Introduction
Sinonasal mucormycosis represents an invasive,
extremely serious fungal infection located in the nasal fossae and
sinuses, with an evolution towards extension to nearby structures
and with potential destructive capacity (1). Its incidence is increasing in
immunosuppressed patients (2), but
it can rarely be observed in patients with a competent immune
system (3). The invasive infection
has a rapid onset, and early diagnosis and treatment can be a ‘life
savior’.
More than 2 million people suffer from diabetes
mellitus in Romania, and the patient numbers are increasing.
Sinonasal infection with mucormycosis is one of the most feared
complications in patients with diabetes mellitus. The fungal agent
can invade the blood vessels and will embolize, which can in turn
lead to distant organs being infected. Usually, the diagnosis of
mucormycosis is not immediately confirmed, and the specific
treatment is commonly delayed. In invasive infection with
mucormycosis, the treatment of choice is tissue debridement until
reaching healthy tissue and administration of amphotericin B
(4,5). If amphotericin B is not available,
treatment can be initiated with posaconazole (6,7).
The rate of fatality for patients with mucormycosis
is extremely high, from 30% to as high as 80% (8), even when the surgical treatment
consisting of accurate debridement of the fungus and medical
treatment with amphotericin B or with posaconazole are utilized
(9). Renal failure and hemodialysis
are also redundant complications of these substances which should
be emphasized. Although the diagnosis is definitely suggested by
the specific clinical and endoscopic evaluation due to the presence
of black crusting, it must be confirmed by biopsy. The time factor
is also extremely important due to the tendency of rapid spread
that characterizes this disease. The management of these patients
represents a team challenge, as it requires a multidisciplinary
team that must treat the associated diseases that have contributed
to the immunocompromised status that led to the onset of the fungal
disease and the contribution of the ENT surgeon, who must resect
all necrotic tissue while preserving as much of the regional
anatomy as possible (10).
For the surgeon, it is crucial to see the boundaries
between necrosis, the infected tissue, and the healthy tissue.
Direct sinonasal assessment includes five stages: i) White light
examination (without magnification); ii) microscopic assessment
(light with magnification); iii) endoscopic assessment (light with
magnification and mobility of the endoscopic image, also giving
details using angulated optic versions with 0˚, 30˚, 70˚); iv)
refined endoscopic assessment consisting of the following: a) with
focus 0 mm meaning video contact endoscopy including deep
magnification with x60 or x150); b) selection of wavelength for
veins and capillary during narrow band imaging (NBI); c) optic
filters (selected from soft), from SPIES (Storz Professional Image
Enhancement System); d) autofluorescence of the normal and
pathologic tissue; e) video contact endoscopy with SPIES
(synchronous); f) video contact endoscopy with NBI; v) endoscopic
3D imaging.
Endoscopic surgery has forced the revision of the
anatomic and surgical landmarks, has revised the notions of
topographic anatomy and has proposed the use of these concepts in
surgical dissection and risk assessment during dissection under the
endoscope.
SPIES filters were developed by The Karl Storz
Company and can be applied directly during endoscopic surgical
interventions. We observed the surgical field using Standard,
CLARA, CHROMA, CLARA + CHROMA association, SPECTRA A and SPECTRA
B.
SPIES consists of Storz Professional Image
Enhancement filters and these can be used on FULL HD camera and 3D
cable as well. Applying these filters allows the surgeon to better
define the boundaries of any lesion, be it a tumor or in the case
of mucormycosis, the necrotic tissue and, more importantly the
limit between infected and healthy tissue.
The CLARA filter aids the surgeon by increasing the
light in dark places of the endoscopic field. CHROMA increases the
contrast of the surgical field. SPECTRA A removes the red color,
and the surgeon is able to obtain an image similar to the NBI
image. SPECTRA B adds a 15% red to the SPECTRA A filter and the
surgeon is able to better visualize the darkened area of the
surgical field.
Materials and methods
The aim of the present article was to reveal the
endoscopic assessment protocol for patients with sinonasal
mucormycosis, as implemented in our clinics.
The objective was to emphasize during endoscopic
surgical assessment and dissection a sign (or more) that may be
characteristic for the histopathology of invasive fungal
rhinosinusitis ‘in vivo and in situ’. These
characteristic aspects during endoscopic surgery may help the
surgeon to better define the area and lesions, to dissect in a more
accurate and careful manner and to follow the profile of the lesion
until the limit of the surrounding healthy tissue is reached.
Reading the lesion in real-time during endoscopic dissection helps
the surgeon to accurately perform all the steps of the surgical
intervention.
At the same time, assessing the endoscopic aspect of
the lesion and defining the sinonasal area affected, the surgeon
can be efficient even when an extemporaneous examination is not
possible to be carried out in every step of the intervention. Thus,
reading the lesion ‘in vivo and in situ’, the entire
area affected and the surrounding regions of the lesion can be
thoroughly examined, and the endoscopic intervention can be carried
out under safe conditions, allowing, at the same time, for all
tissues presenting with fungal invasion to be excised.
According to our previous observations, the main
objective of this study was to identify characteristic aspects of
the affected tissue by mucormycosis and to define these aspects.
The method consisted of sinonasal endoscopic assessment with a
special endoscopic technology (SPIES) based on software filters,
used in patients with invasive sinonasal mucormycosis. We wish to
underline that patients that presented to our clinic with
mucormycosis presented with important comorbidities and important
immunodeficiency, most often due to poorly controlled diabetes
mellitus.
The anesthetic and surgical assessment (ASA) are
always difficult and sometimes dramatic because of the powerful
inflammatory and infectious complications surrounding the lesion
induced by the invasive fungus.
The results according to the research hypothesis are
the identification of a characteristic endoscopic design (image)
visible during the endoscopic surgical assessment of the sinonasal
area affected by this invasive type of fungus.
The final result was similar to our expectations and
was based on observations during the assessment of multiple cases
of invasive fungal sinusitis in immunosuppressed patients that were
referred to our clinics, patients that presented with associated
various comorbidities, such as diabetes mellitus, neutropenia or a
history of organ transplant. We attempted to make a comparison
between the endoscopic image projected as a landscape and a
‘battlefield’.
The two enemies (mucormycosis and the yet remaining
healthy tissue) are separated by a neutral area (a pale territory)
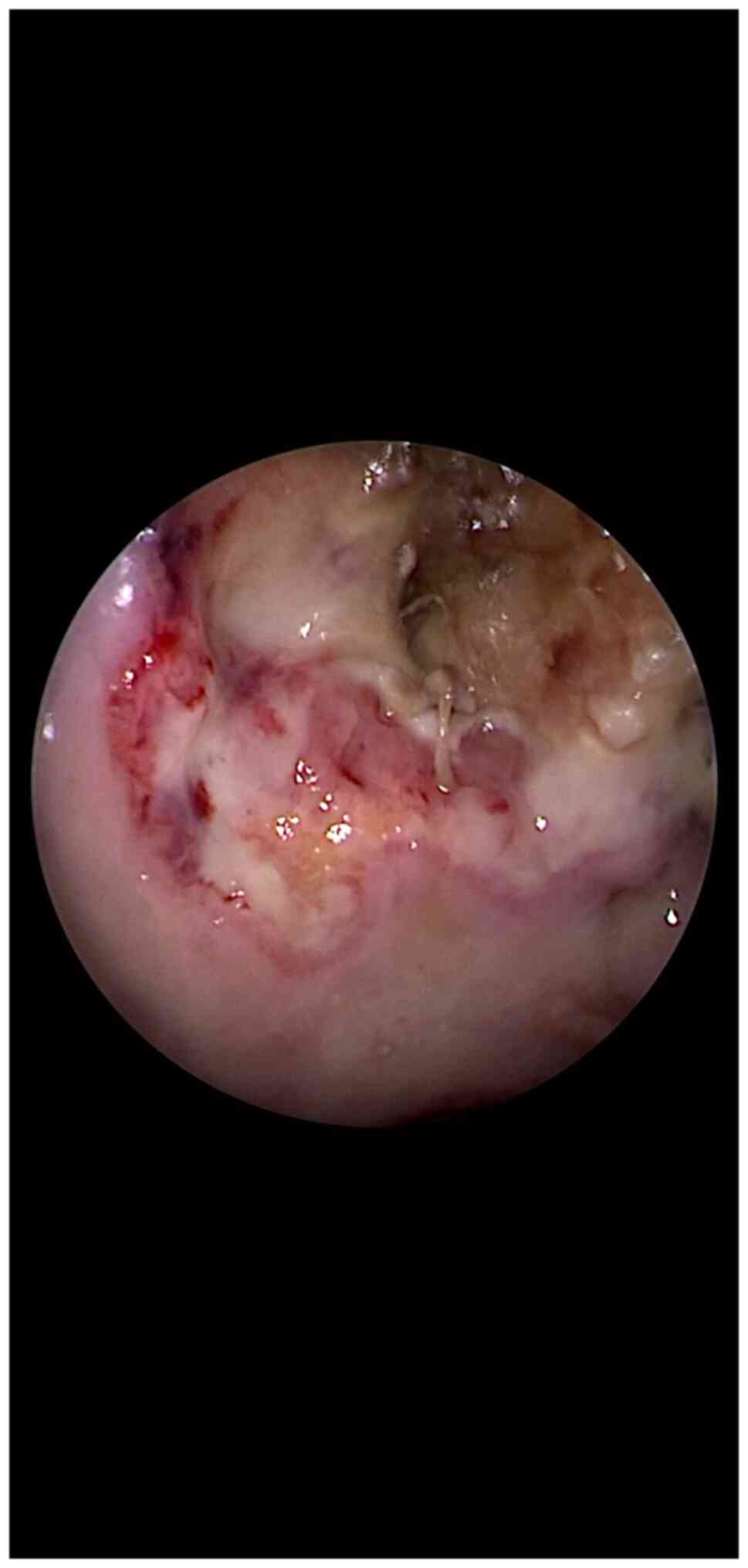
(Fig. 1). This ‘battlefield’ sign
evokes the 3 lines of a ‘battlefield’ as follows: 1) Dark green
mixed with black color (mucormycosis and tissue necrosis) which
defines the mucormycosis area; 2) a pale-colored area (tissue
ischemia which is undergoing necrosis) is to be noted surrounding
the area described above (point 1). It can be defined as a ‘neutral
field of the battle’; and 3) a pale pink area with enforced
vascular design (the advancing front of the surrounding healthy and
reactive tissue) consisting of an inflammatory reaction with a
protective purpose.
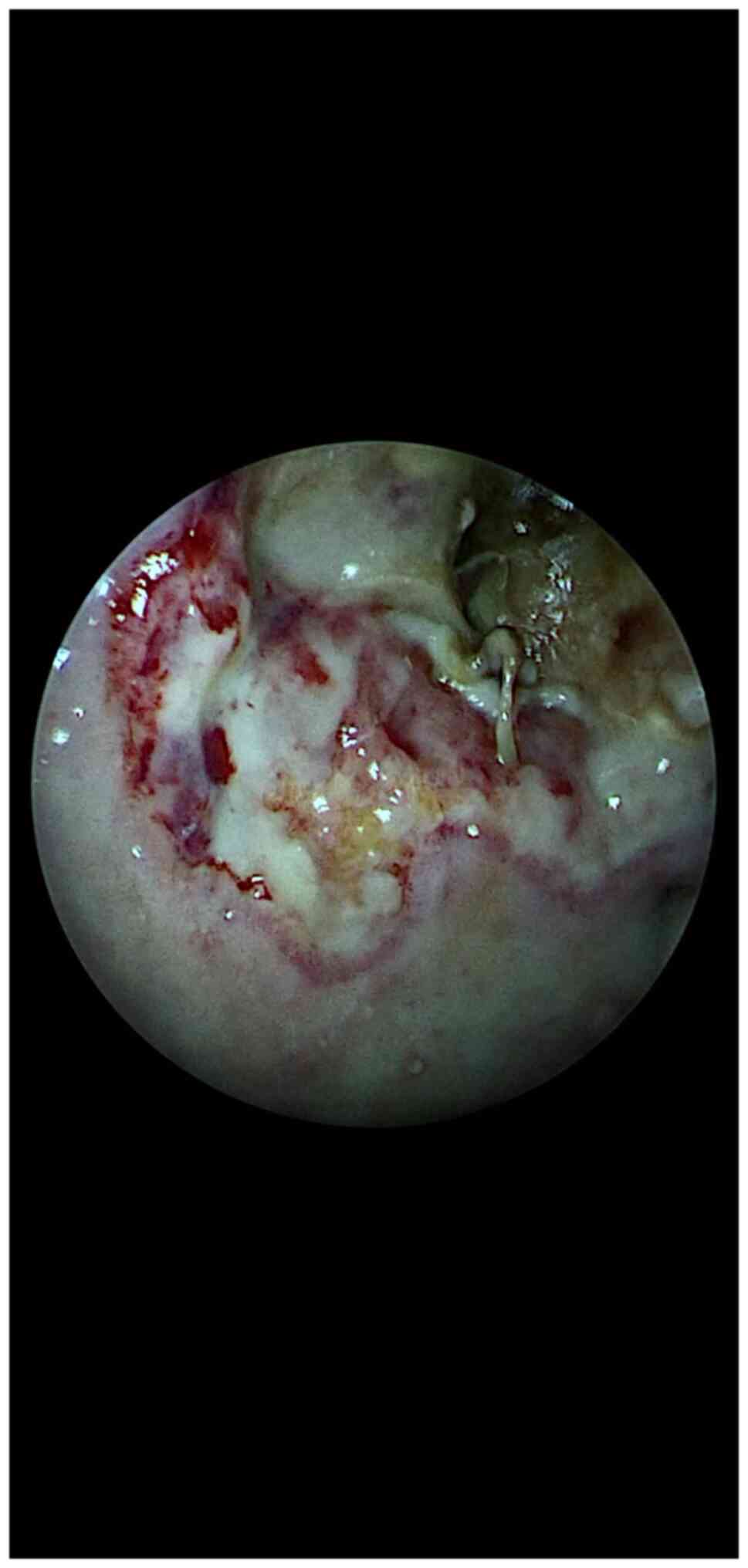
Such an endoscopic surgical image with the 3
‘battlefield’ areas is not so difficult to observe during the
operation if the technologic endoscopic system based on filters is
mainly used in Chroma and Spectra B modes (Fig. 2). These images show the presence of
invasive sinonasal fungus, the area of attachment, the progression
front of the lesions, the reaction of the surrounding healthy
tissue, the intermediate area between the two ‘enemies’
(mucormycosis and healthy tissue). All of these landmarks help the
surgeon to establish the strategy and make possible the procedure
of debridement of the necrotic tissue up to the point of safe
margins.
In this case, safe margins define exclusively
healthy tissue (mucosal line, clear bone and total macroscopic
removal of the sinonasal mucormycosis). Thus, the term ‘safe
margins’ is used here similar to oncologic surgery principles.
We tried to select suggestive endoscopic filtered
images obtained during endoscopic surgery performed for patients
with invasive mucormycosis which underline the ‘battlefield’ sign.
These images and the boundaries defined with their help were later
compared with images from the histopathological examination.
We consider that the best filters to be utilized to
assess the ‘battlefield’ sign in mucormycosis infection are CLARA +
CHROMA and SPECTRA B. Using only the CLARA filter will provide the
surgeon with an increased luminosity of the surgical field, but the
boundaries will not be well visualized. Using the SPECTRA A filter
will darken the image too profoundly and the image will not be
reliable.
Results
This paper aims to point out an endoscopic sign
evidenced with the aid of SPIES technology during endoscopic
surgical interventions performed for patients with invasive fungal
rhinosinusitis with mucormycosis.
The meaning of this endoscopic sign consists of the
evidence of the three components of a ‘battlefield’: 1)
Mucormycosis, an aggressive agent which is confirmed by evidence of
mycologic infection. For a correct assessment and clear diagnosis
computed tomography (CT), Magnetic Resonance Imaging (MRI) and
sampling (biopsies) must also be performed; 2) neutral line which
is tissue ischemia marking the start of the undergoing necrosis;
and 3) reactive surrounding tissue.
The endoscopic surgeon must always look for this
sign on the monitor during the progressing surgery, aiming to
identify the border of the lesions, the neutral and reactive areas
and finally to make a complete debridement and removal of the
lesions induced by the fungal agent up to the healthy tissue (‘safe
margins’).
The best filters to assess ‘safe margins’ include
CLARA + CHROMA and SPECTRA B. Use of these filters allows the
surgeon to better see the affected and healthy tissue. The SPIES
technology is a valuable tool for assessing the limit between the
healthy and the infected tissue. It is simple to apply during
surgery and can be applied to any Hopkins type rigid endoscope with
any angulation.
No matter how useful this method is in assuring a
good outcome for the surgical intervention, the importance of the
complex management of such patients must be emphasized. Although
the complete removal of the necrotic tissue is essential for a
positive evolution, these patients also require antifungal
treatment and management of the underlying conditions that favored
the infection with mucormycosis. One of the most frequently
encountered systemic disease that is associated with mucormycosis
is diabetes mellitus, patients that have trouble with maintaining
adequate blood sugar levels (11).
The long-term imbalance of glycemia levels causes poor local
defense mechanisms and a more difficult recovery, overall
decreasing the survival chances of our patients (12). When evaluating a diabetic patient
with a significant aggravation of the general status who is
suspected of presenting with a fungal infection, we should consider
the possibility of mucormycosis infection (13). A poor nutritional status may also
lead to a significantly poor evolution (14). However, this is not the only
condition that may be associated with mucormycosis. Patients with
neoplasms, liver failure, neurologic disorders or hematological
illnesses are at risk for opportunistic infections and may also
present with such fungal infections (15). We must also keep in mind that
patients must be made aware of the severity of the disease and the
importance of their compliance to treatment, which may result in an
overall increased survival (16).
Intensive care units will provide the best standard
of care for patients with mucormycosis. Life support, systemic
treatment and thorough monitoring are essential in the days
following surgery. Only by maintaining such standards of care
associated with the surgical intervention do such patients have a
chance at recovery.
Discussion
During endoscopic interventions, currently surgeons
have advanced endoscopic technology based on software using image
filters which can better detect and point out the limits of any
lesion, ranging from malignant tumors to invasive fungal
rhinosinusitis (17).
This type of technology using selected filters,
especially STANDARD, CLARA + CHROMA and SPECTRA B, can help the
surgeon identify during surgery the so-called ‘battlefield sign’ as
presented above with photo documentation. The ‘battlefield’ sign
consists in identifying on the endoscopic landscape (screen) the
aforementioned 3 areas, each with its characteristic color, from
dark green-almost black to pale pink.
The advantage of identifying this sign during
endoscopic surgery for invasive fungal rhinosinusitis with
mucormycosis is that it facilitates the progress of the steps of
the operation until clear margins (safe margins) are reached as is
targeted in oncologic surgery.
We always verify and compare the ‘battlefield’ sign
with the specimens directly sampled from the tissue. It must be
emphasized that the biopsy of the affected tissue still remains the
gold standard of histologic diagnosis and it is always more
accurate than the endoscopic examination, even with advanced SPIES
filters (18).
The mycologic, CT and MRI examinations are always
mandatory for a complete diagnosis (8). The management of these patients
remains a multidisciplinary challenge (19), with the aim of the ENT surgeon to
resect all infected tissue, while preserving as much of the normal
anatomy as possible and to protect the vital areas, while treating
all the underlying disease of the patient and administering the
relevant antifungal treatment. This endoscopic method of evaluation
will aid the ENT surgeon is assessing the lesion and performing a
thorough excision, while preserving as much of the healthy tissue
as possible in a narrow area with vital structures and adding no
extra risks to these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The information used and/or analyzed during the
current study is available from the corresponding author on
reasonable request.
Authors' contributions
All authors (VZ, IGI, SP, CP, AR, CDS, FG, FA, DP
and RH) contributed to the acquisition of the data and critical
revision of the manuscript for intellectual content. VZ and CDS
were responsible for the research design and manuscript drafting.
CP and AR were responsible for the language editing. FG and FA were
responsible for editing the article and images. DP and SP
contributed to the literature data analysis and the critical
interpretation and IGI was a major contributor in writing the
manuscript. RH reviewed the manuscript. All authors read and
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
All patients have given their consent to participate
in this study, and the study was conducted according to the
principles of the Declaration of Helsinki. Ethics committee
approval was not necessary.
Patient consent for publication
All patients provided their consent for the
publication of the data; the privacy data regulation was
followed.
Competing interests
The authors declare no competing interests.
References
|
1
|
Battikh R, Labbene I, Ben Abdelhafidh N,
Bahri M, Jbali A, Louzir B, M'saddek F, Ferjani M, Gargouri S,
Dhahri MA, et al: Rhinofacial mucormycosis: 3 cases. Med Mal
Infect. 33:427–430. 2003.
|
|
2
|
Soler ZM and Schlosser RJ: The role of
fungi in diseases of the nose and sinuses. Am J Rhinol Allergy.
26:351–358. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hussain S, Salahuddin N, Ahmad I,
Salahuddin I and Jooma R: Rhinocerebral invasive mycosis:
Occurrence in immunocompetent individuals. Eur J Radiol.
20:151–155. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouza E, Munoz P and Guinea J:
Mucormycosis: An emerging disease? Clin Microbiol Infect. 12:7–23.
2006.
|
|
5
|
Brown J: Zygomycosis: An emerging fungal
infection. Am J Health Syst Pharm. 24:2593–2596. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rutar T and Cockerham KP: Periorbital
zygomycosis (mucormycosis) treated with posaconazole. Am J
Ophthalmol. 142:187–188. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Notheis G, Tarani L, Costantino F, Jansson
A, Rosenecker J, Friederici D, Belohradsky BH, Reinhardt D, Seger
V, Schweinitz DV and Wintergerst U: Posaconazole for treatment of
refractory invasive fungal disease. Mycoses. 49 (Suppl 1):S37–S41.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh VP, Bansal C and Kaintura M:
Sinonasal mucormycosis: A to Z. Indian J Otolaryngol Head Neck
Surg. 71 (Suppl 3):S1962–S1971. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Greenberg RN, Mullane K, Van Burik JA,
Raad I, Abzug MJ, Anstead G, Herbrecht R, Langston A, Marr KA,
Schiller G, et al: Posaconazole as salvage therapy for zygomycosis.
Antimicrob Agents Chemother. 50:126–133. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
DeShazo RD, O'Brien M, Chapin K,
Soto-Aguilar M, Gardner L and Swain R: A new classification and
diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol
Head Neck Surg. 123:1181–1188. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hainarosie R, Zainea V, Rusescu A, Iana
RO, Ghindea T, Suceveanu AP, Stefanescu DC, Ionita IG, Pietrosanu C
and Stoian AP: Management of infectious complications in diabetes
mellitus mellitus patients. Romanian J Military Med. 122:46–51.
2019.
|
|
12
|
Ditu G, Voiculescu DC, Bodnarescu M,
Pantea Stoian A, Hainarosie R, Stefan S and Serafinceanu C:
Mucormycosis and Diabetes Mellitus. Proceedings of National ENT,
Head and Neck Surgery Conference: Arad, Romania, June 06-09, 2018,
pp. 199-204, 2018.
|
|
13
|
Pantea Stoian A, Hainarosie R, Stoica R,
Nitipir C, Paduraru DN, Andronache L, Oros M, Pituru S and
Serafinceanu C: MOE-A Severe Complication in Diabetes Mellitus.
Proceedings of National ENT, Head and Neck Surgery Conference,
Arad, Romania, June 6-9, 2018, pp401-405.
|
|
14
|
Drăgoi CM, Moroșan E, Dumitrescu IB,
Nicolae AC, Arsene AL, Drăgănescu D, Lupuliasa D, Ioniță AC, Pantea
Stoian A, Nicolae C, et al: Insight into chrononutrition: The
innermost interplay amongst nutrition, metabolism and the circadian
clock, in the context of epigenetic reprogramming. Farmacia.
67:557–571. 2019.
|
|
15
|
Ioacara S, Tiu C, Panea C, Nicolae H, Sava
E, Martin S and Fica S: Stroke mortality rates and trends in
Romania, 1994-2017. J Stroke Cerebrovasc Dis.
28(104431)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Țânțu MM, Stan GM, Rogozea L, Păunescu A,
Pleșa CF, Nemeș RM, Nicolae C and Bisoc A: Drug use, a valid
indicator for effective implementation of medical protocols.
Revista de Chimie. 70:859–862. 2019.
|
|
17
|
Chandra RK, Conley DB and Kern RC:
Evaluation of the endoscope and endoscopic sinus surgery.
Oolaryngol Clin North Am. 42:747–52. 2009.
|
|
18
|
Skiada A, Lanternier F, Groll AH, Pagano
L, Zimmerli S, Herbrecht R, Lortholary O and Petrikkos GL: European
Conference on Infections in Leukemia. Diagnosis and treatment of
mucormycosis in patients with hematological malignancies:
guidelines from the 3rd European Conference on Infections in
Leukemia (ECIL 3). Haematologica. 98:492–504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Paleiwala SK, Zangeneh TT, Goldstein SA
and Lemole GM: An aggressive multidisciplinary approach reduces
mortality in rhinocerebral. mucormycosis. 7(61)2016.PubMed/NCBI View Article : Google Scholar
|