Introduction
The complete mechanism of hypertension is complex
and remains unclear. Clinical treatments [such as diet
modification, increased exercise and a selection of appropriate
blood pressure lowering medications (diuretics combined with
angiotensin receptor antagonists or angiotensinase I inhibitors)]
can effectively control the increase in high blood pressure in
patients with hypertension; however, complications of hypertension
can still occur, including stroke, heart failure and kidney damage
(1-3).
Emerging evidence has indicated that increased oxidative stress is
involved in the pathogenesis of hypertension, which results in an
abundance of reactive oxygen species (ROS) (4). During this process, nitric oxide
(NO), synthesized by one of the NO synthase isoforms, inducible NO
synthase, is a marker of oxidative stress (5). As a key enzyme in NO release,
endothelial NO synthase (eNOS) also exerts a notable role in
mediating oxidative stress (6).
The development of hypertension is influenced by the interaction of
several components, the most important being the abnormal
renin-angiotensin system (RAS) (7). Angiotensin II (AngII), an important
component of the RAS, can promote vasoconstriction, increase
peripheral resistance and cause vascular dysfunction (8). Therefore, effective inhibition of
AngII-induced vascular smooth muscle dysfunction could prevent and
help the treatment of cardiovascular diseases such as
hypertension.
Estrogen receptor modulators can play a
vasoprotective role similar to that of estrogen (9). It has been reported that both
tamoxifen and raloxifene can decrease the concentration of total
serum cholesterol and low-density lipoprotein (10). Based on the reduction of lipids and
inflammatory markers, tamoxifen is effective at reducing the burden
of coronary heart disease (11).
Following tamoxifen treatment, the mortality rate of myocardial
infarction and ischemic heart disease is decreased (12). In a small group of women with high
cardiovascular risk during menopause, raloxifene is associated with
a reduced incidence of cardiovascular disease (13). In addition, tamoxifen can reduce
the risk of heart disease and stroke; however, the risk of deep
venous thrombosis development is similar to that with other
selective estrogen receptor modulators, mainly increasing the risk
of femoral vein thrombosis, pulmonary embolism and retinal venous
thrombosis, although the relative incidence is very low (14).
Bazedoxifene, an estrogen receptor modulator, can
alleviate cardiac hypertrophy induced by blood pressure overload
in vivo by inhibiting interleukin (IL)-6 cytokine family
signal transducer signaling transduction and can reduce myocardial
fibrosis induced by AngII in mice (15,16).
Furthermore, bazedoxifene can inhibit arterial aging and the
development of atherosclerosis by increasing the expression level
of sirtuin 1 (SIRT1) (17).
Furthermore, SIRT1 activation can be used to treat hypertension by
enhancing AMP-activated protein kinase activity (18). Therefore, the present study
explored whether bazedoxifene may have the ability to attenuate
AngII-induced vascular endothelial cell dysfunction by targeting
the activation of SIRT1.
Materials and methods
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were
purchased from the American Type Culture Collection (cat. no.
PCS-100-010). The cells were cultured in DMEM (HyClone; Cytiva)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and placed at 37˚C in a humidified incubator with 5%
CO2.
The experiments were performed 24 h after induction
of cells with 1 µM AngII (19).
Cells in the treatment group were treated with bazedoxifene (2, 4,
6, 8 and 10 µM) for 1 h prior to AngII induction for 2 h (20). To block SIRT1, cells were incubated
with 1 µM of the inhibitor EX527 for 48 h prior to bazedoxifene
treatment (21). All drugs were
purchased from Sigma-Aldrich, Merck KGaA.
Cell transfection
SIRT1 small interfering (si)RNA (si-SIRT1) and siRNA
negative control (si-NC) were synthesized by Shanghai GenePharma
Co., Ltd. Cells were seeded into 6-well plates at a density of
3x105 cells/well and cultured for 24 h at 37˚C. In
strict accordance with the manufacturer's instructions, the siRNAs
(100 nM) were transfected into the cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfection efficiency was detected using
reverse transcription quantitative (RT-q)PCR 48 h following
transfection. The sequences of the siRNAs were as follows:
si-SIRT1-1, 5'-GCTAAGAATTTCAGGATTA-3'; si-SIRT1-2,
5'-ACTTTGCTGTAACCCTGTA-3'; si-NC, 5'-ACUUUCAUAAGUCUUCGUGGG-3'.
Cell Counting Kit (CCK)-8 assay
Trypsin (Beyotime Institute of Biotechnology) was
used to digest HUVECs and prepare a single cell suspension.
Subsequently, cells were seeded into 96-well plates at the density
of 2x103 cells/well for 24 h. A total of 10 µl CCK-8
solution (Dojindo Molecular Technologies, Inc., Japan) was then
added to the each well of the 96-well plates. Cells were further
incubated for 1 h and the absorbance at 450 nm was detected using a
spectrophotometer (Thermo Fisher Scientific, Inc.).
NO and ROS determination
NO and ROS production were evaluated using NO assay
kit (cat. no. BC1475) and ROS (CA1410-100T) assay kit (Beijing
Solarbio Science & Technology). Briefly, for NO determination,
cells were treated with the extract from the kit, sonicated in an
ice bath for 3 min and centrifuged at 4˚C for 15 min at 12,000 x g.
The absorbance of the centrifuged supernatant at 550 nm was
measured using a spectrophotometer to determine NO production. For
ROS determination, DCFH-DA was diluted 1:1,000 with serum-free
medium (HyClone; Cytiva) to a final concentration of 10 µmol/l.
After removing the cell culture medium, enough diluted DCFH-DA was
added to cover the volume of cells. They were then incubated for 20
min at 37˚C in a cell incubator. DCFH-DA was removed, and the cells
were washed with serum-free medium. Finally, the fluorescence
intensity at 525 nm emission wavelength (the absorbance) was
measured.
TUNEL assay
Apoptosis was detected using a TUNEL Apoptosis Assay
kit (Beyotime Institute of Biotechnology). Briefly, HUVECs were
washed with PBS, fixed with 4% paraformaldehyde for 20 min and
treated with 0.1% Triton X-100 for 10 min, all at room temperature.
Cells were incubated with TUNEL assay solution (TdT enzyme:
Fluorescent labeling solution=1:9) at 37˚C for 1 h. Cell nucleus
were stained with 10 µg/ml DAPI for 5 min at 37˚C. Fluorescence
expression of the apoptotic cells was observed under an EVO
fluorescence microscope (Advanced Microscopy Group), and five
fields of view were randomly observed in each group. The green
fluorescence was considered to stain apoptotic cells and the blue
stained nuclei, and the cells were counted using ImageJ
(V1.8.0.112; National Institutes of Health). The apoptotic rate was
calculated as follows: Apoptosis rate = (average number of
apoptotic cells/average number of total cells) x100%.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). A
reverse transcription kit (Takara Bio, Inc.) was subsequently used
to reverse transcribe the RNA into cDNA according to the
manufacturer's instructions. RT-qPCR reactions were performed using
the ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: 95˚C for 30
sec, followed by 40 cycles of 95˚C for 10 sec and 60˚C for 30 sec.
The relative expression levels were normalized to endogenous
control and were expressed as 2-ΔΔCq (22). The sequences of the primers were as
follows: SIRT1 forward, 5'-TATGGCTGACTTCGCTTTGG-3', reverse,
5'-TCGGGGCACTGATTTCTGTA-3'; and GAPDH forward,
5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'.
Western blotting
Cells were lysed with RIPA lysate (Beyotime
Institute of Biotechnology) containing 1% protease inhibitor and 1%
phosphatase inhibitor (both from Solarbio), centrifuged at 4˚C for
5 min with 3,220 x g and BCA (Beyotime Institute of Biotechnology)
was used to quantify the protein concentration in the supernatant.
Proteins (40 µg/lane) were separated by 10% SDS-PAGE (Beyotime
Institute of Biotechnology) and transferred onto PVDF membranes
(MilliporeSigma). Subsequently, membranes were blocked with 5%
skimmed milk for 2 h at room temperature and incubated with primary
antibodies against phosphorylated-eNOS (p-eNOS; 1:300; cat. no.
ab215717; Abcam), eNOS (1:400; cat. no. ab252439; Abcam), p47 phox
(1:300; cat. no. ab181090; Abcam), Bcl-2 (1:400; cat. no. ab182858;
Abcam), Bax (1:400; cat. no. ab32503; Abcam), cleaved caspase-3
(1:500; cat. no. ab32042; Abcam), caspase 3, (1:500; cat. no.
ab32351; Abcam), SIRT1 (1:400; cat. no. ab189494; Abcam), β-actin
(1:1,000; cat. no. ab8227; Abcam) and GAPDH (1:1,000; cat. no.
ab181602; Abcam) overnight at 4˚C. Subsequently, the membranes were
washed with PBS-10% Tween-20 three times and were incubated with
the goat anti-rabbit IgG H&L (HRP; 1:1,000; cat. no. ab7090;
Abcam) for 2 h at room temperature. Pierce Western Blotting
Substrate (Thermo Fisher Scientific, Inc.) was used to detect the
signal on the membrane. The data were analyzed via densitometry
using ImageJ software and normalized to expression of the internal
controls β-actin or GAPDH.
Statistical analysis
Each experiment was repeated independently at least
three times. The data are presented as the means ± standard
deviation. Statistical analyses were performed using SPSS v19.0
software (IBM Corp.). One-way ANOVA followed by Tukey's post hoc
test was used for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Bazedoxifene promotes the
proliferation of HUVECs induced by AngII
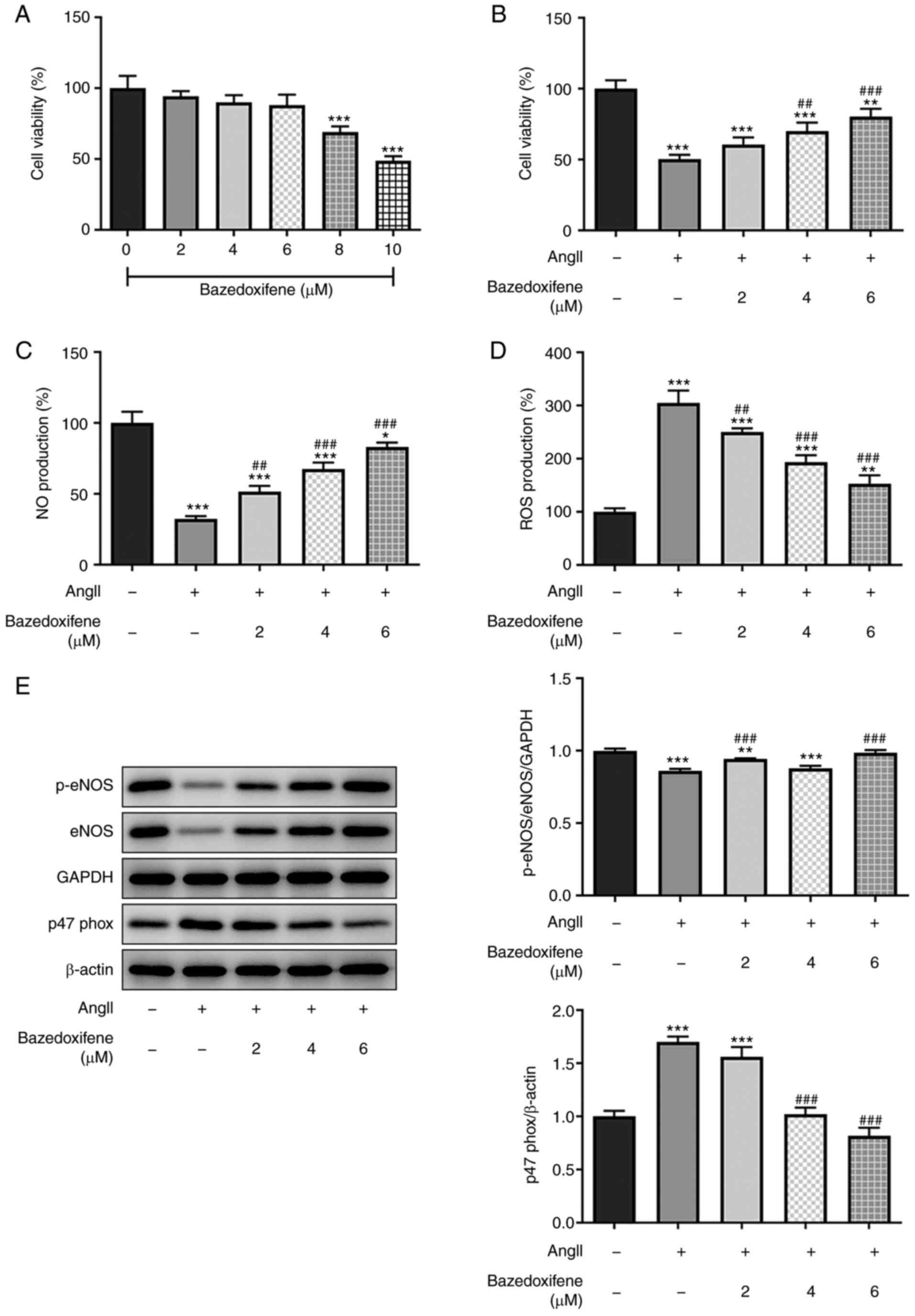
The effect of bazedoxifene on normal endothelial
cells was determined, as shown in Fig.
1A. The results from CCK-8 assay demonstrated that low
concentrations of bazedoxifene had little effect on cell viability.
However, when the concentration of bazedoxifene was >6 µM, cell
viability was significantly decreased, demonstrating a cytotoxicity
of bazedoxifene. Subsequently, the concentrations of 2-6 µM
bazedoxifene were selected in subsequent experiments. The cells
were then pretreated with bazedoxifene for 1 h, followed by
treatment with 1 µM AngII for 24 h. The results from CCK-8 assay
showed that bazedoxifene promoted the viability of HUVECs induced
by AngII (Fig. 1B).
Bazedoxifene reduces AngII-induced
oxidative stress in endothelial cells
The effect of bazedoxifene on AngII-induced
endothelial cells was further investigated, and NO content was
detected using a NO kit. As presented in Fig. 1C, bazedoxifene upregulated the
decreased NO content in Ang-II-induced HUVECs in a
concentration-dependent manner. Furthermore, bazedoxifene treatment
decreased the ROS content in AngII-induced endothelial cells
(Fig. 1D). Subsequently, the
expression levels of oxidative stress-related proteins were
detected using western blotting. The protein expression levels of
p-eNOS and eNOS were decreased, while the protein expression levels
of p47 phox were increased in AngII-induced endothelial cells.
Furthermore, the protein expression levels of p-eNOS and eNOS were
increased, while the protein expression level of p47 phox was
decreased following the addition of bazedoxifene (Fig. 1E). These findings indicated that
bazedoxifene may reduce AngII-induced oxidative stress in
endothelial cells.
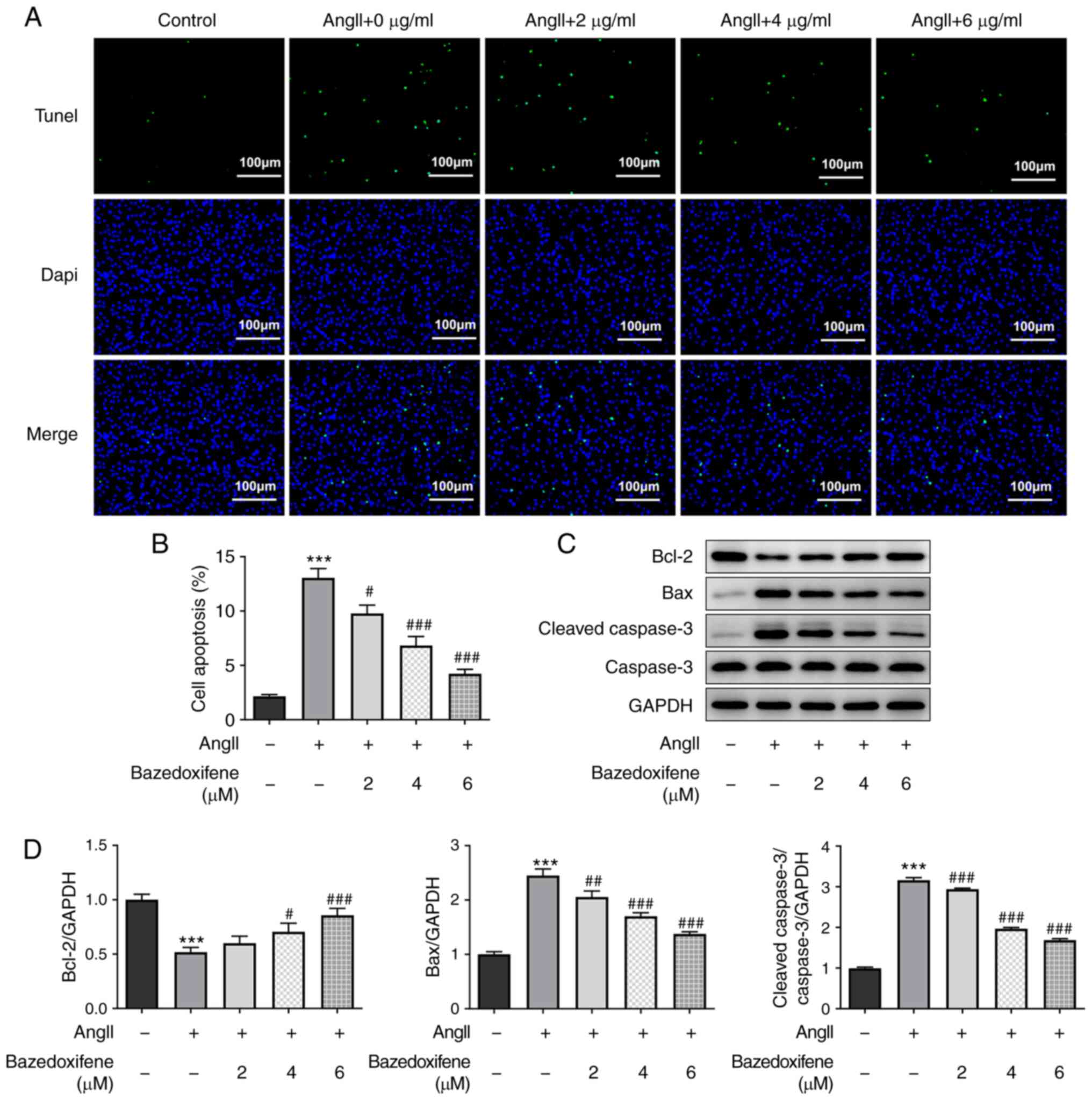
Bazedoxifene inhibits AngII-induced
apoptosis of endothelial cells
To determine the effect of bazedoxifene on the
apoptosis of AngII-induced HUVECs, TUNEL staining was performed.
The results demonstrated that the apoptotic rate in the
AngII-treated alone group was significantly higher compared with
that in the control group; however, bazedoxifene treatment could
decrease cell apoptosis (Fig. 2A
and B). These results were further
confirmed by the detection of apoptosis-related protein expression
levels. As presented in Fig. 2C
and D, bazedoxifene could decrease
Bax and cleaved caspase-3 protein expression levels in the
AngII-induced HUVECs compared with the AngII-induced HUVECs alone
group; however, bazedoxifene increased the expression level of the
anti-apoptosis protein Bcl-2. These findings demonstrated that
bazedoxifene may inhibited the AngII-induced apoptosis of
endothelial cells.
Bazedoxifene promotes AngII-induced
endothelial cell proliferation and inhibits oxidative stress by
activating SIRT1
It has been reported that SIRT1 activation can be
used to limit hypertension by enhancing AMP-activated protein
kinase activity (23). In the
present study, si-SIRT1 was transfected into HUVECs and the results
from western blotting (Fig. 3A)
and RT-qPCR (Fig. 3B) demonstrated
that the transfection efficiency of si-SIRT1-1 was higher than of
si-SIRT1-2. si-SIRT1-1 was therefore selected for use in subsequent
experiments. The expression level of SIRT1 was decreased in
AngII-induced endothelial cells, while the expression level of
SIRT1 was increased with bazedoxifene in a concentration-dependent
manner. The results showed that when the concentration of
bazedoxifene was 4 µM, the inhibitory effect of AngII on SIRT1 was
counteracted (Fig. 3C and D). Therefore, this concentration was
selected for use in subsequent experiments.
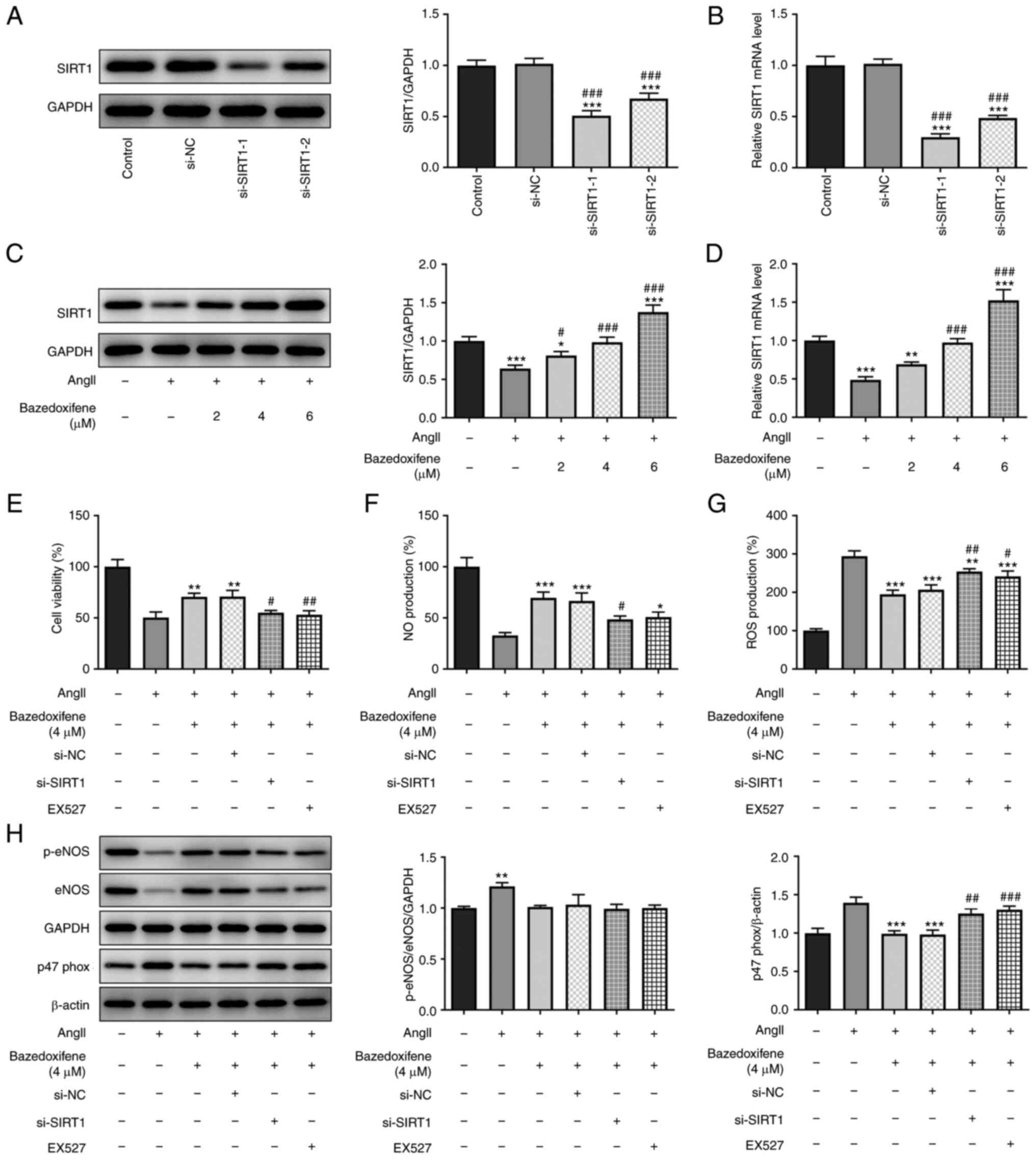 | Figure 3Bazedoxifene stimulates AngII-induced
HUVEC proliferation and inhibits oxidative stress by activating
SIRT1. (A and B) Cell transfection efficiency with si-SIRT1 was
confirmed by (A) western blotting (A) and (B) RT-qPCR. (C and D)
Expression of SIRT1 in AngII-induced endothelial cells was detected
by (C) western blotting and (D) RT-qPCR. (E) Cell Counting Kit-8
assay was used to determine the cell viability. (F) NO content was
detected using a NO kit. (G) ROS content was detected using a ROS
kit. (H) Expression of oxidative stress related proteins was
detected by western blotting. *P<0.05,
**P<0.01 and ***P<0.001 vs. Control or
AngII + 0 µg/ml; #P<0.05, ##P<0.01 and
###P<0.001 vs. si-NC or AngII + 4 µg/ml + si-NC.
RT-qPCR, reverse transcription quantitative PCR; ANGII, angiotensin
II; SIRT1, sirtuin 1; NO, nitric oxide; ROS, reactive oxygen
species; eNOS, endothelial nitric oxide synthase; p,
phosphorylated; si, small interfering; NC, negative control. |
The SIRT1 inhibitor, EX527, was added to the
AngII-induced endothelial cells. The results from CCK-8 assay
demonstrated that si-SIRT1 could partially counteract the promoting
effect of bazedoxifene on the proliferation of AngII-induced
endothelial cells (Fig. 3E). These
results indicated that bazedoxifene may activate SIRT1 and promote
AngII-induced endothelial cell proliferation.
Furthermore, as presented in Fig. 3F, bazedoxifene could increase the
concentration of NO, while si-siRT1 and EX527 could partially
reduce the concentration of NO. Furthermore, bazedoxifene could
decrease ROS content, whereas both si-SIRT1 and EX527 partially
increased the concentration of ROS (Fig. 3G). The results from western
blotting further demonstrated that bazedoxifene could activate
SIRT1 to inhibit AngII-induced oxidative stress in endothelial
cells (Fig. 3H).
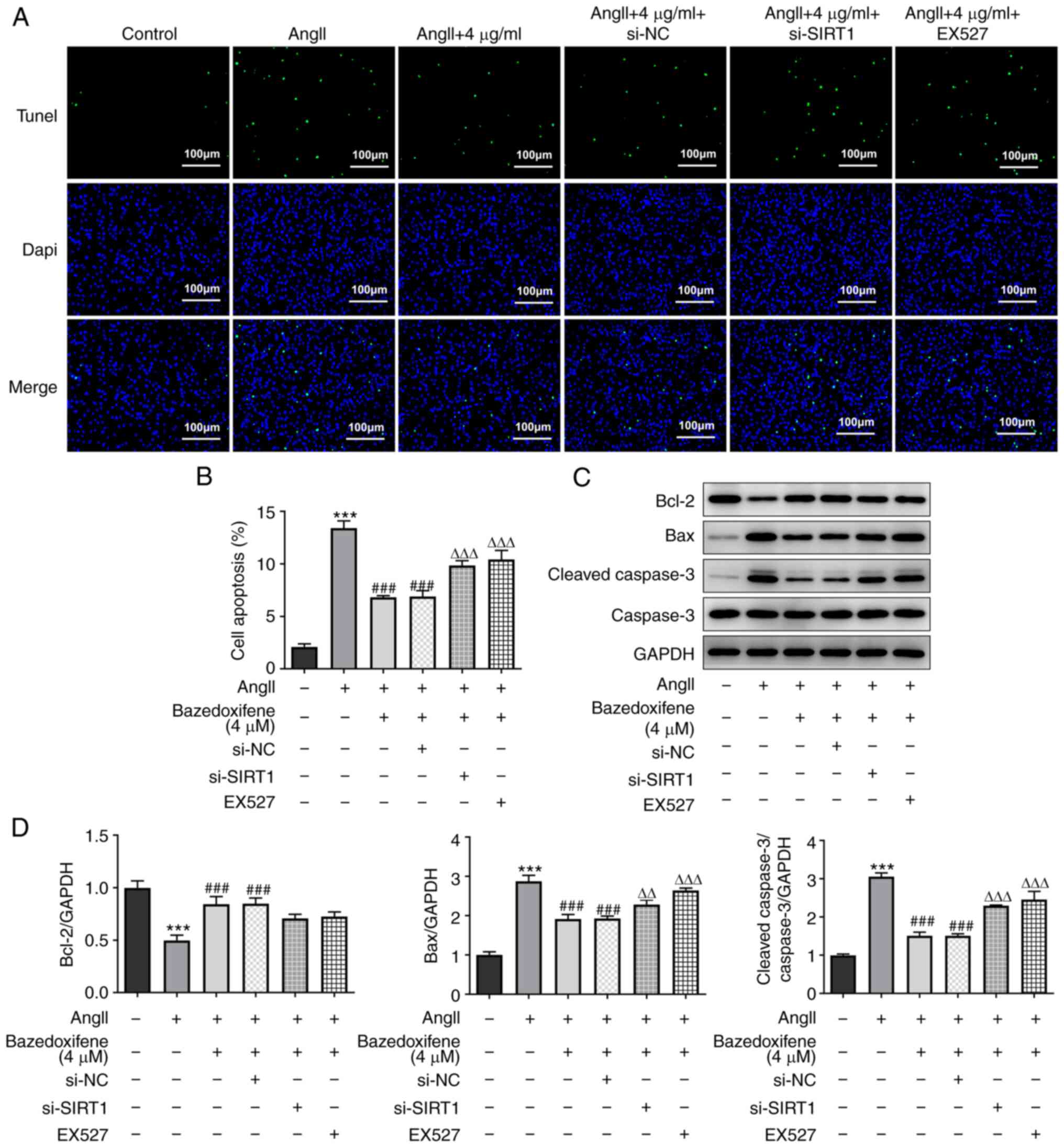
Bazedoxifene activates SIRT1 to
inhibit AngII-induced apoptosis of endothelial cells
As presented in Fig.
4A and B, the cell apoptosis
rate in the bazedoxifene treatment group was significantly
decreased compared with that in the AngII group, and both si-SIRT1
and EX527 could partially increase the apoptosis rate. These
results were further confirmed by detecting the expression levels
of apoptosis-related proteins (Fig.
4C and D). Both si-SIRT1 and
EX527 partially increased the protein expression of the
pro-apoptotic protein Bax, cleaved caspase 3/caspase 3, while
promoting the degradation of the anti-apoptotic protein Bcl-2 in
endothelial cells in comparison with the AngII + 4 µg/ml + si-NC
goup and the AngII + 4 µg/ml group, respectively. These findings
suggested that bazedoxifene may activate SIRT1 to inhibit the
AngII-induced apoptosis of endothelial cells.
Discussion
AngII is the main active peptide of RAS, and AngII
produced by both classical and non-classical pathways can reduce
endothelial cell function (24).
Endothelial cells are the main components of the vascular system,
which serve a crucial role in vascular homeostasis by secreting and
releasing vasodilators (such as NO and prostaglandins) and
vasoconstrictors (such as AngII and endothelin) (25-27).
The shift of vascular function to vasoconstriction, proinflammatory
state, oxidative stress and NO deficiency may lead to endothelial
dysfunction and injury. This shift is a key event in the
pathophysiological process of cardiovascular diseases, including
hypertension, diabetes, atherosclerosis, arterial hypertension and
pulmonary hypertension (28,29).
In the present study, HUVECs were treated with 1 µM AngII for 24 h
to establish an AngII-induced endothelial cell model. The results
demonstrated that endothelial cell viability was decreased, while
oxidative stress levels and apoptosis were increased in
AngII-induced cells, which was consistent with a previous report
(30).
Statins are mainly used to reduce blood lipid and
cholesterol levels and treat patients with cardiovascular diseases
(31). At present, the commonly
used statins in clinical practice include atorvastatin,
fluvastatin, lovastatin, simvastatin and pravastatin. Recent
studies have reported that statins exert anti-inflammatory and
anti-angiogenesis effects and can inhibit endothelial cell
migration (32,33). The results from the present study
demonstrated that bazedoxifene could promote AngII-induced HUVEC
proliferation and inhibit cell apoptosis, indicating that
bazedoxifene could enhance the viability of AngII-induced
HUVEC.
When the endothelium suffers damage and dysfunction
(such as ischemia and oxygen deprivation reperfusion), the
excessive production of oxidants exceeds the antioxidant capacity
of the cell and the balance between oxidants and antioxidants is
disrupted, resulting in increased oxidative stress (34). The endothelium releases NO, which
relaxes the surrounding smooth muscle to increase blood flow and
relax the vessels. NO is synthesized by e-NOS, with L-arginine,
oxygen and NADPH as endogenous sources, and is capable of
vasodilating, which can be balanced with vasoconstriction produced
by the sympathetic nervous system and RAS (35). AngII stimulates NADPH oxidase,
which then increases the formation of superoxide anions in the
blood vessels, resulting in increased oxidative stress. Excessive
ROS can easily inactivate NO via the formation of peroxynitrite,
thus further damaging vascular function (36). The present study demonstrated that
bazedoxifene could decrease the oxidative stress of endothelial
cells induced by AngII, suggesting that it could protect
AngII-induced endothelial cells and enhance the function of damaged
endothelial cells. It has been reported that bazedoxifene
stimulates the activation of eNOS and upregulates SIRT1 in
bilateral ovariectomy mice and delays the development of
atherosclerosis and vascular senescence (37). In addition, SIRT1 can reduce
systolic blood pressure elevation and inhibit AngII-induced
vascular remodeling in mice (38).
These results might indicate whether bazedoxifene mechanism of
action could be mediated by targeted agonism of SIRT1. Therefore,
to further investigate bazedoxifene underlying mechanism, the
present study determined the effect of bazedoxifene on
AngII-induced cells using STIR1 inhibitor and si-SIRT1. The results
demonstrated that both EX527 and si-SIRT1 could counteract the
apoptosis-inhibiting effect and anti-oxidative stress effect
observed with bazedoxifene. Subsequently, our study suggested that
bazedoxifene may be able to counteract AngII-induced endothelial
cell apoptosis, increased oxidative stress and NO release
dysfunction by agonizing SIRT1. However, the present study only
focused on the protective effect of bazedoxifene on AngII-induced
cells by inhibiting or downregulating STIR1. Future investigation
will involve animal experiments to confirm these results, refine
our understanding and provide an experimental basis for the
application of bazedoxifene in the treatment of AngII-induced
diseases.
In summary, the present study demonstrated that
bazedoxifene may promote AngII-induced HUVEC proliferation and
inhibit cell apoptosis and oxidative stress by activating SIRT1,
thereby playing a protective role. These findings could provide an
important basis for the clinical treatment of AngII-induced
hypertension with estrogen.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY and BL designed the study and wrote the
manuscript. QY and BL confirm the authenticity of all the raw data.
QY, JZ and BL performed experiments and participated in data
collection and analysis. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brumback LC, Andrews LIB, Jacobs DR Jr,
Duprez DA, Shah SJ, Dougherty CM, Denenberg JO and Allison MA: The
association between indices of blood pressure waveforms (PTC1 and
PTC2) and incident heart failure. J Hypertens. 39:661–666.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harrison N, Pang P, Collins S and Levy P:
Blood pressure reduction in hypertensive acute heart failure. Curr
Hypertens Rep. 23(11)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hering D, Esler MD, Krum H, Mahfoud F,
Böhm M, Sobotka PA and Schlaich MP: Recent advances in the
treatment of hypertension. Expert Rev Cardiovasc Ther. 9:729–744.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rodrigo R, González J and Paoletto F: The
role of oxidative stress in the pathophysiology of hypertension.
Hypertens Res. 34:431–440. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pierini D and Bryan NS: Nitric oxide
availability as a marker of oxidative stress. Methods Mol Biol.
1208:63–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang ZM, Zhang JX, Li B, Gao X, Liu Y, Mao
W and Chen SL: Resveratrol ameliorates low shear stress-induced
oxidative stress by suppressing ERK/eNOS-Thr495 in endothelial
cells. Mol Med Rep. 10:1964–1972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li XC, Zhang J and Zhuo JL: The
vasoprotective axes of the renin-angiotensin system: Physiological
relevance and therapeutic implications in cardiovascular,
hypertensive and kidney diseases. Pharmacol Res. 125:21–38.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fyhrquist F and Saijonmaa O:
Renin-angiotensin system revisited. J Intern Med. 264:224–236.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee HS, Park HK, Kim KH, Ko JH, Kim YJ, Yi
KH and Hwang JS: Estrogen receptor α gene analysis in girls with
central precocious puberty. J Pediatr Endocrinol Metab. 26:645–649.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Demissie S, Cupples LA, Shearman AM,
Gruenthal KM, Peter I, Schmid CH, Karas RH, Housman DE, Mendelsohn
ME and Ordovas JM: Estrogen receptor-alpha variants are associated
with lipoprotein size distribution and particle levels in women:
The Framingham heart study. Atherosclerosis. 185:210–218.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chlebowski RT, Anderson GL, Geller M and
Col N: Coronary heart disease and stroke with aromatase inhibitor,
tamoxifen, and menopausal hormone therapy use. Clin Breast Cancer.
6 (Suppl 2):S58–S64. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nordenskjold B, Rosell J, Rutqvist LE,
Malmström PO, Bergh J, Bengtsson NO, Hatschek T, Wallgren A and
Carstensen J: Coronary heart disease mortality after 5 years of
adjuvant tamoxifen therapy: Results from a randomized trial. J Natl
Cancer Inst. 97:1609–1610. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharma D, Sharma U, Bhatnagar VB and Singh
VS: A study of the effect of tamoxifen on serum lipoprotein
profiles in premenopausal and postmenopausal women with breast
carcinoma and associated risk of cardiovascular disease. Indian J
Med Sci. 55:359–365. 2001.PubMed/NCBI
|
|
14
|
McLean BA, Zhabyeyev P, Patel VB, Basu R,
Parajuli N, DesAulniers J, Murray AG, Kassiri Z, Vanhaesebroeck B
and Oudit GY: PI3Kalpha is essential for the recovery from
Cre/tamoxifen cardiotoxicity and in myocardial insulin signalling
but is not required for normal myocardial contractility in the
adult heart. Cardiovasc Res. 105:292–303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shi W, Ma H, Liu T, Yan D, Luo P, Zhai M,
Tao J, Huo S, Guo J, Li C, et al: Inhibition of
Interleukin-6/glycoprotein 130 signalling by Bazedoxifene
ameliorates cardiac remodelling in pressure overload mice. J Cell
Mol Med. 24:4748–4761. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McKeand W, Ermer J and Korth-Bradley J:
Assessment of the effects of age and renal function on
pharmacokinetics of bazedoxifene in postmenopausal women. Clin
Pharmacol Drug Dev. 7:920–926. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Clarkson TB, Ethun KF, Pajewski NM, Golden
D, Floyd E and Appt SE: Effects of bazedoxifene, conjugated equine
estrogens, and a tissue-selective estrogen complex containing both
bazedoxifene and conjugated equine estrogens on cerebral artery
atherosclerosis in postmenopausal monkeys. Menopause. 21:8–14.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Martinez-Arroyo O, Ortega A, Galera M,
Solaz E, Martinez-Hervas S, Redon J and Cortes R: Decreased urinary
levels of SIRT1 as non-invasive biomarker of early renal damage in
hypertension. Int J Mol Sci. 21(6390)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang R, Fang W, Liang J, Lin C, Wu S, Yan
S, Hu C and Ke X: Apelin/APJ axis improves angiotensin II-induced
endothelial cell senescence through AMPK/SIRT1 signaling pathway.
Arch Med Sci. 14:725–734. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ishibashi Y, Matsui T, Ueda S, Fukami K,
Okuda S, Ohta H and Yamagishi S: Bazedoxifene blocks
AGEs-RAGE-induced superoxide generation and MCP-1 level in
endothelial cells. Climacteric. 18:426–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang XY, Li XY, Wu CH, Hao Y, Fu PH, Mei
HX, Chen F, Gong YQ, Jin SW and Li H: Protectin conjugates in
tissue regeneration 1 restores lipopolysaccharide-induced pulmonary
endothelial glycocalyx loss via ALX/SIRT1/NF-kappa B axis. Respir
Res. 22(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expres- sion data using real-time quantitative PCR
and the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H
and Sun Z: Activation of SIRT1 attenuates klotho deficiency-induced
arterial stiffness and hypertension by enhancing AMP-activated
protein kinase activity. Hypertension. 68:1191–1199.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ding Y, Chen J, Cui G, Wei Y, Lu C, Wang L
and Diao H: Pathophysiological role of osteopontin and angiotensin
II in atherosclerosis. Biochem Biophys Res Commun. 471:5–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei F, Liu S, Luo L, Gu N, Zeng Y, Chen X,
Xu S and Zhang D: Anti-inflammatory mechanism of ulinastatin:
Inhibiting the hyperpermeability of vascular endothelial cells
induced by TNF-α via the RhoA/ROCK signal pathway. Int
Immunopharmacol. 46:220–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamawaki H, Kameshima S, Usui T, Okada M
and Hara Y: A novel adipocytokine, chemerin exerts
anti-inflammatory roles in human vascular endothelial cells.
Biochem Biophys Res Commun. 423:152–157. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan X, Wang E, He J, Zhang L, Zeng X, Gui
Y, Sun Q, Song Y and Yuan H: Ligustrazine protects
homocysteine-induced apoptosis in human umbilical vein endothelial
cells by modulating mitochondrial dysfunction. J Cardiovasc Transl
Res. 12:591–599. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chan P, Chen YC, Lin LJ, Cheng TH, Anzai
K, Chen YH, Liu ZM, Lin JG and Hong HJ: Tanshinone IIA attenuates
H(2)O(2)-induced injury in human umbilical vein endothelial cells.
Am J Chin Med. 40:1307–1319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bik E, Mielniczek N, Jarosz M, Denbigh J,
Budzynska R, Baranska M and Majzner K: Tunicamycin induced
endoplasmic reticulum changes in endothelial cells investigated in
vitro by confocal Raman imaging. Analyst. 144:6561–6569.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wassmann S, Laufs U, Stamenkovic D, Linz
W, Stasch JP, Ahlbory K, Rösen R, Böhm M and Nickenig G: Raloxifene
improves endothelial dysfunction in hypertension by reduced
oxidative stress and enhanced nitric oxide production. Circulation.
105:2083–2091. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. 60:175–199.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mostafa TM, Hegazy SK, Elshebini EM, Saif
DS and Elabd AH: A comparative study on the anti-inflammatory
effect of angiotensin-receptor blockers & statins on rheumatoid
arthritis disease activity. Indian J Med Res. 152:393–400.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peppas S, Piovani D, Peyrin-Biroulet L,
Danese S and Bonovas S: Statins and inflammatory bowel disease:
Where do we stand? Eur J Intern Med. 75:10–14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Orellana-Urzúa S, Rojas I, Líbano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vanhoutte PM: Nitric oxide: From good to
bad. Ann Vasc Dis. 11:41–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li H and Forstermann U: Nitric oxide in
the pathogenesis of vascular disease. J Pathol. 190:244–254.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y,
Akasaki Y and Ohishi M: Estrogen-SIRT1 axis plays a pivotal role in
protecting arteries against menopause-induced senescence and
atherosclerosis. J Atheroscler Thromb. 27:47–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li L, Gao P, Zhang H, Chen H, Zheng W, Lv
X, Xu T, Wei Y, Liu D and Liang C: SIRT1 inhibits angiotensin
II-induced vascular smooth muscle cell hypertrophy. Acta Biochim
Biophys Sin (Shanghai). 43:103–109. 2011.PubMed/NCBI View Article : Google Scholar
|