Introduction
Endometritis is an inflammatory reaction of the
uterine endometrial lining, which typically occurs due to infection
(1). There are two types of
endometritis, namely acute and chronic (2). The former is frequently triggered by
miscarriage, abortion, parturition or ascending infection of the
uterine cavity, whilst the latter is closely associated with
infertility or problematic pregnancy (3,4).
Initiation of the inflammatory response caused by Gram-negative
bacteria that cause endometritis mainly occurs by the recognition
of lipopolysaccharide (LPS) by Toll-like receptor 4 (TLR4) in the
epithelial cells of the endometrium (5). It has been previously reported that
treatment of primary bovine endometrial epithelial cells (bEECs)
with LPS induces a rapid inflammatory response (6). LPS is considered to be a trigger of
the inflammatory response in bEECs in an in vitro
endometritis cell model (7). In
addition, previous in vivo and in vitro studies have
revealed that endoplasmic reticulum (ER) stress is involved in the
inflammatory response and apoptosis of endometrial stromal cells
and mouse uterine tissues (8,9).
Honokiol (HKL) is a natural phenol that can be
extracted from the oriental herb Magnolia officinalis and
has been demonstrated to protect against ER stress-related
apoptosis in a rat model of testicular injury (10). The clinical use of Magnolia
officinalis in traditional Chinese medicine for
anti-inflammatory and antibacterial purposes has been documented in
Chinese culture for >1,800 years. HKL is an active component of
this extract that has also been shown to exhibit anti-inflammatory
and anti-oxidant properties in previous studies of cardiovascular
diseases, kidney injury and asthma (11-13).
However, to the best of our knowledge, whether and how HKL may be
of benefit in endometritis has not been elucidated to date.
In the present study, it was inferred that HKL may
also confer beneficial effects on inflammation in endometritis.
Lipopolysaccharide (LPS) is a classic endotoxin that has been used
widely in inflammatory model establishment, including the
establishment of an endometritis model (7,14). In
particular, HKL has been demonstrated to exert protective effects
against LPS-induced inflammation in the respiratory system and at
the cellular level (15-17).
Therefore, the present study conducted an
experimental analysis of the possible role of HKL in an in
vitro model of LPS-induced endometritis, in the hope that the
subsequent findings will provide another therapeutic approach to
endometritis.
Materials and methods
Cell culture and treatment
BEND bovine endometrial epithelial cells (bEECs;
ATCC® CRL-2398™) were acquired from American
Type Culture Collection. The cells were grown in a 1:1 mixture of
Ham's F12 and Eagle's Minimal Essential medium (Gibco; Thermo
Fisher Scientific, Inc.) with Earle's balanced salts with 1.5 mM
L-glutamine (MilliporeSigma) adjusted to contain 1.5 g/l sodium
bicarbonate (MilliporeSigma) supplemented with 0.034 g/l D-valine
(MilliporeSigma), 10% FBS (Biowest LLC) and 10% horse serum (Gibco;
Thermo Fisher Scientific, Inc.) in an environment containing 5%
CO2 at 37˚C. HKL (purity, ≥98%) was purchased from
Shanghai Aladdin Biochemical Technology Co., Ltd., where its
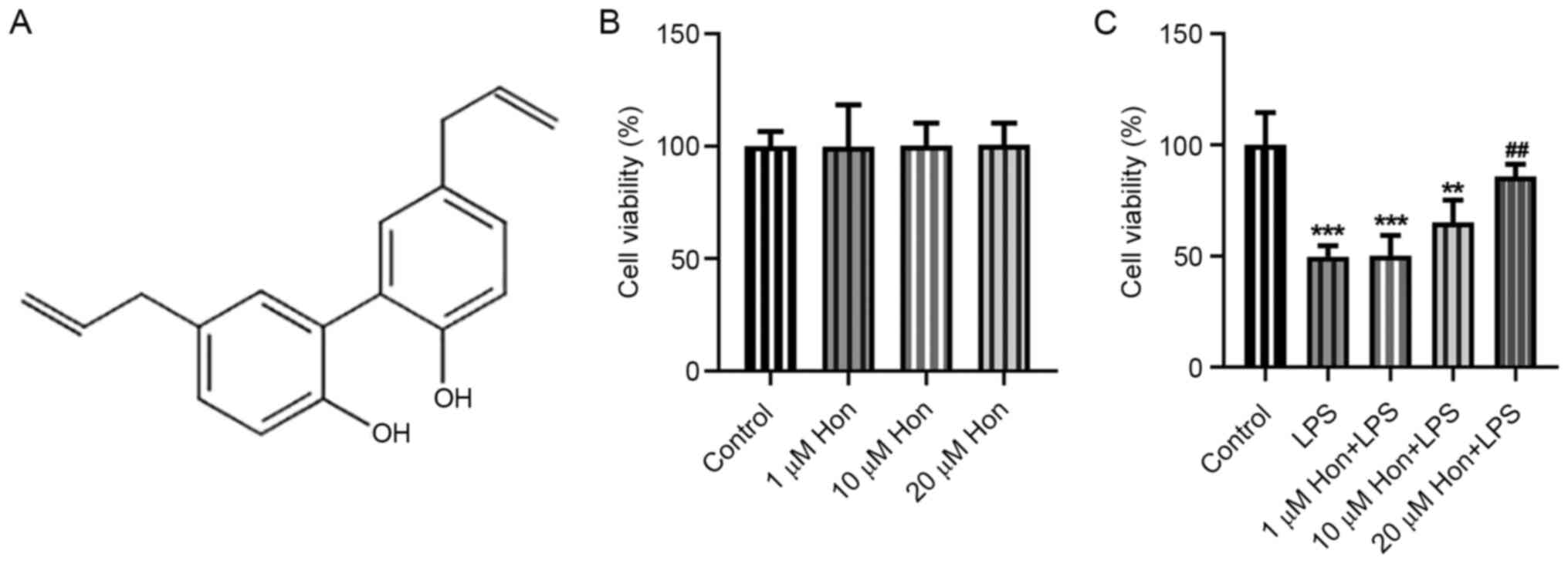
structural formula is shown in Fig.
1A.
Pre-treatment of the cells was performed with HKL at
doses of 1, 10 and 20 µM for 1 h at 37˚C, followed by stimulation
with 1 µg/ml LPS for 12 h at 37˚C. In addition, 1 µg/ml Tunicamycin
(an ER stress inducer; purchased from MedChemExpress), was added
into cells for 2 h at 37˚C followed by LPS treatment. Cells without
any treatment were considered to be the control group.
MTT assay
Cells in the logarithmic growth phase were
collected. Subsequently, 100 µl cell suspension was added to each
well of a 96-well plate to a final density of 1x103
cells/well. Following corresponding treatment, the cells were
incubated with 5% CO2 for 12 h at 37˚C. Next, 20 µl 0.5%
MTT solution (Shanghai Aladdin Biochemical Technology Co., Ltd.)
was added to each well, and the incubation was continued for
another 4 h at 37˚C. Subsequently, 150 µl DMSO solution (EMD
Millipore) was added to each well, followed by gentle oscillation
on a shaking rocker to fully dissolve the crystals. A microplate
reader was used to measure the optical density at a wavelength of
480 nm.
ELISA
ELISA kits were used to detect the expression levels
of TNF-α (cat. no. DY2279; R&D Systems, Inc.), IL-1β (cat. no.
ESS0027; Thermo Fisher Scientific, Inc.) and IL-6 (cat. no. DY8190;
R&D Systems, Inc.) in bEECs in different treatment groups. The
supernatant was centrifuged at 14,000 x g for 10 min. Preparation
of the solution was performed in accordance with the manufacturer's
instructions. A total of 90 µl of the sample was added to each well
and the wells were sealed and incubated in the dark for 120 min at
37˚C. Subsequently, the plate was washed three times and dried with
absorbent paper, followed by the addition of 100 µl
3,3',5,5'-tetramethylbenzidine solution and incubation in the dark
for20 min at 37˚C. After termination of the reaction, the
absorbance was detected in each well at 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). RNA
quality was examined by ultraviolet spectroscopy. Reverse
transcription was performed to synthesize cDNA by PrimeScript RT
Master Mix (Takara Bio, Inc.) and the reaction was incubated at
25˚C for 5 min, 42˚C for 30 min, 85˚C for 5 min, and then
maintained at 4˚C for 5 min. Subsequently, PCR reactions were
conducted with the SYBR Premix ExTaq kit (Takara Bio, Inc.) and the
DNA was amplified for 5 min at 95˚C; followed by 40 cycles of
denaturation for 20 sec at 95˚C, annealing for 20 sec at 55˚C and
extension for 20 sec at 72˚C. After termination of PCR and natural
cooldown to 60˚C, the temperature was increased up to 95˚C to
denature the DNA products. The primer sequences for PCR were as
follows: TNF-α forward, 5'-GCCTCCCTCTCATCAGTTCTA-3' and reverse,
5'-GGCA GCCTTGTCCCTTG-3'; IL-1β forward, 5'-ACCTGTGTCTTT CCCGTGG-3'
and reverse, 5'-TCATCTCGGAGCCTGT AGTG-3'; IL-6 forward,
5'-AGTTGTGCAATGGCAAT TCTGA-3' and reverse,
5'-CCCCAGCATCGAAGGTAGA-3'; and GAPDH forward,
5'-TGCTGTCCCTGTATGCCTCT-3' and reverse, 5'-TTTGATGTCACGCACGATTT-3'.
The 2-ΔΔCq method was applied for the relative
quantification of the data using GAPDH as the normalization control
(18).
TUNEL staining
The Colorimetric TUNEL Apoptosis Assay Kit (Beyotime
Institute of Biotechnology) was purchased for the cell apoptosis
assay. The cells were washed twice with Dulbecco's PBS and fixed
with 4% paraformaldehyde at room temperature for 30 min in the
dark. Then the cells were incubated with proteinase K for 15 min at
37˚C and placed in 3% H2O2 for 15 min at room
temperature, followed by staining with the TUNEL detection kit. A
total of 50 µl labeling solution was added to the samples for
incubation in the dark for 60 min at 37˚C. The cells were then
incubated with 0.1 ml termination solution at room temperature for
10 min before being washed three times with PBS (Thermo Fisher
Scientific, Inc.). After washing, cells were co-labeled with DAPI
working solution (1 µg/ml) for 10 min at 37˚C. The staining could
be observed after the cells were washed three times with PBS under
a fluorescence microscope (Nikon Eclipse80i; Nikon Corporation;
magnification, x200), and at least 10 fields per section for each
sample were examined. TUNEL apoptosis rate (%) = number of
TUNEL-positive podocytes/total number of podocytes x100%.
Western blotting
Protein samples were collected using IP lysis buffer
(cat. no G2038; Wuhan Servicebio Technology Co., Ltd.) and protein
concentration was measured using a BCA kit (Beyotime Institute of
Biotechnology). The samples were sufficiently denatured after
boiling in a water bath at 100˚C for 3-5 min. A total of 30 µg
protein samples per well were separated by 10% SDS-PAGE and
electrophoretically transferred onto PVDF membranes. After being
blocked with 5% non-fat milk in TBS with 0.1% Tween-20 for 1 h at
room temperature, the membranes were then incubated with the
specific primary antibody [anti-Bcl-2 (1:1,000; cat. no. ab32124;
Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-cleaved
caspase 3 (1:500; cat. no. ab32042; Abcam), anti-cleaved caspase 9
(1:1,000; cat. no. ab2324; Abcam), anti-activating transcription
factor 6 (ATF6; 1:1,000; cat. no. ab37149; Abcam),
anti-CCAAT-enhancer-binding protein homologous protein (CHOP;
1:1,000; cat. no. ab11419; Abcam), anti-inositol-requiring enzyme 1
(IRE1; 1:1,000; cat. no. ab96481; Abcam), anti-caspase 12 (1:1,000;
cat. no. ab8117; Abcam), anti-cleaved caspase 12 (1:1,000; cat. no.
ab62484; Abcam) and anti-GAPDH (1:3,000; ab125247; Abcam)] diluted
with TBS-0.05% Tween-20 (TBST; Cell Signaling Technology, Inc.) on
a shaking bed at 4˚C overnight. The membrane was then incubated
with the specific secondary antibody goat anti-mouse IgG H&L
(HRP) (1:1,000; ab6789; Abcam) or goat anti-rabbit IgG H&L
(HRP) (1:1,000; ab6721; Abcam) for 2 h at 37˚C. TBST was used to
wash the membrane before photographic development using an ECL kit
(Beyotime Institute of Biotechnology) and the band density was
analyzed using ImageJ software (version 1.49; National Institutes
of Health). GAPDH was used as the internal reference.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc.) was
utilized for data graphing and analysis. All data are presented as
the mean ± SD. All experiments were performed independently three
times. One-way ANOVA followed by Bonferroni post hoc test was
applied for the comparisons among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
HKL treatment improves the viability
of LPS-stimulated bEECs
To investigate how HKL affects the viability of
bEECs, cells were treated with different doses of HKL. The MTT
assay results demonstrated that 0, 1, 10 and 20 µM HKL treatment
conferred no cytotoxicity to bEECs (Fig. 1B). However, after LPS induction, it
was revealed that it significantly reduced bEEC viability, which
was in turn improved by HKL treatment in a dose-dependent manner
(Fig. 1C). This suggest that HKL
treatment may improve the viability of LPS-stimulated bEECs.
HKL treatment inhibits LPS-induced
inflammation and apoptosis of bEECs
ELISA was utilized to examine the effect of HKL
treatment on the inflammation and apoptosis of LPS-stimulated
bEECs. Significantly increased secretion of proinflammatory
cytokines (TNF-α, IL-1β and IL-6) in bEECs was found after LPS
stimulation, which were markedly suppressed by HKL treatment in a
dose-dependent manner (Fig. 2A).
The mRNA expression levels of these cytokines were detected by
RT-qPCR, which were significantly higher in LPS-stimulated bEECs
compared with those in cells in the control group. However, they
were gradually decreased by treatment with increasing doses of HKL
(Fig. 2B). Furthermore, TUNEL
staining revealed that LPS-induced apoptosis was significantly
alleviated as bEECs were treated with increasing doses of HKL
(Fig. 2C and D). Western blotting also detected
significantly decreased expression levels of the anti-apoptotic
protein Bcl-2 and significantly increased expression levels of
pro-apoptotic proteins Bax, cleaved caspase-3 and cleaved caspase-9
following LPS treatment (Fig. 2E
and 2F). By contrast, these effects
aforementioned were all marked reversed after HKL treatment,
especially at the high dose (20 µM; Fig. 2E and 2F). These results suggest that HKL
treatment inhibited the LPS-induced inflammatory response and
apoptosis in bEECs.
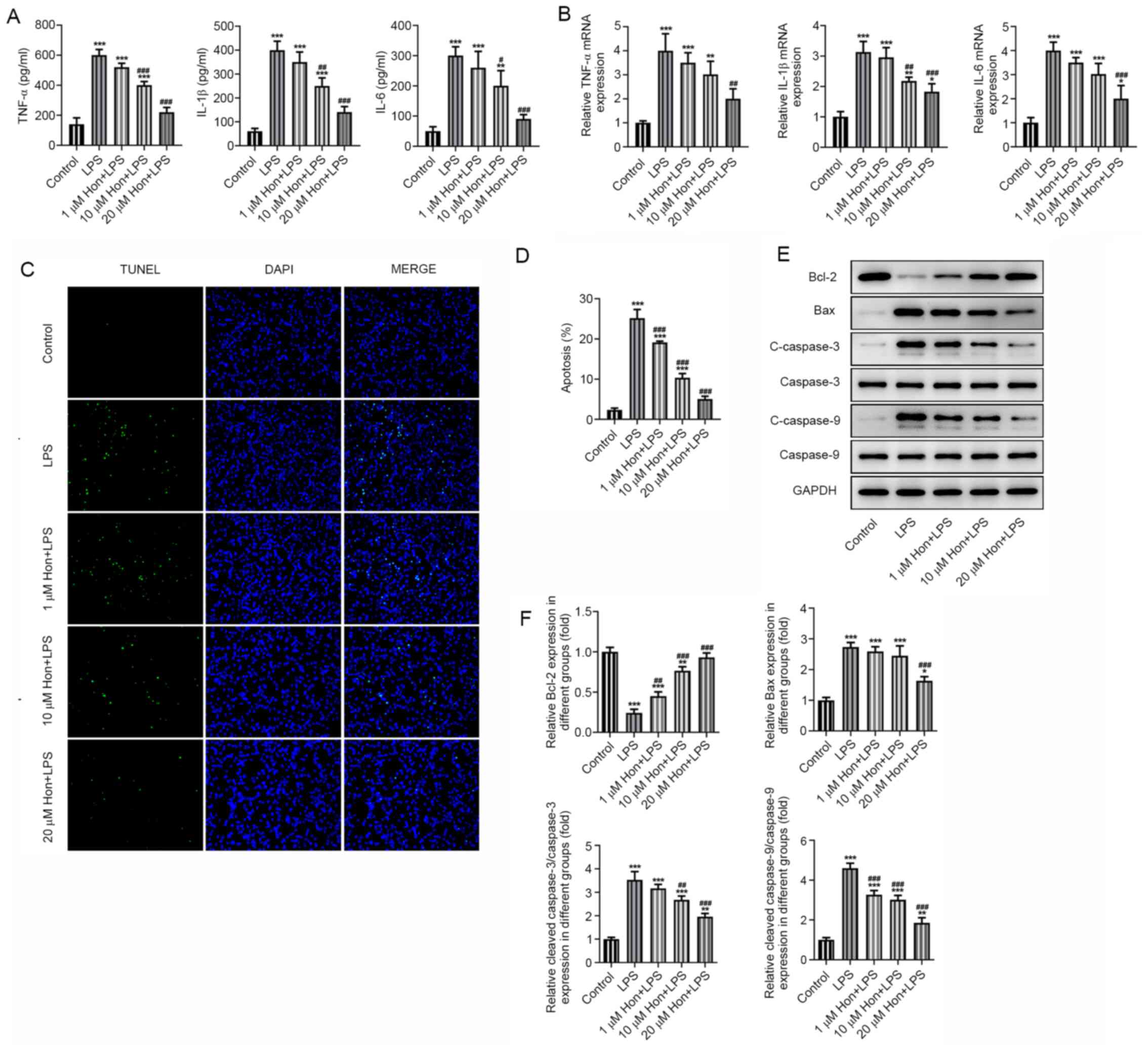 | Figure 2Honokiol treatment inhibits
LPS-induced inflammation and apoptosis in bEECs. (A) The expression
levels of proinflammatory cytokines TNF-α, IL-1β and IL-6 in
LPS-stimulated bEECs following treatment with different doses of
HKL as detected by ELISA. (B) The mRNA expression of
proinflammatory cytokines TNF-α, IL-1β and IL-6 in LPS-stimulated
bEECs following treatment with different doses of HKL as detected
by reverse transcription-quantitative PCR. (C) Apoptosis level of
LPS-stimulated bEECs following treatment with different doses of
HKL as observed by TUNEL staining, (D) which was quantified. (E)
The expression of apoptosis markers Bcl-2, Bax, cleaved caspase-3
and cleaved caspase-9 in LPS-stimulated bEECs following treatment
with different doses of HKL as detected by western blotting, (F)
which was quantified. *P<0.05,
**P<0.01, ***P<0.001 vs. Control.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS. HKL or Hon, honokiol; LPS,
lipopolysaccharide; bEECs, bovine endometrial epithelial cells. |
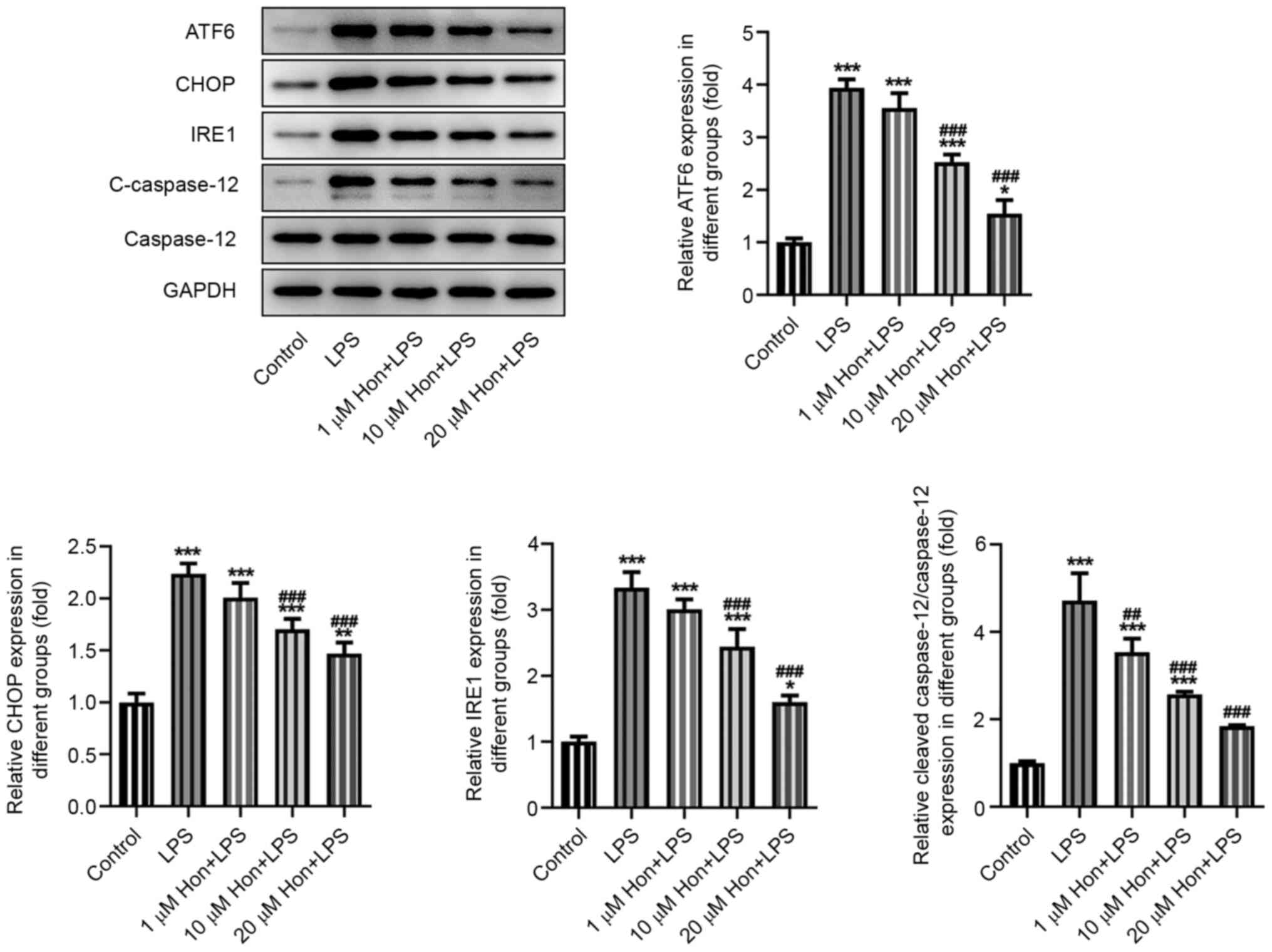
HKL inhibits LPS-induced ER stress in
bEECs
The expression levels of ER stress-related proteins
activating transcription factor 6, CCAAT-enhancer-binding protein
homologous protein, inositol-requiring enzyme 1 and cleaved
caspase-12 were also detected by western blotting, which revealed a
significantly increase in their expression levels after LPS
stimulation and a marked reversal by HKL co-treatment in a
dose-dependent manner (Fig. 3).
This suggested an inhibitory effect of HKL on LPS-induced ER stress
in bEECs.
HKL mitigates ER stress to inhibit the
LPS-induced inflammatory response and apoptosis in bEECs
To identify whether ER stress is a link between HKL
treatment and its effects on bEEC inflammation and apoptosis, bEECs
were treated with the ER stress inducer tunicamycin (1 µg/ml)
(19), followed by treatment with
20 µM HKL. Results from ELISA demonstrated that while the
expression levels of proinflammatory TNF-α, IL-1β and IL-6 in
LPS-stimulated bEECs were significantly suppressed by HKL
treatment, subsequent treatment with tunicamycin significantly
reversed this effect (Fig. 4A). The
same trend in the mRNA expression levels of these cytokines was
also observed according to data from RT-qPCR (Fig. 4B). Additionally, it was observed by
TUNEL staining that HKL significantly reduced the apoptosis levels
of LPS-stimulated bEECs, but co-treatment with tunicamycin
significantly elevated these apoptosis levels again (Fig. 4C and D). Consistently, western blotting revealed
that tunicamycin significantly decreased the expression levels of
the anti-apoptotic protein Bcl-2 whilst significantly increasing
the expression levels of the pro-apoptotic proteins Bax, cleaved
caspase-3 and cleaved caspase-9 in LPS and HKL-treated bEECs,
compared with those in the group with LPS and HKL treatment but
without tunicamycin treatment (Fig.
4E and F). These results
collectively suggest that HKL inhibited the LPS-induced
inflammatory response and apoptosis by mitigating ER stress in
bEECs.
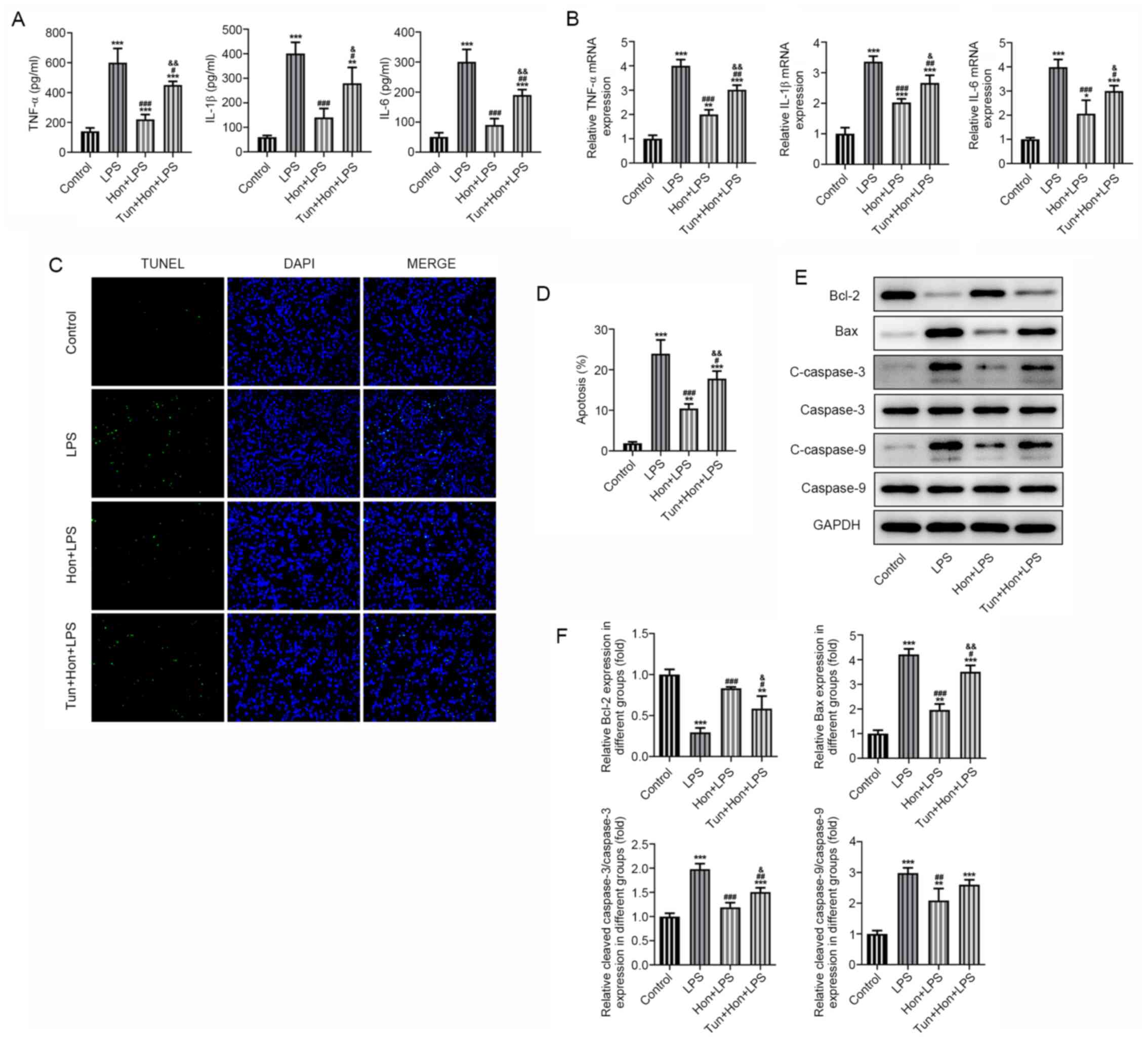 | Figure 4Honokiol mitigates ER stress to
inhibit LPS-induced inflammation and apoptosis in bEECs. (A) The
expression level of proinflammatory cytokines TNF-α, IL-1β and IL-6
in LPS-stimulated bEECs treated with 20 µM HKL in the absence or
presence of tunicamycin as detected by ELISA. (B) The mRNA
expression of proinflammatory cytokines TNF-α, IL-1β and IL-6 in
LPS-stimulated bEECs treated with HKL in the absence or presence of
tunicamycin as detected by reverse transcription-quantitative PCR.
(C) The apoptosis level of LPS-stimulated bEECs treated with HKL in
the absence or presence of tunicamycin as observed by TUNEL
staining, (D) which was quantified. (E) The expression level of
apoptosis-related proteins Bcl-2, Bax, cleaved caspase-3 and
cleaved caspase-9 in LPS-stimulated bEECs treated with HKL in the
absence or presence of tunicamycin as detected by western blotting,
(F) which was quantified. *P<0.05,
**P<0.01 and ***P<0.001 vs. control.
#P<0.05, ##P<0.01,
###P<0.001 vs. the LPS group.
&P<0.01, &&P<0.001 vs. the
Hon + LPS group. HKL or Hon, honokiol; LPS, lipopolysaccharide;
bEECs, bovine endometrial epithelial cells; tun, tunicamycin. |
Discussion
Endometritis is caused by inflammation in the
endometrium, either in the form of acute attacks or in a chronic
setting (18). Endometritis is
typically caused by bacterial infection or lesions in the uterine
cavity (20), which may lead to
subfertility or infertility, fetal malformations, premature
delivery or miscarriage (2,21,22).
Currently available therapeutic strategies for endometritis are
limited to antibiotic treatment, intrauterine drug administration,
dilation and curettage, which are not favorable either due to the
lack of efficacy or are deeply unpleasant for the patient (23-26).
Therefore, a milder but more effective treatment option for this
condition remain urgently in demand.
HKL is a natural polyphenol that is derived from the
traditional Chinese herb Magnolia officinalis, also known as
Mulan in China and has anti-inflammatory and antioxidant properties
(27). It has been previously
demonstrated to alleviate oxidative stress and block inflammatory
signals to protect against sepsis-induced acute kidney injury
(11,28). Chen et al (29) showed that HKL modulates the function
of the pulmonary microvascular endothelial barrier and ameliorates
LPS-induced acute respiratory distress syndrome by the activating
sirtuin 3/AMP-activated protein kinase pathway to inhibit
angiopoietin 2. Although there is a substantial number of studies
on the therapeutic potential of HKL, to the best of our knowledge,
its role in endometritis has not been previously reported (12,30).
Therefore, the present study was performed to verify this
hypothesis.
The in vitro model of endometritis in the
present study was established through LPS stimulation of bEECs. LPS
treatment can induce an inflammatory response that may contribute
to the injury and death of cells (31). In addition, since drug treatment
require a period of time before it can exert its effect, bEECs were
treated with HKL prior to LPS stimulation (32,33).
The present study revealed that the viability of bEECs without LPS
stimulation was not affected by HKL treatment at any dose,
suggesting that HKL exerted no cytotoxic effects on bEECs.
Additionally, LPS-stimulated bEECs treated with low to high doses
of HKL exhibited increased viability to varying degrees, which
provided preliminary proof for the aforementioned hypothesis.
Furthermore, this suppressive action of HKL on IL-1β-induced
inflammation has been corroborated by a previous study, where the
survival and chondrogenesis of human umbilical-cord-derived
mesenchymal stem cells was improved by HKL (34). In fact, the anti-inflammatory
activity of HKL has been reported in studies on other
inflammation-associated diseases. For example, HKL demonstrated
protective potential against smoking-induced inflammation,
senescence and apoptosis of keratinocytes (35). Liu et al (12) also revealed that the levels of
proinflammatory markers TNF-α, IL-6 and IL-1β, in addition to those
of reactive oxygen species, declined after intraperitoneal
injection of HKL into mice following the induction of carotid
artery atherosclerotic lesions. Furthermore, the present results
demonstrated that HKL effectively inhibited the expression of
proinflammatory cytokines TNF-α, IL-1β and IL-6, as well as that of
pro-apoptotic proteins, in LPS-stimulated bEECs. By contrast, the
expression levels of the anti-apoptotic protein Bcl-2 were markedly
upregulated by HKL treatment in a dose-dependent manner. This
demonstrated the prominent anti-inflammatory and anti-apoptotic
potential of HKL for treating endometritis. In an in vivo
study in which cognitive disorders and behaviors similar to
depression were observed in mice exposed to restraint stress, HKL
treatment (10 mg/kg) conferred marked improvements in these
symptoms, whereas inflammation and ER stress levels were both
markedly inhibited (36).
ER stress has been previously implicated in the
LPS-induced apoptosis of endometrial stromal cells and
overexpression of inflammatory cytokines in the goat uterus
(8). A close association between ER
stress and the activation of thioredoxin-interacting protein/NLR
family pyrin domain-containing 3 inflammasome has also been
validated in a mouse model of LPS-induced endometritis (9). Therefore, the present study
investigated the possible involvement of ER stress in endometritis.
LPS enhanced the levels of ER stress-related proteins ATF6, CHOP,
IRE1 and cleaved caspase 12 but a notable reduction in the levels
of these proteins was observed in bEECs after HKL treatment.
Furthermore, LPS and HKL-treated bEECs were treated with the ER
stress inducer tunicamycin, which resulted in the reversal of the
effects mediated by HKL, revealing the potential regulatory effects
of HKL on ER stress in the present endometritis model.
In conclusion, findings of the present study
suggested that HKL effectively suppressed ER stress, thereby
inhibiting the inflammatory response and apoptosis induced by LPS
in bEECs. These findings may further improve our understanding on
both the therapeutic potential of HKL and the role of ER stress in
the pathogenic process of endometritis. Although the molecular
mechanism underlying HKL-mediated protection against endometritis
require further study, HKL may be considered as a potentially
effective strategy for endometritis treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and JW designed the study, drafted and revised
the manuscript. SZ and XL analyzed the data and searched the
literature. WC, JW and SZ performed the experiments. SZ and XL
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitaya K, Takeuchi T, Mizuta S,
Matsubayashi H and Ishikawa T: Endometritis: New time, new
concepts. Fertil Steril. 110:344–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kimura F, Takebayashi A, Ishida M,
Nakamura A, Kitazawa J, Morimune A, Hirata K, Takahashi A, Tsuji S,
Takashima A, et al: Review: Chronic endometritis and its effect on
reproduction. J Obstet Gynaecol Res. 45:951–960. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kiviat NB, Wølner-Hanssen P, Eschenbach
DA, Wasserheit JN, Paavonen JA, Bell TA, Critchlow CW, Stamm WE,
Moore DE and Holmes KK: Endometrial histopathology in patients with
culture-proved upper genital tract infection and laparoscopically
diagnosed acute salpingitis. Am J Surg Pathol. 14:167–175.
1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Park HJ, Kim YS, Yoon TK and Lee WS:
Chronic endometritis and infertility. Clin Exp Reprod Med.
43:185–192. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Herath S, Fischer DP, Werling D, Williams
EJ, Lilly ST, Dobson H, Bryant CE and Sheldon IM: Expression and
function of Toll-like receptor 4 in the endometrial cells of the
uterus. Endocrinology. 147:562–570. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gärtner MA, Bondzio A, Braun N, Jung M,
Einspanier R and Gabler C: Detection and characterisation of
Lactobacillus spp. in the bovine uterus and their influence on
bovine endometrial epithelial cells in vitro. PLoS One.
10(e0119793)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang H, Wu ZM, Yang YP, Shaukat A, Yang
J, Guo YF, Zhang T, Zhu XY, Qiu JX, Deng GZ, et al: Catalpol
ameliorates LPS-induced endometritis by inhibiting inflammation and
TLR4/NF-κB signaling. J Zhejiang Univ Sci B. 20:816–827.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mohamed AAA, Yang D, Liu S, Lin P, Mohamad
OAA and Jin Y: Endoplasmic reticulum stress is involved in
lipopolysaccharide-induced inflammatory response and apoptosis in
goat endometrial stromal cells. Mol Reprod Dev. 86:908–921.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hu X, Li D, Wang J, Guo J, Li Y, Cao Y,
Zhang N and Fu Y: Melatonin inhibits endoplasmic reticulum
stress-associated TXNIP/NLRP3 inflammasome activation in
lipopolysaccharide-induced endometritis in mice. Int
Immunopharmacol. 64:101–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang KH, Weng TI, Huang HY, Huang KD, Lin
WC, Chen SC and Liu SH: Honokiol attenuates
torsion/detorsion-induced testicular injury in rat testis by way of
suppressing endoplasmic reticulum stress-related apoptosis.
Urology. 79:967.e5–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xia S, Lin H, Liu H, Lu Z, Wang H, Fan S
and Li N: Honokiol Attenuates Sepsis-Associated Acute Kidney Injury
via the Inhibition of Oxidative Stress and Inflammation.
Inflammation. 42:826–834. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Y, Cheng P and Wu AH: Honokiol
inhibits carotid artery atherosclerotic plaque formation by
suppressing inflammation and oxidative stress. Aging (Albany NY).
12:8016–8028. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hong T, Min H, Hui Z, Yuejian L, Lixing Y
and Liang XZ: Oral administration of honokiol attenuates airway
inflammation in asthmatic mouse model. Pak J Pharm Sci.
31:1279–1284. 2018.PubMed/NCBI
|
|
14
|
Li R, Maimai T, Yao H, Liu X, He Z, Xiao
C, Wang Y and Xie G: Protective effects of polydatin on LPS-induced
endometritis in mice. Microb Pathog. 137(103720)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen L, Li W and Wang D: [Honokiol
attenuates lipopolysaccharide-induced acute respiratory distress
syndrome via activation of mitochondrion-dependent Sirt3/AMPK
pathway]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 43:1075–1082.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rickert U, Cossais F, Heimke M, Arnold P,
Preusse-Prange A, Wilms H and Lucius R: Anti-inflammatory
properties of Honokiol in activated primary microglia and
astrocytes. J Neuroimmunol. 323:78–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee SY and Cho JY: Inhibitory effects of
honokiol on LPS and PMA-induced cellular responses of macrophages
and monocytes. BMB Rep. 42:574–579. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li W, Li W, Leng Y, Xiong Y and Xia Z:
Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion
Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol.
39:210–225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Puente E, Alonso L, Laganà AS, Ghezzi F,
Casarin J and Carugno J: Chronic Endometritis: Old Problem, Novel
Insights and Future Challenges. Int J Fertil Steril. 13:250–256.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vitagliano A, Saccardi C, Noventa M, Di
Spiezio Sardo A, Saccone G, Cicinelli E, Pizzi S, Andrisani A and
Litta PS: Effects of chronic endometritis therapy on in vitro
fertilization outcome in women with repeated implantation failure:
A systematic review and meta-analysis. Fertil Steril.
110:103–112.e1. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaku S, Kubo T, Kimura F, Nakamura A,
Kitazawa J, Morimune A, Takahashi A, Takebayashi A, Takashima A,
Kushima R, et al: Relationship of chronic endometritis with chronic
deciduitis in cases of miscarriage. BMC Womens Health.
20(114)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meaney-Delman D, Bartlett LA, Gravett MG
and Jamieson DJ: Oral and intramuscular treatment options for early
postpartum endometritis in low-resource settings: A systematic
review. Obstet Gynecol. 125:789–800. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mackeen AD, Packard RE, Ota E and Speer L:
Antibiotic regimens for postpartum endometritis. Cochrane Database
Syst Rev. 2015(2)(CD001067)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mustafa-Mikhail S, Assaraf S, Abecassis P,
Dabaja H, Jarrous S, Hadad S and Lowenstein L: Comparison between
Lornoxicam and Paracetamol for Pain Management after Dilation and
Curettage for Abortion. Isr Med Assoc J. 19:543–546.
2017.PubMed/NCBI
|
|
26
|
Liu H, Song J, Zhang F, Li J, Kong W, Lv
S, Zhang L and Yan L: A New Hysteroscopic Scoring System for
Diagnosing Chronic Endometritis. J Minim Invasive Gynecol.
27:1127–1132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo Y, Lv X, Wang Y, Zhou Y, Lu N, Deng X
and Wang J: Honokiol Restores Polymyxin Susceptibility to
MCR-1-Positive Pathogens both In Vitro and In Vivo. Appl Environ
Microbiol. 86:e02346–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang T and Xiang L: Honokiol alleviates
sepsis-induced acute kidney injury in mice by targeting the
miR-218-5p/heme oxygenase-1 signaling pathway. Cell Mol Biol Lett.
24(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen L, Li W, Qi D, Lu L, Zhang Z and Wang
D: Honokiol protects pulmonary microvascular endothelial barrier
against lipopolysaccharide-induced ARDS partially via the
Sirt3/AMPK signaling axis. Life Sci. 210:86–95. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rauf A, Patel S, Imran M, Maalik A, Arshad
MU, Saeed F, Mabkhot YN, Al-Showiman SS, Ahmad N and Elsharkawy E:
Honokiol: An anticancer lignan. Biomed Pharmacother. 107:555–562.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ren Q, Guo F, Tao S, Huang R, Ma L and Fu
P: Flavonoid fisetin alleviates kidney inflammation and apoptosis
via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways
in septic AKI mice. Biomed Pharmacother. 122(109772)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yin P, Zhang Z, Li J, Shi Y, Jin N, Zou W,
Gao Q, Wang W and Liu F: Ferulic acid inhibits bovine endometrial
epithelial cells against LPS-induced inflammation via suppressing
NK-κB and MAPK pathway. Res Vet Sci. 126:164–169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shen Y, Liu B, Mao W, Gao R, Feng S, Qian
Y, Wu J, Zhang S, Gao L, Fu C, et al: PGE2 downregulates
LPS-induced inflammatory responses via the TLR4-NF-κB signaling
pathway in bovine endometrial epithelial cells. Prostaglandins
Leukot Essent Fatty Acids. 129:25–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu H, Yin Z, Wang L, Li F and Qiu Y:
Honokiol improved chondrogenesis and suppressed inflammation in
human umbilical cord derived mesenchymal stem cells via blocking
nuclear factor-κB pathway. BMC Cell Biol. 18(29)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Costa A, Facchini G, Pinheiro ALTA, da
Silva MS, Bonner MY, Arbiser J and Eberlin S: Honokiol protects
skin cells against inflammation, collagenolysis, apoptosis, and
senescence caused by cigarette smoke damage. Int J Dermatol.
56:754–761. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jangra A, Dwivedi S, Sriram CS, Gurjar SS,
Kwatra M, Sulakhiya K, Baruah CC and Lahkar M: Honokiol abrogates
chronic restraint stress-induced cognitive impairment and
depressive-like behaviour by blocking endoplasmic reticulum stress
in the hippocampus of mice. Eur J Pharmacol. 770:25–32.
2016.PubMed/NCBI View Article : Google Scholar
|