Introduction
Retinoblastoma (RB) typically occurs in pediatric
patients under the age of 6 years and is the most common type of
childhood intraocular malignancy (1). RB is an uncommon pediatric cancer,
with a global incidence of 1 in every 15,000-18,000 live births
(2,3). In recent years, with earlier diagnosis
and the development of multimodal treatments, the survival rates of
patients with RB have markedly improved (4). However, numerous survivors of RB
experience blindness or eye loss. Furthermore, individuals with
hereditary RB continue to have an increased risk of developing
metastases, trilateral RB and secondary malignant neoplasms, which
leads to high mortality rates (2,5).
Therefore, it is essential to identify novel specific markers for
RB, elucidate its molecular mechanisms and develop better, targeted
therapeutic strategies for RB.
Long noncoding RNAs (lncRNAs) are a class of
mRNA-like transcripts that are longer than 200 nucleotides and lack
protein-coding capability (6). In
the past decade, lncRNAs have been implicated in the pathogenesis
of a variety of diseases and biological processes, such as
metabolic disorders, tumorigenesis, cellular differentiation,
X-chromosome imprinting and even metastasis (7-9).
Furthermore, lncRNAs are widely expressed in various cancer tissues
and may be exploited as diagnostic or prognostic indicators for
various cancer types, including RB (10-12).
However, the full lncRNA expression profile in RB has not been
determined and the detailed lncRNA regulatory mechanisms in RB have
remained elusive. The present study aimed to explore the lncRNA
expression profile in RB and identify mechanistically relevant
lncRNAs by bioinformatics analysis of the Gene Expression Omnibus
(GEO) dataset GSE111168, which may allow for the identification of
novel biomarkers for the diagnosis and treatment of RB.
Materials and methods
Microarray data
The gene expression dataset GSE111168 was downloaded
from GEO (http://www.ncbi.nlm.nih.gov/geo) (13). The dataset includes the mRNA
expression profiles of three RB samples and three adjacent normal
tissue samples determined using the GeneChip Human Genome U133 Plus
2.0 platform (Affymetrix; Thermo Fisher Scientific, Inc.). The
annotation files were downloaded from the platform.
Data preprocessing and determination
of differential expression
Based on the annotation information, the probe
levels were converted into lncRNA or mRNA expression values and
preprocessed using log2 transformation and Z-score
normalization in R software 3.6 (R Core Team) for each study.
Thereafter, the differentially expressed RNAs in the RB and healthy
retina samples were selected using the linear models for microarray
analysis package in R (Affymetrix; Thermo Fisher Scientific, Inc.;
http://www.affymetrix.com/analysis/)
(14). The cutoff values for the
differentially expressed lncRNAs (DELs) and differentially
expressed mRNAs (DEMs) were P<0.01 and
|log2(fold-change)|≥2.
Functional enrichment analysis
To predict potential biological functions of
DEL-targeted and other DEMs in RB, Gene Ontology (GO) analysis
(www.geneontology.org) was performed
using GO Association tools (www.github.com/tanghaibao/GOatools). Functional
annotations were performed and terms in the categories biological
process, cellular component or molecular function were determined.
To predict the signaling pathways in which DEL-targeted and other
DEMs participated, the Kyoto Encyclopedia of Genes and Genomes
(KEGG) Orthology-Based Annotation System was used and KEGG
(www.genome.ad.jp/kegg) pathway
enrichment analysis was performed. The GO and KEGG items with
P<0.05 were considered statistically significant.
DEL-DEM coexpression network
analysis
Weighted correlation network analysis (www.genetics.ucla.edu/labs/horvath/CoexpressionNetwork/Rpackages/WGCNA)
was used to construct the coexpression network of DELs and DEMs. In
brief, Pearson's correlation coefficients (PCCs) for comparisons
between random single DELs and DEMs were calculated. The DEL-DEM
pairs were filtered for network construction using PCCs; PCCs ≥0.99
were considered meaningful values. The b parameter was simulated to
weigh the network and a meaningful biological coexpression network
was established. Cytoscape (http://cytoscape.org/) software was used to visualize
the network.
Cell culture
The human retinal pigment epithelial cell line
ARPE19 (The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences) was cultured in Dulbecco's modified Eagle's
medium/F12 medium (Gibco; Thermo Fisher Scientific, Inc.). The RB
cell lines Y79, SO-RB50 (The Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences) and WERI-RB1 (Zhejiang Ruyao
Biotechnology Co., Ltd.) were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). The media were supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin and cells were incubated at 37˚C in a
humidified atmosphere with 5% CO2.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Complementary
DNA was generated by RT of total RNA using the PrimeScript RT
reagent kit (Takara Biotechnology, Co. Ltd) according to the
manufacturer's protocol. qPCR was performed using SYBR Premix Ex
Taq II (Takara Biotechnology, Co. Ltd) on a 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 45 cycles of 95˚C for 7
sec, 57˚C for 10 sec and 72˚C for 15 sec. Relative expression
levels of DELs were calculated using the 2-ΔΔCq method
(15) and GAPDH was used as an
internal control. The PCR primers used in the present study are
listed in Table SI.
Statistical analysis
RT-qPCR experiments were performed in triplicate and
the data are presented as the mean ± standard deviation. The
differences between two groups were analyzed using one-way ANOVAs
and Scheffe tests were performed for the post-hoc tests. P<0.05
was considered to indicate statistical significance.
Results
DELs and DEMs in RB
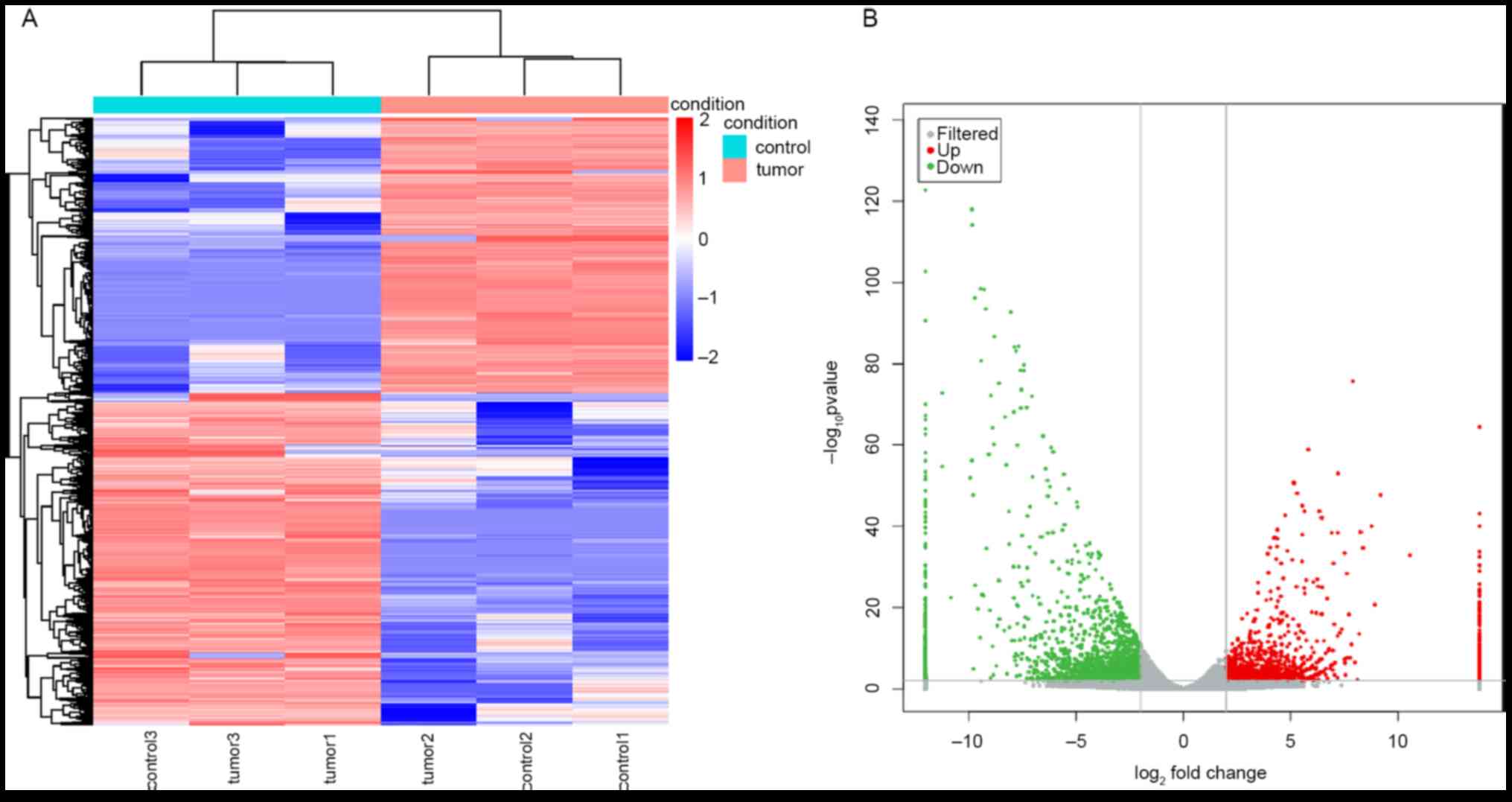
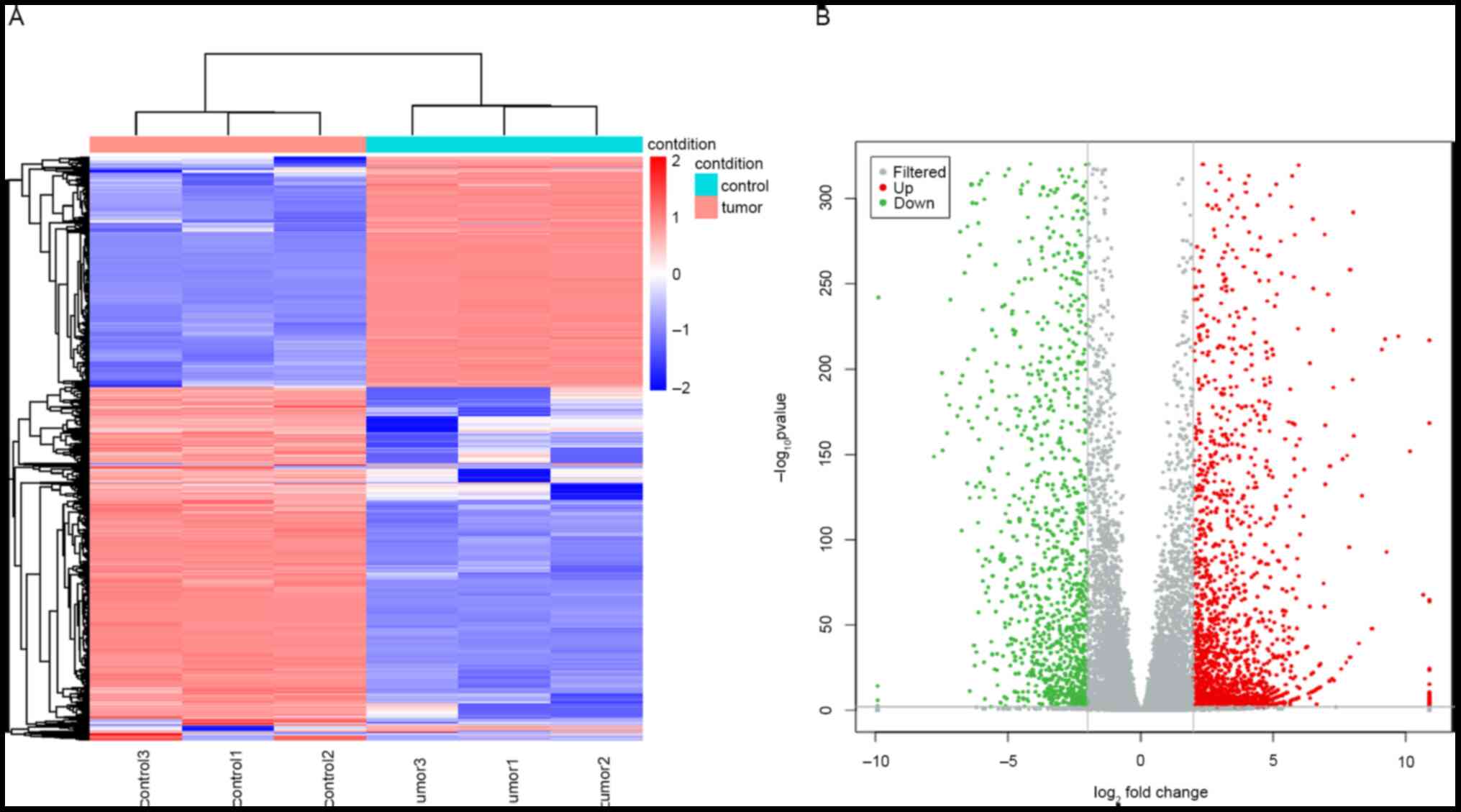
Simultaneous genome-wide analyses of lncRNA and mRNA
expression profiles were performed and 3,915 DELs and 3,715 DEMs
were identified between the RB and adjacent normal tissue samples.
Among the DELs, 1,774 were upregulated and 2,141 were downregulated
in RB tissues compared to those in healthy tissues (Fig. 1A and B). Among the DEMs, 1,492 were upregulated
and 2,223 were downregulated in RB tissues compared to those in
healthy tissues (Fig. 2A and
B). The top 10 up- and
downregulated DELs are listed in Table
I. TCONS_00008015 was the most significantly upregulated DEL
and lnc-LRRC59-3:3 was the most significantly downregulated
DEL.
 | Table ITop 10 most up and downregulated
mapped differentially expressed lncRNAs and mRNAs. |
Table I
Top 10 most up and downregulated
mapped differentially expressed lncRNAs and mRNAs.
| ID | Chromosome | Position |
log2FC | P-value |
|---|
| TCONS_00008015 | 12 |
116405401-116463531 | Inf |
3.61x10-27 |
| lnc-DAZ1-161:1 | Y |
56675831:56678602 | Inf |
3.07x10-23 |
| lnc-BICRA-12:1 | 19 |
47840709:47843109 | Inf |
1.44x10-21 |
| miR124-2HG:15 | 8 |
64379148:64382668 | Inf |
5.20x10-19 |
| lnc-NEPRO-36:2 | 3 |
114314500:114329714 | Inf |
8.59x10-18 |
| TCONS_00024597 | 3 |
32716474-32835950 | Inf |
2.75x10-17 |
|
lnc-OTUD7A-24:1 | 15 |
32765545:32767369 | Inf |
2.41x10-16 |
| lnc-HDAC7-21:1 | 12 |
48688463:48692507 | Inf |
1.48x10-15 |
|
lnc-CCDC39-11:1 | 3 |
179900672:180037035 | Inf |
2.15x10-15 |
|
Lnc-CCDC85C-26:2 | 14 |
100361702:100375473 | Inf |
2.33x10-15 |
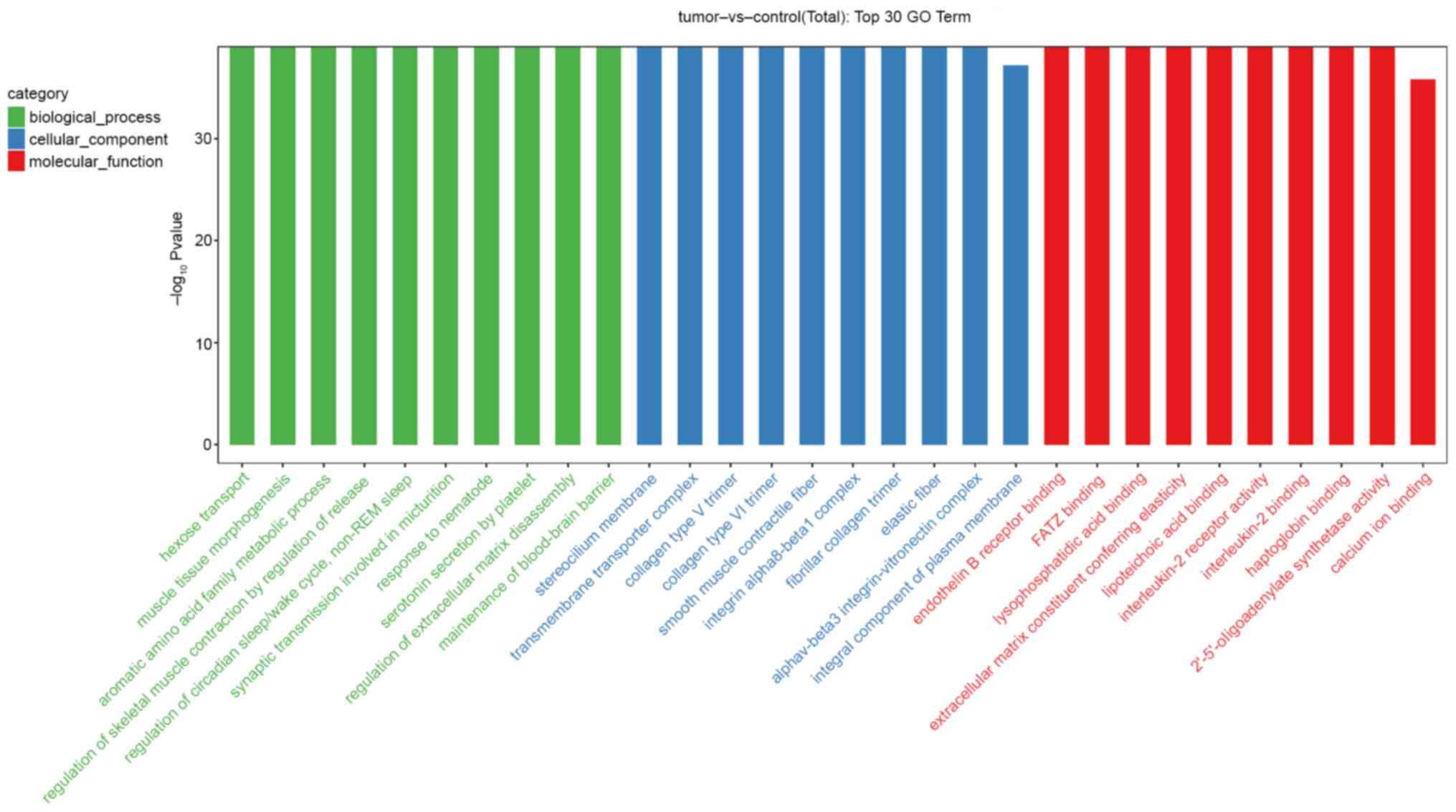
Functional and pathway enrichment
analyses
To explore potential biological functions and
signaling pathways associated with the DEL-targeted DEMs, GO and
KEGG enrichment analyses were performed. The results indicated that
the DEL-targeted DEMs were highly enriched by genes associated with
hexose transport (GO:0008645, biological process), muscle tissue
morphogenesis (GO:0060415, biological process), the stereocilium
membrane (GO:0060171, cellular component), endothelin B receptor
binding (GO:0031708, molecular function) and γ-filamin/ABP-L,
α-actinin and telethonin binding protein of the Z-disc binding
(GO:0051373, molecular function) (Fig.
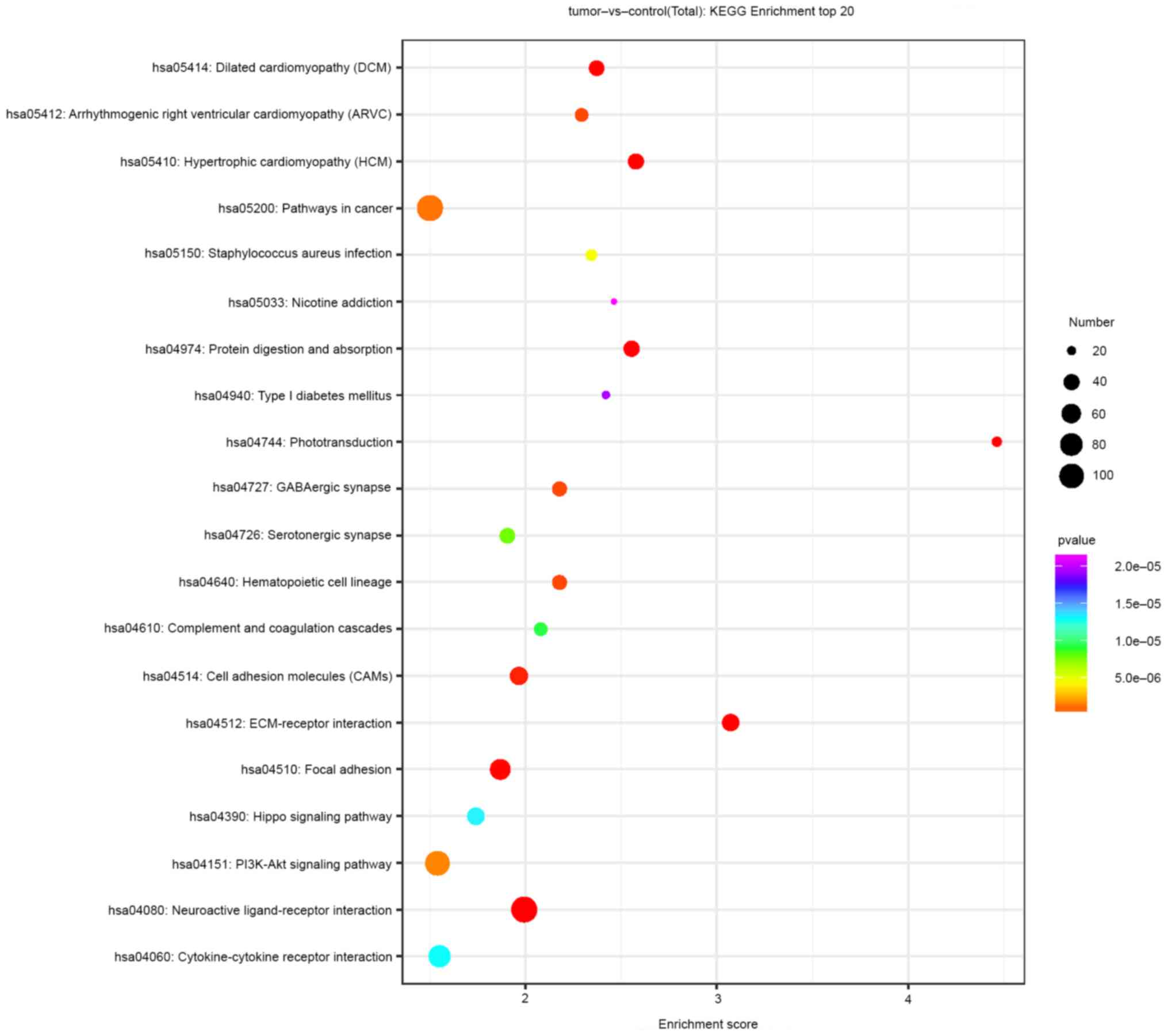
3). The KEGG pathway analysis suggested that the DEL-targeted
DEMs were most enriched in pathways such as the PI3K/AKT, Hippo and
cancer pathways, as well as extracellular matrix (ECM)-receptor
interaction and neuroactive ligand-receptor interaction pathways
(Fig. 4).
DEL-DEM coexpression network
analysis
Next, a DEL-DEM coexpression network was constructed
to investigate the potential roles of lncRNAs in RB. The
coexpression network consisted of 152 DELs and 285 DEMs and
comprised 671 nodes (Table SII).
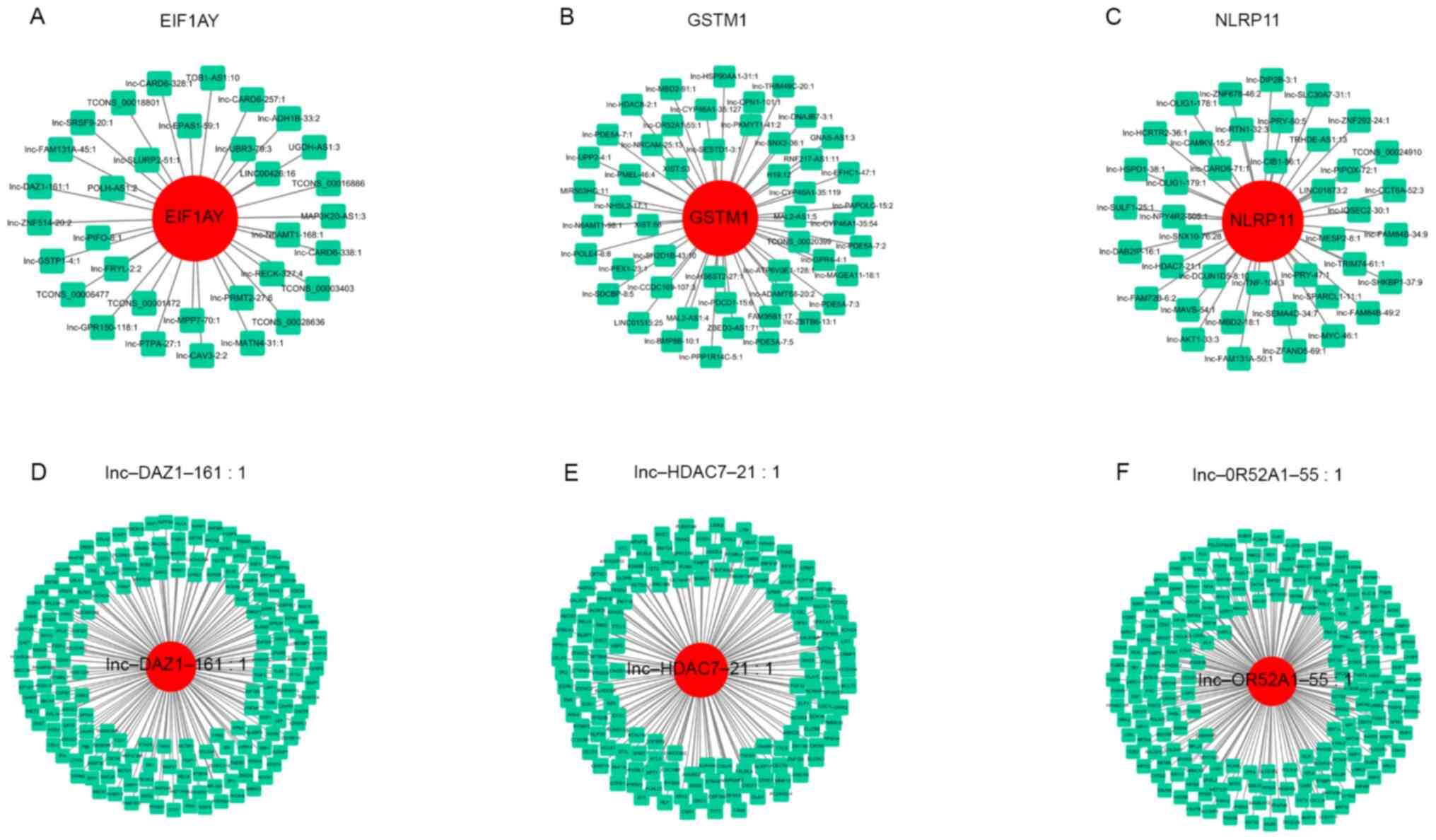
The top three DEMs, namely, EIF1AY, GSTM1 and NLRP11, formed
coexpression modules with 33, 50 and 41 DELs, respectively
(Fig. 5A-C). The top three DELs,
namely, lnc-DAZ1-161, lnc-HDAC7-21 and lnc-OR52A1-55, formed
coexpression modules with 181, 156 and 210 DEMs, respectively
(Fig. 5D-F). The expression of each
DEL correlated with that of several DEMs and the expression of each
DEM correlated with that of multiple DELs.
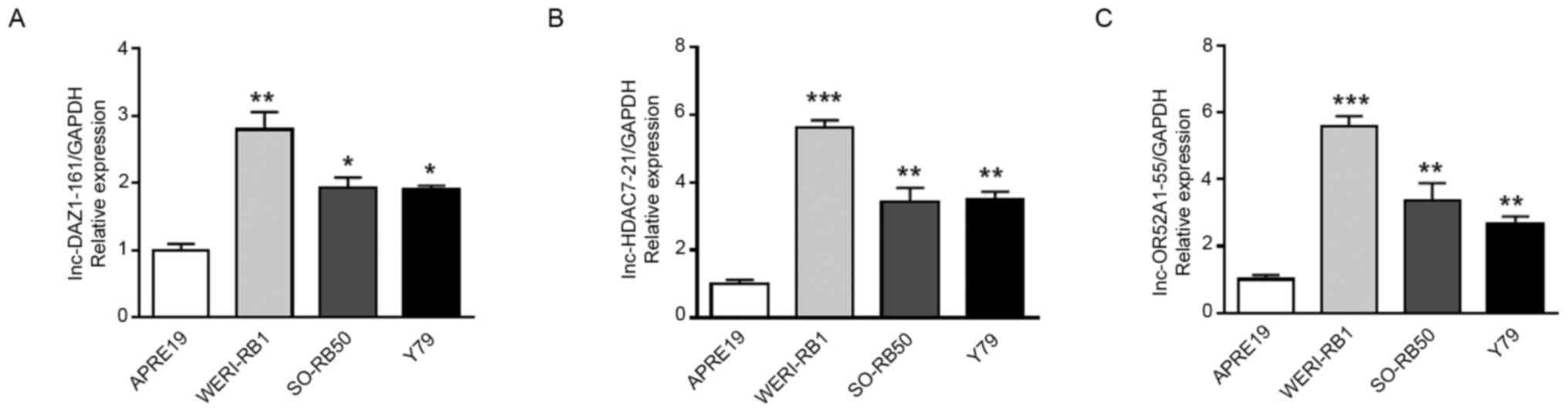
RT-qPCR validation of DELs
To validate the accuracy of the microarray data from
the GEO database, RT-qPCR was used to evaluate the expression
levels of lnc-DAZ1-161, lnc-HDAC7-21 and lnc-OR52A1-55 in human
retinal pigment epithelial cells and RB cells. Among these lncRNAs,
lnc-DAZ1-161 and lnc-HDAC7-21 were the top upregulated DELs and
lnc-OR52A1-55 was a downregulated DEL, as obtained through
bioinformatics analysis. Both lnc-DAZ1-161 and lnc-HDAC7-21 were
significantly overexpressed in RB cells, which was consistent with
their expression pattern in the database (Fig. 6A and B). However, lnc-OR52A1-55 was also
significantly overexpressed in RB cells, which was inconsistent
with its expression pattern in the database (Fig. 6C).
Discussion
RB is the leading global pediatrics eye malignancy
based on the mortality rate of patients (16); therefore, its underlying molecular
mechanisms have been widely investigated and the treatment of RB
has evolved significantly in the past half-century (4). However, as a rare childhood cancer,
the pathogenesis of RB has remained largely elusive. Recent studies
have demonstrated that numerous lncRNAs are dysregulated in RB and
drive its development and progression. Hu et al (7) reported that the lncRNA XIST promoted
RB progression, in part by modulating the miR-124/STAT3 axis. Shang
(11) indicated that the lncRNA
THOR was upregulated in RB, acting as an oncogene by enhancing the
expression of the MYC mRNA and IGF2BP1 protein. Yang and Peng
(17) demonstrated that ANRIL
depletion suppressed RB progression by activating the ATM/E2F1
signaling pathway. However, comprehensive analyses of lncRNA and
related mRNA expression profiles, as well as potential biological
functions of these RNAs in RB, have not been previously reported,
to the best of our knowledge. In the present study, a comprehensive
analysis of GEO datasets was performed to identify lncRNAs and
mRNAs that are potentially involved in the pathogenesis of RB. A
total of 1,774 upregulated DELs, 2,141 downregulated DELs, 1,492
upregulated DEMs and 2,223 downregulated DEMs were identified
between the RB and para-tumor samples, which comprise the
lncRNA/mRNA profile of RB.
lncRNAs mainly participate in various biological
processes by regulating their target mRNAs. To gain insight into
potential functions of the DELs in RB, GO and KEGG analyses of the
pathways that were most highly represented among the differentially
regulated RNAs were performed. The results indicated that the
highly differentially expressed RNAs were commonly associated with
PI3K/AKT signaling, Hippo signaling, cancer pathways, ECM-receptor
interactions and neuroactive ligand-receptor interactions. The
PI3K/AKT signaling pathway has a central role in regulating
apoptosis, cell proliferation, metabolism and migration, as well as
in maintaining the biological characteristics of malignant cells,
affecting carcinogenesis and tumor progression (18). The PI3K/AKT signaling pathway also
has an important role in RB (19,20).
The Hippo signaling pathway is an evolutionarily conserved network
that promotes uncontrolled cell proliferation, impairs
differentiation and is associated with cancer (14). Furthermore, biologically active ECM
fragments are able to regulate tissue injury, remodeling and cell
growth and induce inflammatory responses, which are characteristic
of carcinogenesis; thus ECM-receptor interactions may have a role
in cancer progression (21-23).
The present results suggested that the potential functions of the
identified DELs were closely related to the development and
pathogenesis of RB.
The lncRNA and mRNA coexpression network consisted
of 671 nodes among 152 DELs and 285 DEMs. Of note, the top three
DELs, lnc-DAZ1-161, lnc-HDAC7-21 and lnc-OR52A1-55, have not been
previously studied. The network analysis indicated that
lnc-DAZ1-161 formed a coexpression module with 181 DEMs, one of
which, EIF1AY, was previously reported to be upregulated in dilated
ischemic cardiomyopathy and during neural differentiation (24,25).
However, the association of EIF1AY with RB has not been previously
reported. Thus, the possibility that lnc-DAZ1-161 has a role in RB
development by regulating EIF1AY expression requires further
investigation. Furthermore, lnc-HDAC7-21 formed a coexpression
module with 156 DEMs, including NLRP11. NLRP11, a primate-specific
member of the NOD-like receptor family, is highly expressed in the
testis, ovary, liver and immune cells (26). Upregulation of NLRP11 has pivotal
roles in attenuating Toll-like receptor signaling and preventing
the dysregulation of inflammatory responses (27). The possibility that lnc-HDAC7-21
regulates NLRP11 expression during RB development requires further
investigation. Furthermore, lnc-OR52A1-55 formed a coexpression
module with 210 DEMs. The null genotype of GSTM1, one of the
coexpressed mRNAs, alters enzymatic activity and exerts indirect
effects on the development of various cancer types (28,29).
In addition, the GSTM1 null genotype appears to be associated with
the risk of glaucoma (30). Further
research is required to elucidate the potential relationship
between lnc-OR52A1-55 and GSTM1 in RB.
Furthermore, RT-qPCR analysis was performed to
compare expression levels of the three representative lncRNAs in
human retinal pigment epithelial cells and RB cells. Among these
lncRNAs, lnc-DAZ1-161 and lnc-HDAC7-21 were the top upregulated
DELs in RB and were indicated to be significantly overexpressed in
RB cells. However, the expression levels of lnc-OR52A1-55 in the
cells were inconsistent with those in the tissue microarray data,
which may be due to an insignificant log2(fold-change)
of lnc-OR52A1-55 in the GEO database that was used in the present
study compared with other top deregulated genes. Thus, the three
lncRNAs require to be further studied in clinical RB tissues.
In conclusion, the present study used bioinformatics
analyses to determine the lncRNA and mRNA expression profiles
associated with RB. The potential pathological roles of the
aberrantly expressed lncRNAs and mRNAs were predicted using GO and
KEGG analyses. Furthermore, RT-qPCR validation confirmed that the
expression of certain representative lncRNAs in RB cells was
consistent with that in the tissue microarray database. These
results may contribute to the elucidation of the molecular
mechanisms of RB and provide novel targets for the development of
improved clinical therapies. However, further studies are required
to determine the biological functions of the dysregulated lncRNAs
in RB.
Supplementary Material
Primer sequences used for PCR.
Supplementary Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE111168).
The experimental data (PCR) used and/or analyzed during the current
study are available from the corresponding author on reasonable
request..
Authors' contributions
XF and FC designed the study and contributed to the
editing and proofreading of the manuscript. JG, QL, CX and JP
retrieved and processed the data. JG, RZ and LZ analyzed the data.
All authors read and approved the final manuscript. XF and FC
checked and approved the authenticity of the raw data in the
present study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H and Corson TW: Retinoblastoma,
the visible CNS tumor: A review. J Neurosci Res. 97:29–44.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tamboli D, Topham A, Singh N and Singh AD:
Retinoblastoma: A SEER dataset evaluation for treatment patterns,
survival, and second malignant neoplasms. Am J Ophthalmol.
160:953–958. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rao R and Honavar SG: Retinoblastoma.
Indian J Pediatr. 84:937–944. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fabian ID, Onadim Z, Karaa E, Duncan C,
Chowdhury T, Scheimberg I, Ohnuma SI, Reddy MA and Sagoo MS: The
management of retinoblastoma. Oncogene. 37:1551–1560.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fabian ID, Puccinelli F, Gaillard MC,
Beckpopovic M and Munier FL: Diagnosis and management of secondary
epipapillary retinoblastoma. Br J Ophthalmol. 101:1412–1418.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ulitsky I: Evolution to the rescue: Using
comparative genomics to understand long non-coding RNAs. Nat Rev
Genet. 17:601–614. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hu C, Liu S, Han M, Wang Y and Xu C:
Knockdown of lncRNA XIST inhibits retinoblastoma progression by
modulating the miR-124/STAT3 axis. Biomed Pharmacother.
107:547–554. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Fan Q, Yang L, Zhang X, Peng X, Wei S, Su
D, Zhai Z, Hua X and Li H: The emerging role of exosome-derived
non-coding RNAs in cancer biology. Cancer Lett. 414:107–115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shang Y: LncRNA THOR acts as a
retinoblastoma promoter through enhancing the combination of c-myc
mRNA and IGF2BP1 protein. Biomed Pharmacother. 106:1243–1249.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shang W, Yang Y, Zhang J and Wu Q: Long
noncoding RNA BDNF-AS is a potential biomarker and regulates cancer
development in human retinoblastoma. Biochem Biophys Res Commun.
497:1142–1148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chai P, Jia R, Jia R, Pan H, Wang S, Ni H,
Wang H, Zhou C, Shi Y, Ge S, et al: Dynamic chromosomal tuning of a
novel GAU1 lncing driver at chr12p13.32 accelerates tumorigenesis.
Nucleic Acids Res. 46:6041–6056. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Misra JR and Irvine KD: The Hippo
Signaling Network and its biological functions. Annu Rev Genet.
52:65–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Esteban-Cardeñosa E, Duran M, Infante M,
Velasco E and Miner C: High-throughput mutation detection method to
scan BRCA1 and BRCA2 based on heteroduplex analysis by capillary
array electrophoresis. Clin Chem. 50:313–320. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ramasubramanian A, Sinha N, Rosenwasser RH
and Shields CL: Regression of advanced group e retinoblastoma with
intraarterial chemotherapy. Retin Cases Brief Rep. 6:406–408.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang Y and Peng XW: The silencing of long
non-coding RNA ANRIL suppresses invasion, and promotes apoptosis of
retinoblastoma cells through ATM-E2F1 signaling pathway. Biosci
Rep. 38(BSR20180558)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adh Migr. 9:317–324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song Z, Du Y and Tao Y: Blockade of sonic
hedgehog signaling decreases viability and induces apoptosis in
retinoblastoma cells: The key role of the PI3K/Akt pathway. Oncol
Lett. 14:4099–4105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meng B, Qu W and Yuan H: Anticancer
effects of gingerol in retinoblastoma cancer cells (RB355 Cell
Line) are mediated via apoptosis induction, cell cycle arrest and
upregulation of PI3K/Akt signaling pathway. Med Sci Monit.
24:1980–1987. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cambier S, Mu DZ, O'Connell D, Boylen K,
Travis W, Liu WH, Broaddus VC and Nishimura SL: A role for the
integrin alphavbeta8 in the negative regulation of epithelial cell
growth. Cancer Res. 60:7084–7093. 2000.PubMed/NCBI
|
|
22
|
Zhang HJ, Tao J, Sheng L, Hu X, Rong RM,
Xu M and Zhu TY: Twist2 promotes kidney cancer cell proliferation
and invasion by regulating ITGA6 and CD44 expression in the
ECM-receptor interaction pathway. Onco Targets Ther. 29:1801–1812.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Misra S, Hascall VC, Markwald RR and
Ghatak S: Interactions between Hyaluronan and its receptors (CD44,
RHAMM) regulate the activities of inflammation and cancer. Front
Immunol. 6(201)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu A, Zhang J, Liu H, Liu B and Meng L:
Identification of nondiabetic heart failure-associated genes by
bioinformatics approaches in patients with dilated ischemic
cardiomyopathy. Exp Ther Med. 11:2602–2608. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vakilian H, Mirzaei M, Sharifi TM, Pooyan
P, Habibi RL, Parker L, Haynes PA, Gourabi H, Baharvand H and
Salekdeh GH: DDX3Y, a male-specific region of Y chromosome gene,
may modulate neuronal differentiation. J Proteome Res.
14:3474–3483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ellwanger K, Becker E, Kienes I, Sowa A,
Postma Y, Cardona Gloria Y, Weber ANR and Kufer TA: The NLR family
pyrin domain containing 11 protein contributes to the regulation of
inflammatory signalling. J Biol Chem. 293:2701–2710.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu C, Su Z, Lin M, Ou J, Zhao W, Cui J and
Wang RF: NLRP11 attenuates Toll-like receptor signalling by
targeting TRAF6 for degradation via the ubiquitin ligase RNF19A.
Nat Commun. 8(1977)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang F, Wu X, Niu J, Kang X, Cheng L, Lv
Y and Wu M: GSTM1 polymorphism is related to risks of
nasopharyngeal cancer and laryngeal cancer: A meta-analysis. Onco
Targets Ther. 10:1433–1440. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rodrigues-Fleming GH, Fernandes GMM, Russo
A, Biselli-Chicote PM, Netinho JG, Pavarino ÉC and Goloni-Bertollo
EM: Molecular evaluation of glutathione S transferase family genes
in patients with sporadic colorectal cancer. World J Gastroenterol.
24:4462–4471. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Malik MA, Gupta V, Shukla S and Kaur J:
Glutathione S-transferase (GSTM1, GSTT1) polymorphisms and JOAG
susceptibility: A case control study and meta-analysis in glaucoma.
Gene. 628:246–252. 2017.PubMed/NCBI View Article : Google Scholar
|