Introduction
Oral cancer is a general term for various malignant
tumors of the mouth. Oral squamous cell carcinoma (OSCC) is one the
most common types of oral cancer, accounting for ~90% of all oral
cancers found in the mouth and lips (1). In addition to being one of the most
common types of oral tumor, is also the 8th leading cause of
cancer-associated mortality worldwide (1,2). The
current treatment of OSCC has been significantly improved and
includes radiotherapy, chemotherapy and surgical resection
(3). However, some patients with
OSCC are ineligible for radiation or chemotherapy (4) and their survival is not better than
that of patients with OSCC. At present, the 5-year survival rate is
~50% (5,6). Further investigation on the mechanism
of OSCC tumorigenesis is therefore crucial.
Pre-B-cell leukemia homeobox protein 1 (PBX1) is a
member of the PBX family. PBX1 can form hetero-oligomeric complexes
with other homeodomain transcription factors, such as Hox
and engrailed, to prevent the development of OSCC (7,8).
Increasing evidence showed that dysregulated expression of PBX1
contributes to the proliferation, survival and metastasis of
various types of cancer, such as breast, lung, gastric and ovarian
cancers. For example, in gastric carcinomas (9) and non-small cell lung cancers
(10), PBX1 can promote the
transformation between the epithelial and mesenchymal cells
associated with chemoresistance. Furthermore, it has recently been
found that PBX1 is upregulated in several OSCC cell lines (8).
Signal transducer and activator of transcription 3
(STAT3) is a cytoplasmic transcription factor, which expression can
be regulated by various cytokines and growth factors, such as IL6,
IL10 and TNF-α (11-14).
Previous studies have demonstrated that STAT3 is an
important protein in tumorigenesis and progression of different
malignancies, such as hepatocellular carcinoma (HCC), clear cell
renal cell carcinoma and non-small cell lung cancer (15-18).
The phosphorylation of janus kinase 2 (JAK2) protein
can activate STAT3 protein (19).
Furthermore, the JAK2/STAT3 signaling pathway plays crucial roles
in cell proliferation and apoptosis of various cancers (20). Activating the JAK2/STAT3 signaling
pathway can promote the stemness of OSCC (21) and certain microRNAs (miRs/miRNAs)
might regulate the expression of JAK/STAT, such as miR-203, miR-409
and miR-365a-3p (20,22-24).
miRs are small non-coding RNAs of ~22 nucleotides in
length. They regulate mRNA translation and deterioration, and gene
expression is modulated following mRNA transcription (25). Some miRNAs associated with cancer
can influence multiple biological processes, such as cell survival,
differentiation, proliferation, apoptosis and migration (26). Certain miRNAs have been
characterized as tumor suppressors and others as oncogenic
(27,28). Previous studies reported that OSCC
progression is affected by numerous miRNAs, including miR-141,
miR-186(29), miR-145 and miR-429
(30,31). miR-141-3p is a member of the
miR-141 family. Previous studies have demonstrated that miR-141-3p
expression is related to several types of tumors, such as
nasopharyngeal carcinoma, esophageal squamous cell carcinoma and
HCC (32-36).
However, the effects of miR-141-3p on OSCC have been rarely
reported, and the function of miR-141-3p and the relationship
between miR-141-3p and JAK2/STAT3 pathway remain unclear in
OSCC.
The present study aimed to investigate the possible
modulation of JAK2/STAT3 pathway by miR-141-3p via targeting PBX1,
and to determine the potential effects of miR-141-3p in OSCC.
Materials and methods
Clinical specimens
A total of 30 pairs of tumor and adjacent normal
tissues were collected from clinical surgical cases of OSCC between
March 2018 and December 2019 from Lishui People's Hospital (Lishui,
China). Adjacent tissues were collected at least 1.5 cm from the
tumor tissue margin. The clinical specimen information of the 30
patients is presented in Table I.
The study complied with the ethics committee regulations and was
approved by the Clinical Ethical Committee of Lishui University
(approval no. 201803). Written informed consent was provided by all
the patients or their relatives for the use of their tissues in
experiments. Following collection, tissue samples were immediately
frozen in liquid nitrogen and preserved at -80˚C for subsequent
experiments.
 | Table IClinicopathological characteristics
of patients with oral squamous cell carcinoma. |
Table I
Clinicopathological characteristics
of patients with oral squamous cell carcinoma.
| Variable | Number (%) |
|---|
| Age, years | |
|
<60 | 11 (36.7) |
|
≥60 | 19 (63.3) |
| Sex | |
|
Male | 14 (46.7) |
|
Female | 16 (53.3) |
| BMI | |
|
<22.5 | 12 (40.0) |
|
≥22.5 | 18 (60.0) |
|
Differentiation | |
|
Poorly
differentiated | 3 (10.0) |
|
Moderate
differentiation | 6 (20.0) |
|
Well
differentiated | 21 (70.0) |
| Clinical stage | |
|
I-II | 16 (53.3) |
|
III-IV | 14 (46.7) |
| Tumor size, cm | |
|
<2.9 | 13 (43.3) |
|
≥2.9 | 17 (56.7) |
| T stage | |
|
T1-2 | 16 (53.3) |
|
T3-4 | 14 (46.7) |
| Infiltration
depth | |
|
Primary
tissue layer | 7 (23.3) |
|
Adjacent
tissue layer | 19 (63.3) |
|
Surrounding
tissue layer | 4 (13.3) |
| Smoking status | |
|
No | 22 (73.3) |
|
Yes | 8 (26.7) |
| Underlying
disease | |
|
No | 17 (56.7) |
|
Yes | 13 (43.3) |
Cell lines and culture
The OSCC cell lines CAL27, SCC-9, SCC-4 and normal
human oral keratinocytes (NHOK; cat. no. PCS-200-014 TM) cells were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. All OSCC cells were cultured in DMEM
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 1%
penicillin-streptomycin and 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) placed at 37˚C in a humidified incubator
containing 5% CO2.
Cell transfection
CAL27 cells were seeded in a six-well plate at
1x105 cells/well for incubation. After 24 h, the
miR-141-3p inhibitor, miR-141-3p mimic, mimic/inhibitor negative
control (NC; 50 nM; Guangzhou RiboBio Co., Ltd.) and small
interfering (si)-PBX1 and si-NC (2 µM; Guangzhou RiboBio Co.,
Ltd.), were transfected into the cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, the cells were incubated in a humidified atmosphere
of 5% CO2 at 37˚C. After 72 h, all transfected cells
were collected, and subsequent experiments were immediately carried
out. CAL27 cells were also co-transfected with 50 nM miR-141-3p
inhibitor and 2 µM si-PBX1. The sequences were as follows: si-NC
sense, 5'-AAUUCUCCGAACGUGUCACGU-3' and antisense,
5'-ACGUGACACGUUCGGAGAAUU-3'; si-PBX1 sense,
5'-AGCUGUCACUGCUACCAAUGU-3' and antisense,
5'-ACAUUGGUAGCAGUGACAGCU-3'; mimic NC sense,
5'-UUCUCCGAACGUGUCACUGUU-3' and antisense,
5'-AACAGUGACACGUUCGGAGAA-3'; miR-141-3p mimic sense,
5'-AACACUGUCUGGUAAAGAUGG-3' and antisense,
5'-CCAUCUUUACCAGACAGUGUU-3' inhibitor NC sense,
5'-CAGUACUUUUGUGUAGUACAA-3' and antisense,
5'-UUGUACUACACAAAAGUACUG-3'; miR-141-3p inhibitor sense,
5'-CCAUCUUUACCAGACAGUGUUA-3' and antisense,
5'-UAACACUGUCUGGUAAAGAUGG-3'.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tumor, adjacent tissues
and cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were the following: Pre-denaturation at
95˚C for 10 min; 40 cycles of denaturation at 95˚C for 10 sec,
annealing at 60˚C for 20 sec. The reaction system contained SYBR
Premix Ex Taq™ II 10 µl, PCR Forward Primer (10 µM) 0.8 µl, PCR
Reverse Primer (10 µM) 0.8 µl, ROX Reference Dye 0.4 µl, DNA
template (2.0 µl) and sterile distilled water (6.0 µl). U6 served
as an internal control. The 2-ΔΔCq method was used to
calculate expression of the relative mRNA (37). The sequences of the primers were as
follows: miR-141-3p forward, 5'-ACACTCCAGCTGGGTAACACTGTCTGGTAA-3'
and reverse, 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCATCTTT-3'; U6
forward, 5'-ATTGGAACGATACAGAGAAGATT-3' and reverse,
5'-GGAACGCTTCACGAATTTG-3'. RT-qPCR reactions were performed using a
RealTime PCR System (Applied Biosystems).
Immunohistochemistry (IHC)
Tissues were fixed in 4% paraformaldehyde at 4˚C for
24 h. Then, the tissues were dehydrated in graded dilutions of
ethanol at room temperature (70% ethanol for 3-4 h; 80% ethanol for
3-4 h; 90% ethanol for 2-3 h; 95% ethanol for 2-3 h, 100% ethanol I
for 1.5-2 h; 100% ethanol II for 1.5-2 h). Then, the tissues were
made transparent in xylene I and xylene II for 0.5-1 h each time at
room temperature. Next, a mixture of low melting point wax (melting
point, 56-58˚C) pre-heated at 60˚C with an equivalent volume of
fresh xylene was prepared in a glass bottle. The tissues were
dipped into the mixture and incubated overnight at room
temperature. Then, the tissues were dipped in low melting point wax
and incubated in a 60-˚C oven for 0.5-1 h three times. Following
this, the tissues were embedded in low melting point wax at room
temperature until the wax concreted. Finally, the waxes of
containing tissue were trimmed and marked at room temperature.
Wax-embedded oral tissues were cut into 5-µm slices. Xylene and
graded dilutions of ethanol (100% I; 100% II; 95% I; 95% II; 85%;
75%) were used to remove wax and rehydrate sections, respectively.
Slices were heated in an autoclave with citric acid buffer (pH 7.0)
for antigen retrieval for 10 min, and then cooled down at room
temperature; they were next incubated with 3%
H2O2 for 30 min at 37˚C. Slices were washed
three times with PBS for 5 min and subsequently incubated with 10%
goat serum (Beyotime Institute of Biotechnology) for 30 min at
37˚C, without subsequent washing. Sections were incubated with an
anti-rabbit PBX1 primary antibody (1:100; cat. no. ab104247; Abcam)
overnight at 4˚C. Sections were then washed three times with PBS
for 5 min. Subsequently, slices were incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:1,000;
cat. no. ab6721; Abcam) at room temperature for 30 min at 37˚C and
with an avidin-biotin HRP complex for 30 min at 37˚C, followed by
three washes with PBS for 5 min. Slices incubated with only an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:1,000;
cat. no. ab6721; Abcam) at room temperature for 30 min at 37˚C
served as a negative control. The slices were subsequently stained
with 3,3'-diaminobenzidine avoid light for 15 min at room
temperature and counterstained with hematoxylin for 15 min at room
temperature. Sections were observed using a light microscope
(Eclipse Ni-U; Nikon Corporation) at x20 magnification and ImageJ
v1.8.0 software (National Institutes of Health) was used to
evaluate the positive area of PBX1 staining. PBX1 expression was
calculated as follows: Average Optical Density = Integrated Optical
Density/Area (Area of target protein distribution).
Western blotting
Tissues and cells were lysed using RIPA buffer
(Thermo Fisher Scientific, Inc.) supplemented with protease and
phosphatase inhibitors (Thermo Fisher Scientific, Inc.) on ice for
20 min. Samples were centrifuged at 1,500 x g at 4˚C for 5 min, and
a BCA protein assay kit (Thermo Fisher Scientific, Inc.) was used
to determine the protein concentration after collecting the
supernatants. A total of 20 µg of total proteins were separated by
8-12% SDS-PAGE and transferred onto a PVDF membrane. Membranes were
blocked with 5% skimmed milk at room temperature for 1 h and were
incubated with primary antibodies anti-rabbit JAK2 (Abcam; cat. no.
ab245303; 1:1,000), anti-rabbit p-JAK2 (Abcam; cat. no. ab195055;
1:1,000), anti-rabbit STAT3 (Abcam; cat. no. ab226942; 1:1,000),
anti-rabbit p-STAT3 (Abcam; 1:1,000), anti-rabbit PBX1 (Abcam; cat.
no. ab104247; 1:1,000), and anti-mouse GAPDH (Abcam; cat. no.
ab181602; 1:1,000) at 4˚C overnight. Membranes were washed three
times with TBST (0.2% Tween) and were incubated with secondary
antibodies: HRP-conjugated goat anti-rabbit IgG (Abcam; cat. no.
ab6721; 1:5,000) and HRP-conjugated goat anti-mouse IgG (Abcam;
cat. no. ab205719; 1:5,000) for 2 h at 37˚C. Membranes were washed
three times with TBST and enhanced chemiluminescence reagent
(Thermo Fisher Scientific, Inc.) was used to detect the signal on
the membrane. The data were analyzed via densitometry using ImageJ
v1.8.0 software (National Institutes of Health) and normalized to
expression of the internal control GAPDH.
MTT assay
CAL27 cells were seeded at the density of
2x103 per well in 96-well plates. MTT solution (20 µl;
Sigma-Aldrich; Merck KGaA) was added at the concentration of 5 g/l.
After 4 h, the supernatant from each well was discarded and 150
µl/well DMSO was added. The samples reacted for 10 min in the dark
at room temperature. A microplate reader (Beckman Coulter, Inc.)
was used to detect the optical density at 490 nm and the cell
proliferation curve was plotted.
Luciferase assay
TargetScanHuman v7.2 (www.targetscan.org) online prediction software was
used to predict the binding sites of miR-141-3p and PBX1. We first
constructed two plasmids wild type (Wt) and mutant type (Mut), and
then we constructed two vectors, Wt-PBX1 and Mut-PBX1.
Subsequently, miR-141-3p mimic, miR-141-3p inhibitor and
mimic/inhibitor-NC were transfected into CAL27 cells using
Lipofectamine® 2000. After 48 h, the cells were
collected and lysed and used for luminescence detection. The
luciferase activity was detected using a dual-luciferase reporter
system (Promega Corporation) which includes the use of two
reporters, firefly and Renilla luciferases. The activity of
the co-transfected control reporter (Renilla luciferase)
provided an internal control to normalize the results.
Transwell assay
Transwell chambers were used for the Transwell assay
(Corning). Matrigel (cat. no. E1270; Sigma-Aldrich; Merck KGaA) and
serum-free DMEM medium were used to solidify the upper chamber.
Matrigel was thawed at 4˚C overnight. Matrigel was then diluted
with serum-free DMEM medium to a Matrigel concentration of 300
µl/ml mixture at 4˚C. Upper chambers were pre-coated with the
mixture, then pre-warmed at 37˚C. After 1 h, 200 µl cell suspension
(2x104) containing 1% FBS was added to the upper
chamber, whereas 500 µl DMEM medium containing 10% FBS was added to
the lower chamber. After 24 h, the non-invading cells were removed
from the upper chamber and cells were fixed with 4% formaldehyde
for 10 min at 4˚C and stained with 0.05% crystal violet for 30 min
at room temperature. Five fields were randomly chosen and observed
under an inverted light microscope (Eclipse Ts2; Nikon Corporation)
at x20 magnification and the number of invasive cells was
calculated.
Wound healing assay
Cells were seeded onto 6-well plates at the density
of 5x105 cells per well. Once the cell density reached
~80%, a 200-µl pipette tip was used to create a vertical wound on
the cell layer, and detached cells were washed away with PBS.
Images were captured at 0 h and at 48 h under a light microscope
(Eclipse Ni-U; Nikon Corporation) at x40 magnification and the cell
migration distances was calculated.
Statistical analysis
Data were presented as the means ± standard
deviation of three independent experiments. Data were analyzed
using SPSS 22.0 software (SPSS, Inc.). Paired t-test was used to
compare tumor group and adjacent normal tissue group and others
significant difference between two groups was compared with
unpaired t-test. Multiple comparison was performed using one-way
ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-141-3p and PBX1 in
OSCC tissues and cell lines
RT-qPCR, western blotting and IHC were used to
analyze the expression of miR-141-3p and the protein expression of
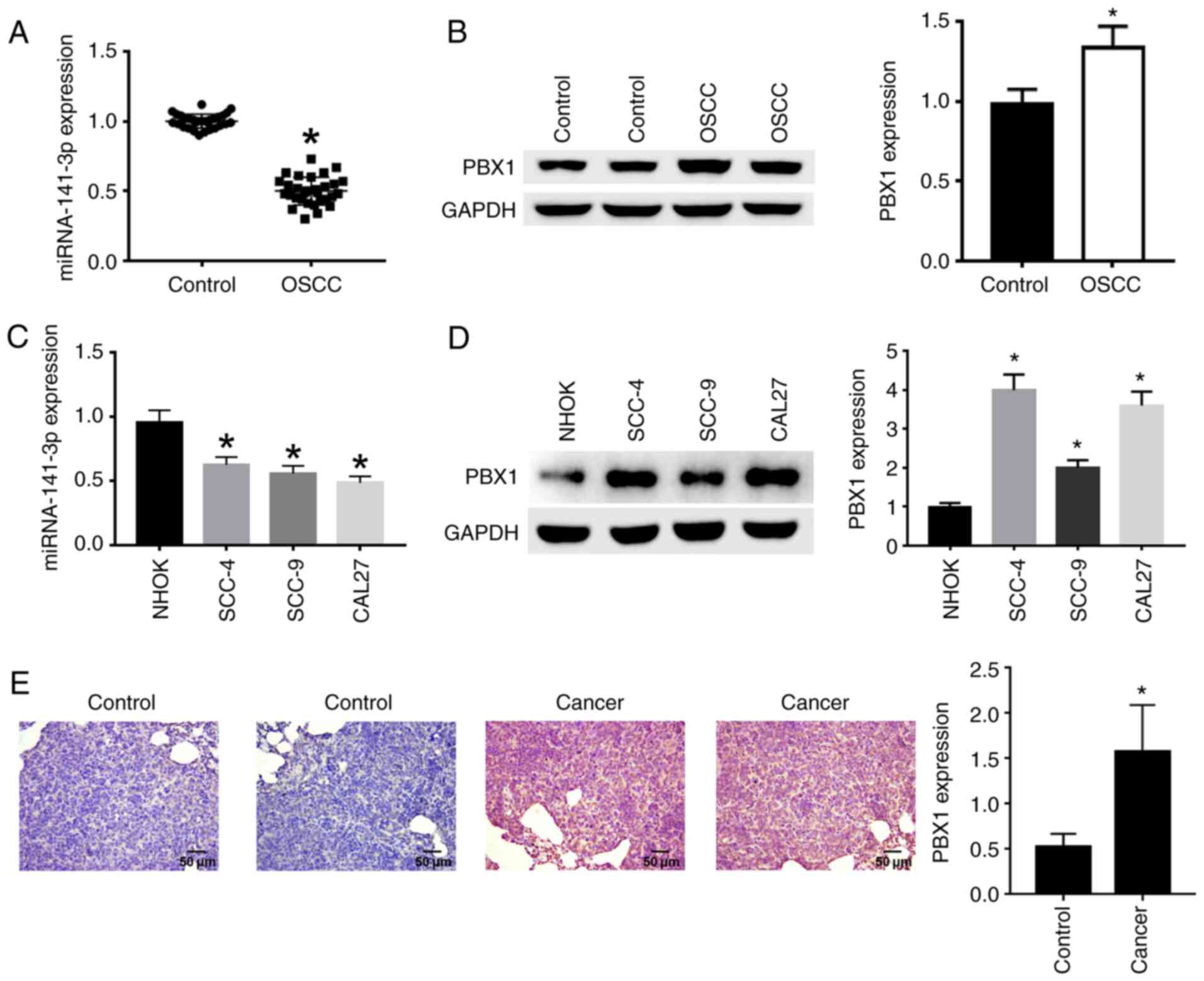
PBX1. The results demonstrated that miR-141-3p expression in OSCC
tissues (Fig. 1A) and cell lines
(Fig. 1C) was significantly
decreased compared with non-cancerous tissues and NHOK cell line,
respectively. In addition, PBX1 protein expression was
significantly increased in OSCC tissues (Fig. 1B) and cell lines (Fig. 1D) compared with non-cancerous
tissues and NHOK cell line. The expression of PBX1 was
significantly higher in CAL27 and SCC-4 cells compared with the
other cell lines (Fig. 1D), and
miR-141-3p expression levels were the lowest in CAL27 cells
compared with the other cell lines (Fig. 1C). Thus, CAL27 cell line was
selected for PBX1 knockdown in subsequent experiments to confirm
the effects of PBX1 in OSCC. Immunohistochemical staining with
anti-PBX1 primary antibody showed that cancer tissues were stained
brown in cytoplasm, corresponding to a positive staining. Control
tissues were stained blue, corresponding to a negative staining
(Fig. 1E). From the above results,
it can be seen that miR-141-3p was downregulated, while PBX1 was
upregulated in OSCC tissues and cell lines.
PBX1 is a potential target of
miR-141-3p in OSCC
TargetScanHuman v7.2 online software was used to
analyze the binding sites of miR-141-3p and PBX1. The results
demonstrated that the sequence of the 3'-untranslated region (UTR)
of the PBX1 gene was complementary to the sequence of miR-141-3p
(Fig. 2A). Furthermore, the
co-existence of PBX1-Wt and miR-141-3p mimic in the cells
significantly inhibited luciferase activity, while the co-existence
of PBX1-Mut and miR-141-3p mimic or co-existence of PBX1-Wt and
miR-141-3p mimic NC had no inhibition effect (Fig. 2B).
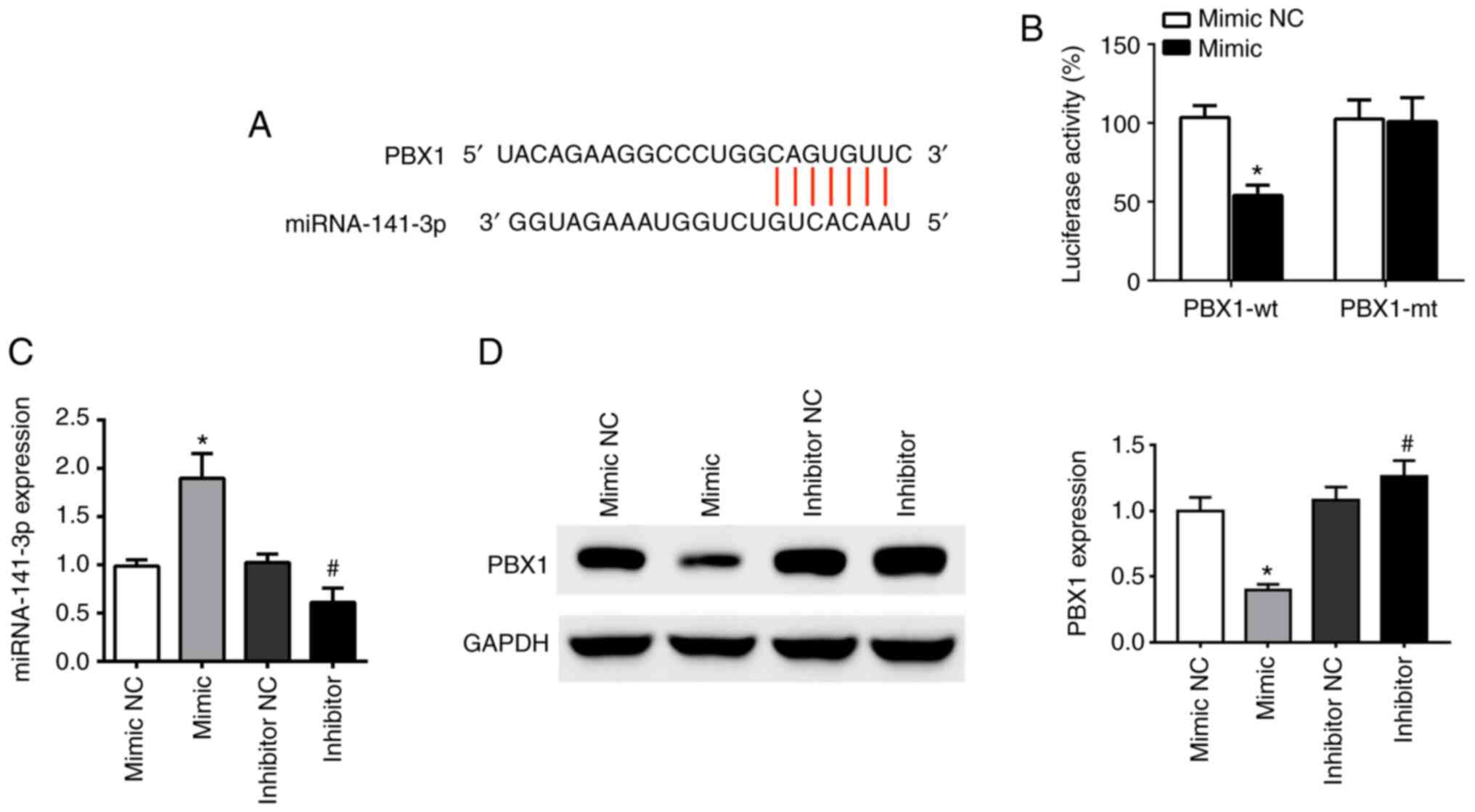 | Figure 2PBX1 is a direct target of miR-141-3p
in CAL27 cells. (A) Predicted duplex formation of PBX1-wt-3'-UTR
and miR-141-3p. (B) Luciferase gene reporter assay results in cells
co-transfected with PBX1-wt and miR-141-3p mimic or mimic NC, or
co-transfected with PBX1-mt and miR-141-3p mimic or mimic NC. (C)
Expression levels of miR-141-3p in cells transfected with
miR-141-3p mimic, mimic NC, miR-141-3p inhibitor or inhibitor NC
was analyzed by RT-qPCR. (D) PBX1 expression in cells transfected
with miR-141-3p mimic, mimic NC, miR-141-3p inhibitor or inhibitor
NC was analyzed by western blotting. *P<0.05 vs.
mimic NC. #P<0.05 vs. inhibitor NC. wt, wild type;
mt, mutant; miRNA/miR, microRNA; NC, negative control; UTR,
untranslated region; PXB1, pre-B-cell leukaemia homeobox-1. |
To determine the relationship between miR-141-3p and
PBX1, the expression of miR-141-3p mRNA and PBX1 protein was
detected using RT-qPCR and western blotting, respectively,
following CAL27 cell transfection miR-141-3p mimic or inhibitor.
The results demonstrated that transfections with miR-mimic and
inhibitor were successful in CAL27 cells. The level of miR-141-3p
was upregulated by miR-141-3p mimic and downregulated by miR-141-3p
inhibitor, compared with corresponding NC (Fig. 2C). Furthermore, PBX1 protein
expression was significantly decreased following transfection with
miR-141-3p mimics, whereas miR-141-3p inhibitor had the opposite
effect (Fig. 2D). These findings
indicated that miR-141-3p could negatively regulate PBX1 protein
expression in CAL27 cells, suggesting that PBX1 may be a target of
miR-141-3p in OSCC cells.
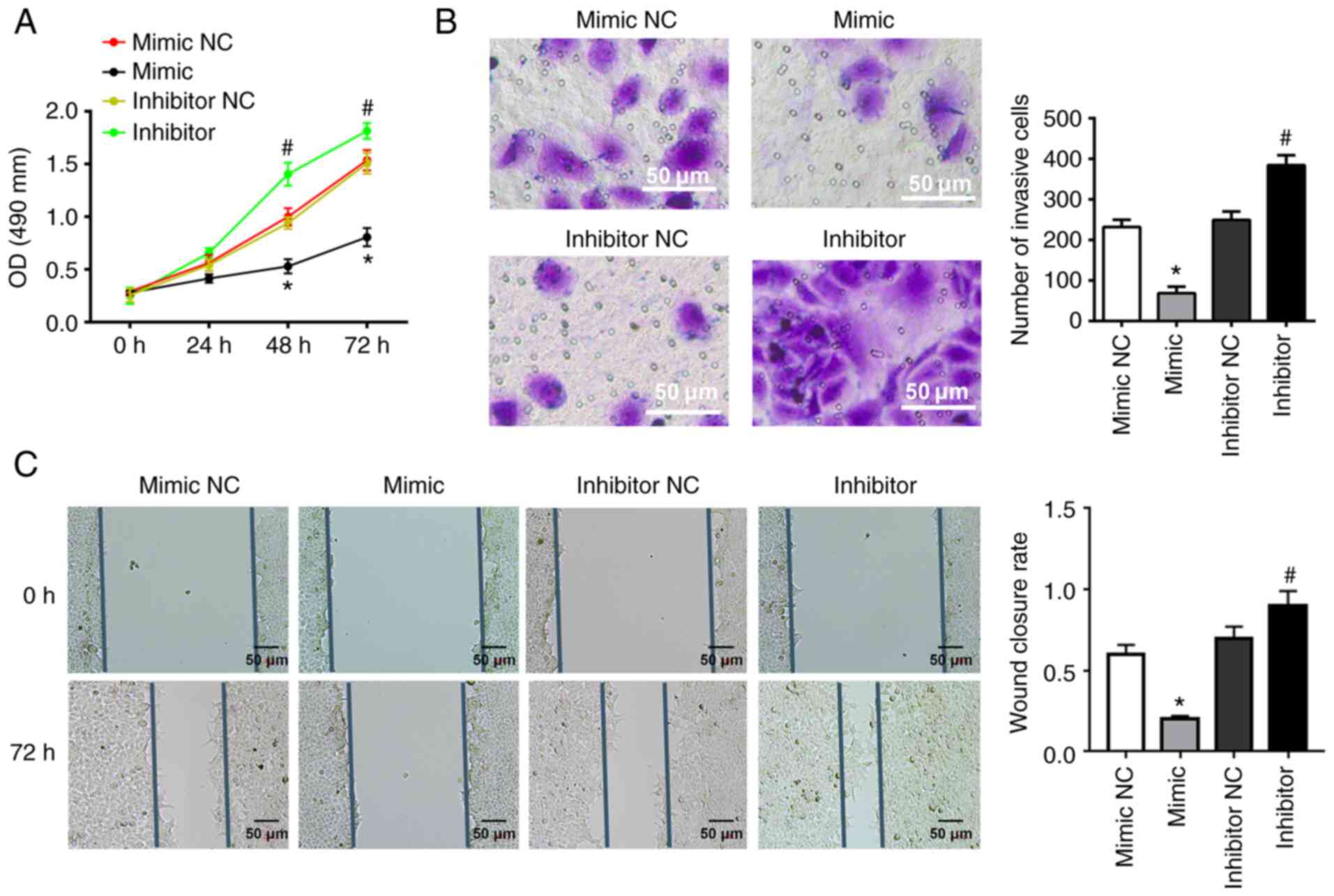
miR-141-3p inhibits CAL27 cell
invasion, proliferation and migration
To determine the effects of miR-141-3p on CAL27
cells, miR-141-3p mimic or inhibitor and corresponding NC were
transfected into cells. miR-141-3p mimic significantly inhibited
the proliferation of CAL27 cells, as determined by MTT assay;
however, miR-141-3p inhibitor had the opposite effect (Fig. 3A). In addition, miR-141-3p mimic
significantly inhibited the invasion and migration of CAL27 cells,
while miR-141-3p inhibitor exhibited the opposite effects,
according to results from Transwell and wound healing assays
(Fig. 3B and C).
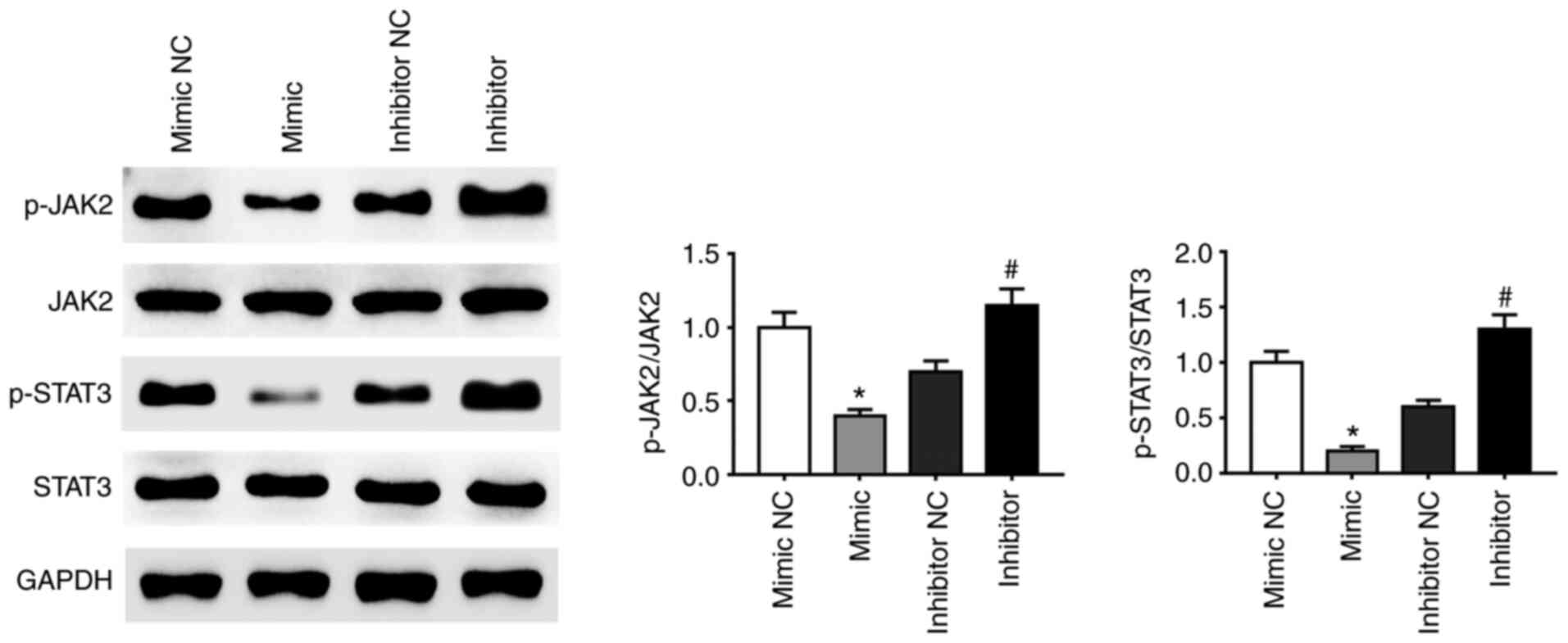
miRNA-141-3p inhibitor activates the
JAK2/STAT3 pathway
A previous study demonstrated that activation of the
JAK2-STAT3 signaling pathway enhanced proliferation, invasion and
metastasis of HCC cells (38). The
present study demonstrated that the phosphorylation of JAK2 and
STAT3 proteins was affected by miR-141-3p mimic and inhibitor.
Expression of p-JAK2 and p-STAT3 was decreased in CAL27 cells
transfected with miRNA-141-3p mimic compared with NC whereas
miR-141-3p inhibitor had the opposite effect (Fig. 4). These findings suggested that the
JAK2/STAT3 signaling pathway may be activated following inhibition
of miR-141-3p expression in CAL27 cells.
si-PBX1 weakens the effects of
miRNA-141-3p inhibitor on JAK2/STAT3 pathway and cell behavior in
CAL27 cells
si-PBX1, miR-141-3p inhibitor and the combination of
PBX1-siRNA + miR-141-3p inhibitor were transfected into CAL27
cells. The results from western blotting confirmed the successful
downregulation of PBX1 in the si-PBX1 group in comparison with the
si-NC group, and also a decreased expression of p-JAK2 and p-STAT3.
The combined transfection of miR-141-3p inhibitor and si-PBX1
increased the expression of p-JAK2, p-STAT3 and PBX1, whereas
si-PBX1 transfection attenuated the increase in constitutive levels
of p-JAK2, p-STAT3 and PBX1 expression induced by miR-141-3p
inhibitor (Fig. 5A).
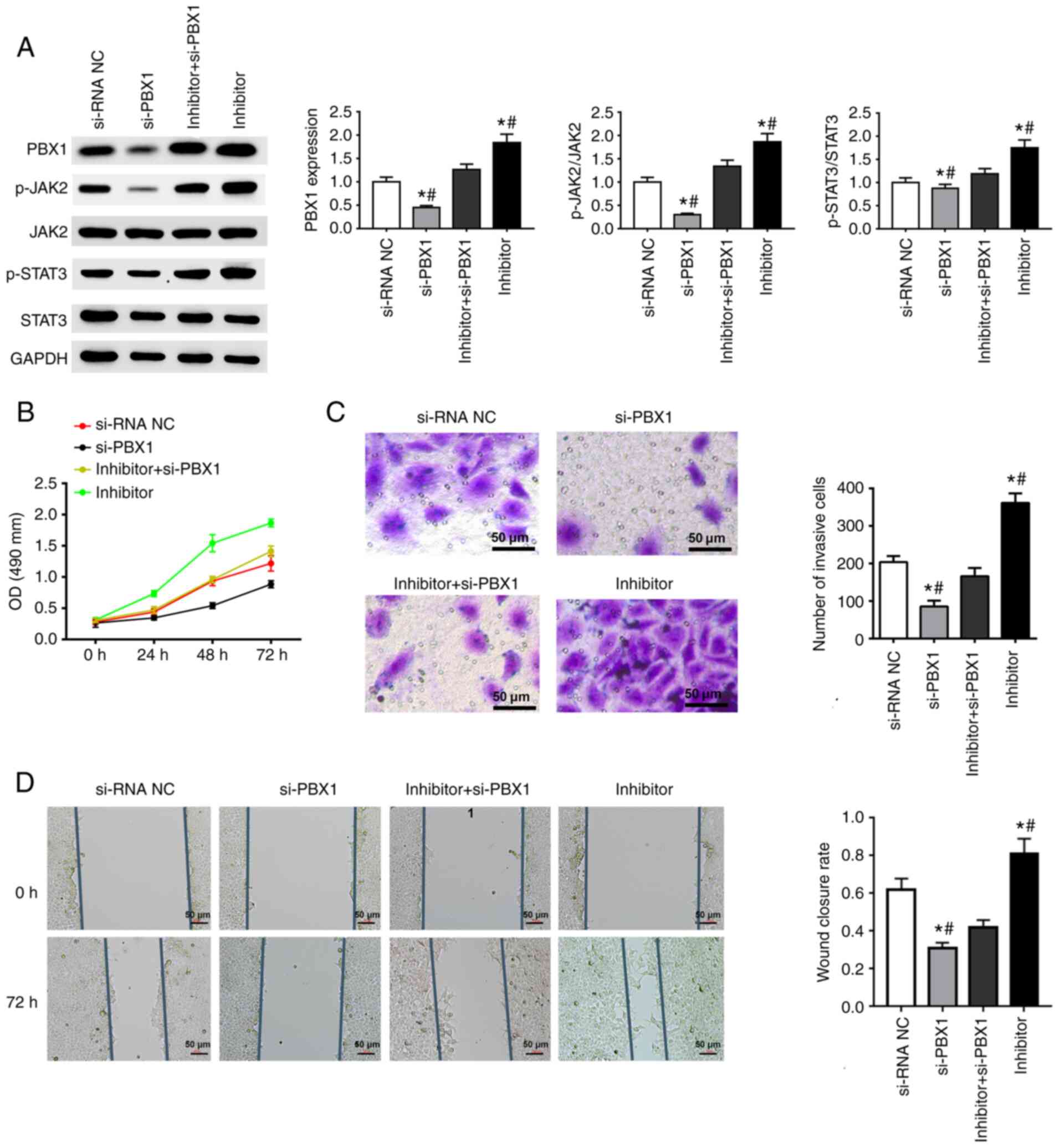 | Figure 5Effect of si-PBX1 and miRNA-141-3p
inhibitor on JAK2/STAT3 signaling pathway and CAL27 cell invasion,
proliferation and migration. (A) Western blotting was used to
determine the expression of PBX1, JAK2, STAT3, p-JAK2 and p-STAT3.
Quantitative data are shown for PBX1, p-JAK2/JAK2 and p-STAT3/STAT3
in CAL27 cells transfected with si-PBX1 or miR-141-3p inhibitor.
(B) Cell proliferation assay was used to detected CAL27 cells
transfected with si-PBX1, siRNA NC, inhibitor + si-PBX1 or
inhibitor. (C) Invasion assay results of CAL27 cells transfected
with si-PBX1, siRNA NC, inhibitor + si-PBX1 or inhibitor. Scale
bar=50 µm. (D) Wound healing assay results of CAL27 cells
transfected with si-PBX1, siRNA NC, inhibitor + si-PBX1 or
inhibitor. Scale bar=50 µm. *P<0.05 vs. si-NC.
#P<0.05 vs. miRNA-141-3p inhibitor + si-PBX1. miR,
microRNA; NC, negative control; si, small interfering; JAK2, janus
kinase 2; STAT3, signal transducer and activator of transcription 3
signaling pathway; PXB1, pre-B-cell leukaemia homeobox-1; OD,
optical density. |
The results from MTT, Transwell and wound healing
assays suggested that miR-141-3p inhibitor could significantly
enhance the cell proliferation, migration and invasion of CAL27
cells. Furthermore, PBX1 downregulation inhibited the invasion,
proliferation and migration of CAL27 cells. In addition, in the
combination group, si-PBX1 notably weakened the promoting effects
of miR-141-3p inhibitor on cell invasion, proliferation and
migration (Fig. 5B-D). Taken
together, these results suggested that si-PBX1 could weaken the
effects of miRNA-141-3p inhibitor on the JAK2/STAT3 pathway and
CAL27 cell behavior.
Discussion
OSCC is considered as a common malignant tumor and
its incidence rate is continuously rising in developing countries
(31). In OSCC, invasion occurs
before metastasis, which is an important reason why OSCC leads to
mortality (39). Determining novel
targeted treatments for OSCC is therefore crucial, and this can be
achieved by exploring the molecular properties of OSCC. The
dysregulation of miRs contributes to the development and metastasis
of many types of tumor, such as lung, breast, gastric, ovarian and
liver cancers (40). Increasing
evidence indicates that certain miRs can regulate the metastasis
and invasion of OSCC cells, such as microRNA-22, microRNA-205-5p
and miRNA-491-5p (41-43).
miR-141-3p is a member of the miR-200 family and exists in two
clusters on chromosomes 1 and 12, named miRs-200b/a/429 and
miRs-200c/141. The five miRs form the miR-200 family (44). miR-141 has the same sequence as
miR-200a, with only one nucleotide difference between miR-141 and
miR-200a (45). Previous studies
have reported that miR-141 is involved in ovarian tumorigenesis,
colon cancer, small cell lung cancer and renal cell carcinoma
(46-48).
However, the effects of miR-141-3p on OSCC remain
unclear. Thus, the effects of miR-141-3p on OSCC were analyzed in
the present study. The results from the present study demonstrated
that miR-141-3p was downregulated in OSCC tissues and cells
compared with non-cancerous controls, indicating its potential role
in OSCC tumorigenesis. Furthermore, consistent with a previous
study, PBX1 expression was increased in OSCC cells and tissues
(8).
miRs are short endogenous single chain RNA molecules
that can bind to 3'UTR to regulate the post-transcriptional
expression of miR of target genes (49). In addition, target genes can be
activated or inhibited by miRs binding to their promoters after
transcription (50,51). The result from luciferase reported
assay demonstrated that miR-141-3p mimic significantly suppressed
the activity of PBX1-Wt, whereas the activity of PBX1-Mut was not
modified by miR-141-3p mimic. PBX1 was predicted to be a direct
target of miR-141-3p. miR-141-3p has been reported to inhibit the
occurrence and development of numerous types of tumors. For
example, miR-141-3p was shown to inhibit the migration and invasion
of HCC cells (52). The present
results demonstrated that miR-141-3p overexpression could inhibit
the invasion, proliferation and migration of OSCC cells.
The present study demonstrated that miR-141-3p
expression was decreased in OSCC cells, thereby promoting cell
invasion, proliferation and migration. It was previously reported
that the JAK2/STAT3 signaling pathway is related to tumor
metastasis (53,54). In OSCC, the JAK2/STAT3 signaling
pathway has also been found to mediate the process of cancer
metastasis (55). Therefore, we
hypothesized that miR-141-3p could inhibit the invasion,
proliferation and migration of OSCC cells via the JAK2/STAT3
signaling pathway. The results demonstrated that miR-141-3p
inhibitor could activate the JAK2/STAT3 signaling pathway in OSCC
cells, indicating that miR-141-3p and the JAK2/STAT3 signaling
pathway may have a role in OSCC tumorigenesis. Furthermore, PBX1
expression has been found to be abnormally high in a variety of
human tissues, and PXB1 gene was shown to play a key role in tumor
proliferation (56). The results
from the present study demonstrated that PBX1 downregulation could
attenuate OSCC cell invasion, proliferation and migration. It has
been previously reported that PBX1 downregulation can inhibit the
expression of STAT3 and p-STAT3 in esophageal squamous cancer
(42). The present study thus
speculated that miR-141-3p could be related to PBX1, and the
interaction between PBX1 and miR-141-3p was subsequently
investigated. The results demonstrated that miR-141-3p exerted its
function in OSCC cells by targeting PBX1, when PBX1 knockdown
attenuated the invasion, proliferation and migration caused by
miR-141-3p knockdown. Taken together, the findings from our study
revealed that miR-141-3p may inhibit the invasion, proliferation
and migration of OSCC cells through the JAK2/STAT3 signaling
pathway by targeting PBX1.
In summary, the present study demonstrated that PBX1
may be considered as a direct target of miR-141-3p, and that
miR-141-3p may interact with JAK2 and its downstream signaling
kinases, such as STAT3, by inhibiting the catalytic activities of
kinases, thus inhibiting the invasion, proliferation and migration
of OSCC cells. This study provided a novel target that may be
useful for monitoring the progression of OSCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC and WS designed the study and performed
experiments. KT and WS performed experiments, confirmed the
authenticity of all the raw data, interpreted the results and wrote
the manuscript. JX and YT interpreted the results and contributed
to the preparation, review and revision of the manuscript. SW
provided research funding, modified the study design, provided
guidance for the experiments and revised the manuscript. All
authors agreed to be accountable for the content of the work. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Clinical Ethical
Committee of Lishui University (approval no. 201803). Written
informed consent was provided by all the patients or their
relatives for the use of their tissues in experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Markopoulos AK: Current aspects on oral
squamous cell carcinoma. Open Dent J. 6:126–130. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wei J and Wu J, Xu W, Nie H, Zhou R, Wang
R, Liu Y, Tang G and Wu J: Salvianolic acid B inhibits glycolysis
in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α
signaling pathway. Cell Death Dis. 9(599)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in
early breast cancer: Meta-analyses of individual patient data from
randomised trials. Lancet. 386:1353–1361. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim SM, Jeong D, Min KK, Sang SL and Lee
SK: Two different protein expression profiles of oral squamous cell
carcinoma analyzed by immunoprecipitation high-performance liquid
chromatography. World J Surg Oncol. 15(151)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Radhika T, Jeddy N, Nithya S and
Muthumeenakshi RM: Salivary biomarkers in oral squamous cell
carcinoma-an insight. J Oral Biol Craniofac Res. 6 (Suppl
1):S51–S54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sasahira T, Kirita T and Kuniyasu H:
Update of molecular pathobiology in oral cancer: A review. Int J
Clin Oncol. 19:431–436. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu N, Zhang Z, Li L, Shen X, Sun B, Wang
R, Zhong H, Shi Q, Wei L, Zhang Y, et al: MicroRNA-181 regulates
the development of ossification of posterior longitudinal ligament
via Epigenetic modulation by targeting PBX1. Theranostics.
10:7492–7509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Platais C, Radhakrishnan R, Niklander
Ebensperger S, Morgan R, Lambert DW and Hunter KD: Targeting
HOX-PBX interactions causes death in oral potentially malignant and
squamous carcinoma cells but not normal oral keratinocytes. BMC
Cancer. 18(723)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He C, Wang Z, Zhang L, Yang L, Li J, Chen
X, Zhang J, Chang Q, Yu Y, Liu B and Zhu Z: A hydrophobic residue
in the TALE homeodomain of PBX1 promotes epithelial-to-mesenchymal
transition of gastric carcinoma. Oncotarget. 8:46818–46833.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Risolino M, Mandia N, Iavarone F, Dardaei
L, Longobardi E, Fernandez S, Talotta F, Bianchi F and Pisati F:
Transcription factor PREP1 induces EMT and metastasis by
controlling the TGF-β-SMAD3 pathway in non-small cell lung
adenocarcinoma. Proc Natl Acad Sci USA. 111:E3775–E3784.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
Oncogene. Cell. 98:295–303. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Degboé Y, Rauwel B, Baron M, Boyer JF,
Ruyssen-Witrand A, Constantin A and Davignon JL: Polarization of
rheumatoid macrophages by TNF targeting through an IL-10/STAT3
mechanism. Front Immunol. 10(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu J, Niu P, Zhao Y, Cheng Y, Chen W, Lin
L, Lu J, Cheng X and Xu Z: Impact of miR-223-3p and miR-2909 on
inflammatory factors IL-6, IL-1β, and TNF-α, and the
TLR4/TLR2/NF-κB/STAT3 signaling pathway induced by
lipopolysaccharide in human adipose stem cells. PLoS One.
14(e0212063)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Wang B, Liu T, Wu JC, Luo SZ, Chen R, Lu
LG and Xu MY: STAT3 aggravates TGF-β1-induced hepatic
epithelial-to-mesenchymal transition and migration. Biomed
Pharmacother. 98:214–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li YL, Wu LW, Zeng LH, Zhang ZY, Wang W,
Zhang C and Lin NM: ApoC1 promotes the metastasis of clear cell
renal cell carcinoma via activation of STAT3. Oncogene.
39:6203–6217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen B and Ling CH: Long noncoding RNA
AK027294 acts as an oncogene in non-small cell lung cancer by
up-regulating STAT3. Eur Rev Med Pharmacol Sci. 23:1102–1107.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou W, Bi X, Gao G and Sun L: miRNA-133b
and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling
pathway in human renal carcinoma cells. Biomed Pharmacother.
84:722–729. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Y, Shao Z, Jiang E, Zhou X, Wang L,
Wang H, Luo X, Chen Q, Liu K and Shang Z: CCL21/CCR7 interaction
promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3
signaling pathway. J Cell Physiol. 235:5995–6009. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin XM, Chen H and Zhan XL: miR-203
regulates JAK-STAT pathway in affecting pancreatic cancer cells
proliferation and apoptosis by targeting SOCS3. Eur Rev Med
Pharmacol Sci. 23:6906–6913. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang CS, Lin Y, Sun FB, Gao J, Han B and
Li SJ: miR-409 down-regulates Jak-Stat pathway to inhibit
progression of liver cancer. Eur Rev Med Pharmacol Sci. 23:146–154.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hong YG, Xin C, Zheng H, Huang ZP, Yang Y,
Zhou JD, Gao XH, Hao L, Liu QZ, Zhang W and Hao LQ: miR-365a-3p
regulates ADAM10-JAK-STAT signaling to suppress the growth and
metastasis of colorectal cancer cells. J Cancer. 11:3634–3644.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yates L, Norbury C and Gilbert RC: The
long and short of microRNA. Cell. 153:516–519. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cai Z, Hao XY and Liu FX: MicroRNA-186
serves as a tumor suppressor in oral squamous cell carcinoma by
negatively regulating the protein tyrosine phosphatase SHP2
expression. Arch Oral Biol. 89:20–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arunkumar G, Deva Magendhra Rao AK,
Manikandan M, Prasanna Srinivasa Rao H, Subbiah S, Ilangovan R,
Murugan AK and Munirajan AK: Dysregulation of miR-200 family
microRNAs and epithelial-mesenchymal transition markers in oral
squamous cell carcinoma. Oncol Lett. 15:649–657. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shao Y, Qu Y, Dang S, Yao B and Ji M:
miR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by
targeting c-Myc and Cdk6. Cancer Cell Int. 13(51)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Della Vittoria Scarpati G, Calura E, Di
Marino M, Romualdi C, Beltrame L, Malapelle U, Troncone G, De
Stefano A, Pepe S, De Placido S, et al: Analysis of differential
miRNA expression in primary tumor and stroma of colorectal cancer
patients. Biomed Res Int. 2014(840921)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Verrando P, Capovilla M and Rahmani R:
Trans-nonachlor decreases miR-141-3p levels in human melanocytes in
vitro promoting melanoma cell characteristics and shows a
multigenerational impact on miR-8 levels in Drosophila.
Toxicology. 368-369:129–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li M, Huang H, Cheng F, Hu X and Liu J:
miR-141-3p promotes proliferation and metastasis of nasopharyngeal
carcinoma by targeting NME1. Adv Med Sci. 65:252–258.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ishibashi O, Akagi I, Ogawa Y and Inui T:
miR-141-3p is upregulated in esophageal squamous cell carcinoma and
targets pleckstrin homology domain leucine-rich repeat protein
phosphatase-2, a negative regulator of the PI3K/AKT pathway.
Biochem Biophys Res Commun. 501:507–513. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hou X, Yang L, Jiang X, Liu Z, Li X, Xie
S, Li G and Liu J: Role of microRNA-141-3p in the progression and
metastasis of hepatocellular carcinoma cell. Int J Biol Macromol.
128:331–339. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qiu X, Ye Q, Sun M, Wang L, Tan Y and Wu
G: Saturated hydrogen improves lipid metabolism disorders and
dysbacteriosis induced by a high-fat diet. Exp Biol Med (Maywood).
245:512–521. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu Y, Yuan T, Wang WW, Ge PL, Gao ZQ,
Zhang G, Tang Z, Dang XW, Zhao YF, Zhang JY and Jiang GZ: Long
noncoding RNA HOST2 promotes epithelial-mesenchymal transition,
proliferation, invasion and migration of hepatocellular carcinoma
cells by activating the JAK2-STAT3 signaling pathway. Cell Physiol
Biochem. 51:301–314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Xu W, Sun D, Wang Y, Zheng X, Li Y, Xia Y
and Teng Y: Inhibitory effect of microRNA-608 on lung cancer cell
proliferation, migration, and invasion by targeting BRD4 through
the JAK2/STAT3 pathway. Bosn J Basic Med Sci. 20:347–356.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Huang WC, Chan SH, Jang TH, Chang JW, Ko
YC, Yen TC, Chiang SL, Chiang WF, Shieh TY, Liao CT, et al:
miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral
squamous cell carcinoma invasion and metastasis. Cancer Res.
74:751–564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nagai H, Hasegawa S, Uchida F, Terabe T,
Ishibashi Kanno N, Kato K, Yamagata K, Sakai S, Kawashiri S, Sato
H, et al: MicroRNA-205-5p suppresses the invasiveness of oral
squamous cell carcinoma by inhibiting TIMP-2 expression. Int J
Oncol. 52:841–850. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Feng X, Luo Q, Wang H, Zhang H and Chen F:
MicroRNA-22 suppresses cell proliferation, migration and invasion
in oral squamous cell carcinoma by targeting NLRP3. J Cell Physiol.
233:6705–6713. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Renthal NE, Williams KC and Mendelson CR:
MicroRNAs-mediators of myometrial contractility during pregnancy
and labour. Nat Rev Endocrinol. 9:391–401. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Olsen PH and Ambros V: The lin-4
regulatory RNA controls developmental timing in Caenorhabditis
elegans by blocking LIN-14 protein synthesis after the initiation
of translation. Dev Biol. 216:671–680. 1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mao S, Lu Z, Zheng S, Zhang H, Zhang G,
Wang F, Huang J, Lei Y, Wang X, Liu C, et al: Exosomal miR-141
promotes tumor angiogenesis via KLF12 in small cell lung cancer. J
Exp Clin Cancer Res. 39(193)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen X, Lou N, Anming R, Qiu B, Yun Y,
Wang X, Du Q, Ruan H, Han W, Wei H, et al: miR-224/miR-141 ratio as
a novel diagnostic biomarker in renal cell carcinoma. Oncol Lett.
16:1666–1674. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Moriya Y, Nohata N, Kinoshita T, Mutallip
M, Okamoto T, Yoshida S, Suzuki M, Yoshino I and Seki N: Tumor
suppressive microRNA-133a regulates novel molecular networks in
lung squamous cell carcinoma. J Hum Genet. 57:38–45.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Janowski BA, Younger ST, Hardy DB, Ram R,
Huffman KE and Corey DR: Activating gene expression in mammalian
cells with promoter-targeted duplex RNAs. Nat Chem Biol. 3:166–173.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Khraiwesh B, Arif MA, Seumel GI, Ossowski
S, Weigel D, Reski R and Frank W: Transcriptional control of gene
expression by microRNAs. Cell. 140:111–122. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu Y, Ding Y, Huang J, Wang S, Ni W, Guan
J, Li Q, Zhang Y, Ding Y, Chen B and Chen L: miR-141 suppresses the
migration and invasion of HCC cells by targeting Tiam1. PLoS One.
9(e88393)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ahn JH, Choi YS and Choi JH: Leptin
promotes human endometriotic cell migration and invasion by
up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol
Hum Reprod. 21:792–802. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu F, Zhang T, Zou S, Jiang B and Hua D:
B7-H3 promotes cell migration and invasion through the
Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med
Rep. 12:5455–5460. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang Y, Jing Y, Ding L, Zhang X, Song Y,
Chen S, Zhao X, Huang X, Pu Y, Wang Z, et al: Epiregulin reprograms
cancer-associated fibroblasts and facilitates oral squamous cell
carcinoma invasion via JAK2-STAT3 pathway. J Exp Clin Cancer Res.
38(274)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yu D, Ma Y, Feng C, Ma Z, Guo J, Chen H,
He T, Guo J, Sun X, Qin Q, et al: PBX1 increases the
radiosensitivity of oesophageal squamous cancer by targeting of
STAT3. Pathol Oncol Res. 26:2161–2168. 2020.PubMed/NCBI View Article : Google Scholar
|