Introduction
Preeclampsia is a common health condition in
pregnant females, which is characterized by hypertension and
multiple organ failure including renal, liver and lung dysfunction
(1). It is a leading cause of
mortality of pregnant females and infants (2). Preeclampsia poses a risk to millions
of pregnant females during pregnancy, among which almost 300,000
females decease along the process and >500,000 infants expire
annually worldwide, including the death prior to birth and
afterwards (3). According to the
severity parameters of preeclampsia, pregnant females with signs of
blood pressure ≥160/110 mmHg, thrombocytopenia [with platelet count
(PLT) <100x109/l], renal insufficiency (serum
creatinine two times higher than the normal limit) and serum
alanine aminotransferase (ALT) more than two times of the normal
limit, are at a higher risk of severe preeclampsia (SPE) (4). Haemolysis, Elevated Liver enzymes and
Low Platelet count (HELLP) syndrome is a high-risk factor for
mortality of pregnant females and infants characterized by signs of
haemolysis, elevation of the liver enzymes and low PLT (5). HELLP syndrome is a serious
complication of pregnancy owing to hypertensive disorder, which is
a cause of preeclampsia, accounting for about 1/4 of preeclampsia
(5). In the past years, researchers
have been concentrating on the cause and the pathological mechanism
of preeclampsia (6). Extensive
research is necessary for the adoption of epidemiological methods
to follow up the prognosis of patients with preeclampsia and
determine risk factors to provide evidence-based medical insight
into assessing the condition and preventing adverse outcomes of
pregnant females and their infants.
Long noncoding RNAs (lncRNAs) are a class of
non-translatable RNA longer than 200 nucleotides; stable in serum
and other biological fluids, they function as a definitive
indicator for evaluating disease and tissue specificity (7). Since the initial discovery of lncRNAs,
accumulating evidence has indicated their significance in the
development and functioning of the placenta, where anomalies in
placental differentiation and function critically affect the
pregnancy outcome and postpartum maternal and infant health
(8). T-cell leukemia/lymphoma 6
(TCL6) is an lncRNA identified in T-cell leukemia; it is located in
the breakpoint cluster region on chromosome 14q32, also known as
TNG1 or TNG2(9). Emerging evidence
has supported the potential role of TCL6 as a tumor suppressor
(10-12).
A study identified an association between lncRNA TCL6 and immune
infiltration and suggested it may be adopted as a molecular marker
for the prognosis of breast cancer (13). A recent study indicated that TCL6 is
highly expressed in the placenta of patients with threatened
abortion and may serve as a modulator of trophoblast function via
the epidermal growth factor receptor signaling pathway (14). In addition, a previous study has
elucidated the involvement of TCL6 in the pathogenesis of
preeclampsia, where its high expression may inhibit the
proliferation of trophoblast cells (15).
Currently, only a limited number of studies have
focused on TCL6 and preeclampsia and the relationship between the
expression of lncRNA TCL6 and the diagnosis, grading and prognosis
of preeclampsia warrants further exploration. The aim of the
present study was to investigate the lncRNA TCL6 expression in the
serum of pregnant females with preeclampsia as compared with that
in normal controls and assess the association of TCL6 with the
diagnosis and severity of preeclampsia and pregnancy outcomes.
Materials and methods
Study subjects
Pregnant women from January 2018 to December 2019 in
obstetrics department of The Affiliated Hospital of Yunnan
University were selected. The purpose, operation process and
possible impact of the clinical study were explained to them
through interview. The case data were reviewed by the medical
professionals to determine whether they were suitable for the
study. The patients who were fully informed and agreed to
participate in the study were included in the study. A total of 120
females during late singleton pregnancy (gestational age ≥28 weeks)
with preeclampsia hospitalized at the Second People's Hospital of
Yunnan Province (Kunming, China) between January 2018 and December
2019 were enrolled as the experimental group of the present study,
including 60 cases of mild preeclampsia (MPE) and 60 cases of SPE.
Another 100 healthy females in late singleton pregnancy were
enrolled as the control group. Healthy subjects during the same
period were matched with preeclampsia patients according to age ± 3
years and gestational age ± 2 weeks. All patients were admitted to
the hospital when they were enrolled until delivery and discharge.
From all participants, whole-blood samples and 24-h urine samples
were collected. The diagnosis of preeclampsia and grouping of
subjects were performed strictly according to the hypertension in
pregnancy published by the American College of Obstetricians and
Gynecologists in 2013(16) and the
Diagnosis and treatment guidelines of hypertensive disorders in
pregnancy published by the Obstetrics and Gynecology Subcommittee
of the Chinese Medical Association in 2015(17).
Inclusion and exclusion criteria
The diagnostic criteria for preeclampsia were as
follows: Normal blood pressure prior to pregnancy with increases at
least twice (≥140/90 mmHg at an interval of >4 h) after 20 weeks
of pregnancy, urinary protein ≥300 mg/24 h or
proteinuria/creatinine ≥0.3 mg/dl, or urine regular protein
2+ and above in random samples. Normal blood pressure
prior to pregnancy was defined as normal blood pressure within 1
year before pregnancy without the history of hypertension.
As the group of normal pregnant females, those in
late singleton pregnancy with no cardiovascular diseases, renal
diseases, endocrine diseases or other chronic diseases complicating
the pregnancy or tumors or other complications were enrolled. The
exclusion criteria for this group were as follows: Participants
with <28 weeks of pregnancy, gestational diabetes mellitus,
pregnancy complicated with chronic hypertension, peripheral
vascular disease history, genital malformation, tumor or any
autoimmune diseases.
The subgroup classification of patients with
preeclampsia was according to the following standards: Patients
with SPE in conformity with the diagnostic criteria of
preeclampsia, with blood pressure ≥160/110 mmHg or PLT
<100x109/l or serum ALT elevated more than twice the
normal limit or serum creatinine twice the normal limit and without
other renal diseases. Patients with MPE met the diagnostic criteria
for preeclampsia but did not meet the diagnostic criteria for SPE.
Early-onset preeclampsia was definitive with the manifestation of
preeclampsia prior to 34 weeks of pregnancy. Late-onset
preeclampsia was definitive with the manifestation of preeclampsia
after 34 weeks of pregnancy. Patients with HELLP syndrome had at
least one of the following abnormal findings: Intravascular
hemolysis, PLT <100x109/l, aspartate aminotransferase
(AST) ≥70 U/l, lactate dehydrogenase (LDH) ≥600 U/l.
Data collection and follow-up
The data of the included subjects were recorded
after introduction into the program, including parameters such as
age, body height and body weight prior to pregnancy, systolic and
diastolic blood pressure and whether assisted reproductive
technology was employed during pregnancy. In the morning of the 2nd
day after enrollment, 8 ml fasting venous blood from the elbows of
the subjects was withdrawn and sub-packaged into EDTA-K2
anticoagulant vacuum tubes. The PLT of 1 ml blood sample was
measured within 1 h and the remaining blood was centrifuged at 769
x g at 4˚C for 3 min. The supernatant was filled in fresh
centrifuge tubes and stored in the refrigerator at -80˚C for
determination of AST, ALT, LDH and the extraction of the total RNA
content. PLT reagent was acquired from the BioRoYee Co. (cat. no.
DA0156) and ELISAs were adopted to test the concentrations of LDH
(Human LDH ELISA kit; cat. no. 48T-QS40354; Gersion Bio-Technology
Co., Ltd.), AST (Human AST ELISA kit; cat. no. 48T-QS41359; Gersion
Bio-Technology Co., Ltd.) and ALT (Human ALT/GPT ELISA kit; cat.
no. 48T-QS40443; Gersion Bio-Technology Co., Ltd.) in the serum.
Urinary protein test kits (Wanlei Bio Co.) were used to determine
the protein content of the urine samples.
The patients were followed up until the time-point
of delivery and the number of gestational weeks was recorded.
Furthermore, the maternal and fetal outcomes were acquired and
recorded after delivery. Adverse pregnancy outcomes were defined as
those occurring during follow-up or delivery and included placental
abruption, cardiac insufficiency, acute renal injury,
cerebrovascular accident, disseminated intravascular coagulation,
premature delivery, postpartum hemorrhage, small fetal size for
gestational age (SGA), neonatal asphyxia and fetal death. SGA was
defined as a birth weight 10% below the normal weight for the same
gestational age. Premature infants were defined as those whose
gestational age was <37 weeks prior to delivery. Neonatal
asphyxia was diagnosed if the 1-minute Apgar score of the fetus was
≤7.
Reverse transcription-quantitative PCR
(RT-qPCR)
The serum samples were preserved in 1.5-ml RNA-free
centrifuge tubes centrifuge tubes in the -80˚C refrigerator. The
concentration of the RNAs was detected within one week. A 0.5-ml
serum sample was incubated with TRIzol reagent (Thermo Fisher
Scientific, Inc.) at room temperature for 5 min, followed by RNA
extraction with 0.2 ml chloroform and centrifugation at 12,000 x g
for 5 min. Subsequently, the total RNA content was purified using
the mirVana PARIS kit (cat. no. AM1556; Thermo Fisher Scientific,
Inc.) in strict accordance with the manufacturer's protocol. The
samples were supplemented with ethanol and passed through a filter
cartridge containing a glass-fiber filter to immobilize the RNA
content. The filter was then repeatedly rinsed and the RNA content
was eluted with a low ionic-strength solution. The concentration
and purity of the RNA was determined using an ultraviolet
spectrophotometer. The PrimeScript RT reagent kit (Takara Bio,
Inc.) was employed to synthesize complementary DNA according to the
manufacturer's protocol. ChamQ™ SYBR qRT-PCR MasterMix (Vazyme
Biotech) was used for qPCR according to the manufacturer's
protocol. The reaction system was as follows: Initial denaturation
of 95˚C for 10 min, followed by 95˚C for 10 sec and 60˚C for 60 sec
for a total of 45 cycles. The 2-∆∆Cq method (18) was adopted to estimate the relative
expression of TCL6 with standardization to β-actin as the internal
control. The primer sequences are listed in Table I.
 | Table ISequences of primers used for PCR. |
Table I
Sequences of primers used for PCR.
| Gene | GenBank accession
no. | Forward (5'-3') | Reverse (5'-3') |
|---|
| LncRNA TCL6 | AF195821 |
GCTGTCTAAGGGCTCATC |
GGAGAAAGGCAAAGAACA |
| β-actin | NM_001101 |
CATGTACGTTGCTATCCAGGC |
CTCCTTAATGTCACGCACGAT |
Statistical analysis
SPSS 21.0 statistical software (IBM Corp.) and
GraphPad Prism 6.0 software (GraphPad Software Inc.) were used to
analyze data and plotting of graphs. The Shapiro-Wilk test was used
to analyze the data for normality of distribution. Continuous
variables with a normal distribution are expressed as the mean ±
standard deviation. An independent-samples t-test was used for
comparison between two groups. Measurement data with a non-normal
distribution were expressed as the mean (interquartile range) and
the Mann-Whitney U-test was used for comparison between groups.
Enumeration data were expressed as n (%) and comparisons between
groups were performed with the χ2 test. Receiver
operating characteristic (ROC) curve analysis was used to evaluate
the diagnostic efficacy of parameters and determine the cut-off
values. The χ2 test and Kaplan-Meier curves with the
log-rank test were used to analyze the effect of the expression of
lncRNA TCL6 on adverse pregnancy. Logistic regression model was
used to evaluate the influencing factors of adverse pregnancy.
Single-factor analysis of each influencing factor was performed,
the factors with P<0.1 were included in the multivariate
logistic regression analysis and for the independent variable
screening method, the Enter method provided by SPSS was selected.
The P-values calculated for all comparisons were two-sided and
P<0.05 was considered to indicate statistical significance.
Results
Clinical baseline characteristics of
included subjects
A total of 100 pregnant women were included in the
normal group with an age of 27.0 years (range, 24.7-28.8 years) and
120 pregnant women were included in the preeclampsia group with an
age of 25.9 years (range, 25.0-28.1 years). No significant
differences were evident in parameters such as age, pre-pregnancy
body mass index and PLT between patients with preeclampsia and
normal controls (P>0.05). In comparison with the normal pregnant
females, the systolic pressure, diastolic pressure, serum AST, ALT
and LDH levels as well as 24-h urinary protein of patients with
preeclampsia were higher. The proportion of pregnant females who
used assisted reproductive technology for their pregnancy was
higher relative to that of pregnant females in the control group
(P<0.05; Table II).
 | Table IIComparison of clinical baseline
characteristics. |
Table II
Comparison of clinical baseline
characteristics.
| Characteristic | Normal value | Normal group
(n=100) | Preeclampsia group
(n=120) | P-value |
|---|
| Baseline maternal age
(years) | - | 27.0 (24.7-28.8) | 25.9 (25.0-28.1) | 0.460 |
| Gestational age at
baseline (weeks) | - | 29.1 (28.1-30.2) | 29.2 (28.0±30.5) | 0.152 |
| Gestational age at
delivery (weeks) | 37-41 | 37.8 (36.0-40.6) | 38.5 (35.1-40.3) | 0.175 |
| Pre-pregnancy BMI
(kg/m2) | - | 24.7 (21.1-28.1) | 23.6 (21.5-26.0) | 0.271 |
| Systolic blood
pressure (mmHg) | 90-140 | 118.9
(108.3-128.5) | 154.6
(147.7-160.1) | <0.001 |
| Diastolic blood
pressure (mmHg) | 60-90 | 76.6 (68.5-83.5) | 101.9
(97.0-105.8) | <0.001 |
| AST (U/l) | 13-35 | 26.7
(19.4-32.4) | 65.9
(35.2-107.7) | <0.001 |
| ALT (U/l) | 7-40 | 15.1
(10.1-18.9) | 42.2
(20.5-80.3) | <0.001 |
| LDH (U/l) | 100-240 | 154.7
(106.9-191.5) | 187.5
(129.0-276.6) | <0.001 |
| PLT
(x109/l) | 125-350 | 230.7
(182.9-270.7) | 200.5
(218.4-235.9) | 0.070 |
| 24-h urinary
protein (g) | <0.03 | 0.01
(0.01-0.02) | 2.41
(1.41-4.57) | <0.001 |
| Assisted
reproductive technology | - | 19 (19.0) | 41 (34.2) | 0.015 |
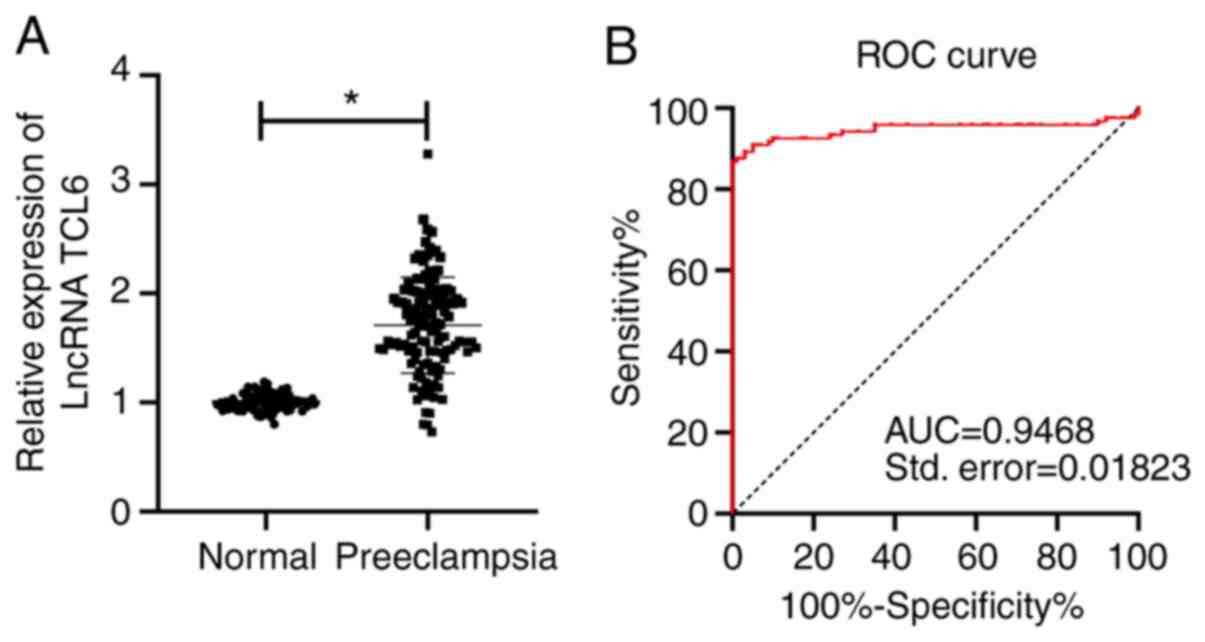
LncRNA TCL6 is upregulated in the
blood of patients with preeclampsia
The expression pattern of lncRNA TCL6 in the serum
of the pregnant women between the normal pregnant females and
patients with preeclampsia was compared in the present study. The
results suggested that the expression level of TCL6 in the normal
pregnant females relative to β-actin was 1.003±0.076, while that in
the preeclampsia group was 1.709±0.439. The expression of TCL6 in
the preeclampsia group was significantly higher compared with that
in the normal control group (P<0.01; Fig. 1A). Furthermore, the ROC curve was
plotted for TCL6 expression to distinguish between patients with
preeclampsia and normal pregnant females (Fig. 1B). The results suggested that the
area under the curve (AUC) was 0.9468 and the cutoff value was
1.206 (the sensitivity was 86.7% and the specificity was 100%).
These results indicated that a serum level of lncRNA TCL6 of
>1.206 relative to β-actin is able to identify patients with
preeclampsia, which may aid the diagnosis of the condition.
LncRNA TCL6 is a biomarker for grading
the severity of preeclampsia
To investigate the involvement of lncRNA TCL6 in
preeclampsia, the patients with preeclampsia were stratified into
the following subgroups: SPE and MPE, early-onset preeclampsia and
late-onset preeclampsia, preeclampsia complicated with HELLP
syndrome and preeclampsia without the complication of the HELLP
syndrome. The classification of the patients with preeclampsia was
as follows: The 120 preeclampsia patients included 60 MPE patients
and 60 SPE patients, including 63 cases of early-onset
preeclampsia, 57 cases of late-onset preeclampsia and 10 cases of
HELLP syndrome. The ROC curves were plotted to examine the ability
of lncRNA TCL6 to distinguish between the different pairs of
groups. The results suggested that the expression of TCL6 in the
SPE group was significantly higher than that in the MPE group
(Fig. 2A) and the ROC curve for
distinguishing between these groups had an AUC of 0.7947 at the
cutoff value of 1.838 (P<0.0001; Fig. 2B), sensitivity of 65% and
specificity of 85%. The expression of TCL6 in patients with
early-onset preeclampsia was significantly higher compared with
that in patients with late-onset preeclampsia (Fig. 2C) and the ROC curve for
distinguishing between these groups had an AUC of 0.7700 at the
cutoff value of 1.776 (P<0.0001; Fig. 2D), sensitivity of 75.4% and
specificity of 66.7%. Compared with that in the patients with
preeclampsia without HELLP syndrome, the level of TCL6 in patients
with HELLP syndrome was significantly higher (Fig. 2E) and the ROC curve for
distinguishing between these groups had an AUC of 0.8309 at the
cutoff value of 1.928 (P<0.001; Fig.
2F), sensitivity of 77.3% and specificity of 90.0%.
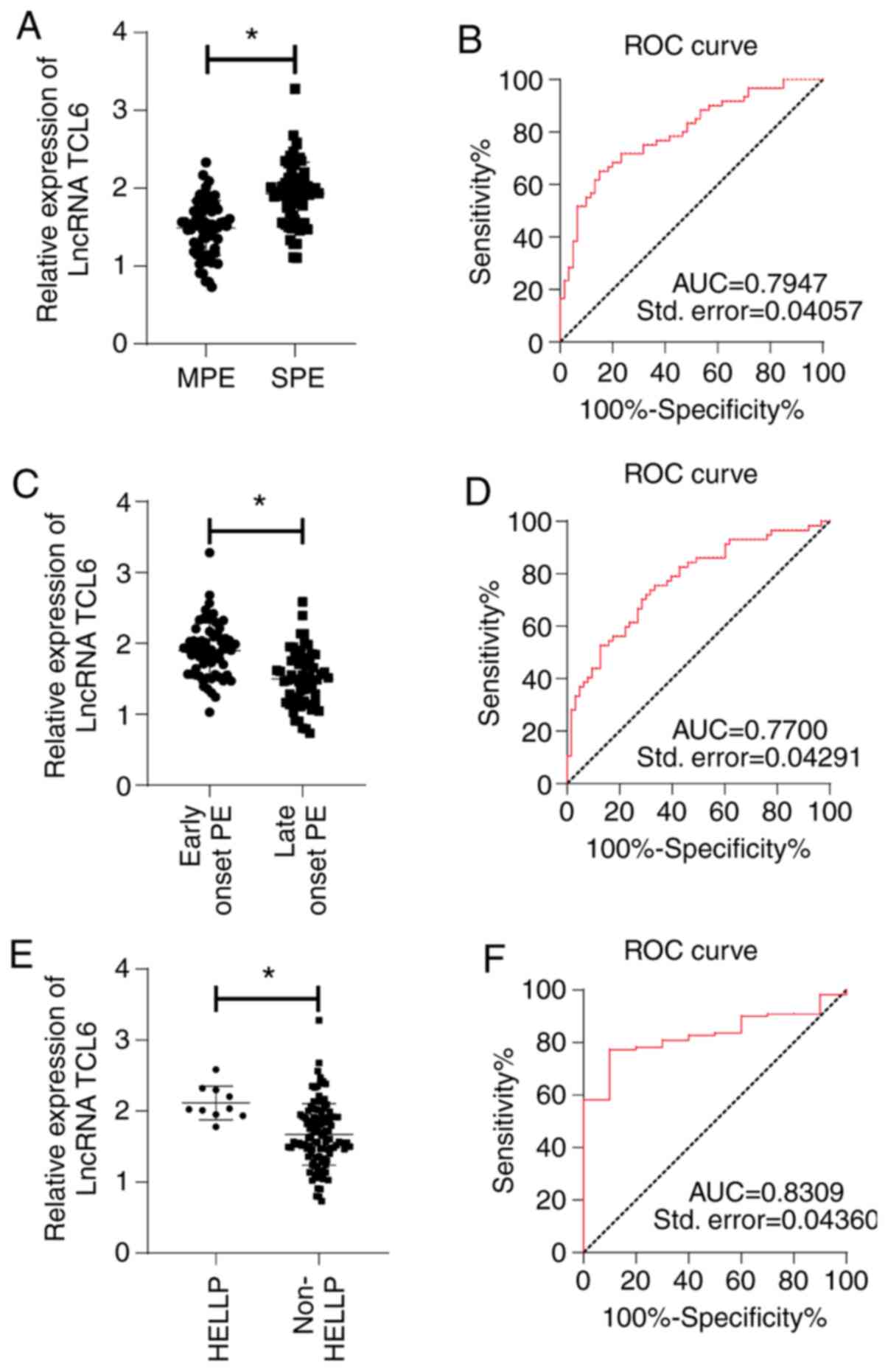 | Figure 2LncRNA TCL6 was upregulated in the
blood of each subgroup of patients with preeclampsia. LncRNA TCL6
expression in different subgroups of preeclampsia was detected by
reverse transcription-quantitative PCR. (A) Difference in TCL6
expression between SPE and MPE groups. (B) ROC curve of lncRNA TCL6
in the diagnosis of severe preeclampsia. (C) Difference in TCL6
expression between early-onset preeclampsia and late-onset
preeclampsia groups. (D) ROC curve of lncRNA TCL6 in the diagnosis
of early-onset preeclampsia. (E) Difference in TCL6 expression
between patients with preeclampsia with and without HELLP syndrome.
(F) ROC curve of lncRNA TCL6 in the diagnosis of HELLP syndrome in
patients with preeclampsia. Independent-samples t-tests were used
to compare the data in panels A, C and E. *P<0.05.
lncRNA, long non-coding RNA; TCL6, T-cell leukemia/lymphoma 6; ROC,
receiver operating characteristic; AUC, area under the ROC curve;
Std., standard error; HELLP, Haemolysis, Elevated Liver enzymes and
Low Platelet count; PE, preeclampsia; SPE, severe PE; MPE, mild
PE. |
High expression of lncRNA TCL6
increases the risk of adverse pregnancy outcomes
Patients with preeclampsia were allocated to a low
expression group and a high expression group according to the
median expression of lncRNA TCL6 in this study. Comparison of the
incidence of adverse pregnancy outcomes between the two groups
indicated that the prognosis of the two groups was significantly
different (χ2=20.89; P<0.0001). The incidence of
adverse pregnancy outcomes in the low expression group was 31.7%,
which was lower than that in the high expression group (73.3%;
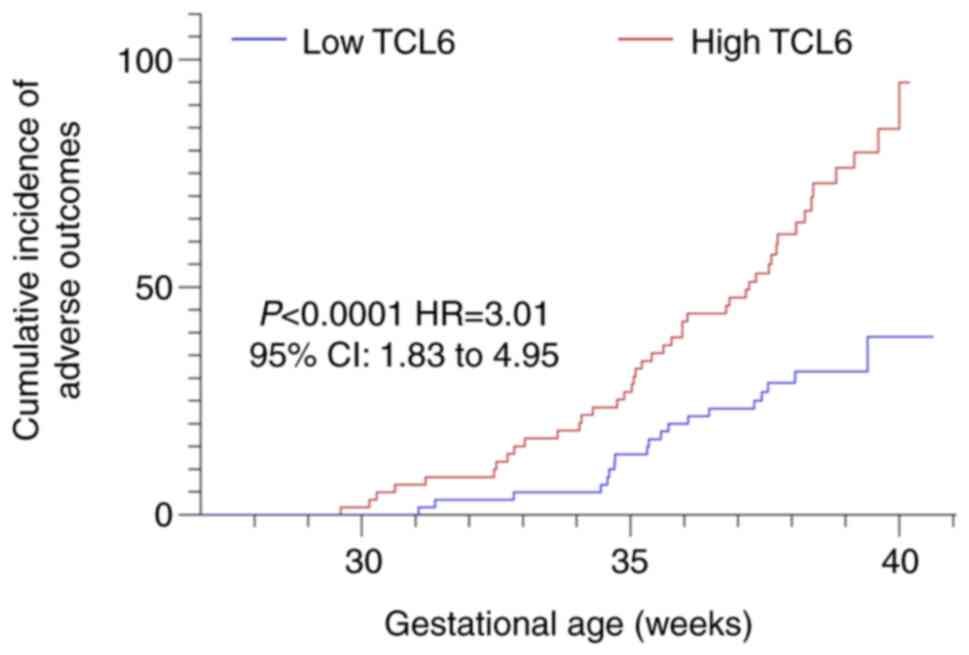
Table III). Kaplan-Meier analysis
indicated a left shift in the curve of the TCL6 high-expression
group (P<0.001; Fig. 3), which
indicated that at the same gestational week, the cumulative
incidence of adverse pregnancy outcomes was higher in the
high-expression group than in the low-expression group. These
results suggested that the increased expression of TCL6 was
associated with adverse pregnancy outcomes.
 | Table IIIComparison of pregnancy outcomes for
patients with preeclampsia with different expression of TCL6. |
Table III
Comparison of pregnancy outcomes for
patients with preeclampsia with different expression of TCL6.
| Expression of
TCL6 | Adverse outcomes, n
(%) | Normal delivery, n
(%) | P-value | Total, n |
|---|
| Low (≥1.708) | 19 (30.2%) | 41 (71.9%) | ≤ | 60 |
| High
(<1.708) | 44 (69.8%) | 16 (28.1%) | 0.001 | 60 |
| Total, n | 63 | 57 | | 120 |
High expression of lncRNA TCL6 is an
independent risk factor for adverse pregnancy outcomes in patients
with preeclampsia
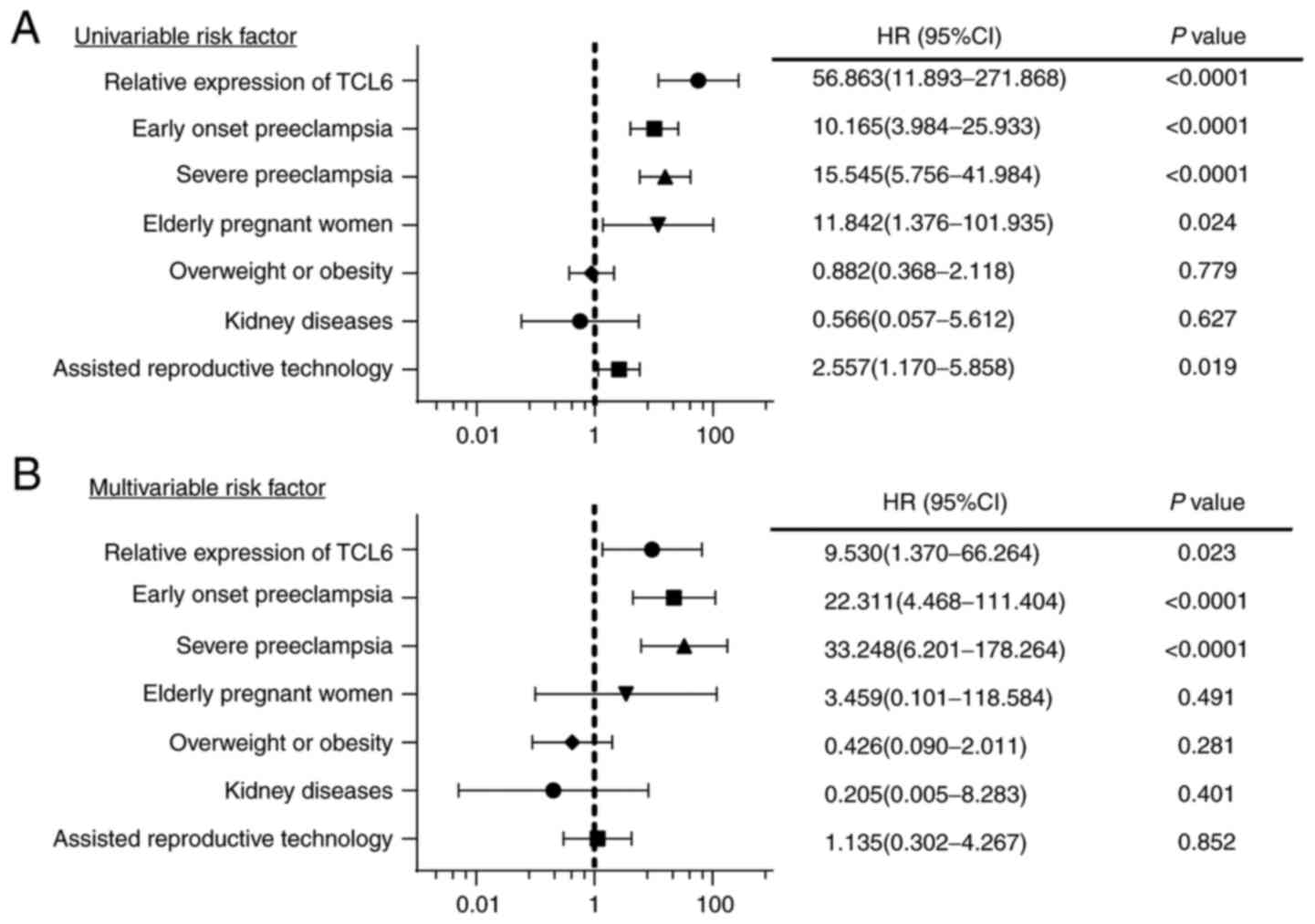
Existing evidence has indicated that indexes such as
advanced age, overweight or obesity, assisted reproductive
technology and kidney disease of the mother are high-risk factors
of adverse outcomes of singleton pregnancies affected by
preeclampsia (19-21).
To provide an accurate evaluation of the effect of TCL6 on
pregnancy outcomes, SPE, early-onset preeclampsia, TCL6 expression,
advanced age, overweight or obesity prior to pregnancy, assisted
reproductive technology and kidney disease were included as
observational indexes in the regression analysis. As indicated in
Fig. 4A, all variables except for
overweight/obesity and kidney disease were significant risk factors
(P<0.1). Multivariate logistic regression analysis then
indicated that SPE, early-onset preeclampsia and TCL6 expression
were independent risk factors for adverse pregnancy outcomes (all
P<0.05; Fig. 4B). For each unit
increase in the relative expression of TCL6, a 9.5-fold increase in
the risk of adverse maternal and fetal outcomes was determined.
Discussion
Preeclampsia is a common syndrome among pregnant
females and is a high risk factor of mortality of pregnant females
and their infants (1). According to
a previous study, lncRNA TCL6 expression was highly expressed in
the placenta of pregnant females with preeclampsia (15). The present study indicated that
maternal plasma levels of lncRNA TCL6 were increased in patients
with preeclampsia, and that determination of TCL6 is supportive in
the diagnosis and grading of preeclampsia and it fundamentally
serve as an independent risk factor for adverse pregnancy
outcomes.
Certain studies have illustrated the importance of
blood pressure in the diagnosis and management of preeclampsia
(22). LDH is able to independently
function as an indicator for the risk of preeclampsia (23). According to the clinical baseline
characteristics of the included subjects, compared with those in
normal pregnant females, the alterations in blood pressure, AST,
ALT and LDH levels and 24-h urinary protein of patients with
preeclampsia were higher. In consistency with this, a study
demonstrated the utility of elevated systolic blood pressure and
diastolic blood pressure with significant proteinuria after the
20th week of pregnancy as potential factors for the diagnosis of
preeclampsia (24). Hence, in the
present study, it was speculated that increased blood pressure,
serum AST, ALT and LDH levels and 24-h urinary protein may serve as
fundamental risk factors of preeclampsia.
Various studies have demonstrated an association
between low expression of TCL6 and the occurrence and prognosis of
renal hyaluronic cell carcinoma, acute B-lymphocyte leukemia and
hepatocarcinoma (10-12).
However, the expression and the diagnostic value of TCL6 in
preeclampsia remained to be fully determined. The present study
indicated that the expression of TCL6 was higher in patients with
preeclampsia. The ROC curve demonstrated that the AUC was 0.9468,
while the cutoff value was 1.206 (the sensitivity was 86.7% and the
specificity was 100%), thereby indicating that relative serum
levels of lncRNA TCL6 >1.206 may aid the diagnosis of
preeclampsia. Consistently, dysregulation of a variety of lncRNAs,
such as lncRNAs MEG3, IGF2/H19, HOTAIR, SPRY4-IT1 and MALAT1, has
been evident in the placenta tissues of pregnant females with
preeclampsia (25). In the present
study, lncRNA TCL6 was upregulated in the blood of pregnant
patients with preeclampsia and detection of high expression of
lncRNA TCL6 was indicated to be able to contribute to the diagnosis
of preeclampsia.
Research has also focused on the concepts of
early-onset preeclampsia and late-onset preeclampsia (6). In the present study, differences in
lncRNA TCL6 expression between subgroups of patients with
preeclampsia according to time of onset and severity were examined.
The results revealed that the expression of TCL6 in the SPE group
was higher than the expression in the MPE group, thereby indicating
that TCL6 may serve as a diagnostic marker for the severity of
preeclampsia. TCL6 levels in patients with early-onset preeclampsia
were also higher than those in patients with late-onset
preeclampsia, indicating the utility of TCL6 as a marker for the
onset time of preeclampsia. In comparison with that in patients
with preeclampsia without HELLP syndrome, TCL6 was significantly
increased in patients with preeclampsia with HELLP syndrome,
indicating the functionality of TCL6 as a factor to diagnose the
preeclampsia complication HELLP syndrome. A previous study reported
that the microcirculatory flow index and perfused vessel density of
pregnant females with preeclampsia complicated with the HELLP
syndrome were lower than those of the patients with preeclampsia
without HELLP syndrome, while the heterogeneity index of pregnant
females with preeclampsia complicated with HELLP syndrome was
higher than the index of patients with preeclampsia without HELLP
syndrome, thereby indicating that patients with HELLP syndrome may
have a worse prognosis (26). To
conclude, lncRNA TCL6 may be adopted as a biomarker for grading the
severity of preeclampsia. As a prospective study, the present study
determined the expression pattern of TCL6 in the serum of patients
with preeclampsia for the first time and explored the role of TCL6
expression in the diagnosis and classification of preeclampsia by
ROC curve analysis.
The diagnosis of preeclampsia poses an associated
risk of developing maternal complications leading to adverse
perinatal outcomes (27). To
identify the role of TCL6 expression in pregnancy outcomes,
pregnant females with preeclampsia were stratified into low and
high TCL6 expression groups in the present study. The results were
indicative of a lower incidence of adverse pregnancy outcomes in
the low-expression group relative to the high-expression group.
Previous studies have indicated the association of factors such as
obesity, assisted reproductive technology, kidney disease with
adverse pregnancy outcomes (19-21).
Early-onset preeclampsia conferred a high risk of fetal death
relative to late-onset preeclampsia (28). To accurately evaluate the effect of
TCL6 on pregnancy outcomes, factors such as SPE, early onset of
preeclampsia, TCL6 expression, advanced maternal age, overweight or
obesity prior to pregnancy, assisted reproductive technology and
kidney disease were included as observation indexes in the
regression analysis. The results indicated that SPE, early-onset
preeclampsia and TCL6 expression were independent risk factors for
adverse pregnancy outcomes. It was determined that for each unit
increase in TCL6 expression, the risk of adverse maternal and fetal
outcomes increased by 9.5-fold.
TCL6 was initially flagged as a candidate gene with
potential involvement in leukemia (9). Studies have illustrated the
participation of TCL6 in clear-cell renal cell carcinoma, breast
cancer, retinoblastoma, liver cirrhosis and threatened abortion
(10,13,14,29,30).
The present results suggested that high expression of lncRNA TCL6
was associated with the pathogenesis, severity and poor prognosis
of cases of preeclampsia and it may be worthwhile to explore the
underlying mechanisms. Following extensive investigation of the
pathogenesis of preeclampsia, Redman (31) proposed the ‘six stages’ of
preeclampsia in 2014: The first stage ranges from fertilization to
embryo implantation, which is a relatively short process. The
second stage is 8-18 weeks of gestation, during which the
trophoblast initiates invasion into the uterine spiral artery,
which is the vital aspect of placentation. The third stage is a
stress response after abnormal placental formation. The fourth
stage is the second half of pregnancy, where various types of
placental injury factors are released into the maternal blood
circulation. In the fifth stage, patients exhibit the clinical
manifestations of rising blood pressure. The sixth stage is the
period of acute aggravation of the disease, during which
atherosclerosis or thrombosis of the spiral artery may occur, and
placenta infarction may also take place. Pathophysiological
alterations in trophoblast cells are vital for the pathogenesis of
preeclampsia. Previous research has validated the involvement of
lncRNAs in the proliferation, migration, invasion, apoptosis and
other biological functions of trophoblasts. In the present study,
it was speculated that lncRNA TCL6 may have a pathogenic role by
regulating the biological behaviors of trophoblast cells. In
addition, in light of the similarity between the biological
behavior of placental trophoblast and tumor cells, a study has
indicated that TCL6 is able to inhibit the proliferation, migration
and invasion of hepatoma cells (12). Therefore, TCL6 may induce abnormal
biological behaviors of trophoblast cells, including proliferation,
migration and invasion. The study of Wu et al (15) validated the ability of
overexpression of lncRNA TCL6 to inhibit the proliferation of
trophoblast cells, thus highlighting its involvement in the
pathogenesis of preeclampsia. The study of Liu and Gong (14) reported that lncRNA-TCL6 inhibited
the proliferation of trophoblast cells by regulating the EGFR
pathway in vitro. Considering the significance of the EGFR
pathway in the proliferation and differentiation of trophoblast
cells and placental development, it was speculated in the present
study whether lncRNA TCL6 serves as a regulator of the biological
behaviors of trophoblast cells by affecting the EGFR pathway, thus
affecting the occurrence of preeclampsia, which requires to be
assessed in further studies.
To conclude, the present study supported that high
expression of lncRNA TCL6 may assist the diagnosis and grading of
preeclampsia and may be functionally utilized as an independent
risk factor for adverse pregnancy outcomes. Since the time of
sample collection in the present study was from the third trimester
of pregnancy to delivery, the determination of TCL6 levels may have
been affected. As the sample size in the present study was small,
further research with a larger sample size is necessary and a
multi-center study should be performed to further clarify the
diagnostic and prognostic value of TCL6. In addition, determination
of the serum levels of TCL6 in the first or second trimester of
pregnancy should be considered to investigate its diagnostic and
prognostic value in the early stage of the disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and HM confirm the authenticity of all the raw
data. HW and HM contributed to the study concepts, study design,
definition of intellectual content, literature research, manuscript
preparation, manuscript editing and manuscript review. HW, GS, ML,
LM and HM contributed to the clinical study, performing experiments
and data acquisition. HW, GS and HM contributed to data analysis
and statistical analysis. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The experiments were authorized by the academic
ethics committee of the Second People's Hospital of Yunnan Province
(Kunming, China; no. 2020040). All procedures were strictly
implemented according to the Declaration of Helsinki. All the
subjects involved were fully informed of the objective of the study
and they consented to being enrolled in the study and provided
written informed consent prior to sampling.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bokslag A, van Weissenbruch M, Mol BW and
de Groot CJ: Preeclampsia; short and long-term consequences for
mother and neonate. Early Hum Dev. 102:47–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pauli JM and Repke JT: Preeclampsia:
Short-term and Long-term Implications. Obstet Gynecol Clin North
Am. 42:299–313. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ahmed A, Rezai H and Broadway-Stringer S:
Evidence-Based Revised View of the Pathophysiology of Preeclampsia.
Adv Exp Med Biol. 956:355–374. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Balogun OA and Sibai BM: Counseling,
Management, and Outcome in Women With Severe Preeclampsia at 23 to
28 Weeks' Gestation. Clin Obstet Gynecol. 60:183–189.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aloizos S, Seretis C, Liakos N, Aravosita
P, Mystakelli C, Kanna E and Gourgiotis S: HELLP syndrome:
Understanding and management of a pregnancy-specific disease. J
Obstet Gynaecol. 33:331–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sones JL and Davisson RL: Preeclampsia, of
mice and women. Physiol Genomics. 48:565–572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagy B: Cell-free nucleic acids in
prenatal diagnosis and pregnancy-associated diseases. EJIFCC.
30:215–223. 2019.PubMed/NCBI
|
|
8
|
McAninch D, Roberts CT and Bianco-Miotto
T: Mechanistic Insight into Long Noncoding RNAs and the Placenta.
Int J Mol Sci. 18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saitou M, Sugimoto J, Hatakeyama T, Russo
G and Isobe M: Identification of the TCL6 genes within the
breakpoint cluster region on chromosome 14q32 in T-cell leukemia.
Oncogene. 19:2796–2802. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Z, Zhuang Q, Cheng K, Ming Y, Zhao Y,
Ye Q and Zhang S: Long non-coding RNA TCL6 enhances preferential
toxicity of paclitaxel to renal cell carcinoma cells. J Cancer.
11:1383–1392. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cuadros M, Andrades Á, Coira IF, Baliñas
C, Rodríguez MI, Álvarez-Pérez JC, Peinado P, Arenas AM, García DJ,
Jiménez P, et al: Expression of the long non-coding RNA TCL6 is
associated with clinical outcome in pediatric B-cell acute
lymphoblastic leukemia. Blood Cancer J. 9(93)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo LH, Jin M, Wang LQ, Xu GJ, Lin ZY, Yu
DD, Yang SL, Ran RZ, Wu G and Zhang T: Long noncoding RNA TCL6
binds to miR-106a-5p to regulate hepatocellular carcinoma cells
through PI3K/AKT signaling pathway. J Cell Physiol. 235:6154–6166.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Li Z, Chen M, Chen H, Zhong Q,
Liang L and Li B: lncRNA TCL6 correlates with immune cell
infiltration and indicates worse survival in breast cancer. Breast
Cancer. 27:573–585. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu LP and Gong YB: LncRNA-TCL6 promotes
early abortion and inhibits placenta implantation via the EGFR
pathway. Eur Rev Med Pharmacol Sci. 22:7105–7112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu JL, Wang YG, Gao GM, Feng L, Guo N and
Zhang CX: Overexpression of lncRNA TCL6 promotes preeclampsia
progression by regulating PTEN. Eur Rev Med Pharmacol Sci.
23:4066–4072. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
No authors listed. Hypertension in
pregnancy. Report of the American College of Obstetricians and
Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet
Gynecol. 122:1122–1131. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hypertensive Disorders in Pregnancy
Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese
Medical Association and Hypertensive Disorders in Pregnancy
Subgroup Chinese Society of Obstetrics and Gynecology Chinese
Medical Association. Diagnosis and treatment guideline of
hypertensive disorders in pregnancy. Zhonghua Fu Chan Ke Za Zhi.
50:721–728. 2015.PubMed/NCBI(In Chinese).
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hui D and Hladunewich MA: Chronic Kidney
Disease and Pregnancy. Obstet Gynecol. 133:1182–1194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marsh CA and Hecker E: Maternal obesity
and adverse reproductive outcomes: Reducing the risk. Obstet
Gynecol Surv. 69:622–628. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reddy UM, Wapner RJ, Rebar RW and Tasca
RJ: Infertility, assisted reproductive technology, and adverse
pregnancy outcomes: Executive summary of a National Institute of
Child Health and Human Development workshop. Obstet Gynecol.
109:967–977. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ashworth DC, Maule SP, Stewart F, Nathan
HL, Shennan AH and Chappell LC: Setting and techniques for
monitoring blood pressure during pregnancy. Cochrane Database Syst
Rev. 8(CD012739)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han Q, Zheng W, Guo XD, Zhang D, Liu HF,
Yu L and Yan JY: A new predicting model of preeclampsia based on
peripheral blood test value. Eur Rev Med Pharmacol Sci.
24:7222–7229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Morris RK, Riley RD, Doug M, Deeks JJ and
Kilby MD: Diagnostic accuracy of spot urinary protein and albumin
to creatinine ratios for detection of significant proteinuria or
adverse pregnancy outcome in patients with suspected pre-eclampsia:
Systematic review and meta-analysis. BMJ. 345 (jul09
1)(e4342)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song X, Luo X, Gao Q, Wang Y, Gao Q and
Long W: Dysregulation of LncRNAs in Placenta and Pathogenesis of
Preeclampsia. Curr Drug Targets. 18:1165–1170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cornette J, Herzog E, Buijs EA, Duvekot
JJ, Rizopoulos D, Hop WC, Tibboel D and Steegers EA:
Microcirculation in women with severe pre-eclampsia and HELLP
syndrome: A case-control study. BJOG. 121:363–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guida JPS, Surita FG, Parpinelli MA and
Costa ML: Preterm Preeclampsia and Timing of Delivery: A Systematic
Literature Review. Rev Bras Ginecol Obstet. 39:622–631.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lisonkova S and Joseph KS: Incidence of
preeclampsia: risk factors and outcomes associated with early-
versus late-onset disease. Am J Obstet Gynecol. 209:544.e1–544.e12.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li LJ, Wu XY, Tan SW, Xie ZJ, Pan XM, Pan
SW, Bai WR, Li HJ, Liu HL, Jiang J, et al: Lnc-TCL6 is a potential
biomarker for early diagnosis and grade in liver-cirrhosis
patients. Gastroenterol Rep (Oxf). 7:434–443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tao S, Wang W, Liu P, Wang H and Chen W:
Long non-coding RNA T-cell leukemia/lymphoma 6 serves as a sponge
for miR-21 modulating the cell proliferation of retinoblastoma
through PTEN. Korean J Physiol Pharmacol. 23:449–458.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Redman C: The six stages of pre-eclampsia.
Pregnancy Hypertens. 4(246)2014.PubMed/NCBI View Article : Google Scholar
|