Introduction
Endometrial stromal sarcoma (ESS) is a rare tumor,
predominantly occurring as a primary tumor of the uterus. Rare
cases of primary extrauterine ESS (EESS) have been reported.
Low-grade ESS (LG-ESS) is more common than high-grade ESS (HG-ESS).
Heterogeneous mixtures of morphologic and genetic features of
LG-ESS and HG-ESS are distinct histopathological entities. The
diagnosis is often made after hysterectomy (1).
ESS represents a rare diagnostic and a high- or
low-grade tumor profile is distinguished by immunohistochemistry
(IHC) tests (2). Up to 30% of
patients have EESS at presentation. The treatment of ESS is
multimodal, its management requiring a multidisciplinary team, and
it is different according to the primary tumor location and tumor
staging. The role of adjuvant radiotherapy remains controversial in
high-grade EESS and due to the rarity of these cases there are
limited data concerning the efficacy of adjuvant external
radiotherapy (EBRT) available from prospective randomized control
clinical trials (3). Here, we
present five cases of ESS and one case of EESS. All cases received
EBRT at the Radiotherapy Department of the Emergency Clinical
Hospital ‘Sfantul Apostol Andrei’ Galati, during 2004-2020.
Case reports
Literature data support radiotherapy use for ESS
with the aim of improving local tumor control, but without
revealing increases in the survival rate with certainty (1,4). We
present five ESS cases and one EESS case. All cases received EBRT
at the Radiotherapy Department of the ‘Sfantul Apostol Andrei’
Emergency Clinical Hospital of Galati, between 2004 and 2020, where
we identified six EES cases. Five of them had ESS histology and
were localized in the uterus [receiving treatment with EBRT in
two-dimensional (2D) technique] and only one had ESS histology with
extrauterine location [receiving palliative treatment with three
dimensional conformational radiotherapy (3DCRT) technique, using
the linear accelerator Elekta Synergy].
Clinical and therapeutic parameters of the EES and
EESS patients are documented in Table
I. The median age was 57.4 years.
 | Table IClinical and therapeutical parameters
of the ESS and EESS patients. |
Table I
Clinical and therapeutical parameters
of the ESS and EESS patients.
| Patient no. | Age (years) | Year | Surgical
intervention | HP | IHC | Risk factors | Treatment intent and
total dose | Survival
(months) |
|---|
| 1 | 55 | 2004 | TH+BSO | ESS G3 | - | Vagina | Adjuvant EBRT TD=50
Gy | |
| 2 | 43 | 2004 | TH+BSO | ESS | - | Vagina | Adjuvant EBRT TD=50
Gy | 168 |
| 3 | 68 | 2006 | TH+BSO | ESS | - | Vagina +
parameters | Adjuvant EBRT TD=50
Gy | |
| 4 | 55 | 2009 | TH+BSO | ESS G3 | - | Parameter,
miometer | Adjuvant EBRT TD=50
Gy | |
| 5 | 66 | 2016 | TH+BSO | ESS | HG-ESS;
ER-, PR- CD10+, VIM+,
Ki-67 30% | | Adjuvant EBRT TD=50
Gy | 23 |
| 6 | 64 | 2020 | | EESS | | | Palliative EBRT
TD=30 Gy | 11 |
Patients 1-5
Only one patient (patient no. 5) had IHC tests
[HG-ESS that was estrogen receptor-negative (ER-),
progesterone receptor-negative (PR-), CD10+,
vimentin-positive (VIM+), Ki-67 30%] because between
2004 and 2009, IHC assays were not performed routinely in our
medical unit.
The surgical intervention, for patient nos. 1-5,
consisted of a total hysterectomy with bilateral
salpingo-oophorectomy. Patients who presented with post-surgical
relapse risk factors such as vaginal or parameter
invasion/extension received adjuvant radiotherapy.
Patient no. 2 presented with a second neoplastic
disease occurring 9 years after the ESS diagnosis; this patient
died in 2018 suffering from lung metastases from breast cancer; the
breast cancer features were: pT2N0M0, invasive ductal carcinoma,
ER=90%, PR-, Ki-67 >14%. Taking into consideration
the long survival and death of breast cancer complications, we
consider that the histological aspects for this patient would have
been LG-ESS.
Patient no. 3 presented for post-therapeutic
controls, up until 2009, evading medical care since then. Patient
no. 5 had a 23-month survival. We could not calculate a median
survival due to the lack of data regarding all of the analyzed
cases.
Patient no. 6
EESS was diagnosed in a 64-year-old patient (patient
no. 6) with a personal history of hysterectomy and bilateral
salpingo-oophorectomy in 1997, with the histopathological diagnosis
of chronic cervicitis, uterine leiomyofibroma and uterine
adenomyosis. In November 2019, the computer tomography (CT) scan
examination revealed solid retroperitoneal lesions with
heterogeneous contrast enhancement and areas of necrosis and
calcification, localized in the left para-aortic region, inferior
from the renal hilum with extension into the left psoas muscle
(58/66 mm) and inferior pelvis, caudal from the common iliac artery
bifurcation (34/42 mm with 34/40 mm on the right and 22/33 mm with
44/62 mm on the left side). The retroperitoneal mass was biopsied,
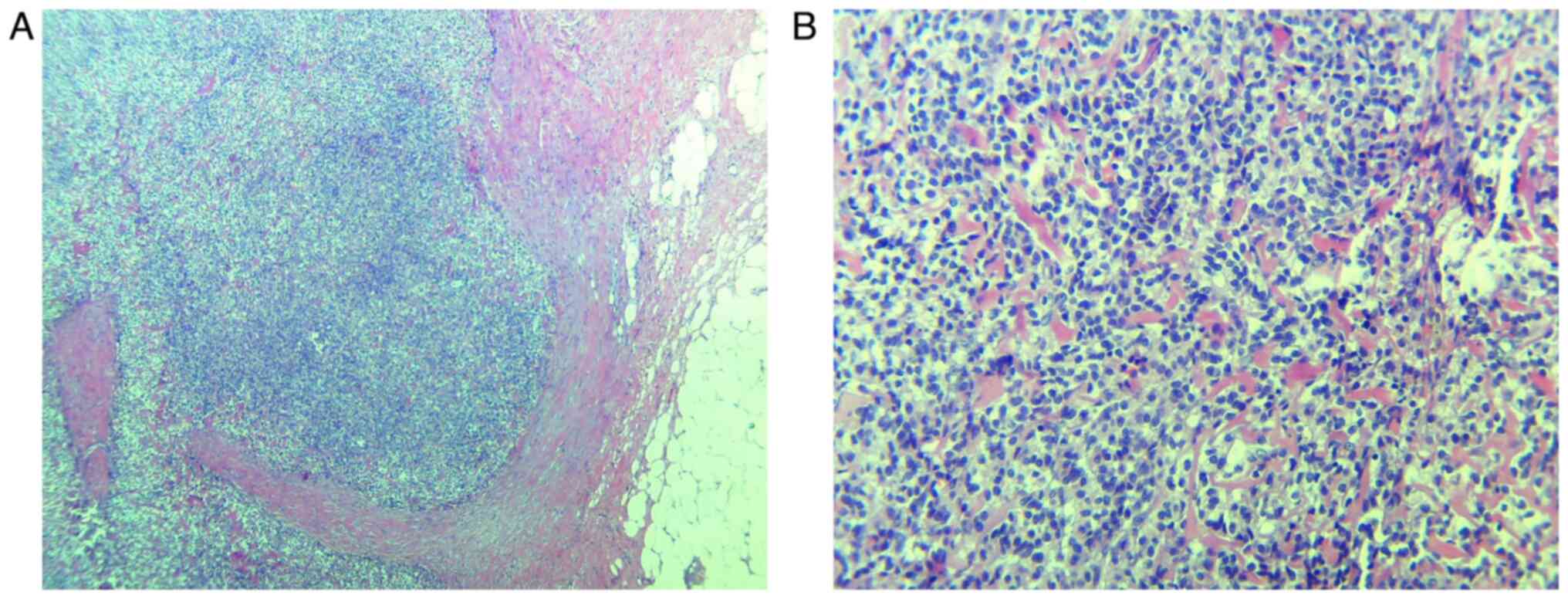
and the microscopic examination revealed a proliferation of
monotonous ovoid to spindle cells, with scant cytoplasm, focally
with ‘clear cell’ aspects, monomorphic nuclei and low mitotic
activity, without any areas of necrosis.
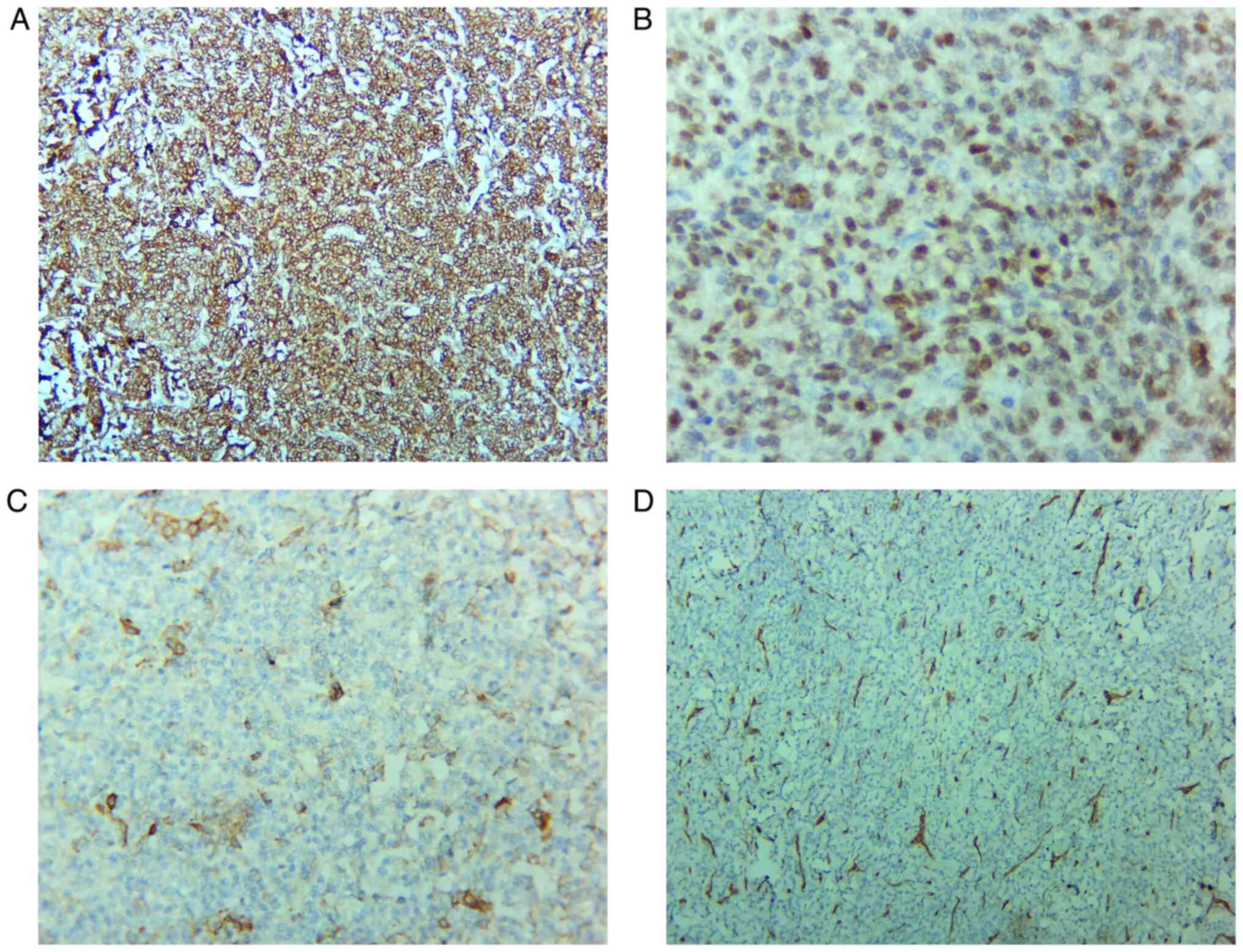
Biomarker IHC studies and hematoxylin and eosin
(H&E) slide staining were necessary (5). Immunohistochemical staining revealed
intense, diffuse positivity for ER isoform α, diffuse positivity
for CD10, focal actin and desmin positivity, and negativity for
CD34, inhibin, calretinin, OCT3/4, PLAP, chromogranin A and
Melan-A. The histopathological aspects of the mesenchymal tumor and
the immunophenotype (ER+, CD10+) revealed the
diagnosis of LG-EESS (Figs. 1 and
2).
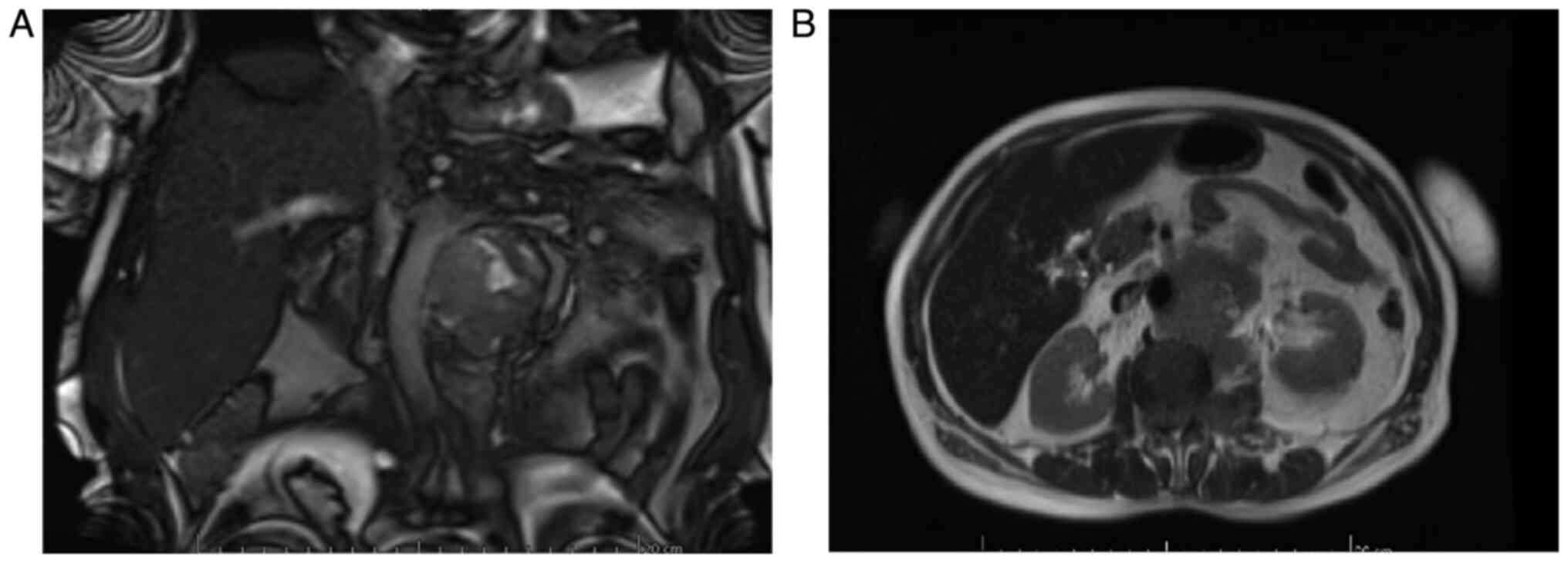
In January 2020, the magnetic resonance imaging
(MRI) examination described a retroperitoneal heterogeneous tissue
mass of 52/62 mm, with contrast enhancement and areas of necrosis,
extending caudally from the left renal hilum having approximately
85 mm in length; the lesion encased the aorta and the left renal
artery (which is still patent), it invaded the left renal vein and
left psoas muscle, extending into the spinal canal (through the L2
left neural foramen in the anterior epidural space, extending
cranially and caudally from L1 to L3). The mass also infiltrated
the left sided para-vertebral muscles (Fig. 3).
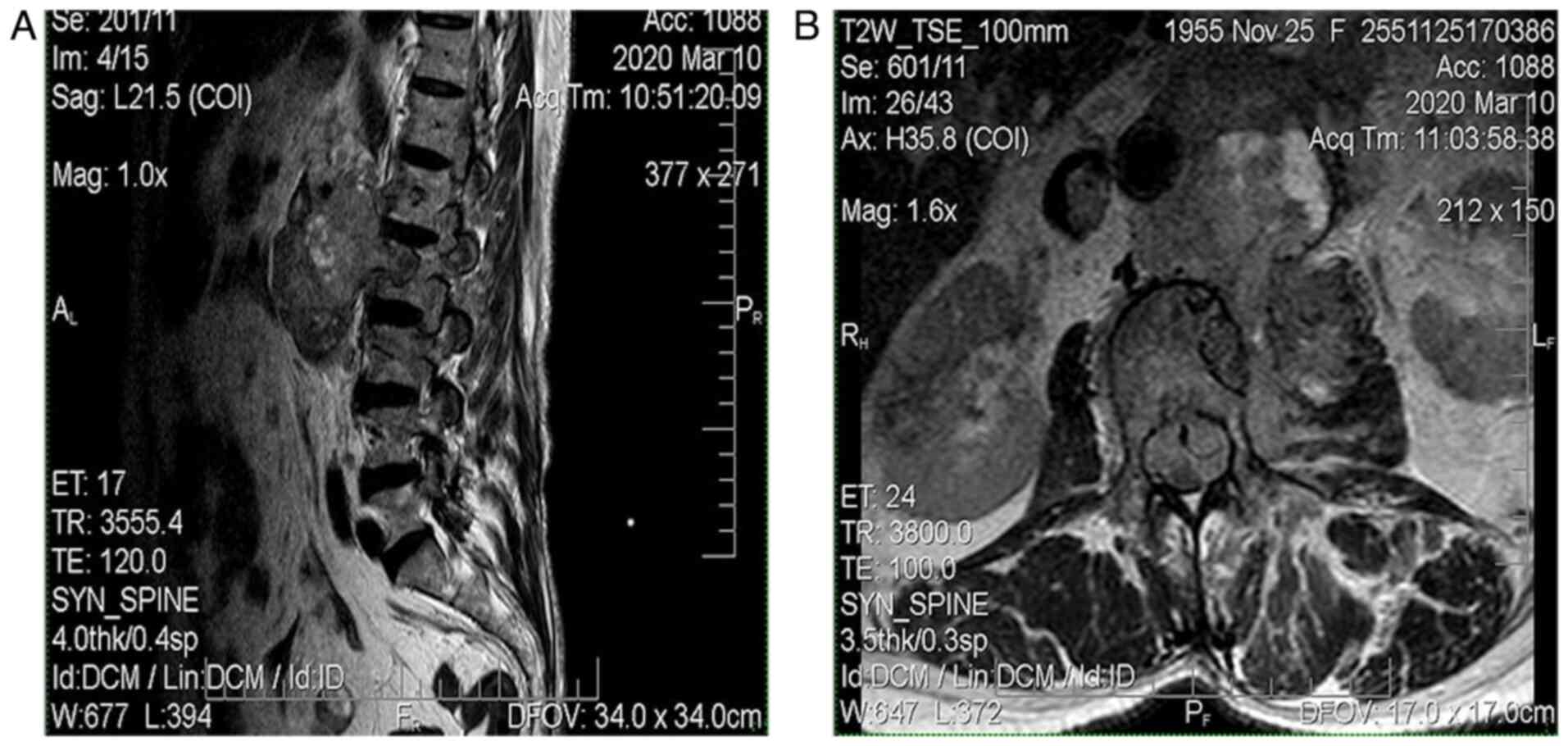
The MRI from March 2020 revealed a solid expansive
gadolinophilic retroperitoneal process, having axial measurements
of 100/75 mm and invading the left psoas muscle, L1, L2 and with
extension in the spinal canal (with extradural location), expanding
across approximately 150 mm, in between D1 and L4. The mass
determined foraminal and spinal canal stenosis, with compressive
effects on the horsetail roots (Fig.
4).
As treatment, the patient started hormone therapy
with letrozol, and the oncology team decided to perform EBRT as
palliative treatment at LINAC in 3DCRT technique, TD=30 Gy/10fr,
D/fr=300 cGy on primary tumor and extension, with good treatment
tolerance.
Discussion
Most of the information concerning uterine sarcomas
available in the medical literature is based on small series or
case reports, which have analyzed the effects of adjuvant
radiotherapy on the main histological subtypes. When grouping all
of the histological subtypes, most studies have shown that adjuvant
radiotherapy reduces the local and regional recurrence rates
without having an overall survival advantage (4,6).
Endometrial stromal sarcoma (ESS) patients receiving adjuvant
radiotherapy have registered improved local control, as compared to
patients undergoing surgical treatment alone (4).
Wang et al (7) retrospectively reviewed 152 patients
with stage I to II resected low-grade (LG)-ESS; the patients were
included and analyzed for 20 years (1998-2018). Forty patients
received adjuvant radiotherapy (EBRT group) and 112 patients did
not receive adjuvant radiotherapy (no EBRT group). For all patients
in the EBRT group, adjuvant radiotherapy significantly improved the
disease-free survival (DFS) (P=0.008) and pelvic failure-free
survival (PFFS) (P=0.004). This study indicated that adjuvant
radiotherapy significantly improved DFS and PFFS with tolerable
adverse effects, especially in patients with stage IB to IIB
disease. Despite several limitations (unequal number of patients in
the two groups; retrospective single-center study), this is the
largest population-based study exploring adjuvant radiotherapy in
resected, early-stage LG-ESS patients and could be a valuable
reference that provides guidance for radiotherapy treatment
selection in specific subgroups (7).
A retrospective analysis was conducted in 2009 and
it included 3,650 patients with uterine sarcoma; it was conducted
using the National Oncology Database and had a median follow-up
period of 59 months, with a 5-year overall survival of 37%. Use of
adjuvant radiotherapy was not predictive for overall survival. For
non-metastatic cancer patients receiving definitive surgery, the
5-year local-regional failure-free survival (LRFFS) was 87%
(8).
ESS is a rare diagnosis, usually presenting as a
leiomyoma of the uterus. The symptoms are nonspecific, patients
complaining most frequently of abnormal uterine bleeding. An early
diagnosis is essential because patient survival is directly related
to the tumor stage. Uterine sarcomas most often affect
postmenopausal women (9).
The gross appearance of LG-ESS is characterized by
several confluent tumor masses having a tan to yellow cut surface,
sometimes presenting tumor plugs with worm-like appearance which
permeate the myometrium or which can be found in blood vessels. The
extrauterine spread of LG-ESS is indicated by some palpable tumor
cords in the parauterine tissues. In contrast, high grade (HG)-ESS
presents as large tumor masses (up to 9 cm in diameter) with
extensive permeation of the myometrium; in some cases, the
extrauterine spread can be more extensive than the uterine tumor
itself (10-13).
The microscopic features of LG-ESS are characterized
by an endometrial stromal nodule-like cytology (small cells,
uniform round-oval nuclei, scant cytoplasm) with
tongue-like/island-like invasion of the myometrium, sometimes also
with lymphovascular invasion. It can have <5 mitoses/10
high-power fields (HPF), with limited, ischemic-type areas of
necrosis and also various types of differentiation: smooth or
skeletal muscle, adipose, sex cord or even glandular elements
(10-12,14).
HG-ESS can develop from a LG-ESS, and it presents
with high-grade round cells (‘small, round, blue-cell tumor’-like
appearance), having scant-to-moderate cytoplasm and round and
slightly irregular nuclei (larger than those of LG-ESS,
approximately 4-6 lymphocytes) which sometimes can be pleomorphic;
some also have low-grade areas with spindle-cell appearance, or
with sex cord differentiation. The tumor cell growth pattern is
frequently characterized by tight nests, or it can be rosette-like,
pseudopapillary or pseudoglandular. The tumor has a fine network of
vessels which are curvilinear or arborizing. The mitotic activity
exceeds that of 10/10 HPF and the tumor mass has frequent areas of
necrosis and lymphovascular invasion. The low-grade areas with
bland spindle-cells can have various morphological aspects ranging
from fibroblastic to fibrous to fibro-myxoid, with a low number of
mitoses (a maximum of 3 mitoses/10 HPF) and no necrosis
(typically). HG-ESS metastases can have either round, spindle-cell
or round and spindle-cell morphology (10-12).
In general, the IHC profile of LG-ESS is
characterized by CD10, ER and PR positivity, with possible positive
markers: keratin, calretinin, and actin. These tumors can also be
positive for other markers, according to the type of cell
differentiation found (desmin, caldesmon positivity in smooth
muscle differentiated cells, calretinin, inhibin, WT1, CD99,
Melan-A in sex cord differentiated areas) (10-12,15).
HG-ESS has a rather similar IHC profile,
characterizing each of the cellular components; thus, the spindle
cells are positive for CD10, ER, PR, while the round cells are
negative for these markers, but are highly positive for cyclin-D1.
Markers such as caldesmon, desmin, SMA, EMA, Melan-A or pankeratin
are negative (10-12,15,16).
Differential diagnosis for uterine LG-ESS includes
cellular leiomyoma, intravascular leiomyomatosis, leiomyosarcoma,
HG-ESS, gastrointestinal stromal tumor (GIST), perivascular
epithelioid cell tumor (PEComa), gland-poor adenomyosis. Proper
diagnosis requires attention to histological aspects and the use of
an adequate panel of IHC markers (10-12,16).
Vroobel et al framed an immunopanel for the differential
diagnosis of extrauterine neoplasms, which can be helpful in
distinguishing LG-ESS from the entities mentioned above, but also
from granulosa cell tumor, desmoplastic small round cell tumor or
even lymphoma (12). HG-ESS needs
to be differentiated from epithelioid leiomyosarcoma, PEComa of
gynecological origin, epithelioid GIST with pelvic location, or
undifferentiated uterine sarcoma (UUS), mainly based on
histomorphology and immunophenotype (17).
In the specialty literature, the ESS relapse pattern
is limited. Zhou et al (18)
demonstrated that relapse of LG-ESS was 12.3% (14 of 114 included
patients) mainly due to distant metastasis (64.3%, 9/14) and only
5/14 were pelvic recurrences. The median time of recurrences was 50
months (range, 6-169 months). In this study, all patients performed
hysterectomy, ovarian preservation was performed in 20/114 of
cases, adjuvant radiotherapy was performed in 31.6% of cases, 49.1%
(56/114) patients received postoperative chemotherapy (median 3
courses) and 9.6% of patients received endocrine therapy. None of
the analyzed factors, such as ovarian preservation, chemotherapy,
adjuvant radiotherapy and endocrine therapy, did not influence DFS.
The benefit of EBRT was not evidenced in this study.
Up to 30% of women with LG-ESS have an extrauterine
disease at presentation. Preoperative diagnosis is often difficult
and approximately 75% are diagnosed as benign leiomyoma.
Endometrial curettage and histopathological examination (HPE) do
not help due to the similarity with normal endometrium (2). A case of ESS was reported in a
30-year-old woman with ultrasound and Doppler findings suggestive
of uterine leiomyoma. Sarcomatous change was suspected in view of
the rapid enlargement of the tumor. Endometrial aspiration revealed
a secretory endometrium with neoplastic cells and thus a
hysterectomy was performed (19).
Another rare presentation of LG-ESS was a low-grade endometrial
sarcoma of the endocervix, clinically presenting as a soft
hemorrhagic mass in the posterior cervix, appearing as a
degenerated leiomyoma (20). Some
recent data on the low numbers of patients with LG-ESS appear to
show an incidence of nodal involvement higher than previously
expected, thus suggesting a purpose for lymphadenectomy in this
malignancy (21). Hormone therapy
with gonadotropin releasing hormone (GnRH) analogues and aromatase
inhibitors are suggested for LG-ESS stage 3-4 and for recurrent
disease (22,23).
In a study by Chu and colleague, 75% of patients
with stage I disease did not recur when treated with adjuvant
medroxyprogesterone acetate compared with 29% similar stage
patients who did not receive treatment (24).
Recommended adjuvant therapy options for stage I ESS
include observation (especially in the case of menopause or prior
bilateral salpingo-oophorectomy) or estrogen blockade.
Postoperative estrogen blockade is recommended for stages II to IV
ESS. Adjuvant EBRT may be added for stage II-IVA. Hormone therapy
includes aromatase inhibitors, megestrol acetate, or
medroxyprogesterone acetate (25).
Case series of patients with ESS suggest long
disease-free intervals in the absence of specific therapy and raise
questions about the use of adjuvant EBRT that has been demonstrated
to reduce local recurrence rates but with limited effect on
survival (6,26,27).
Regarding HG-ESS, the role of adjuvant EBRT in
non-metastatic disease is controversial (3).
The treatment is multimodal, with a management
requiring a multidisciplinary team, and a difference regarding the
primary tumor location and staging of the tumor. Rarely ESS is
initially present in an extrauterine site. IHC will help in the
detection of the specific tumor markers. In our cases, the IHC of
LG-ESS and HG-ESS revealed a completely different biological
aggressiveness and clinical behavior. Management was different
regarding the primary tumor location and staging of the tumor.
Initial surgical approach is considered as the optimal treatment,
but the aim of post-operative radiation treatment remains
uncertain. EBRT may have broader indications than what is currently
accepted in clinical practice. The purpose of adjuvant EBRT in
non-metastatic disease is controversial.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The information generated and analyzed in regards to
the case reports is available from the corresponding author on
reasonable request.
Authors' contributions
LFR, DF, RMA, LG, AMI, MED, MC, EN, ALT, MSC, ML and
AIN contributed to the acquisition, analysis and interpretation of
the data; they made substantial contributions to the conception and
design of the work, and supervised and substantially revised this
work. All authors had equal contributions, equal participation and
the same rights to this article. All authors have read and agreed
to the published version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of ‘Sfantul Apostol Andrei’
Emergency Clinical Hospital of Galati has granted approval for this
study with the decision number 8540 from 09.04.2021.
Patient consent for publication
Consent for participation was granted by all of the
patients and is part of the personal observation sheet.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sampath S and Gaffney DK: Role of
radiotherapy treatment of uterine sarcoma. Best Pract Res Clin
Obstet Gynaecol. 25:761–772. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jin Y, Pan L, Wang X, Dai Z, Huang H, Guo
L, Shen K and Lian L: Clinical characteristics of endometrial
stromal sarcoma from an academic medical hospital in China. Int J
Gynecol Cancer. 20:1535–1539. 2010.PubMed/NCBI
|
|
3
|
Reed NS, Mangioni C, Malmström H, Scarfone
G, Poveda A, Pecorelli S, Tateo S, Franchi M, Jobsen JJ, Coens C,
et al: Phase III randomised study to evaluate the role of adjuvant
pelvic radiotherapy in the treatment of uterine sarcomas stages I
and II: An European organisation for research and treatment of
cancer gynaecological cancer group study (protocol 55874). Eur J
Cancer. 44:808–818. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Valduvieco I, Rovirosa A, Colomo L, De San
Juan A, Pahisa J and Biete A: Endometrial stromal sarcoma. Is there
a place for radiotherapy? Clin Transl Oncol. 12:226–230.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bacinschi XE, Zgura A, Safta I and Anghel
R: Biomolecular factors represented by Bcl-2, p53, and
tumor-infiltrating lymphocytes predict response for adjuvant
anthracycline chemotherapy in patients with early triple-negative
breast cancer. Cancer Manag Res. 12:11965–11971. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Weitmann HD, Knocke TH, Kucera H and
Pötter R: Radiation therapy in the treatment of endometrial stromal
sarcoma. Int J Radiat Oncol Biol Phys. 49:739–748. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang W, Sun S, Miao Z, Hou X, Zhang F and
Hu K: Adjuvant radiotherapy improved survival in stage I to II
low-grade endometrial stromal sarcoma: A retrospective study of 152
cases. Front Oncol. 10(608152)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sampath S, Schultheiss TE, Ryu JK and Wong
JY: The role of adjuvant radiation in uterine sarcomas. Int J
Radiat Oncol Biol Phys. 76:728–734. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jain R, Batra S, Ahmad A, Elahi AA, Gupta
M and Saith P: Low grade endometrial stromal sarcoma: A case
report. Iran J Med Sci. 40:81–84. 2015.PubMed/NCBI
|
|
10
|
Hoang L, Chiang S and Lee CH: Endometrial
stromal sarcomas and related neoplasms: New developments and
diagnostic considerations. Pathology. 50:162–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lipsich F, Causa Andrieu PI, Wernicke A,
Patrono MG, Napoli MN, Chacon CRB and Nicola R: Extra-uterine
endometrial stromal sarcoma arising from deep infiltrating
endometriosis. Clin Imaging. 67:250–254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vroobel KM, Karawita TS and Nafisa
Wilkinson: New developments in endometrial stromal sarcoma. Diagn
Histopathol. 23:311–322. 2017.
|
|
13
|
Ashraf-Ganjoei T, Behtash N, Shariat M and
Mosavi A: Low grade endometrial stromal sarcoma of uterine corpus,
a clinico-pathological and survey study in 14 cases. World J Surg
Oncol. 4(50)2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Seagle BL, Shilpi A, Buchanan S, Goodman C
and Shahabi S: Low-grade and high-grade endometrial stromal
sarcoma: A national cancer database study. Gynecol Oncol.
146:254–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Farhood AI and Abrams J:
Immunohistochemistry of endometrial stromal sarcoma. Hum Pathol.
22:224–230. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu XQ, Shi YF, Cheng XD, Zhao CL and Wu
YZ: Immunohistochemical markers in differential diagnosis of
endometrial stromal sarcoma and cellular leiomyoma. Gynecol Oncol.
92:71–79. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ali RH and Rouzbahman M: Endometrial
stromal tumours revisited: An update based on the 2014 WHO
classification. J Clin Pathol. 68:325–332. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J, Zheng H, Wu SG, He ZY, Li FY, Su
GQ and Sun JY: Influence of different treatment modalities on
survival of patients with low-grade endometrial stromal sarcoma: A
retrospective cohort study. Int J Surg. 23:147–151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Puliyath G, Nair VR and Singh S:
Endometrial stromal sarcoma. Indian J Med Paediatr Oncol. 31:21–23.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hasiakos D, Papakonstantinou K,
Kondi-Paphiti A and Fotiou S: Low-grade endometrial stromal sarcoma
of the endocervix. Report of a case and review of the literature.
Eur J Gynaecol Oncol. 28:483–486. 2007.PubMed/NCBI
|
|
21
|
Gadducci A, Cosio S, Romanini A and
Genazzani AR: The management of patients with uterine sarcoma: A
debated clinical challenge. Crit Rev Oncol Hematol. 65:129–142.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Palombaa S, Falboa A, Mocciaroa R, Russob
T and Zulloa F: Laparoscopic treatment for endometrial cancer: A
meta-analysis of randomized controlled trials (RCTs). Gynecol
Oncol. 112:415–421. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lindner T, Pink D, Kretzschmar A, Mrozek
A, Thuss-Patience PC and Reichardt P: Hormonal treatment of
endometrial stromal sarcoma: A possible indication for aromatase
inhibitors. J Clin Oncol. 23 (Suppl 16)(S9057)2005.
|
|
24
|
Chu MC, Mor G, Lim C, Zheng W, Parkash V
and Schwartz PE: Low grade endometrial stromal sarcoma: Hormonal
aspects. Gynaecol Oncol. 90:170–176. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Amant F, Coosemans A, Debiec-Rychter M,
Timmerman D and Vergote I: Clinical management of uterine sarcomas.
Lancet Oncol. 10:1188–1198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mansi JL, Ramachandra S, Wiltshaw E and
Fisher C: Endometrial stromal sarcomas. Gynecol Oncol. 36:113–118.
1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Berchuck A, Rubin SC, Hoskins WJ, Saigo
PE, Pierce VK and Lewis JL Jr: Treatment of endometrial stromal
tumors. Gynecol Oncol. 36:60–65. 1990.PubMed/NCBI View Article : Google Scholar
|