Introduction
Diabetes mellitus (DM) is a disease characterized by
hyperglycemia induced by absolute or relative insufficiency of
insulin (1). The total number of
diabetic patients in China ranks the first in the world, and 95% of
diabetic patients have type 2 DM (T2DM) (2). If blood glucose levels cannot be
effectively controlled after the diagnosis of diabetes, there will
be extensive microvascular and macrovascular lesions during the
course of the disease (3-5).
The long-term existence of various pathogenic
factors in T2DM can damage the vascular endothelium, lead to
abnormal secretion function, and interrupt the normal proliferation
and apoptosis balance of smooth muscle cells (6). In the disease, atherosclerosis,
decreased compliance with various vasoactive substances, abnormal
diastolic and systolic functions, and finally stenosis,
obstruction, and complications may occur (6). At present, it is believed that the
molecular mechanism of vascular lesions in T2DM is very complex,
being closely associated with gene susceptibility, oxidative
stress, advanced glycation end products, aldose reductase and
inflammation (7).
Nitric oxide synthase 2 (NOS2) is a key enzyme for
endogenous NO synthesis in the human body. An appropriate amount of
NO can relax blood vessels, maintain local blood flow, and play a
protective role in body tissues (8). However, with the prolongation of
ischemia, NOS2 will induce a large amount of NO, which produces
more toxic superoxide radicals that damage vascular tissues
(9). At present, the regulation of
NOS2 is a hot spot in research on vascular lesions, and regulations
by microRNA (miR) have attracted increasing attention. During the
preliminary bioinformatics prediction, it was discovered that
miR-185 is closely associated with NOS2 and may be an upstream
miRNA regulating NOS2. It is also confirmed that miR-185 plays an
important role in the circulatory system. For example, miR-185 is
reported to reverse myocardial hypertrophy through a variety of
signaling pathways (10). However,
the regulatory association between miR-185 and NOS2 has not been
fully clarified in diabetic angiopathy.
In the present study, the aim was to investigate the
association between NOS2 and miR-185, and the expression level of
NOS2 was examined in the blood and vascular tissues from diabetic
patients and rat models.
Materials and methods
Subjects
A total of 33 patients with T2DM, including 17 males
and 16 females, participated in the present study between December
2017 and July 2019. The ages of the patients ranged from 38 to 60
years, and the mean age was 50.3 years. In addition, 33 age-matched
healthy subjects (age match by power analysis), including 17 males
and 16 females, were included in the control group (age range,
37-62 years; mean age, 51.7 years). Blood was drawn from all
patients on the day of diagnosis and healthy subjects on the day of
physical examination, and stored in tubes containing EDTA. After
centrifugation at 1,200 x g at 4˚C for 10 min, plasma was separated
and stored at -20˚C. All procedures performed in the current study
were approved by the Ethics Committee of Lishui People's Hospital.
Written informed consent was obtained from all patients or their
families.
Animals
A total of 40 male SD rats [certificate no.
SCXK(Yu)2018-0011; Tengxin] weighing between 150 and 200 g were
used for animal experiments. Before conducting the experiments, all
rats had adaptive feeding for one week and were free to eat and
drink.
The rats were firstly fasted overnight. Under
anesthesia by intraperitoneal injection of chloral hydrate (300
mg/kg), alloxan was injected via the tail vein at a dose of 50
mg/kg body weight. After 48 h, the diabetic rat model was
established. The number of rats in the diabetic group was 20 and
that in control group was also 20.
Blood was collected from the caudal vein of all
rats, and stored in the presence of EDTA. Following centrifugation
at 1,200 x g at 4˚C for 10 min, plasma was separated and stored at
-20˚C. After the model construction was completed, and blood was
drawn from the animals, the animals were euthanized by decapitation
after anesthesia by intraperitoneal injection of chloral hydrate
(300 mg/kg). After sacrifice, aortic vascular tissues were
collected from the rats and stored in liquid nitrogen. All animal
experiments were conducted according to the ethical guidelines of
Lishui People's Hospital.
Extraction of RNA and reverse
transcription-quantitative PCR (RT-qPCR)
Tissues (100 mg) were ground into powder in liquid
nitrogen and lysed with 1 ml TRIzol® (cat. no. R0016;
Beyotime Institute of Biotechnology), and liquid samples (100 µl)
were directly lysed with 1 ml TRIzol. Total RNA was extracted using
an RNA extraction kit (R0016; Beyotime Institute of Biotechnology).
The integrity of RNA bands was detected by gel electrophoresis. The
ratio of absorbance at 260 nm over absorbance at 280 was measured
by spectrophotometer to detect the purity of RNA. Total RNA (1 µg)
was reverse-transcribed into template cDNA using the TIANScript II
RT kit (Tiangen Biotech Co., Ltd.) according to the manufacturer's
protocol, which was stored at -20˚C.
For RT-qPCR, the primer sequences were as follows:
human U6 (upstream), 5'-GCTTCGGCAGCACATATACTAAAAT-3'; human U6
(universal downstream), 5'-CGCTTCACG AATTTGCGTGTCAT-3'; human
miR-185 (upstream), 5'-TGGAGAGAAAGGCAGTTCCTGA-3'; human miR-185
(universal downstream), 5'-CGCTTCACGA ATTTGCGTGTCAT-3'; human NOS2
(upstream), 5'-GGT AGAGGCCTGGAAAACCC-3'; human NOS2 (downstream),
5'-AGCTCATCCCCTTCTCCCAT-3'; human β-actin (upstream),
5'-CTCCATCCTGGCCTCGCTGT-3'; and human β-actin (downstream),
5'-GCTGTCACCTTCACCGTTCC-3'. According to the manufacturer's
instructions (2xSYBR Green qPCR Master Mix; EZBioscience), the qPCR
reaction system (20 µl) contained RT-qPCR-Mix (10 µl), upstream
primer (0.5 µl), downstream primer (0.5 µl), cDNA (2 µl) and
ddH2O (7 µl). The reaction protocol of RTq-PCR was:
Initial denaturation, 95˚C and 30 sec; denaturation, 95˚C and 5
sec; annealing, 60˚C and 20 sec. A total of 39 cycles were
performed. The results were analyzed by the 2-∆∆Cq
method (11). The ratio of
NOS2/β-actin was calculated.
The primer sequences for rat NOS2 were
5'-ATCCCGAAACGCTACACTT-3' (upstream) and 5'-GGTCTGGCGAAGAACAATC-3'
(downstream). The primer sequences for rat β-actin were
5'-CGTGCGTGACATTAAAGAG-3' (upstream) and 5'-CTGGAAGGTGGACAGTGAG-3'
(downstream). The primer sequences for rat U6 were 5'-
CTCGCTTCGGCAGCACA-3' (upstream) and 5'-AACGCTTCACGAATTTGCGT-3'
(downstream). The primer sequences for rat miR-185 were
5'-ACACTCCAGCTGGGTGGAGAGAAAGGCAGT-3' (upstream) and
5'-TGGTGTCGTGGAGTCG-3' (downstream). Real-time PCR reaction system
(20 µl) for rat was the same with that for human described above.
The reaction protocol of RT-qPCR for rat: initial denaturation,
95˚C and 10 min; denaturation, 95˚C and 45 sec; annealing, 52˚C and
45 sec; elongation, 72˚C and 45 sec. A total of 35 cycles were
performed. The results were analyzed by 2-∆∆Cq method
(11). The ratio of NOS2/β-actin
was calculated.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of human NOS2 (cat. no. ab253217;
Abcam) and rat NOS2 (cat. no. SBJ-R0012; SenBeiJia Biological
Technology) were measured by ELISA according to the protocols
provided by the kit manufacturers.
Western blotting
After extraction of proteins using RIPA buffer
(Beyotime Institute of Biotechnology), BCA protein concentration
detection kit (cat. no. P0009; Beyotime Institute of Biotechnology)
was used to measure the concentrations of target proteins. The
protein samples were mixed with SDS-PAGE loading buffer before
boiling for 5 min. Then, 20 µg protein samples were loaded for 10%
SDS-PAGE. On ice bath, the samples were transferred onto PVDF
membrane at 100 V for 2 h, and then blocked with 5% skimmed milk at
room temperature for 1 h. Then, primary antibodies of NOS2 (cat.
no. ab3523; 1:800; rabbit anti-rat, polyclonal; Abcam) and internal
reference β-actin (cat. no. ab129348; 1:5,000; rabbit anti-rat,
Abcam) were used to incubate the membrane at 4˚C overnight.
Afterwards, secondary antibody (cat. no. ab6721; 1:3,000; goat
anti-rabbit; Abcam) was used to incubate the membrane at room
temperature for 1 h. The membrane was then soaked in
electrochemiluminescence liquid (cat. no. ab65623; Abcam), and
imaged in gel imaging system. Then, Image Lab (version 3.0; Bio-Rad
Laboratories, Inc.) was used to analyze the protein bands, and the
relative expression of target protein was expressed as the ratio of
target protein greyscale against β-actin greyscale.
Bioinformatics prediction
Bioinformatics prediction is the basis and main clue
for miRNA function research. The miRwalk 3.0 target gene prediction
software (http://mirwalk.umm.uni-heidelberg.de) was used to
predict genes that might regulate NOS2, following the instructions
published on the website.
Cells and transfection
HMEC-1 and 293T cells were purchased from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences,
and cultured at 37˚C and 5% CO2.
Before transfection, 1 ml medium containing
1x105 cells was added to 6-well plates, which were
incubated at 37˚C and 5% CO2. When the cells reached
40-60% confluence, Lipofectamine® 3000 was used to
transfect the cells following the manufacturer's protocols (cat.
no. L3000001; Thermo Fisher Scientific, Inc.). Subsequent
experiments were performed 48 h after transfection.
Dual-luciferase reporter assay
Wild-type (WT) and mutant seed regions of miR-185 in
the 3'-untranslated region (UTR) of NOS2 gene were chemically
synthesized in vitro, and Spe-1 and HindIII
restriction sites were attached to the two ends. Then, the two
types of DNA fragments were cloned into pMIR-REPORT luciferase
reporter plasmids (Ambion; Thermo Fisher Scientific,Inc.), using
the mutant 3'-UTR seeding region as control. Using Lipofectamine
3000 (Thermo Fisher Scientific, Inc.), plasmids (0.8 µg) with WT or
mutant 3'-UTR DNA sequences were co-transfected with human or rat
agomiR-185 (100 nM; Sangon Biotech, Co. Ltd.) into 293T cells. For
control, 293T cells were transfected with agomiR-negative control
(agomiR-NC). After being cultured for 24 h, the cells were lysed
using dual-luciferase reporter assay kit (E1980; Promega
Corporation) according to the manufacturer's manual. The
luminescence intensity was measured using GloMax 20/20 luminometer
(Promega Corporation). Using renilla luminescence activity as an
internal reference, the luminescence values of each group of cells
were measured.
Statistics
Data are expressed as mean ± SD. SPSS v.18.0
software (SPSS, Inc.) was used for statistical analysis. One-way
ANOVA was performed for group comparison, with LSD post hoc test.
Comparison between two groups was carried out using unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
NOS2 level in patients with diabetes
is elevated
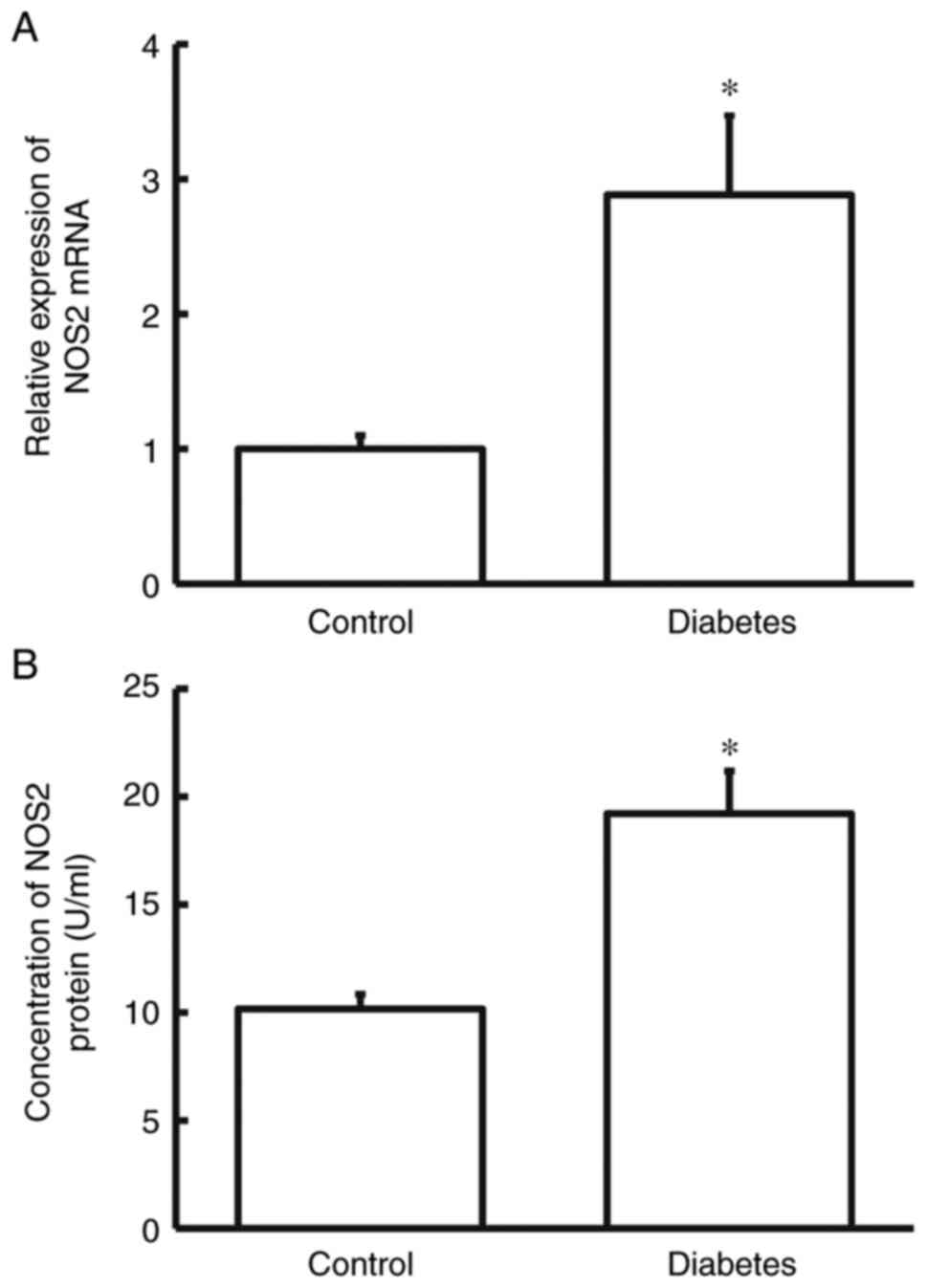
Firstly, RT-qPCR and ELISA were used to measure the
levels of NOS2 in the blood. Compared with the control group, NOS2
mRNA and protein levels in the blood from diabetes group were
notably higher compared with those in the control group (P<0.05
for both; Fig. 1A and B). The result suggests that NOS2 level in
patients with diabetes is elevated.
Decreased miR-185 expression and
elevated NOS2 expression in the blood of patients with T2DM meet
the regulatory mode between miRNA and mRNA
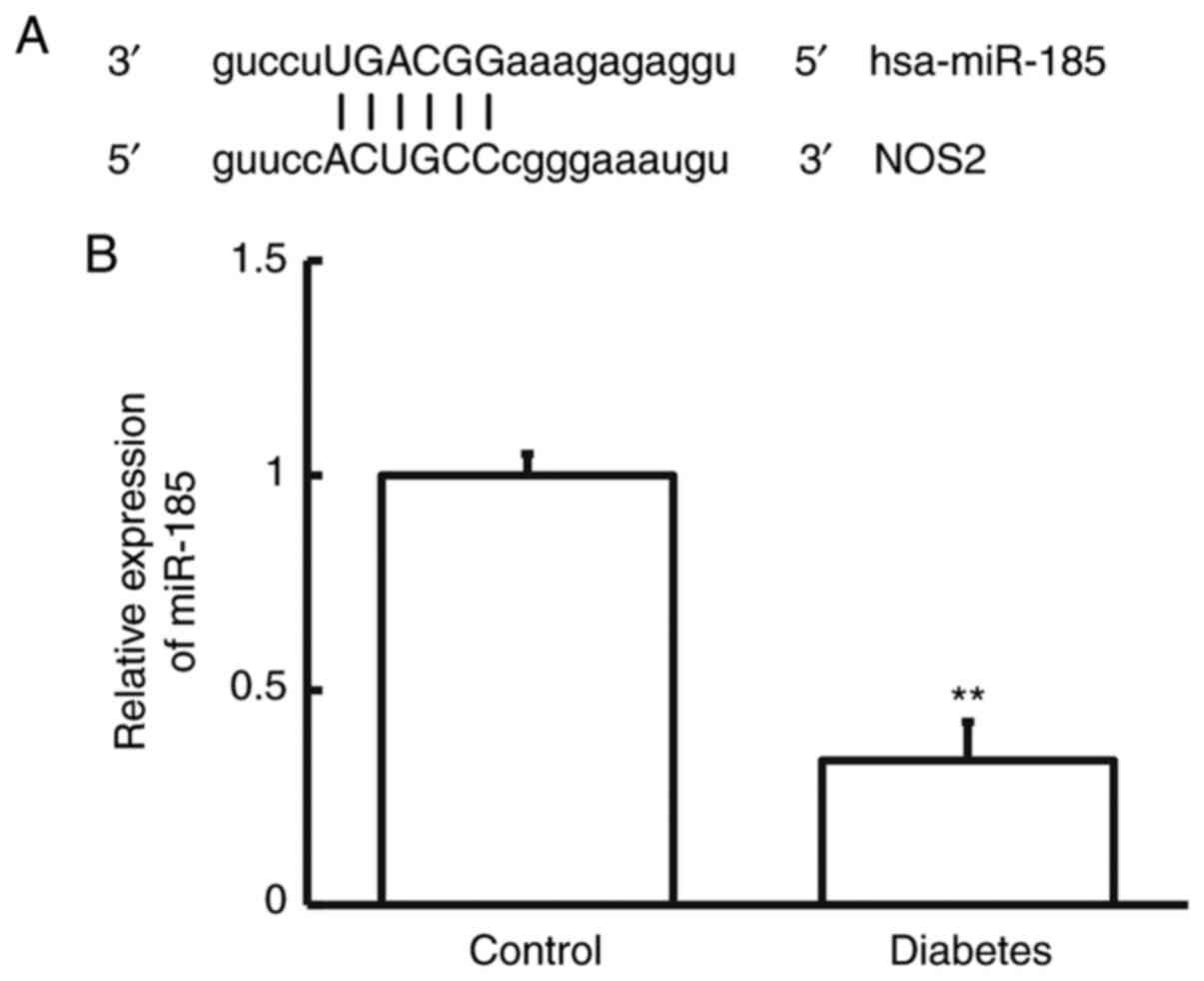
Using bioinformatics, miR-185 was predicted to be an
upstream regulatory gene for NOS2 (Fig. 2A). Using RT-qPCR, the level of
miR-185 in the blood from patients with T2DM was determined. The
data showed that the expression of miR-185 in patients with T2DM
was notably decreased compared with that in the control group
(P<0.05; Fig. 2B). The result
indicates that decreased miR-185 expression and elevated NOS2
expression in the blood of patients with T2DM meet the regulatory
mode between miRNA and mRNA.
NOS2 levels in vascular tissues and
blood from rats with diabetes are elevated compared with normal
rats
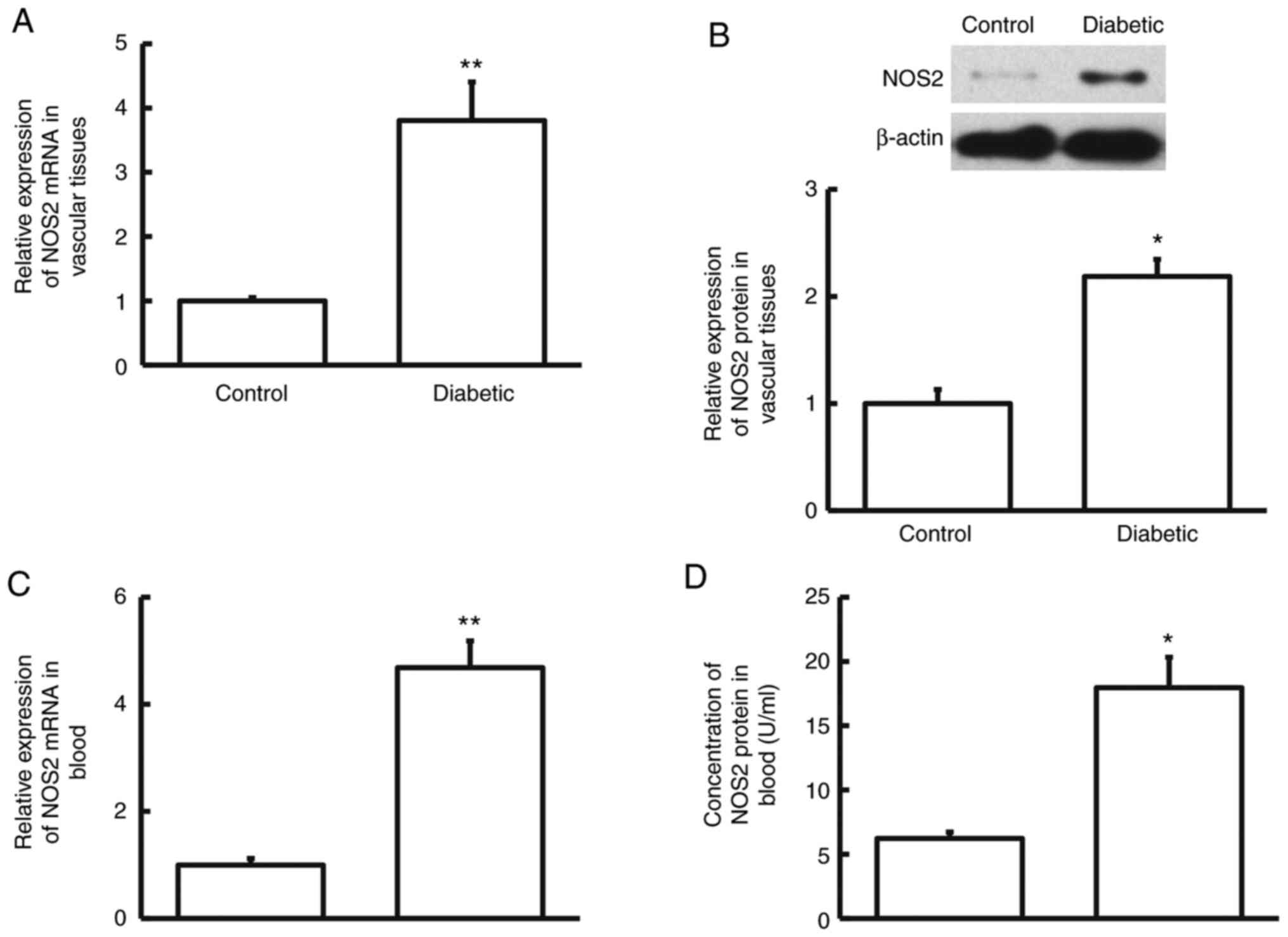
After construction of diabetic rats, the
diabetes-associated biochemical indices were examined in the rats.
The data showed that the levels of HbA1c and glucose in the
diabetic group were notably elevated compared with the control
group (P<0.05), while the concentration of insulin in the
diabetic group was notably decreased compared with the control
group (P<0.05; Table I). Then,
mRNA and protein levels of NOS2 in vascular tissues and blood were
determined. RT-qPCR and western blotting showed that the expression
level of NOS2 mRNA and protein in vascular tissues from diabetic
group was significantly higher compared with that from the control
group (P<0.05; Fig. 3A and
B). Similarly, RT-qPCR and ELISA
showed that NOS2 mRNA and protein levels in the blood from diabetic
group were obviously higher compared with those from the control
group (P<0.05; Fig. 3C and
D). The results demonstrate that
NOS2 levels in vascular tissues and blood from rats with diabetes
are elevated compared with normal rats.
 | Table IBiochemical indexes of diabetic
rats. |
Table I
Biochemical indexes of diabetic
rats.
| Groups | N | HbA1c, % | Glucose, mmol/l | Insulin, µU/l |
|---|
| Control | 20 | 6.17±1.05 | 5.28±0.16 | 17.55±4.81 |
| Diabetic | 20 |
10.21±3.56a |
22.18±5.83b |
6.13±1.66a |
Rats with diabetes have downregulated
levels of miR-185 in vascular tissues and blood compared with
normal rats
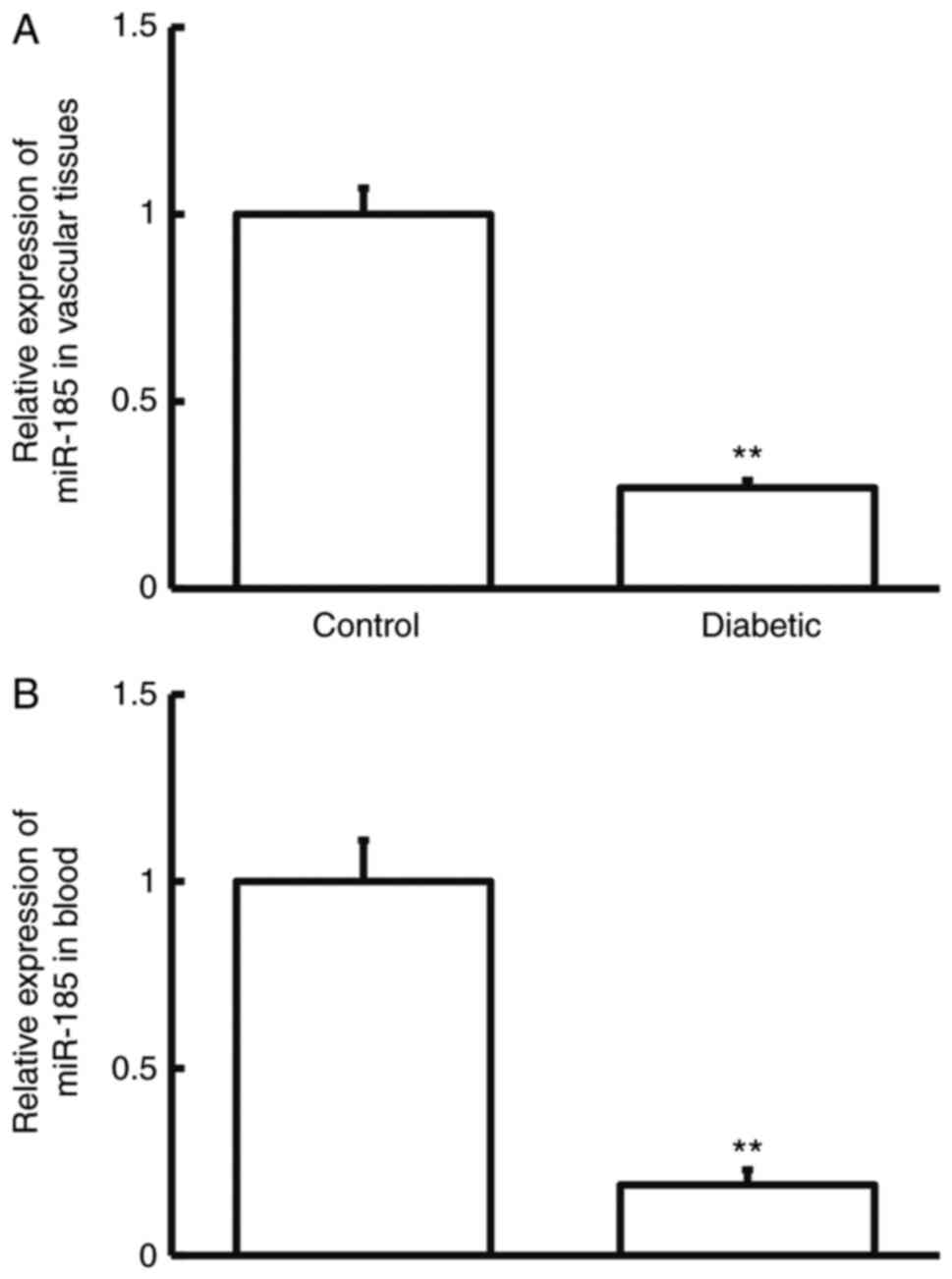
Moreover, the expression of miR-185 was detected in
rats. RT-qPCR showed that the levels of miR-185 in vascular tissues
and blood from diabetic group were markedly decreased compared with
those from the control group (P<0.01; (Fig. 4A and B). The results suggest that rats with
diabetes have downregulated levels of miR-185 in vascular tissues
and blood compared with normal rats.
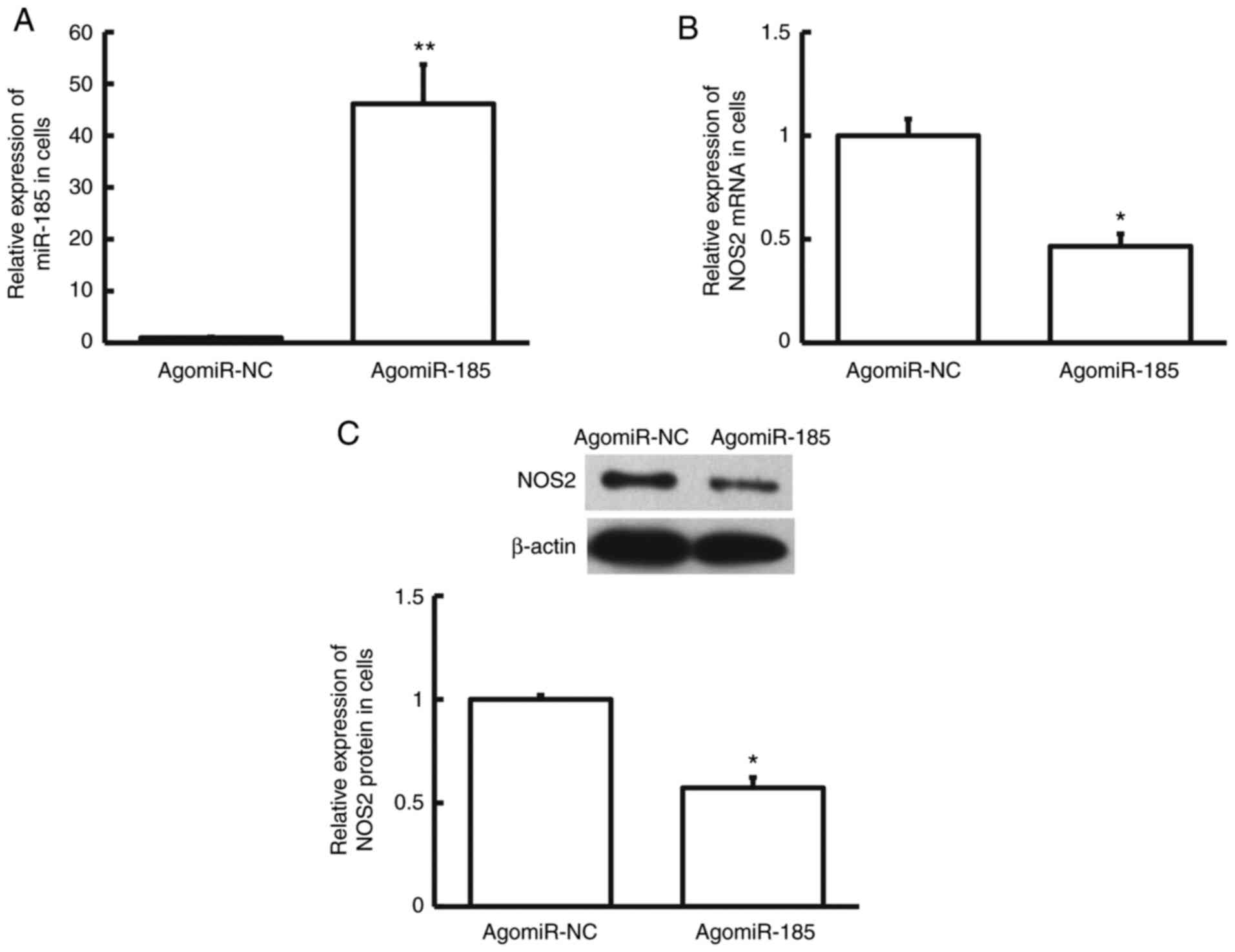
Overexpression of miR-185
downregulates the expression of NOS2 in vitro
In cell experiments, HMEC-1 cells were transfected
with agomiR-NC or agomiR-185. RT-qPCR showed that the expression of
miR-185 in agomiR-185 group was markedly elevated compared with
that in the agomiR-NC group (P<0.01; Fig. 5A). Furthermore, NOS2 mRNA and
protein levels in agomiR-185 group were obviously decreased
compared with those in the agomiR-NC group (P<0.05; Fig. 5B and C). These results elucidate that
overexpression of miR-185 downregulates the expression of NOS2
in vitro.
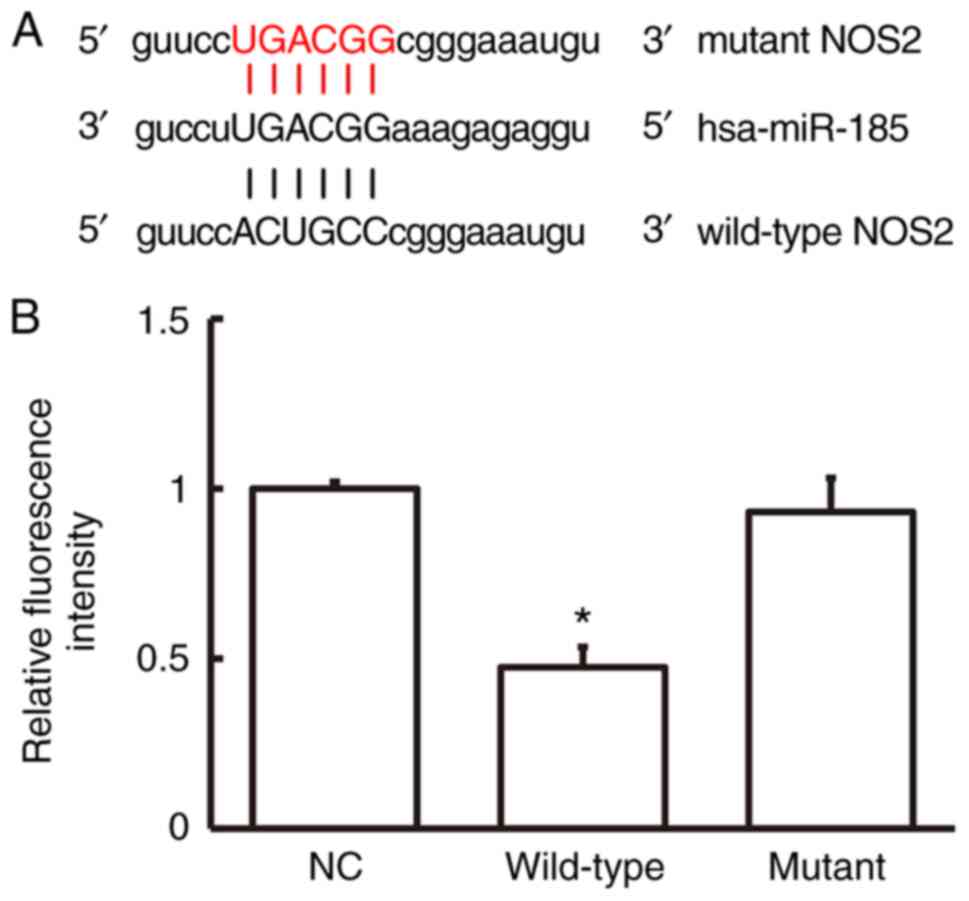
miR-185 directly binds with the 3'-UTR
of NOS2 gene to regulate NOS2 expression
Dual-luciferase reporter showed that the
fluorescence intensity of 293T cells co-transfected with wild-type
agomiR-185 and pMIR-REPORT was markedly decreased compared with
that of negative control group (P<0.05). By contrast, the
fluorescence intensity of 293T cells co-transfected with mutant
agomiR-185 and pMIR-REPORT was not different from that of negative
control group (P>0.05; Fig. 6A
and B). The result indicates that
miR-185 directly binds with the 3'-UTR of NOS2 gene to regulate
NOS2 expression.
Discussion
Hyperglycemia leads to chronic damage and
dysfunction of various tissues and organs, especially eyes,
kidneys, heart, blood vessels and nerves (12). However, the pathogenesis of
diabetes is not yet fully understood. The incidence rate of
vascular diseases in diabetic patients is 2-4 times higher than
that of non-diabetic patients (13,14).
The pathological basis of diabetic angiopathy is atherosclerosis
and irregular thickening, fibrosis and calcification of media and
adventitia of arteries caused by the proliferation of vascular
smooth muscle cells and fibroblasts (14).
Angiogenesis is a complexed, dynamic and coordinated
process involving a variety of cytokines and cell components, in
which the state of vascular endothelial cells plays a leading role
(15). The central process of
angiogenesis is the proliferation, migration, differentiation and
lumen formation of vascular endothelial cells (15). Inhibition of apoptosis and
promotion of survival for endothelial cells are the basic
mechanisms of angiogenesis. Exogenous inducers can accelerate the
establishment of collateral circulation, and improve the symptoms
of myocardial ischemia and hypoxia.
Furchgott et al (16) discovered that under the action of
acetylcholine, a substance was produced in blood vessels to relax
the smooth muscle of the vessels and named it endothelium-derived
relaxing factor, which was later proven to be No. NO plays a dual
role in cerebral ischemia-reperfusion injury, and can be
synthesized by NOS2. NOS2 is not expressed under physiological
state, but can be induced by LPS, IFN-γ, TNF-α and IL-1β (17). NOS2 has important clinical
significance in the occurrence and development of several diseases.
It decreases the survival rate by promoting tumor metastasis in
patients with triple-negative breast cancer (18). In patients with nasopharyngeal
carcinoma, IL-6 and NOS2 are highly expressed and involved in the
regulation of MMP9- and MMP2-dependent metastasis (19). High circulating nitrite levels may
be a prognostic marker for survival (19). In addition, the expression of NOS2
is increased in hypertensive rats (20). In the present study, elevated NOS2
levels were observed in the blood from patients with T2DM and the
vascular tissues and blood from T2DM rats. The aforementioned data
indicated that NOS2 plays important regulatory roles in T2DM.
miRNAs, ~18-22 base pairs in length, are a class of
endogenous non-coding single-stranded RNA molecules, which
negatively regulate gene expression at posttranscriptional level by
binding to the 3'-UTR of target genes (21). miRNAs are widely involved in the
pathophysiological processes of cardiovascular diseases (22,23).
Mian et al (24) discovered
that the expression of >140 miRNAs was changed in arterial
vessels of patients with arteriosclerosis obliterans of lower
extremity. Knockout of Dicer, an enzyme required for miRNA
production, can inhibit vascular budding, endothelial cell
migration and angiogenesis (25).
These studies suggest that miRNAs play important roles in arterial
injury and angiogenesis. According to bioinformatics prediction, it
was found that miR-185 was closely associated with NOS2 and might
be an upstream miRNA that regulates NOS2. It is reported that
miR-185 can inhibit the proliferation of clear cell renal cell
carcinoma cells and induce their apoptosis by targeting VEGFA
(26). In the meantime, miR-185
can inhibit β cell dysfunction caused by diabetes mellitus through
targeting SOCS3 gene (27). In
breast cancer, the target of miR-185 is VEGFA, which plays an
important role in angiogenesis and biological functions (28). The data in the present study
demonstrated that expression of miR-185 was decreased in the blood
of patients with T2DM and the vascular tissues and blood from
diabetic rats. This matched the regulatory mechanism between
microRNA and its target gene. Then, miR-185 was overexpressed in
vascular endothelial cells, and discovered decreased expression of
NOS2 in these cells. Dual-luciferase reporter assay demonstrated
that miR-185 could directly bind to NOS2 mRNA. All the
aforementioned results indicated that miR-185 plays a regulatory
role on NOS2 in the process of diabetes. Although NO produced by
eNOS is one of the key factors on vascular homeostasis, the
prediction and experimental results of the present study showed
that mi-R185 does not regulate eNOS (data not shown).
To summarize, the present study demonstrates that
the downregulation of miR-185 expression in vascular tissues and
blood of diabetic patients leads to the upregulation of NOS2
expression, which results in a series of vascular lesions and
eventually vascular injury. As a regulatory factor of NOS2, miR-185
may become a new foothold in the study of diabetic angiopathy. The
present study reveals some mechanisms of miRNA-mRNA regulatory
network in the course of type 2 diabetes, which may play a positive
role in the prevention, early diagnosis and intervention of the
disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and HW contributed to the design of the study.
YZ, LG and JT performed the experiments. YZ and LG analyzed the
data. YZ and HW interpreted the results and prepared the
manuscript. YZ and HW confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Lishui People's Hospital
(approval no. IACUC-20160201-01). Written informed consent was
obtained from all patients or their families. All animal
experiments were conducted according to the ethical guidelines of
Lishui People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA.
317:2515–2523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ma RCW: Epidemiology of diabetes and
diabetic complications in China. Diabetologia. 61:1249–1260.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi Y and Vanhoutte PM: Macro- and
microvascular endothelial dysfunction in diabetes. J Diabetes.
9:434–449. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cattin L: Diabetes Mellitus: etiology,
pathophysiology and clinical classification. G Ital Entomol.
33(gin/33.S68.6)2016.PubMed/NCBI(In Italian).
|
|
5
|
Guthrie RA and Guthrie DW: Pathophysiology
of diabetes mellitus. Crit Care Nurs Q. 27:113–125. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goligorsky MS: Vascular endothelium in
diabetes. Am J Physiol Renal Physiol. 312:F266–F275.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oleson BJ and Corbett JA: Dual role of
nitric oxide in regulating the response of β cells to DNA damage.
Antioxid Redox Signal. 29:1432–1445. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Incalza MA, D'Oria R, Natalicchio A,
Perrini S, Laviola L and Giorgino F: Oxidative stress and reactive
oxygen species in endothelial dysfunction associated with
cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim JO, Song DW, Kwon EJ, Hong SE, Song
HK, Min CK and Kim DH: miR-185 plays an anti-hypertrophic role in
the heart via multiple targets in the calcium-signaling pathways.
PLoS One. 10(e0122509)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng S, Tan H, Ling H and Yuan X:
Detecting overexpression level of HER2 gene in NSCLC by real-time
quantitative PCR and the 2[-Delta Delta C(T)] method. Zhongguo Fei
Ai Za Zhi. 14:938–942. 2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
12
|
Elena C, Chiara M, Angelica B, Chiara MA,
Laura N, Chiara C, Claudio C, Antonella F and Nicola G:
Hyperglycemia and diabetes induced by glucocorticoids in
nondiabetic and diabetic patients: Revision of literature and
personal considerations. Curr Pharm Biotechnol. 19:1210–1220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lechner J, O'Leary OE and Stitt AW: The
pathology associated with diabetic retinopathy. Vision Res.
139:7–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yahagi K, Kolodgie FD, Lutter C, Mori H,
Romero ME, Finn AV and Virmani R: Pathology of human coronary and
carotid artery atherosclerosis and vascular calcification in
diabetes mellitus. Arterioscler Thromb Vasc Biol. 37:191–204.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duran CL, Howell DW, Dave JM, Smith RL,
Torrie ME, Essner JJ and Bayless KJ: Molecular regulation of
sprouting angiogenesis. Compr Physiol. 8:153–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376.
1980.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Frodl T and Amico F: Is there an
association between peripheral immune markers and
structural/functional neuroimaging findings? Prog
Neuropsychopharmacol Biol Psychiatry. 48:295–303. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Basudhar D, Glynn SA, Greer M,
Somasundaram V, No JH, Scheiblin DA, Garrido P, Heinz WF, Ryan AE,
Weiss JM, et al: Coexpression of NOS2 and COX2 accelerates tumor
growth and reduces survival in estrogen receptor-negative breast
cancer. Proc Natl Acad Sci USA. 114:13030–13035. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zergoun AA, Zebboudj A, Sellam SL, Kariche
N, Djennaoui D, Ouraghi S, Kerboua E, Amir-Tidadini ZC, Chilla D,
Asselah F, et al: IL-6/NOS2 inflammatory signals regulate MMP-9 and
MMP-2 activity and disease outcome in nasopharyngeal carcinoma
patients. Tumour Biol. 37:3505–3514. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
da Cunha NV, Lopes FN, Panis C, Cecchini
R, Pinge-Filho P and Martins-Pinge MC: iNOS inhibition improves
autonomic dysfunction and oxidative status in hypertensive obese
rats. Clin Exp Hypertens. 39:50–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Q, Wu DH, Han L, Deng JW, Zhou L, He
R, Lu CJ and Mi QS: Roles of microRNAs in psoriasis: Immunological
functions and potential biomarkers. Exp Dermatol. 26:359–367.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zaccagnini G, Maimone B, Di Stefano V,
Fasanaro P, Greco S, Perfetti A, Capogrossi MC, Gaetano C and
Martelli F: Hypoxia-induced miR-210 modulates tissue response to
acute peripheral ischemia. Antioxid Redox Signal. 21:1177–1188.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mian C, Pennelli G, Fassan M, Balistreri
M, Barollo S, Cavedon E, Galuppini F, Pizzi M, Vianello F and
Pelizzo MR: , et al: MicroRNA profiles in familial and
sporadic medullary thyroid carcinoma: preliminary relationships
with RET status and outcome. Thyroid. 22:890–896. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen YS, Meng F, Li HL, Liu QH, Hou PF,
Bai J and Zheng JN: Dicer suppresses MMP-2-mediated invasion and
VEGFA-induced angiogenesis and serves as a promising prognostic
biomarker in human clear cell renal cell carcinoma. Oncotarget.
7:84299–84313. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ma X, Shen D, Li H, Zhang Y, Lv X, Huang
Q, Gao Y, Li X, Gu L, Xiu S, et al: MicroRNA-185 inhibits cell
proliferation and induces cell apoptosis by targeting VEGFA
directly in von Hippel-Lindau-inactivated clear cell renal cell
carcinoma. Urol Oncol. 33:169.e1–169.e11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bao L, Fu X, Si M, Wang Y, Ma R, Ren X and
Lv H: MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction
in diabetes. PLoS One. 10(e0116067)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang R, Tian S, Wang HB, Chu DP, Cao JL,
Xia HF and Ma X: MiR-185 is involved in human breast carcinogenesis
by targeting Vegfa. FEBS Lett. 588:4438–4447. 2014.PubMed/NCBI View Article : Google Scholar
|