Introduction
Perfluorooctane sulfonate (PFOS) is a degradation
product of perfluorinated compounds and is characterized by
widespread use and environmental stability (1). This chemical compound has been used in
a large variety of industrial and commercial material, such as
plastic packaging bags, cosmetics and textiles (2). It is globally distributed and can be
detected in soil, air, water, wildlife and in humans (1,2). It
can accumulate biologically through the food chain, having a
half-life of ~5 years in human serum (3). Therefore, the potential toxicity of
PFOS is concerning. The chief manufacturer of PFOS in the United
States, The 3M Company, is to halt production (4), and a series of international
regulations were set out to restrict usage of polyfluoroalkyl
substances in 2016(5). However,
emission of PFOS still persists in Asian markets (6,7). A
recent study reported that PFOS concentrations in serum are
significantly increased with age in the general Chinese population,
suggesting that they may have common exposure sources (8).
As previously reported, PFOS involves several toxic
effects in the cardiovascular (9)
and reproductive systems (10),
effecting immunological (11) and
hepatic functions (12). Notably,
the heart exhibits the second greatest bioaccumulation of PFOS
after the liver, and it is also a target organ for PFOS. Our
previous study indicated that several marker proteins involved in
cardiovascular development, such as Brachyury, GATA4, myocyte
enhancer factor 2C and α-actinin, were downregulated when exposed
to PFOS (13). In a marine medaka
model and in embryonic stem cell (ESC)-derived cardiomyocytes,
prenatal PFOS exposure disrupts the expression of genes associated
with cardiac development, and affects the function of the heart
(9,14). PFOS has been considered to induce
cardiac mitochondrial damage and gene transcript disorder (15), which may be one possible toxicity
mechanism. Nevertheless, to our knowledge, the possible impact of
PFOS on cardiac dysfunction in adult rats has not been
investigated, and the underlying toxicity mechanism has not yet
been fully elucidated.
Apoptosis is a type of programmed cell death that
participates in various pathological events (16). It has been widely reported that
excessive apoptosis is responsible for structural abnormality and
dysfunction of the heart (17,18).
During all stages of heart development, PFOS possesses the
potential to alter key genes, reduce ATP production, stimulate
reactive oxygen species (ROS) generation and induce apoptosis
(19). In a zebrafish embryo model,
PFOS exposure induced apoptosis and upregulated gene expression
levels of P53 and Bax, which are associated with apoptosis. These
genes are also closely associated with the JNK and p38 signaling
pathway (20,21). In addition, a potential association
between PFOS and inflammation has been revealed in a previous study
(22); however, the specific
mechanism is unclear. PFOS can modulate the inflammatory factors,
such as TNF-α and IL-6, in vivo and in vitro
(22). Therefore, it was
hypothesized that PFOS toxicity in the cardiac tissue of adult rats
might be associated with inflammation and apoptosis.
The current study aimed to explore whether PFOS
exposure would induce heart impairment and a degree of pathological
change in rats. Moreover, the level of apoptosis and inflammatory
infiltration in cardiomyocytes was investigated to provide evidence
for further research on PFOS-induced cardiac toxicology.
Materials and methods
Ethics statement
Animal experiments in the current study were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (23). Procedures of animal experiments were
approved by The Ethics Committee of the Laboratory Animal Care and
Welfare, Zhejiang Academy of Medical Sciences (Zhejiang,
China).
Animals
A total of 48 male Sprague Dawley (SD) rats (220±5
g) in 8-week old were purchased from the Laboratory Animal Center
of Zhejiang Province (Hangzhou, China) and bred in house. All rats
were housed in a specific pathogen free facility under a 12-h
light/dark cycle in an ambient temperature of 22-26˚C and relative
humidity of ~55%. Animals were fed standard laboratory rat chow.
Animals were provided food and water ad libitum.
Experimental protocols
SD rats were divided into three groups (each, n=6)
that received 0 (control), 1 and 10 mg/kg PFOS (Sigma Aldrich;
Merck KGaA). The rats were intraperitoneally (I.P) injected at 1 cm
to the left of the midline of the lower abdomen with PFOS every
other day for 14 days. The dose, period and drug-delivery way of
PFOS was selected based on previous reports (24,25).
On day 14, all animals were placed on the operating table and
immobilized with ketamine (40 mg/kg; intramuscular injection in the
front of the thigh) and xylazine (5 ml/kg; I.P) anesthesia, after
which 5 ml blood was obtained from the aorta abdominalis. After
blood and heart tissues were obtained, rats were sacrificed by
exsanguination from the carotid artery. Rat hearts and blood were
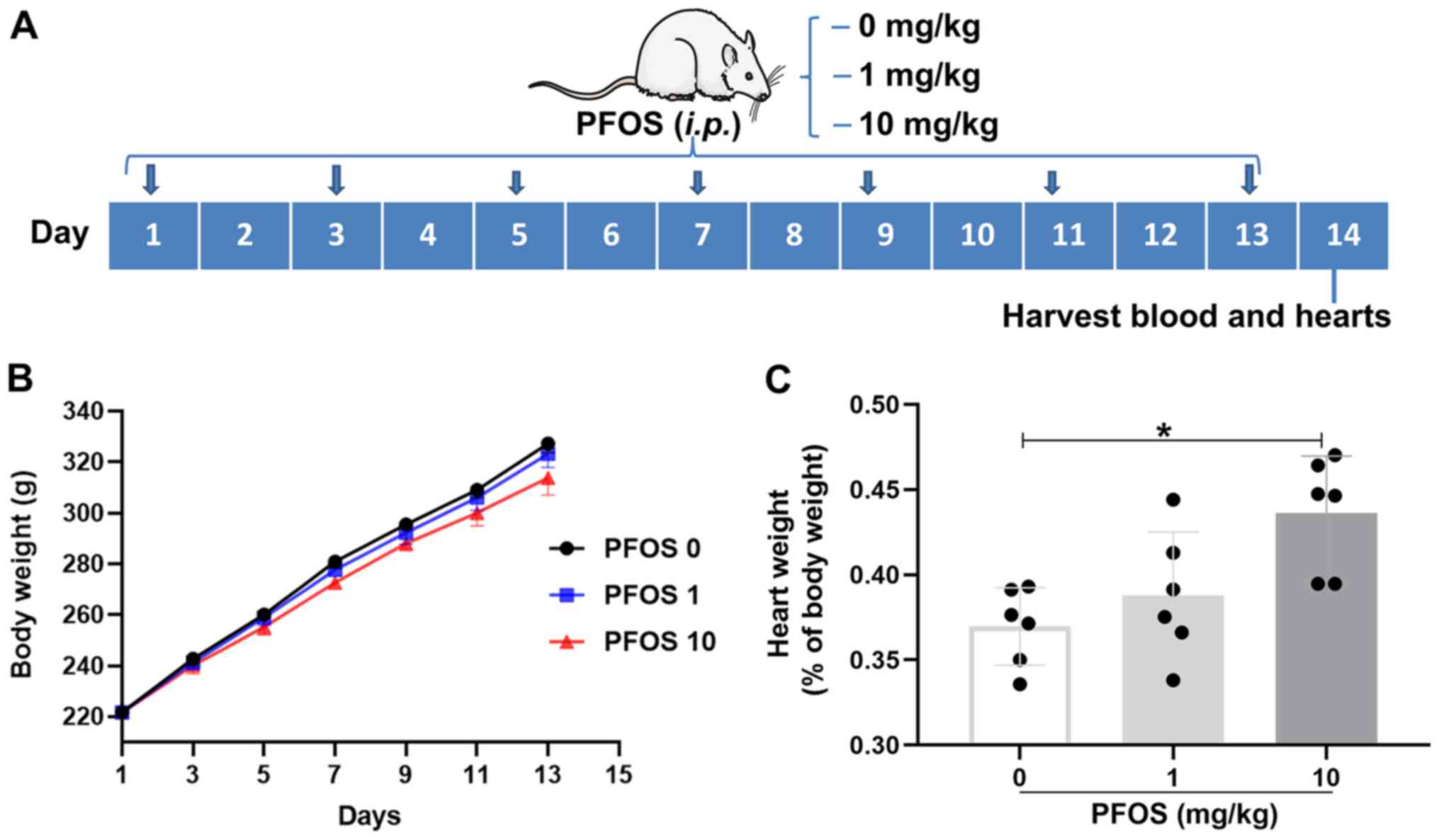
used for biochemical and pathological assays (Fig. 1A). One sample from each group was
used for preliminary experiments.
Body weight and hearts weight
determinations
Changes of body weight in each group were measured
every other day until the 14th day. At the end of the PFOS
treatment, the rats were sacrificed and hearts were quickly moved,
carefully blotted dry and weighed. The percentage of heart weight
to body weight was calculated as follow: (heart weight/body weight)
x100%.
Heart tissues and blood biochemical
assays
Blood samples were obtained from the abdominal aorta
and left to stand at room temperature for 1 h, and then at 4˚C for
2 h, followed by 3,000 x g centrifugation for 10 min at 4˚C. The
supernatant was used for serum lactic dehydrogenase (cat. no.
201902; LDH), creatine kinase (cat. no. 201823; CK) and creatine
kinase-isoenzyme-MB (cat. no. 201811; CK-MB) measurements. The
collected heart tissues were homogenized and centrifuged at 3,200 x
g for 30 min at 4˚C. The supernatant was harvested for cardiac
troponin-T (cat. no. 201904; cTn-T) measurement. All biomarkers
were determined using commercial ELISA kits (BD Biosciences).
Histological analysis
The isolated hearts were fixed in 4%
paraformaldehyde for 24 h at room temperature and embedded in
paraffin for histological analysis. Next, samples were cut into
5-µm sections and heated at 65˚C for 20 min. Slides were then
deparaffinized with xylene and dehydrated in a grade series of
ethanol through 70, 80, 90, 95 and 100%. The slices were stained
with Masson for 10 min at room temperature to evaluate fibrosis and
stained with wheat germ agglutinin (WGA) for 15 min at 37˚C to
analyze myocardial hypertrophy. Images were captured
(magnification, x200 or x400) under a light microscope (Leica
Microsystems GmbH). The fibrosis area of heart tissues and the
cardiomyocyte cross-sectional area were measured using Image-Pro
Plus software version 6.0 (Media Cybernetics, Inc.). The percentage
of fibrotic areas were calculated.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
For all groups, 5-µm sections were stained using the
In Situ Cell Death kit-TMR (cat. no. MK1012; Wuhan Boster
Biological Technology, Ltd.) overnight at 4˚C. The process of TUNEL
staining were performed according to the manufacturer's
instructions. The slides were incubated with TUNEL reaction mixture
for 1 h at 37˚C before being rinsed 3 times with PBS. The nucleus
was stained by DAPI (1 µg/ml; cat. no. C1002; Beyotime Institute of
Biotechnology) for 10 min at room temperature. A drop of antifade
mounting medium (cat. no. P0098; Beyotime Institute of
Biotechnology) was added for 5 min at room temperature. Images were
taken with a fluorescence microscope and the number of
TUNEL-positive cells in five random views were selected in each
sample. Cells were counted by ImageJ analysis software (version
1.51; National Institutes of Health).
Immunohistochemical analysis
For immunohistochemical assay, heart tissues were
fixed in 4% paraformaldehyde for 24 h at room temperature and
embedded in paraffin for immunohistochemical analysis. Sections
were blocked using 5% goat serum (cat. no. G1209; Wuhan Boster
Biological Technology, Ltd.) and incubated with specific primary
antibodies overnight at 4˚C, such as IL-1β Rabbit mAb (1:800; cat.
no. SRP8033; Sigma-Aldrich; Merck KGaA) and TNF-α Rabbit mAb
(1:500; cat. no. ab6671, Abcam). The sections were washed with PBS
three times, followed by staining with horseradish
peroxidase-conjugated secondary anti-rabbit IgG (1:5,000; cat. no.
GB23303; Wuhan Boster Biological Technology, Ltd.) for 2 h at room
temperature and visualizing with substrate DAB. Images were
obtained and captured (magnification, x200) using a fluorescent
microscope (Leica Microsystems GmbH). The number of positive cells
was analyzed by ImageJ analysis software (version 1.51; National
Institutes of Health).
Western blotting
Protein from myocardial tissue was extracted using
the cell and tissue total protein extraction kit (cat. no. KC415;
Shanghai Kang Cheng Bioengineering Co., Ltd.). Protein
concentration was quantified using the bicinchoninic acid (BCA)
protein assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology) and was diluted to the same concentration with 5X
loading buffer (cat. no. P0015L; Beyotime Institute of
Biotechnology). A total of 50 µg protein was separated using 15%
SDS-PAGE. Protein was transferred to polyvinylidene fluoride
membrane and blocked in 5% skimmed milk in Tris-buffered saline for
90 min at room temperature. After blocking, the membranes were
incubated overnight with primary antibodies against p53 mouse mAb
(1:1,000; cat. no. 2524; Cell Signaling Technology, Inc.), anti-Bax
antibody (1:800; cat. no. A00183; Wuhan Sanying Biotechnology) and
GAPDH mouse mAb (1:3,000; cat. no. G3214; Bioworld Technology,
Inc.) overnight at 4˚C. Following washing with Tris-buffered saline
containing 1% Tween, membranes were incubated for 2 h with
horseradish peroxidase-conjugated IgG (1:5,000, cat. no. GB23303;
Wuhan Boster Biological Technology, Ltd.) secondary antibodies at
room temperature for 2 h. The specific protein bands were
visualized using an enhanced chemiluminescence detection kit (cat.
no. 33021; Boster Biological Technology, Ltd.). The intensity of
each band was quantified using Quantity One software version 6
(Bio-Rad Laboratories, Inc.).
Statistical analyses
Data are presented as mean ± standard deviation.
Values from three groups were analyzed using one-way ANOVA followed
by Student-Newman-Keuls multiple comparison post hoc test.
Statistical analysis was performed in GraphPad Software (Prism
Version 8.01; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of PFOS on body weight and
associated heart weight in rats
Body weight in 0 (control), 1 and 10 mg/kg PFOS
groups were recorded every two days for 14 days. There was no
significant difference in the body weight of rats among the three
groups, though a slight reduction was observed between control and
10 mg/kg PFOS group (Fig. 1A). As
presented in Fig. 1C, the
percentages ratio of heart weight to body weight demonstrated no
significant increase in 1 mg/kg group (P>0.05), but were
significantly increased in the 10 mg/kg PFOS group compared with
control group (0.44±0.03% vs. 0.37±0.02%; P<0.05; Fig. 1C).
PFOS-induced myocardial injury in
rats
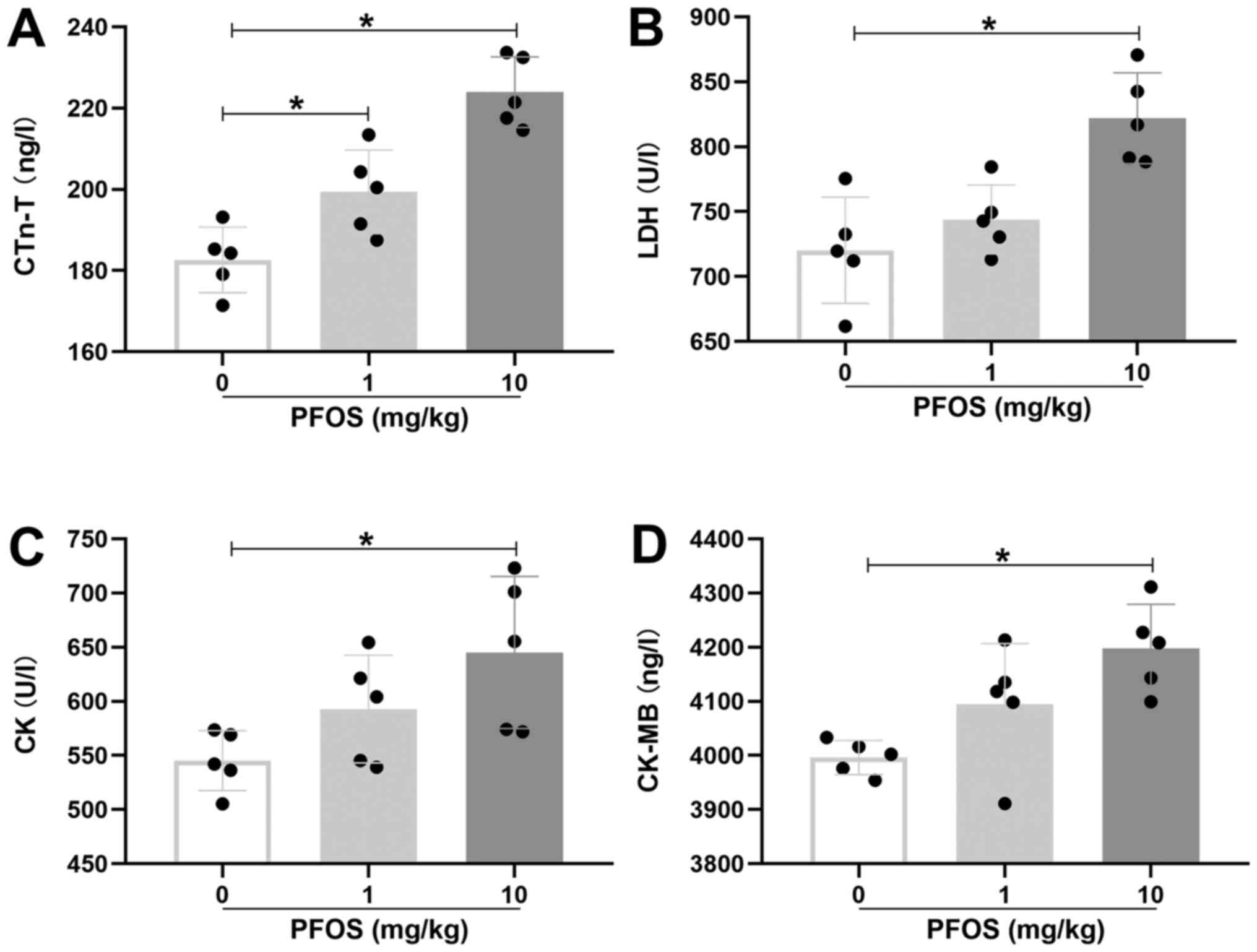
In order to detect the toxic effect of PFOS on the
heart, cTn-T, LDH, CK and CK-MB, which are markers for myocardial
injury, were measured in rats. In the 1 mg/kg PFOS group, only the
level of cTn-T in heart tissues was significantly elevated compared
with control group (P<0.05; Fig.
2A). Whereas in the 10 mg/kg PFOS group, cTn-T, LDH, CK and
CK-MB levels were all significantly increased when compared with
the control group (P<0.05; Fig.
2A-D). These results suggested that 10 mg/kg PFOS could induce
significant myocardial injury in rats. In addition, gavage
administration was selected to test whether PFOS could elevate
myocardial injury in rats. There were no significant differences
between gavage administration and intraperitoneal injection
administration on cTn-T, LDH, CK and CK-MB expression levels among
the three groups (P>0.05; Fig.
S1).
To determine the toxicological effect of PFOS on rat
hearts over a longer duration, PFOS was administrated every other
day for 28 days to model sub-chronic exposure. The levels of cTn-T,
LDH, CK and CK-MB were examined after 28 days of PFOS exposure. The
10 mg/kg PFOS group demonstrated significantly elevated levels of
cTn-T, LDH, CK and CK-MB compared with the 0 mg/kg PFOS group
(P<0.05; Fig. S2). The
expression level of cTn-T was also significantly increased in the 1
mg/kg PFOS group compared with the 0 mg/kg PFOS group (P<0.05;
Fig. S2A). However, there were no
significant differences in expression levels of LDH, CK and CK-MB
between the 1 mg/kg PFOS group and 0 mg/kg PFOS group (P>0.05,
Fig. S2B-D).
PFOS is associated with cardiac
fibrosis and myocardiac hypertrophy in rats
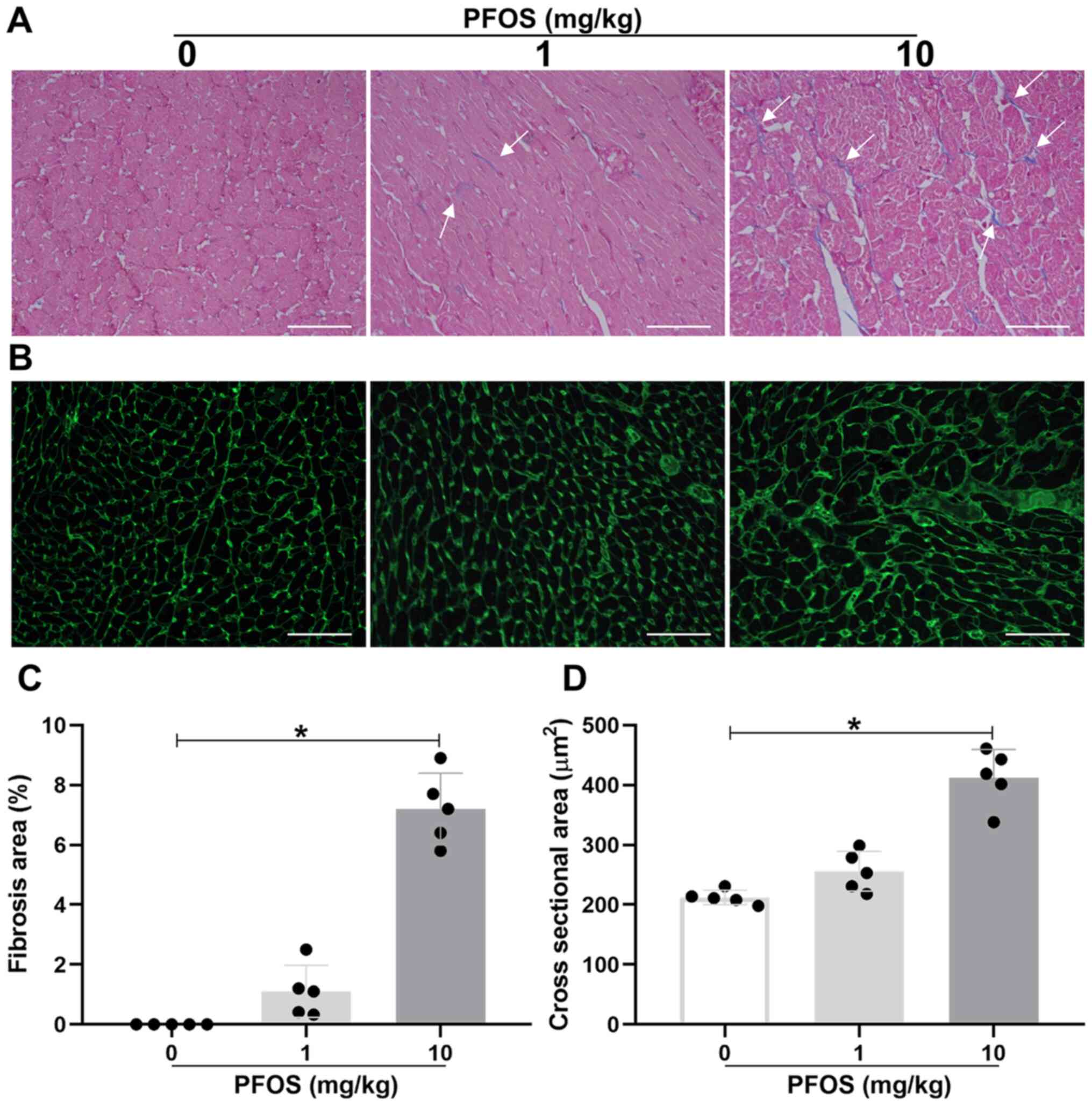
To further determine the toxic influence of PFOS on
heart tissues in rats, Masson and WGA staining were performed. The
rats in the 1 mg/kg PFOS group exhibited no significant increase in
cardiac fibrosis and hypertrophy when compared with the control
group (P>0.05; Fig. 3A and
B). However, the fibrosis area and
the myocyte cross-sectional area were markedly upregulated in rat
hearts exposed to 10 mg/kg PFOS compared with the control group
(fibrosis area, 7.2±1.2% vs. 0.0±0.0%; P<0.05; Fig. 3A and C; cross-sectional area, 412.6±47.4
µm2 vs. 212.4±12.0 µm2; P<0.05; Fig. 3B and D). These results were matched by the
aforementioned changes in the percentage of heart weight to body
weight.
PFOS is associated with myocardial
apoptosis in rats
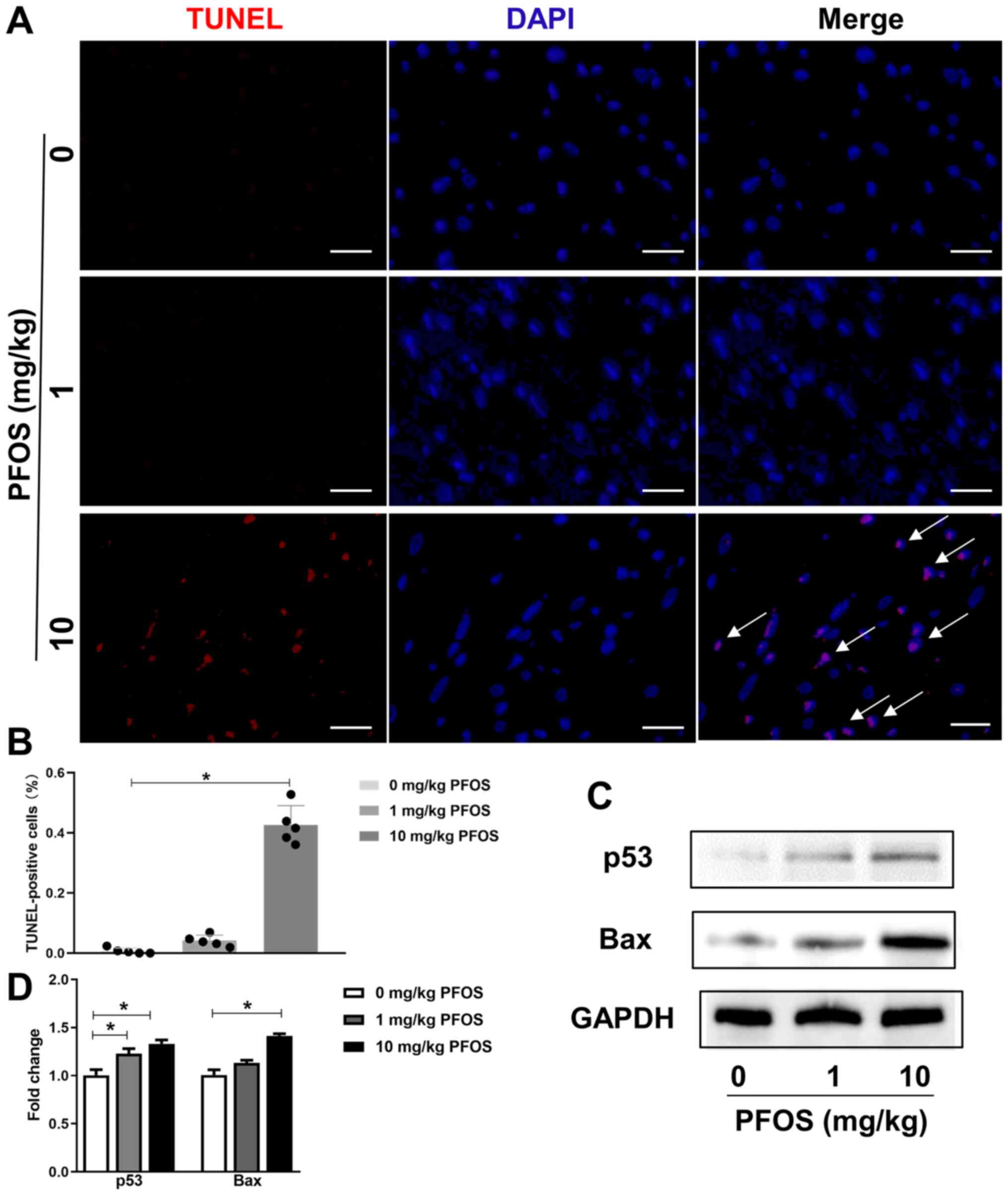
The cardiotoxicity effect of PFOS on apoptosis was
explored using TUNEL staining. As presented in Fig. 4A and B, rats subjected to 1 mg/kg PFOS exhibited
no difference in myocardial apoptosis compared with the control
group (P>0.05). In addition, in the 10 mg/kg PFOS group,
the percentage of TUNEL-positive nuclei were significantly
increased compared with the control group (P<0.05). Furthermore,
the expression levels of p53 and Bax were significantly upregulated
in the 10 mg/kg PFOS group compared with the control group
(P<0.05; Fig. 4C and D), and p53 protein was also significantly
upregulated in the 1 mg/kg PFOS group (P<0.05; Fig. 4C and D).
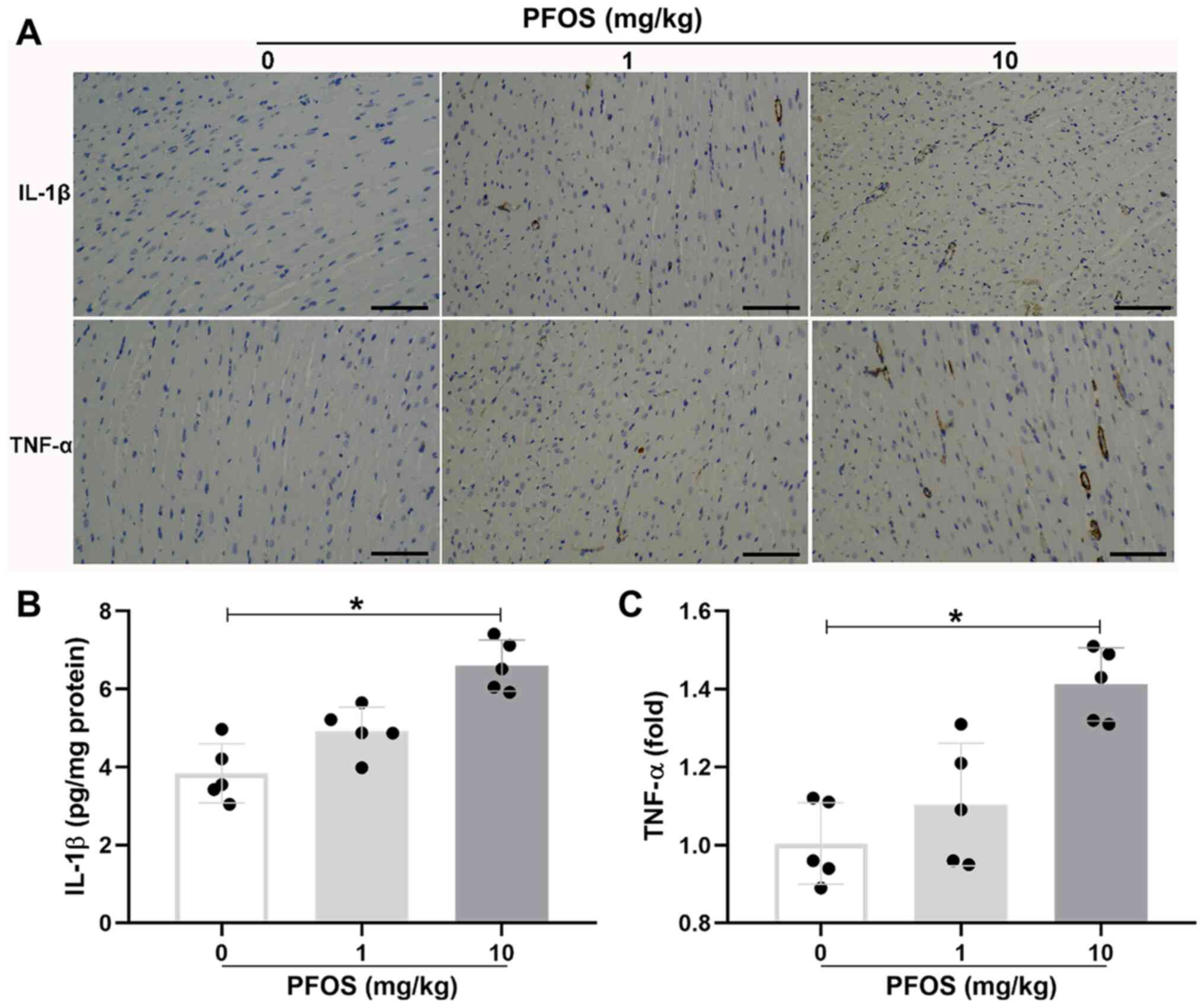
PFOS is associated with inflammatory
infiltration in rat heart tissues
The influence of PFOS on the cardiac inflammation in
rats was investigated. Immunohistochemical staining for
pro-inflammatory cytokine IL-1β indicated that the expression
profile of IL-1β was significantly increased in the 10 mg/kg PFOS
group compared with the control group (6.60±0.65 vs. 3.84±0.76
pg/mg protein; P<0.05; Fig. 5A
and B). Consistent with the
apparent IL-1β accumulation, there was also a significant
upregulation of TNF-α expression in the 10 mg/kg PFOS group
compared with the control group (1.41±0.09 vs. 1.00±0.10;
P<0.05; Fig. 5A and C). In addition, there was little
expression of IL-1β and TNF-α in heart tissues exposed to 1 mg/kg
PFOS (P>0.05).
Discussion
The current study revealed several main findings.
Firstly, exposure of PFOS at the dosage of 10 mg/kg caused
myocardial damage in rats. Secondly, 10 mg/kg PFOS significantly
increased cardiac fibrosis and myocardiac hypertrophy in rats.
Finally, it was demonstrated that 10 mg/kg PFOS treatment
upregulated myocardial apoptosis and expression levels of IL-1β and
TNF-α in heart tissue of rats.
PFOS, a type of fluorine-saturated eight-carbon
compound, is a persistent, bioaccumulative and organic pollutant as
a result of its ubiquitous distribution and extreme stability
(26). For humans, dietary intake
is the main source of exposure to PFOS. Pollution data by the
National Health and Nutrition Examination Survey has demonstrated
that serum concentrations of PFOS range from 0.8-0.9 µM, while an
increased concentration (0.3-6.9 µM) has been detected in Minnesota
Mining and Manufacturing company employees (3,27).
Serum PFOS concentrations are generally far lower compared with 1
mg/kg in this study. However, based on the average weight of adults
in China (60 kg) and their average blood content (~6,400 ml), the
serum PFOS concentration of fluorination plant workers is 1,386
ng/ml (28); therefore, the average
concentration of PFOS has been calculated to reach 0.1 mg/kg body
weight. Studies have demonstrated that concentrations of PFOS that
are lower than the oral PFOS LD50 of 250 µg/g (29) were associated with significant
injury in rats or in mice (4,25). In
the current study, the dosage of 10 mg/kg was a little higher
compared with the accumulated doses in human blood.
The cardiovascular toxicity of PFOS has been
rudimentarily studied in vitro and in vivo. Harada
et al (30) reported that
the action potential duration and peak potential were markedly
reduced when exposed to PFOS in guinea-pig ventricular myocytes
(30). The sinus venosus-bulbus
arteriosus distance was also demonstrated to be increased in a
marine medaka when exposed to PFOS (9). Moreover, PFOS can enlarge the right
atrium of mice and rats (31). PFOS
not only affects heart malformation, but also heart function. PFOS
exposure also changes heart rates in zebrafish embryos (32). Our previous study demonstrated that
PFOS induced toxicity of ESCs through mitochondrial structure
injury and abnormal Ca2+ shuttle (33). However, there is only a small amount
of research performed to assess the cardiovascular toxicity of PFOS
in rats. To investigate what effects PFOS exposure exerted on
cardiac toxicity, the present study detected biochemical indices
and pathological changes in rats. It was demonstrated that PFOS
treatment augmented the percentage of heart to body weight in rats.
Moreover, 10 mg/kg PFOS induced significant cardiac fibrosis and
myocardiac hypertrophy that was matched by increased biochemical
indices associated with myocardial damage in rats. The results of
the present study suggested that PFOS at a dosage of 10 mg/kg could
exert a pronounced cardiotoxicity in rats; however the mechanism
underlying PFOS toxicity in the cardiovascular system remains
unclear.
Apoptosis is an important process in various human
diseases and has been implicated in PFOS toxicity (34). A small number of basal studies have
been carried out to examine the toxicity of PFOS associated with
apoptosis (35-37).
PFOS treatment can induce cell apoptosis in murine N9 cells
(35) and in hepatoma Hep G2 cells
(36). It can also upregulate the
number of apoptotic cells in liver of adult rats (37). However, PFOS exposure has been
reported to influence the protein and mRNA expression levels of Bax
and Bcl-2 in adults (38). In
addition, the genes associated with apoptosis, such as p53 and Bax,
were significantly upregulated in zebrafish embryos that were
raised in an environment with PFOS, the mechanism of which was
associated with ROS generation and MAPK activity (21). A previous study revealed that PFOS
exposure results in the apoptosis of rat cardiocytes via TUNEL
analysis (18). In line with this
study, the current study revealed that 10 mg/kg PFOS induced
increases of TUNEL-positive cells, p53 and Bax protein expression,
suggesting an increase in apoptosis in the heart tissues of adult
rats.
Increasing evidence has demonstrated that
inflammation serves a notable role in Polyfluoroalkyl
chemical-induced toxicity (22,39).
Perfluorononanoic acid is hypothesized to increase liver weight and
upregulate large quantities of IL-1β and TNF-α (39). In addition, it is reported that PFOS
exposure leads to hepatocyte proliferation accompanied by an
increase of serum IL-6 and TNF-α (22). On the contrary, clinical research
has revealed that lower gut inflammation is closely associated with
higher perfluoroalkyl substances exposure, which can be regarded as
a risk factor for inflammatory bowel disease (40). However, whether pro-inflammatory
cytokines are involved in PFOS-stimulated cardiac toxicity remains
unclear. Notably, the results of the current study revealed that
proinflammatory cytokines IL-1β and TNF-α were significantly
elevated in rat heart tissues after exposure to 10 mg/kg PFOS.
Previous studies have reported that the exposure of
female rats to perfluorooctane sulfonate increased the estrogen
receptor α (ERα) expression, suggesting that PFOS acts as
estrogenic compounds to activate ERα (41,42).
In adult male and female B6C3F1 mice, daily PFOS exposure could
induce immunotoxicity, with certain differences between male mice
and female mice being identified in regards to immune parameters
(43). Further study should be
conducted to provide evidence towards the difference of male and
female rats in cardiac toxicity. In addition, as an organic
compound, the concentration of PFOS in heart tissue needs to be
detected with high performance liquid chromatography in future
experiments.
In summary, the current study demonstrated that PFOS
exposure caused pathological changes, reflected by cardiac fibrosis
and myocardial hypertrophy in the hearts of adult rats, which was
possibly associated with an increase in apoptosis and
proinflammatory cytokines, such as IL-1β and TNF-α. The present
study provided preliminary data for further study of cardiovascular
system subjected to a PFOS challenge.
Supplementary Material
Effect of PFOS administration by
gavage or intraperitoneal injection on myocardial injury in rats.
Sprague-Dawley rats were administered PFOS by gavage or
intraperitoneal injection once every two days for 14 days. Doses of
PFOS were 0 mg/kg (normal saline), 1 and 10 mg/kg. (A) cTn-T in
heart tissues, and (B) LDH, (C) CK and (D) CK-MB in serum were
detected. n=5. PFOS, perfluorooctane sulfonate; cTn-T, cardiac
troponin-T; LDH, lactic dehydrogenase; CK, creatine kinase; MB,
isoenzyme-MB.
PFOS induces myocardial injury in rats
under a subchronic exposure. Sprague-Dawley rats were administered
PFOS at 0, 1 and 10 mg/kg every 2 days for 28 days. On day 28,
blood was collected and the markers of myocardial damage in cardiac
tissues and serum were detected. (A) Levels of cTn-T in the cardiac
tissues were measured. (B) contents of LDH, (C) CK and (D) CK-MB in
the serum of rats were determined. n=5. *P<0.05 as
indicated. PFOS, perfluorooctane sulfonate; cTn-T, cardiac
troponin-T; LDH, lactic dehydrogenase; CK, creatine kinase; MB,
isoenzyme-MB.
Acknowledgements
Not applicable.
Funding
This study was supported by The Medical Health Science and
Technology Project of Zhejiang provincial Health Commission (grant
no. 2018KY148) and The Medical Health Science and Technology
Program of Hangzhou (grant no. 20190551).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and MG designed the current study and wrote the
manuscript. DX, LL and LT performed experiments and analyzed data.
DX and JY confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Procedures of animal experiments were approved by
The Ethics Committee of Laboratory Animal Care and Welfare,
Zhejiang Academy of Medical Sciences (Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang G, Sun S, Wu X, Yang S, Wu Y, Zhao J,
Zhang H and Chen W: Intestinal environmental disorders associate
with the tissue damages induced by perfluorooctane sulfonate
exposure. Ecotoxicol Environ Saf. 197(110590)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gao Y, Guo X, Wang S, Chen F, Ren X, Xiao
H and Wang L: Perfluorooctane sulfonate enhances mRNA expression of
PPARγ and ap2 in human mesenchymal stem cells monitored by
long-retained intracellular nanosensor. Environ Pollut.
263(114571)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Olsen GW, Burris JM, Ehresman DJ,
Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR: Half-life of
serum elimination of perfluorooctanesulfonate,
perfluorohexanesulfonate, and perfluorooctanoate in retired
fluorochemical production workers. Environ Health Perspect.
115:1298–1305. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lau C, Thibodeaux JR, Hanson RG, Rogers
JM, Grey BE, Stanton ME, Butenhoff JL and Stevenson LA: Exposure to
perfluorooctane sulfonate during pregnancy in rat and mouse. II:
Postnatal evaluation. Toxicol Sci. 74:382–392. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
UNEP: The stockholm convention-national
implementation plans-addressing COP 4 amendments. http://chm.pops.int/Implementation/NIPs/NIPTransmission/tabid/253/Default.aspx.
(accessed on September 7th 2019), 2016.
|
|
6
|
Xie S, Wang T, Liu S, Jones KC, Sweetman
AJ and Lu Y: Industrial source identification and emission
estimation of perfluorooctane sulfonate in China. Environ Int.
52:1–8. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang S, Huang J, Yang Y, Hui Y, Ge Y,
Larssen T, Yu G, Deng S, Wang B and Harman C: First report of a
Chinese PFOS alternative overlooked for 30 years: Its toxicity,
persistence, and presence in the environment. Environ Sci Technol.
47:10163–10170. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jin H, Lin S, Dai W, Feng L, Li T, Lou J
and Zhang Q: Exposure sources of perfluoroalkyl acids and influence
of age and gender on concentrations of chlorinated polyfluorinated
ether sulfonates in human serum from China. Environ Int.
138(105651)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang Q, Fang C, Wu X, Fan J and Dong S:
Perfluorooctane sulfonate impairs the cardiac development of a
marine medaka (Oryzias melastigma). Aquat Toxicol. 105:71–77.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chan E, Burstyn I, Cherry N, Bamforth F
and Martin JW: Perfluorinated acids and hypothyroxinemia in
pregnant women. Environ Res. 111:559–564. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elcombe CR, Elcombe BM, Foster JR, Chang
SC, Ehresman DJ and Butenhoff JL: Hepatocellular hypertrophy and
cell proliferation in sprague-dawley rats from dietary exposure to
potassium perfluorooctanesulfonate results from increased
expression of xenosensor nuclear receptors PPARα and CAR/PXR.
Toxicology. 293:16–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang L, He L, Xue J, Ma Y, Xie Z, Wu L,
Huang M and Zhang Z: Persulfate-based degradation of
perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS)
in aqueous solution: Review on influences, mechanisms and
prospective. J Hazard Mater. 393(122405)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang YY, Tang LL, Zheng B, Ge RS and Zhu
DY: Protein profiles of cardiomyocyte differentiation in murine
embryonic stem cells exposed to perfluorooctane sulfonate. J Appl
Toxicol. 36:726–740. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Cheng W, Yu Z, Feng L and Wang Y:
Perfluorooctane sulfonate (PFOS) induced embryotoxicity and
disruption of cardiogenesis. Toxicol In Vitro. 27:1503–1512.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xia W, Wan Y, Li YY, Zeng H, Lv Z, Li G,
Wei Z and Xu SQ: PFOS prenatal exposure induce mitochondrial injury
and gene expression change in hearts of weaned SD rats. Toxicology.
282:23–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tomei LD and Umansky SR: Apoptosis and the
heart: A brief review. Ann N Y Acad Sci. 946:160–168.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeng HC, He QZ, Li YY, Wu CQ, Wu YM and Xu
SQ: Prenatal exposure to PFOS caused mitochondia-mediated apoptosis
in heart of weaned rat. Environ Toxicol. 30:1082–1090.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Beesoon S, Zhu L and Martin JW:
Isomers of perfluorooctanesulfonate and perfluorooctanoate and
total perfluoroalkyl acids in human serum from two cities in North
China. Environ Int. 53:9–17. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi X, Du Y, Lam PK, Wu RS and Zhou B:
Developmental toxicity and alteration of gene expression in
zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol.
230:23–32. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi X and Zhou B: The role of Nrf2 and
MAPK pathways in PFOS-induced oxidative stress in zebrafish
embryos. Toxicol Sci. 15:391–400. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han R, Zhang F, Wan C, Liu L, Zhong Q and
Ding W: Effect of perfluorooctane sulphonate-induced Kupffer cell
activation on hepatocyte proliferation through the
NF-κB/TNF-α/IL-6-dependent pathway. Chemosphere. 200:283–294.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
National Research Council: Guide for the
Care and Use of Laboratory Animals: Eighth Edition. The National
Academies Press, Washington, DC, 2011.
|
|
24
|
Chou HC, Wen LL, Chang CC, Lin CY, Jin L
and Juan SH: From the cover: l-carnitine via PPARγ- and
sirt1-dependent mechanisms attenuates epithelial-mesenchymal
transition and renal fibrosis caused by perfluorooctanesulfonate.
Toxicol Sci. 160:217–229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wen LL, Lin CY, Chou HC, Chang CC, Lo HY
and Juan SH: Perfluorooctanesulfonate mediates renal tubular cell
apoptosis through PPARgamma inactivation. PLoS One.
11(e0155190)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L, Duan X, Sun W and Sun H:
Perfluorooctane sulfonate acute exposure stimulates insulin
secretion via GPR40 pathway. Sci Total Environ.
726(138498)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Calafat AM, Wong LY, Kuklenyik Z, Reidy JA
and Needham LL: Polyfluoroalkyl chemicals in the U.S. population:
Data from the national health and nutrition examination survey
(NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ
Health Perspect. 115:1596–1602. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yan G: Environmental behavior and human
exposure to perfluoroalkyl acids around a manufacturing facility in
China. Graduate University of the Chinese Academy of Sciences,
2017.
|
|
29
|
3M: Perfluorooctane Sulfonate: Current
Summary of Human Sera, Health and Toxicology Data. 1st edition.
U.S. Environmental Protection Agency, St. Paul, MN, pp1-129,
1999.
|
|
30
|
Harada K, Xu F, Ono K, Iijima T and
Koizumi A: Effects of PFOS and PFOA on L-type Ca2+ currents in
guinea-pig ventricular myocytes. Biochem Biophys Res Commun.
329:487–494. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thibodeaux JR, Hanson RG, Rogers JM, Grey
BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA and Lau C:
Exposure to perfluorooctane sulfonate during pregnancy in rat and
mouse. I: Maternal and prenatal evaluations. Toxicol Sci.
74:369–381. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang H, Huang C, Wang L, Ye X, Bai C,
Simonich MT, Tanguay RL and Dong Q: Toxicity, uptake kinetics and
behavior assessment in zebrafish embryos following exposure to
perfluorooctanesulphonicacid (PFOS). Aquat Toxicol. 98:139–147.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tang LL, Wang JD, Xu TT, Zhao Z, Zheng JJ,
Ge RS and Zhu DY: Mitochondrial toxicity of perfluorooctane
sulfonate in mouse embryonic stem cell-derived cardiomyocytes.
Toxicology. 382:108–116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu M, Liu G, Li M, Huo M, Zong W and Liu
R: Probing the cell apoptosis pathway induced by perfluorooctanoic
acid and perfluorooctane sulfonate at the subcellular and molecular
levels. J Agric Food Chem. 68:633–641. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang L, Li YY, Zeng HC, Li M, Wan YJ,
Schluesener HJ, Zhang ZY and Xu SQ: Perfluorooctane sulfonate
induces apoptosis in N9 microglial cell line. Int J Toxicol.
30:207–215. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu XZ and Hu DC: Effects of
perfluorooctanoate and perfluorooctane sulfonate exposure on
hepatoma Hep G2 cells. Arch Toxicol. 83:851–861. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kim HS, Jun Kwack S, Sik Han E, Seok Kang
T, Hee Kim S and Young Han S: Induction of apoptosis and CYP4A1
expression in sprague-dawley rats exposed to low doses of
perfluorooctane sulfonate. J Toxicol Sci. 36:201–210.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shen Y and White E: p53-dependent
apoptosis pathways. Adv Cancer Res. 82:55–84. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fang X, Zou S, Zhao Y, Cui R, Zhang W, Hu
J and Dai J: Kupffer cells suppress perfluorononanoic acid-induced
hepatic peroxisome proliferator-activated receptor α expression by
releasing cytokines. Arch Toxicol. 86:1515–1525. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu Y, Li Y, Scott K, Lindh CH, Jakobsson
K, Fletcher T, Ohlsson B and Andersson EM: Inflammatory bowel
disease and biomarkers of gut inflammation and permeability in a
community with high exposure to perfluoroalkyl substances through
drinking water. Environ Res. 181(108923)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qiu Z, Qu K, Luan F, Liu Y, Zhu Y, Yuan Y,
Li H, Zhang H, Hai Y and Zhao C: Binding specificities of estrogen
receptor with perfluorinated compounds: A cross species comparison.
Environ Int. 134(105284)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu C, Jiang ZY, Liu Q, Liu H and Gu AH:
Estrogen receptor beta mediates hepatotoxicity induced by
perfluorooctane sulfonate in mouse. Environ Sci Pollut Res Int.
24:13414–13423. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peden-Adams MM, Keller JM, Eudaly JG,
Berger J, Gilkeson GS and Keil DE: Suppression of humoral immunity
in mice following exposure to perfluorooctane sulfonate. Toxicol
Sci. 104:144–154. 2008.PubMed/NCBI View Article : Google Scholar
|