Introduction
Gestational diabetes mellitus (GDM) is a form of
diabetes first recognized during pregnancy, which is characterized
by glucose intolerance and insulin resistance (IR) (1). Epidemiological studies have reported
that GDM affects ~15.5-19.9% of all pregnant women in China
(2,3). GDM is associated with several adverse
events, including stillbirth, fetal macrosomia and development of
type 2 DM later in life (4-6).
The activation of inflammation in placenta and adipose tissue plays
key roles in IR during the pathogenesis of GDM (7,8).
Several inflammatory cytokines derived from placenta and adipose
tissue participate in the activation of inflammation and initiate
or aggravate IR during pregnancy (9-11).
The placenta is a highly specialized organ during pregnancy that
releases various cytokines and hormones, and contributes to the
maternal IR (12-14).
Since IR significantly improves immediately after delivery in GDM
women (15,16), it is speculated that the placenta
may play a key role in the activation of inflammation and
initiation of IR during GDM pathogenesis. However, the mechanism
responsible for the regulation of inflammation in GDM placenta
remains unclear.
Interleukin (IL)-1β and IL-18 are important
inflammatory cytokines in the initiation of maternal IR during GDM
(17-19).
The animal experimental study by Schulze et al (20) reported that treatment with an
anti-IL-1β antibody improved glucose-tolerance of GDM mice. The
nucleotide binding and oligomerization domain-like receptor family
pyrin domain-containing 3 (NLRP3) inflammasome participates in the
regulation of IL-1β and IL-18 production (21,22).
The NLRP3 inflammasome can be activated by a wide range of
pathogens and cellular damages, resulting in the generation of
cleaved caspase-1, and produces IL-1β and IL-18 (21,22).
Previous studies have demonstrated that activation of the NLRP3
inflammasome is significantly elevated in patients with obesity,
dyslipidemia and diabetes (23-25).
According to the animal experimental study by Zhang et al
(26), the expression levels of
NLRP3 and caspase-1 are elevated in the placenta tissues of GDM
mice. However, given that the expression of the NLRP3 inflammasome
has not yet been investigated in clinical GDM placenta samples,
further studies are required to determine the mechanism of the
excessive activation of the NLPR3 inflammasome in placenta of
GDM.
Known as ‘the third endogenous gaseous signaling
transmitter’, hydrogen sulfide (H2S) exerts biological
functions, including anti-inflammatory, anti-oxidative stress and
anti-apoptosis (27,28). Our previous study demonstrated that
H2S suppresses activation of the NLPR3 inflammasome in
adipocytes (29). Teng et
al (30) reported that
H2S concentration significantly decreases in parturient
women with GDM, suggesting that decreasing H2S may be
involved in the pathogenesis of GDM. H2S is synthesized
by L-cysteine in a range of mammalian tissues mainly by
cystathionine-γ-lyase (CSE) and cystathionine-β-synthetase (CBS)
(31). Our previous study
demonstrated that human placenta samples express H2S
synthetase, CSE and CBS, and deficiency of CSE and CBS in the
placenta is associated with preeclampsia (32). Previous studies have also reported
that deficiency in H2S synthetase is associated with
other pregnancy complications, including premature labor (33) and fetal growth restriction
(34). Thus, H2S may
participate in the pathogenesis of GDM by regulating activation of
the NLPR3 inflammasome in placentas. The present study aimed to
investigate the expression of the NLPR3 inflammasome and
H2S synthetases, CSE and CBS in clinical GDM placenta
samples. In addition, the regulatory effect of H2S on
the NLPR3 inflammasome in the cultured extravillous trophoblast
cell line, HTR-8/SVneo was investigated.
Materials and methods
Clinical samples
Human placenta tissues were collected from pregnant
women with GDM (n=16) and healthy pregnant women at term (n=16) who
underwent elective cesarean section between January 2019 and
December 2020 at the Chinese PLA 903rd Hospital and Women's
Hospital School of Medicine Zhejiang University. The clinical
characteristics of the pregnant women are presented in Table I. The present study was approved by
the Medical Ethics Committee of the Chinese PLA 903rd Hospital
(ethics approval data and no. 2017/03/05) and written informed
consent was provided by all participants prior to the study start.
Clinical placenta samples were collected within 30 min of cesarean
birth, and three small pieces of tissues from separate lobules were
randomly taken from each placenta. The tissues were washed with
normal saline, immediately frozen in liquid nitrogen and
subsequently stored at -80˚C until subsequent experimentation.
 | Table IClinical characteristics of the
pregnant women enrolled in the present study. |
Table I
Clinical characteristics of the
pregnant women enrolled in the present study.
| Clinical
characteristic | Normal pregnant
women (n=16) | GDM pregnant women
(n=16) | P-value |
|---|
| Maternal age,
years | 31.10±3.28 | 32.42±4.51 | 0.300 |
| Gestational age,
week | 38.60±0.94 | 38.05±1.22 | 0.125 |
| BMI,
kg/m2 | 26.75±1.75 | 27.60±3.34 | 0.326 |
| Blood pressure,
mmHg | | | |
|
Systolic | 116.05±11.17 | 117.58±10.65 | 0.665 |
|
Diastolic | 73.45±9.67 | 73.37±9.17 | 0.979 |
| Blood glucose,
mmol/l | | | |
|
OGTT 0
h | 4.46±0.31 | 4.88±0.56 | 0.043a |
|
OGTT 1
h | 6.59±1.13 | 10.94±2.12 |
<0.001b |
|
OGTT 2
h | 5.89±0.75 | 9.66±1.92 |
<0.001b |
| HbA1c, % | 4.95±0.27 | 5.28±0.45 | 0.014a |
| Infant birth
weight, g | 3407.75±349.45 | 3315.79±417.34 | 0.459 |
Human placental cell culture and
treatment
The human first trimester extravillous trophoblast
cell line, HTR-8/SVneo was gifted by Professor Xin Ni at the
Research Center for Molecular Metabolomics, Xiangya Hospital
Central. Cells were recovered and incubated in RPMI-1640 media
supplemented with 10% fetal bovine serum (both purchased from
Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 and 95% air, until they reached ~90% confluence.
Cells were subsequently digested with 0.25% trypsin.
Subsequently, 1x105 cells seeded into 12-wells plates.
To investigate the role of H2S in regulating the NLPR3
inflammasome, cells were treated with different concentrations of
NaHS (0, 10, 25 and 50 nmol/l; (Sigma-Aldrich; Merck KGaA) or
L-cysteine (0, 0.25, 0.50 and 1.00 mmol/l; Sigma-Aldrich; Merck
KGaA) for 24 h. The present study also investigated the role of the
NLPR3 inflammasome in the production of IL-1β and IL-18, using the
NLPR3 inflammasome inhibitor,
N-acetyl-tyrosyl-valyl-alanyl-aspartyl chloromethyl ketone
(Ac-YVAD-CMK; Sigma-Aldrich; Merck KGaA).
Western blotting
Placental tissues (~30-40 mg) were homogenized using
RIPA lysis buffer (Beyotime Institute of Biotechnology) containing
protease inhibitor cocktail tablet (Roche Diagnostics). Cultured
human placental cells were scraped off the plate using RIPA lysis
buffer containing protease inhibitor cocktail tablet (Roche
Diagnostics). The lysates were subsequently centrifuged in the
speed of 12,000 x g at 4˚C for 15 min and the supernatant was
collected. The concentration of protein in the supernatant was
determined using the BCA kit (Beyotime Institute of Biotechnology).
According to the concentration of protein, samples containing 30 µg
of protein were used for western blot analysis. The protein samples
were separated via 4 and 10% SDS-PAGE, transferred onto
nitrocellulose membranes and blocked by 5% skim milk at room
temperature for 2 h. The membranes were incubated with primary
antibodies against NLRP3 (1:1,000; ab263899; Abcam), cleaved
caspase-1 (1:1,000; ab179515; Abcam) and β-actin (1:8,000; cat. no.
A5441; Sigma-Aldrich; Merck KGaA) overnight at 4˚C. Following the
primary incubation, membranes were incubated with a goat
anti-rabbit secondary HRP-conjugated antibody (1:5,000; cat. no.
BA1054; Wuhan Boster Biological Technology, Ltd.) at room
temperature for 1 h. Protein bands were visualized using the
enhanced chemiluminescence substrate kit (Merck KGaA) and
ChemiScope 6000EXP and the band intensities were calculated by
ImageJ (version 1.51b; National Institutes of Health). Then ratio
of band intensities to β-actin was obtained to quantify the
relative protein expression levels.
ELISA
Following treatment, the culture media of the human
placental cells was collected and IL-1β and IL-18 production was
determined using the IL-1β ELISA kit (cat. no. F10770) and IL-18
ELISA kit (cat. no. F10920) (both Shanghai Westang Biotech),
according to the manufacturer's instructions. All experiments were
performed in duplicate.
Statistical analysis
Data are presented as the mean ± SEM in SPSS
(version 20; IBM Corp.). Each experiment in HTR-8/SVneo was
repeated four times. All data were tested for homogeneity of
variance using the Bartlett's test before analyzing the
significance. Unpaired Student's t-test was used to compare
differences between two groups, while one-way ANOVA followed by
Bonferroni's post hoc test was used to compare differences between
multiple groups. Pearson's analysis was used to analyze the
correlation between two indexes. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of NLRP3, cleaved
caspase-1, CBS and CSE in GDM and healthy placentas
To investigate the role of H2S in the
excessive activation of the NLPR3 inflammasome in GDM placenta, the
expression levels of NLRP3, cleaved caspase-1, and the
H2S synthetases CBS and CSE in placentas were
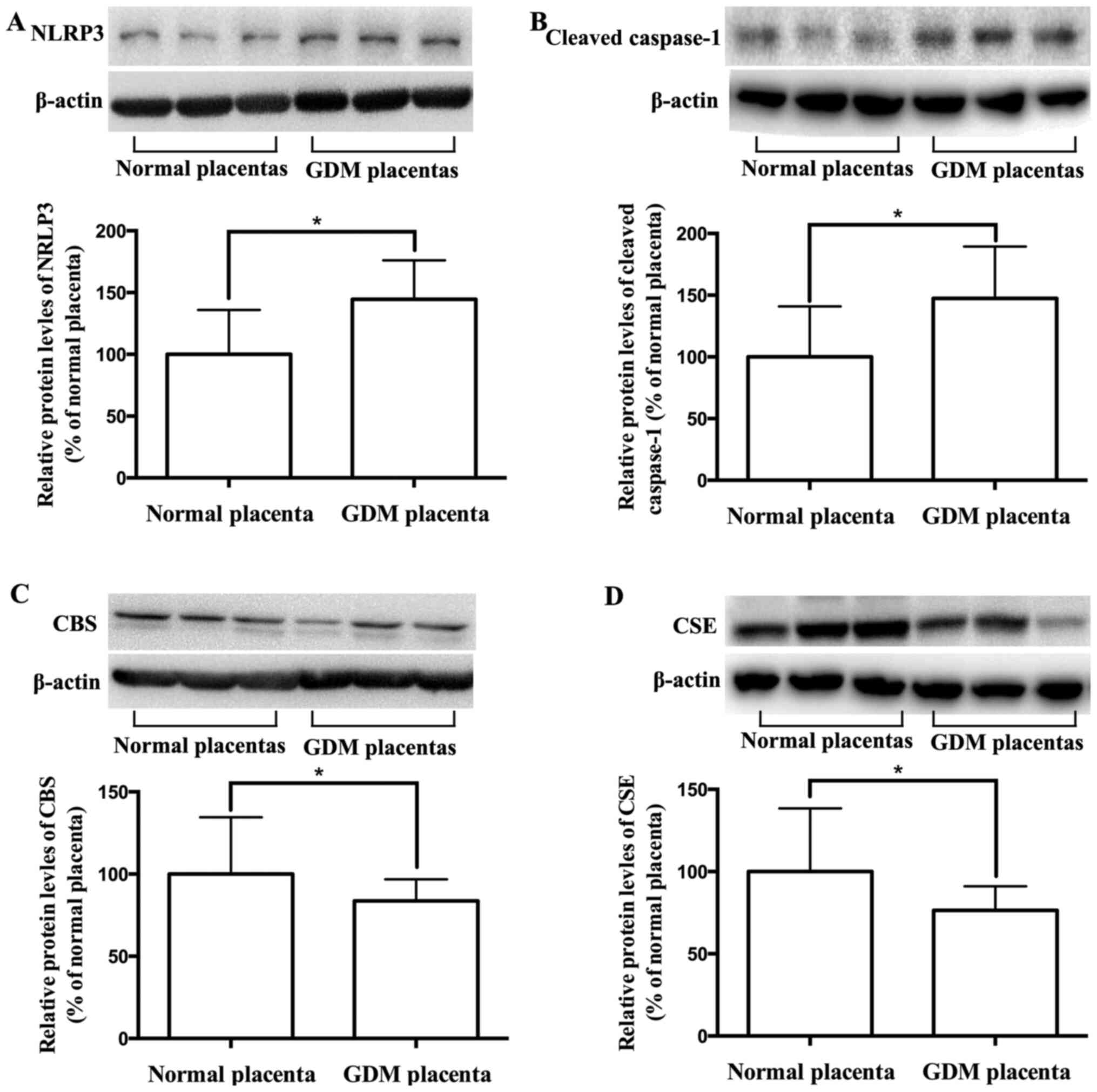
determined. As presented in Fig.
1A-D, the expression levels of NLRP3 and cleaved caspase-1
increased, while the expression levels of CBS and CSE decreased in
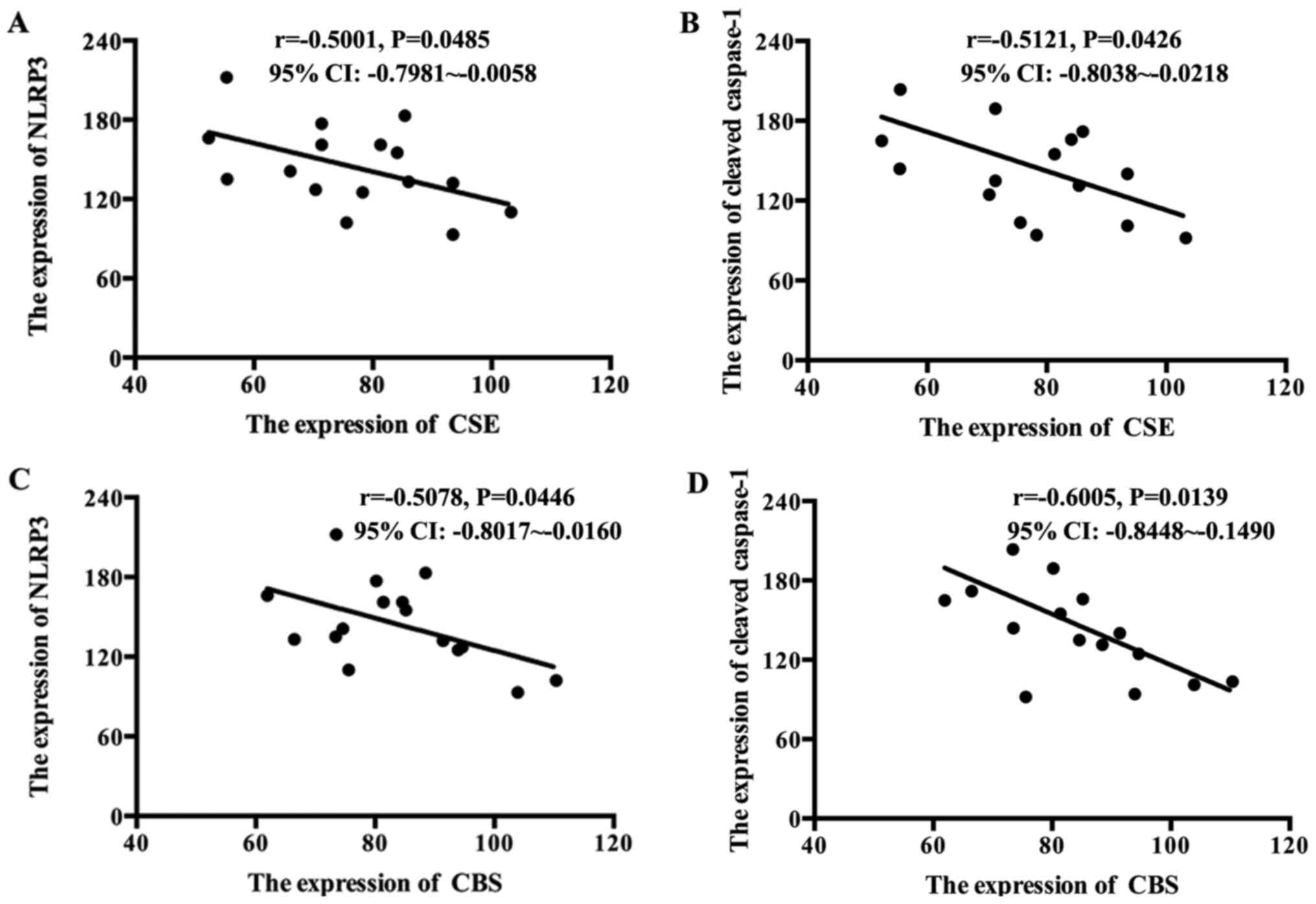
GDM placentas compared with healthy placentas. The correlation
between NLRP3 and cleaved caspase-1 with the H2S
synthetases were analyzed. As presented in Fig. 2A-D, the levels of CBS and CSE were
inversely correlated with NLRP3 and cleaved caspase-1 in GDM
placentas.
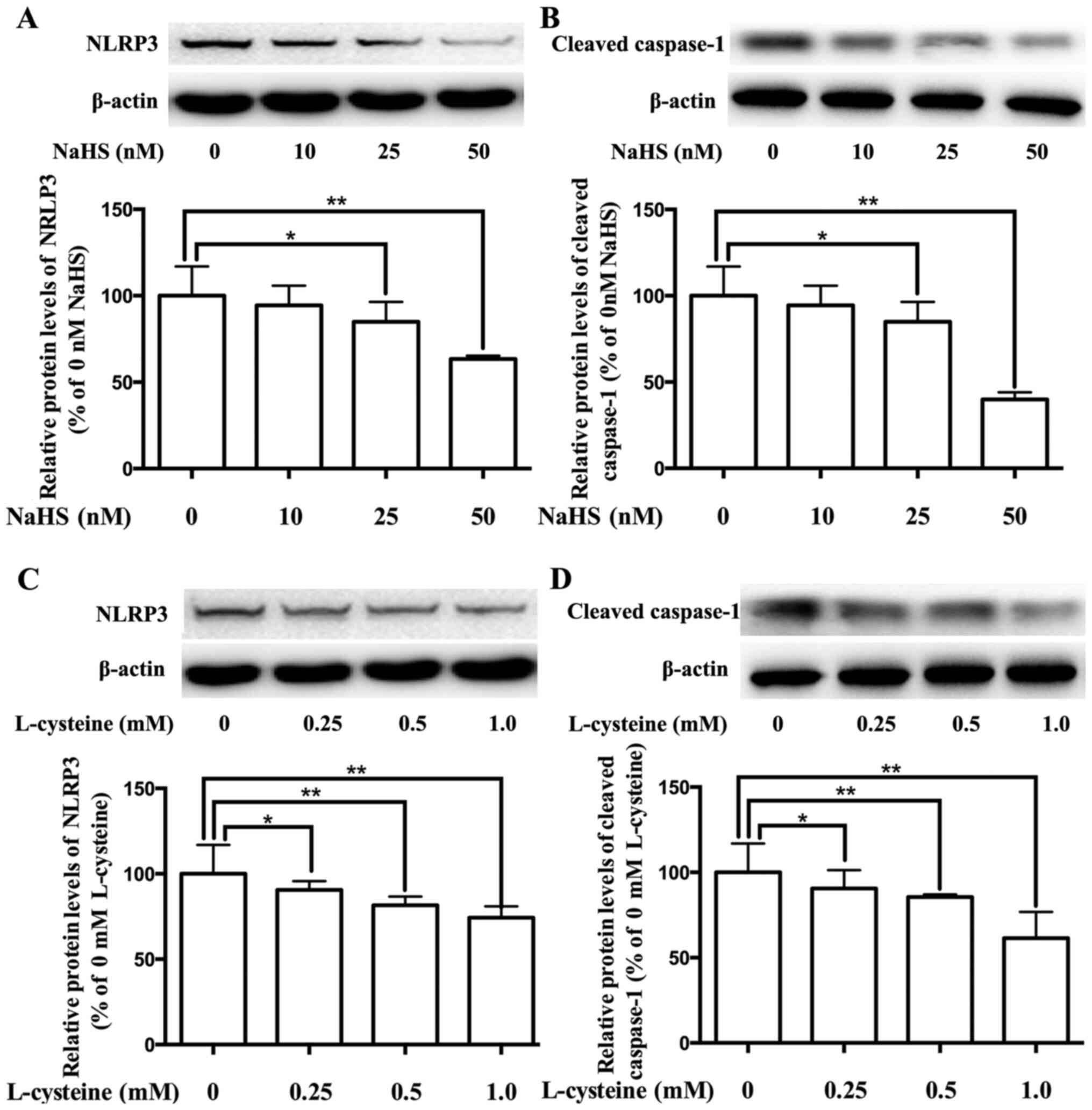
Effect of H2S on the
expression of the NLPR3 inflammasome in placental cells
Our previous study demonstrated that the expression
of the NLPR3 inflammasome decreases via H2S in
adipocytes (29). To investigate
the role of H2S in the regulation of the NLPR3
inflammasome in placenta, placental cells were cultured and treated
with H2S donor NaHS or H2S precursor
L-cysteine. As presented in Fig.
3A-D, treatment with NaHS and L-cysteine significantly
inhibited the expression levels of NLRP3 and cleaved caspase-1, in
dose-dependent manners.
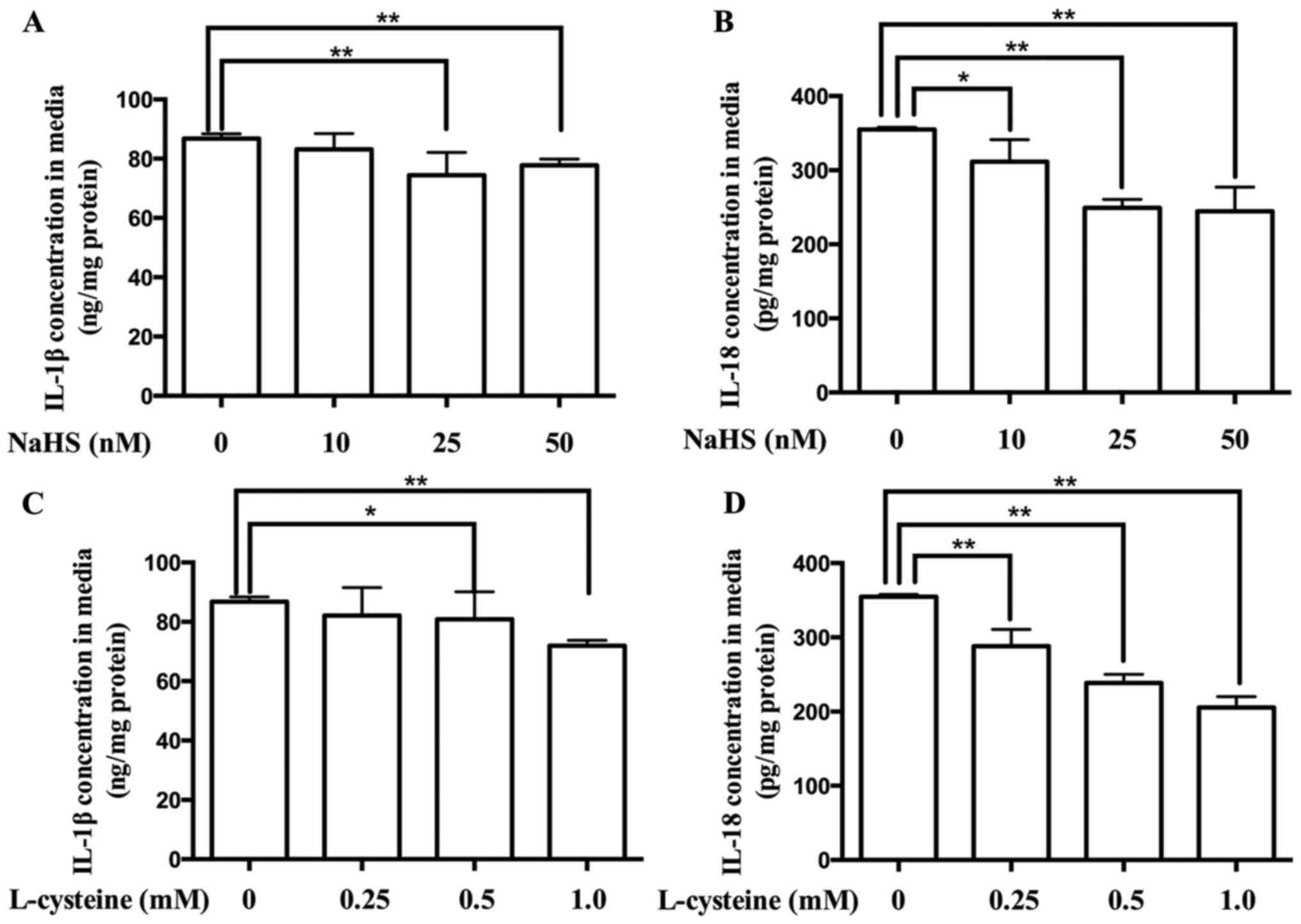
Effect of H2S on the
production of IL-1β and IL-18 in placental cells
Activation of the NLPR3 inflammasome releases IL-1β
and IL-18 (21,22). To confirm the role of
H2S in the regulation of the NLPR3 inflammasome in
placenta, the contents of IL-1β and IL-18 in the culture media of
placental cells were determined. As presented in Fig. 4, treatment with NaHS and L-cysteine
decreased the production of IL-1β and IL-18, in dose-dependent
manners.
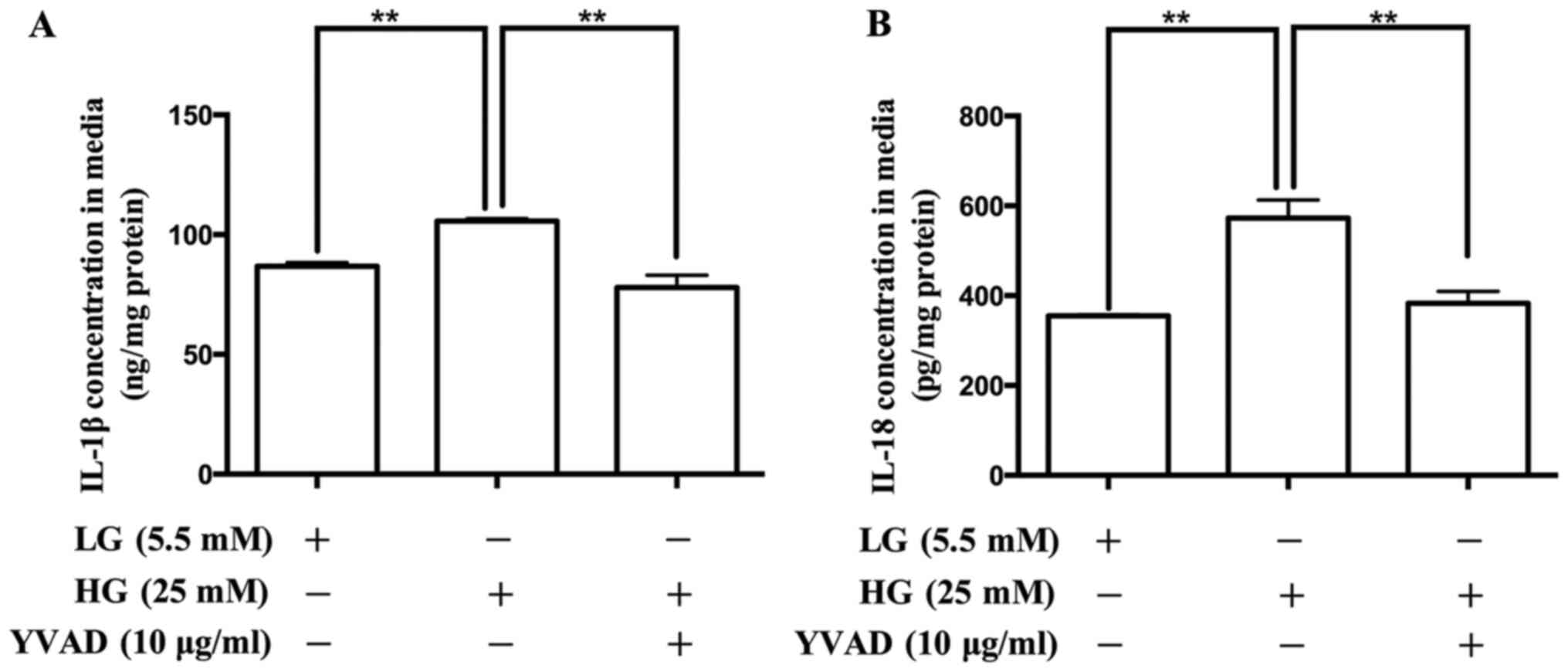
Effect of the NLRP3 inflammasome
inhibitor on the production of IL-1β and IL-18 in placental
cells
To confirm the role of the NLRP3 inflammasome in the
production of IL-1β and IL-18 in placental cells, the NLRP3
inflammasome inhibitor, Ac-YVAD-CMK was used. As presented in
Fig. 5, treatment with Ac-YVAD-CMK
decreased the release of IL-1β and IL-18.
Discussion
The results of the present study demonstrated that
the reduced expression of H2S synthetases, CSE and CBS
was correlated with the excessive activation of the NLPR3
inflammasome in GDM placenta. H2S significantly
suppressed the activation of the NLPR3 inflammasome in human
placental cells in vitro. Furthermore, the NLPR3
inflammasome was involved in the production of IL-1β and IL-18 in
human placental cells.
Known as a highly specialized organ during
pregnancy, the placenta serves as the interface between maternal
and fetal circulation (35). In
recent years, the key role of the placenta in the occurrence and
development of GDM has been reported by multiple studies (36-38).
Currently, IR is the critical pathophysiological characteristic of
GDM, which is also found during normal pregnancy. Placenta derived
hormones, cytokines and gaseous signaling transmitter can induce IR
by interfering with insulin receptor signal transduction (12-14).
Furthermore, the dysregulation of hormones, cytokines and gaseous
signaling transmitter in placenta may aggravate IR and trigger
abnormal glucose metabolism (12-14).
Thus, the present study investigated the key molecules in the
placenta responsible for the pathogenesis of GDM.
The overactive inflammatory response may be the
initiating factor for IR. Cytokines of the IL-1 family critically
regulate the inflammatory response by controlling several
inflammation processes (39,40).
Both IL-1β and IL-18, which are classic pro-inflammatory cytokines
of the IL-1 family, participate in the initiation of IR of GDM and
type 2 DM (17-19).
The production of IL-1β and IL-18 is regulated by the NLRP3
inflammasome in different types of tissues and cells. The NLRP3
inflammasome complex is composed of NLRP3, ASC and pro caspase-1.
Activation of the inflammasome recruits and cleaves pro caspase-1,
which results in the formation of cleaved caspase-1. Subsequently,
cleaved caspase-1 converts pro-IL-1β and pro-IL-18 into the mature
forms, IL-1β and IL-18 (21,22).
According to the animal experimental study by Zhang et al
(26), the expression levels of
NLRP3 and cleaved caspase-1 are elevated in the placenta tissues of
GDM mice. The results of the present study demonstrated that the
expression levels of NLRP3 and cleaved caspase-1 were elevated in
the clinical placenta samples collected from pregnant women with
GDM. Taken together, the results of the present study suggest that
excessive activation of the NLRP3 inflammasome in the placenta may
be involved in the development of GDM.
Further research on the mechanism of the regulation
of the NLRP3 inflammasome in the placenta is required.
H2S is a lately identified gaseous signaling transmitter
that mediates a variety of biological activities, including,
anti-apoptotic and anti-oxidative stress (27,28).
During pregnancy, the abnormal production of H2S and the
dysregulation of the H2S synthetases, CBS and CSE are
associated with various pregnancy complications (32,41,42).
The results of the present study demonstrated that the expression
of the H2S synthetases, CBS and CSE were significantly
downregulated in GDM placenta samples, which was consistent with
the findings reported by Teng et al (30). Our previous study investigated the
regulatory effect of H2S on the NLRP3 inflammasome in
the pathogenesis of vascular complications of type 2 DM, and the
results demonstrated that H2S significantly suppressed
activation of the NLRP3 inflammasome in adipocyte (23). Other studies have also reported the
role of H2S in regulating the NLRP3 inflammasome. For
example, Jia et al (43),
Zheng et al (44) and Su
et al (45) reported the
inhibitory effect of H2S on the NLRP3 inflammasome in
diabetic myocardial injury model, diabetes-accelerated
atherosclerosis model and renal injury model.
The results of the present study demonstrated an
inverse correlation between the H2S synthetases and the
NLRP3 inflammasome in GDM placentas, suggesting that H2S
may participate in regulating the NLRP3 inflammasome in placenta.
The effect of H2S on the NLRP3 inflammasome in
vitro was also investigated. In human placental cells, both the
H2S donor and precursor decreased the expression levels
of NLRP3 and cleaved caspase-1, as well as the production of IL-1β
and IL-18. In addition, the NLRP3 inflammasome inhibitor decreased
the production of IL-1β and IL-18 in human placental cells.
Collectively, these results suggest that H2S plays a
regulatory role in the activation of the NLRP3 inflammasome, and
H2S synthetase deficiency results in excessive
activation of the NLRP3 inflammasome and excessive production of
IL-1β and IL-18 in GDM placenta.
Most previous studies focused on the downstream
biological effects of H2S (29,32,43-45);
however, the mechanism responsible for the upstream regulatory
factor for the expression of CBS and CSE, and the production of
H2S remains unclear. Recently, several studies
investigated the upstream regulatory mechanism for the expression
of CBS and CSE, and the production of H2S, and the
results demonstrated that high fat (46,47),
high salt (48), hypoxia (49) and oxidative stress (50) inhibited the expression of CBS and
CSE, and the production of H2S. Conversely, vitamin D
supplementation increased CSE expression and the production of
H2S (51). Other
clinical studies have reported that high-fat and high-salt diet,
vitamin D deficiency during pregnancy (52-54),
hypoxia and oxidative stress in the placenta (55,56)
are associated with the pathogenesis of GDM. Taken together, these
results suggest that high-fat and high-salt diet, vitamin D,
hypoxia and oxidative stress may be upstream regulatory factors for
the expression of CBS and CSE, and the production of H2S
in GDM. However, further studies are required to determine the
specific mechanism responsible for the expression of CBS and CSE,
and the production of H2S in GDM.
In conclusion, the results of the present study
demonstrated the role of the NLRP3 inflammasome and H2S
in the occurrence and development of GDM. Excessive activation of
the NLRP3 inflammasome may be induced by the H2S
synthetase deficiency in the placenta, and activation of the NLRP3
inflammasome mediates the elevated production of IL-1β and IL-18,
thus initiating maternal IR and causing abnormal glucose
metabolism.
Acknowledgements
The authors of the present study would like to thank
Professor Xin Ni (Research Center for Molecular Metabolomics,
Xiangya Hospital Central, Changsha, Hunan 410008, China) for
valuable comments on the manuscript.
Funding
Funding: The present study was supported by the Natural Science
Foundation of China (grant no. 81701481) and the Medical and Health
Science and Technology Project of Zhejiang Province (grant no.
2019RC253).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TXH and XBD were involved in the overall structuring
and designing of the study, drafting and revising the manuscript
and obtaining funding. WW and QYT contributed to the major cell
experiments, including cell culture, protein expression and
cytokine contents determination. FFX and QL collected the clinical
samples and data. YR and JW contributed to the analysis of data.
All authors reviewed the initial manuscript and revised it
critically for important intellectual content. All authors have
confirmed the authenticity of all the raw data and read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Chinese PLA 903rd Hospital (ethics approval data
and no. 2017/03/05) and performed in accordance with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Written informed consent was provided by all
participants prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Catalano PM: Trying to understand
gestational diabetes. Diabet Med. 31:273–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yan B, Yu Y, Lin M, Li Z, Wang L, Huang P,
Song H, Shi X, Yang S, Li X, et al: High, but stable, trend in the
prevalence of gestational diabetes mellitus: A population-based
study in Xiamen, China. J Diabetes Investig. 10:1358–1364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang M, Hu RY, Gong WW, Pan J, Fei FR,
Wang H, Zhou XY, Zhong JM and Yu M: Trends in prevalence of
gestational diabetes mellitus in Zhejiang Province, China,
2016-2018. Nutr Metab (Lond). 18(12)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gualdani E, Di Cianni G, Seghieri M,
Francesconi P and Seghieri G: Pregnancy outcomes and maternal
characteristics in women with pregestational and gestational
diabetes: A retrospective study on 206,917 singleton live births.
Acta Diabetol. 58:1169–1176. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Sousa RAL, de Lima EV, da Silva TP, de
Souza RV, Figueiredo CP, Passos GF and Clarke JR: Late Cognitive
Consequences of Gestational Diabetes to the Offspring, in a New
Mouse Model. Mol Neurobiol. 56:7754–7764. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kajantie E, Osmond C and Eriksson JG:
Gestational hypertension is associated with increased risk of type
2 diabetes in adult offspring: the Helsinki Birth Cohort Study. Am
J Obstet Gynecol. 216:281 e281–281 e287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cinkajzlová A, Anderlová K, Šimják P,
Lacinová Z, Kloučková J, Kratochvílová H, Krejčí H, Pařízek A, Mráz
M, Kršek M, et al: Subclinical Inflammation and Adipose Tissue
Lymphocytes in Pregnant Females With Gestational Diabetes Mellitus.
J Clin Endocrinol Metab. 105(dgaa528)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olmos-Ortiz A, Flores-Espinosa P, Díaz L,
Velázquez P, Ramírez-Isarraraz C and Zaga-Clavellina V:
Immunoendocrine Dysregulation during Gestational Diabetes Mellitus:
The Central Role of the Placenta. Int J Mol Sci.
22(8087)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rancourt RC, Ott R, Ziska T, Schellong K,
Melchior K, Henrich W and Plagemann A: Visceral Adipose Tissue
Inflammatory Factors (TNF-Alpha, SOCS3) in Gestational Diabetes
(GDM): Epigenetics as a Clue in GDM Pathophysiology. Int J Mol Sci.
21(479)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Keckstein S, Pritz S, Amann N, Meister S,
Beyer S, Jegen M, Kuhn C, Hutter S, Knabl J, Mahner S, et al: Sex
Specific Expression of Interleukin 7, 8 and 15 in Placentas of
Women with Gestational Diabetes. Int J Mol Sci.
21(8026)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsiotra PC, Halvatsiotis P, Patsouras K,
Maratou E, Salamalekis G, Raptis SA, Dimitriadis G and Boutati E:
Circulating adipokines and mRNA expression in adipose tissue and
the placenta in women with gestational diabetes mellitus. Peptides.
101:157–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Simpson S, Smith L and Bowe J: Placental
peptides regulating islet adaptation to pregnancy: Clinical
potential in gestational diabetes mellitus. Curr Opin Pharmacol.
43:59–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hill DJ: Placental control of metabolic
adaptations in the mother for an optimal pregnancy outcome. What
goes wrong in gestational diabetes? Placenta. 69:162–168.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ngala RA, Fondjo LA, Gmagna P, Ghartey FN
and Awe MA: Placental peptides metabolism and maternal factors as
predictors of risk of gestational diabetes in pregnant women. A
case-control study. PLoS One. 12(e0181613)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Skajaa GO, Fuglsang J, Knorr S, Møller N,
Ovesen P and Kampmann U: Changes in insulin sensitivity and insulin
secretion during pregnancy and post partum in women with
gestational diabetes. BMJ Open Diabetes Res Care.
8(e001728)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Waters TP, Kim SY, Sharma AJ, Schnellinger
P, Bobo JK, Woodruff RT, Cubbins LA, Haghiac M, Minium J, Presley
L, et al: Longitudinal changes in glucose metabolism in women with
gestational diabetes, from late pregnancy to the postpartum period.
Diabetologia. 63:385–394. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu T, Deng JM, Liu YL, Chang L and Jiang
YM: The relationship between gestational diabetes mellitus and
interleukin 1beta gene polymorphisms in southwest of China.
Medicine (Baltimore). 99(e22679)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fatima SS, Alam F, Chaudhry B and Khan TA:
Elevated levels of chemerin, leptin, and interleukin-18 in
gestational diabetes mellitus. J Matern Fetal Neonatal Med.
30:1023–1028. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gomes CP, Torloni MR, Gueuvoghlanian-Silva
BY, Alexandre SM, Mattar R and Daher S: Cytokine levels in
gestational diabetes mellitus: A systematic review of the
literature. Am J Reprod Immunol. 69:545–557. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schulze F, Wehner J, Kratschmar DV,
Makshana V, Meier DT, Häuselmann SP, Dalmas E, Thienel C, Dror E,
Wiedemann SJ, et al: Inhibition of IL-1beta improves Glycaemia in a
Mouse Model for Gestational Diabetes. Sci Rep.
10(3035)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou F, Li C and Zhang SY: NLRP3
inflammasome: A new therapeutic target for high-risk reproductive
disorders? Chin Med J (Engl). 134:20–27. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fang X, Wang Y, Zhang Y, Li Y, Kwak-Kim J
and Wu L: NLRP3 Inflammasome and Its Critical Role in Gynecological
Disorders and Obstetrical Complications. Front Immunol.
11(555826)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gora IM, Ciechanowska A and Ladyzynski P:
NLRP3 Inflammasome at the Interface of Inflammation, Endothelial
Dysfunction, and Type 2 Diabetes. Cells. 10(10)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu ZW, Zhang J, Li X, Wang Y, Fu YH and
Gao XY: A new research hot spot: The role of NLRP3 inflammasome
activation, a key step in pyroptosis, in diabetes and diabetic
complications. Life Sci. 240(117138)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mastrocola R, Aragno M, Alloatti G,
Collino M, Penna C and Pagliaro P: Metaflammation: Tissue-Specific
Alterations of the NLRP3 Inflammasome Platform in Metabolic
Syndrome. Curr Med Chem. 25:1294–1310. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang R, Zhang X, Xing B, Zhao J, Zhang P,
Shi D and Yang F: Astragaloside IV attenuates gestational diabetes
mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod
Biol Endocrinol. 17(77)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen CQ, Xin H and Zhu YZ: Hydrogen
sulfide: Third gaseous transmitter, but with great pharmacological
potential. Acta Pharmacol Sin. 28:1709–1716. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang C, Li X and Du J: Hydrogen sulfide as
a new endogenous gaseous transmitter in the cardiovascular system.
Curr Vasc Pharmacol. 4:17–22. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu TX, Zhang NN, Ruan Y, Tan QY and Wang
J: Hydrogen sulfide modulates high glucose-induced NLRP3
inflammasome activation in 3T3-L1 adipocytes. Exp Ther Med.
19:771–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teng Y, Xuan S, Jiang M, Tian L, Tian J
and Chang Q: Expression of H2S in Gestational Diabetes
Mellitus and Correlation Analysis with Inflammatory Markers IL-6
and TNF-α. J Diabetes Res. 2020(3085840)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Renga B: Hydrogen sulfide generation in
mammals: The molecular biology of cystathionine-β- synthase (CBS)
and cystathionine-γ-lyase (CSE). Inflamm Allergy Drug Targets.
10:85–91. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu T, Wang G, Zhu Z, Huang Y, Gu H and Ni
X: Increased ADAM10 expression in preeclamptic placentas is
associated with decreased expression of hydrogen sulfide production
enzymes. Placenta. 36:947–950. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun Q, Chen Z, He P, Li Y, Ding X, Huang
Y, Gu H and Ni X: Reduced Expression of Hydrogen Sulfide-Generating
Enzymes Down-Regulates 15-Hydroxyprostaglandin Dehydrogenase in
Chorion during Term and Preterm Labor. Am J Pathol. 188:63–71.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu L, Kingdom J, Burton GJ and
Cindrova-Davies T: Placental Stem Villus Arterial Remodeling
Associated with Reduced Hydrogen Sulfide Synthesis Contributes to
Human Fetal Growth Restriction. Am J Pathol. 187:908–920.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ganguly E, Hula N, Spaans F, Cooke CM,
Davidge ST and Ganguly E: Placenta-targeted treatment strategies:
An opportunity to impact fetal development and improve offspring
health later in life. Pharmacol Res. 157(104836)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsai K, Tullis B, Jensen T, Graff T,
Reynolds P and Arroyo J: Differential expression of mTOR related
molecules in the placenta from gestational diabetes mellitus (GDM),
intrauterine growth restriction (IUGR) and preeclampsia patients.
Reprod Biol. 21(100503)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Awamleh Z, Butcher DT, Hanley A,
Retnakaran R, Haertle L, Haaf T, Hamilton J and Weksberg R:
Exposure to Gestational Diabetes Mellitus (GDM) alters DNA
methylation in placenta and fetal cord blood. Diabetes Res Clin
Pract. 174(108690)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sarina Li DF, Feng ZQ, Du J, Zhao WH,
Huang N, Jia JC, Wu ZY, Alamusi Wang YY, Ji XL and Yu L: Mechanism
of Placenta Damage in Gestational Diabetes Mellitus by
Investigating TXNIP of Patient Samples and Gene Functional Research
in Cell Line. Diabetes Ther. 10:2265–2288. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dinarello CA: Overview of the IL-1 family
in innate inflammation and acquired immunity. Immunol Rev.
281:8–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ballak DB, Stienstra R, Tack CJ, Dinarello
CA and van Diepen JA: IL-1 family members in the pathogenesis and
treatment of metabolic disease: Focus on adipose tissue
inflammation and insulin resistance. Cytokine. 75:280–290.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zochio GP, Possomato-Vieira JS, Chimini
JS, da Silva MLS and Dias-Junior CA: Effects of fast versus
slow-releasing hydrogen sulfide donors in hypertension in pregnancy
and fetoplacental growth restriction. Naunyn Schmiedebergs Arch
Pharmacol. 392:1561–1568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
You X, Chen Z, Zhao H, Xu C, Liu W, Sun Q,
He P, Gu H and Ni X: Endogenous hydrogen sulfide contributes to
uterine quiescence during pregnancy. Reproduction. 153:535–543.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jia Q, Mehmood S, Liu X, Ma S and Yang R:
Hydrogen sulfide mitigates myocardial inflammation by inhibiting
nucleotide-binding oligomerization domain-like receptor protein 3
inflammasome activation in diabetic rats. Exp Biol Med (Maywood).
245:221–230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zheng Q, Pan L and Ji Y: H 2S protects
against diabetes-accelerated atherosclerosis by preventing the
activation of NLRP3 inflammasome. J Biomed Res. 34:94–102.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Su Y, Wang Y, Liu M and Chen H: Hydrogen
sulfide attenuates renal I/R-induced activation of the inflammatory
response and apoptosis via regulating Nrf2-mediated NLRP3 signaling
pathway inhibition. Mol Med Rep. 24(518)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nguyen TTP, Kim DY, Lee YG, Lee YS, Truong
XT, Lee JH, Song DK, Kwon TK, Park SH, Jung CH, et al: SREBP-1c
impairs ULK1 sulfhydration-mediated autophagic flux to promote
hepatic steatosis in high-fat-diet-fed mice. Mol Cell.
81:3820–3832.e7. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Peh MT, Anwar AB, Ng DS, Atan MS, Kumar SD
and Moore PK: Effect of feeding a high fat diet on hydrogen sulfide
(H2S) metabolism in the mouse. Nitric Oxide. 41:138–145.
2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Moreira AM, Grisote SA, Francescato HDC,
Coimbra TM, Elias LLK, Antunes-Rodrigues J and Ruginsk SG: Effects
of endogenous H2S production inhibition on the
homeostatic responses induced by acute high-salt diet consumption.
Mol Cell Biochem. 476:715–725. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zheng W and Liu C: The cystathionine
γ-lyase/hydrogen sulfide pathway mediates the trimetazidine-induced
protection of H9c2 cells against hypoxia/reoxygenation-induced
apoptosis and oxidative stress. Anatol J Cardiol. 22:102–111.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bibli SI and Fleming I: Oxidative
Post-Translational Modifications: A Focus on Cysteine
S-Sulfhydration and the Regulation of Endothelial Fitness. Antioxid
Redox Signal: Sep 29, 2021 (Epub ahead of print). doi:
10.1089/ars.2021.0162.
|
|
51
|
Manna P and Jain SK: Vitamin D
up-regulates glucose transporter 4 (GLUT4) translocation and
glucose utilization mediated by cystathionine-γ-lyase (CSE)
activation and H2S formation in 3T3L1 adipocytes. J Biol
Chem. 287:42324–42332. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Brown J, Alwan NA, West J, Brown S,
McKinlay CJ, Farrar D and Crowther CA: Lifestyle interventions for
the treatment of women with gestational diabetes. Cochrane Database
Syst Rev. 5(CD011970)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jiang XC, Liang ZD, Chen DL, Jia JP, Hu JR
and Hu L: Correlation of Homocysteine, AHSG, CRP with Insulin
Resistance, 25-(OH)2-VitD, Blood Lipids in Gestational Diabetes
Patients. Clin Lab. 67(67)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen W, Li Y, Gao B, Li J, Zheng M and
Chen X: Serum 25-hydroxyvitamin D levels in relation to lipids and
clinical outcomes in pregnant women with gestational diabetes
mellitus: An observational cohort study. BMJ Open.
10(e039905)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Akarsu S, Bagirzade M, Omeroglu S and Büke
B: Placental vascularization and apoptosis in Type-1 and
gestational DM. J Matern Fetal Neonatal Med. 30:1045–1050.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kasture V, Sahay A and Joshi S: Cell death
mechanisms and their roles in pregnancy related disorders. Adv
Protein Chem Struct Biol. 126:195–225. 2021.PubMed/NCBI View Article : Google Scholar
|