Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a
common complication of neonatal asphyxia that may lead to cerebral
hypoxic-ischemic damage, cerebral palsy, intellectual retardation
and epilepsy and is a leading cause of neonatal mortality and
disability (1-3).
The pathogenesis of HIE is complex and has been proposed to be
associated with abnormal hemorheology (4). Compromised integrity of the
blood-brain barrier (BBB) caused by ischemia/reperfusion (IR)
injury may lead to secondary brain damage after infarction, with
symptoms including brain edema and hemorrhage (5). Preserving the integrity of the BBB is
considered to be one of the most important therapeutic strategies
for alleviating cerebral infarction injury (6). Cerebrovascular endothelial cells
normally create the BBB between brain and the rest of the
circulation and exhibit a variety of secretory functions (7). Therefore, microvascular endothelial
cell injury is closely associated with the occurrence and
development of cerebrovascular diseases (8,9).
A previous study revealed a significant difference
either in the upregulation or downregulation of the expression
levels of 255 long non-coding RNAs (lncRNAs) in serum samples
isolated from rats that underwent middle cerebral artery occlusion,
compared with a sham group (10).
Hox transcript antisense intergenic RNA (HOTAIR) is an important
lncRNA that is encoded by the antisense chain of the HOXC11 gene in
the 2q13.13 chromosomal region and transcribed by RNA polymerase
(11). Emerging evidence has
suggested that lncRNAs can serve important roles in a number of
diseases, including atherosclerosis, stroke and aneurysm (12,13). A
previous study demonstrated that the expression level of lncRNA
HOTAIR was upregulated in neurons following cerebral IR injury,
compared with healthy neurons (1).
In addition, lncRNA HOTAIR downregulation was found to promote
burn-induced angiogenesis in umbilical vein endothelial cells
(14). However, to the best of our
knowledge, the role of HOTAIR in oxygen-glucose
deprivation/reperfusion (OGD/R)-induced brain endothelial cell
injury has not been previously studied.
Enhancer of zeste homolog 2 (EZH2) is a histone
methyltransferase that has been documented to be involved in IR
injury (15). Previous studies have
demonstrated that EZH2 knockdown conferred a neuroprotective role
in ischemic brain injury (16-18).
Another study revealed that EZH2 inhibition can regulate microglial
activation and inflammation following hypoxic-ischemic brain
injury, where it could promote autophagy through the PTEN/AKT/mTOR
signaling pathway (19).
Furthermore, following IR injury, the expression level of EZH2 was
found to be upregulated in microglia, compared with a sham group
(16). It has also been proposed
that the protein stability of EZH2 is regulated by several lncRNAs
including lncRNA ANCR and lncRNA FAM83C-AS1(20). Previous studies demonstrated that
the interaction between EZH2 and different lncRNAs, such as lncRNA
ANCR and lncRNA HERES can suppress cancer cell invasion, including
breast cancer and esophageal squamous cell carcinoma (21,22).
Therefore, the present study aimed to investigate
the role of HOTAIR in neonatal HIE and its association with EZH2 in
OGD/R-induced human brain microvascular endothelial cells
(hBMVECs).
Materials and methods
Clinical data
In the present study children who met the diagnostic
criteria of neonatal HIE as described previously (23) were enrolled between January 2020 and
January 2021, including 2 males and 3 females (age, <24 h), with
a gestational age of 38-42 weeks and a birth weight of 2.6-3.9 kg.
All children had a history of asphyxia, caused by umbilical cord
prolapse and compression and around the neck. All children were
delivered in the Huazhong University of Science and Technology
Union Shenzhen Hospital (Shenzhen, China).
In addition, five normal-term newborns, including
two males and three females, who were delivered in the Huazhong
University of Science and Technology Union Shenzhen Hospital
(Shenzhen, China) during the same time period, were also enrolled.
In the five HIE cases aforementioned, the respective mothers did
not experience intrauterine infections or intrauterine or
postpartum asphyxia, and did not receive immune agents or blood
products, including various human plasma protein products during
gestation.
A total of 3 ml venous blood was collected from
neonates in both groups after birth. Following centrifugation at
400 x g for 40 min at 25˚C, the serum was collected and stored in a
-70˚C refrigerator for subsequent experiments. The present study
was approved by the Ethics Committee of Huazhong University of
Science and Technology Union Shenzhen Hospital. Written informed
consent for blood collection was obtained from the legal guardians
of each child.
Cell culture
Human brain microvascular endothelial cells
(hBMVECs; cat. no. CP-H124; Procell Life Science & Technology
Co., Ltd.) were cultured in Procell Life Science & Technology
Co., Ltd. medium supplemented with 10% FBS and 100 µl/ml
penicillin/streptomycin (all Procell Life Science & Technology
Co., Ltd.) at 37˚C in a water-saturated atmosphere of 5%
CO2 and 95% air. The cells were dispersed with 0.25%
trypsin and were then sub-cultured. Cells in the logarithmic growth
phase were selected for subsequent experiments.
Establishment of OGD/R injury in vitro
model
hBMVECs were first seeded into 35-mm diameter dishes
at a density of 1x106 cells/well. After 24 h at 37˚C,
the cells adhered to the dish walls. Following washing with
sugar-free Earle's balanced salt solution (sugar-free OGD solution;
Thermo Fisher Scientific, Inc.), the medium was replaced with
glucose-free, serum-free culture medium at 37˚C. The cells were
then cultured in a modified closed OGD tank at an atmosphere of 1%
O2, 5% CO2 and balanced N2 for 2 h
at 37˚C before being incubated in Procell Life Science &
Technology Co., Ltd. medium in a humidified incubator at 37˚C for
re-oxygenation for 12, 24 or 48 h. The cells in the control were
cultured in serum-free medium at 37˚C with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR assay was performed to measure the
expression levels of the target genes in each group after RNA
extraction using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) for 10 min, followed by centrifugation at 4˚C and
13,514 x g (centrifugation radius, 10 cm) for 15 min. Subsequently,
the cell-TRIzol® mixture was transferred into 1.5-ml
microcentrifuge tubes without RNase. The reverse transcription
process was performed with a First Strand cDNA Synthesis Kit (cat.
no. K1073; APExBIO Technology LLC) and then qPCR was performed
using a qPCR kit (Sigma-Aldrich; Merck KGaA). Thermocycling
conditions were as following: Initial denaturation at 95˚C for 3
min, followed by 39 cycles of 95˚C for 30 sec, 55˚C for 20 sec and
72˚C for 20 sec. GAPDH served as the internal reference gene and
results were analyzed using the 2-ΔΔCq method (24). The primers sequences used in this
study are as follows: HOTAIR forward, 5'-ATAGGCAAATGTCAGAGGGTT-3'
and reverse, 5'-ATTCTTAAATTGGGCTGGGTC-3'; EZH2 forward,
5'-AATCAGAGTACATGCGACTGAGA-3' and reverse,
5'-GCTGTATCCTTCGCTGTTTCC-3'; zonula occludens-1 (ZO-1) forward,
5'-CAACATACAGTGACGCTTCACA-3' and reverse,
5'-CACTATTGACGTTTCCCCACTC-3'; occludin forward,
5'-ACAAGCGGTTTTATCCAGAGTC-3' and reverse,
5'-GTCATCCACAGGCGAAGTTAAT-3'; Claudin-5 forward,
5'-CTCTGCTGGTTCGCCAACAT-3' and reverse, 5'-CAGCTCGTACTTCTGCGACA-3';
vascular endothelial (VE)-cadherin forward,
5'-TTGGAACCAGATGCACATTGAT-3' and reverse,
5'-TCTTGCGACTCACGCTTGAC-3' and GADPH forward,
5'-AAAGATGTGCTTCGAGATGTGT-3' and reverse,
5'-CACTTTGTCAGTTACCAACGTCA-3'.
Western blot analysis
hBMVECs were lysed using RIPA lysis buffer (Beyotime
Institute of Biotechnology). The protein concentration was
calculated using a BCA assay and adjusted at a final concentration
of 0.5 mg/ml. The protein extracts (20 µg) were separated by 10%
SDS-PAGE and subsequently blocked with 5% BSA at room temperature
for 1 h. The expression of the target proteins was detected using
the corresponding antibodies against ZO-1 (cat. no. ab276131;
1:1,000; Abcam), Occludin (cat. no. ab167161; 1:1,000; Abcam),
Claudin-5 (cat. no. ab131259; 1:1,000; Abcam), VE-cadherin
(1:1,000; cat. no. ab232880, Abcam), Bcl-2, Bax (cat. no. ab32503;
1:1,000; Abcam), Cleaved caspase-3 (cat. no. ab2302; 1:500; Abcam),
cleaved poly-ADP ribose polymerase (PARP; cat. no. ab32064;
1:1,000; Abcam), EZH2 (cat. no. ab191080; 1:500; Abcam), GADPH
(cat. no. ab9485; 1:1,000; Abcam) at 4˚C overnight, followed by the
use of HRP-conjugated goat anti rabbit secondary antibody at room
temperature for 2 h (cat. no. ab7090; 1:10,000; Abcam). Finally,
the protein bands were visualized utilizing an ECL reagent (Thermo
Fisher Scientific, Inc.). The quantitative analysis of protein
levels was performed using ImageJ software 1.46r (National
Institutes of Health).
RNA immunoprecipitation (RIP)
assay
The potential interaction between HOTAIR and EZH2
was evaluated using a RIP (RNA Binding Protein Immunoprecipitation
Assay) kit (cat. no. KT102-01; Guangzhou Saicheng Biological
Technology Co., Ltd.) according to the manufacturer's protocols.
hBMVECs were resuspended in RIP lysis buffer on ice for 5 min. The
supernatant was collected following cell lysate preparation using
centrifugation at 4˚C for 10 min. Part of the supernatant was used
as the Input group. Antibodies against EZH2 (5 µg; cat. no.
ab191250; Abcam) or IgG (5 µg; cat. no. ab6715; Abcam) were
incubated with magnetic beads for 2 h at room temperature.
Subsequently, this antibody-magnetic bead complex was incubated in
the presence of the supernatant collected. The immunoprecipitated
complexes were collected following centrifugation at 14,000 x g at
4˚C for 5 sec. The beads were re-suspended in Proteinase K for
digestion at 55˚C for 10 min, and RNA were extracted using TRIzol
method and analyzed using RT-qPCR.
Transfections
At 24 h prior to transfection, hBMVECs
(5x104 cells/ml) were seeded into six-well plates. When
the cells reached 70-90% confluence, hBMVECs were transfected with
small interfering (si)RNAs (75 pmol) against HOTAIR (si-HOTAIR-1,
5'-CAGCCCAAUUUAAGAAUUATT-3'; si-HOTAIR-2,
5'-GGAGUACAGAGAGAAUAAUTT-3') and its negative control
(5'-CUAUGAUACCCAAGUAAUATT-3'), EZH2 overexpressing plasmids
(pEGFP-N1-EZH2; 2500 ng/well) or with the corresponding control
(empty plasmids, NC) vectors (Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 3000, according to manufacturer's
instructions (Thermo Fisher Scientific, Inc.). After 24 h, cells
were used to perform further experiment.
Cell Counting Kit-8 (CCK-8) assay
hBMVECs (3x104 cells/well) were seeded
into 96-well plates and after incubation at 37˚C for 24 h, 10 µl
CCK-8 solution was added into each well (Thermo Fisher Scientific,
Inc.). Following incubation for an additional 2 h at 37˚C, the
absorbance at a wavelength of 450 nm was measured in each well
using a microplate reader (Thermo Fisher Scientific, Inc.).
Lactate dehydrogenase (LDH)
activity
hBMVECs (3x104 cells/well) were seeded
into 96 well plate. The cell culture plates were centrifuged for 5
min at 37˚C at 400 x g. Subsequently, a total of 120 µl supernatant
from each well was added into the corresponding well of a 96-well
plate. The levels of LDH were measured using a LDH cytotoxicity
assay kit (cat. no. C0017; Beyotime Institute of Biotechnology).
Each well was supplemented with 60 µl detection solution followed
by incubation at 37˚C for 30 min. The absorbance in each well was
measured using a microplate reader (Thermo Fisher Scientific, Inc.)
at a wavelength of 490 nm.
Endothelial monolayer cell
permeability assay
hBMVECs from each treatment group were seeded into a
24-well Transwell chamber (pore size, 0.4 µm; Corning, Inc.) at a
density of 1x105 cells/well. The cells were then
incubated in Procell Life Science & Technology Co., Ltd. medium
for 8 h in a humidified atmosphere containing 5% CO2 at
37˚C to form a confluent cell monolayer. The upper chamber was
supplemented with FITC-albumin solved in Procell Life Science &
Technology Co., Ltd. medium containing no serum (0.5 mg/ml,
Sigma-Aldrich; Merck KGaA), whilst the bottom chamber with D-Hank's
solution (Beijing Solarbio Science & Technology, Co., Ltd.).
The cells were then incubated at 37˚C and 5% CO2 for 45
min before samples were aspirated from both chambers. The optical
density was detected at 488 nm using a fluorospectrophotometer. The
membrane permeability coefficient of monolayer endothelial was
calculated as described by a previous study (25).
TUNEL staining
hBMVECs (1x106 cells) were washed with
PBS and fixed with 4% paraformaldehyde for 30 min at room
temperature. Following incubation with PBS containing 0.3% Triton
X-100 for 5 min, cells were rinsed with PBS and supplemented with a
TUNEL solution (Beyotime Institute of Biotechnology) and incubated
for 60 min at 37˚C. DAPI (0.1 ug/ml) was used to stain nuclei for 5
min at room temperature in the dark. After Antifade Mounting Medium
(Beyotime Institute of Biotechnology) was added to the sections,
four random fields were chosen and the apoptotic cells were
observed under a fluorescence microscope (magnification, x200;
Olympus Corporation).
Transwell assay
The invasive ability of hBMVECs was evaluated using
Transwell assays (Costar; Corning Inc.). Matrigel (Costar; Corning
Inc.) was first melted overnight at 4˚C and diluted in Procell Life
Science & Technology Co., Ltd. medium with serum-free at a
ratio of 1:8. Subsequently, the diluted Matrigel (40 µl/well) was
coated onto the upper chamber of the Transwell chamber. The
Transwell chamber was then placed in an incubator at 37˚C for 60
min for solidification. Subsequently, 1x104 hBMVECs
suspended in serum-free Procell Life Science & Technology Co.,
Ltd. medium were added into the upper chamber. A total of 600 µl
Procell Life Science & Technology Co., Ltd. medium containing
10% FBS was added to the lower chamber. Following incubation for 24
h at 37˚C, the invasive cells were fixed with 4% paraformaldehyde
for 20 min at room temperature followed by staining with 0.1%
crystal violet for 15 min at room temperature. Five fields were
randomly selected and the invaded cells were observed under an
inverted light microscope (magnification, x100; Olympus
Corporation).
Tube formation assay
Matrigel was melted at 4˚C and subsequently used to
coat 96-well plates at 37˚C (80 µl Matrigel/well; 10 mg/ml).
Following incubation for 30 min, 200 µl of the cell suspension
(Procell Life Science & Technology Co., Ltd. medium) was added
into each well (1x104 cells/well). After 4 h at 37˚C,
the formed tubes were observed under an inverted microscope
(magnification, x40; Olympus Corporation). Four randomly selected
fields were chosen and the tube quantities were calculated as the
number of branch points in which at least 3 tubes joined.
Statistical analysis
All data were statistically analyzed using GraphPad
Prism 7 software (GraphPad Software, Inc.). All experimental data
are expressed as the mean ± SD. Each experiment was repeated at
least three times. The differences among multiple groups were
compared by one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HOTAIR expression is upregulated in
the plasma of patients with neonatal HIE and in an vitro model of
OGD/R injury
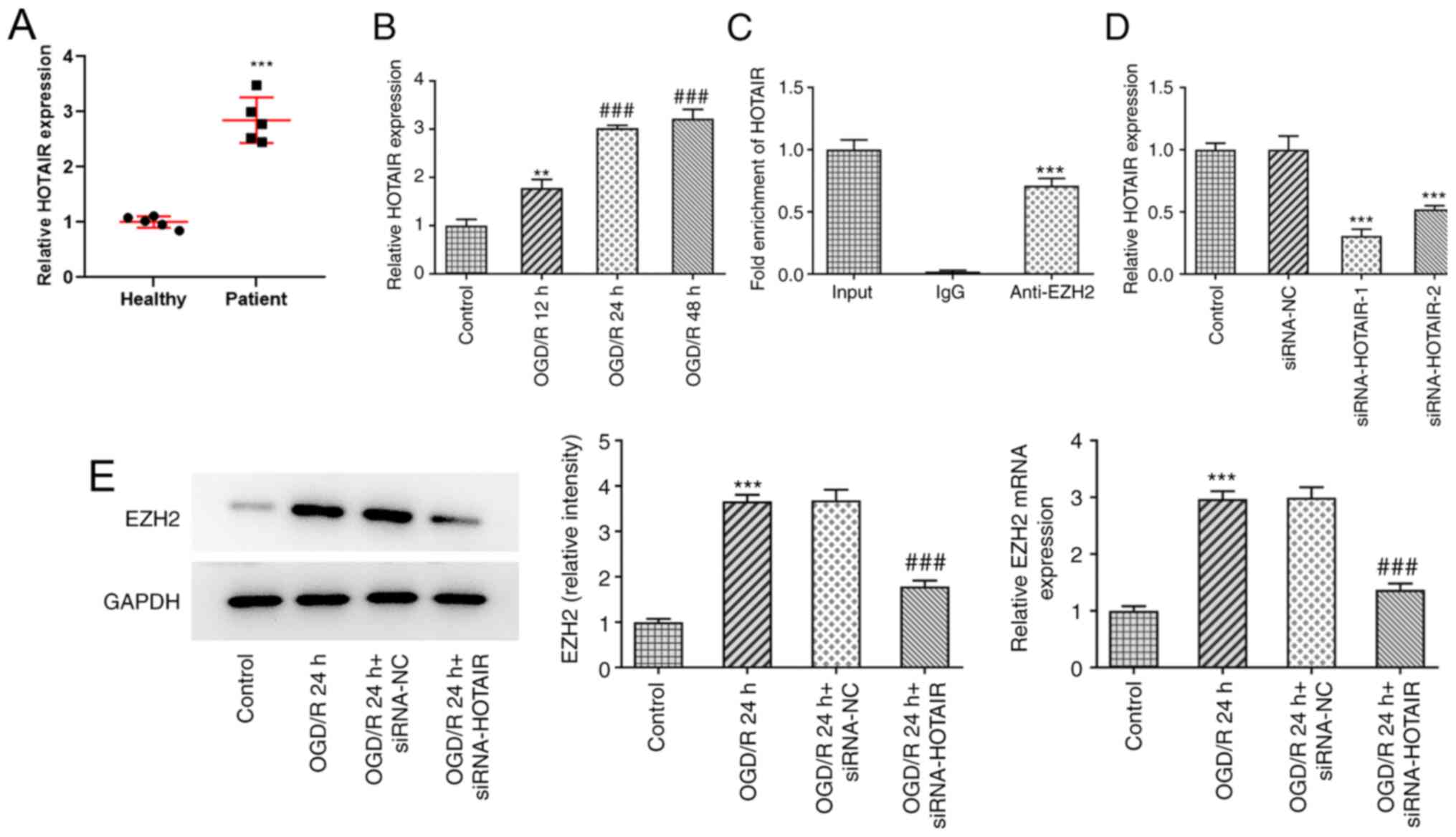
HOTAIR expression was found to be markedly
upregulated in the plasma of patients with neonatal HIE compared
with that in healthy neonates (Fig.
1A), suggesting a role for HOTAIR in HIE.
To assess the effects of IR on HOTAIR expression,
hBMVECs were exposed to OGD/R before the expression levels of
HOTAIR were determined by RT-qPCR (Fig.
1B). The results demonstrated that OGD/R exposure increased the
expression level of HOTAIR in a time-dependent manner, compared
with control group. It has been previously reported that HOTAIR can
interact with EZH2 (26,27). Therefore, the potential interaction
between HOTAIR and EZH2 was assessed by RIP assays. The results
demonstrated that HOTAIR was markedly enriched in the samples
immunoprecipitated using the EZH2 antibody, compared with the
control IgG, according to results from the RT-qPCR assay (Fig. 1C). The transfection efficacy of
siRNA-HOTAIR-1 or -2 was then evaluated using RT-qPCR. As shown in
Fig. 1D, both siRNA-HOTAIR-1 and -2
significantly reduced HOTAIR expression compared with that in the
siRNA-NC group. However, the effects of siRNA-HOTAIR-1 were
superior compared with those mediated by siRNA-HOTAIR-2. To further
investigate whether EZH2 could mediate the effects of HOTAIR on
HIE, hBMVECs were transfected with si-HOTAIR before the expression
levels of EZH2 were detected. RT-qPCR and western blot assays
revealed that EZH2 expression was significantly upregulated in
OGD/R-treated cells compared with that in control cells (Fig. 1E). However, these effects were
significantly reversed following HOTAIR silencing.
EZH2 overexpression attenuates the
effects of HOTAIR knockdown on cell viability and LDH release
To investigate if EZH2 overexpression can modulate
the effects of HOTAIR silencing, an in vitro model of OGD/R
injury was established to measure the changes in endothelial cell
viability and LDH release following the transfection and subsequent
OGD/R induction with an EZH2 overexpression plasmid. As shown in
Fig. 2A, EZH2 was significantly
overexpressed in cells transfected with EZH2 overexpression
plasmids compared with that in cells transfected with the negative
control plasmid. Although HOTAIR knockdown significantly enhanced
cell viability whilst also significantly attenuating the release of
LDH from OGD/R-treated hBMVECs, EZH2 overexpression significantly
reversed both of the effects aforementioned (Fig. 2B and C). These findings suggest that EZH2
overexpression can regulate cell viability and LDH release from
OGD/R-treated hBMVECs by interacting with HOTAIR.
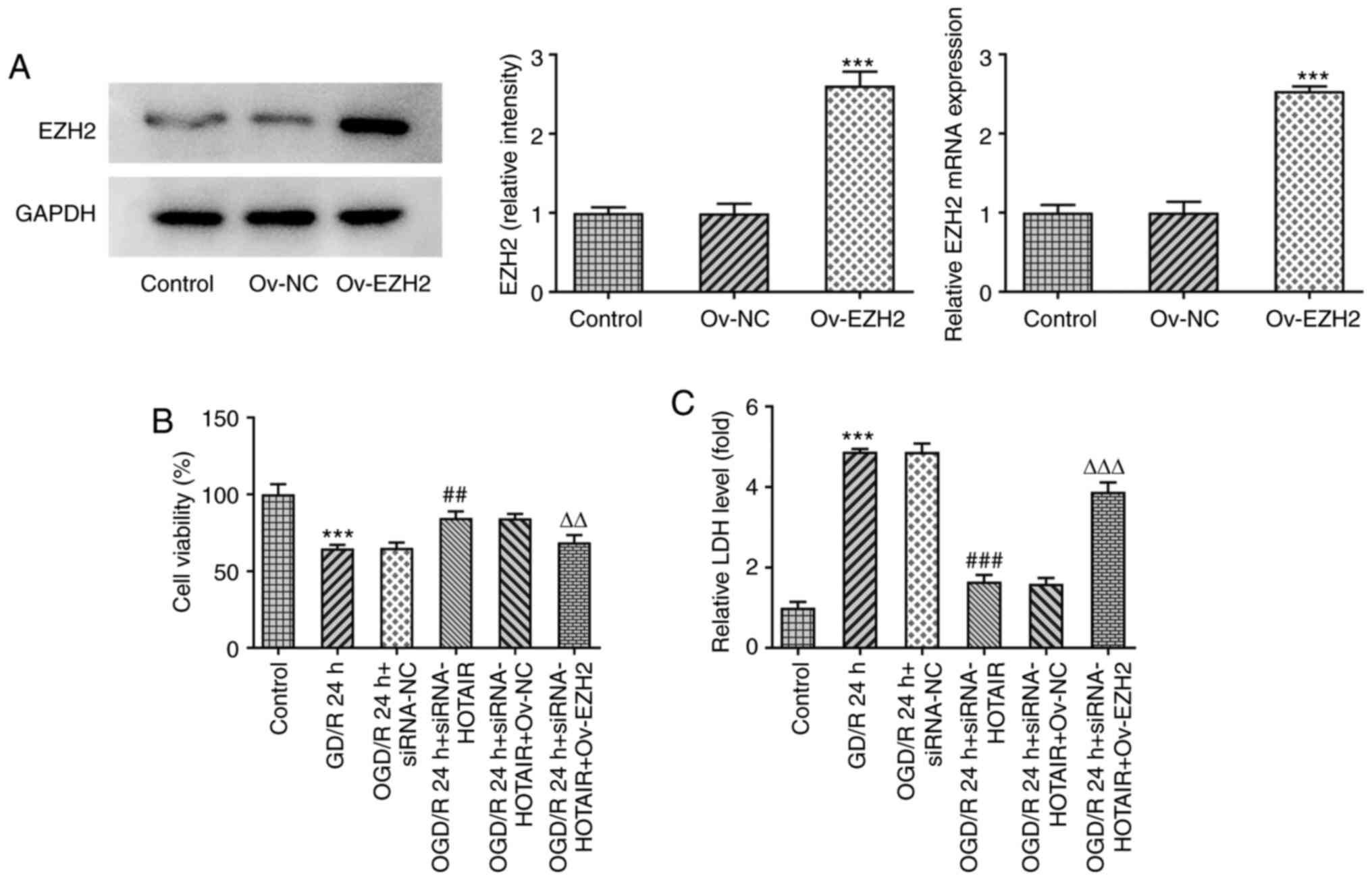 | Figure 2HOTAIR silencing alleviates human
brain microvascular endothelial cells injury in an EZH2-dependent
manner. (A) The expression of EZH2 after Ov-EZH2 transfection was
measured by western blotting. ***P<0.001 vs. Ov-NC.
(B) Analysis of cell viability using Cell Counting Kit-8 assay.
***P<0.001 vs. Control, ##P<0.01 vs.
OGD/R 24 h + siRNA-NC, ∆∆P<0.01 vs. OGD/R 24 h +
siRNA-HOTAIR + Ov-NC. (C) LDH release levels.
***P<0.001 vs. Control, ###P<0.001 vs.
OGD/R 24 h + siRNA-NC, ∆∆∆P<0.001 vs. OGD/R 24 h +
siRNA-HOTAIR + Ov-NC.. HOTAIR, Hox transcript antisense intergenic
RNA; EZH2, enhancer of zeste homolog 2; si, small interfering RNA;
OGD/R, oxygen-glucose deprivation/reperfusion; NC, negative
control; Ov, overexpression; NC, negative control; LDH, lactate
dehydrogenase. |
HOTAIR silencing maintains the
integrity of BBB via EZH2
BBB injury and aberrant changes in its permeability
are considered to be processes that occur during cerebral IR injury
and one of the main causes of mortality (28). To investigate whether EZH2 could
mediate the effects of HOTAIR, an in vitro model of BBB was
established to detect changes in the endothelial barrier integrity
of OGD/R-induced hBMVECs following HOTAIR silencing and EZH2
overexpression. Subsequently, cell permeability and the expression
levels of tight junction-proteins were measured by RT-qPCR and
western blot analysis. The results demonstrated that HOTAIR
silencing significantly reduced endothelial cell permeability
compared with that in si-NC-transfected cells following OGD/R
induction (Fig. 3A). In addition,
HOTAIR silencing significantly increased the expression of zonula
occludens-1 (ZO-1), occludin, claudin-5 and VE-cadherin in
OGD/R-treated hBMVECs (Fig. 3B and
C). However, all of the
aforementioned effects were significantly reversed by EZH2
overexpression.
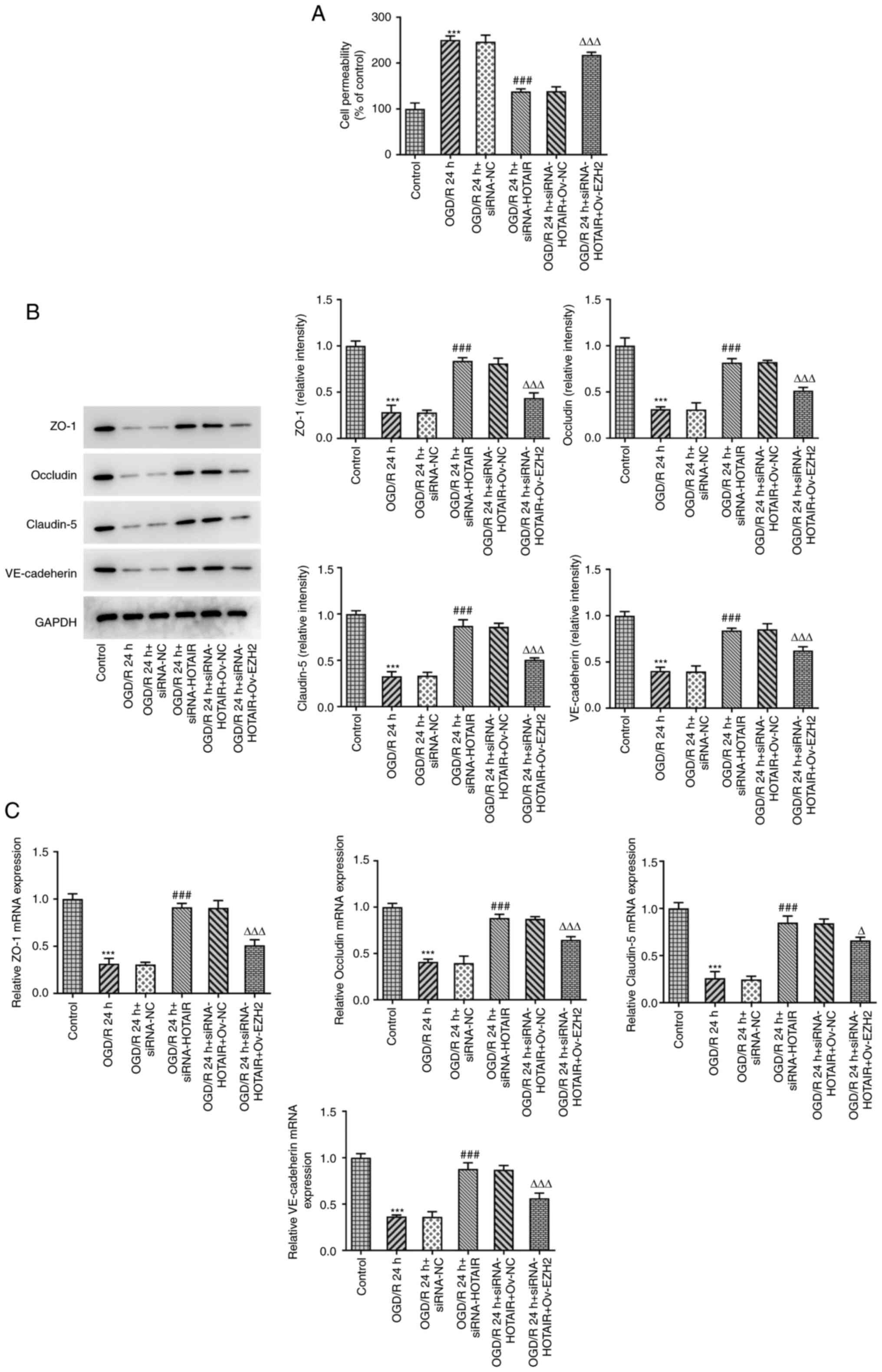 | Figure 3HOTAIR knockdown reduces the
permeability of the hBMVEC layer and increases the expression of
tight function proteins. (A) Analysis of HBMVECs permeability using
Transwell assay. The expression of tight function proteins ZO-1,
occludin, claudin-5 and VE cadherin were measured using (B) Western
blotting and (C) reverse transcription-quantitative PCR.
***P<0.001 vs. Control, ###P<0.001 vs.
OGD/R 24 h + siRNA-NC, ∆P<0.05 and
∆∆∆P<0.001 vs. OGD/R 24 h + siRNA-HOTAIR + Ov-NC.
HOTAIR, Hox transcript antisense intergenic RNA; EZH2, enhancer of
zeste homolog 2; si, small interfering RNA; OGD/R, oxygen-glucose
deprivation/reperfusion; NC, negative control; Ov, overexpression;
NC, negative control; hBMVECs, human brain microvascular
endothelial cells; ZO-1, zonula occludens-1; VE, vascular
endothelial. |
Since HOTAIR silencing appears to have improved the
endothelial barrier integrity of OGD/R-induced hBMVECs, the effect
of HOTAIR knockdown on hBMVEC apoptosis was then investigated. As
shown in Fig. 4, HOTAIR knockdown
significantly attenuated cell apoptosis compared with that in
si-NC-transfected cells following OGD/R induction according to
TUNEL assay. HOTAIR knockdown also significantly decreased the
expression levels of the apoptosis-related proteins, Bax, cleaved
caspase-3 and cleaved poly (adenosine diphosphate-ribose)
polymerase whilst significantly increasing those of Bcl-2 (Fig. 5). All of these effects
aforementioned were significantly reversed by EZH2
overexpression.
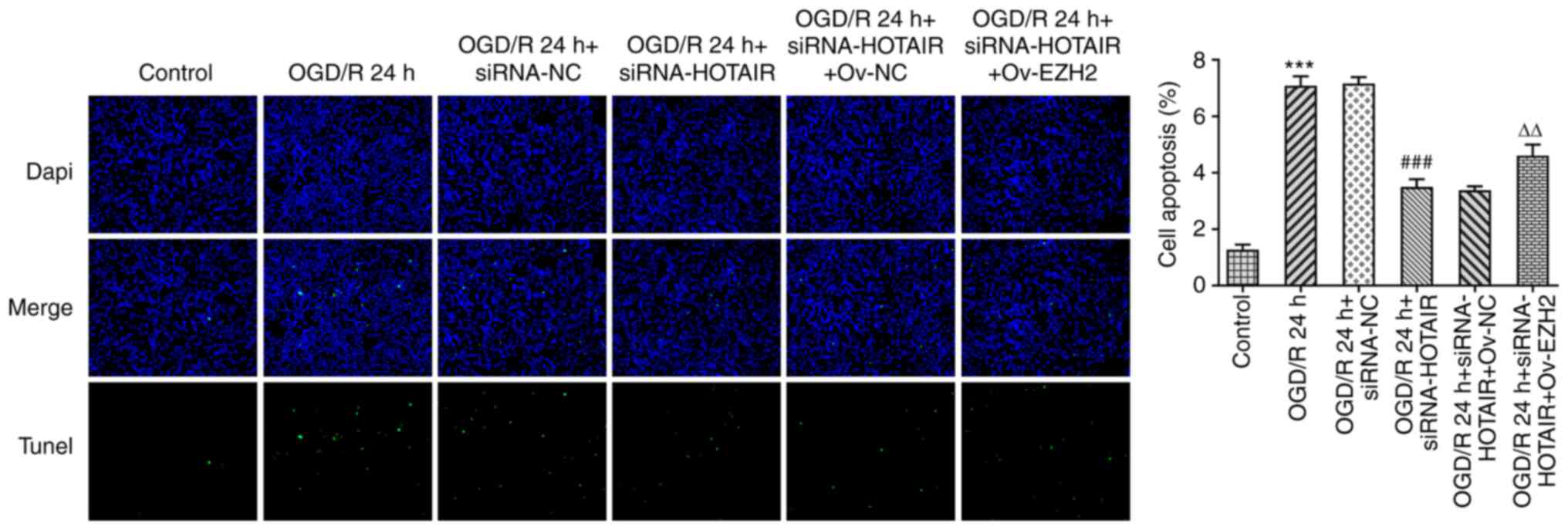 | Figure 4Effects of HOTAIR silencing and/or
EZH2 overexpression on hBMVEC apoptosis. Evaluation of HBMVECs
apoptosis using TUNEL assay. Magnification, x200.
***P<0.001 vs. Control, ###P<0.001 vs.
OGD/R 24 h + siRNA-NC and ∆∆P<0.01 vs. OGD/R 24 h +
siRNA-HOTAIR + Ov-NC. HOTAIR, Hox transcript antisense intergenic
RNA; EZH2, enhancer of zeste homolog 2; si, small interfering RNA;
OGD/R, oxygen-glucose deprivation/reperfusion; NC, negative
control; Ov, overexpression; NC, negative control; hBMVECs, human
brain microvascular endothelial cells. |
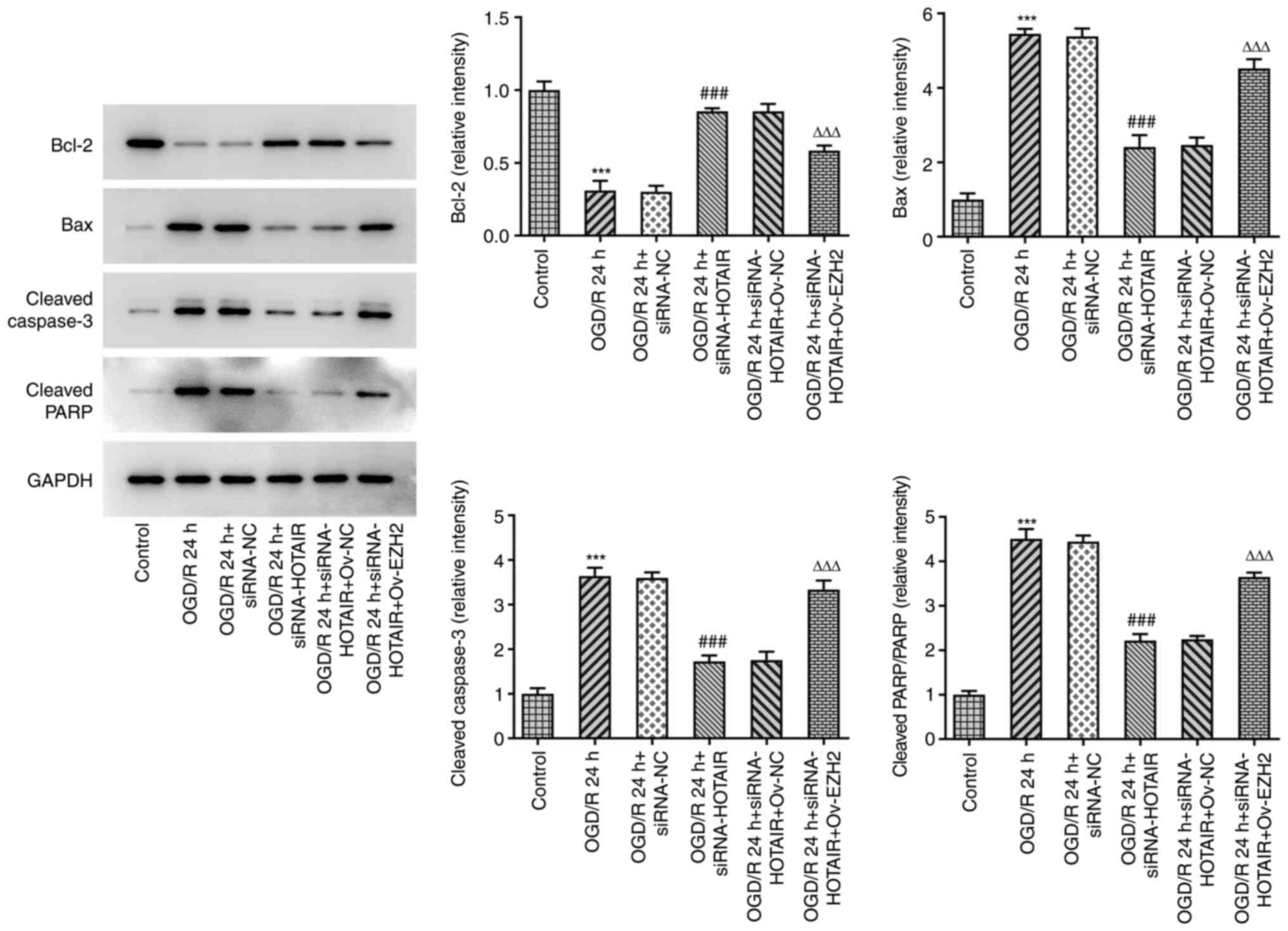 | Figure 5Effects of HOTAIR silencing and/or
EZH2 overexpression on the expression of proteins associated with
apoptosis in hBMVECs. Evaluation of HBMVECs apoptosis-related
proteins Bcl-2, Bax, cleaved caspase-3 and cleaved PARP were
measured by western blotting assay. ***P<0.001 vs.
Control, ###P<0.001 vs. OGD/R 24 h + siRNA-NC and
∆∆∆P<0.001 vs. OGD/R 24 h + siRNA-HOTAIR + Ov-NC.
HOTAIR, Hox transcript antisense intergenic RNA; EZH2, enhancer of
zeste homolog 2; si, small interfering RNA; OGD/R, oxygen-glucose
deprivation/reperfusion; NC, negative control; Ov, overexpression;
NC, negative control; hBMVECs, human brain microvascular
endothelial cells; PARP, poly-ADP ribose polymerase. |
HOTAIR knockdown attenuates the
migratory and angiogenic abilities of OGD/R-induced hBMVECs via
EZH2
Since the regulatory effect of HOTAIR and EZH2 on
OGD/R-induced hBMVEC layer integrity and apoptosis was uncovered,
the present study next aimed to further investigate the role of
HOTAIR in the migratory and tube formation capabilities of hBMVECs.
It was found that transfection with si-HOTAIR significantly
enhanced the migratory and tube formation abilities of hBMVECs
compared with those in the si-NC group (Fig. 6A). However, these effects were
significantly reversed following EZH2 overexpression (Fig. 6B). Overall, these results suggest
that EZH2 can mediate the downstream physiological effects of
HOTAIR on the migratory and tube formation abilities of
hBMVECs.
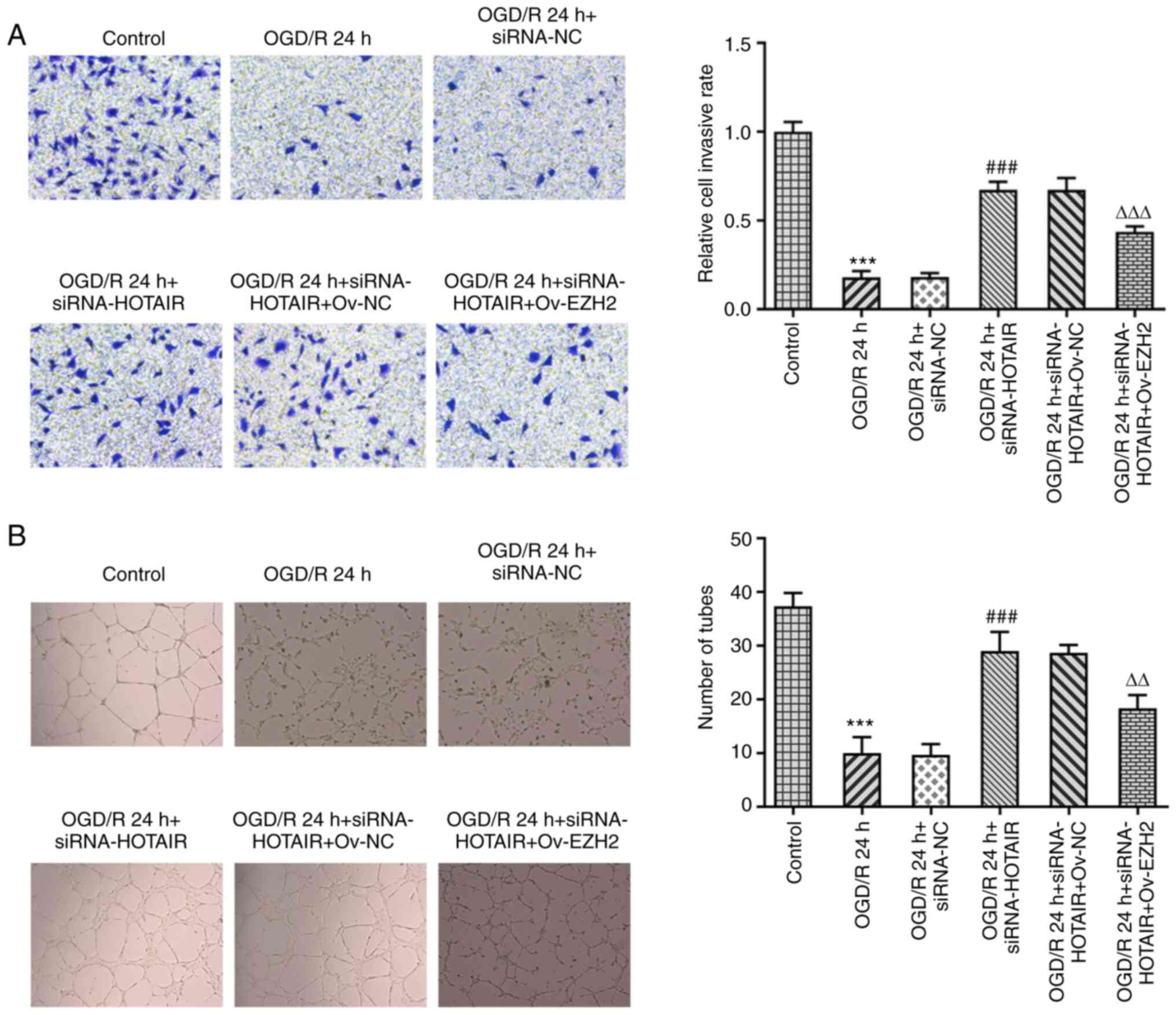 | Figure 6EZH2 overexpression reverses the
increased cell migration and angiogenesis induced by HOTAIR
silencing. (A) Analysis of hBMVEC invasion using a Transwell assay.
Magnification, x100. (B) Measurement of tube formation capabilities
of hBMVECs. Magnification, x40. ***P<0.001 vs.
Control, ###P<0.001 vs. OGD/R 24 h + siRNA-NC, and
∆∆P<0.01 and ∆∆∆P<0.001 vs. OGD/R 24 h
+ siRNA-HOTAIR + Ov-NC. HOTAIR, Hox transcript antisense intergenic
RNA; EZH2, enhancer of zeste homolog 2; si, small interfering RNA;
OGD/R, oxygen-glucose deprivation/reperfusion; NC, negative
control; Ov, overexpression; NC, negative control; hBMVECs, human
brain microvascular endothelial cells. |
Discussion
Neonatal HIE is a type of cerebral hypoxic-ischemic
injury that is caused by perinatal and is associated with poor
prognosis (29). Among children
with HIE, the mortality rate is typically 15-20% during the
neonatal period in China (30).
This relatively high mortality rate of HIE has been reported to be
mainly due to delayed diagnosis and treatment of this condition
(31). Therefore, early diagnosis
and assessment of HIE severity and prognosis is of great clinical
significance for improving the survival rate and quality of life of
neonates. However, reperfusion is frequently accompanied by
reperfusion injury (32). BMVECs
normally maintain the structure of the BBB and express tight
junction-related proteins, including VE-cadherin, ZO-1 and occludin
(33,34). The present study revealed that
HOTAIR was significantly upregulated in patients with neonatal HIE
and an in vitro model of IR injury, which was consistent
with those from previous studies (35-39),
supporting a potential role of HOTAIR in IR injury.
Although there were statistical differences in
HOTAIR expression between patients with neonatal HIE and healthy
individuals, the relatively small sample size is a limitation of
the present study. Results of a previous study demonstrated that
HOTAIR interacts with EZH2(40);
thus, further experiments were performed to evaluate whether this
interaction was also present in OGD/R-induced hBMVECs. The results
showed that within hours following OGD/R, HOTAIR silencing could
attenuate hBMVEC injury, as evidenced by the reduced hBMVEC
permeability and apoptosis observed, in addition to the enhanced
cell migration and angiogenesis. In addition, results from the RIP
assays verified the interaction between HOTAIR and EZH2 in hBMVECs
exposed to OGD/R. However, EZH2 overexpression reversed the effects
of therapeutic effects of HOTAIR silencing on cell injury and tube
formation ability of OGD/R-stimulated hBMVECs. By silencing HOTAIR
in hBMVECs induced by OGD/R, the expression of EZH2 was reduced.
Furthermore, hBMVECs exhibited higher EZH2 levels compared with
control following OGD/R exposure. Therefore, there may possibly be
a positive association between EZH2 and HOTAIR expression
underlying the pathological process of patients with neonatal HIE,
which warrants further study. HOTAIR has been documented to be an
EZH2-binding lncRNA (40), where it
regulates EZH2 expression and recruits EZH2 to MYC promoter sites
(23). A previous report showed
that lncRNA HERES regulates EZH2 protein levels through an
interaction between the G-rich motif on the lncRNA and the
N-terminal region of EZH2(20).
However, it remains unclear if there exists a direct site of
interaction between HOTAIR and EZH2, which also require further
study. Additionally, the effects of EZH2 silencing on HOTAIR
overexpression in OGD/R induced hBMVECs remains unknown, which is
another limitation of the present study.
It has been previously reported that HOTAIR exerts a
regulatory role in IR injury by regulating cell apoptosis and
oxidative stress in the ischemic myocardium of rats (41). Notably, miR-130a-3p mediated the
effects of HOTAIR on IR injury (41). To the best of our knowledge. the
present study was the first to demonstrate that the HOTAIR/EZH2
axis may be involved in OGD/R-mediated endothelial dysfunction.
ZO-1 was the first tight junction adhesion protein reported and is
an important structural protein in the tight junction complex that
is frequently used for evaluating BBB damage and function (42,43). A
previous study demonstrated that the expression levels of ZO-1,
occludin and claudin 5 mediated the tight junction, and were
associated with cerebral IR injury in terms of BBB permeability
(35). VE-cadherin is an adherens
junction-associated protein that serves an important role in
maintaining vascular integrity in vascular endothelial cells
(44,45). The present study revealed that the
HOTAIR/EZH2 axis could damage the structure of tight and adherens
junctions by downregulating ZO-1, occludin, claudin 5 and
VE-cadherin expression. However, a lack of in vivo data to
validate this observation further is a limitation of the present
study.
In conclusion, the present study revealed that the
lncRNA HOTAIR may be involved in hBMVEC injury and apoptosis to
mediate BBB damage through EZH2.
Acknowledgements
Not applicable.
Funding
Funding: The present study was approved by Science and
Technology Innovation Bureau Foundation of Nanshan District
Shenzhen (grant no. 2020118).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPW, JYM, XDL, BBW and XGZ made substantial
contributions to the conception and design of the study, performed
the experiments and interpreted the data, and drafted and revised
the manuscript for important intellectual content. YPW and XGZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Huazhong University of Science and Technology Union
Shenzhen Hospital (Shenzhen, China). Written informed consent for
blood collection was obtained from the legal guardian of each
child.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dumbuya JS, Chen L, Wu JY and Wang B: The
role of G-CSF neuroprotective effects in neonatal hypoxic-ischemic
encephalopathy (HIE): Current status. J Neuroinflammation.
18(55)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ohshima M, Tsuji M, Taguchi A, Kasahara Y
and Ikeda T: Cerebral blood flow during reperfusion predicts later
brain damage in a mouse and a rat model of neonatal
hypoxic-ischemic encephalopathy. Exp Neurol. 233:481–489.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lv H, Wang Q, Wu S, Yang L, Ren P, Yang Y,
Gao J and Li L: Neonatal hypoxic ischemic encephalopathy-related
biomarkers in serum and cerebrospinal fluid. Clin Chim Acta.
450:282–297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hua C, Ju WN, Jin H, Sun X and Zhao G:
Molecular chaperones and hypoxic-ischemic encephalopathy. Neural
Regen Res. 12:153–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong MX, Hu QC, Shen P, Pan JX, Wei YD,
Liu YY, Ren YF, Liang ZH, Wang HY, Zhao LB and Xie P: Recombinant
tissue plasminogen activator induces neurological side effects
independent on thrombolysis in mechanical animal models of focal
cerebral infarction: A systematic review and meta-analysis. PLoS
One. 11(e0158848)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arba F, Leigh R, Inzitari D, Warach SJ,
Luby M and Lees KR: STIR/VISTA Imaging Collaboration. Blood-brain
barrier leakage increases with small vessel disease in acute
ischemic stroke. Neurology. 89:2143–2150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Graves SI and Baker DJ: Implicating
endothelial cell senescence to dysfunction in the ageing and
diseased brain. Basic Clin Pharmacol Toxicol. 127:102–110.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Doll DN, Hu H, Sun J, Lewis SE, Simpkins
JW and Ren X: Mitochondrial crisis in cerebrovascular endothelial
cells opens the blood-brain barrier. Stroke. 46:1681–1689.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tarafdar A and Pula G: The role of NADPH
oxidases and oxidative stress in neurodegenerative disorders. Int J
Mol Sci. 19(3824)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu C, Yang J, Zhang C, Liu M, Geng X, Ji
X, Du H and Zhao H: Analysis of long non-coding RNA expression
profiles following focal cerebral ischemia in mice. Neurosci Lett.
665:123–129. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Soudyab M, Iranpour M and Ghafouri-Fard S:
The role of long non-coding RNAs in breast cancer. Arch Iran Med.
19:508–517. 2016.PubMed/NCBI
|
|
12
|
Huang T, Zhao HY, Zhang XB, Gao XL, Peng
WP, Zhou Y, Zhao WH and Yang HF: LncRNA ANRIL regulates cell
proliferation and migration via sponging miR-339-5p and regulating
FRS2 expression in atherosclerosis. Eur Rev Med Pharmacol Sci.
24:1956–1969. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang W, Chen Y, Liu P, Chen J, Song L,
Tang Y, Wang Y, Liu J, Hu FB and Hui R: Variants on chromosome
9p21.3 correlated with ANRIL expression contribute to stroke risk
and recurrence in a large prospective stroke population. Stroke.
43:14–21. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang B, Tang Y, Wang H, Chen C, Yu W, Sun
H, Duan M, Lin X and Liang P: Down-regulation of long non-coding
RNA HOTAIR promotes angiogenesis via regulating miR-126/SCEL
pathways in burn wound healing. Cell Death Dis.
11(61)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jin D, Wei W, Song C, Han P and Leng X:
Knockdown EZH2 attenuates cerebral ischemia-reperfusion injury via
regulating microRNA-30d-3p methylation and USP22. Brain Res Bull.
169:25–34. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Zhang M, Zhang X, Fan L, Liu P, Yu
L, Cao X, Qiu S and Xu Y: EZH2 inhibitor DZNep modulates microglial
activation and protects against ischaemic brain injury after
experimental stroke. Eur J Pharmacol. 857(172452)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luo Y, Fang Y, Kang R, Lenahan C, Gamdzyk
M, Zhang Z, Okada T, Tang J, Chen S and Zhang JH: Inhibition of
EZH2 (enhancer of zeste homolog 2) attenuates neuroinflammation via
H3k27me3/SOCS3/TRAF6/NF-κB (trimethylation of histone 3 lysine
27/suppressor of cytokine signaling 3/tumor necrosis factor
receptor family 6/nuclear factor-κB) in a rat model of subarachnoid
hemorrhage. Stroke. 51:3320–3331. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu YL, Chou RH, Shyu WC, Hsieh SC, Wu CS,
Chiang SY, Chang WJ, Chen JN, Tseng YJ, Lin YH, et al:
Smurf2-mediated degradation of EZH2 enhances neuron differentiation
and improves functional recovery after ischaemic stroke. EMBO Mol
Med. 5:531–547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xue H, Xu Y, Wang S, Wu ZY, Li XY, Zhang
YH, Niu JY, Gao QS and Zhao P: Sevoflurane post-conditioning
alleviates neonatal rat hypoxic-ischemic cerebral injury via
Ezh2-regulated autophagy. Drug Des Devel Ther. 13:1691–1706.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Z, Hou P, Fan D, Dong M, Ma M, Li H,
Yao R, Li Y, Wang G, Geng P, et al: The degradation of EZH2
mediated by lncRNA ANCR attenuated the invasion and metastasis of
breast cancer. Cell Death Differ. 24:59–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xue W, Wang F, Han P, Liu Y, Zhang B, Gu
X, Wang Y, Li M, Zhao Y and Cui B: The oncogenic role of lncRNA
FAM83C-AS1 in colorectal cancer development by epigenetically
inhibits SEMA3F via stabilizing EZH2. Aging (Albany NY).
12:20396–20412. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
You BH, Yoon JH, Kang H, Lee EK, Lee SK
and Nam JW: HERES, a lncRNA that regulates canonical and
noncanonical Wnt signaling pathways via interaction with EZH2. Proc
Natl Acad Sci USA. 116:24620–24629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Papazian O: Neonatal hypoxic-ischemic
encephalopathy. Medicina (B Aires). 78 (Suppl 2):36–41.
2018.PubMed/NCBI(In Spanish).
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ing NH, Berghman L, Abi-Ghanem D, Abbas K,
Kaushik A, Riggs PK and Puschett JB: Marinobufagenin regulates
permeability and gene expression of brain endothelial cells. Am J
Physiol Regul Integr Comp Physiol. 306:R918–R924. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qian L, Fei Q, Zhang H, Qiu M, Zhang B,
Wang Q, Yu Y, Guo C, Ren Y, Mei M, et al: lncRNA HOTAIR promotes
DNA repair and radioresistance of breast cancer via EZH2. DNA Cell
Biol: Nov 2, 2020 (Epub ahead of print).
|
|
27
|
Zhao YH, Liu YL, Fei KL and Li P: Long
non-coding RNA HOTAIR modulates the progression of preeclampsia
through inhibiting miR-106 in an EZH2-dependent manner. Life Sci.
253(117668)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bernardo-Castro S, Sousa JA, Brás A,
Cecília C, Rodrigues B, Almendra L, Machado C, Santo G, Silva F,
Ferreira L, et al: Pathophysiology of blood-brain barrier
permeability throughout the different stages of ischemic stroke and
its implication on hemorrhagic transformation and recovery. Front
Neurol. 11(594672)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakamura S, Koyano K, Jinnai W, Hamano S,
Yasuda S, Konishi Y, Kuboi T, Kanenishi K, Nishida T and Kusaka T:
Simultaneous measurement of cerebral hemoglobin oxygen saturation
and blood volume in asphyxiated neonates by near-infrared
time-resolved spectroscopy. Brain Dev. 37:925–932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK,
Im GH, Choi SJ and Chang YS: Hypothermia augments neuroprotective
activity of mesenchymal stem cells for neonatal hypoxic-ischemic
encephalopathy. PLoS One. 10(e0120893)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khan RH, Islam MS, Haque SA, Hossain MA,
Islam MN, Khaleque MA, Chowdhury B and Chowdhury MA: Correlation
between grades of intraventricular hemorrhage and severity of
hypoxic ischemic encephalopathy in perinatal asphyxia. Mymensingh
Med J. 23:7–12. 2014.PubMed/NCBI
|
|
32
|
Catanese L, Tarsia J and Fisher M: Acute
ischemic stroke therapy overview. Circ Res. 120:541–558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Méresse S, Dehouck MP, Delorme P, Bensaïd
M, Tauber JP, Delbart C, Fruchart JC and Cecchelli R: Bovine brain
endothelial cells express tight junctions and monoamine oxidase
activity in long-term culture. J Neurochem. 53:1363–1371.
1989.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Uwamori H, Ono Y, Yamashita T, Arai K and
Sudo R: Comparison of organ-specific endothelial cells in terms of
microvascular formation and endothelial barrier functions.
Microvasc Res. 122:60–70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Z, Liu Y, Zhu Y and Gong J:
HOTAIR/miRNA-1/Cx43: A potential mechanism for treating myocardial
ischemia-reperfusion injury. Int J Cardiol. 308(11)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma NH, Zhang MH, Yang JX, Sun ZJ, Yuan F
and Qiu XL: Long noncoding RNA HOTAIR sponging miR-211 regulates
cerebral ischemia-reperfusion injury. J Biol Regul Homeost Agents.
34:2209–2214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Meng K, Jiao J, Zhu RR, Wang BY, Mao XB,
Zhong YC, Zhu ZF, Yu KW, Ding Y, Xu WB, et al: The long noncoding
RNA hotair regulates oxidative stress and cardiac myocyte apoptosis
during ischemia-reperfusion injury. Oxid Med Cell Longev.
2020(1645249)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sun Y and Hu ZQ: LncRNA HOTAIR aggravates
myocardial ischemia-reperfusion injury by sponging microRNA-126 to
upregulate SRSF1. Eur Rev Med Pharmacol Sci. 24:9046–9054.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang B, Bao N, He G and Wang J: Long
noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7
axis in hepatic ischemia/reperfusion injury. Gene. 686:56–62.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Y, Xie Y, Li L, He Y, Zheng D, Yu P,
Yu L, Tang L, Wang Y and Wang Z: EZH2 RIP-seq identifies
tissue-specific long non-coding RNAs. Curr Gene Ther. 18:275–285.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fang J, Zheng W, Hu P and Wu J:
Investigating the effect of lncRNA HOTAIR on apoptosis induced by
myocardial ischemia-reperfusion injury. Mol Med Rep.
23(169)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jiao H, Wang Z, Liu Y, Wang P and Xue Y:
Specific role of tight junction proteins claudin-5, occludin, and
ZO-1 of the blood-brain barrier in a focal cerebral ischemic
insult. J Mol Neurosci. 44:130–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Reinhold AK and Rittner HL: Barrier
function in the peripheral and central nervous system-a review.
Pflugers Arch. 469:123–134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lampugnani MG, Dejana E and Giampietro C:
Vascular endothelial (VE)-cadherin, endothelial adherens junctions,
and vascular disease. Cold Spring Harb Perspect Biol.
10(a029322)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Giannotta M, Trani M and Dejana E:
VE-cadherin and endothelial adherens junctions: Active guardians of
vascular integrity. Dev Cell. 26:441–454. 2013.PubMed/NCBI View Article : Google Scholar
|