Introduction
Glioma is the most common primary brain tumor
worldwide (1). Despite efforts to
facilitate its early detection, as well as the use of surgical
resection combined with radiotherapy and chemotherapy, the majority
of patients with glioma are diagnosed at a late stage and their
prognosis is poor (2,3). In the last decade, the molecular
alterations that occur during glioma progression have been the
focus numerous studies and several oncogenes and tumor suppressors
have been identified (3-10).
An improved understanding of the underlying molecular mechanisms of
glioma progression would be beneficial for the identification of
novel therapeutic targets.
MicroRNAs (miRNAs/miRs) are endogenous
single-stranded non-coding RNA molecules comprising 18-25
nucleotides (11). They are able to
bind complementary sites within the 3'-untranslated regions
(3'-UTRs) of target genes, suppressing translation or promoting
mRNA degradation (11). Since a
single miRNA can regulate the expression of numerous target genes,
miRNAs serve as key regulators of various physiological and
pathological processes, including cell survival, differentiation,
proliferation, apoptosis, metabolism and tumorigenesis (12,13).
In previous years, various studies have reported deregulation of
miRNAs in various types of human cancer, including glioma (14,15).
Furthermore, numerous miRNAs have been demonstrated to serve
promotional or tumor suppressive roles in glioma. For instance,
miR-365 has been demonstrated to inhibit proliferation, migration
and invasion in glioma by targeting PIK3R3(16), whereas miR-599 does so by targeting
periostin (17).
Previously, the aberrant expression of miR-25 was
indicated to serve tumor promoting and suppressive roles in
different types of human cancer, including liver cancer (18). miR-25 was also revealed to enhance
cell migration and the invasiveness of non-small-cell lung cancer
cells through the MAPK signalling pathway by inhibiting
Krüppel-like factor 4(19).
Additionally, miR-25 contributed to cisplatin resistance in gastric
cancer cells by inhibiting forkhead box O3a (20). Moreover, miR-25 may promote
glioblastoma cell proliferation and invasiveness by directly
targeting neurofilament light polypeptide (21). Zhang et al (22) reported that miR-25 promoted glioma
cell proliferation by targeting CDK inhibitor 1C (CDKN1C). These
results indicated that miR-25 may serve an oncogenic role in
glioma. However, the function of miR-25 in glioma cell migration
and its underlying mechanism remain unknown. Therefore, the present
study aimed to investigate the function and underlying mechanism of
miR-25 in the progression of glioma.
Materials and methods
Tissue samples
The present study was approved by the Ethics
Committee of Xiangya Hospital of Central South University
(Changsha, China). A total of 15 normal brain tissues and 53
primary glioma tissues were obtained between March 2010 and May
2014. Patients were included in the current study if they were
diagnosed with primary glioma that was confirmed by an independent
pathologist at Xiangya Hospital. Patients were excluded if patients
with glioma had received chemotherapy or radiotherapy prior to
surgery. The normal brain tissues were collected from 15 patients
without malignancy who underwent surgery to reduce increased
intracranial pressure or to treat a severe head injury. The
histomorphology of these tissues was confirmed by the hospital's
Department of Pathology. These 15 patients included 9 males and 6
females (age range, 43-66 years; mean age, 55.1 years) and the 53
patients with glioma included 31 males and 22 females (age range,
34-68 years; mean age, 53.5 years). Glioma was pathologically
confirmed and was staged according to the TNM classification.
Written informed consent was obtained from all participants.
Cell culture
Human glioma cell lines (U-373MG Uppsala, U-87MG
Uppsala, U251MG and T98G) were obtained from the Cell Bank of the
Chinese Academy of Sciences. Human normal brain cells were
purchased from ScienCell Research Laboratories, Inc. The cells were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
incubator with 5% CO2.
Transfection
Cell transfection was performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. T98G and U251MG cells
(100,000 cells/well) were transfected with 100 nM negative control
(NC) inhibitors (cat. no. NCSTUD001; Sigma-Aldrich; Merck KGaA),
miR-25 inhibitors (cat. no. HSTUD0414; Sigma-Aldrich; Merck KGaA),
miR-NCs (cat. no. HMC0002; Sigma-Aldrich; Merck KGaA), miR-25
mimics (cat. no. HMI0414; Sigma-Aldrich; Merck KGaA), NC small
interfering (si)RNA (siNC; cat. no. sc-37007; Santa Cruz
Biotechnology, Inc.) or cell adhesion molecule 2 (CADM2) siRNA
(siCADM2; cat. no. sc-78228; Santa Cruz Biotechnology Inc.). The
sequences used for all transfection experiments were not
commercially available. After 48 h of transfection, subsequent
experiments were performed as described below.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissues or T98G and
U251MG cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). The total RNA was then reverse transcribed using
PrimeScript II 1st Strand cDNA Synthesis kit (Takara Bio, Inc.),
according to the manufacturer's protocol. The cDNA was amplified by
qPCR using SensiFAST™ SYBR® Hi-ROX Mix (Bioline), with
U6 and GAPDH as internal references to normalize expression data.
The thermocycling conditions were as follows: Initial denaturation
at 95˚C for 3 min; 40 cycles at 95˚C for 30 sec and 60˚C for 30
sec. The primer sequences were as follows: miR-25 forward,
5'-CTGGTAGGCATTGCACTTGTCT-3' and reverse, 5'-TCAACTGGTGTCGTGGAG-3';
U6 forward, 5'-CTCGCTTCGGCAGCACATATACT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGT-3'; GAPDH forward,
5'-CTGGGCTACACTGAGCACC-3' and reverse, 5'-AAGTGGTCGTTGAGGGCAATG-3';
CADM2 forward, 5'-AAACTTCCAAGGCATATCTCACC-3' and reverse,
5'-TGCGATTTGCATCCTCTTCTT-3'. Relative gene expression levels were
analyzed according to the 2-IICq method (23).
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was used to determine the cell
proliferation rate. The transfected T98G and U251MG cells were
seeded (5x103 cells/well) into 96-well plates and 20 µl
CCK-8 reagent (Beyotime Institute of Biotechnology) was added to
each well. The cells were incubated in a humidified incubator at
37˚C (5% CO2) for 24, 48 and 72 h time intervals
(14,15). The optical density at a 450 nm
absorbance was determined using a microplate reader.
Wound healing analysis
Transfected T98G and U251MG cells were seeded in
24-well plates and cultured at 37˚C until ~90% confluence. A wound
was scratched across the center of each well using a 10 µl sterile
pipette tip. The cells were then washed twice with PBS and cultured
in serum-free DMEM at 37˚C for 24 h. Images were captured under a
light microscope (magnification, x40) at 0 and 24-h time
points.
Transwell assay
For the Transwell assay, 24-well Transwell chambers
(8-mm pore size; Corning Inc.) pre-coated with Matrigel Basement
Membrane Matrix (BD Biosciences) at room temperature for 1 h were
used. Briefly, DMEM with 10% FBS was added to the lower chambers
and transfected T98G and U251MG cells (1x104 cells/ml)
in 300 ul serum-free DMEM were added to the upper chamber.
Following incubation at 37˚C for 24 h, the cells on the upper
surface of the insert were removed using a cotton-tipped swab. The
invaded cells were then fixed in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 20 min and stained with 0.5%
crystal violet (Sigma-Aldrich; Merck KGaA) for 5 min at room
temperature and images were captured under an inverted light
microscope (magnification, x200; Olympus Corporation).
Bioinformatics analysis
TargetScan 7.1 software (www.targetscan.org) was used to analyze the putative
target genes of miR-25. The resultant data revealed that CADM2 was
a putative target gene of miR-25 and as such was selected in the
search for further mutual verification.
Luciferase reporter assay
The wild-type (WT) and mutant (MT) 3'-UTR sequences
of CADM2 were synthesized by Shanghai GenePharma Co., Ltd. and
cloned into PGL3 luciferase reporter plasmids (Ambion; Thermo
Fisher Scientific, Inc.). T98G and U251MG cells (100,000
cells/well) seeded in 24-well plates were transfected with 100 nM
WT or MT luciferase reporter plasmids, and either 100 nM miR-25 or
miR-NC using Lipofectamine® 2000. The sequences used
were mentioned previously. After 24 h, the firefly and
Renilla luciferase activities were detected using the
Luc-Pair miRNA Luciferase Assay kit (GeneCopoeia, Inc.), according
to the manufacturer's instructions.
Western blot analysis
Total protein was extracted from cell lines using
RIPA lysis buffer (Thermo Fisher Scientific, Inc.). The protein
concentration was determined using a bicinchoninic acid assay kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The proteins (50 µg/lane) were separated by SDS-PAGE
using 10% gels and transferred to PVDF membranes (Thermo Fisher
Scientific, Inc.). The membranes were blocked in 5% skimmed milk at
room temperature for 3 h and subsequently washed three times with
PBS and 0.05% Tween-20 (PBST) prior to incubation with rabbit
anti-human CADM2 (1:300 dilution; cat. no. ab64873; Abcam) or
rabbit anti-human GAPDH antibodies (1:500 dilution; cat. no.
ab9485; Abcam) at room temperature for 3 h. After three washes in
PBST, the membranes were incubated with an HRP-conjugated goat
anti-rabbit secondary antibody (1:5,000 dilution; cat. no. ab7090;
Abcam) at room temperature for 1 h. The blots were visualized using
Western Blotting Luminol reagent (Santa Cruz Biotechnology, Inc.)
and protein expression was semi-quantified using Image-Pro Plus
software 6.0 (Media Cybernetics, Inc.). GAPDH was used for the
normalization of protein expression levels.
Statistical analysis
Experiments were performed in triplicate. Prism 5
software (GraphPad Software, Inc.) was used for all statistical
analyses and the data are presented as the mean ± standard
deviation. An unpaired Student's t-test was used to analyze the
differences between two groups, whilst one-way ANOVA followed by
Tukey's post-hoc test was used to analyze differences among
multiple groups. χ2 test analysis was conducted to
determine the association between miR-25 expression levels and the
clinicopathological features of glioma. Spearman's rank correlation
was used to analyze the correlation between the expression levels
of CADM2 and miR-25 in glioma tissues. P<0.05 was considered to
indicate a statistically significant difference.
Results
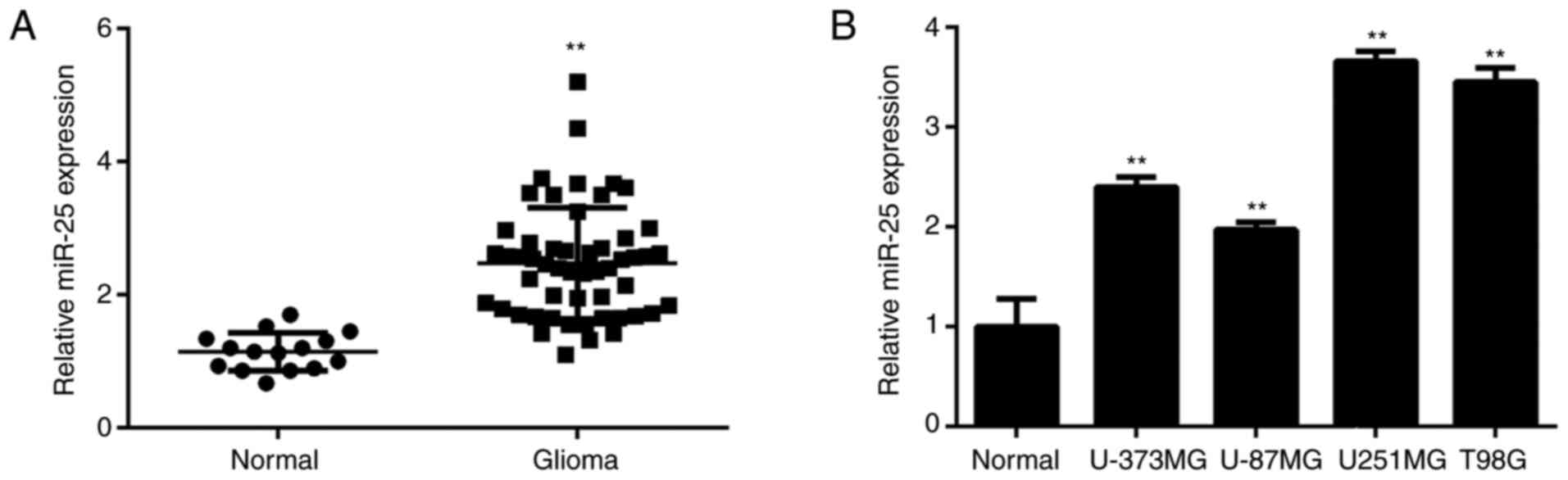
miR-25 is upregulated in glioma
In the present study, RT-qPCR was conducted to
determine miR-25 expression levels in glioma. The expression level
of miR-25 was significantly upregulated in glioma tissues compared
with normal brain tissues (Fig.
1A). The patients with glioma were subsequently divided into
high and low miR-25-expression groups according to the mean miR-25
expression level. As presented in Table
I, a high expression level of miR-25 was significantly
associated with advanced clinical stage in patients with glioma.
Additionally, miR-25 expression was significantly increased in
glioma cell lines compared with normal brain tissue cells (Fig. 1B).
 | Table IAssociation between miR-25 expression
level and clinicopathological characteristics of patients with
glioma. |
Table I
Association between miR-25 expression
level and clinicopathological characteristics of patients with
glioma.
| Variable | Number of patients
(n=53) | Low miR-25 expression
(n=27) | High miR-25
expression (n=26) | P-value |
|---|
| Age | | | | 0.275 |
|
<50
years | 28 | 12 | 16 | |
|
≥50
years | 25 | 15 | 10 | |
| Sex | | | | 0.583 |
|
Male | 31 | 17 | 14 | |
|
Female | 22 | 10 | 12 | |
| TNM stage | | | | 0.028 |
|
I-II | 29 | 19 | 10 | |
|
III-IV | 24 | 8 | 16 | |
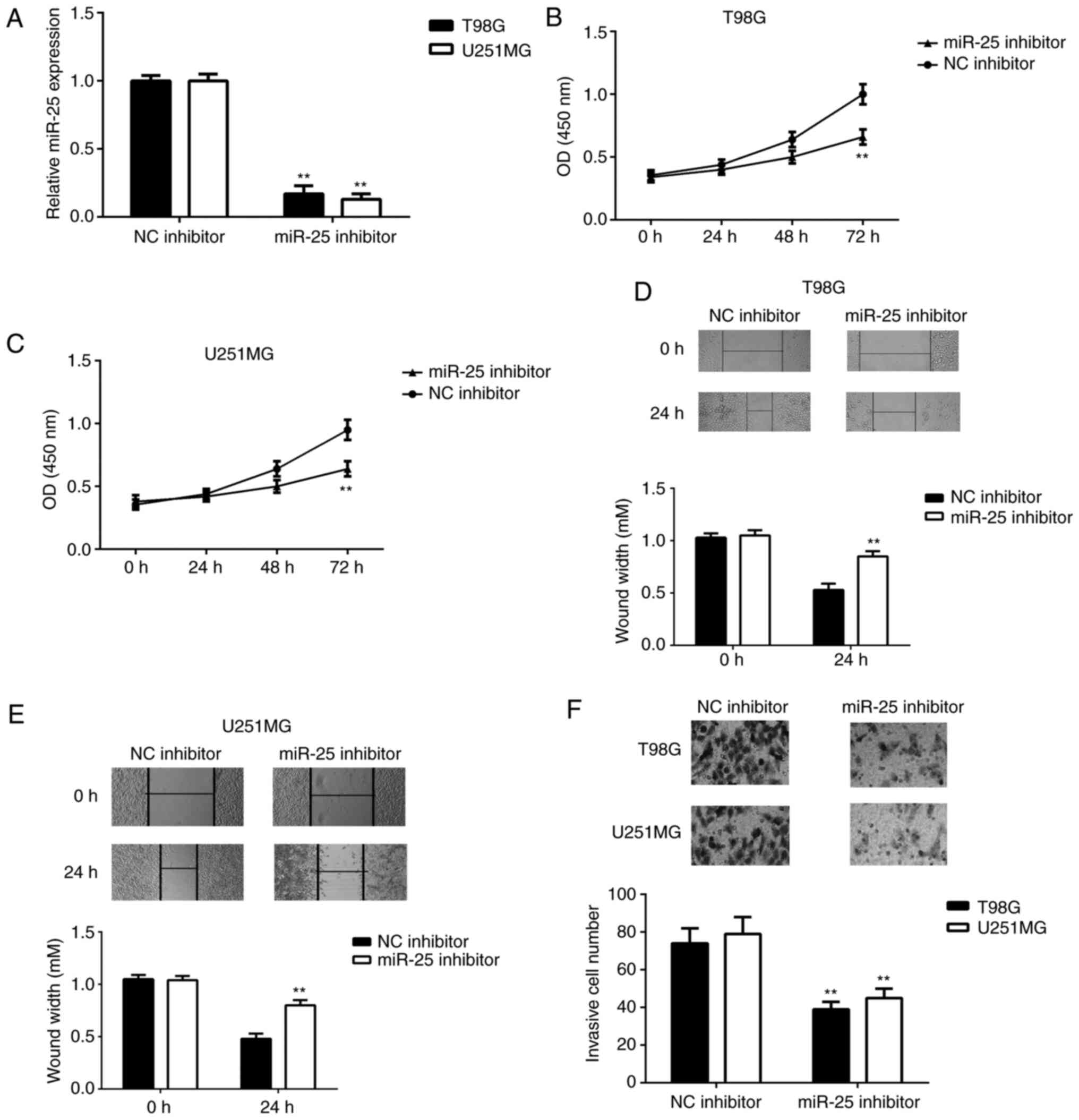
Knockdown of miR-25 suppresses the
proliferation, migration and invasiveness of glioma cells
The function of miR-25 in glioma cells was
subsequently investigated. To knock down miR-25 expression, U251MG
and T98G cells were transfected with an miR-25 inhibitor. These
cell lines were chosen for subsequent experiments as they exhibited
the highest expression levels of miR-25. Cells that were
transfected with the NC inhibitor served as the control group. The
miR-25 expression level was significantly downregulated in T98G and
U251MG cells transfected with miR-25 inhibitor compared with the
control group (Fig. 2A). As
presented in Fig. 2B-C, CCK-8 assay
data showed that the inhibition of miR-25 significantly reduced the
proliferation rate of U251MG and T98G cells at 72 h
post-transfection. Wound healing and Transwell assays were then
performed to investigate the role of miR-25 in glioma cell
migration and invasion. As demonstrated by Fig. 2D-F, downregulation of miR-25
markedly reduced the migratory and invasive capacities of U251MG
and T98G cells, indicated that the knockdown of miR-25 inhibited
the proliferation and migration of glioma cells.
CADM2 is a target of miR-25 in glioma
cells
Bioinformatics analysis was performed to predict the
potential target genes of miR-25. The data revealed that CADM2 was
a putative target gene of miR-25 (Fig.
3A) and luciferase reporter gene analysis verified this
prediction. The overexpression of miR-25 significantly inhibited
the luciferase activity of the WT 3'-UTR of CADM2, but not that of
the MT 3'-UTR in T98G and U251MG cells (Fig. 3B and C). Additionally, it was revealed that
miR-25 knockdown significantly increased the mRNA and protein
expression levels of CADM2 in both cell lines (Fig. 3D and E). By contrast, transfection with miR-25
mimics significantly enhanced the expression of miR-25 and reduced
the expression level of CADM2 in glioma cells (Fig. 3F-H). Taken together, these findings
suggested that CADM2 is a direct target gene of miR-25 in glioma
cells.
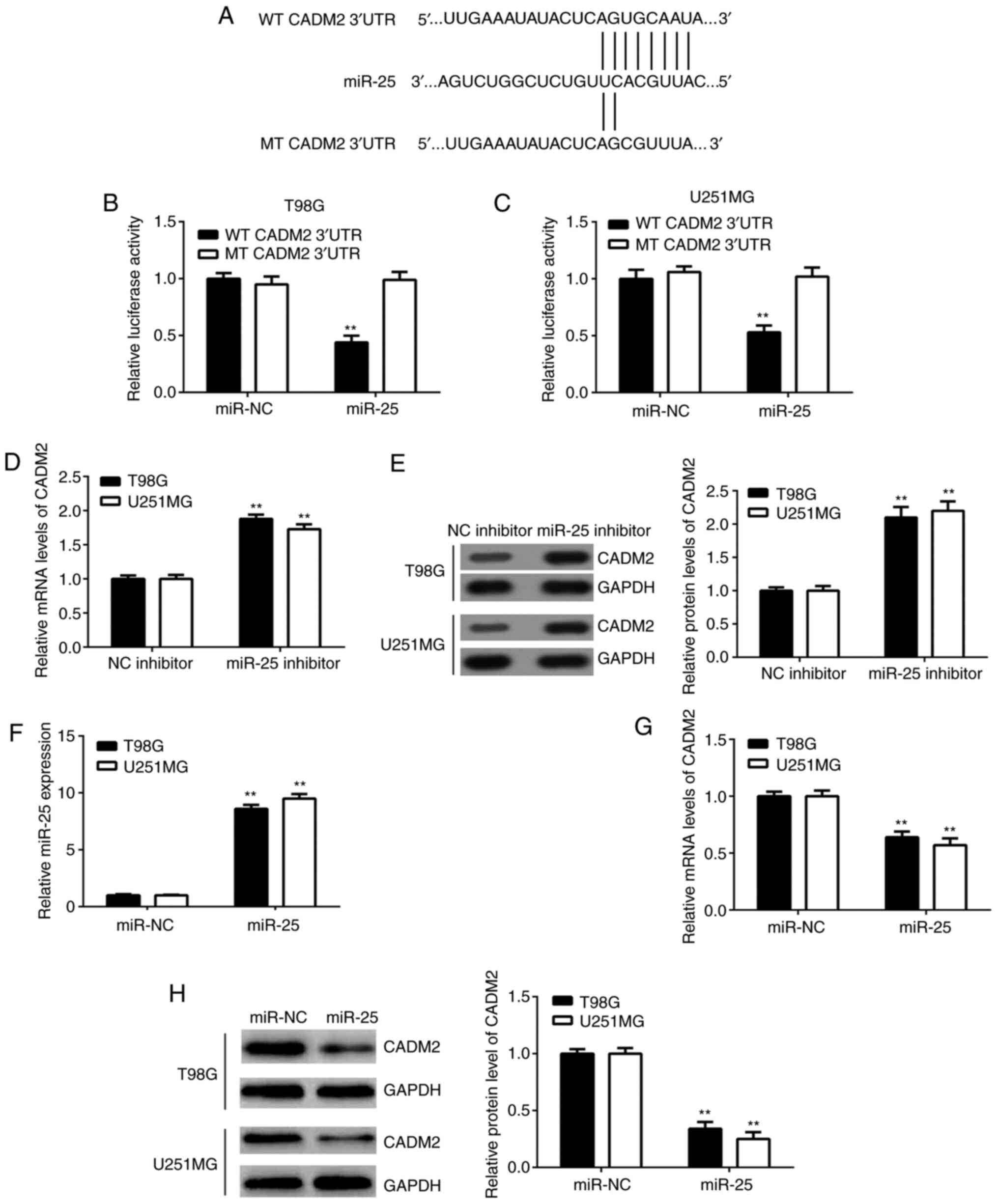 | Figure 3CADM2 is a target gene of miR-25 in
glioma. (A) Putative WT and MT binding sequences in the 3'-UTR of
CADM2. (B) T98G and (C) U251MG cells were co-transfected with
miR-25 mimics or miR-NC and WT-CADM2 or MT-CADM2 reporter gene
plasmids. Following transfection for 48 h, dual luciferase reporter
assays were conducted. **P<0.01 vs. miR-NC. The (D)
mRNA and (E) protein expression levels of CADM2 were detected using
RT-qPCR and western blotting, respectively, in T98G and U251MG
cells transfected with miR-25 inhibitor or NC inhibitor.
**P<0.01 vs. NC inhibitor. The expression levels of
(F) miR-25 and (G and H) CADM2 were detected using RT-qPCR and
western blotting in T98G and U251MG cells transfected with miR-25
mimics or miR-NC. **P<0.01 vs. miR-NC. CADM2, cell
adhesion molecule 2; miR, microRNA; MT, mutant type; NC, negative
control; RT-qPCR, reverse transcription-quantitative PCR; UTR,
untranslated region; WT, wild-type. |
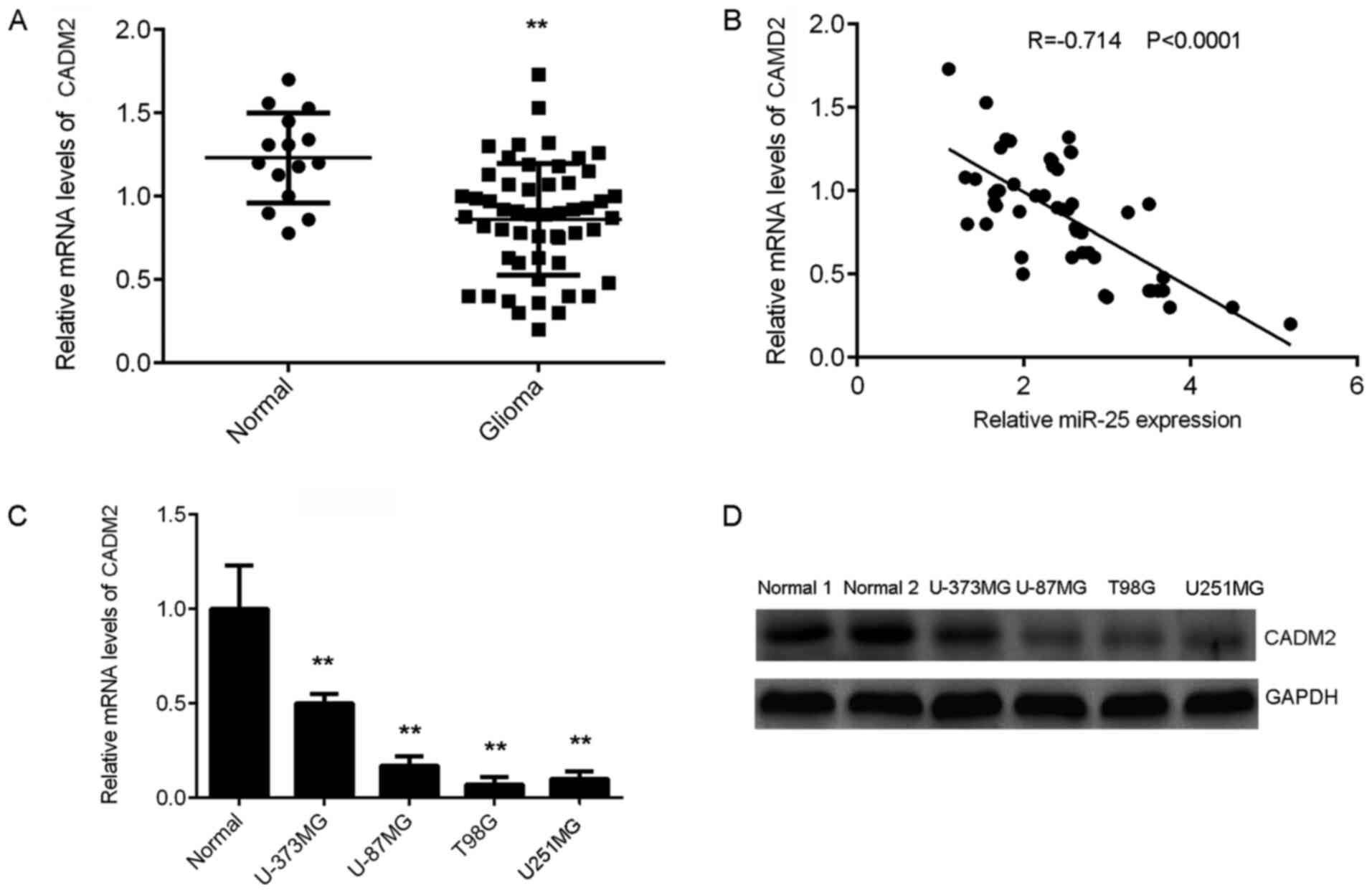
CADM2 is downregulated in glioma
The expression of CADM2 in glioma was also
determined. RT-qPCR results demonstrated that CADM2 was
significantly downregulated in glioma tissues compared with normal
brain tissues (Fig. 4A). Notably,
the CADM2 expression level was negatively correlated with that of
miR-25 in glioma tissues (Fig. 4B).
Consistently, the mRNA and protein levels of CADM2 were also
significantly lower in glioma cell lines compared with normal brain
tissues (Fig. 3C and D, respectively). Thus, CADM2 was
downregulated in glioma, which may be due to the upregulation of
miR-25.
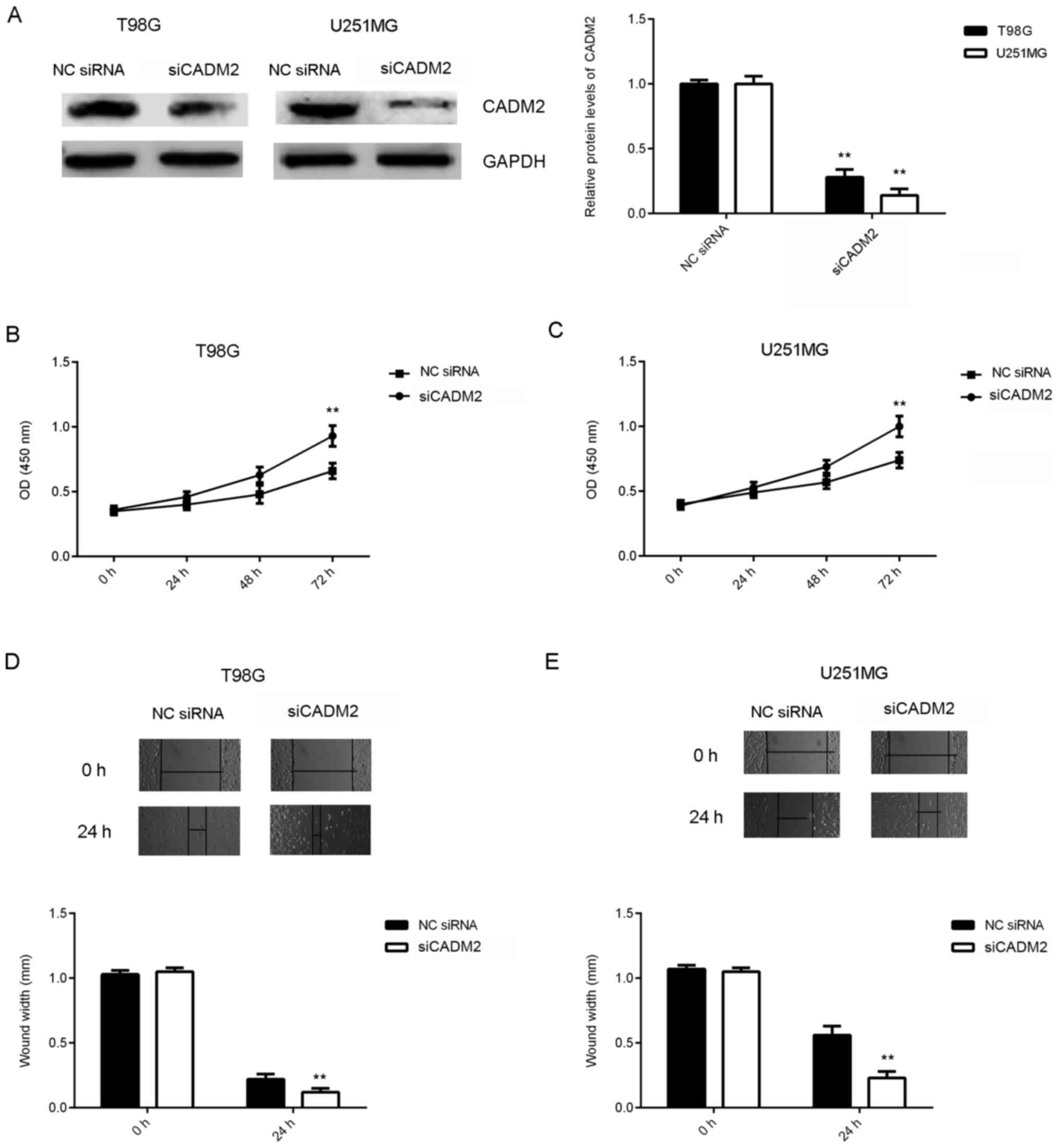
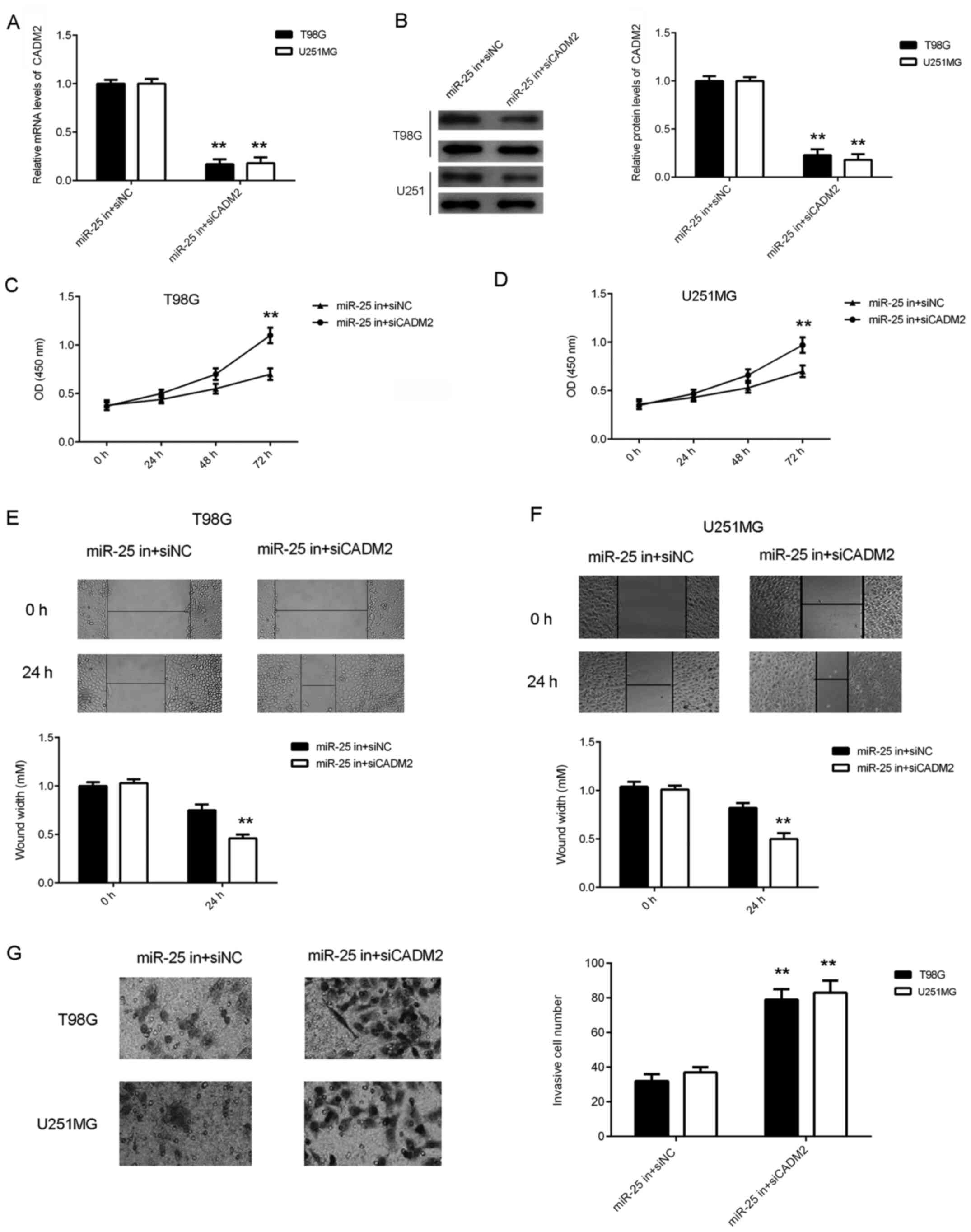
Knockdown of CADM2 reverses the
inhibitory effects of miR-25 downregulation on the proliferation,
migration and invasion of glioma cells
Based on the aforementioned findings, it was
hypothesized that CADM2 may be involved in the miR-25-mediated
proliferation and migration of glioma cells. To test this
hypothesis, T98G and U251MG cells were transfected with NC siRNA or
siCADM2. After transfection, the protein expression levels of CADM2
were significantly reduced in the siCADM2 group compared with the
NC siRNA group (Fig. 5A). CCK-8 and
wound healing assays were performed to assess cell proliferation
and migration. As shown in Fig.
5B-E, the knockdown of CADM2 significantly increased the
proliferation and migration of glioma cells. These findings
suggested that CADM2 serves a suppressive role in glioma cell
proliferation and migration.
After that, T98G and U251MG cells were
co-transfected with a miR-25 inhibitor and CADM2 siRNA or NC siRNA.
Following transfection, the CADM2 mRNA and protein expression
levels were significantly reduced in the miR-25 inhibitor + siCADM2
group compared with the miR-25 inhibitor + siNC group (Fig. 6A and B). Data from the CCK-8, wound healing and
Transwell assays further demonstrated that the proliferation (at 72
h), migration and invasiveness of glioma cells were increased in
the miR-25 inhibitor + siCADM2 group compared with the miR-25
inhibitor + siNC group (Fig. 6C-G),
suggesting that the knockdown of CADM2 reversed the inhibitory
effects of miR-25 downregulation on the proliferation, migration
and invasiveness of glioma cells.
Discussion
The present study aimed to investigate the
underlying molecular mechanism of miR-25 in the progression of
glioma. The expression of miR-25 was significantly upregulated in
glioma tissues and cell lines, and a high expression level of
miR-25 was associated with advanced clinical stage. The knockdown
of miR-25 expression significantly reduced glioma cell
proliferation, migration and invasion. CADM2 was identified as a
direct target of miR-25 in glioma cells. Moreover, the expression
of level CADM2 was significantly reduced in glioma tissues and was
inversely correlated with miR-25 expression. Furthermore, the
expression of CADM2 was negatively regulated by miR-25 in glioma
cells and CADM2 knockdown reversed the effects of miR-25 inhibition
and promoted glioma cell proliferation, migration and invasion.
An increasing number of studies have reported that
the expression levels of miR-25 are frequently upregulated in
numerous common types of human cancer and that miR-25 serves an
oncogenic role by promoting the malignant phenotype of tumor cells.
For example, Xiang et al (24) demonstrated that miR-25 was
upregulated in non-small cell lung cancer (NSCLC) tissues and cell
lines, and that this promoted NSCLC cell proliferation and motility
by suppressing the expression of F-box/WD repeat-containing protein
7. Feng et al (25) revealed
that miR-25 promoted ovarian cancer cell proliferation and motility
by directly targeting the serine/threonine-protein kinase LATS2.
Zhao et al (26) reported
that miR-25 enhanced the proliferation and motility of gastric
cancer cells by inhibiting the expression of reversion-inducing
cysteine-rich protein with Kazal motifs. The results of the present
study indicated that the expression level of miR-25 was
significantly increased in glioma tissues compared with normal
brain tissues and that a high miR-25 expression level was
significantly associated with advanced clinical stage. These data
suggested that the upregulation of miR-25 may promote glioma
progression. Additionally, miR-25 expression level was increased in
glioma cell lines compared with normal brain tissues. Therefore,
two glioma cell lines were transfected with a miR-25 inhibitor to
decrease its expression. This revealed that the knockdown of miR-25
significantly reduced glioma cell proliferation, migration and
invasion. Similarly, Zhang et al (22) showed that miR-25 was upregulated in
91% of human glioma tissues and four out of six human glioma cell
lines examined and that miR-25 promoted glioma cell
proliferation.
miRNAs function by regulating the expression of
their target genes, a number of which have been identified for
miR-25, including BTG2(27), cyclic
GMP-AMP synthase (28),
transcription factor SOX4(29) and
CDKN1C (22). In the present study,
a bioinformatics prediction showed that CADM2 was a putative target
gene of miR-25. Luciferase reporter gene analysis further confirmed
this targeting relationship. CADM2, a member of the synaptic cell
adhesion molecule 1 family, contains three Ig-like domains and a
cytosolic protein 4.1 binding site near the C-terminus, through
which it crosslinks spectrin and interacts with other cytoskeletal
proteins (30). It has been
reported that CADM2 acts as a tumor suppressor in several common
types of human cancer. He et al (30) reported that aberrant methylation and
loss of CADM2 expression were associated with the progression of
renal cell carcinoma. Furthermore, Yang et al (31) revealed that a low CADM2 expression
level predicted a high risk of recurrence following hepatectomy in
patients with hepatocellular carcinoma. The present study revealed
that CADM2 was significantly downregulated in glioma tissues and
cell lines compared with normal brain tissues. Moreover, a negative
correlation was observed between the expression levels of CADM2 and
miR-25 in glioma tissues and the expression of CADM2 was negatively
regulated by miR-25 in glioma cell lines. These findings suggested
that the increased expression level of miR-25 may contribute to a
decrease in that of CADM2 in glioma. It was further revealed that
the inhibition of CADM2 expression rescued the suppressive effects
of miR-25 downregulation on the proliferation, migration and
invasion of glioma cells, confirming that CADM2 acted as a
downstream effector of miR-25 in glioma cells.
To the best of our knowledge, this is the first
study to report that miR-25 promoted glioma cell proliferation,
migration and invasion, at least in part by targeting CADM2. These
findings expand the understanding of the underlying molecular
mechanisms of glioma progression.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Nature Science
Foundation of Hunan province (no. 2021JJ70074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS designed the study and revised the manuscript. GP
collected the clinical tissues, conducted the statistical analysis
and wrote the manuscript. YL and CY performed the experiments. GP
and CS have confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiangya Hospital, Central South University (Changsha,
China). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marumoto T and Saya H: Molecular biology
of glioma. Adv Exp Med Biol. 746:2–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma Z: Downregulation of SETD8 by miR-382
is involved in glioma progression. Pathol Res Pract. 214:356–360.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan Z, Zhao J and Jiang Y: miR-634
sensitizes glioma cells to temozolomide by targeting CYR61 through
Raf-ERK signaling pathway. Cancer Med. 7:913–921. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu L, Liu Z, Wang H, Chen L, Ruan F,
Zhang J, Hu Y, Luo H and Wen S: 14-3-3β exerts glioma-promoting
effects and is associated with malignant progression and poor
prognosis in patients with glioma. Exp Ther Med. 15:2381–2387.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peng Z, Liu C and Wu M: New insights into
long noncoding RNAs and their roles in glioma. Mol Cancer.
17(61)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dai S, Yan Y, Xu Z, Zeng S, Qian L, Huo L,
Li X, Sun L and Gong Z: SCD1 confers temozolomide resistance to
human glioma cells via the Akt/GSK3β/β-catenin signaling axis.
Front Pharmacol. 8(960)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Che F, Xie X, Wang L, Su Q, Jia F, Ye Y,
Zang L, Wang J, Li H, Quan Y, et al: B7-H6 expression is induced by
lipopolysaccharide and facilitates cancer invasion and metastasis
in human gliomas. Int Immunopharmacol. 59:318–327. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song H, Zhang Y, Liu N, Wan C, Zhang D,
Zhao S, Kong Y and Yuan L: miR-92b regulates glioma cells
proliferation, migration, invasion, and apoptosis via PTEN/Akt
signaling pathway. J Physiol Biochem. 72:201–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stojcheva N, Schechtmann G, Sass S, Roth
P, Florea AM, Stefanski A, Stühler K, Wolter M, Müller NS, Theis
FJ, et al: MicroRNA-138 promotes acquired alkylator resistance in
glioblastoma by targeting the Bcl-2-interacting mediator BIM.
Oncotarget. 7:12937–12950. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu Y, Zhao H, Rao M and Xu S:
MicroRNA-365 inhibits proliferation, migration and invasion of
glioma by targeting PIK3R3. Oncol Rep. 37:2185–2192.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang T, Ma G, Zhang Y, Huo H and Zhao Y:
miR-599 inhibits proliferation and invasion of glioma by targeting
periostin. Biotechnol Lett. 39:1325–1333. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Tan W, Neo TW, Aung MO, Wasser S,
Lim SG and Tan TM: Role of the miR-106b-25 microRNA cluster in
hepatocellular carcinoma. Cancer Sci. 100:1234–1242.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ding X, Zhong T, Jiang L, Huang J, Xia Y
and Hu R: miR-25 enhances cell migration and invasion in
non-small-cell lung cancer cells via ERK signaling pathway by
inhibiting KLF4. Mol Med Rep. 17:7005–7016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He J, Qi H, Chen F and Cao C: MicroRNA-25
contributes to cisplatin resistance in gastric cancer cells by
inhibiting forkhead box O3a. Oncol Lett. 14:6097–6102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng G, Yuan X, Yuan J, Liu Q, Dai M, Shen
C, Ma J, Liao Y and Jiang W: miR-25 promotes glioblastoma cell
proliferation and invasion by directly targeting NEFL. Mol Cell
Biochem. 409:103–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang J, Gong X, Tian K, Chen D, Sun J,
Wang G and Guo M: miR-25 promotes glioma cell proliferation by
targeting CDKN1C. Biomed Pharmacother. 71:7–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiang J, Hang JB, Che JM and Li HC: MiR-25
is up-regulated in non-small cell lung cancer and promotes cell
proliferation and motility by targeting FBXW7. Int J Clin Exp
Pathol. 8:9147–9153. 2015.PubMed/NCBI
|
|
25
|
Feng S, Pan W, Jin Y and Zheng J: MiR-25
promotes ovarian cancer proliferation and motility by targeting
LATS2. Tumour Biol. 35:12339–12344. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhao H, Wang Y, Yang L, Jiang R and Li W:
MiR-25 promotes gastric cancer cells growth and motility by
targeting RECK. Mol Cell Biochem. 385:207–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Pan H, Qian Y, Zhou W and Liu X:
MiR-25-3p promotes the proliferation of triple negative breast
cancer by targeting BTG2. Mol Cancer. 17(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu MZ, Cheng WC, Chen SF, Nieh S, O'Connor
C, Liu CL, Tsai WW, Wu CJ, Martin L, Lin YS, et al: miR-25/93
mediates hypoxia-induced immunosuppression by repressing cGAS. Nat
Cell Biol. 19:1286–1296. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Chen B, Liu J, Qu J, Song Y, Li Y and Pan
S: MicroRNA-25 suppresses proliferation, migration, and invasion of
osteosarcoma by targeting SOX4. Tumour Biol.
39(1010428317703841)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
He W, Li X, Xu S, Ai J, Gong Y, Gregg JL,
Guan R, Qiu W, Xin D, Gingrich JR, et al: Aberrant methylation and
loss of CADM2 tumor suppressor expression is associated with human
renal cell carcinoma tumor progression. Biochem Biophys Res Commun.
435:526–532. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang S, Yan HL, Tao QF, Yuan SX, Tang GN,
Yang Y, Wang LL, Zhang YL, Sun SH and Zhou WP: Low CADM2 expression
predicts high recurrence risk of hepatocellular carcinoma patients
after hepatectomy. J Cancer Res Clin Oncol. 140:109–116.
2014.PubMed/NCBI View Article : Google Scholar
|