Introduction
Diabetic retinopathy (DR) is a microvascular
complication associated with diabetes mellitus (DM) that is usually
caused by hyperglycemia-induced leakage of the retinal capillary
wall (1). Diabetes can cause
various physiological and pathological changes to eye structure and
function, including cataracts, glaucoma and optic nerve atrophy
(2). Pathophysiological changes in
the retina are important causes of visual function loss in DM
patients (3). According to the
results of previous studies, impaired physiological function of the
retina in patients with DM is most often caused by blockage of the
microcirculation (4-7).
It has been established that altered microvessel structure, as is
the case where increased basement membrane thickness reduces the
inner diameter of the blood vessel, represents one of the causes of
microcirculation blockage (8).
Retinopathy is often detected in patients with DM and the risk of
these patients developing proliferative diabetic retinopathy
(PDR)-induced vision loss is increased (9), which should be distinguished from DR,
the generalized diseases.
Clinical studies have indicated that there is an
elevated concentration of various inflammatory cytokines in the
serum and vitreous humor of patients with PDR, including monocyte
chemotactic protein-1, interleukin (IL)-1α, IL-6, tumor necrosis
factor-α and vascular endothelial growth factor (VEGF) (10,11).
There have also been reports investigating the roles of VEGF in DR
(12-14).
Jain et al (15) have
suggested that high expression levels of VEGF and intercellular
adhesion molecule-1 in DM patients are associated with destruction
of the retinal outer membrane and the internal and external
photoreceptor segments, which is positively correlated with the
degree of retinopathy. As an important risk factor for promoting
PDR neovascularization (thus enhancing irregular growth of blood
vessels and increasing microcirculation burden) (16,17),
VEGF can not only directly bind to vascular endothelial cells to
promote pathological proliferation, but also induce the secretion
of other inflammatory factors, contributing to disease progression
(18). To the best of our
knowledge, the mechanism underlying VEGF regulation has not been
fully elucidated.
MicroRNAs (miRNAs) are a class of 18-22-nucleotide
non-coding small RNA molecules in eukaryotes, which regulate gene
expression at the mRNA level (19,20).
Altered expression levels of multiple miRNAs and proteins have been
observed in the pathogenesis of DR, suggesting that miRNAs may play
important roles in regulating the levels of genes and proteins in
disease pathogenesis (21,22). miRNA-23a has been indicated to be
involved in angiogenesis in lung cancers and nasopharyngeal
carcinomas (23,24). In cardiovascular diseases, such as
the coronary heart disease, miRNA-23a inhibits VEGF expression and
regulates angiogenic processes (25). The regulation of VEGFA by microRNA
is closely related to angiogenesis. For example, miRNA-134 has been
suggested to inhibit angiogenesis and osteosarcoma proliferation
through targeting the VEGFA/VEGFR1 pathway, and miRNA-29b inhibits
the angiogenesis of endometrial cancer by targeting VEGFA (26,27).
However, to the best of our knowledge, there have been no reports
concerning the effects of miRNA-23a on VEGF in the pathogenesis of
DR.
In the present study, the roles of miRNA-23a and
VEGF in the pathogenesis of DR were investigated. The expression
levels of VEGF and miRNA-23a in blood and tear samples from
patients with PDR and in a rat DR model were determined and
analyzed. The relationship and interaction between miRNA-23a and
VEGF were also investigated and discussed.
Materials and methods
Study subjects
In total 33 patients with proliferative type 2 DR
(PDR), 15 males and 18 females with a median age of 54.6 years old
(age range, 38 to 62 years), who were admitted to The Third
Affiliated Hospital of Jinzhou Medical University (Liaoning, China)
between June and December 2013, were included in this study. A
further 28 healthy subjects, 13 males and 15 females, with a median
age of 55.2 years old (age range, 39-63), were also included as the
control group during the same period and from the same hospital.
Blood and tear samples were collected from these subjects. All
patients were diagnosed by the same fundus specialist, based on
dilated fundus examination and photography, as well as binocular
B-ultrasound. Inclusion criteria for patients with PDR was as
follows: i) Subject met the WHO diagnostic criteria for type 2
diabetes 1999(28); and ii)
patients whose findings from the fluorescein fundus angiography
under fully dilated condition met the diagnostic criteria for PDR
according to the DR diagnosis and treatment 2014 guidelines from
the Chinese Fundus Group (29).
Among the PDR patients, 8 cases were at the early stage of
hyperplasia, 20 cases at the fibrous hyperplasia-stage and 5 cases
at the late hyperplasia stage. All patients were treated in
accordance with Chinese PDR treatment guidelines. In the control
group, diabetes and retinopathy were clearly excluded according to
the examination. The study was approved by The Ethics Committee of
The Third Affiliated Hospital of Jinzhou Medical University, and
written informed consent was obtained from each subject.
Sample preparation
For the collection of peripheral blood serum,
density gradient centrifugation combined with the adherent
separation was conducted. Peripheral venous blood samples (10-15
ml; anti-coagulated with EDTA, 1.5 mg/ml) were obtained from each
subject and placed at 4˚C for 1-2 h. The upper supernatant in the
tube was collected and centrifuged at 400 x g for 10 min and then
stored at -70˚C. The tear samples were harvested from the patients
and healthy subjects on the day of the physical examination and
stored at -70˚C. Before sampling, the skin of the lower eyelid was
smeared with alcohol. When the reflective tears appeared, a
capillary was used to suck the tears from the outer canthus.
Study animals and model
establishment
A total of 30 male specific pathogen free
Sprague-Dawley rats, 50-days-old, weighing 180-200 g, were provided
by Chongqing Tengxinbier Experimental Animal Co., Ltd. [animal
approval number: SCXK (Yu) 2016-0012; Chongqing, China]. Before
experiments, all rats were acclimatized for one week, in a 12-h
light/dark cycle, at the constant temperature of 24±2˚C, with a
humidity of 55±5%, and free access to food and water. The 3R animal
welfare principle (30) was
followed during the animal experiments.
For the model establishment, after fasting for 12 h,
the SD rats were intraperitoneally injected with streptozotocin
(STZ; Sigma-Aldrich; Merck KGaA; in 0.1 mol/l sodium citrate
buffer, pH 4.6), at 60 mg/kg body weight. Rats in the control group
were injected with an equivalent volume of sodium citrate buffer.
After 72 h, venous blood samples were collected through the tail
vein, for random blood glucose monitoring. Model establishment was
considered to be successful when the random blood glucose level
exceeded the level of 16.7 mmol/l, which was maintained for one
week. After successful model establishment, animal body weights and
the random blood glucose levels were recorded every week (31). Before sacrifice, animals were
injected with 50 mg/kg body pentobarbital and then subject to the
decapitation. All animal experiment were performed in accordance
with the guidelines of the Ethics Committee of Guangxi Medical
University.
Reverse transcription-quantitative-PCR
(RT-qPCR)
Total RNA was extracted from the hRMECs with
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
cDNA was obtained from the total RNA with reverse-transcription
with the TIANScript II cDNA First Strand Synthesis kit (cat. no.
KR107; Tiangen Biotech Co., Ltd.) at 42˚C. The qPCR was performed
with the miRcute miRNA Fluorescence Quantification kit (cat. no.
FP401; Tiangen Biotech. Co., Ltd.) with the SuperReal PreMix (SYBR
Green) (cat. no. FP204; Tiangen Biotech. Co., Ltd.) on the iQ5
qRT-PCR instrument (Bio-Rad, Laboratories, Inc.).
For the determination of human VEGF mRNA levels, the
following primer sequences were used: human VEGF, forward
5'-TTGCCTTGCTGCTCTACCTC-3' and reverse 5'-AAATGCTTTCTCCGCTCTGA-3';
and human β-actin, forward 5'-TGACGTGGACATCCGCAAAG-3' and reverse
5'-CTGGAAGGTGGACAGCGAGG-3'. The 25-µl system consisted of 12.5 µl
SYBR Premix EXTaqTM, 10 µM primer each, 1 µl cDNA, and 0.5 µl
ddH2O. The reaction conditions were as follows: 94˚C for
2 min, followed by 34 cycles of 94˚C for 30 sec, 55˚C for 30 sec
and 71˚C for 1 min followed by a final extension at 71˚C for 2
min.
For the determination of rat VEGF mRNA expression
levels, the following primer sequences were used: miRNA-23a,
forward 5'-CCATGAACTTTCTGCTCTTC-3' and reverse
5'-GGTGAGAGGTCTAGTTCCCGA-3'; and GAPDH, forward
5'-CCCTCAATGACCACTTTGTG-3' and reverse 5'-GGTTTGAGGGCTCTTACTCCT-3'.
The reaction conditions were set as follows: 95˚C for 10 min
followed by 40 cycles of 95˚C for 30 sec, 55˚C for 20 sec and 72˚C
for 30 sec.
For the determination of miRNA-23a expression levels
in the tissues, the following primer sequences were used:
miRNA-23a, forward 5'-ATCACATTGCCAGGGA-3' and reverse
5'-CAGTGCGTGTCGTGGAGT-3'; and U6, forward 5'-CTCGCTTCGGCAGCACA-3'
and reverse 5'-AACGCTTCACGAATTTGCGT-3'. The reaction conditions
were set as follows: 95˚C for 5 min followed by 40 cycles of 95˚C
for 10 sec, 60˚C for 35 sec and 72 for 30 sec. Relative expression
levels of the target genes were calculated with the
2-ΔΔCq method (32).
β-actin, GAPDH and U6 were used as internal references.
Western blot analysis
Tissue samples and cells were lysed with the RIPA
lysis solution (cat. no. P0013B; Beyotime Institute of
Biotechnology). Protein concentration was determined with the BCA
method (cat. no. RTP7102; ZhongKeRuitai Biotechnology Co, Ltd.). A
total of 20 µg of each protein was separated by 10% SDS-PAGE and
then transferred onto the PDVF membrane. After blocking with 5%
non-fat milk at room temperature for 1 h, the membrane was
incubated with rabbit anti-human anti-VEGF polyclonal primary
antibody (1:1,000 dilution; cat. no. ab46154; Abcam) or
anti-β-actin primary antibody (1:5,000 dilution; cat. no. ab129348;
Abcam) at 4˚C overnight. The membrane was then incubated with goat
anti-rabbit secondary antibody (1:3,000 dilution; cat. no. ab6721;
Abcam) at room temperature for 1 h. Blots were visualized using ECL
reagent (cat. no. ab65623; Abcam). Protein bands was acquired and
analyzed using Image lab 3.0 software (Bio-Rad Labratories, Inc.).
β-actin was used as internal reference.
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples were centrifuged at 1,000 x g, at 4˚C,
for 10 min to obtain the serum, and the tear samples were directly
harvested. The VEGF levels were determined with ELISA kits (Human
VEGF ELISA kit; cat. no. ab222510; and Rat VEGF ELISA kit; cat. no.
ab100786; Abcam). According to the manufacturer's instructions, 50
µl standard samples at indicated concentrations were added into the
standard wells, while 10 µl serum or tear samples were added into
the sample wells, followed by 40 µl of diluting solution.
HRP-labeled detection antibody (100 µl) was added into the standard
and sample wells. The plate was sealed and incubated for 1 h. After
washing for 5 times, substrates A and B (50 µl each) were added
into the wells and the plate was incubated at 37˚C for 15 min. A
total of 50 µl of stopping solution was added into each well and
the OD values at 450 nm were read within 15 min.
Bioinformatics analysis
Based on retrieved references (33-36),
bioinformatics analysis was performed to predict the up-stream
miRNA that would interact with VEGF, to further investigate the
regulatory mechanism underlying the DR pathogenesis. miRanda
software (2010 version; http://www.microma.org/rnicroma/home.do) was used for
the gene prediction, according to the online software operating
instructions.
Cell transfection
For cell transfection, the hRMECs or 293 cells were
seeded onto 24-well plates at the density of 3x105
cells/well with F12/DMEM medium containing 10% FBS without
antibiotics, which were incubated at 37˚C. The cell culture
duration varied from cell to cell, to achieve the cell growth
confluence of 70%, which was generally 24-48 h. Cell transfection
was performed when 70% confluence was achieved. The
plasmid/siRNA/agomiR (designed and synthesized by Sangon Biotech.
Co., Ltd.) sequences were: AgomiR-23a, forward
5'-GGGGUUCCUGGGGAUGGGAUUU-3', and reverse,
5'-AAAUCCCAUCCCCAGGAACCCC-3'; agomiR-NC forward,
5'-UUUGUACUACACAAAAGUACUG-3', and reverse,
5'-CAGUACUUUUGUGUAGUACAAA-3'). Plasmid (0.8 µg)/siRNA (100
nM)/agomiR (100 nM) and lipofectamine® 2000 (1 µl) were
added to the EP tubes containing 50 µl Opti Memi medium (Thermo
Fisher Scientific, Inc.), respectively. After 5 min, the solution
in these two tubes were mixed together. After a further 20 min at
room temperature, the mixture was used to incubate the cells for 6
h and then the medium was replaced with F12/DMEM medium (Thermo
Fisher Scientific, Inc.). After 48 h, the cells were collected for
analysis.
Dual luciferase reporter assay
The wild-type and mutant sequence regions in the
VEGF 3'-untranslated region (UTR) for miRNA-23a were chemically
synthesized by the Sangon, Biotech Co., Ltd. with the Spe-1 and
HindIII restriction sites at each end, respectively. These two DNA
fragments were cloned into the pMIR-REPORT luciferase reporter
plasmid from the Dual-Luciferase® Reporter Assay System
(Promega Corporation), and the mutant 3'-UTR region was used as a
control. The sequences were as follows: hsa-miR-23a,
3'-CCUUUAGGGACCGUUACACUA-5'; VEGFA, 5'-AUGUUUAUGUAUAUAUGUGAU-3';
rno-miR-23a, 3'-CCUUUAGGGACCG-UUACACUA-5'; and Vegfa,
5'-CCUGCAGCAUAGCAGAUGUGAA-3'.
The plasmids (0.8 µg) containing the wild-type and
mutant 3'-UTR DNA sequences were transfected into 293T cells (Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences)
using Lipofectamine® for 24 h in a 37˚C, 5%
CO2 incubator, in 12/DMEM medium. Then 100 nM
agomiRNA-23a was transfected into the cells for 24 h. The cells
were then lysed, and the luciferase was detected using a GloMax
20/20 sluminometer. Renilla was used as internal
reference.
MTT assay
hRMECs (human retinal microvascular endothelial
cells) were purchased from the Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences and were seeded onto 96-well
plates at a density of 2x103/well. At 24, 48, and 72 h,
respectively, 20 µl MTT (5 g/l) was added into each well. At each
indicated time point, MTT was added into the wells and incubated at
37˚C for 4 h, and then 150 µl of DMSO was added. The absorbance at
490 nm was detected, and the cell proliferation curve was plotted
accordingly.
Statistical analysis
Data are presented as the mean ± SD. SPSS 18.0
(SPSS, Inc.) was used for statistical analysis. After the normality
test, the one-way ANOVA was used for multiple group comparison,
with the LSD and SNK tests for data with homogeneous variance, or
the Tamhane's T2 or Dunnett's T3 test for data with non-homogeneous
variance. P<0.05 was considered statistically significant.
Results
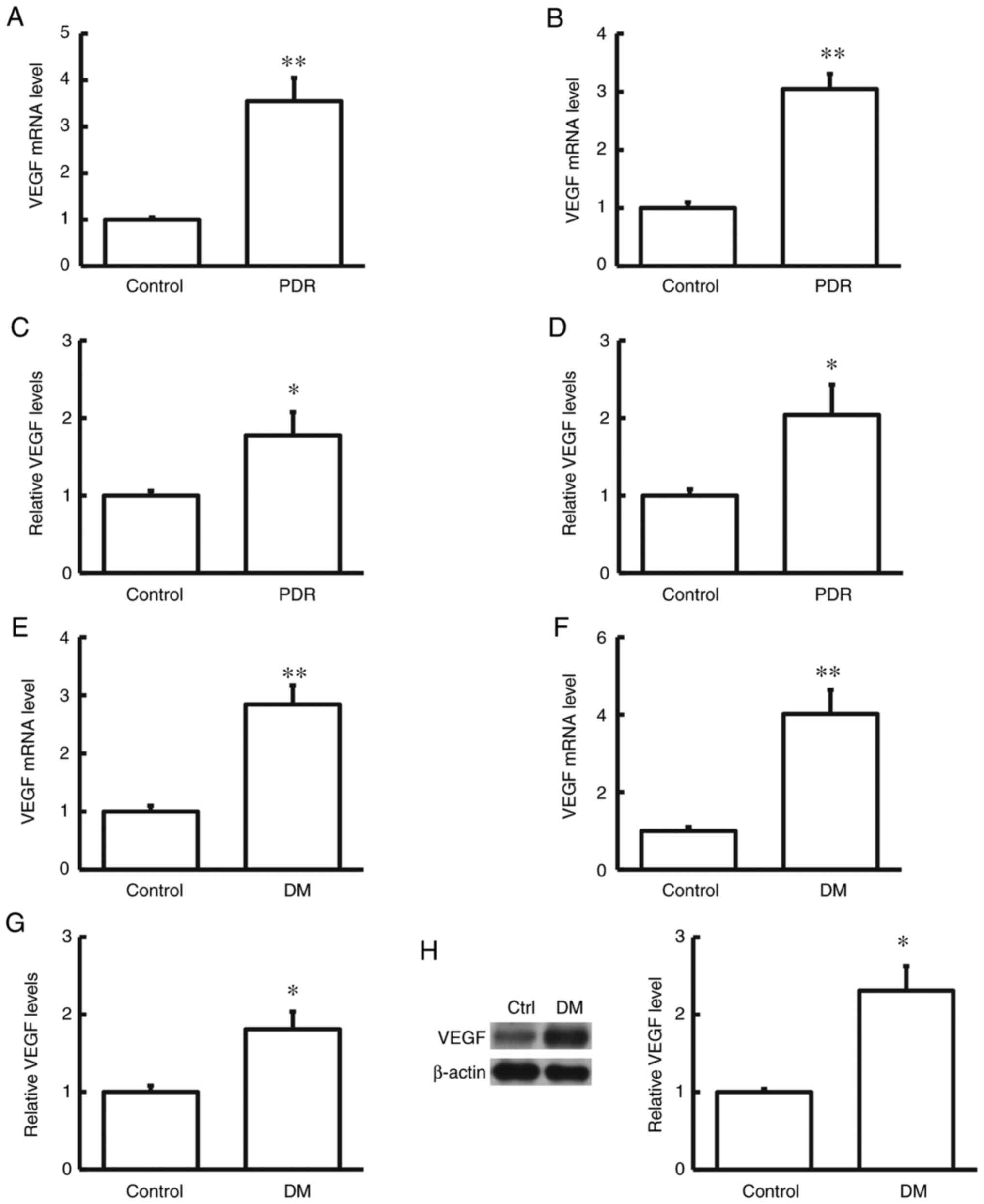
VEGF mRNA and protein expression
levels in human samples and rat model samples
To determine the mRNA and protein expression levels
of VEGF in the human serum and tear samples, RT-qPCR and ELISA were
performed. The results indicated that in comparison with the
control group, both the mRNA and protein expression levels of VEGF
in the human serum and tear samples were significantly increased in
patients with DR (both P<0.05; Fig.
1).
mRNA and protein expression levels of VEGF in the
serum and retina samples of diabetic rat models were determined by
RT-qPCR, ELISA and western blot analysis. The results showed that
in comparison with the control group, the mRNA and protein
expression levels of VEGF in the serum and retina of diabetic rats
was significantly increased (both P<0.05; Fig. 1). Taken together, these results
suggest that VEGF may play a regulatory role in the disease
pathogenesis of DR.
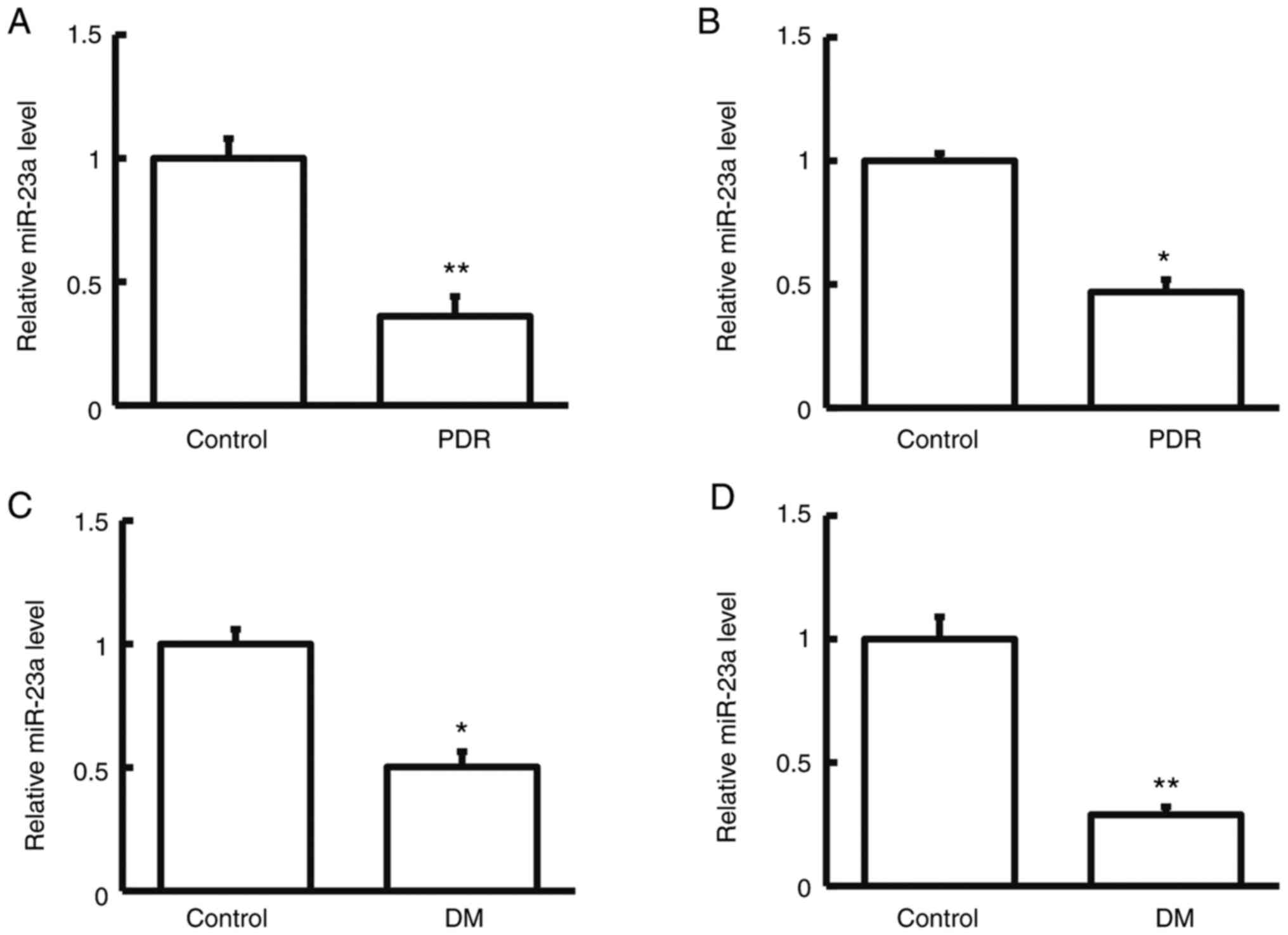
Expression of miRNA-23a in human
samples and rat model samples
To determine the miRNA-23a expression levels in the
human serum and tear samples RT-qPCR was performed. The results
showed that in comparison with the control group, miRNA-23a
expression levels were significantly reduced in the human serum and
tear samples in the patients with DR (P<0.05; Fig. 2).
miRNA-23a expression levels in the serum and retina
samples of diabetic rats were determined by RT-qPCR. The results
indicated that in comparison with the control group, the miRNA-23a
expression levels in the serum and retina samples of diabetic rats
were significantly reduced (P<0.05; Fig. 2). Taken together, these results
suggest that miRNA-23a may be involved in the pathogenesis and
development of DR.
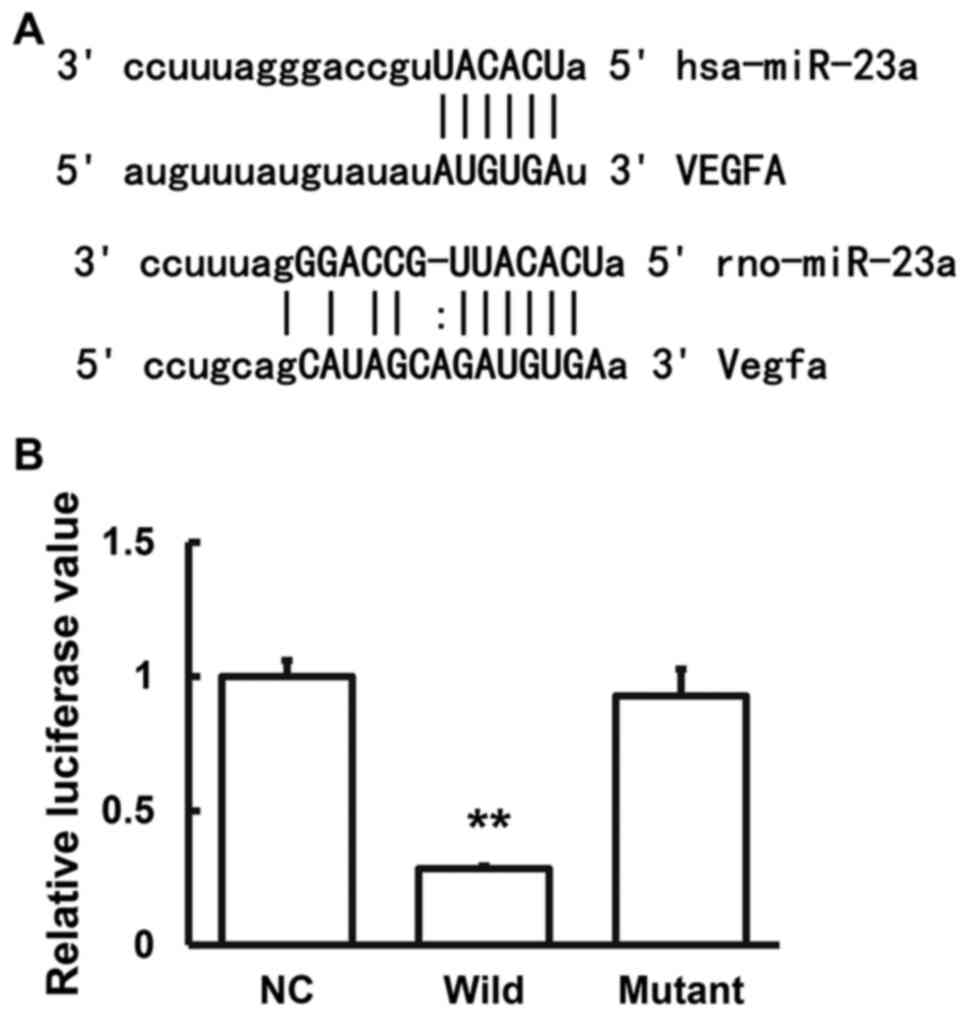
Prediction and confirmation of
interaction between miRNA-23a and VEGF
miRanda software (http://www.microma.org/rnicroma/home.do) was used to
predict the possible VEGF regulatory genes. The results showed that
miRNA-23a could be a probable regulatory gene (Fig. 3). Dual luciferase reporter assay was
performed to confirm the interaction between the miRNA-23a and
VEGF. The results indicated that after co-transfection with the
agomiRNA-23a and pMIR-REPORT luciferase reporter plasmids, the
luciferase values were significantly reduced (P<0.05), while
there were no significant changes in the mutant group (P>0.05;
Fig. 3). These results suggested
that miRNA-23a binds to the 3'-UTR of VEGF to regulate the gene
expression.
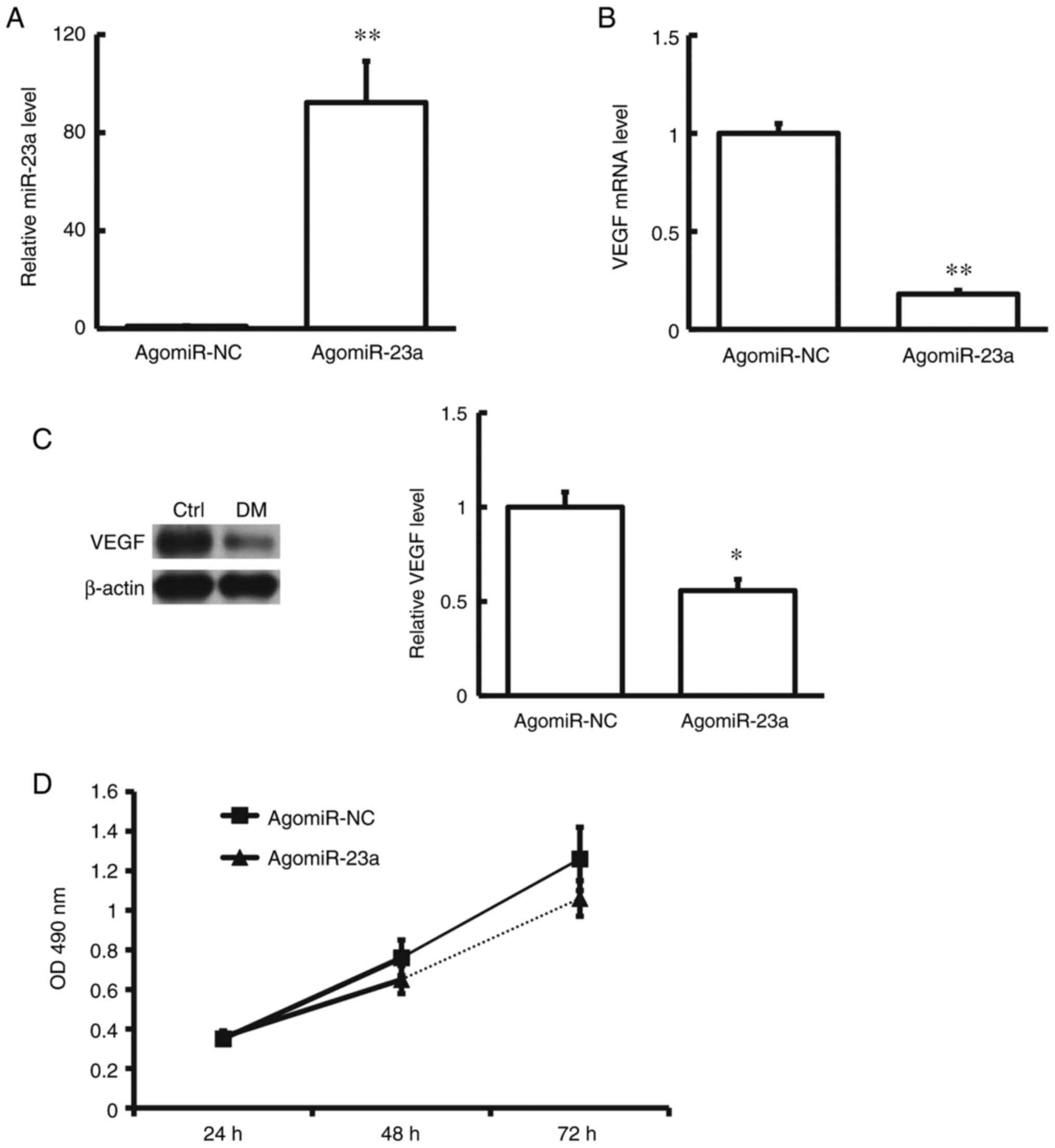
Effects of agomiRNA-23a transfection
on hRMECs
hRMECs were transfected with agomiRNA-23a and cell
proliferative ability was assessed with the MTT assay. The results
showed that after transfection the VEGF expression levels in the
hRMECs were significantly down-regulated. Moreover, after
transfection with agomiRNA-23a, the cell proliferation ability was
significantly declined (P<0.05) (Fig. 4). These results suggest that
agomiRNA-23a would influence the VEGF to inhibit the proliferation
ability of hRMECs.
Discussion
In the present study the mRNA and protein expression
levels of VEGF in the serum and tear samples of patients with DR
were determined as were the expression levels of its upstream
regulatory gene miRNA-23a. Moreover, a rat model of diabetes was
established and the expression levels of VEGF and miRNA-23a in the
blood and retina tissues of diabetic rats were detected. Direct
binding between miRNA-23a and VEGF, as well as related biological
functions, were preliminarily explored and the molecular mechanism
underlying the regulatory effects of miRNA-23a on VEGF in the
pathogenesis of DR were studied.
DR is a multifactorial chronic progressive disease,
which is characterized by altered retinal microvascular blood flow,
impaired function and apoptosis of pericytes and endothelial cells
and increased vascular permeability, further aggravating the
occlusion of capillary vessels (37). The formation of non-perfused areas
results in neovascularization, leading to repeated retinal
hemorrhage and fibroproliferation, recognized as the proliferative
diabetic retinopathy (PDR) (38).
With the progression of DR, the degrees of ischemia and hypoxia are
further aggravated, which may increase the secretion of various
growth-promoting factors (such as fibroblast growth factor 21 and
VEGF) in the vitreous cavity, further leading to the imbalance
between the intraocular angiogenesis and inhibitory factors, and
promoting the disease into the proliferative phase, ending with the
development of refractory neovascular glaucoma (39). The typical pathological features of
PDR include neovascularization and fibroproliferation, which are
also the most important pathological factors leading to visual loss
in patients with DR (40).
Persistent inflammatory responses and up-regulated expression
levels of angiogenic factors are closely related to the
pathogenesis of PDR. Cytokines such as VEGF, interleukin and growth
factors play important roles in pathological process (41). As a potent angiogenic agent, VEGF is
a core factor in PDR development. The biological activity of VEGF
is closely related to its expression level. In normal retinal
tissues, VEGF is expressed at low levels, regulating physiological
angiogenesis (18) and exerting
neuroprotective effects (41). Jain
et al (15) found that in
comparison with normal healthy people, the serum VEGF levels in
diabetic patients were significantly increased along with the
disease progression, in the following order: PDR>NPDR>DM with
no retinopathy>healthy people. Moreover, the VEGF expression
levels in the aqueous humor and vitreous humor are associated with
retinopathy in patients with DM (42). In the present study the VEGF
expression levels in the serum and tear samples from the patients
with DR were significantly increased in comparison with healthy
individuals. In line with this, results from the rat diabetic
models showed that, the VEGF expression levels in the serum and
retina tissues were increased in comparison with normal rats. VEGF
and angiogenesis are closely related, and angiogenesis has been
proven to be an important feature for the progression of PDR
(43). Therefore, VEGF was a focus
of the present investigation. Taken together, the results of the
present study suggest an important role for VEGF in the
pathogenesis and development of DR.
The mechanism underlying the regulatory effects of
VEGF has not been fully elucidated. A correlation between VEGF and
miR-23 has been previously reported (44). In the present study, results from
bioinformatics prediction indicated that miRNA-23a was an upstream
miRNA regulating VEGF, with similar and highly conservative
sequences within the targeted regions in human and rat models.
miRNA-23a is widely involved in the regulation of various
physiological and pathological processes in the body, which plays
an important role in the cellular differentiation (45-47).
miRNA-23a has been shown to be involved in the formation of
skeletal muscle, hematopoietic cell differentiation and bone
metabolism (48). miRNA-23a
expression levels in the skeletal muscle are relatively high, which
has a negative correlation with the process of cell differentiation
(49). miRNA-23a can form a
positive feedback loop with Kruppel like factor 3 to promote the
expression of β-like globulin genes involved in erythrocyte
formation (50). Moreover,
miRNA-23a could reduce the expression of alkaline phosphatase and
osteocalcin genes, thus participating in the regulation of bone
metabolism (51). Furthermore,
miRNA-23a could promote the hypertrophy of cardiomyocytes, which is
involved in the pathogenesis and development of atherosclerotic
coronary heart diseases (52).
These findings suggest that miRNA-23a may be involved in
angiogenesis. The present study results indicated that miRNA-23a
was significantly reduced in the serum and tear samples of patients
with DR in comparison with controls. Similarly, the miRNA-24a
expression levels in the serum and retina tissues of rat diabetic
models were also significantly reduced. These results suggest that
miRNA-23a may play an important role in the pathogenesis of DR. In
the retina and serum samples from diabetic mice, abnormal
expression levels of miRNA-20a-5p, miRNA-20a-3p, miRNA-20b,
miRNA-106a-5p, miRNA-27a-5p, miRNA-27b-3p, miRNA-206-3p and
miR-381-3p were observed. Moreover, the expression levels of VEGF,
BDNF, PPAR-α and CREB1 have also been shown to be altered,
indicating that there is a molecular interaction network (53).
To further investigate the molecular mechanism of
miRNA-23a regulation of VEGF, in the present study hRMECs cells
were cultured and transfected with agomiRNA-23a and the cell
proliferation was analyzed. The results suggested that miRNA-23a
could slow down the cell proliferation of hRMECs. Moreover, the
VEGF mRNA expression levels were reduced after miRNA-23a
transfection. In order to confirm the direct binding of miRNA-23a
and VEGF mRNA a dual luciferase reporter assay was performed. The
results indicated that miRNA-23a could bind to the VEGF mRNA 3'-UTR
seed region and regulate its expression levels.
There were several limitations to the present study.
First, no longitudinal analysis of VEGF and miR-23 concentration
with time was performed due to limited specimen availability, nor
the association analysis had been performed. Additionally, it was
not possible to assess all downstream targets of miR-23a, though
these will be the subject of further research.
In conclusion, the results of the present study
indicated that during DR pathogenesis miRNA-23a expression levels
are reduced which increased the expression levels of VEGF and
enhanced the proliferation of human retinal microvascular cells,
promoting angiogenesis. These results suggest that miRNA-23a and
VEGF play important roles in the pathogenesis and development of
DR.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural
Science Foundation of China (grant no. 81571383) and the China
Postdoctoral Science Foundation (grant no. 2017M612870).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LS, XL and ZZ contributed to the study design,
performing the experiments, data collection and analysis and
manuscript preparation. LS, XL and ZZ confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
the Third Affiliated Hospital of Jinzhou Medical University, and
written informed consent was obtained from each subject.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pan WW, Gardner TW and Harder JL:
Integrative biology of diabetic retinal disease: Lessons from
diabetic kidney disease. J Clin Med. 10(1254)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sosna T: History of diagnosis and therapy
of diabetic retinopathy. Vnitr Lek. 62 (11 Suppl 4):S136–S141.
2016.PubMed/NCBI(In Czech).
|
|
4
|
Liu Y and Wu N: Progress of nanotechnology
in diabetic retinopathy treatment. Int J Nanomedicine.
16:1391–1403. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Inanc M, Tekin K, Kiziltoprak H, Ozalkak
S, Doguizi S and Aycan Z: Changes in retinal microcirculation
precede the clinical onset of diabetic retinopathy in children with
type 1 diabetes mellitus. Am J Ophthalmol. 207:37–44.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forst T, Weber MM, Mitry M, Müller L,
Forst S, Tanis M, Pfützner A and Michelson G: Retinal
microcirculation in type 1 diabetic patients with and without
peripheral sensory neuropathy. J Diabetes Sci Technol. 8:356–361.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vujosevic S, Toma C, Villani E, Gatti V,
Brambilla M, Muraca A, Ponziani MC, Aimaretti G, Nuzzo A, Nucci P
and De Cilla' S: Early detection of microvascular changes in
patients with diabetes mellitus without and with diabetic
retinopathy: Comparison between different swept-source OCT-A
instruments. J Diabetes Res. 2019(2547216)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Heng LZ, Comyn O, Peto T, Tadros C, Ng E,
Sivaprasad S and Hykin PG: Diabetic retinopathy: Pathogenesis,
clinical grading, management and future developments. Diabet Med.
30:640–650. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zheng Y, He M and Congdon N: The worldwide
epidemic of diabetic retinopathy. Indian J Ophthalmol. 60:428–431.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bromberg-White JL, Glazer L, Downer R,
Furge K, Boguslawski E and Duesbery NS: Identification of
VEGF-independent cytokines in proliferative diabetic retinopathy
vitreous. Invest Ophthalmol Vis Sci. 54:6472–6480. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koleva-Georgieva DN, Sivkova NP and
Terzieva D: Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha
and VEGF have influence on the development of diabetic retinopathy.
Folia Med (Plovdiv). 53:44–50. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amadio M, Bucolo C, Leggio GM, Drago F,
Govoni S and Pascale A: The PKCbeta/HuR/VEGF pathway in diabetic
retinopathy. Biochem Pharmacol. 80:1230–1237. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
The American Association of Neurological
Surgeons (AANS), American Society of Neuroradiology (ASNR),
Cardiovascular and Interventional Radiology Society of Europe
(CIRSE), Canadian Interventional Radiology Association (CIRA),
Congress of Neurological Surgeons (CNS), European Society of
Minimally Invasive Neurological Therapy (ESMINT), European Society
of Neuroradiology (ESNR), European Stroke Organization (ESO),
Society for Cardiovascular Angiography and Interventions (SCAI),
Society of Interventional Radiology (SIR)et al. Multisociety
consensus quality improvement revised consensus statement for
endovascular therapy of acute ischemic stroke. Int J Stroke.
13:612–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Platania CBM, Leggio GM, Drago F, Salomone
S and Bucolo C: Computational systems biology approach to identify
novel pharmacological targets for diabetic retinopathy. Biochem
Pharmacol. 158:13–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jain A, Saxena S, Khanna VK, Shukla RK and
Meyer CH: Status of serum VEGF and ICAM-1 and its association with
external limiting membrane and inner segment-outer segment junction
disruption in type 2 diabetes mellitus. Mol Vis. 19:1760–1768.
2013.PubMed/NCBI
|
|
16
|
Lu ES, Cui Y, Le R, Zhu Y, Wang JC, Laíns
I, Katz R, Lu Y, Zeng R, Garg I, et al: Detection of
neovascularisation in the vitreoretinal interface slab using
Widefield swept-source optical coherence tomography angiography in
diabetic retinopathy. Br J Ophthalmol. 2020(317983)2020.PubMed/NCBI(Epub ahead of print). doi:
10.1136/bjophthalmol-2020-317983.
|
|
17
|
Song S, Yu X, Zhang P and Dai H: Increased
levels of cytokines in the aqueous humor correlate with the
severity of diabetic retinopathy. J Diabetes Complications.
34(107641)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Behl T and Kotwani A: Exploring the
various aspects of the pathological role of vascular endothelial
growth factor (VEGF) in diabetic retinopathy. Pharmacol Res.
99:137–148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Inoue K and Ogura A: Cloning mice: From
aspects of donor cells. Tanpakushitsu Kakusan Koso. 52 (Suppl
16):S2189–S2196. 2007.PubMed/NCBI(In Japanese).
|
|
20
|
Williams AE, Moschos SA, Perry MM, Barnes
PJ and Lindsay MA: Maternally imprinted microRNAs are
differentially expressed during mouse and human lung development.
Dev Dyn. 236:572–580. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gomaa AR, Elsayed ET and Moftah RF:
MicroRNA-200b expression in the vitreous humor of patients with
proliferative diabetic retinopathy. Ophthalmic Res. 58:168–175.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Q, Qiu F, Zhou K, Matlock HG,
Takahashi Y, Rajala RVS, Yang Y, Moran E and Ma JX: Pathogenic role
of microRNA-21 in diabetic retinopathy through downregulation of
PPARα. Diabetes. 66:1671–1682. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bao L, You B, Shi S, Shan Y, Zhang Q, Yue
H, Zhang J, Zhang W, Shi Y, Liu Y, et al: Metastasis-associated
miR-23a from nasopharyngeal carcinoma-derived exosomes mediates
angiogenesis by repressing a novel target gene TSGA10. Oncogene.
37:2873–2889. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC,
Tsai PH, Wu CY and Kuo PL: Hypoxic lung cancer-secreted exosomal
miR-23a increased angiogenesis and vascular permeability by
targeting prolyl hydroxylase and tight junction protein ZO-1.
Oncogene. 36:4929–4942. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di Y, Zhang D, Hu T and Li D: miR-23
Regulate the pathogenesis of patients with coronary artery disease.
Int J Clin Exp Med. 8:11759–11769. 2015.PubMed/NCBI
|
|
26
|
Zhang L, Lv Z, Xu J, Chen C, Ge Q, Li P,
Wei D, Wu Z and Sun X: MicroRNA-134 inhibits osteosarcoma
angiogenesis and proliferation by targeting the VEGFA/VEGFR1
pathway. FEBS J. 285:1359–1371. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen HX, Xu XX, Tan BZ, Zhang Z and Zhou
XD: MicroRNA-29b inhibits angiogenesis by targeting VEGFA through
the MAPK/ERK and PI3K/Akt signaling pathways in endometrial
carcinoma. Cell Physiol Biochem. 41:933–946. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vijan S: In the clinic. Type 2 diabetes.
Ann Intern Med. 162:ITC1–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu X: Ocular fundus disease in China: The
current situation, progression, and issues to be resolved. Zhonghua
Yan Ke Za Zhi. 50:801–803. 2014.PubMed/NCBI(In Chinese).
|
|
30
|
Wellenberg A, Weides L, Kurzke J, Hennecke
T, Bornhorst J, Crone B, Karst U, Brinkmann V, Fritz G and Honnen
S: Use of C. elegans as a 3R-compliant in vivo model for the
chemoprevention of cisplatin-induced neurotoxicity. Exp Neurol.
341(113705)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ighodaro OM and Akinloye OA: Anti-diabetic
potential of Sapium ellipticum (Hochst) Pax leaf extract in
Streptozotocin(STZ)-induced diabetic Wistar rats. BMC Complement
Altern Med. 17(525)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Czescik A, Trzcinska A, Dunal-Szczepaniak
M and Siennicka J: The use of real-time RT-PCR method for the
determination of Toll-like genes expression at mRNA level. Med Dosw
Mikrobiol. 66:17–22. 2014.PubMed/NCBI(In Polish).
|
|
33
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tang H, Zhang S, Huang C, Li K, Zhao Q and
Li X: MiR-448-5p/VEGFA axis protects cardiomyocytes from hypoxia
through regulating the FAS/FAS-L signaling pathway. Int Heart J.
62:647–657. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yan Z, Hong S, Song Y and Bi M:
microR-4449 Promotes colorectal cancer cell proliferation via
regulation of SOCS3 and activation of STAT3 signaling. Cancer Manag
Res. 13:3029–3039. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu X, Lei J, Zhou B, Sun Q, Gao Y, Shi F
and Yang W: MiR-628-5p inhibits cervical carcinoma proliferation
and promotes apoptosis by targeting VEGF. Am J Med Sci.
361:499–508. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nishinaka A, Nakamura S, Tanaka M, Masuda
T, Inoue Y, Yamamoto T, Imai T, Hidaka Y, Shimazawa M and Hara H:
Excess adiponectin in eyes with progressive ocular vascular
diseases. FASEB J. 35(e21313)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang W, Liu H, Al-Shabrawey M, Caldwell
RW and Caldwell RB: Inflammation and diabetic retinal microvascular
complications. J Cardiovasc Dis Res. 2:96–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kovacs K, Marra KV, Yu G, Wagley S, Ma J,
Teague GC, Nandakumar N, Lashkari K and Arroyo JG: Angiogenic and
inflammatory vitreous biomarkers associated with increasing levels
of retinal ischemia. Invest Ophthalmol Vis Sci. 56:6523–6530.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Umeda N, Ozaki H, Hayashi H, Kondo H,
Uchida H and Oshima K: Non-paralleled increase of hepatocyte growth
factor and vascular endothelial growth factor in the eyes with
angiogenic and nonangiogenic fibroproliferation. Ophthalmic Res.
34:43–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Calvo PM, de la Cruz RR and Pastor AM:
Synaptic loss and firing alterations in Axotomized Motoneurons are
restored by vascular endothelial growth factor (VEGF) and VEGF-B.
Exp Neurol. 304:67–81. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chernykh VV, Varvarinsky EV, Smirnov EV,
Chernykh DV and Trunov AN: Proliferative and inflammatory factors
in the vitreous of patients with proliferative diabetic
retinopathy. Indian J Ophthalmol. 63:33–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kianersi F, Ghanbari H, Naderi Beni Z and
Naderi Beni A: Intravitreal vascular endothelial growth factor
(VEGF) inhibitor injection in patient during pregnancy. J Drug
Assessment. 10:7–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ang WJ, Zunaina E, Norfadzillah AJ,
Raja-Norliza RO, Julieana M, Ab-Hamid SA and Mahaneem M: Evaluation
of vascular endothelial growth factor levels in tears and serum
among diabetic patients. PLoS One. 14(e0221481)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu F, Wang F, Yang Q, Zhang Y, Cai K, Liu
L, Li S, Zheng Y, Zhang J, Gui Y, et al: Upregulation of
miRNA-23a-3p rescues high glucose-induced cell apoptosis and
proliferation inhibition in cardiomyocytes. In Vitro Cell Dev Biol
Anim. 56:866–877. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Huang Y, Huang J, Qi R, Wang Q, Wu Y and
Wang J: Effects of MicroRNA-23a on differentiation and gene
expression profiles in 3T3-L1 adipocytes. Genes (Basel).
7(92)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shen L, Chen L, Zhang S, Zhang Y, Wang J
and Zhu L: MicroRNA-23a reduces slow myosin heavy chain isoforms
composition through myocyte enhancer factor 2C (MEF2C) and
potentially influences meat quality. Meat Sci. 116:201–206.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xu Y, Jiang Y, Jia B, Wang Y and Li T:
Icariin stimulates osteogenesis and suppresses adipogenesis of
human bone mesenchymal stem cells via miR-23a-mediated activation
of the Wnt/β-catenin signaling pathway. Phytomedicine.
85(153485)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hernandez-Torres F, Aranega AE and Franco
D: Identification of regulatory elements directing
miR-23a-miR-27a-miR-24-2 transcriptional regulation in response to
muscle hypertrophic stimuli. Biochim Biophys Acta. 1839:885–897.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ma Y, Wang B, Jiang F, Wang D, Liu H, Yan
Y, Dong H, Wang F, Gong B, Zhu Y, et al: A feedback loop consisting
of microRNA 23a/27a and the β-like globin suppressors KLF3 and SP1
regulates globin gene expression. Mol Cell Biol. 33:3994–4007.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang Y, Xie RL, Croce CM, Stein JL, Lian
JB, van Wijnen AJ and Stein GS: A program of microRNAs controls
osteogenic lineage progression by targeting transcription factor
Runx2. Proc Natl Acad Sci USA. 108:9863–9868. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Han H, Qu G, Han C, Wang Y, Sun T, Li F,
Wang J and Luo S: MiR-34a, miR-21 and miR-23a as potential
biomarkers for coronary artery disease: A pilot microarray study
and confirmation in a 32 patient cohort. Exp Mol Med.
47(e138)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Platania CBM, Maisto R, Trotta MC, D'Amico
M, Rossi S, Gesualdo C, D'Amico G, Balta C, Herman H, Hermenean A,
et al: Retinal and circulating miRNA expression patterns in
diabetic retinopathy: An in silico and in vivo approach. Br J
Pharmacol. 176:2179–2194. 2019.PubMed/NCBI View Article : Google Scholar
|