Introduction
Age-related hearing loss (ARHL), also known as
presbycusis, is described as a gradual loss of hearing that is
linked to aging. ARHL is characterized by impaired speech
discrimination, deteriorated hearing sensitivity, slowed central
acoustic signal processing and impaired localization of sound
sources. ARHL is the most common sensory disorder in the elderly
population (1), with a prevalence
of 25-40% of individuals aged 65 years or above. The prevalence
increases with age, ranging from 40 to 66% in individuals over 75
years of age and >80% in those over 85 years of age (2).
ARHL is thought to be the result of aging,
mitochondrial dysfunction, oxidative damage and environmental
factors (3). It has been widely
recognized that aging is a process of cumulative oxidative damage
caused by free radicals (4,5).
Free radical production increases with age and it is recognized
that oxidative stress and related mitochondrial dysfunction have an
important role in aging and age-related diseases (6,7).
Numerous studies have demonstrated that diminishing oxidative
stress may extend an organism's life span (8). Therefore, an effective way to slow
the progression of age-related damage to the body may be to improve
its antioxidant defense against oxidative stress.
Apoptosis, or programmed cell death, is essential
for maintaining the balance of cell life and death. It has been
known for a long time that apoptosis is involved in aging and
age-related diseases (9). In
addition, it has been demonstrated that apoptotic cell death occurs
earlier in mice with presbycusis (10).
Resveratrol (3,5,4'-trihydroxystilbene; RSV), which
is known to be a potent antioxidant, is a natural phytoalexin and
polyphenol that is present in a wide range of plants, such as
grapes, berries, mulberries and peanuts (11). The biological effects of RSV
include scavenging of free radicals, inhibition of lipid
peroxidation, anti-inflammatory effects, copper chelation,
modification of eicosanoid synthesis, inhibition of platelet
aggregation, vasodilatation, modulation of lipid metabolism,
vasorelaxant activity, anticancer activity and estrogenic activity
(11,12).
Resveratrol has protective effects against
cisplatin-induced (13),
aminoglycoside-induced (14) and
noise-induced hearing losses (15). Furthermore, it was reported that a
mixture of polyphenols, including RSV, have a beneficial effect on
presbycusis (16).
The aim of the present study was to evaluate the
efficacy of RSV in the prevention and treatment of ARHL as assessed
by auditory brainstem response (ABR) testing and evaluation of the
changes in the mRNA expression levels of inducible nitric oxide
synthase (iNOS), cyclooxygenase-2 (COX-2) and NF-κB
genes, which are effective in inflammation, and Bcl-2, Bcl-xL,
Bax, Bcl-2 homologous antagonist/killer (Bak), caspase-3 and
caspase-9 genes, which all have a role in apoptosis, in
cochlear samples of C57BL/6 mice.
Materials and methods
Animals
A total of 32 male C57BL/6 mice were used for the
present study. The animals were obtained from the Gazi University
Laboratory Animal Raising and Experimental Research Center at 12
weeks of age (mean weight, 28.6±1.9 g) and the study was carried
out in the same place (Ankara, Turkey). These mice have been
extensively studied as a model of ARHL (17). Hearing loss begins at approximately
five to six months of age and progresses to near-complete hearing
loss by the age of 18 months in this strain (18). The animals were housed in plastic
cages at 23±2˚C with a relative humidity of 55%±5, with water and
food provided ad libitum and maintained under a 12-h
light/dark cycle. The Animal Care and Use Committee of Gazi
University (Ankara, Turkey) approved the study protocol (approval
no. 66332047-604.01.02-7347). The study was performed in accordance
with the EU Directive 2010/63/EU.
Anesthesia
Mice were anesthetized by i.p. injection of ketamine
(90 mg/kg) and xylazine (10 mg/kg) cocktail during ABR
measurements. At the end of the study, animals were painlessly
sacrificed by cervical dislocation under the same anesthetic
protocol.
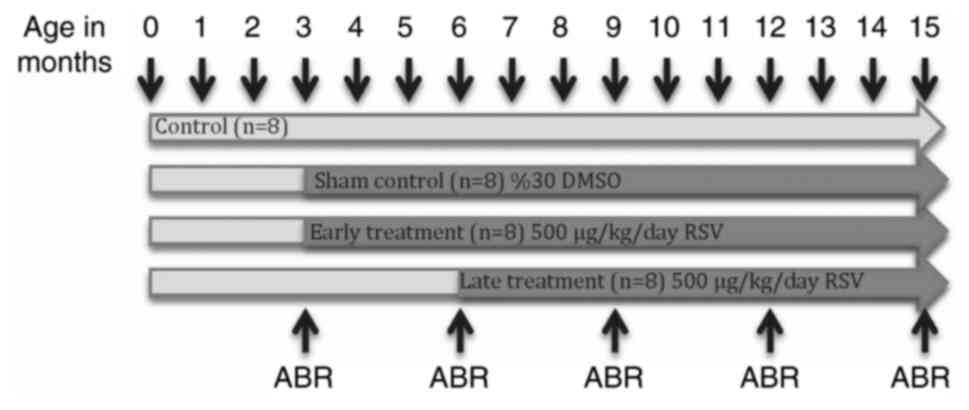
Study protocol
Power analysis was used to determine the sample
size. A desired power of 0.80 and a desired α of 0.05 were set with
an estimated size effect δ of ~1.4, based on preliminary data. Mice
were randomized into four groups and treated as follows: Control
group (n=8), with no medication; sham control group (n=8), with
DMSO administration initiated at three months of age; early
treatment group (n=8), with RSV treatment initiated at three months
of age; and late treatment group (n=8), with RSV treatment
initiated at six months of age. The timeline of the study protocol
is presented in Fig. 1.
Trans-resveratrol (Sigma-Aldrich; Merck KGaA),
dissolved in 30% DMSO (20 mg/ml) (Sigma-Aldrich; Merck KGaA) and
added to the drinking water of mice so that the approximate daily
dosage of 500 µg/kg for each mouse was maintained (16). Drinking water was changed every day
to ensure the required dose was taken by the mice. In addition, new
solution was prepared weekly during the study period to ensure the
stability of RSV. The mice were weighed once every week and the
dose of the RSV was adjusted according to the weights of the mice,
if necessary. 30% DMSO was also prepared freshly on a weekly basis
and added to the drinking water of the sham control group. The sham
control group was created to evaluate the possible effects of DMSO,
the substance that RSV was dissolved in, on the hearing of the
mice.
ABR
ABR measurements were performed with a two-channel
Neuro-audio® ABR device (Neurosoft; Ivanova). After
anesthesia, ABR measurements were performed for both the right and
left ears of every mouse; two traces were recorded for each ear to
control repeatability of the response. ER-2, 10 ohm insert
earphones with a frequency spectrum of 125-16,000 Hz were used for
auditory stimuli and subdermal disposable needle electrodes (Bionen
Medical Devices) were used for recording of the ABR. Single-channel
ipsilateral recording was performed to prevent a possible short
circuit between electrodes. The non-inverting electrode was placed
at the vertex, the invert electrode was placed on the ipsilateral
mastoid and the ground electrode was placed on the contralateral
mastoid. The stimuli consisted of frequency-specific tone bursts
over a 4,000-16,000 Hz range, with a 21/sec stimulus rate and 1,024
presentations averaged for each intensity.
Thresholds were determined by reducing the intensity
of the stimulus in 5-dB steps starting from 110 decibels sound
pressure level (dB SPL). The ABR threshold was defined as the
lowest dB SPL level at which waveforms lost their reproducible
morphology.
Baseline ABRs measurements were performed when the
mice were 3 months of age, prior to the initiation of all
treatments, before the start of all treatments. ABRs were recorded
every three months till the end of the study, when the mice were 6,
9, 12 and 15 months of age (Fig.
1).
RNA isolation, cDNA synthesis and
quantitative PCR (qPCR)
At the end of the study period, all mice were
sacrificed under anesthesia after the last ABR testing. The
cochleae of both ears were removed under a dissecting microscope
and crushed. Total RNA was isolated from fresh cochlear tissues by
using TRIzol® reagent (Thermo Fisher Scientific, Inc.),
in accordance with the manufacturer's instructions and then stored
at -80˚C until use. The RNA concentration was measured at 260/280
nm, using the NanoDrop® 1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). Total RNA (1 µg) was used for
gene-specific reverse transcription (RT) with a Transcriptor High
Fidelity cDNA Synthesis Kit (Roche Diagnostics), following the
manufacturer's protocol.
qPCR reactions were performed using the
LightCycler® 480 instrument (Roche Diagnostics),
according to the manufacturer's instructions. Gene-specific intron
spanning primers and universal probe library (UPL) numbers for each
gene were designed using the online UPL Assay Design Center
(https://www.universalprobe library.com) and are
provided in Table I. The following
thermal cycling conditions were applied on the
LightCycler® 480 Instrument: Denaturation at 95˚C for 10
min, 45 cycles of amplification, 95˚C for 10 sec, 60˚C for 20 sec
and cooling at 40˚C for 30 sec. The results obtained were
normalized to a housekeeping gene b-actin (ACTB). Fold changes of
the gene expression of iNOS, COX-2, NF-κB, Bcl-2, Bcl-xL, Bax,
Bak, caspase-3 and caspase-9 were calculated using the
2-ΔΔCT method (19).
All experiments were performed in triplicate in 3 independent
experiments.
 | Table IGene-specific primer sequences and
UPL probe numbers. |
Table I
Gene-specific primer sequences and
UPL probe numbers.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') | UPL Probe No |
|---|
| ACTB |
CTAAGGCCAACCGTGAAAAG |
ACCAGAGGCATACAGGGACA | 64 |
| iNos |
CTTTGCCACGGACGA |
TCATTGTACTCTGAG | 13 |
| Cox-2 |
GATGCTCTTCCGAGC-3' |
5'-GGATTGGAACAGCAA-3' | 45 |
| NF-κB |
CTCTCGACGTCAGTGGGAAT |
CTCGTCCTCTCTCGCTCACT | 20 |
| Bcl-2 |
AGTACCTGAACCGGCATCTG |
GGGGCCATATAGTTCCACAAA | 75 |
| Bcl-xL |
TGACCACCTAGAGCCTTGGA |
GCTGCATTGTTCCCGTAGA | 2 |
| Bax |
AGTGTCTCCGGCGAATTG |
CCACGTCAGCAATCATCCT | 56 |
| Bak |
AGAGGGAGCTGGTCATTGC |
AAACCTCGCGACTTTGTGAC | 33 |
|
Caspase-3 |
ACTCGTGAAGACATTTTGGAATTA |
TCACCATGGCTTAGAATCACA | 68 |
|
Caspase-9 |
AAGAAGACCGGAGTGCAATG |
GGCACAATCCCTAACCACAG | 27 |
Statistical analysis
The differences in iNOS, COX-2, NF-κB, Bcl-2,
Bcl-xL, Bax, Bak, caspase-3 and caspase-9 expression
between groups determined by RT-qPCR analysis were analyzed by Pair
Wise Fixed Reallocation Randomization Test© included in
the Relative Expression Software Tool (REST©, 2009
v2.0.13) statistical software developed for group-wise comparison
and statistical analysis of relative expression results (20). ANOVA was used to determine any
statistically significant differences in ABR thresholds across
groups. To confirm statistical significance, Tukey's post-hoc test
was performed with SPSS v22® (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
General observations
All mice survived until the end of the study. There
were no significant differences in body weight of the mice among
groups both at the beginning and at the end of the study (P=0.520,
data not shown).
ABR
Baseline ABR measurements that were obtained at the
beginning of the study, when the mice were three months of age,
were subjected to ANOVA and no significant difference among the
groups was obtained (Table
II).
 | Table IIMean auditory brainstem response
thresholds by frequency of stimulus at the beginning of the study
(values in decibels sound pressure level). |
Table II
Mean auditory brainstem response
thresholds by frequency of stimulus at the beginning of the study
(values in decibels sound pressure level).
| Frequency
(kHz) | Control | Sham control | Early | Late | P-value |
|---|
| 4 | 39.375±4.886 | 40.000±4.978 | 40.625±4.673 | 38.750±5.342 | 0.510 |
| 8 | 16.875±3.578 | 14.375±3.876 | 15.000±4.312 | 16.250±3.632 | 0.492 |
| 12 | 15.000±3.578 | 16.250±4.312 | 15.625±3.876 | 16.250±3.578 | 0.476 |
| 16 | 23.125±6.214 | 25.000±5.983 | 24.375±5.834 | 22.500±6.121 | 0.520 |
Similarly, when the mean ABR thresholds of the mice
at the third month of the study (when the early treatment group had
received three months of RSV therapy, while no therapy had been
provided to the late and control groups) were compared, no
significant difference was present among groups at any frequency
(P=0.825; data not shown).
The ABR results at the sixth month are presented in
Table III. While no significant
difference was obtained between the control, sham control and late
treatment groups, the average thresholds of the early treatment
group were significantly lower than those of the control groups and
late treatment group.
 | Table IIIMean auditory brainstem response
thresholds of the mice at the sixth month of the study by frequency
of stimulus (values in decibels sound pressure level). |
Table III
Mean auditory brainstem response
thresholds of the mice at the sixth month of the study by frequency
of stimulus (values in decibels sound pressure level).
| Frequency
(kHz) | Control | Sham control | Early | Late |
P-valuea |
P-valueb |
|---|
| 4 | 51.875±8.421 | 51.25±9.883 | 49.375±10.883 | 42.5±10.033 | 0.006 | 0.320 |
| 8 | 25.625±4.323 | 26.25±3.679 | 20.625±4.412 | 24.375±4.873 | 0.008 | 0.254 |
| 12 | 24.375±3.362 | 23.75±4.543 | 19.375±3.354 | 25±3.612 | 0.007 | 0.465 |
| 16 | 35±5.873 | 34.375±4.173 | 30±4.246 | 35.625±3.644 | 0.005 | 0.312 |
Regarding the ABR results at 9 months, while the
average thresholds for the early treatment group were determined to
be 11.875-19.25 dB lower than those of the control groups
(P<0.001), the average thresholds for the late treatment group
were 1.875-8.75 dB lower than those of the control groups
(P>0.05). The mean thresholds of the early treatment group were
also lower when compared to those of the late treatment group
(8.125-11.25 dB). This difference was determined to be
statistically significant at 12 and 16 kHz (P=0.003 and 0.005,
respectively), while no significant difference was obtained at 4
and 8 kHz (P>0.05; data not shown).
The last ABR threshold data obtained at the end of
the study, just before the mice were sacrificed, are presented in
Table IV. The mice were 15 months
of age and the early treatment group had received twelve months of
RSV therapy, while the late treatment group had received nine
months of RSV therapy at this time-point. The mean hearing
thresholds of the early treatment group were significantly better
than those of the late treatment group and control groups
(P<0.001). When the thresholds of the late treatment group and
control groups were compared, although the hearing thresholds of
the late treatment group were lower than those of the controls at
all frequencies, the difference was not statistically significant
(P>0.05).
 | Table IVMean auditory brainstem response
thresholds of the mice at the end of the study by frequency of
stimulus (values in decibels sound pressure level). |
Table IV
Mean auditory brainstem response
thresholds of the mice at the end of the study by frequency of
stimulus (values in decibels sound pressure level).
| Frequency
(kHz) | Control | Sham control | Early | Late |
P-valuea |
P-valueb |
|---|
| 4 | 74.375±8.766 | 76.25±10.967 | 55.625±8.932 | 68.125±8.463 | <0.001 | 0.090 |
| 8 | 60.625±9.256 | 59.375±10.112 | 30.625±9.102 | 51.875±7.732 | 0.001 | 0.112 |
| 12 | 58.125±9.324 | 57.5±9.646 | 31.875±8.321 | 48.125±8.653 | <0.001 | 0.070 |
| 16 | 63.125±8.725 | 62.5±8.354 | 40.625±9.627 | 54.375±9.372 | <0.001 | 0.102 |
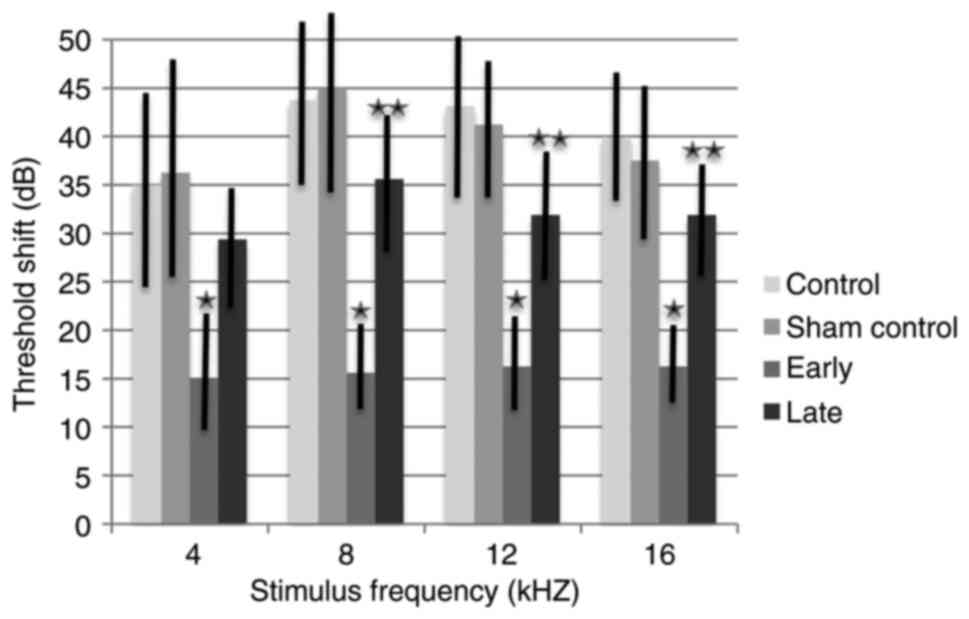
The ABR threshold shifts from baseline to 15 months
were also analyzed (Fig. 2). Mean
ABR threshold shifts for the control and sham control groups ranged
between 35.000 and 43.750 dB according to frequencies, with no
significant difference between these two groups (P=0.895). While
the threshold shifts of the early treatment group ranged between
15.000 and 16.250 dB, the threshold shifts of the late treatment
group ranged between 29.375 and 35.625 dB. The threshold shifts for
the early treatment group were significantly lower than those of
the control groups at all frequencies (P<0.001). The final mean
threshold shift differences between the early and the late
treatment groups ranged from 14.375 to 20.000 dB; this difference
was not statistically significant at 4 kHz (P=0.785). However, at
8, 12 and 16 kHz, the threshold shift was significantly lower in
the early treatment group (P<0.001).
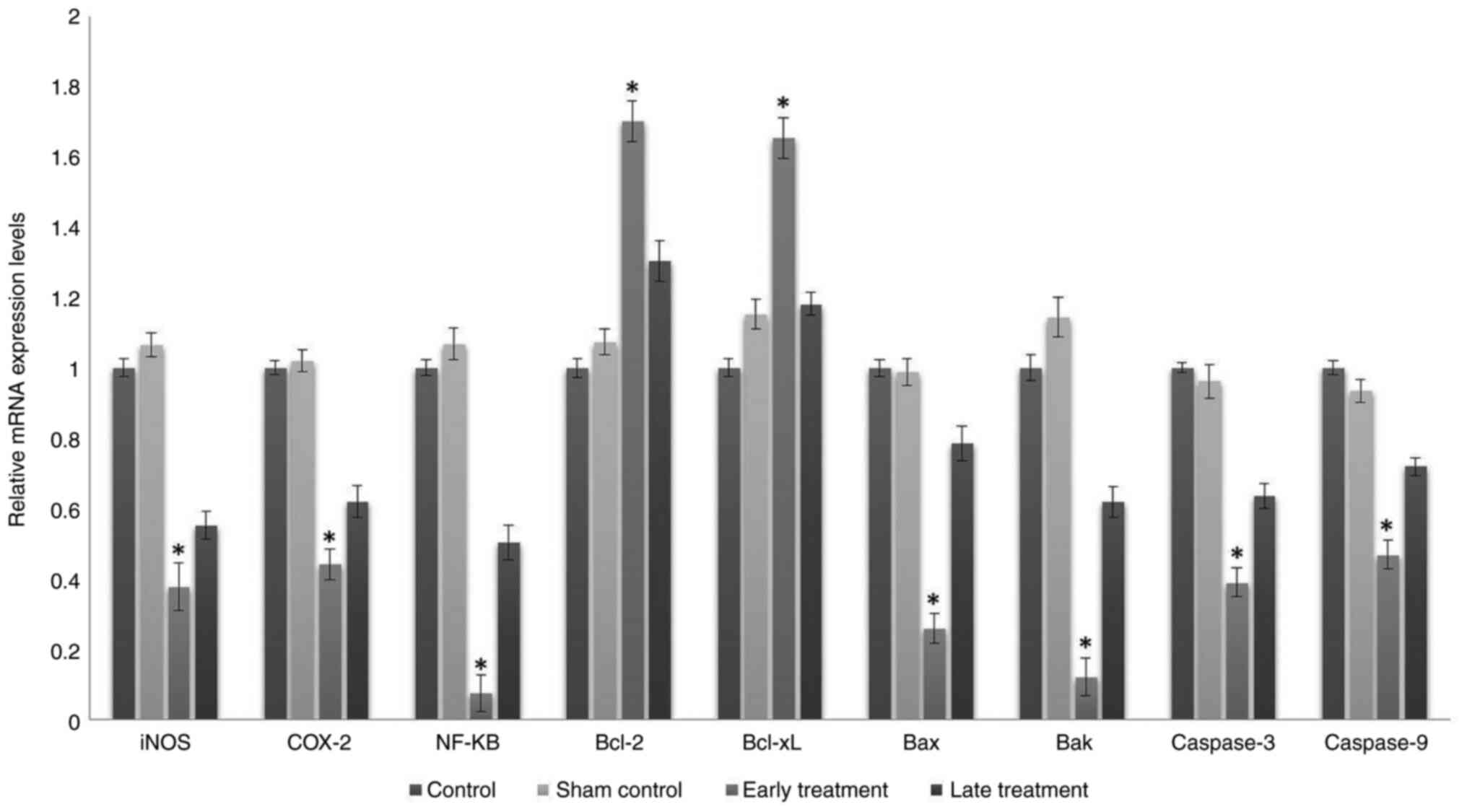
Gene expression levels
In the early treatment group, mRNA expression levels
of pro-apoptotic genes such as Bax and Bak, which may
be responsible for increased apoptosis in cells, were significantly
decreased (P<0.001), mRNA expression levels of anti-apoptotic
genes such as Bcl-2 and Bcl-xL were significantly
increased (P<0.001), mRNA expression levels of caspase-3
and caspase-9 genes, which have a vital role in apoptosis,
were significantly decreased (P<0.001) and mRNA expression
levels of inflammatory mediators such as NF-κB, COX-2
and iNOS genes were significantly decreased (P<0.001),
when compared to the control and sham control groups (Fig. 3).
Similarly, in the late treatment group, the mRNA
expression levels of the Bax and Bak genes were
decreased, the mRNA expression levels of Bcl-2 were
increased and the mRNA expression levels of caspase-3,
caspase-9 NF-κB, COX-2 and iNOS genes were decreased
when compared with the control and sham control groups. However,
these changes were not statistically significant (P>0.05;
Fig. 3). The mRNA levels of the
Bcl-xL gene were increased when compared with the control
group; however, they did not change when compared with the sham
control group. These changes were not statistically significant
(P>0.05).
Discussion
ARHL is a complex degenerative disease affecting
millions of individuals worldwide. As a major type of sensory
impairment, ARHL may cause patients to withdraw from social life
and become isolated and depressed (21). The high prevalence of ARHL, its
substantial impact on the well-being of elderly individuals and the
cost of the rehabilitation by hearing aids or implants, either to
the patient or health care system, makes it a major public health
concern.
The primary cause of age-related pathology in cell
aging is probably oxidative injury caused by free radical damage.
It is generally accepted that mitochondria are a major source of
free radicals [reactive oxygen species (ROS) and reactive nitrogen
species (RNS)] and a major site of ROS/RNS-induced oxidative damage
(16).
Several rat studies have demonstrated the favorable
effects of RSV on mitochondria. In particular, RSV supplementation
causes enhancement of several mitochondrial functions (respiratory
enzyme activity, oxygen consumption and activity of lipid-oxidizing
enzymes) (22,23). RSV decreases ROS by acting as a
strong scavenger of superoxide anions, hydrogen peroxide and
hydroxyl radicals (24).
Sánchez-Rodríguez et al (16) reported that a mixture of
polyphenols, of which RSV is also a potent member, exerts a
significant protective effect against ARHL by reversing the
destructive effects of aging on the integrity of the cochlea in
rats. This protection against aging damage in the inner ear was
caused by its antioxidant properties. Similarly, Heman-Ackah et
al (17) determined that a
combination of antioxidants (ascorbic acid, vitamin B12, folate,
ribose-cysteine, NW-nitro-L-arginine methyl ester and
L-cysteine-glutathione mixed disulfide) effectively decreased
threshold shifts of ABR in an animal model. The present study
indicated that RSV alone also has protective effects against ARHL,
particularly when started prior to the onset of the hearing loss.
This protective effect is due to its antioxidant properties, which
were proven by molecular studies. RSV decreased the mRNA expression
levels of the pro-apoptotic genes Bax and Bak, the
mRNA expression levels of caspase-3 and caspase-9,
which have a vital role in apoptosis, and the mRNA expression
levels of inflammatory mediators such as NF-κB, COX-2
and iNOS genes, while increasing anti-apoptotic genes such
as Bcl-2 and Bcl-xL. Similarly, Someya et al
(18) reported that ARHL in
C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis
and that oral supplementation of mitochondrial antioxidants
decreased Bak expression in the cochlea, mitigated cochlear
cell death and prevented ARHL.
Chronic inflammation has an important role in
age-related intracellular damage in mammalian cells (25). The present study indicated that the
expression levels of the NF-κB, COX-2 and iNOS
genes, which have critical roles on different levels of the
inflammatory process, were significantly higher in the cochlear
tissues of the mice in the early treatment group. The expression
levels of these genes in the late treatment group were also lower
than those of the control groups, but these differences were not
statistically significant. These results suggested that RSV slows
down chronic inflammatory processes, which are responsible for
aging, if it is initiated prior to the onset of these processes. It
may also suppress the inflammatory processes if it is used after
these processes become active, but no significant impact was
observed on either the ABR thresholds of the mice or at the
molecular level.
Different antioxidant molecules have been studied
for the prevention of ARHL, with variable results. In animal
studies, when C57BL/6 mice were fed with a diet containing one of
17 antioxidant agents, ARHL was almost entirely prevented by
α-lipoic acid and coenzyme Q10 and partially by
N-acetyl-L-cysteine, but not by any other agent (26). While addition of vitamin C to the
diet did not increase vitamin C levels in the cochlea or decelerate
ARHL in C57BL/6 mice (27),
Fischer 344 rats given vitamin E, C, melatonin or lazaroid had
better auditory sensitivities and fewer mitochondrial DNA deletions
in comparison with placebo subjects (28). Similarly, Fischer 344 rats
supplemented orally with lecithin for six months had significantly
better hearing sensitivities compared with their controls (29).
These results suggested that the prevention and
attenuation of ARHL by antioxidant supplements may be affected by
numerous factors such as the type and dosage of antioxidant
compounds, the timing and duration of the treatment and the species
and strains that are studied.
The present study demonstrated that daily oral RSV
treatment was able to attenuate the manifestation of ARHL in
C57BL/6 mice. This benefit was observed to be significant in the
early treatment group, in which the treatment was initiated prior
to the beginning of hearing loss. The late treatment group also
exhibited slightly improved ABR results, but neither the changes at
the molecular level nor the hearing thresholds were significantly
better than those in the control groups. These results suggested
that antioxidant treatment, in this case RSV, may be more
beneficial when initiated prior to the beginning of degenerative
changes that are responsible for ARHL. When the treatment is
initiated after the onset of ARHL, RSV may be less effective due to
the already ongoing apoptotic process and inflammation at the
molecular level. These results are compatible with those of
previous human studies, which have consensus regarding that the
antioxidants are effective in the prevention of ARHL but not as a
remedy, since hair cells in the ear do not regenerate and hearing
loss may not be restored (30).
ARHL usually begins at high frequencies and most,
but not all, of the previous animal studies focusing on ARHL
include 32 kHz ABR measurements (17,31).
Due to technical limitations, it was not possible to use an ABR
device that covers 32 kHz in the present study, and this is its
major limitation. In addition, even though the present study was
one of the very few studies on this context to date, only the
expression levels of the genes in cochlear tissues were analyzed,
providing an investigation of only the peripheral components of
ARHL at the molecular level. The lack of pathologic examination and
protein expression analysis are also potential limitations to the
present study. Advanced histopathological and molecular studies are
required to be performed to further elucidate the effects of RSV on
the central mechanisms of ARHL. Also, the present study revealed
that RSV prevents oxidation-associated aging in the ear to prevent
ARHL before it develops. In addition, considering the many
beneficial anti-aging effects of RSV, further human studies in
elderly patients are needed to evaluate the effects of RSV on
hearing loss.
In conclusion, RSV was indicated to have a
beneficial role in ARHL, with its potent antioxidant and
anti-inflammatory properties, particularly when started prior to
the onset of hearing loss. However, even with the promising
potential of different preventive drugs, minimizing noise exposure
and maintaining a healthy lifestyle remain the most effective
accepted routes for limiting ARHL in the human population.
Acknowledgements
This work was presented at the 39th Turkish
Otolaryngology Head and Neck Surgery Meeting (abstract no: SB-143;
November 8-12, 2017; Antalya, Turkey) and awarded with the prize
for the best original research in otology.
Funding
Funding: This work was supported by the Scientific and
Technological Research Council of Turkey (grant no. 114S938).
Availability of the data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM: Conceptualization, funding acquisition,
investigation, writing and original draft preparation. ASYS:
Conceptualization, methodology, resources, validation. DU: Data
curation, methodology. SM: Software, data curation, resources. ES:
Resources, funding acquisition, data curation. HK: Methodology,
supervision. TM, DU, SM and ES performed the ABR's. TM, SM and ES
removed the cochleae. ASYS prepared the solutions and performed PCR
testing. TM, DU and ASYS confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Gazi University Animal Care and Use Committee
(Ankara, Turkey) approved the study protocol (approval no.
66332047-604.01.02-7347).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gates GA and Mills JH: Presbycusis.
Lancet. 366:1111–1120. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yamasoba T, Someya S, Yamada C, Weindruch
R, Prolla TA and Tanokura M: Role of mitochondrial dysfunction and
mitochondrial DNA mutations in age-related hearing loss. Hear Res.
226:185–193. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu XZ and Yan D: Ageing and hearing loss.
J Pathol. 211:188–197. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perez P and Bao J: Why do hair cells and
spiral ganglion neurons in the cochlea die during aging? Aging Dis.
2:231–241. 2011.PubMed/NCBI
|
|
5
|
Bielefeld EC, Tanaka C, Chen GD and
Henderson D: Age-related hearing loss: Is it a preventable
condition? Hear Res. 264:98–107. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fujimoto C and Yamasoba T: Oxidative
stresses and mitochondrial dysfunction in age-related hearing loss.
Oxid Med Cell Longev. 2014(582849)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen H and Tang J: The role of
mitochondria in age-related hearing loss. Biogerontology. 15:13–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Tower J: Programmed cell death in aging.
Ageing Res Rev. 23:90–100. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park SN, Back SA, Park KH, Kim DK, Park
SY, Oh JH, Park YS and Yeo SW: Comparison of cochlear morphology
and apoptosis in mouse models of presbycusis. Clin Exp
Otorhinolaryngol. 3:126–135. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ozgová S, Hermánek J and Gut I: Different
antioxidant effects of polyphenols on lipid peroxidation and
hydroxyl radicals in the NADPH-, Fe-ascorbate- and Fe-microsomal
systems. Biochem Pharmacol. 66:1127–1137. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yumusakhuylu AC, Yazici M, Sari M,
Binnetoglu A, Kosemihal E, Akdas F, Sirvanci S, Yuksel M, Uneri C
and Tutkun A: Protective role of resveratrol against cisplatin
induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol.
76:404–408. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bonabi S, Caelers A, Monge A, Huber A and
Bodmer D: Resveratrol protects auditory hair cells from gentamicin
toxicity. Ear Nose Throat J. 87:570–573. 2008.PubMed/NCBI
|
|
15
|
Seidman M, Babu S, Tang W, Naem E and
Quirk WS: Effects of resveratrol on acoustic trauma. Otolaryngol
Head Neck Surg. 129:463–470. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sánchez-Rodríguez C, Martin-Sanz E,
Cuadrado E, Granizo JJ and Sanz-Fernández R: Protective effect of
polyphenols on presbycusis via oxidative/nitrosative stress
suppression in rats. Exp Gerontol. 83:31–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Heman-Ackah SE, Juhn SK, Huang TC and
Wiedmann TS: A combination antioxidant therapy prevents age-related
hearing loss in C57BL/6 mice. Otolaryngol Head Neck Surg.
143:429–434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Someya S, Xu J, Kondo K, Ding D, Salvi RJ,
Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M
and Prolla TA: Age-related hearing loss in C57BL/6J mice is
mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad
Sci USA. 106:19432–19437. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30(e36)2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kalayam B, Meyers BS, Kakuma T,
Alexopoulos GS, Young RC, Solomon S, Shotland R, Nambudiri D and
Goldsmith D: Age at onset of geriatric depression and sensorineural
hearing deficits. Biol Psychiatry. 38:649–658. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Murase T, Haramizu S, Ota N and Hase T:
Suppression of the aging-associated decline in physical performance
by a combination of resveratrol intake and habitual exercise in
senescence-accelerated mice. Biogerontology. 10:423–434.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hart N, Sarga L, Csende Z, Koltai E, Koch
LG, Britton SL, Davies KJ, Kouretas D, Wessner B and Radak Z:
Resveratrol enhances exercise training responses in rats
selectively bred for high running performance. Food Chem Toxicol.
61:53–59. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lorenz P, Roychowdhury S, Engelmann M,
Wolf G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chung HY, Kim DH, Lee EK, Chung KW, Chung
S, Lee B, Seo AY, Chung JH, Jung YS, Im E, et al: Redefining
chronic inflammation in aging and age-related diseases: Proposal of
the senoinflammation concept. Aging Dis. 10:367–382.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Someya S and Prolla TA: Mitochondrial
oxidative damage and apoptosis in age-related hearing loss. Mech
Ageing Dev. 131:480–486. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kashio A, Amano A, Kondo Y, Sakamoto T,
Iwamura H, Suzuki M, Ishigami A and Yamasoba T: Effect of vitamin C
depletion on age-related hearing loss in SMP30/GNL knockout mice.
Biochem Biophys Res Commun. 390:394–398. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Seidman MD: Effects of dietary restriction
and antioxidants on presbyacusis. Laryngoscope. 110:727–738.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seidman MD, Khan MJ, Tang WX and Quirk WS:
Influence of lecithin on mitochondrial DNA and age-related hearing
loss. Otolaryngol Head Neck Surg. 127:138–144. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tavanai E and Mohammadkhani G: Role of
antioxidants in prevention of age-related hearing loss: A review of
literature. Eur Arch Otorhinolaryngol. 274:1821–1834.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Marie A, Meunier J, Brun E, Malmstrom S,
Baudoux V, Flaszka E, Naert G, Roman F, Cosnier-Pucheu S and
Gonzalez-Gonzalez S: N-acetylcysteine treatment reduces age-related
hearing loss and memory impairment in the senescence-accelerated
prone 8 (SAMP8) mouse model. Aging Dis. 9:664–673. 2018.PubMed/NCBI View Article : Google Scholar
|