Introduction
Liver fibrosis is a chronic repair response caused
by various stimulus injuries, including ethanol, viruses, toxins or
various drugs (1). Due to an
imbalance between the degradation and synthesis of the
extracellular matrix (ECM), normal hepatic structures are destroyed
by progressively increasing levels of ECM in the liver parenchyma
(1). Liver fibrosis is a common
and frequently occurring clinical disease that can further develop
into liver cirrhosis or hepatocellular carcinoma (2). However, this process is reversible
(3,4), and thus it is critical to prevent
chronic liver disease from developing into cirrhosis and liver
cancer.
Hepatic stellate cells (HSCs) serve a key role in
the development of liver fibrosis, as they are the primary target
cell affected by a range of fibrotic factors, such as
Platelet-derived growth factor (PDGF) and transforming growth
factor β (TGF-β) (5-7).
The hedgehog (Hh) signaling pathway is the key mediator of several
basic processes in embryonic development and serves a vital role in
controlling cell fate, differentiation, survival and proliferation
(8,9). It has been demonstrated that Hh
ligands are released by injured liver cells in the process of liver
fibrosis, which bind to the membrane receptor protein patched
homolog 1 (Ptch1) on the surface of HSCs, releasing the inhibitory
effect of Ptch1 on the membrane receptor Smoothened (Smo). The
entry of the full-length glioma-associated oncogene homolog 1 (Gli;
a nuclear transcription factor) into the nucleus is promoted by
activated Smo, which in turn activates HSCs to promote
transformation into myofibroblasts (MFBs); MFBs then secrete
numerous collagen fibers, leading to liver fibrosis (10).
Salvianolic acid B (Sal B; an aromatic acid
compound) is the primary water-soluble component of Salvia
miltiorrhiza, which has been revealed to possess strong
antioxidant activity (11) and
exert protective effects on blood vessels, the pancreas and other
tissues (12). Sal B has been
demonstrated to improve rat vascular endothelial function by
inhibiting endothelial cell apoptosis and reducing pancreatic
morphological injury by decreasing the concentration of pancreatic
tissue malondialdehyde (12,13).
Sal B has also been used in the treatment of chronic liver
diseases, particularly in the treatment of liver fibrosis (14). Currently, to the best of our
knowledge, only the study by Yu et al (15) has used liver fibrotic mice as a
model to study whether Sal B inhibits the activation and
proliferation of HSCs by interfering with the Hh signaling pathway.
Additionally, the study only assessed the levels of Ptch1, Smo and
Gli2 genes in the Hh signaling pathway using reverse
transcription-quantitative (RT-q)PCR. Thus, it is necessary to
conduct further studies to evaluate whether Sal B can affect rat
liver fibrosis through the Hh signaling pathway.
In the present study, a rat model of liver fibrosis
was induced to evaluate the roles of Sal B in liver fibrosis and
its influence on the Hh signaling pathway. The research of this
experiment may help to provide a new perspective and target for
anti-hepatic fibrosis, whilst providing more experimental and
theoretical basis for the clinical use of Sal B for liver fibrosis
and protect liver function.
Materials and methods
Establishment of a rat model of liver
fibrosis
All animal care and experimental procedures were
approved by the Ethics Committee of Wannan Medical College (Anhui,
China) and performed in accordance with the committee guidelines. A
total of 32 male Sprague Dawley rats (6-7 weeks old with an average
body weight of 172.3±5 g) were provided by the Laboratory Animal
Center of Zhejiang Academy of Medical Sciences [license no. SCXK
(Zhejiang) 2019-0002]. All rats were placed under standard
conditions at a stable temperature of (24±2˚C) with a relative
humidity 45-60% and 12-h light/dark cycle. They also had free
access to water and standard food. After 1 week of adaptive
feeding, rats were randomly divided into four groups as follows
(n=8 each group): i) Normal control; ii) model; iii) positive
control; and iv) Sal B group. Rats of the model, positive control
and Sal B groups were injected subcutaneously in the abdomen region
with 50% carbon tetrachloride (CCl4)/corn oil solution
at 1 ml/kg for 12 weeks (twice per week). An equivalent volume of
normal saline was injected subcutaneously into the rats in the
normal control group. From the 7th week onwards, 0.1 mg/kg
colchicine (cat. no. C90100; Shanghai Jizhi Biochemical Technology
Co., Ltd.) (16) was administered
to the positive control group by oral gavage for 6 weeks (once per
day) and 60 mg/kg Sal B (cat. no. JKBm0226; Shanghai Jingke
Chemical Technology Co., Ltd.) (17) was administered to the Sal B group
by oral gavage once a day for 6 weeks. During the experiments,
animals were weighed once a week and doses were adjusted based on
body weight (according to the administration standard of colchicine
0.1 mg/kg or Sal B 60 mg/kg). At 12 h after the final
administration, rats were anesthetized by intraperitoneal injection
of sodium pentobarbital (40 mg/kg). After taking 3 ml of blood from
the abdominal aorta with a blood sampling needle, the abdominal
aorta was cut to allow for sacrifice of the rats by exsanguination.
Finally, after the death of the rats was confirmed by respiratory
and cardiac arrest, the liver tissues were collected.
Histopathological examination
Tissues were fixed with 4% paraformaldehyde using a
portion of liver tissues from each group for 24 h at 4˚C. The fixed
tissues were then dehydrated and paraffin embedded. Next, a
Leica-RM2235 microtome (Leica Microsystems GmbH) was used to slice
the tissues to a thickness of 5 µm. The staining of three slices
from each liver was performed using hematoxylin and eosin
(H&E), Sirius red and immunohistochemistry (IHC). For the
H&E staining procedure, paraffin slices were dewaxed in xylene
for 6-10 min and then rehydrated with a descending ethanol
gradient. At room temperature, the slices were placed in the
hematoxylin solution for 8-12 min, then rinsed with running water
for 1-2 min, differentiated with 1% HCl-alcohol for 10-20 sec,
rinsed again with running water and incubated with the eosin dye
solution for 1-3 min. After ascending gradient alcohol dehydration,
the slices were hyalinized in xylene and sealed with neutral gum.
Sirius red staining was performed according to the protocols of the
Sirius Red Stain Kit (cat. no. AG1470; Shanghai Jizhi Biochemical
Technology Co., Ltd.).
For the IHC staining procedure, after dewaxing and
hydration (identical to the H&E staining procedure), the tissue
sections were incubated in 3% H2O2-methanol
at room temperature for 30 min. After washing with PBS, the
sections were blocked with 5% goat serum (cat. no. SL038; Beijing
Solarbio Science & Technology Co., Ltd.) for 12 min at room
temperature and then incubated with the α-SMA antibody (1:200; cat.
no. bs-0189R; BIOSS) overnight at 4˚C. After washing with PBS, the
sections were incubated with the secondary antibody (1:500;
HRP-conjugated goat anti-rabbit IgG polymer; cat. no. 18001;
Chengdu Zhengneng Biotechnology Co., Ltd.; https://en.zen-bio.cn/prod_view.aspx?TypeId=115&Id=408364&FId=t3:115:3)
at room temperature for 40 min. Subsequently, at room temperature,
the sections were stained with DAB (Enhanced DAB chromogenic Kit;
cat. no. DA1016; Beijing Solarbio Science & Technology Co.,
Ltd.) for 3 min and then counterstained with hematoxylin for 4 min
at room temperature. The sections were then dehydrated with an
ascending alcohol gradient and sealed using neutral gum. Finally, a
light microscope was used to observe the sections and images
(magnification, x100) were captured with an Olympus microscope
(IXplore; Olympus Corporation).
Serum biochemistry
Blood that was collected from the abdominal aortas
of anesthetized rats was centrifuged to separate the serum at 4˚C
at 1,000 x g for 15 min. An automatic biochemical analyzer Chemray
800 (Rayto Life and Analytical Sciences Co., Ltd.) was subsequently
used to detect serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), total bilirubin (TBIL) and albumin (ALB)
levels.
Western blot analysis
After total protein was extracted by using RIPA
buffer (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) from the rat liver tissue (50 mg) obtained from each
group, the protein concentration was measured using a BCA Protein
Assay Kit (cat. no. A53226; Thermo Fisher Scientific, Inc.). A
total of 10 µg protein was loaded onto a gel (10% separated gel)
for SDS-PAGE and transferred to PVDF membranes. The membranes were
subsequently blocked with 5% skimmed milk for 2 h at 37˚C. After
washing with TBS-Tween 20 (TBST, containing 0.05% Tween-20), the
membranes were incubated with TGF-β1 antibody (cat. no. 3711;
1:1,000; Cell Signaling Technology, Inc.) and β-actin antibody
(cat. no. 4970; 1:1,000; Cell Signaling Technology, Inc.) overnight
at 4˚C. The following day, HRP-conjugated Anti-rabbit lgG antibody
(cat. no. 7074; 1:2,000; Cell Signaling Technology, Inc.) were
applied to the TBST-washed membranes and incubated at 37˚C for 2 h.
After washing with TBST, Clarity™ Western ECL Substrate (cat. no.
1705060; Bio-Rad Laboratories, Inc.) was added to the membranes,
and protein bands were analyzed using ImageJ software (version
1.8.0; National Institutes of Health).
RT-qPCR
A Total RNA Kit I (cat. no. R683 4-01; Omega
Bio-Tek, Inc.) was used to extract total RNA from the liver tissues
of rats in each group, after which the concentration was measured
using a microplate reader. According to the instructions of the
Universal RT-PCR Kit (cat. no. RP1105-50T; Beijing Solarbio Science
& Technology Co., Ltd.), 1 µg total RNA was reverse transcribed
into cDNA. The temperature protocol of reverse transcription was as
follows: 42˚C for 60 min followed by 70˚C for 10 min. Subsequently,
according to the instructions of the SYBR green fluorescent
quantitative kit (cat. no. BL698A; Biosharp Life Sciences), the
mRNA expression levels of Sonic hedgehog (Shh), Ptch1, Smo and Gli1
were detected using qPCR. The StepOnePlus™ Real-Time PCR System
(Thermo Fisher Scientific, Inc.) was then used to quantify mRNA
levels. β-actin was used as a control to measure the mRNA
expression levels of genes related to the Hh signaling pathway via
the comparative 2-ΔΔCq method (18). The thermocycling conditions for
qPCR were as follows: One cycle at 95˚C for 5 min; followed by 40
cycles at 95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 30 sec. The
primer sequences of this study are shown in Table I.
 | Table IPrimer Information. |
Table I
Primer Information.
| Gene | Accession number | Sequence (5'→3') | Tm | PL (bp) |
|---|
| Shh | NM_017221.1 | F:
TCAAGTCCAGCTGAAGTCCG | 59.68 | 88 |
| | | R:
TTTCCCGGTTGCTTATCTGG | 57.88 | |
| Ptch1 | NM_001389256 | F:
ACGCTCCTTTCCTCTTGAGAC | 59.73 | 168 |
| | | R:
TGAACTGGGCAGCTATGAAGT | 59.37 | |
| Smo | NM_012807.1 | F:
TCCATTTCTCCCTGGTGCCT | 60.85 | 122 |
| | | R:
AGTCCGAGTCTGCATCCAAG | 59.47 | |
| Gli1 | NM_001191910.1 | F:
AACTCCACGAGCACACAGG | 59.93 | 106 |
| | | R:
GGCAGTCCGTCTCATACACA | 59.47 | |
| β-actin | NM_031144.3 | F:
TTGCTGACAGGATGCAGAA | 56.96 | 101 |
| | | R:
ACCAATCCACACAGAGTACTT | 56.57 | |
Statistical analysis
Data were analyzed using GraphPad Prism 6 software
(GraphPad Software, Inc.) and are presented as the mean ± SD of
three repeats. One-way ANOVA with Bonferroni post hoc test was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sal B treatment inhibits the
CCL4-induced histopathological deterioration of the
liver
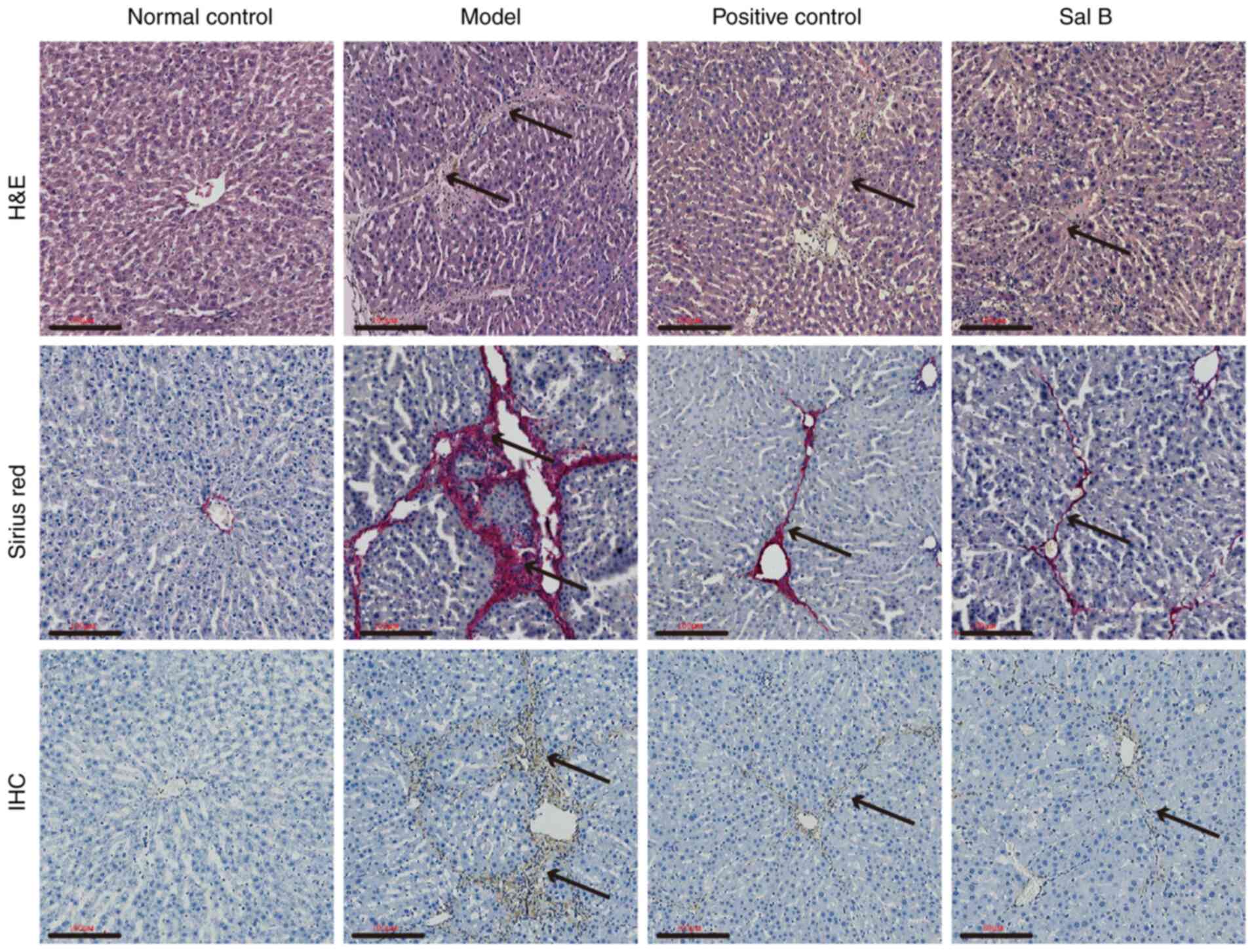
The results of H&E and sirius red staining
revealed no liver fibrous tissue hyperplasia in the normal control
group. However, the fibrotic liver tissue of the model group
exhibited a large amount of hyperplasia. When compared with the
model group, there was a marked reduction in fibrous tissue
hyperplasia present in the positive control and Sal B groups. IHC
results demonstrated that α-smooth muscle actin (SMA) was not
expressed in the liver tissues of the normal control group, whereas
positive expression was present in the model, positive control and
Sal B groups. Furthermore, in comparison with that in the Sal B and
positive control groups, higher α-SMA expression levels were
present in the liver tissues of the model group (Fig. 1). These results suggest that
CCL4-induced liver histological changes can be inhibited
by Sal B.
Sal B treatment reduces liver damage
caused by CCl4
Liver function serum marker analysis demonstrated
that the serum levels of ALT, TBIL and AST were all significantly
increased, while ALB levels were significantly decreased (all
P<0.05) in the Sal B, model and positive control groups compared
with the normal control group. In addition, when compared with the
Sal B and positive control groups, significantly higher serum ALT,
TBIL and AST levels, and significantly lower ALB levels, were
observed in the model group (all P<0.05). Serum ALT, AST, TBIL
and ALB levels did not significantly differ between the positive
control group and the Sal B group (Table II). The analysis of serum markers
suggests that Sal B could protect and improve liver function.
 | Table IIEffects of Sal B on the serum indices
of liver function in rats with liver fibrosis. |
Table II
Effects of Sal B on the serum indices
of liver function in rats with liver fibrosis.
| Group | N | ALT, U/l | AST, U/l | TBIL, µmol/l | ALB, g/l |
|---|
| Normal control | 8 | 38.84±4.38 | 121.42±16.14 | 0.76±0.27 | 37.72±6.10 |
| Model | 8 |
193.48±12.15a |
378.97±53.58a |
25.47±5.96a |
21.79±3.18a |
| Positive
control | 8 |
91.14±9.86a,b |
222.82±42.92a,b |
16.74±4.36a,b |
31.13±4.92a,b |
| Sal B | 8 |
87.68±9.21a,b |
196.62±37.71a,b |
14.79±3.91a,b |
27.92±2.85a,b |
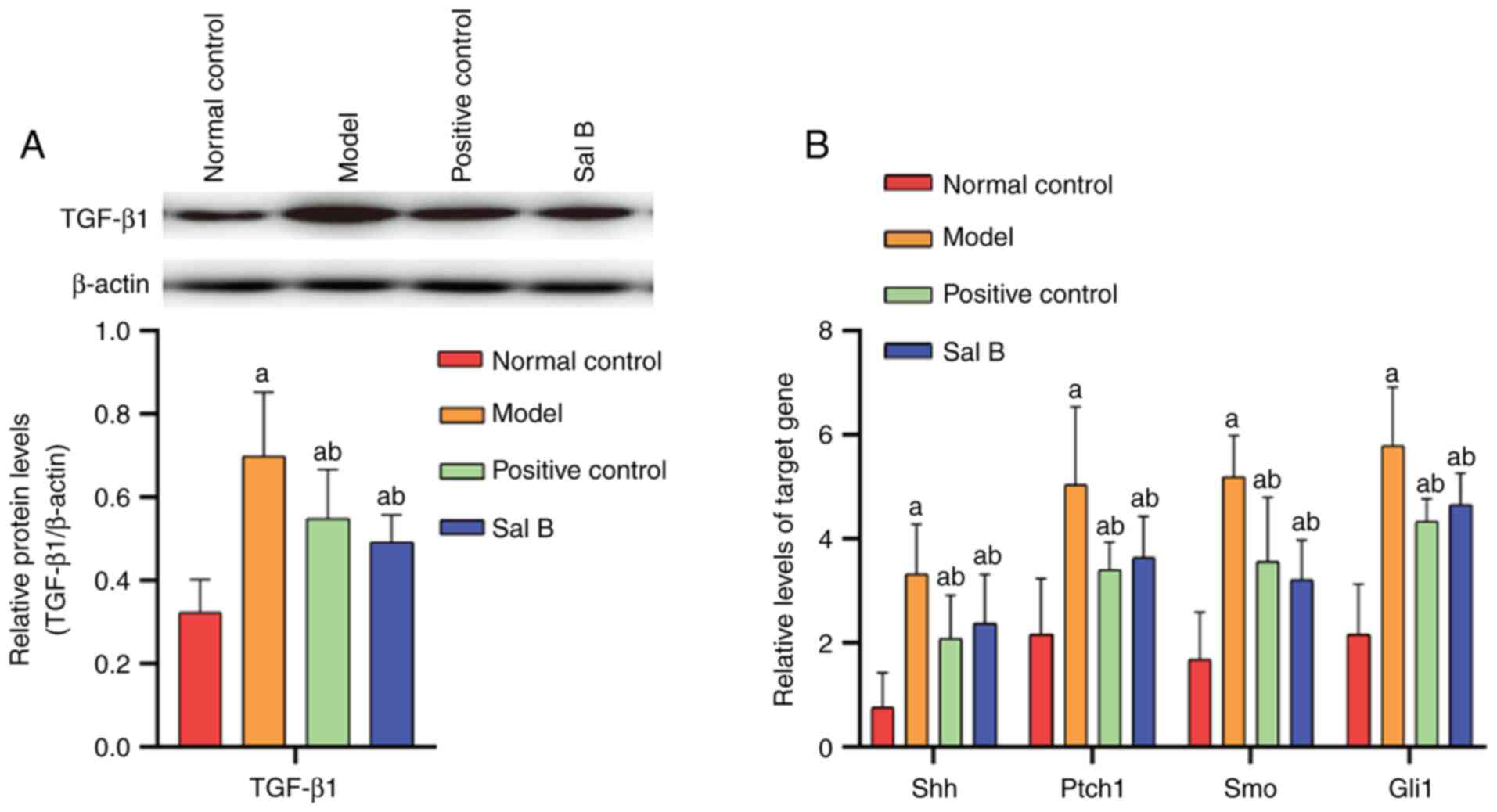
TGF-β1 protein expression levels
The protein expression levels of TGF-β1 were
analyzed using western blotting. The results demonstrated that when
compared with the normal control group, TGF-β1 expression levels
were increased in the model, positive control and Sal B groups (all
P<0.05). Significantly higher TGF-β1 levels were also observed
in the model group compared with the positive control and Sal B
groups (both P<0.05). No marked differences in TGF-β1 levels
were present between the positive control group and the Sal B
control group (Fig. 2A). This
study further confirmed that TGF-β1 is an effective cytokine in
causing liver fibrosis.
Sal B inhibits the
CCl4-induced activation of the Hh signaling pathway
The results of qPCR analysis indicated that compared
with that in the normal control group, there was a significant
increase in Shh, Ptch1, Smo and Gli1 expression levels in the
model, positive control and Sal B groups (all P<0.05).
Furthermore, increased levels of Shh, Ptch1, Smo and Gli1
expression levels were observed in the model group compared with
the Sal B and positive control groups (all P<0.05). No
significant differences in Shh, Ptch1, Smo and Gli1 mRNA levels
were observed between the positive control group and the Sal B
group (Fig. 2B). The
aforementioned results revealed that Hh signaling pathway is
involved in the process of liver fibrosis, where Sal B can inhibit
the activation of Hh signaling pathway.
Discussion
Liver fibrosis is a serious health problem, which
can further develop into liver cirrhosis or even hepatocellular
carcinoma if it is not treated in a timely and effective manner
(19,20). However, as this process is
reversible (4,19), it is vitally important to slow or
reverse the development of liver fibrosis when treating various
chronic liver diseases, such as autoimmune hepatitis, cholestatic
liver diseases and non-alcoholic fatty liver disease, and to reduce
the occurrence of cirrhosis. This has therefore become an important
topic in current medical research.
The incidence and progression of liver fibrosis is a
complex pathological process that involves a series of cytokines
and multiple signaling pathways (21). For example, oxidative stress is an
important factor in promoting inflammation and fibrosis (22). A previous study revealed that
during liver fibrosis, the mitochondrial function of liver cells is
impaired and the regulatory abilities of AMP-activated protein
kinase/peroxisome proliferator-activated receptor α are decreased
(23). Additional studies have
also demonstrated that mitochondria produce excessive superoxide
anions, hydrogen peroxide and other reactive oxygen species, with
the accumulation of the latter causing further damage to
mitochondria and oxidative stress damage to liver tissue (23,24).
Oxidative stress can activate NF-κB, nuclear
factor-erythroid-related factor 2 and other pathways to regulate
gene transcription, protein expression, cell apoptosis and the
activation of HSCs (25-27).
HSC activation is critical to the incidence of liver fibrosis
(5,28). When the liver is stimulated by
various pathogenic factors, such as ethanol, drugs, toxins or
viruses, HSCs are activated and transform into MFBs that secrete
large quantities of collagen to disrupt the balance of the ECM.
This causes liver parenchyma to be replaced by progressively
increasing quantities of ECM, and gradually leads to the formation
of liver fibrosis (29,30). A previous study has demonstrated
that upregulated levels of α-SMA (a marker of HSC activation) are
present in the liver tissues of fibrotic rats and that Sal B can
inhibit HSC activation, thereby reducing the expression of α-SMA
(14). In the present study,
immunohistochemical staining was performed for α-SMA in the liver
tissue of each group of rats. The results revealed no α-SMA
staining in the normal control group, whereas positive staining was
observed in the model, positive control and Sal B groups.
Furthermore, compared with those in the positive control and Sal B
groups, evidently higher α-SMA expression levels were observed in
the model group, further confirming that activated HSCs were
present in the pathological liver tissues of rats. HSC activation
and α-SMA expression were also suppressed by Sal B, which hindered
liver fibrosis.
Sal B exhibits strong antioxidant activity and has
been widely studied in the treatment of chronic liver disease
(31). For example, in the livers
of rats with non-alcoholic steatohepatitis, Sal B can improve the
structure and function of the mitochondria by upregulating the
expression of mitofusin-2, so as to reduce the liver tissue damage
induced by oxidative stress (31).
It has also been reported that Sal B can inhibit the progression of
liver fibrosis in rats by regulating the NF-κB/IκBα signaling
pathway, whilst significantly reducing the serum levels of ALT,
TBIL and AST in rats with liver fibrosis (32). The present study determined that
Sal B could significantly reduce serum ALT, TBIL and AST levels,
and increase ALB levels, in the rat of model of liver fibrosis.
These results are consistent with those already mentioned,
indicating that Sal B plays an important role in protecting liver
function.
The highly conserved Hh signaling pathway is
critical to tissue renewal, damage repair and embryonic development
(33). Studies have demonstrated
that abnormal activation of the Hh signaling pathway is intricately
associated with liver cancer, liver fibrosis and non-alcoholic
fatty liver (34,35). However, there are few studies on
the effects of Sal B on the Hh signaling pathway in liver fibrosis.
In the present study, Sal B was used for the treatment of liver
fibrosis in rats. The results revealed that Sal B reduced Shh,
Ptch1, Smo and Gli1 mRNA expression levels in the liver tissues in
the model group, thereby inhibiting HSC activation.
HSCs can be activated by TGF-β1, which is regarded
as the most effective cytokine involved in the development of liver
fibrosis (36). A previous study
has demonstrated that the levels of TGF-β1 in liver tissues is
positively associated with activation of the Hh signaling pathway
(37). The present study
demonstrated that upregulated Hh signaling pathway-associated genes
and TGF-β1 expression levels were present in the liver tissue of
fibrotic rats, with Sal B treatment negatively regulating their
expression.
Currently, to the best of our knowledge, only the
study by Yu et al (15) has
investigated whether Sal B can inhibit HSC activation and
proliferation by interfering with the Hh signaling pathway. The
results revealed that Sal B induced microRNA-152, leading to the
silencing of DNA methyltransferase 1-mediated Ptch1 demethylation,
thereby inhibiting liver fibrosis. In the study, a mouse model of
hepatic fibrosis was established and the effects of Sal B on the
histological structure of the liver, along with the expression
levels of a-SMA and type I collagen, were detected. The effect of
Sal B on the expression of Ptch1, Smo, and Gli2 genes in the HSCs
of mice with hepatic fibrosis was also detected by qPCR (15). In the current study, a rat model of
hepatic fibrosis was established and the effects of Sal B on the
histological structure of the liver, the expression levels of Shh,
Ptch1, Smo and Gli1 genes associated with the Hh signaling pathway,
and serum markers of liver injury were detected. By assessing the
whole Hh signaling pathway, the present study aimed to clarify
whether Sal B can reverse liver fibrosis in rats through the Hh
signaling pathway.
In summary, the anti-fibrotic effect of Sal B may be
partly mediated through the downregulation of TGF-β1 and by
decreasing the activation of the Hh signaling pathway, thereby
inhibiting the activation of HSCs, which are the key cells involved
in the development of the fibrotic environment. The results may
provide an experimental basis for the treatment of liver fibrosis.
In addition, the potential clinical value of Sal B for the
treatment of liver fibrosis and its protective effects on liver
function were theoretically confirmed by the present study. As
aforementioned, both mitochondrial dysfunction and oxidative stress
are involved in the process of liver fibrosis. Therefore, future
studies should explore the effect of Sal B on liver mitochondrial
function and the oxidative stress response in liver fibrosis
through in vivo and in vitro experiments.
Additionally, it should be determined whether there is an
association between the Hh signaling pathway, mitochondrial
dysfunction and the oxidative stress response.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Young and Middle-Aged
Research Foundation (grant no. WK201810) and the Key Scientific
Research Foundation of Wannan Medical College (grant no.
WK2021Z11).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST, RD and TL conceived and designed the study. ST,
RD, TX, JH, WG and AL performed the experiments. ST wrote the
manuscript. LL, HH and JL performed the statistical analysis and
drafted the manuscript. RD and TL confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures were
performed in accordance with the guidelines of the Ethics Committee
of Wannan Medical College (Anhui, China). The same committee
provided ethical approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellan M, Castello LM and Pirisi M:
Candidate biomarkers of liver fibrosis: A concise,
pathophysiology-oriented review. J Clin Transl Hepatol. 6:317–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wan SZ, Liu C, Huang CK, Luo FY and Zhu X:
Ursolic acid improves intestinal damage and bacterial dysbiosis in
liver fibrosis mice. Front Pharmacol. 10(1321)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Atta HM: Reversibility and heritability of
liver fibrosis: Implications for research and therapy. World J
Gastroenterol. 21:5138–5148. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Trautwein C, Friedman SL, Schuppan D and
Pinzani M: Hepatic fibrosis: Concept to treatment. J Hepatol. 62
(Suppl 1):S15–S24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Elpek GO: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Phaosri M, Jantrapirom S, Takuathung MN,
Soonthornchareonnon N, Sireeratawong S, Buacheen P, Pitchakarn P,
Nimlamool W and Potikanond S: Salacia chinensis L. stem
extract exerts antifibrotic effects on human hepatic stellate cells
through the inhibition of the TGF-β1-induced SMAD2/3 signaling
pathway. Int J Mol Sci. 20(6314)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gandhi CR: Hepatic stellate cell
activation and pro-fibrogenic signals. J Hepatol. 67:1104–1105.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh R and Lauth M: Emerging roles of
DYRK kinases in embryogenesis and hedgehog pathway control. J Dev
Biol. 5(13)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abramyan J: Hedgehog signaling and
embryonic craniofacial disorders. J Dev Biol. 7(9)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Michelotti GA, Xie G, Swiderska M, Choi
SS, Karaca G, Krüger L, Premont R, Yang L, Syn WK, Metzger D and
Diehl AM: Smoothened is a master regulator of adult liver repair. J
Clin Invest. 123:2380–2394. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Zheng Z, Wang Y, Yu H, Li W, Wu J, Cai C
and He Y: Salvianolic acid B inhibits ototoxic drug-induced
ototoxicity by suppression of the mitochondrial apoptosis pathway.
J Cell Mol Med. 24:6883–6897. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ren Y, Tao S, Zheng S, Zhao M, Zhu Y, Yang
J and Wu Y: Salvianolic acid B improves vascular endothelial
function in diabetic rats with blood glucose fluctuations via
suppression of endothelial cell apoptosis. Eur J Pharmacol.
791:308–315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lu XL, Dong XY, Fu YB, Cai JT, Du Q, Si JM
and Mao JS: Protective effect of salvianolic acid B on chronic
pancreatitis induced by trinitrobenzene sulfonic acid solution in
rats. Pancreas. 38:71–77. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang W, Ping J, Zhou Y, Chen G and Xu L:
Salvianolic acid B inhibits activation of human primary Hepatic
stellate cells through downregulation of the myocyte enhancer
factor 2 signaling pathway. Front Pharmacol. 10(322)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu F, Lu Z, Chen B, Wu X, Dong P and Zheng
J: Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis
by attenuating DNMT1-mediated Patched1 methylation. J Cell Mol Med.
19:2617–2632. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim S, Jung ES, Lee J, Heo NJ, Na KY and
Han JS: Effects of colchicine on renal fibrosis and apoptosis in
obstructed kidneys. Korean J Intern Med. 33:568–576.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu H, Liu W, Qiu H, Zou D, Cai H, Chen Q,
Zheng C and Xu D: Salvianolic acid B protects against myocardial
ischaemia-reperfusion injury in rats via inhibiting high mobility
group box 1 protein expression through the PI3K/Akt signalling
pathway. Naunyn Schmiedebergs Arch Pharmacol. 393:1527–1539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun M and Kisseleva T: Reversibility of
liver fibrosis. Clin Res Hepatol Gastroenterol. 39 (Suppl
1):S60–S63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bansal R, Nagorniewicz B and Prakash J:
Clinical advancements in the targeted therapies against liver
fibrosis. Mediators Inflamm. 2016(7629724)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Josan S, Billingsley K, Orduna J, Park JM,
Luong R, Yu L, Hurd R, Pfefferbaum A, Spielman D and Mayer D:
Assessing inflammatory liver injury in an acute CCl4 model using
dynamic 3D metabolic imaging of hyperpolarized [1-(13)C]pyruvate.
NMR Biomed. 28:1671–1677. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Cheresh P, Kim SJ, Tulasiram S and Kamp
DW: Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta.
1832:1028–1040. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Echeverría F, Valenzuela R, Bustamante A,
Álvarez D, Ortiz M, Espinosa A, Illesca P, Gonzalez-Mañan D and
Videla LA: High-fat diet induces mouse liver steatosis with a
concomitant decline in energy metabolism: Attenuation by
eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation
and the additive effects upon EPA and HT co-administration. Food
Funct. 10:6170–6183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ortiz M, Soto-Alarcón SA, Orellana P,
Espinosa A, Campos C, López-Arana S, Rincón MA, Illesca P,
Valenzuela R and Videla LA: Suppression of high-fat diet-induced
obesity-associated liver mitochondrial dysfunction by
docosahexaenoic acid and hydroxytyrosol co-administration. Dig
Liver Dis. 52:895–904. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Valenzuela R and Videla LA: Impact of the
Co-Administration of N-3 fatty acids and olive oil components in
preclinical nonalcoholic fatty liver disease models: A mechanistic
view. Nutrients. 12(499)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ni YH, Huo LJ and Li TT: Antioxidant axis
Nrf2-keap1-ARE in inhibition of alcoholic liver fibrosis by IL-22.
World J Gastroenterol. 23:2002–2011. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Valenzuela R and Videla LA: Crosstalk
mechanisms in hepatoprotection: Thyroid hormone-docosahexaenoic
acid (DHA) and DHA-extra virgin olive oil combined protocols.
Pharmacol Res. 132:168–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang JX, Qu XF, Zeng WT, Zhu KL, Zhang H
and Wei JJ: Mechanism of oxymatrine in preventing hepatic fibrosis
formation in patients with chronic hepatitis B. Nan Fang Yi Ke Da
Xue Xue Bao. 30:1871–1873. 2010.PubMed/NCBI(In Chinese).
|
|
29
|
Seki E and Brenner DA: Recent advancement
of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat
Sci. 22:512–518. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Li D, He L, Guo H, Chen H and Shan H:
Targeting activated hepatic stellate cells (aHSCs) for liver
fibrosis imaging. EJNMMI Res. 5(71)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang YC, Kong WZ, Jin QM, Chen J and Dong
L: Effects of salvianolic acid B on liver mitochondria of rats with
nonalcoholic steatohepatitis. World J Gastroenterol.
21:10104–10112. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang R, Yu XY, Guo ZY, Wang YJ, Wu Y and
Yuan YF: Inhibitory effects of salvianolic acid B on CCl(4)-induced
hepatic fibrosis through regulating NF-κB/IκBα signaling. J
Ethnopharmacol. 144:592–598. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Skoda AM, Simovic D, Karin V, Kardum V,
Vranic S and Serman L: The role of the Hedgehog signaling pathway
in cancer: A comprehensive review. Bosn J Basic Med Sci. 18:8–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kumar V, Dong Y, Kumar V, Almawash S and
Mahato RI: The use of micelles to deliver potential hedgehog
pathway inhibitor for the treatment of liver fibrosis.
Theranostics. 9:7537–7555. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gao L, Zhang Z, Zhang P, Yu M and Yang T:
Role of canonical Hedgehog signaling pathway in liver. Int J Biol
Sci. 14:1636–1644. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ghafoory S, Varshney R, Robison T,
Kouzbari K, Woolington S, Murphy B, Xia L and Ahamed J: Platelet
TGF-β1 deficiency decreases liver fibrosis in a mouse model of
liver injury. Blood Adv. 2:470–480. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
El-Agroudy NN, El-Naga RN, El-Razeq RA and
El-Demerdash E: Forskolin, a hedgehog signalling inhibitor,
attenuates carbon tetrachloride-induced liver fibrosis in rats. Br
J Pharmacol. 173:3248–3260. 2016.PubMed/NCBI View Article : Google Scholar
|