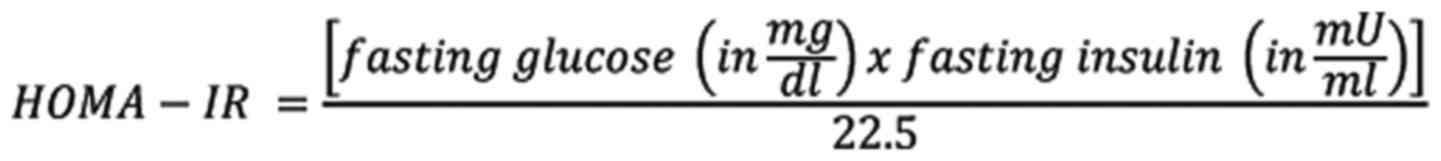Introduction
Cardiovascular disease (CVD) is recognized as a
leading cause of death globally. Obesity, dyslipidemia, insulin
resistance (IR), and interconnected pathological conditions are
considered risk factors closely associated with CVD. IR is defined
as a reduction in insulin sensitivity in peripheral tissues
(1). It is manifested especially in
the tissues that depend on the action of insulin for intracellular
transport of glucose: liver, muscle and adipose tissue. The effects
of IR are, however, multiple, as insulin exerts physiological
actions on carbohydrate, lipid and protein metabolism, as well as
on endothelial function (2).
Excess adipose tissue is not an inert tissue, it can
directly produce and release inflammatory cytokines and other
atherogenic molecules and it is associated with a low insulin
response (3). Similarly, the liver
takes up increased amounts of lipids from increased endogenous
production and absorption of fatty acids from the blood, the
consequence being the appearance of IR at this level.
Insulin-resistant liver increases gluconeogenesis and decreases
glycogen synthesis, perpetually increasing blood glucose levels
(4). The combination of hepatic IR
and high levels of circulating fatty acids also leads to increased
very low-density lipoprotein (VLDL) production, lower high-density
lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL)
particles, all of which are associated with an increased risk of
heart disease (5,6).
IR increases the release of free fatty acids from
adipocytes, which increases circulating levels of free fatty acids,
which stimulates the synthesis of triglyceride-rich VLDL particles
in the liver, leading to increased HDL and triglyceride (TG)-rich
LDL particles. The increase in triglycerides in lipid particles
changes their metabolism. HDL particles are hydrolyzed more rapidly
and HDL levels decrease. LDL particles are further subjected to
lipolysis leading to the formation of small and dense LDL
particles. The resulting dyslipidemia is extremely atherogenic and
represents at least part of the increased risk of CVD in
insulin-resistant individuals (7,8).
Increased accumulation of lipids in the pancreas affects its
response to increased plasma glucose levels and reduces insulin
secretion (9).
At the level of the skeletal muscle, IR causes a
decrease in peripheral glucose utilization, contributing to chronic
hyperglycemia and/or hyperinsulinism (10). In addition to these organ-specific
effects, hyperinsulinemia (itself a consequence of IR) increases
blood pressure by activating the sympathetic nervous system
(11). IR also decreases the
synthesis and release of nitric oxide, which can directly affect
vascular function thereby increasing cardiovascular risk (CVR)
(12,13). In addition, hyperglycemia has been
associated with the increased production of reactive oxygen species
in several organ systems, which has been independently linked to
CVD (14,15).
Taken together, these mechanisms suggested various
pathways by which IR, hyperinsulinemia, and hyperglycemia directly
contribute to the development and progression of atherosclerotic
disease (16,17). The aim of the present study was to
emphasize the association of IR with CVR.
Patients and methods
Study population
The epidemiological, cross-sectional,
non-interventional study was conducted over 12 months (1 October,
2019-30 September, 2020) and included 400 subjects divided into 2
groups: group 1 (control) subjects did not have diabetes (n=200)
and group 2 had type 2 diabetes (T2DM) (n=200). Subject
characteristics are provided in Table
I.
 | Table IGeneral characteristics of the study
participantsa. |
Table I
General characteristics of the study
participantsa.
| Variables | Total (n=400) | Group 1 (n=200) | Group 2 (n=200) | P-value |
|---|
| Age (years) | 62±10.261 | 61.83±10.231 | 62.16±10.313 | 0.728 |
| Sex (%) | | | | |
|
Women | 50 | 50 | 50 | |
|
Men | 50 | 50 | 50 | |
| SBP (mmHg) | 139.16±22.09 | 139.88±20.09 | 138.44±23.95 | 0.515 |
| The presence of IR
(%) | | 35.7 | 79 | |
| HOMA-IR index | 3.259±2.586 | 2.116±1.725 | 4.402±2.794 | <0.001 |
| FRS (%) | 19.75±9.48 | 16.23±9.6 | 23.20±8 | <0.001 |
| Smoker (%) | 12 | 17 | 7 | |
| HTN (%) | 69.5 | 59 | 80 | |
| DM (%) | 50 | - | 100 | |
| HDL-C (mg/dl) | 52.71±16.10 | 56.19±18.44 | 49.23±12.47 | <0.001 |
| TC (mg/dl) | 207.54±49.70 | 221.04±54.00 | 194.04±40.86 | <0.001 |
Caucasian subjects without a history of carbohydrate
metabolism and patients diagnosed with T2DM at the beginning of the
study were included, according to the criteria of the American
Diabetes Association (ADA) with minor modifications (18). Subjects with acute metabolic
imbalance, acute or chronic diseases, drug treatments that
potentially experience a secondary disruption of carbohydrate
metabolism were excluded.
Informed consent was signed, in full knowledge of
the facts, by each participant in the study, after all the
necessary aspects were communicated to take a decision for or
against participation in the study. This study was approved by the
Academic and Scientific Ethics and Deontology Committee of the
University of Medicine and Pharmacy in Craiova (registration no.
18/2019) in accordance with the European Union Guidelines
(Declaration of Helsinki), in accordance with good clinical
practice, respecting the right to integrity, confidentiality, or
the option of the subject to withdraw from the study at any
time.
Demographic data (age, sex), personal pathological
history (diabetes, treated/untreated hypertension), lifestyle data
aimed at identifying smoker status were collected. In this regard,
the patients completed a questionnaire, the answers being
subsequently grouped into the categories: current smoker, former
smoker and non-smoker. Respondents admitting to consuming ≥100
cigarettes during their lifetime and who, at the time of the
survey, smoked daily, or several days or weeks were defined as
current smokers. Respondents who reported smoking ≥100 cigarettes
during their lifetime and who, at the time of the survey, no longer
smoked were regarded as former smokers. Respondents who stated that
they smoked <100 cigarettes during their lifetime were
interpreted as non-smokers (19).
Blood pressure was measured using an automated sphygmomanometer
after the subjects were relaxed and seated for >10 min.
Laboratory tests
Subjects were required to fast for ≥12 h and avoid a
high-fat diet or alcohol consumption prior to blood sampling. The
blood samples obtained were stored in a refrigerator at 4˚C and
subsequently sent to the hospital laboratory. Clinical biochemical
tests measured were represented by the fasting plasma glucose
(FPG); fasting insulin; fasting lipid profiles, especially HDL
cholesterol and total cholesterol (TC).
Assessment of IR
IR was assessed via HOMA-IR (20), which was expressed as:
Subjects were considered to have IR if the value of
HOMA-IR index was ≥1.9.
CVR assessments
The 2008 Framingham risk score (FRS) assessments
were employed to determine the risk of CVD (21). CVR stratification was performed in
the 3 categories: low risk (<10%), moderate risk (10-20%), and
high risk (>20%).
Statistical analysis
Data were analyzed using the Statistical Package for
the Social Sciences software, version 26.0 (IBM Corp.). Patient
characteristics and clinical data are expressed as means ± standard
deviations for continuous variables and countable with percentages
for discrete variables. Descriptive statistics were provided.
Differences among groups with varying HOMA-IR levels were compared
via one-way ANOVA and Post Hoc Multiple Comparisons for continuous
data and the Chi-square test was used for categorical data.
Pearson's analysis was used to evaluate the correlation of CVD risk
factors and HOMA-IR levels. Multiple logistic regression analysis
was used to adjust for covariates. Receiver operating
characteristic (ROC) curve was used to assess the utility of the
HOMA-IR to predict a high FRS. In addition, a favorable cut-off
point and the corresponding sensitivity, specificity, and area
undercurve (AUC) were determined. P<0.05 (two-sided) was
regarded as statistically significant.
Results
Patient characteristics
The general characteristics of the participants are
summarized in Table I. The groups
were constructed without significant differences in terms of sex,
age, mean of systolic blood pressure (SBP), personal history of
hypertension, HDL-C and TC values. Evaluating the average value of
HOMA-IR in the two groups, an average value of 2.116±1.725 was
obtained for the group without DM. For the group with T2DM, an
average value of 4.402±2.794 was obtained, with a statistically
significant difference (P<0.001) between the two groups. This
confirms the close association of IR with T2DM.
Following evaluation of CVR for the two groups, an
average value of 16.23±9.6 was obtained for the group without
diabetes and 23.20±8 for the group with T2DM (Table I). The results indicated a
statistically significant difference (P<0.001), confirming
diabetes as an important factor in increasing CVR. Analyzing the
groups regarding the presence of IR, the following data were
obtained: in group 1 IR was registered in 35.7% of patients, while
in group 2 it was 79% (Table I).
The results indicated a statistically significant difference
(P<0.001) in favor of patients with diabetes.
Stratifying CVR by groups, in group 1 there were no
significant differences among the CVR categories, unlike group 2
where most patients had a moderate or increased CVR (24 or 68%,
respectively), with a statistically significant difference
(P<0.001) in favor of the T2DM (Table II).
 | Table IIStratification of cardiovascular risk
in the two groups. |
Table II
Stratification of cardiovascular risk
in the two groups.
| Framingham risk
score | Group 1 (%) | Group 2 (%) | P-value |
|---|
| Low | 34.67 | 8 | P<0.001 |
| Moderate | 29.6 | 24 | P<0.005 |
| High | 35.7 | 68 | P<0.001 |
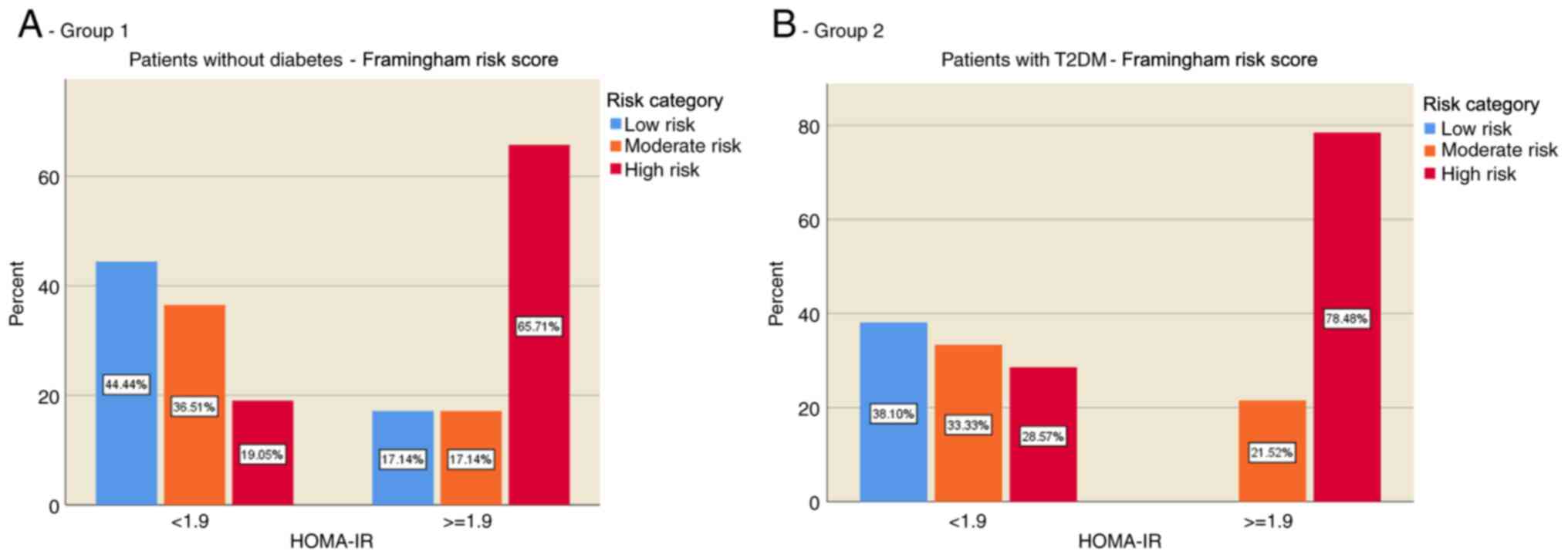
The distribution of CVR in the two groups according
to the presence of IR was evaluated. The results showed that most
of the individuals without diabetes (group 1) and without IR were
in the low CVR category (44.44%), while individuals with IR were
predominantly in the high CVR category (65.71%) (Fig. 1A). In patients with T2DM (group 2),
the risk was evenly distributed in those without IR, but for those
with IR the present risk was predominantly increased (78.48%)
(Fig. 1B).
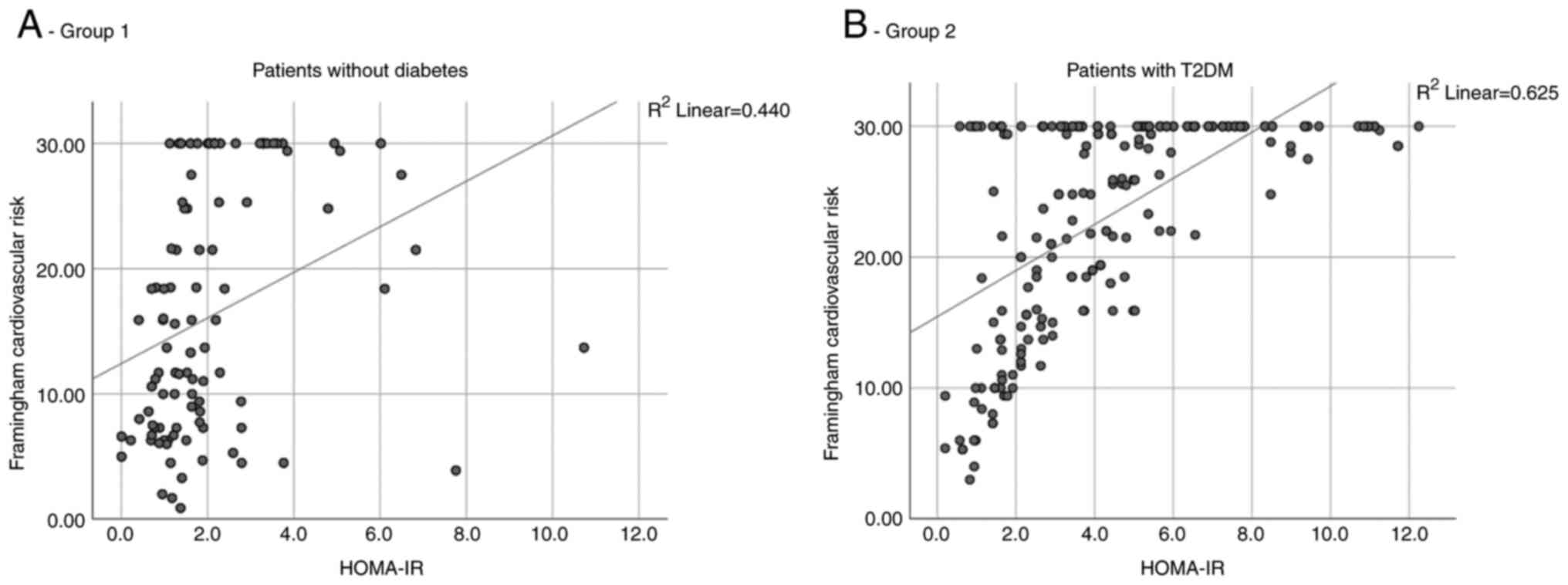
After correlating the value of HOMA-IR with the
degree of CVR it was observed in both groups that as the IR
increased, the CVR also increased. However, in group 2, the
correlation was higher, quantified by a value of the Spearman
correlation coefficient of 0.625, compared to group 1 where this
coefficient had a value of 0.440 (Fig.
2A and B).
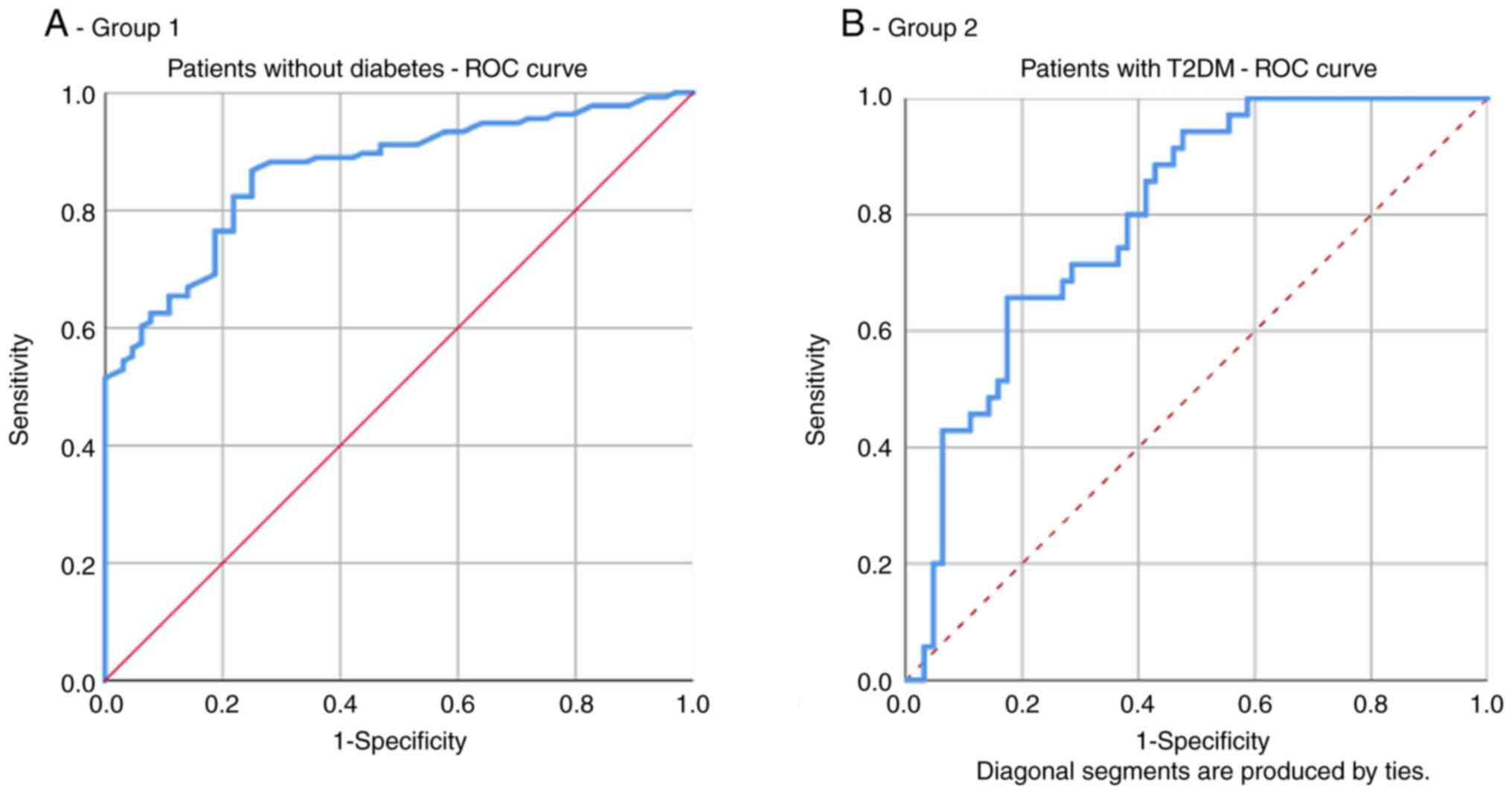
In group 1, the area under the ROC curve for HOMA-IR
as a predictor of increased CVR (≥20%) was 0.797. The optimal
cut-off value of HOMA-IR for high CVR prediction was 1.965 with a
sensitivity of 65.7% and specificity of 82.5% (Fig. 3A and Table III).
 | Table IIIAUC, sensitivity, and specificity of
the optimized cut-off points for the HOMA-IR index in predicting
high Framingham risk score (FRS >20%). |
Table III
AUC, sensitivity, and specificity of
the optimized cut-off points for the HOMA-IR index in predicting
high Framingham risk score (FRS >20%).
| Variable | Group | AUC (95% CI) | P-value | Cut-off point | Sensitivity (%) | Specificity (%) |
|---|
| HOMA-IR | 1 | 0.797 | 0.001 | 1.965 | 65.7 | 82.5 |
| | 2 | 0.867 | 0.001 | 2.926 | 82.4 | 75 |
In group 2, the area under the ROC curve for HOMA-IR
as a predictor of increased CVR (≥20%) was 0.867. The optimal
cut-off value of HOMA-IR for high CVR prediction was 2.926 with a
sensitivity of 82.4% and specificity of 75% (Fig. 3B and Table III).
Discussion
As expected, in the present study, IR was found at a
higher percentage among patients with diabetes when compared with
individuals without diabetes. In addition, the CVR was
statistically significantly higher among patients with diabetes vs.
individuals without diabetes. CVR was significantly higher among
people with IR with or without DM providing that IR is a
pathological condition with adverse cardiovascular influences.
Early identification of IR, lifestyle and dietary
interventions could decrease the development and progression of IR
and its detrimental consequences on the liver, kidney, blood
pressure, heart and endothelium (22-27).
Lifestyle interventions include body weight loss of >5% of
initial weight, physical activity for 150 min/week or more, or a
regular exercise program. Dietary interventions include daily
calorie restriction by reducing 500 Kcal from regular food intake,
fructose restriction and choosing a Mediterranean diet.
Supplementation with antioxidants, amino acids,
vitamins, n-3 polyunsaturated fatty acids and prebiotics/probiotics
may have benefits; however, further studies are needed to confirm
these benefits (22). Nevertheless,
the present study has some limitations. First, the study was
cross-sectional; therefore, a cause-and-effect relationship could
not be established between the FRS and IR. Second, we did not have
a direct measure outcome of CVD. Finally, the size of our sample
was relatively small; thus, further studies are required.
In conclusion, a high FRS (FRS >20%) was
significantly associated with IR. The homeostasis model assessment
of insulin resistance (HOMA-IR) is an independent risk factor for
high FRS. New therapies focusing on decreasing IR may contribute to
a decreased CVD.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the by dates obtained from
the grant (research contract no. 49/09.09.2019) with the research
topic: Cardiovascular risk assessment in patients with type 2
diabetes conducted by Ionela Mihaela Vladu, as project director, in
collaboration with the ‘The Holy Apostles Medical Center’ and the
University of Medicine and Pharmacy Craiova.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IMV, MF, DC, MCF, RP, LR, ȘTȚ, PMR and VP
contributed equally to acquisition, analysis and systematization of
data, manuscript writing and critical revision of it for important
intellectual content. The authenticity of all raw data was assessed
to ensure their legitimacy by IMV and MF. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Scientific Ethics and
Deontology Committee of the Craiova Municipal Hospital
(registration no. 17283/2019) in accordance with the European Union
Guidelines (Declaration of Helsinki). Written informed consent was
obtained for patient participation.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ascaso JF, Pardo S, Real JT, Lorente RI,
Priego A and Carmena R: Diagnosing insulin resistance by simple
quantitative methods in subjects with normal glucose metabolism.
Diabetes Care. 26:3320–3325. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grigorescu ED, Lăcătușu CM, Crețu I,
Floria M, Onofriescu A, Ceasovschih A, Mihai BM and Șorodoc L:
Self-reported satisfaction to treatment, quality of life and
general health of type 2 diabetes patients with inadequate glycemic
control from north-eastern Romania. Int J Environ Res Public
Health. 18(3249)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Gaal LF, Mertens IL and De Block CE:
Mechanisms linking obesity with cardiovascular disease. Nature.
444:875–880. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adiels M, Olofsson SO, Taskinen MR and
Borén J: Overproduction of very low-density lipoproteins is the
hallmark of the dyslipidemia in the metabolic syndrome.
Arterioscler Thromb Vasc Biol. 28:1225–1236. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Michael MD, Kulkarni RN, Postic C, Previs
SF, Shulman GI, Magnuson MA and Kahn CR: Loss of insulin signaling
in hepatocytes leads to severe insulin resistance and progressive
hepatic dysfunction. Mol Cell. 6:87–97. 2000.PubMed/NCBI
|
|
6
|
Borén J, Taskinen M-R, Olofsson S-O and
Levin M: Ectopic lipid storage and insulin resistance: A harmful
relationship. J Intern Med. 274:25–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ginsberg HN: Insulin resistance and
cardiovascular disease. J Clin Invest. 106:453–458. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Reaven GM: Insulin resistance/compensatory
hyperinsulinemia, essential hypertension, and cardiovascular
disease. J Clin Endocrinol Metab. 88:2399–2403. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ashcroft FM and Rorsman P: Diabetes
mellitus and the β cell: The last ten years. Cell. 148:1160–1171.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
DeFronzo RA and Tripathy D: Skeletal
muscle insulin resistance is the primary defect in type 2 diabetes.
Diabetes Care. 32 (Suppl):S157–S163. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ferrannini E and Cushman WC: Diabetes and
hypertension: The bad companions. Hypertension. 380:601–610.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Steinberg HO, Brechtel G, Johnson A,
Fineberg N and Baron AD: Insulin-mediated skeletal muscle
vasodilation is nitric oxide dependent. A novel action of insulin
to increase nitric oxide release. J Clin Invest. 94:1172–1179.
1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Clenciu D, TeneaCojan TS, Dijmarescu A,
Ene CG, Davitoiu DV, Baleanu VD, Ciora CA, Socea B, Voiculescu D,
NedelcuŢă RM, et al: Diabetic retinopathy in relation with eGDR
value in patients with type 1 diabetes mellitus. Rev Chim
(Bucharest). 70:1434–1438. 2019.
|
|
14
|
Laakso M: Heart in diabetes: A
microvascular disease. Diabetes Care. 34 (Suppl):S145–S149.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
The International Expert Committee.
International Expert Committee report on the role of the A1C assay
in the diagnosis of diabetes. Diabetes Care. 32:1327–1334.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Socea B, Radu L, Clenciu D, TeneaCojan TS,
Baleanu VD, Ene CG, Girgavu SR and Vladu IM: The utility of
visceral adiposity index in prediction of metabolic syndrome and
hypercholesterolemia. RevChim (Bucharest). 69:3112–3114. 2018.
|
|
17
|
Vladu IM, Radu L, Girgavu SR, Baleanu V,
Clenciu D, Ene CG, Socea B, Mazen E, Cristea OM, Mota M, et al: An
easy way to detect cardiovascular risk. Rev Chim (Bucharest).
69:3229–3232. 2018.
|
|
18
|
American Diabetes Association: 2.
Classification and diagnosis of diabetes. Standards of medical care
in diabetes-2021. Diabetes Care. 44 (Suppl):S15–S33. 2021.
|
|
19
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and β-cell function from fasting plasma glucose
and insulin concentrations in man. Diabetologia. 28:412–419.
1985.PubMed/NCBI View Article : Google Scholar
|
|
20
|
D'Agostino RB Sr, Vasan RS, Pencina MJ,
Wolf PA, Cobain M, Massaro J and Kannel WB: General cardiovascular
risk profile for use in primary care: The Framingham heart study.
Circulation. 117:743–753. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hernandez-Rodas MC, Valenzuela R and
Videla LA: Relevant aspects of nutritional and dietary
interventions in non-alcoholic fatty liver disease. Int J Mol Sci.
16:25168–25198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Forțofoiu MC, Popescu DM, Pădureanu V,
Dobrinescu AC, Dobrinescu AG, Mită A, Foarfă MC, Bălă VS, Muşetescu
AE, Ionovici N and ForŢofoiu M: Difficulty in positive diagnosis of
ascites and in differential diagnosis of a pulmonary tumor. Rom J
Morphol Embryol. 58:1057–1064. 2017.PubMed/NCBI
|
|
23
|
Valenzuela R and Videla LA: The importance
of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in
development of non-alcoholic fatty liver associated with obesity.
Food Funct. 2:644–648. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Grigorescu ED, Sorodoc V, Floria M, Anisie
E, Popa AD, Onofriescu A, Ceasovschih A and Sorodoc L: The
inflammatory marker hsCRP as a predictor of increased insulin
resistance in type 2 diabetics without atherosclerotic
manifestations. Rev Chim (Bucharest). 70:1791–1794. 2019.
|
|
25
|
Forţofoiu M, Forţofoiu MC, Comănescu V,
Dobrinescu AC, Pădureanu V, Vere CC, Streba CT and Ciurea PL:
Hepatocellular carcinoma and metabolic diseases-histological
perspectives from a series of 14 cases. Rom J Morphol Embryol.
56:1461–1465. 2015.PubMed/NCBI
|
|
26
|
Mihailovici AR, Padureanu V, Albu CV,
Dinescu VC, Pirlog MC, Dinescu SN, Malin RD and Calborean V:
Myocardial Noncompaction. Rev Chim (Bucharest). 69:2209–2212.
2018.
|
|
27
|
Vladu M, Clenciu D, Efrem IC, Forţofoiu
MC, Amzolini A, Micu ST, Mota M and Fortofoiu M: Insulin resistance
and chronic kidney disease in patients with type 1 diabetes
mellitus. J Nutr Metab. 2017(6425359)2017.PubMed/NCBI View Article : Google Scholar
|