proteins in the epithelial regions of ovarian tissues
using IHC
Compared with that in the normal group, the
expression levels of Bcl-2 in the model group tissues were
decreased, whilst that of Bax was significantly increased
(P<0.001; Fig. 6A and B), suggesting that the high expression of
Bax protein and the low expression of Bcl-2 protein in the model
group may accelerate the apoptosis of follicles and result in the
decline in ovarian reserve. Compared with that in the model group,
the expression of Bcl-2 protein in the ovaries of the Pue
intervention group was significantly increased, whilst the
expression of Bax protein was significantly decreased (P<0.05;
Fig. 6A and B). In addition, there was a significant
dose-dependent effect on the expression of Bcl-2 and Bax among the
Pue groups (P<0.05; Fig. 5A and
B).
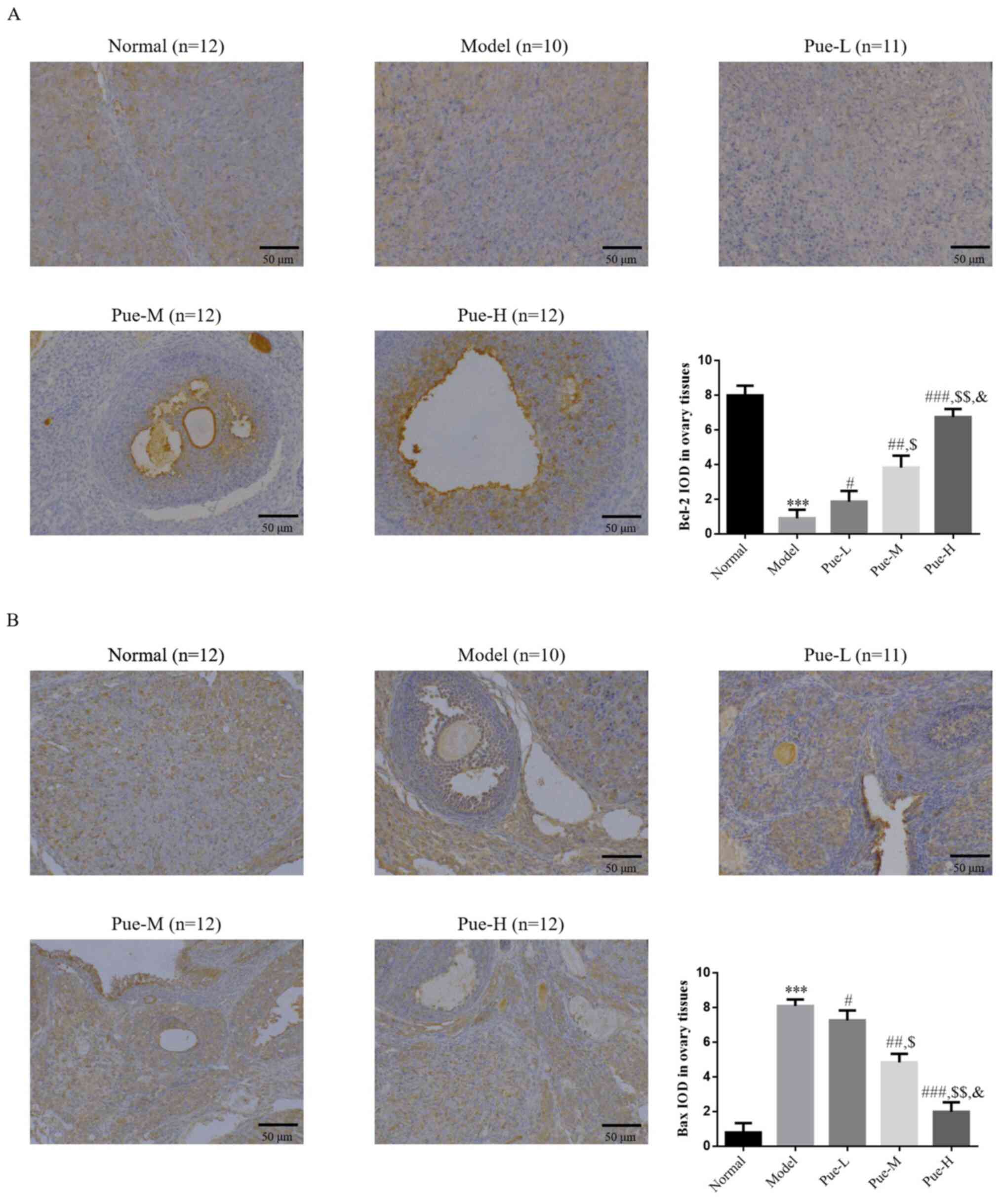 | Figure 6Bcl-2 and Bax protein expression in
ovaries as determined using IHC. Rats were divided into normal,
model, Pue-L, Pue-M and Pue-H groups. (A) Bcl-2 and (B) Bax protein
expression was analyzed using IHC assays. Scale bars, 50 µm.
***P<0.001 vs. normal; #P<0.05,
##P<0.01, ###P<0.001 vs. model;
$P<0.05, $$P<0.01 vs. Pue-L;
&P<0.05 vs. Pue-M. DOR, diminished ovarian
reserve; Pue, pueratin; L, low dose; M, medium dose; IHC,
immunohistochemistry; IOD, integrated optical density. |
Detection of the expression levels of
Bcl-2 and Bax proteins in ovarian tissues using WB
Compared with that in the normal group, the
expression of Bcl-2 protein in the model group was significantly
decreased whereas the expression of Bax was significantly increased
(P<0.001; Fig. 7). In addition,
compared with that in the model group, there was a significant
upregulation in the Bcl-2/Bax ratio (P<0.001; Fig. 7), suggesting that the high
expression of Bax protein and the low expression of Bcl-2 protein
in the model group may accelerate the apoptosis of follicles and
lead to the decline in ovarian reserve. Compared with that in the
model group, the protein expression of Bcl-2 in the ovaries of the
Pue intervention groups were significantly increased (P<0.05;
Fig. 7), whereas that of Bax was
significantly decreased (P<0.05; Fig. 7). Accordingly, the Bcl-2/Bax ratio
was also significantly increased in the Pue intervention groups
compared with that in the model group, where a dose-dependent
effect was observed (P<0.05; Fig.
7).
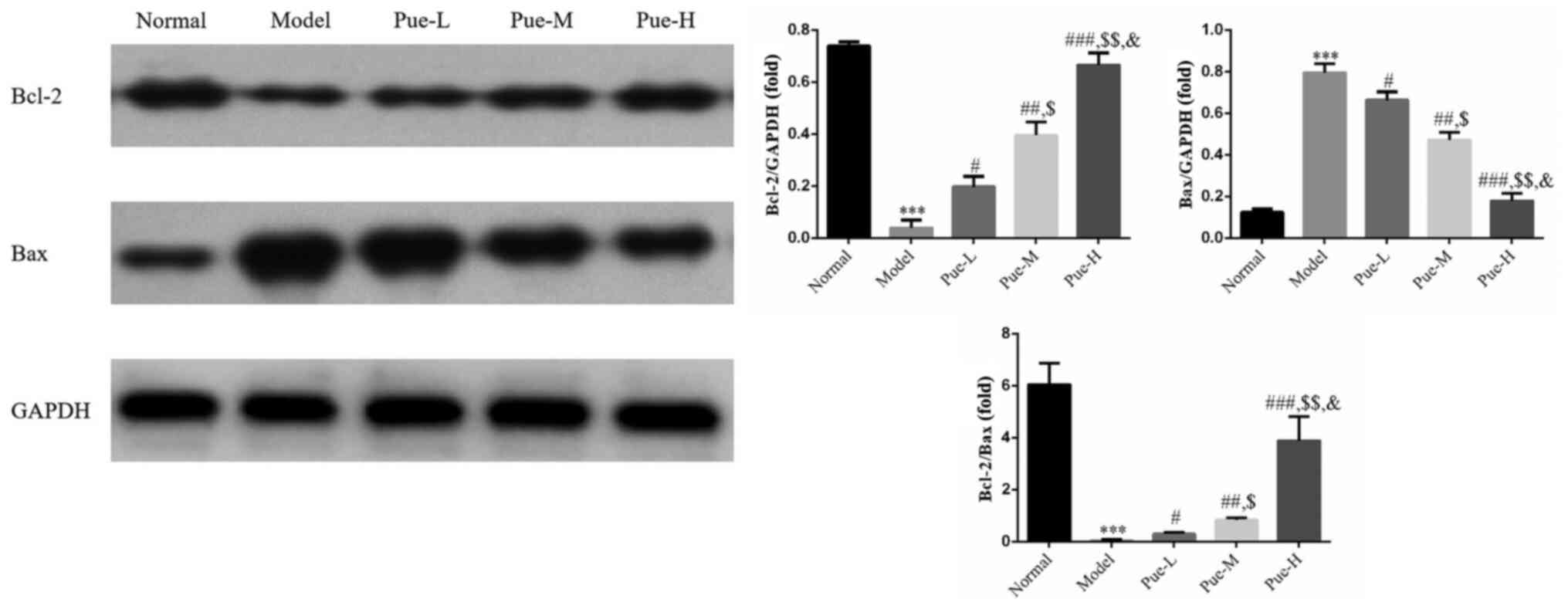 | Figure 7Bcl-2 and Bax protein expression as
determined using western blotting. Rats were divided into normal,
model, Pue-L, Pue-M and Pue-H groups. ***P<0.001 vs.
normal; #P<0.05, ##P<0.01,
###P<0.001 vs. model; $P<0.05,
$$P<0.01 vs. Pue-L; &P<0.05 vs.
Pue-M. DOR, diminished ovarian reserve; Pue, pueratin; L, low dose;
M, medium dose. |
Discussion
As early as the fetal stage, most human ovaries have
follicles (17). After 6-8 weeks
of embryonic development, mitosis occurs in the primordial germ
cells, where the number and volume of cells increases (18). Following development, the number of
oogonia is ~600,000(19).
Following growth of the fetus for 11-12 weeks, oogonia become
oocytes through meiosis (20).
After 16-20 weeks, the number of germ cells reaches its peak at 6-7
million, where oocytes account for ~33% and primary oocytes account
for ~66% (20). As the fetus
continues to develop, the number of cells decreases gradually, such
that by 40 weeks of gestation, >66% of the oocytes die due to
apoptosis and the number remaining is reduced to ~2 million oocytes
(21). Primary oocytes are
normally surrounded by granulosa cells to form the primordial
follicle, which is the basic reproductive unit of women and the
only form of oocyte reserve (12).
Numerous ovarian follicles die after birth (22). Follicular atresia is the gradual
degradation of ovarian follicles at all stages of development
(23). By adolescence, typically
~75% of primordial follicles would have died (24). Generally, only one dominant
follicle can develop and mature completely for ovulation at each
menstrual cycle in women of child-bearing age (25). This suggests that most follicles
cannot develop and mature in the ovary (25). Most of follicles undergo
degradation and atresia through apoptosis (26). The basic conditions required for
follicular development and maturation depend on the proliferation
and differentiation states of granulosa cells in the follicles,
where apoptosis of granulosa cells is a prerequisite for follicular
atresia (27). This directly
affects the quantity and quality of follicles.
The pathophysiology of DOR is complex, where its
etiology remains unclear. The generally accepted mechanism of DOR
include follicular atresia caused by the rapid depletion of
oocytes, abnormal proliferation and low differentiation of
granulosa cells or the apoptosis of granulosa cells (28-30).
The aim of the present study was to evaluate the atresia of
primordial and primary follicles and the associated apoptosis of
ovarian granulosa cells. Therefore, an animal model of DOR was
established using the toxic chemical VCD. VCD was previously found
to accelerate the natural apoptosis of follicles (31), where primary follicles are the main
targets of VCD in the ovary. Abnormal VCD secretion is associated
with a reduction in the number of viable follicles in the ovaries
of patients with DOR (32). In the
present study, the results of modeling were evaluated using vaginal
smears, which suggested that VCD treatment was ideal for the study
of DOR in animal models. Results from the present study revealed
that apoptosis of oocytes and granulosa cells in the follicles was
increased, resulting in the increase of follicular atresia and
reduction of the ovarian reserve. Therefore, these results suggest
that the number of primordial follicles and the number of viable
follicles in the model group were lower than those in the normal
group. In addition, since the apoptosis rate of ovarian granulosa
cells in the model group was significantly higher compared with
that in the normal group, establishment of the DOR rat model
appears to be successful.
Apoptosis is the process of self-destruction that
occurs in both normal physiological conditions and in disease
states (33). Under normal
conditions, the balance between granulosa cell mitosis and
apoptosis is synchronized (34).
When the apoptosis rate of granulosa cells reaches >10%,
follicular atresia occurs (4),
suggesting that apoptosis serves as the key event during follicular
atresia. Since the number of primordial follicles in the ovary is
fixed at birth, follicular atresia serves an important
physiological function in the ovary, since this involves the
removal of redundant tissues (35). Therefore, atresia of a small number
of follicles does not normally affect the normal development of
surrounding follicles (26). By
contrast, DOR occurs when the speed of atresia in a large number of
follicles is faster than the physiological metabolic rate (36).
Bcl-2 and Bax proteins are members of the Bcl-2
family of proteins, which can be sub-divided into the following two
categories: i) Proteins represented by Bcl-2, which inhibit
apoptosis; and ii) proteins represented by Bax, which promote
apoptosis (37-39).
Results from the present study suggested that VCD treatment led to
an increase in FSH and LH levels, a decrease in the ovarian
secretion of E2, a reduction in Bcl-2 expression and an
elevation in Bax expression, which ultimately promoted the
apoptosis of follicular granulosa cells. As pro-caspase 3 does not
accurately reflect apoptotic activity, this may reflect another
limitation of the present study. FSH and LH regulate the apoptosis
and secretion of ovarian granulosa cells, which serve an important
role in the regulation of follicular development and ovulation
(28). The present study
demonstrates that Pue may inhibit the apoptosis of follicles by
downregulating FSH and LH levels whilst upregulating E2
levels, in addition to upregulating the expression levels of Bcl-2
protein whilst downregulating the expression of Bax protein. The
purpose of the present study was to test the effects of Pue on the
pathophysiology and Bax and Bcl-2 expression in rats with DOR.
However, there are limitations in the present study, as it remains
unclear what the correlation is among Bcl-2, Bax expression and
hormone levels during DOR.
In conclusion, the present study demonstrated that
Pue treatment downregulated FSH and LH levels, stimulated
E2 secretion and regulated the expression of the Bcl-2
family of proteins, Bcl-2 and Bax. By upregulating the expression
of Bcl-2 protein and downregulating the expression of Bax protein,
Pue was observed to inhibit apoptosis to preserve ovarian reserves,
which may prove to be useful for the clinical prevention and
treatment of DOR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 2016A030310318).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW drafted the manuscript. QQ conceived the study
and participated in the manuscript preparation. HW, XZ, YC and LY
assisted in the literature search and edited the manuscript. YC
revised the manuscript and checked the data of our experiment. HW,
XZ, YC and LY performed the experiment. QQ and YC confirm the
authenticity of all the raw data and checked the grammar in
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The experiments and operations related to the
animals involved in this study were performed with the approval of
the Animal Ethics Committee of Guangdong Women and Children
Hospital (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jaillard S, Sreenivasan R, Beaumont M,
Robevska G, Dubourg C, Knarston IM, Akloul L, van den Bergen J,
Odent S, Croft B, et al: Analysis of NR5A1 in 142 patients with
premature ovarian insufficiency, diminished ovarian reserve, or
unexplained infertility. Maturitas. 131:78–86. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tanaka Y, Hsueh AJ and Kawamura K:
Surgical approaches of drug-free in vitro activation and
laparoscopic ovarian incision to treat patients with ovarian
infertility. Fertil Steril. 114:1355–1357. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kawamura K, Kawamura N and Hsueh AJ:
Activation of dormant follicles: A new treatment for premature
ovarian failure? Curr Opin Obstet Gynecol. 28:217–222.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gleicher N and Barad DH:
Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian
reserve (DOR). Reprod Biol Endocrinol. 9(67)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Quaas AM and Legro RS: Pharmacology of
medications used for ovarian stimulation. Best Pract Res Clin
Endocrinol Metab. 33:21–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cohen J, Chabbert-Buffet N and Darai E:
Diminished ovarian reserve, premature ovarian failure, poor ovarian
responder - a plea for universal definitions. J Assist Reprod
Genet. 32:1709–1712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen C, Li S, Hu C, Cao W, Fu Q, Li J,
Zheng L and Huang J: Protective Effects of Puerarin on Premature
Ovarian Failure via Regulation of Wnt/β-catenin Signaling Pathway
and Oxidative Stress. Reprod Sci. 28:982–990. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chlebowski RT, Manson JE, Anderson GL,
Cauley JA, Aragaki AK, Stefanick ML, Lane DS, Johnson KC,
Wactawski-Wende J, Chen C, et al: Estrogen plus progestin and
breast cancer incidence and mortality in the Women's Health
Initiative Observational Study. J Natl Cancer Inst. 105:526–535.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Worsley R, Davis SR, Gavrilidis E, Gibbs
Z, Lee S, Burger H and Kulkarni J: Hormonal therapies for new onset
and relapsed depression during perimenopause. Maturitas.
73:127–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Jiang M, Yun Q, Niu G, Gao Y, Shi F and Yu
S: Puerarin prevents inflammation and apoptosis in the neurocytes
of a murine Parkinson's disease model. Genet Mol Res: Oct 5, 2016
(Epub ahead of print). doi: 10.4238/gmr.15047501.
|
|
12
|
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen
C, Shan G and Yao Q: Puerarin attenuates renal fibrosis by reducing
oxidative stress induced-epithelial cell apoptosis via MAPK signal
pathways in vivo and in vitro. Ren Fail. 39:423–431.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xue Q, Liu Y, He R, Yang S, Tong J, Li X,
Chen Y and Xu X: Lyophilized Powder of Catalpol and Puerarin
Protects Neurovascular Unit from Stroke. Int J Biol Sci.
12:367–380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ma Y, Gai Y, Yan J, Li J and Zhang Y:
Puerarin Attenuates Anoxia/Reoxygenation Injury Through Enhancing
Bcl-2 Associated Athanogene 3 Expression, a Modulator of Apoptosis
and Autophagy. Med Sci Monit. 22:977–983. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gaumer S, Guénal I, Brun S, Théodore L and
Mignotte B: Bcl-2 and Bax mammalian regulators of apoptosis are
functional in Drosophila. Cell Death Differ. 7:804–814.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin H, Zhang XM, Chen C and Chen BD:
Apoptosis of Mo7e leukemia cells is associated with the cleavage of
Bcl-2 into a shortened fragment that is not functional for
heterodimerization with Bcl-2 and Bax. Exp Cell Res. 261:180–186.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li L, Dong J, Yan L, Yong J, Liu X, Hu Y,
Fan X, Wu X, Guo H, Wang X, et al: Single-Cell RNA-Seq Analysis
Maps Development of Human Germline Cells and Gonadal Niche
Interactions. Cell Stem Cell. 20:858–873.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu M, Che L, Yang Z, Zhang P, Shi J, Li J,
Lin Y, Fang Z, Che L, Feng B, et al: Proteomic Analysis of Fetal
Ovaries Reveals That Primordial Follicle Formation and Transition
Are Differentially Regulated. BioMed Res Int.
2017(6972030)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Birt JA, Taylor KH, Davis JW and
Sharpe-Timms KL: Developmental exposure of fetal ovaries and fetal
germ cells to endometriosis in an endometriosis model causes
differential gene expression in the preimplantation embryos of the
first-generation and second-generation embryos. Fertil Steril.
100:1436–1443. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu H, Zhang X, Zhong X, Li Z, Cai S, Yang
P, Ou C and Chen M: Puerarin inhibits vascular calcification of
uremic rats. Eur J Pharmacol. 855:235–243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lei L, Zhang H, Jin S, Wang F, Fu M, Wang
H and Xia G: Stage-specific germ-somatic cell interaction directs
the primordial folliculogenesis in mouse fetal ovaries. J Cell
Physiol. 208:640–647. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Juneja SC, Barr KJ, Enders GC and Kidder
GM: Defects in the germ line and gonads of mice lacking connexin43.
Biol Reprod. 60:1263–1270. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pepling ME: From primordial germ cell to
primordial follicle: Mammalian female germ cell development.
Genesis. 44:622–632. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vaskivuo TE and Tapanainen JS: Apoptosis
in the human ovary. Reprod Biomed Online. 6:24–35. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Perez GI, Maravei DV, Trbovich AM,
Cidlowski JA, Tilly JL and Hughes FM Jr: Identification of
potassium-dependent and -independent components of the apoptotic
machinery in mouse ovarian germ cells and granulosa cells. Biol
Reprod. 63:1358–1369. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang S, Sun M, Yu L, Wang Y, Yao Y and
Wang D: Niacin Inhibits Apoptosis and Rescues Premature Ovarian
Failure. Cell Physiol Biochem. 50:2060–2070. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Silva JRV, Lima FEO, Souza ALP and Silva
AWB: Interleukin-1β and TNF-α systems in ovarian follicles and
their roles during follicular development, oocyte maturation and
ovulation. Zygote. 28:270–277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang S, Lin S, Zhu M, Li C, Chen S, Pu L,
Lin J, Cao L and Zhang Y: Acupuncture Reduces Apoptosis of
Granulosa Cells in Rats with Premature Ovarian Failure Via
Restoring the PI3K/Akt Signaling Pathway. Int J Mol Sci.
20(E6311)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kappeler CJ and Hoyer PB:
4-vinylcyclohexene diepoxide: A model chemical for ovotoxicity.
Syst Biol Reprod Med. 58:57–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chauhan P, Sodhi A and Tarang S:
Cisplatin-treated murine peritoneal macrophages induce apoptosis in
L929 cells: Role of Fas-Fas ligand and tumor necrosis factor-tumor
necrosis factor receptor 1. Anticancer Drugs. 18:187–196.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Almarzoug MHA, Ali D, Alarifi S, Alkahtani
S and Alhadheq AM: Platinum nanoparticles induced genotoxicity and
apoptotic activity in human normal and cancer hepatic cells via
oxidative stress-mediated Bax/Bcl-2 and caspase-3 expression.
Environ Toxicol. 35:930–941. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Roosa KA, Mukai M and Place NJ:
4-Vinylcyclohexene diepoxide reduces fertility in female Siberian
hamsters when treated during their reproductively active and
quiescent states. Reprod Toxicol. 51:40–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luciano AM, Modina S, Gandolfi F, Lauria A
and Armstrong DT: Effect of cell-to-cell contact on in vitro
deoxyribonucleic acid synthesis and apoptosis responses of bovine
granulosa cells to insulin-like growth factor-I and epidermal
growth factor. Biol Reprod. 63:1580–1585. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gosden RG and Faddy MJ: Biological bases
of premature ovarian failure. Reprod Fertil Dev. 10:73–78.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Klein JL, Adams SM, De Moura AF, Alves
Filho DC, Maidana FM, Brondani IL, Cocco JM, Rodrigues LDS, Pizzuti
LAD and Da Silva MB: Productive performance of beef cows subjected
to different nutritional levels in the third trimester of
gestation. Animal. 15(100089)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang H, Jiao H, Jiang Z and Chen R:
Propofol inhibits migration and induces apoptosis of pancreatic
cancer PANC-1 cells through miR-34a-mediated E-cadherin and
LOC285194 signals. Bioengineered. 11:510–521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Erfani S, Moghimi A, Aboutaleb N and
Khaksari M: Protective Effects of Nucleobinding-2 After Cerebral
Ischemia Via Modulating Bcl-2/Bax Ratio and Reducing Glial
Fibrillary Acid Protein Expression. Basic Clin Neurosci.
10:451–459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fakhrabadi HG, Rabbani-Chadegani A, Ghadam
P and Amiri S: Protective effect of bleomycin on 5-azacitidine
induced cytotoxicity and apoptosis in mice hematopoietic stem cells
via Bcl-2/Bax and HMGB1 signaling pathway. Toxicol Appl Pharmacol.
396(114996)2020.PubMed/NCBI View Article : Google Scholar
|





















