Introduction
Sevoflurane (Sev), a common inhaled anesthetic in
pediatrics, has the advantages of low airway irritation and a low
blood gas distribution coefficient (1,2).
Despite these advantages, reports have indicated that Sev
significantly increased the incidence of learning deficits in
adolescents (3,4). In neonatal animals, Sev induced
neurological impairment, including cognitive decline and abnormal
social behaviors in adulthood (5,6).
Unfortunately, few interventions or treatments prevent these
neurological defects (7).
Brain-derived neurotrophic factor (BDNF) servesan
important role in neuronal survival, growth and differentiation
(8). BDNF is located in the
hippocampus, cerebral cortex and basal forebrain, which are
critical areas for learning and memory (9). cAMP response element binding protein
(CREB) regulates the expression of several genes, including BDNF,
that promote synapse formation and neural plasticity (10,11).
Furthermore, there are several CREB binding sites in the promoter
region of the BDNF gene (12). CREB
phosphorylation is essential for its function (13).
Several protein kinases, including protein kinase A
(PKA), extracellular receptor kinase and phosphatidylinositol-3
kinases, are known to phosphorylate and activate CREB (14,15).
Since activation of PKA/CREB/BDNF signaling is closely associated
with memory formation, the current study investigated whether Sev
influences cognition via the PKA/CREB/BDNF pathway in the
hippocampus in vivo. The present study tested the hypothesis
that intraperitoneal injections of bucladesine (Buc; also called
DB-cAMP), a membrane permeable selective activator of PKA, can
cause an improvement in cognition.
Materials and methods
Materials
The following anesthetics, substances and kits were
used: sevoflurane (Sev; Abbott GmbH), Buc (Abbott GmbH), anti-PKA
(cat. no. ab5815; Abcam), anti-CREB (cat. no. ab31387; Abcam),
anti-phosphorylated (p-) CREB (cat. no. ab10564; Abcam), anti-BDNF
(cat. no. ab226843; Abcam), anti-actin (cat. no. ab179467; Abcam),
goat anti-rabbit IgG H&L (HRP conjugated; cat. no. ab205718;
Abcam) and BDNF ELISA kits (cat. no. NI-0035; Beijing North
Institute of Biotechnology Co., Ltd.).
Animals
A total of 30 Sprague-Dawley rats (male, 10; female,
20; weight, 220±20 g), 10-12 weeks old, were used. Animals were
purchased from Jinfeng Laboratory Animal Co., Ltd. Animals were
housed with free access to food and water at a temperature of
22±2˚C and 55±5% humidity with 12-h light/dark cycles. Male and
female rats were caged at a ratio of 1:2. Female rats were housed
in individual cages after they were confirmed to be pregnant until
they delivered naturally. Day of birth was noted as postnatal day 0
(P0). The experimental protocol was approved by the Institutional
Animal Care and Use Committee of Zhangqiu District Maternal and
Child Health Care Hospital (Jinan China).
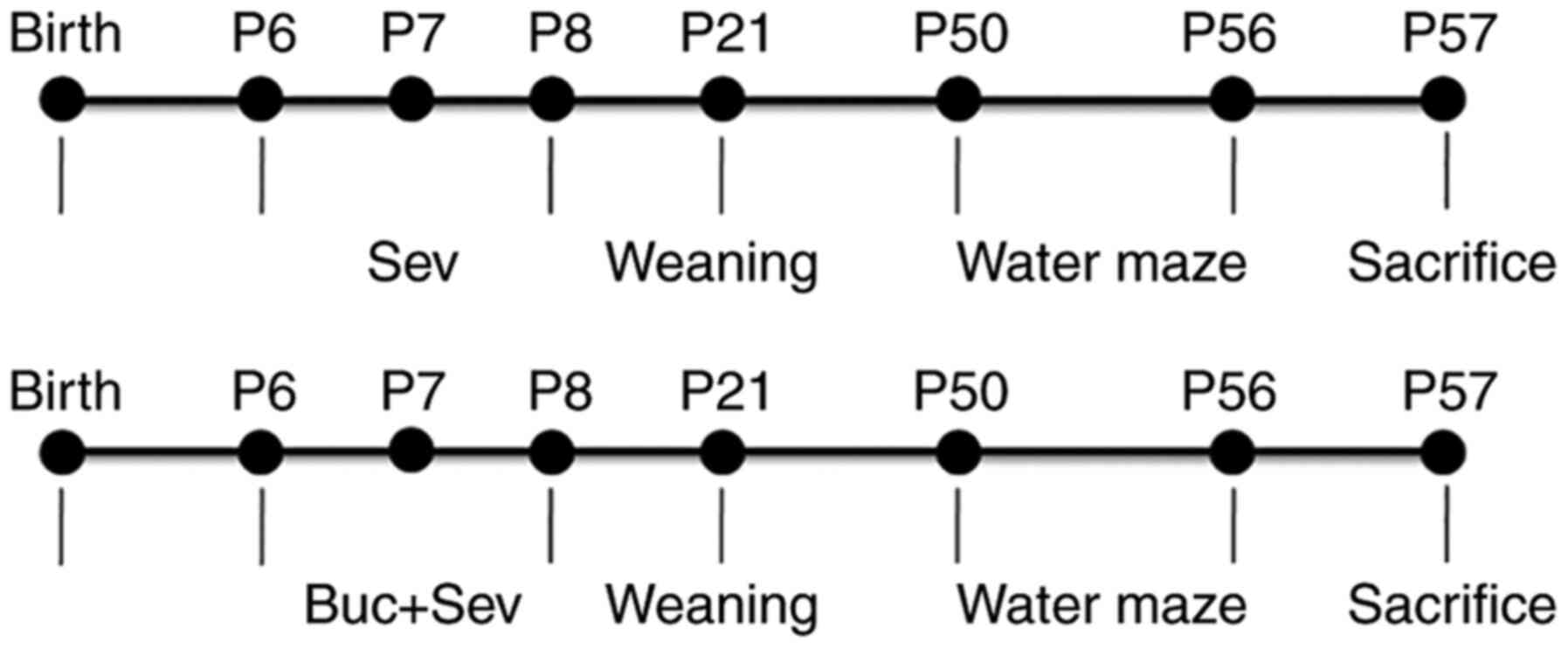
Anesthetic exposure
In total,55 P6-P8 rat pups (male, 28; female, 27)
were placed in a glass chamber (20x12x10 cm) and rested in a water
bath to maintain a constant environmental temperature of 37-38˚C.
The Con group (male, 8; female, 7) was not exposed to Sev. The
Sevgroup (male, 10; female, 10) were exposed to 3% Sev in 30%
oxygen carrier gas (in balance with nitrogen) and exposed to 2 h
daily for 3 consecutive days in the chamber (16). Following anesthesia, pups were
allowed to recover and were returned to their mothers. For the
intervention studies, Buc, a selective PKA agonist (17), was administered to the rats via an
intraperitoneal injection at a concentration of 300 nM/rat 2 h
prior to Sev anesthesia (male, 10; female, 10) (18). The Con group did not undergo Buc
intraperitoneal injection. All the experiments performed were
blinded. At P50, rats underwent behavioral tests. Study protocol is
presented in Fig. 1.
Water maze
Three groups of animals were tested for water maze:
Con (n=10), Sev (n=10) and Buc + Sev (n=10). Rats were trained in
an open water maze (diameter, 1.5 m) filled with water
(temperature, 26˚C) made opaque with latex liquid. Prominent
extra-maze visual cues around the room remained in fixed positions
throughout the experiments. In the behavioral tests, rats were
required to locate a hidden submerged platform (diameter, 10 cm;
1.5 cm below the surface), which remained in the same position for
individual animals; Four equally spaced points (north, south, east
and west) at the edge of the pool were used as starting positions.
The whole process was divided into two parts: the first 4 days were
training tests and day 5 was the probe test. During the training
tests, trials began when rats were placed in the pool facing the
side wall at a start position and ended once the rats found the
platform. If rats had not found the platform within 120 sec, they
were guided there by hand. In the probe tests, the swimming
duration was set to not exceed 120 sec. If the rat did not find the
platform in 120 sec, the task was considered a failure. A Morris
water maze video analysis system was purchased from Chinese Academy
of Medical Sciences. A video camera was mounted to the ceiling
directly above the center of the maze was used in conjunction with
the animal tracking system (19).
Nissl staining
Rats were deeply anesthetized with pentobarbital
sodium (Shanghai Chemical Reagent Company) at the dose of 50 mg/kg
and perfused transcardially with 0.9% NaClfor 5 min. Rats were then
perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer
(pH 7.4) for 20 min. Brains were removed, fixed in 4% PFA for 7
days at room temperature and embedded in paraffin. Following this,
3 µM-thick paraffin sections were excised from the dentate gyrus
(DG) of the hippocampus and Nissl staining (56˚C for 30 min) was
successfully optimized onto paraffin sections. Morphological
changes of neurons were detected using a light microscope
(magnifications, x40, x100 and x200).
Measurement of BDNF levels
Rats were decapitated and sacrificed following 50
mg/kg pentobarbital sodium anesthesia via intraperitoneal
injections. The hippocampus were removed,homogenized and
centrifuged at 10,000 x g at 4˚C for 10 min to obtain hippocampal
homogenates. BDNF levels in hippocampal homogenates were measured
using commercially available BDNF ELISA kits (cat. no. NI-1200;
Beijing North Institute of Biotechnology Co., Ltd.).
Western blotting
Hippocampus were rinsed twice with cold PBS and
dissolved on ice with a RIPA buffer containing 1 Mm
Phenylmethanesulfonyl fluoride (Promega Corporation) for 30 min,
followed by centrifugation at 12,000 x g for 10 min at 4˚C. Protein
concentrations were determinate using the BCA protein assay. Total
proteins (50 µg/well) were separated via 10% SDS-PAGE and
transferred via electrophoresis onto PVDF membranes. Membranes were
blocked with 5% skimmed milk at room temperature for 1 h and
incubated overnight at 4˚C with anti-PKA (1:1,500), anti-CREB
(1:600), anti-phosphorylated-CREB (p-CREB; 1:1,000), anti-BDNF
(1:1,200) and anti-actin (1:3,000). Actin was used as the loading
control. Subsequently, membranes were incubated with corresponding
secondary antibodies (1:1,000) at 37˚C for 2 h and reactions were
visualized with chemiluminescence reagents provided by an ECL kit
(Bioworld Technology, Inc.) and exposed to X-ray film. Blot
intensities were quantified with densitometry by Quantity One
v4.6.6 (Bio-Rad Laboratories).
Statistical analysis
Paired T-tests and one-way ANOVA followed by
Bonferroni's correction were performed to compare differences
between groups. SAS software (Wuhan Oriental Seth Software Co.,
Ltd.) was used for statistical analysis. Data are presented as mean
± standard error of the mean. P<0.05 was considered to indicate
a statistically significant difference.
Results
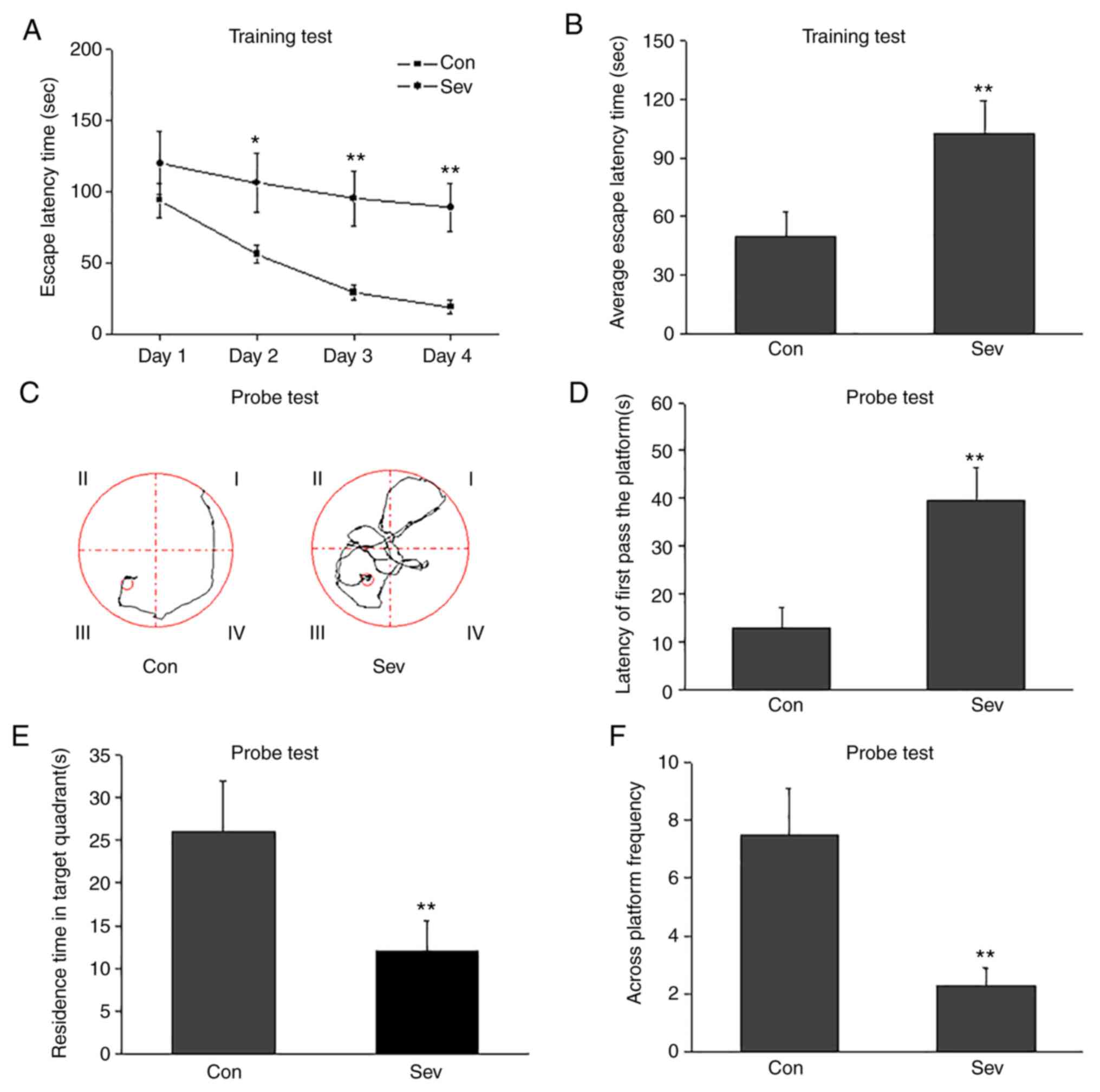
Sev induces cognitive impairment
Morris water maze tests were used to evaluate the
effect of Sev on cognition. In the training tests, escape latency
times were significantly increased following repeated exposure to
Sev (Fig. 2A and B). In the probe tests, Sev significantly
increased latency to first pass the platform (Fig. 2C and D) and decreased residence times in target
quadrants (Fig. 2E) and across
platform frequencies (Fig. 2F).
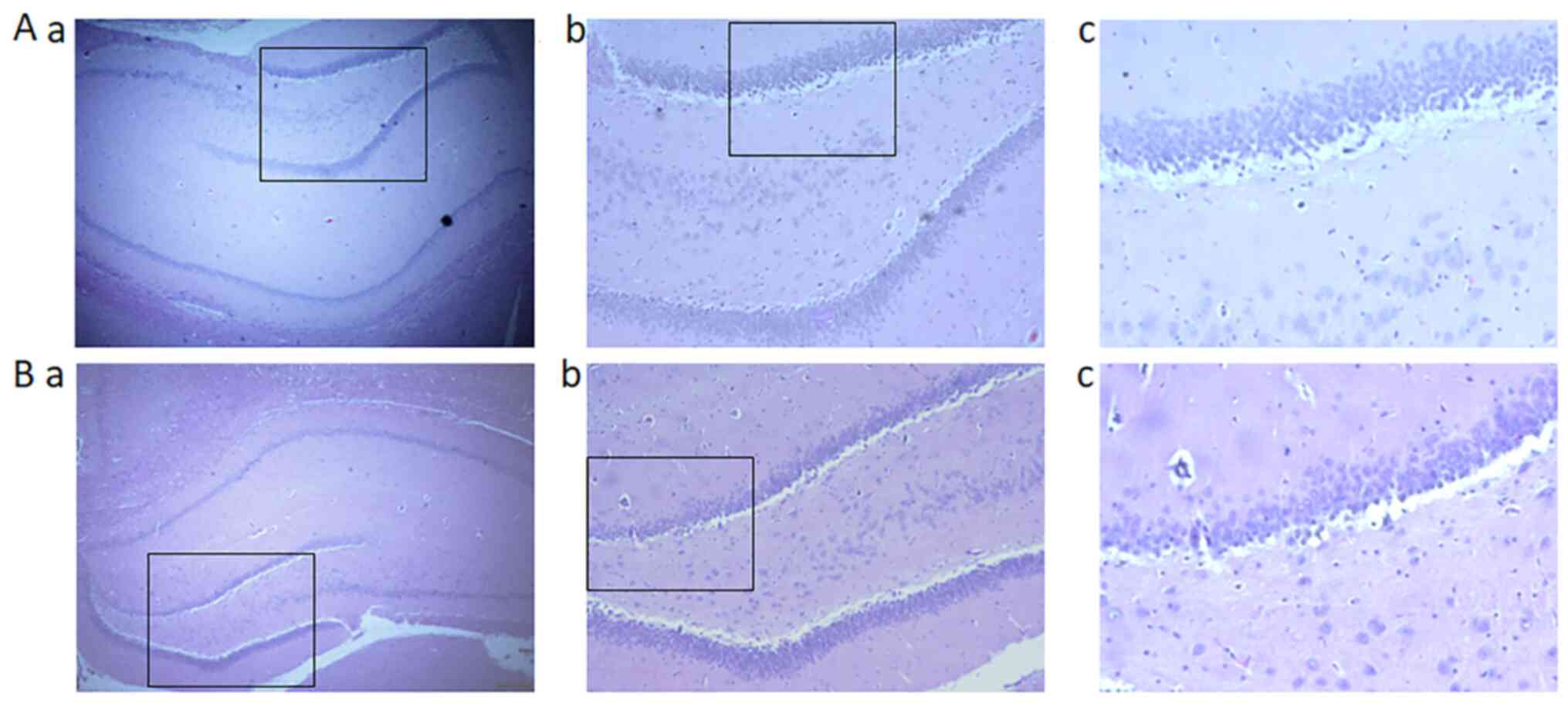
Sev reduces neuron numbers in the DG
of hippocampus
Nissl staining was used to examine the numbers of DG
neurons. In the control group, granule neurons exhibited round
nuclei, which werelocated in the center of the perikaryon and
surrounded by a pale cytoplasm (Fig.
3A). Decreased numbers of granule neurons and vacuoles were
observed in the Sev group (Fig.
3B).
Sev decreases BDNF levels and inhibits
PKA/CREB/BDNF signaling in the hippocampus
The level of hippocampal BDNF was measured via
ELISA.BDNF levels were significantly decreased following repeated
exposure to Sev (Fig. 4A). BDNF
levels in the control group were7.2±1.6 pg/mg protein, which was
significantly higher compared with the Sev-treated group at 5.9±0.8
pg/mg protein.
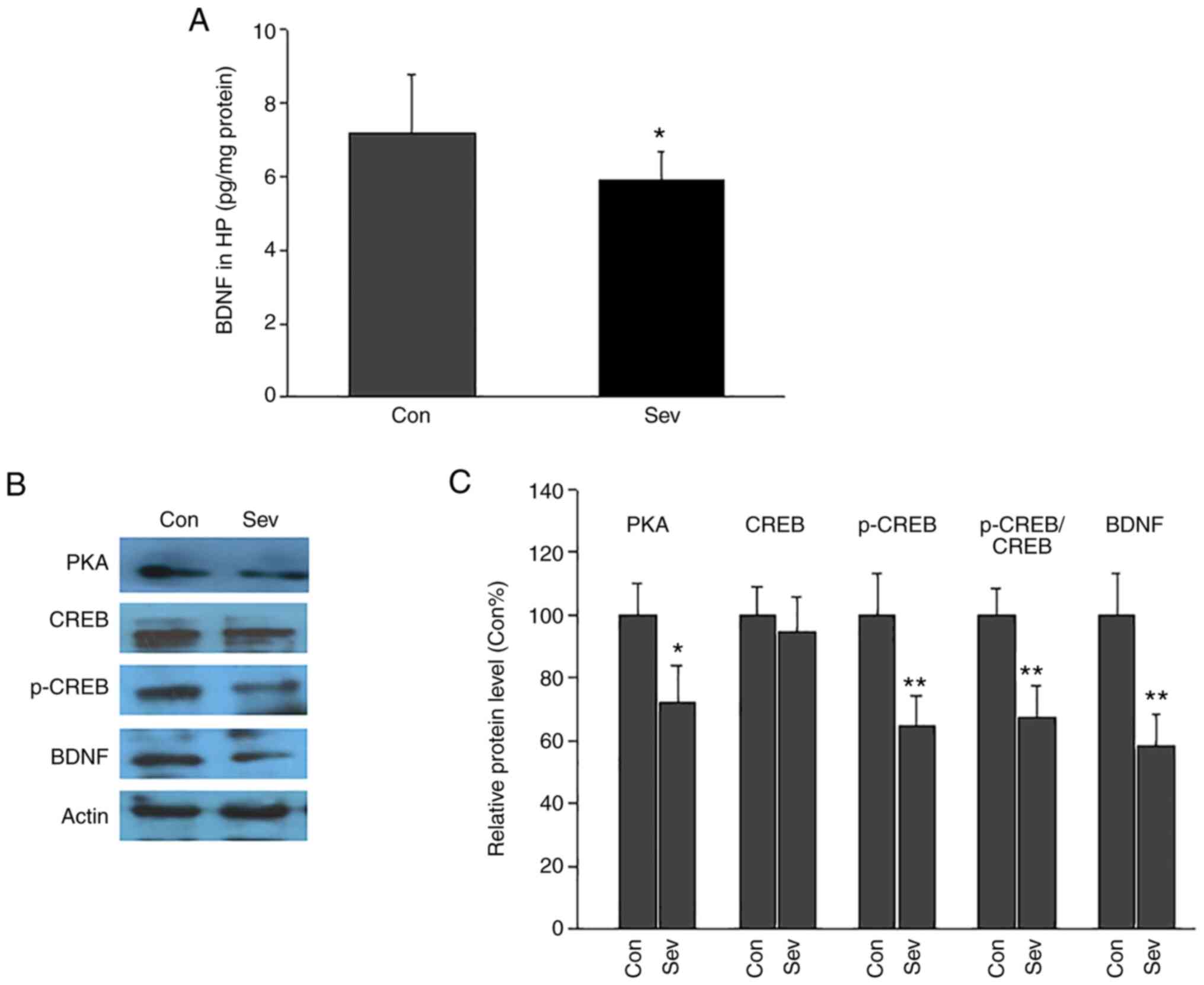 | Figure 4Effect of repeated neonatal exposure
to Sev on hippocampal BDNF levels and PKA-CREB-BDNF signaling in
adult rats. (A) BDNF levels in hippocampus in Con and Sev group.
Repeated Sev exposure significantly decreased hippocampal BDNF
levels. (B) Representative western blotting images of PKA, CREB,
p-CREB and BDNF. (C) Semi-quantitative analysis of the western
blotting results. Expression of PKA, p-CREB, BDNF and the
p-CREB/CREB ratio was decreased in Sev group compared with the
control group. n=5/group. *P<0.05;
**P<0.01 vs. Con. Sev, sevoflurane; BDNF,
brain-derived neurotrophic factor; PKA, protein kinase A; CREB,
cAMP response element binding protein; p-, phosphorylated; Con,
control; HP, hippocampus. |
Expression of PKA, CREB, p-CREB and BDNF in the
hippocampus was quantified via western blotting (Fig. 4B and C). The relative protein levels of PKA,
p-CREB and BDNF in Sev group were significantly decreased compared
with the Con group. CREB expression was not significantly different
between the Con and Sev groups. Furthermore, the p-CREB/CREB ratio
was calculated. The p-CREB/CREB ratio in Sev group was lower than
that in the Con group. These results demonstrated that Sev
inhibited the activation of the PKA/CREB/BDNF signaling
pathway.
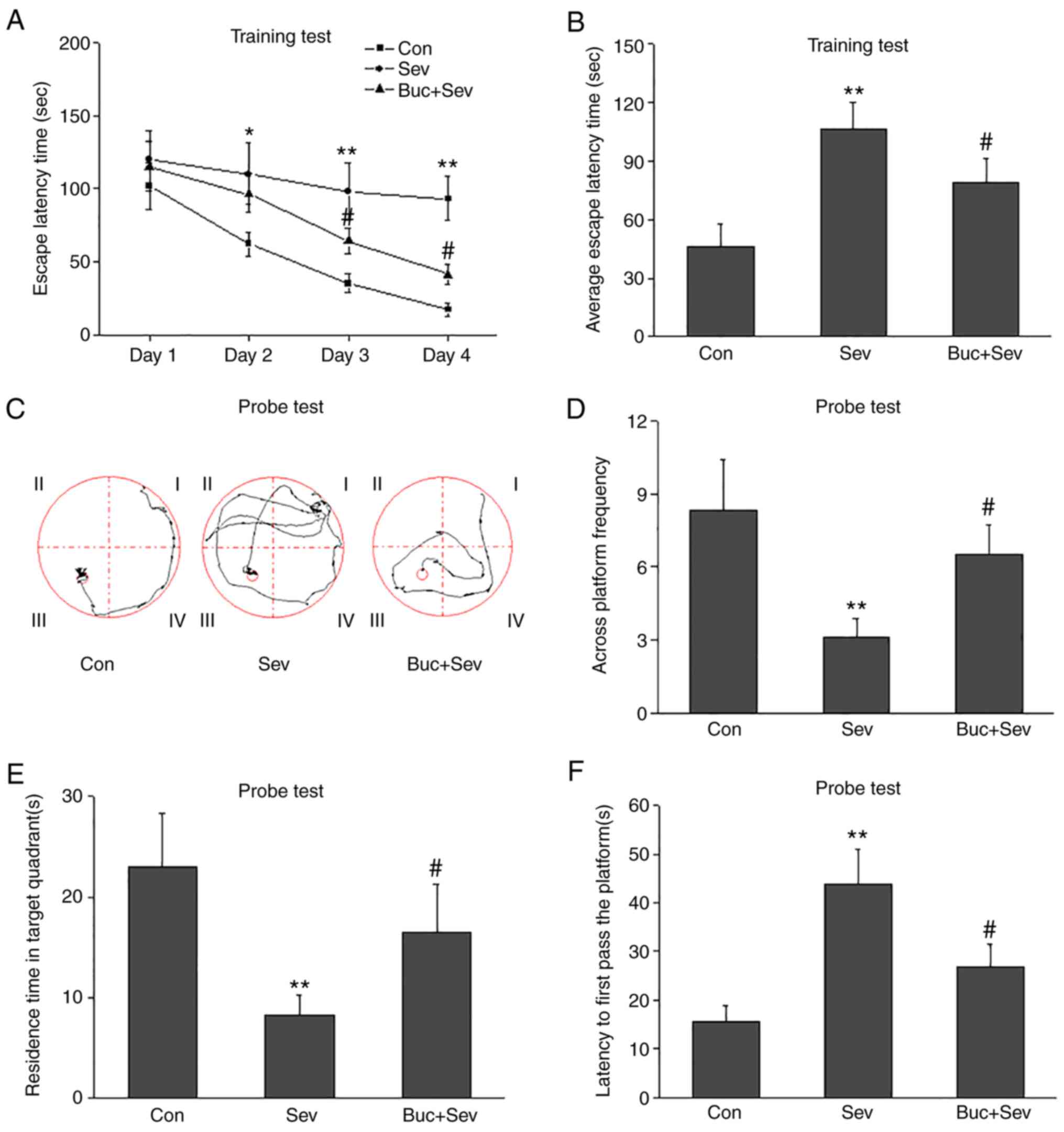
Activation of PKA-CREB signaling
improves cognition and restores BDNF levels
To examine the underlying pathogenesis caused by
Sev, rats were treated with the PKA-selective agonist Buc.
Cognition was evaluated using Morris water maze tests. In the
training tests, escape latency times (Fig. 5A) and the average escape latency
times (Fig. 5B) were significantly
decreased in Buc-treated rats compared with only Sev-treated rats.
In probe tests, the across platform frequency (Fig. 5C and D) and residence time in target quadrants
(Fig. 5E) were significantly
increased in Buc-treated rats compared with only Sev-treated rats.
Furthermore, the latency to first pass the platform was
significantly decreased in Buc-treated rats compared with rats
treated solely with Sev (Fig.
5F).
Additionally, the effect of Buc on expression of
PKA, CREB, p-CREB and BDNF was examined via western blotting. Buc
activated PKA/CREB/BDNF signaling. Following the administration of
Buc, the expression of PKA, p-CREB and BDNF in the hippocampus were
notably increased compared with rats treated solely with Sev
(Fig. 6A and B). CREB expression was not significantly
different between the groups. These results indicated that the
cognitive impairment caused by Sev was dependent on the
PKA/CREB/BDNF pathway.
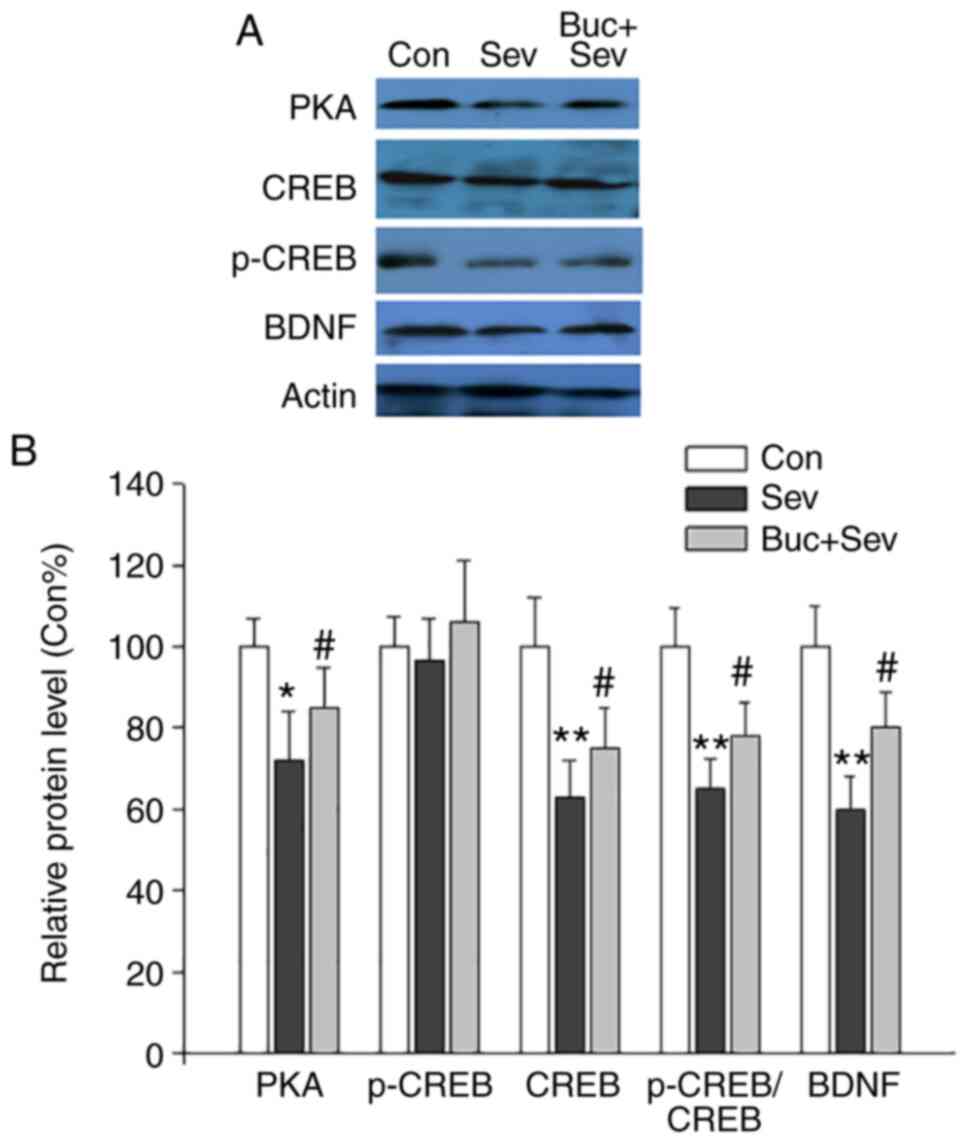 | Figure 6Buc activates the PKA-CREB-BDNF
signaling pathway. (A) Representative western blotting images of
PKA, CREB, p-CREB and BDNF. (B) Semi-quantitative analysis of the
western blotting results. Buc upregulated the protein expression of
PKA, p-CREB and BDNF and the ratio of p-CREB/CREB. n=5/group.
*P<0.05; **P<0.01 vs. Con;
#P<0.05 vs. Sev. Buc, bucladesine; PKA, protein
kinase A; CREB, cAMP response element binding protein; p,
phosphorylated; BDNF, brain-derived neurotrophic factor; Con,
control; Sev, sevoflurane. |
Discussion
Sev is a commonly used inhalational anesthetic in
pediatric surgery and exhibits minimal airway reactivity and a low
blood/gas partition coefficient (2). Although Sev has various advantages, it
is crucial to elucidate its effects on brain development,
particularly on safety issues arising from the use of anesthesia in
children (20). The current study
evaluated the effect of Sev on cognition in rats. The main finding
of the current study was that repeated neonatal exposure to Sev
induced cognitive impairment in adulthood. The results demonstrated
that hippocampal BDNF levels were significantly decreased in rats
treated with Sev, with the loss of granule neurons and inhibition
of the PKA/CREB/BDNF signaling pathway. Cognitive impairment caused
by Sev was partially reversed by the activation of the
PKA/CREB/BDNF signaling pathway following the administration of the
PKA agonist Buc (21). These
results indicated that cognitive deficiencies caused by Sev may be
restored by activation of the PKA/CREB/BDNF pathway.
Rats were treated at P6-8 days to investigate
whether repeated neonatal exposure to Sev caused cognitive
dysfunction in adult rats. The results revealed that Sev treatment
in newborn rats resulted in behavioral changes in later life, as
demonstrated by the Morris water maze tests. Repeated exposure to
Sev caused cognitive impairment in the training and probe
tests.
The DG of the hippocampus is known to serve an
important role in cognitive function (22). The results demonstrated that
repeated exposure to Sev reduced the numbers of granule cells in
the DG. These reductions in cell numbers may be associated with
BDNF expression. BDNF is expressed in multiple areas of the brain
in mammals and is critical for neuronal survival, plasticity and
morphogenesis (23). The binding of
BDNF to tropomyosin receptor kinase B (TrkB) is known to activate
multiple intracellular signaling pathways (24). In cultured neurons, sustained TrkB
activation promotes neuronal dendritic arborization and
spinogenesis, whereas transient TrkB activation facilitates
dendritic growth and spine morphogenesis. In hippocampal slices,
slow delivery of BDNF facilitates LTP, whereas fast application of
BDNF enhances basal synaptic transmission in Schaffer collateral
synapses. High-frequency stimulation of neurons converts
BDNF-induced TrkB signaling from a transient to a sustained mode
(25). Furthermore, Sev has been
reported to down-regulate BDNF/TrkB signaling in neonatal mice
(26).
CREB is a component of multiple intracellular
signaling pathways and serves an important role in the nervous
system. CREB modulates transcription factors via phosphorylation
(27) and CREB signaling in the
hippocampus is associated with emotional and cognitive behaviors
(28). The phosphorylation of CREB
by PKA serves a role in various nervous system diseases such as
Alzheimer's disease and craniocerebral trauma (29,30),
and BDNF is a downstream target of PKA/CREB signaling (31). Several studies have reported that
BDNF down-regulation contributes to structural damage and
functional impairment in the central nervous system (32-35).
The results of the current study revealed that the expression of
PKA, p-CREB and BDNF was down-regulated in the hippocampus
following Sev treatment. It has been hypothesized that the
activation of PKA/CREB/BDNF signaling pathways may improve
cognition (36). Therefore, rats
were treated with Buc, a selective PKA agonist (20), and the results demonstrated that the
activation of PKA significantly improved cognition in Sev-treated
rats. Furthermore, when Buc was administered to rats, the
expression of PKA, p-CREB and BDNF in the hippocampus was increased
compared with rats treated only with Sev. In conclusion, the
results of the current study indicated that Sev induces cognitive
impairment in rats via the PKA-CREB-BDNF signaling pathway.
Acknowledgements
Not applicable.
Funding
This study was financially supported by Key Research and
Development Projects in Shandong (grant no. 2018GSF118216) and
Development Plan of Medical and Health Science and Technology in
Shandong (grant no. 2017WS524).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, JR, SL, YG and PM made substantial contributions
to the conception and design of the study, as well as the
acquisition and analysis of data. HT and YC confirm the
authenticity of all the raw data, conceived the study, performed
the experiments, drafted the manuscript, revised it critically for
important intellectual content and provided final approval of the
version to be published. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Animal
Experimental Ethical Inspection of Laboratory Animal Center,
Zhangqiu District Maternal and Child Health Care Hospital (Jinan
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim EH, Song IK, Lee JH, Kim HS, Kim HC,
Yoon SH, Jang YE and Kim JT: Desflurane versus sevoflurane in
pediatric anesthesia with a laryngeal mask airway: A randomized
controlled trial. Medicine (Baltimore). 96(e7977)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Noga ML, Yarr JE and Chen PE: Evaluation
of sevoflurane as an anesthetic agent for voiding
cystourethrography in pediatric patients. Can Assoc Radiol J.
63:222–227. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Satomoto M, Sun Z, Adachi YU and Makita K:
Neonatal sevoflurane exposure induces adulthood fear-induced
learning disability and decreases glutamatergic neurons in the
basolateral amygdala. J Neurosurg Anesthesiol. 30:59–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Karaman T, Karaman S, Doğru S, Tapar H,
Şahin A and Süren M: Short-term and long-term effects of
dexamethasone on cognitive dysfunction induced by sevoflurane in
adult rats. Turk J Anaesthesiol Reanim. 45:158–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun GY, Xie K, Sun ZY, Sun MY and Li N:
Sevoflurane induces temporary spatial working memory deficits and
synaptic ultrastructure impairments in the hippocampus of neonatal
rats. Eur Rev Med Pharmacol Sci. 23:2620–2629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng SQ, An LX, Cheng X and Wang YJ:
Sevoflurane causes neuronal apoptosis and adaptability changes of
neonatal rats. Acta Anaesthesiol Scand. 57:1167–1174.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Andropoulos DB: Effect of anesthesia on
the developing brain: Infant and fetus. Fetal Diagn Ther. 43:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Popova NK, Ilchibaeva TV and Naumenko VS:
Neurotrophic factors (BDNF and GDNF) and the serotonergic system of
the brain. Biochemistry (Mosc). 82:308–317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leal G, Bramham CR and Duarte CB: BDNF and
hippocampal synaptic plasticity. Vitam Horm. 104:153–195.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tao X, Finkbeiner S, Arnold DB, Shaywitz
AJ and Greenberg ME: Ca2+ influx regulates BDNF
transcription by a CREB family transcription factor-dependent
mechanism. Neuron. 20:709–726. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye
J, Wu D, Fang L, Pi G, Yang Y, et al: Tau accumulation induces
synaptic impairment and memory deficit by calcineurin-mediated
inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci
USA. 113:E3773–E3781. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
West AE, Chen WG, Dalva MB, Dolmetsch RE,
Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X and Greenberg ME:
Calcium regulation of neuronal gene expression. Proc Natl Acad Sci
USA. 98:11024–11031. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharma N, Lopez DI and Nyborg JK: DNA
binding and phosphorylation induce conformational alterations in
the kinase-inducible domain of CREB. Implications for the mechanism
of transcription function. J Biol Chem. 282:19872–19883.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo Y and Feng P: OX2R activation induces
PKC-mediated ERK and CREB phosphorylation. Exp Cell Res.
318:2004–2013. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Al Rahim M, Nakajima A, Saigusa D, Tetsu
N, Maruyama Y, Shibuya M, Yamakoshi H, Tomioka Y, Iwabuchi Y,
Ohizumi Y and Yamakuni T: 4'-Demethylnobiletin, a bioactive
metabolite of nobiletin enhancing PKA/ERK/CREB signaling, rescues
learning impairment associated with NMDA receptor antagonism via
stimulation of the ERK cascade. Biochemistry. 48:7713–7721.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bi C, Cai Q, Shan Y, Yang F, Sun S, Wu X
and Liu H: Sevoflurane induces neurotoxicity in the developing rat
hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1
pathway. Biomed Pharmacother. 108:1469–1476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharifzadeh M, Zamanian AR, Gholizadeh S,
Tabrizian K, Etminani M, Khalaj S, Zarrindast MR and Roghani A:
Post-training intrahippocampal infusion of nicotine-bucladesine
combination causes a synergistic enhancement effect on spatial
memory retention in rats. Eur J Pharmacol. 562:212–220.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hosseini-Zare MS, Salehi F, Seyedi SY,
Azami K, Ghadiri T, Mobasseri M, Gholizadeh S, Beyer C and
Sharifzadeh M: Effects of pentoxifylline and H-89 on epileptogenic
activity of bucladesine in pentylenetetrazol-treated mice. Eur J
Pharmacol. 670:464–470. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang Y, Wang L, Wu Y, Su D, Wang N, Wang
J, Shi C, Lv L and Zhang S: Tanshinol suppresses inflammatory
factors in a rat model of vascular dementia and protects
LPS-treated neurons via the MST1-FOXO3 signaling pathway. Brain
Res. 1646:304–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu M, Han C, Zhou Q, Liu C and Ding Z:
Clinical effects of sevoflurane anesthesia induction with a
portable inhalational anesthetic circuit in pediatric patients.
Arch Med Sci. 11:796–800. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Salehi F, Hosseini-Zare MS, Aghajani H,
Seyedi SY, Hosseini-Zare MS and Sharifzadeh M: Effect of
bucladesine, pentoxifylline, and H-89 as cyclic adenosine
monophosphate analog, phosphodiesterase, and protein kinase A
inhibitor on acute pain. Fundam Clin Pharmacol. 31:411–419.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brickman AM, Khan UA, Provenzano FA, Yeung
LK, Suzuki W, Schroeter H, Wall M, Sloan RP and Small SA: Enhancing
dentate gyrus function with dietary flavanols improves cognition in
older adults. Nat Neurosci. 17:1798–1803. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Numakawa T, Suzuki S, Kumamaru E, Adachi
N, Richards M and Kunugi H: BDNF function and intracellular
signaling in neurons. Histol Histopathol. 25:237–258.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Andero R, Choi DC and Ressler KJ:
BDNF-TrkB receptor regulation of distributed adult neural
plasticity, memory formation, and psychiatric disorders. Prog Mol
Biol Transl Sci. 122:169–192. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo W, Nagappan G and Lu B: Differential
effects of transient and sustained activation of BDNF-TrkB
signaling. Dev Neurobiol. 78:647–659. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ding ML, Ma H, Man YG and Lv HY:
Protective effects of a green tea polyphenol,
epigallocatechin-3-gallate, against sevoflurane-induced neuronal
apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR
signalling pathways in neonatal mice. Can J Physiol Pharmacol.
95:1396–1405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, Hu XH, Huang ZX, Nie Q, Chen ZG,
Xiang JW, Qi RL, Yang TH, Xiao Y, Qing WJ, et al: Regulation of
CREB functions by phosphorylation and sumoylation in nervous and
visual systems. Curr Mol Med. 16:885–892. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dong W, Xu D, Hu Z, He X, Guo Z, Jiao Z,
Yu Y and Wang H: Low-functional programming of the CREB/BDNF/TrkB
pathway mediates cognitive impairment in male offspring after
prenatal dexamethasone exposure. Toxicol Lett. 283:1–12.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo Y, Kuang S, Li H, Ran D and Yang J:
cAMP/PKA-CREB-BDNF signaling pathway in hippocampus mediates
cyclooxygenase 2-induced learning/memory deficits of rats subjected
to chronic unpredictable mild stress. Oncotarget. 8:35558–35572.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu H, Jin X, Yin X, Jin N, Liu F and Qian
W: PKA-CREB signaling suppresses tau transcription. J Alzheimers
Dis. 46:239–248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Han C, Yang Y, Ruan S, Guo L, Zhang X and
Guan Q: The predictive value of serum p-CREB level on secondary
cognitive impairment in patients with mild-to-moderate
craniocerebral trauma. Neurosurg Rev. 42:715–720. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rosa E and Fahnestock M: CREB expression
mediates amyloid β-induced basal BDNF downregulation. Neurobiol
Aging. 36:2406–2413. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu B, Nagappan G and Lu Y: BDNF and
synaptic plasticity, cognitive function, and dysfunction. Handb Exp
Pharmacol. 220:223–250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rahmani M, Rahmani F and Rezaei N: The
brain-derived neurotrophic factor: Missing link between sleep
deprivation, insomnia, and depression. Neurochem Res. 45:221–231.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang R and Holsinger RMD: Exercise-induced
brain-derived neurotrophic factor expression: Therapeutic
implications for Alzheimer's dementia. Ageing Res Rev. 48:109–121.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shin MS, Kim TW, Park SS, Ko IG, Kim CJ,
Kim M, Roh SY, Kim KT and Kim KH: Long-term surgical and chemical
castration deteriorates memory function through downregulation of
PKA/CREB/BDNF and c-Raf/MEK/ERK pathways in hippocampus. Int
Neurourol J. 23:116–124. 2019.PubMed/NCBI View Article : Google Scholar
|