Introduction
Ischemic heart disease, particularly acute
myocardial infarction (AMI), causes 2-4 million deaths in the USA,
>4 million deaths in Europe and northern Asia (1) and is responsible for >33% deaths in
developed nations annually (2). AMI
is a severe heart condition that is caused by sudden interruptions
of the blood circulation in part of the cardiac muscle, which in
turn results in the lack of sufficient oxygen to this key organ
(3-5).
The main pathological processes of MI include ischemia and hypoxia
(6). MI is characterized by severe
and persistent thoracic pain, fever, increased white blood cell
count, increased red blood cell sedimentation rate, increased serum
cardiac enzyme (creatine kinase MB and cardiac troponin I) levels
and electrocardiographic changes (such as ST segment elevation),
which may lead to arrhythmia, shock or heart failure (7-9).
Therefore, effective restoration of blood flow is crucial (10). Previous studies have reported that
several types of stem cells, particularly endothelial progenitor
cells (EPCs), can improve new blood vessel formation in local
ischemic areas safely and effectively (11,12).
EPCs has been used for AMI investigation in vitro (13-15).
For these reasons, EPCs were used in the present study.
MicroRNAs (miRNAs/miRs) are a class of small RNAs
that are 20-22 nucleotides in length and regulate
post-transcriptional gene expression in plants and animals
(16,17). miRNAs serve a variety of important
regulatory functions (18). Each
miRNA may have multiple target genes, whereas several different
miRNAs can regulate the same gene (19). This complex regulatory network can
regulate the expression of multiple genes through a single miRNA or
a combination of several miRNAs to fine-tune the expression profile
(19). Previous studies have
suggested roles for miRNAs in numerous human diseases, including
cardiovascular, gynecological, neurological and urinary system
diseases, as well as cancer (20-22).
An increasing number of studies have found that miRNAs serve an
important role in processes of blood vessel formation and repair,
which are crucial for angiogenesis (23,24).
Recently, miR-126, which participates in endothelial cell function
and angiogenesis, was reported to be highly expressed in EPCs
(25). Previous studies have found
that miR-126 acts by directly regulating the expression of negative
regulatory factors of the VEGF pathway, such as Spred-1 protein and
PI3K regulation sub-base 2 (PIK3R2) (26-28).
Therefore, upregulating the expression of Spred-1 or suppressing
the expression of VEGF may mediate similar effects to miR-126
knockout (26,29). In addition, another study previously
reported that miR-126 can negatively regulate VEGF expression in
hypoxia-induced monkey chorioretinal vessel endothelial cells
(30). However, whether miR-126 can
regulate angiogenesis and/or VEGF expression in AMI has not been
elucidated.
Therefore, the present study aimed to investigate
the effects of the miR-126/PIK3R2/VEGF axis in EPCs under hypoxic
conditions. In addition, the present study explored the possible
underlying molecular mechanism to provide a theoretical basis for
the development of novel clinical strategies for the treatment of
AMI.
Materials and methods
Cell culture and establishment of a
hypoxia EPC model
EPCs from human peripheral blood (cat. no. CC-H163;
Shanghai Enzyme Research Biotechnology Co., Ltd.; http://www.elisakits.cn/Index/productInfo/cid/148/id/769.html)
were cultured in endothelial cell growth medium-2 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and incubated at 37˚C with 5%
CO2. EPCs were exposed to hypoxia
(O2/N2/CO2, 1/94/5%) at 37˚C for
72 h to establish the cell model of hypoxic injury. EPCs cultured
under normoxic conditions served as the control. The study was
approved by the Ethics Committee of Chongqing Emergency Medical
Center (Fourth People's Hospital of Chongqing; Chongqing,
China).
Luciferase reporter analysis
Previous studies have reported the binding sites
between PIK3R2 and miR-126 (27,28).
To verify the binding sites between miR-126 and PIK3R2,
dual-luciferase reporter assay was performed. Briefly, the
3'-untranslated region (UTR) of PIK3R2, which contained the miR-126
binding site or a mutated target site, was synthesized by reverse
transcription (RT) PCR using a PrimeScript™ RT reagent kit (cat.
no. RR037A; Takara Bio, Inc.). The temperature protocol was 5 min
at 25˚C followed by 60 min at 42˚C. The wild type (WT-PIK3R2) and
mutant (MUT-PIK3R2) 3'-untranslated regions (UTR) of PIK3R2 were
cloned into the pmiR-RB-Report™ dual-luciferase reporter gene
plasmid (Guangzhou RiboBio Co., Ltd.) following the manufacturer's
protocols. 293T cells [ATCC; cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS at 37˚C with 5%
CO2] were co-transfected with 1 µg WT-PIK3R2 or 1 µg
MUT-PIK3R2 and 50 nM miR-126 mimic (5'-UCGUACCGUGAGUAAUAAUGCG-3';
Guangzhou RiboBio Co., Ltd.) or 50 nM mimic control
(5'-UAGUCAACGAGUCUAUGAGUCG-3'; Guangzhou RiboBio Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). In total, 48 h after transfection, the relative
luciferase activity was assessed using the Dual-luciferase reporter
assay system (Promega Corporation) and normalized to Renilla
luciferase, according to the manufacturer's protocols.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR analysis
Total cellular RNA was obtained using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and then the RNA was reverse-transcribed to cDNA via a
RevertAid First Strand cDNA Synthesis Kit (cat. no. K1621;
Invitrogen; Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: 70˚C for 5 min, 37˚C for 5 min and 42˚C
for 60 min. In accordance with the manufacturer's protocol, all
reactions were performed using the ABI Prism 7000 Real Time PCR
system with SYBR Green ER™ qPCR SuperMix Universal (cat. no.
11762100; Invitrogen; Thermo Fisher Scientific, Inc.) to quantify
the relative gene expression. The themocycling conditions consisted
of the following steps: 5 min at 95˚C, followed by 40 cycles at
95˚C for 10 sec and 60˚C for 30 sec. U6 or GAPDH was used as the
internal control. Primers were supplied by Sangon Biotech Co., Ltd
and listed as following: miR-126 forward,
5'-TATGGTTGTTCTCGACTCCTTCAC-3' and reverse,
5'-TCGTCTGTCGTACCGTGAGTAAT-3'; U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; PIK3R2 forward,
5'-GCACCACGAGGAACGCACTT-3' and reverse, 5'-CGTCCACTACCACGGAGCAG-3';
AKT forward, 5'-TAAAGAAGGAGGTCATCGTGG-3' and reverse,
5'-CGGGACAGGTGGAAGAAAA-3' and GAPDH forward,
5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'. The related mRNA expression levels of
miR-126, PIK3R2 and AKT were calculated by using the
2-ΔΔCq method (31).
Western blot analysis
EPCs were lysed using RIPA buffer (Beyotime
Institute of Biotechnology). The cell lysates were centrifuged at
10,000 x g at 4˚C for 15 min to obtain total protein. Subsequently,
the protein was quantified by using a bicinchoninic acid protein
kit (Beyotime Institute of Biotechnology) and equal amount of
proteins (40 µg per lane) was separated by 10% SDS-PAGE, followed
by transfer to PVDF membranes. The membranes were then blocked at
room temperature for 1 h in PBST (0.1% % Tween-20) solution
containing 5% non-fat milk. Subsequently, the membranes were
incubated with PIK3R2 (cat. no. ab180967; dilution: 1:2,000;
Abcam), phosphorylated (p-)-AKT (cat. no. 4060; dilution: 1:1,000;
Cell Signaling Technology, Inc.), AKT (cat. no. 4685; dilution:
1:1,000; Cell Signaling Technology, Inc.) or GAPDH (cat. no. 5174;
dilution: 1:1,000; Cell Signaling Technology, Inc.) primary
antibodies at 4˚C overnight. The membranes were then washed three
times with PBST and incubated with the goat anti-rabbit IgG H&L
(HRP) secondary antibody (cat. no. ab6721; dilution: 1:3,000;
Abcam) for 1 h at room temperature. The protein bands were
visualized by an electrochemiluminescence (ECL) luminescent
substrate (Pierce; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols. ImageJ version 2.0 software (National
Institutes of Health) was used to quantify the band
intensities.
Cell transfection
EPCs during the logarithmic growth phase were
inoculated into 6-well plates overnight and were transiently
transfected with 1 µg Control CRISPR Activation Plasmid
(control-plasmid; cat. no. sc-437275; Santa Cruz Biotechnology,
Inc.), 1 µg PI 3-kinase p85β CRISPR Activation Plasmids
(PIK3R2-plasmid; cat. no. sc-401991-ACT; Santa Cruz Biotechnology,
Inc.), 1 µg VEGF CRISPR Activation Plasmids (VEGF-plasmid; cat. no.
sc-400019-ACT; Santa Cruz Biotechnology, Inc.), 50 nM mimic control
or 50 nM miR-126 mimic using the Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. Following 72 h of transfection under
hypoxic conditions at 37˚C, the cells were collected to detect the
transfection efficiency by RT-qPCR analysis and following
experiments were performed. Cells were divided into the following
groups: i) Control group, where cells were cultured for 72 h under
hypoxic conditions at 37˚C; ii) control-plasmid group, where cells
were transfected with the control-plasmid for 72 h under hypoxic
conditions at 37˚C; iii) VEGF-plasmid group, where cells were
transfected with the VEGF-plasmid for 72 h under hypoxic conditions
at 37˚C; iv) mimic control group, where the cells were transfected
with the mimic control for 72 h under hypoxic conditions at 37˚C;
v) miR-126 mimic group, where cells were transfected with the
miR-126 mimic for 72 h under hypoxic conditions at 37˚C; vi)
miR-126 mimic + control-plasmid group, where cells were
co-transfected with the miR-126 mimic and control-plasmid for 72 h
under hypoxic conditions at 37˚C; and vii) miR-126 mimic +
VEGF-plasmid group, where cells were co-transfected with miR-126
mimic and VEGF-plasmid for 72 h under hypoxic conditions at
37˚C.
MTT assay
MTT assay was performed to evaluate cell viability.
The cells (1x104 cells/well) were seeded into 96-well
plates after transfection for 72 h, followed by addition of 5 mg/ml
MTT (Beyotime Institute of Biotechnology) to each well and culture
at 37˚C for 4 h. Subsequently, 100 µl DMSO was added into each well
to solubilize the formazan crystals after the culture medium was
removed. The absorbance was recorded at 570 nm using a microplate
reader (Bio-Rad Laboratories, Inc.).
Transwell migration assay
The migration ability of the EPCs was detected using
Transwell assay. The cells (2x104) were transferred onto
upper chambers (8-µm pore size; BD Biosciences) suspended in a
serum-free endothelial cell growth medium-2 after transfection for
72 h. At the same time, endothelial cell growth medium-2
supplemented with 10% FBS was added into the lower chambers.
Following 24 h at 37˚C, cells that did not migrate and persisted on
the upper surface of the filter were removed, before cells that had
migrated to the lower surface of the membrane were fixed with 4%
paraformaldehyde at room temperature for 30 min, stained with 0.1%
crystal violet solution at room temperature for 15 min and imaged
using a light microscope at x200 magnification. A total of three
independent experiments were conducted.
Tube formation assay
The tube-forming ability of the EPCs was quantified
by using Cultrex® In Vitro Angiogenesis Assay
Tube Formation Kit (cat. no. 3470-096-K; Trevigen, Inc.) according
to the manufacturer's protocols. For Basement Membrane Extract
(BME) coating, 50 µl BME solution (contained within the kit) per
well was added into the 96-well plate and the plate was incubated
at 37˚C for 60 min. In total, 3x104 EPCs were seeded
into each well containing gelled BME and incubated in Endothelial
Growth Medium-2 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 30 ng/ml FGF-2 (Trevigen, Inc.) at 37˚C for 6 h. The mean
number of complete tubes (a tube was defined as a linear sequence
of cells linking two nodes) formed by EPCs was counted in five
random fields of view under inverted light microscope at x100
magnification (TS100; Nikon Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments. The statistical significance of
the differences among groups were analyzed either using unpaired
Student's t test or one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate statistically
significant differences.
Results
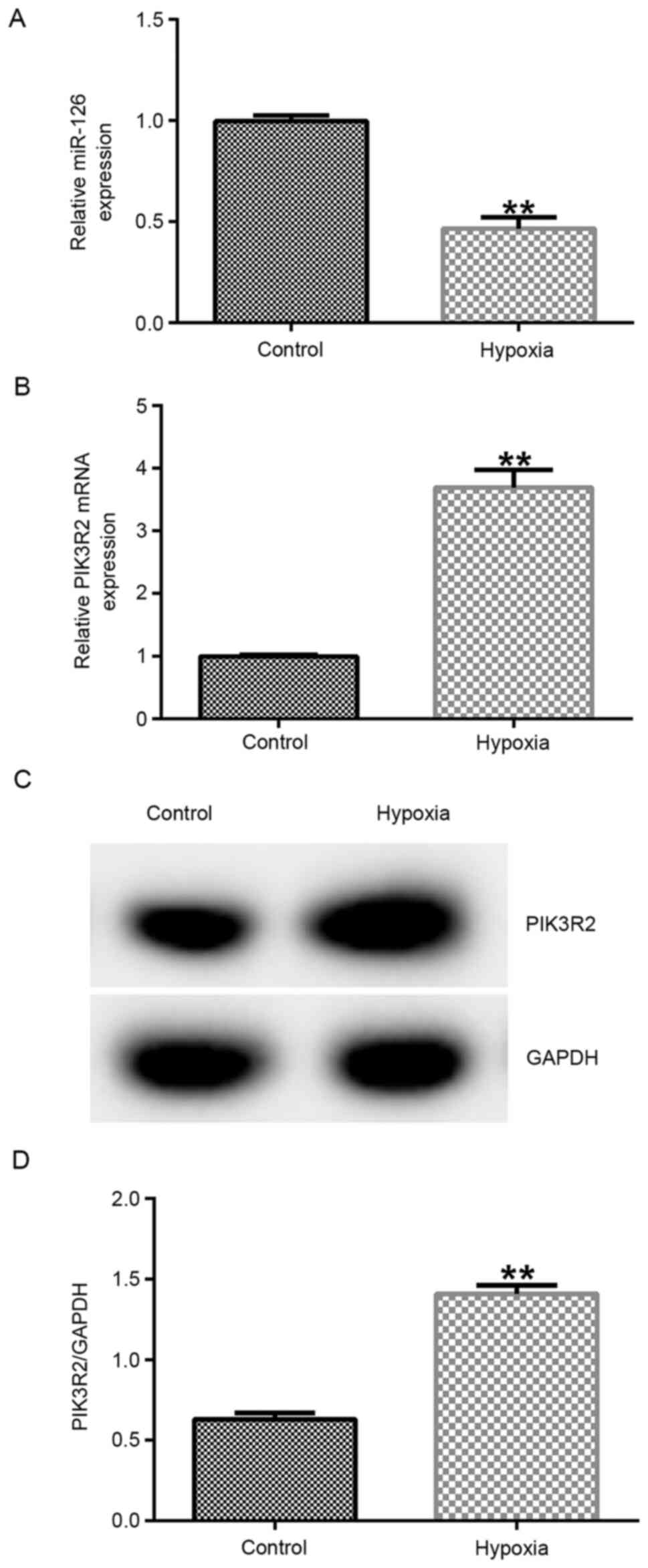
miR-126 expression is downregulated
whereas PIK3R2 expression is upregulated in hypoxia-induced
EPCs
The results demonstrated that the expression of
miR-126 was significantly downregulated in the hypoxia group
compared with that in the control group (Fig. 1A; P<0.01). Previous studies have
shown that PIK3R2 is the target gene of miR-126 (27,28).
Therefore, PIK3R2 expression was measured by RT-qPCR and western
blot analyzes, which found it to be significantly increased at both
mRNA (P<0.01) and protein levels in the hypoxia group compared
with that in the control group (Fig.
1B-D).
miR-126 negatively regulates the
expression of PIK3R2 in EPCs under hypoxic conditions
The possible binding sites between miR-126 and
PIK3R2 were predicted (Fig. 2A) and
verified using dual-luciferase reporter assay. Compared with that
in cells transfected with the mimic control, miR-126 mimic
transfection significantly enhanced miR-126 expression in 293T
cells (Fig. 2B). Subsequently,
compared with that in cells co-transfected with WT-PIK3R2 and mimic
control, the luciferase activity of cells co-transfected with
WT-PIK3R2 and miR-126 mimic significantly reduced (Fig. 2C). However, the luciferase activity
of cells co-transfected with MUT-PIK3R2 and mimic control and cells
co-transfected with MUT-PIK3R2 and miR-126 mimic exhibited no
significant changes (Fig. 2C).
Compared with that in their respective control plasmid and mimic
groups, transfection with PIK3R2-plasmid or miR-126 mimic
significantly increased the expression of PIK3R2 and miR-126 in
EPCs under hypoxic conditions, respectively (Fig. 2D and E). In addition, miR-126 mimic transfection
significantly reduced the expression of PIK3R2 at both mRNA and
protein levels, whilst this reduction was significantly reversed by
PIK3R2-plasmid co-transfection (Fig.
2F-H).
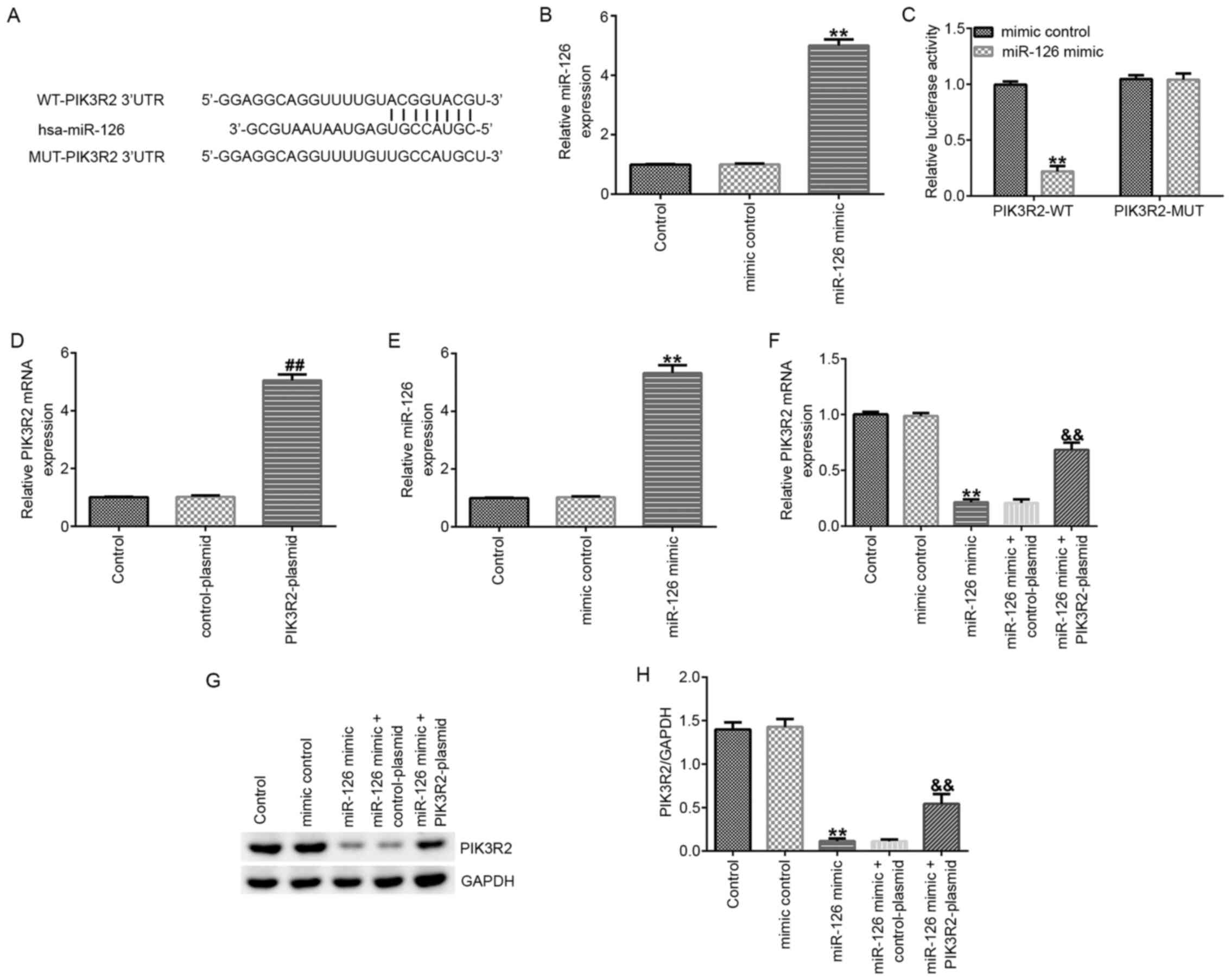 | Figure 2miR-126 overexpression negatively
regulates PIK3R2 expression in EPCs. (A) Possible binding sites
between miR-126 and PIK3R2 3'UTR. (B) 293T cells were transfected
with the mimic control or miR-126 mimic for 48 h, before miR-126
expression was detected using RT-qPCR. (C) Potential interactions
between miR-126 and PIK3R2 were assessed using dual-luciferase
reporter assay. (D-H) EPCs were transfected with control-plasmid,
PIK3R2-plasmid, mimic control, miR-126 mimic, miR-126 mimic +
control-plasmid or miR-126 mimic+PIK3R2-plasmid under hypoxic
conditions. (D) PIK3R2 and (E) miR-126 mRNA expression in EPCs was
measured by RT-qPCR after plasmid or mimic transfection,
respectively. (F) PIK3R2 mRNA expression in EPCs was measured by
RT-qPCR after mimic and plasmid co-transfection. (G) PIK3R2 protein
expression in EPCs was measured by western blot analysis, (H) which
was quantified. **P<0.01 vs. mimic control.
##P<0.01 vs. control-plasmid.
&&P<0.01 vs. miR-126 mimic + control-plasmid.
miR, microRNA; UTR, untranslated region; WT, wild-type; MUT,
mutant; RT-qPCR, reverse transcription-quantitative PCR; EPCs,
endothelial progenitor cells; PIK3R2, PI3K regulation subunit
2. |
miR-126 affects the cell viability,
migration and tube-forming ability of EPCs by targeting PIK3R2
Compared with those in the control group, the cell
viability (Fig. 3A), tube-forming
ability (Fig. 3B and C) and migration (Fig. 3D and E) of EPCs in the hypoxia group were
significantly decreased. In addition, compared with those in the
hypoxia + mimic control group, the proliferation (Fig. 3A), tube-forming ability (Fig. 3B and C) and migration (Fig. 3D and E) of EPCs in the hypoxia + miR-126 mimic
group were significantly increased, which were all significantly
reversed by co-transfection with the PIK3R2-plasmid (Fig. 3A-E).
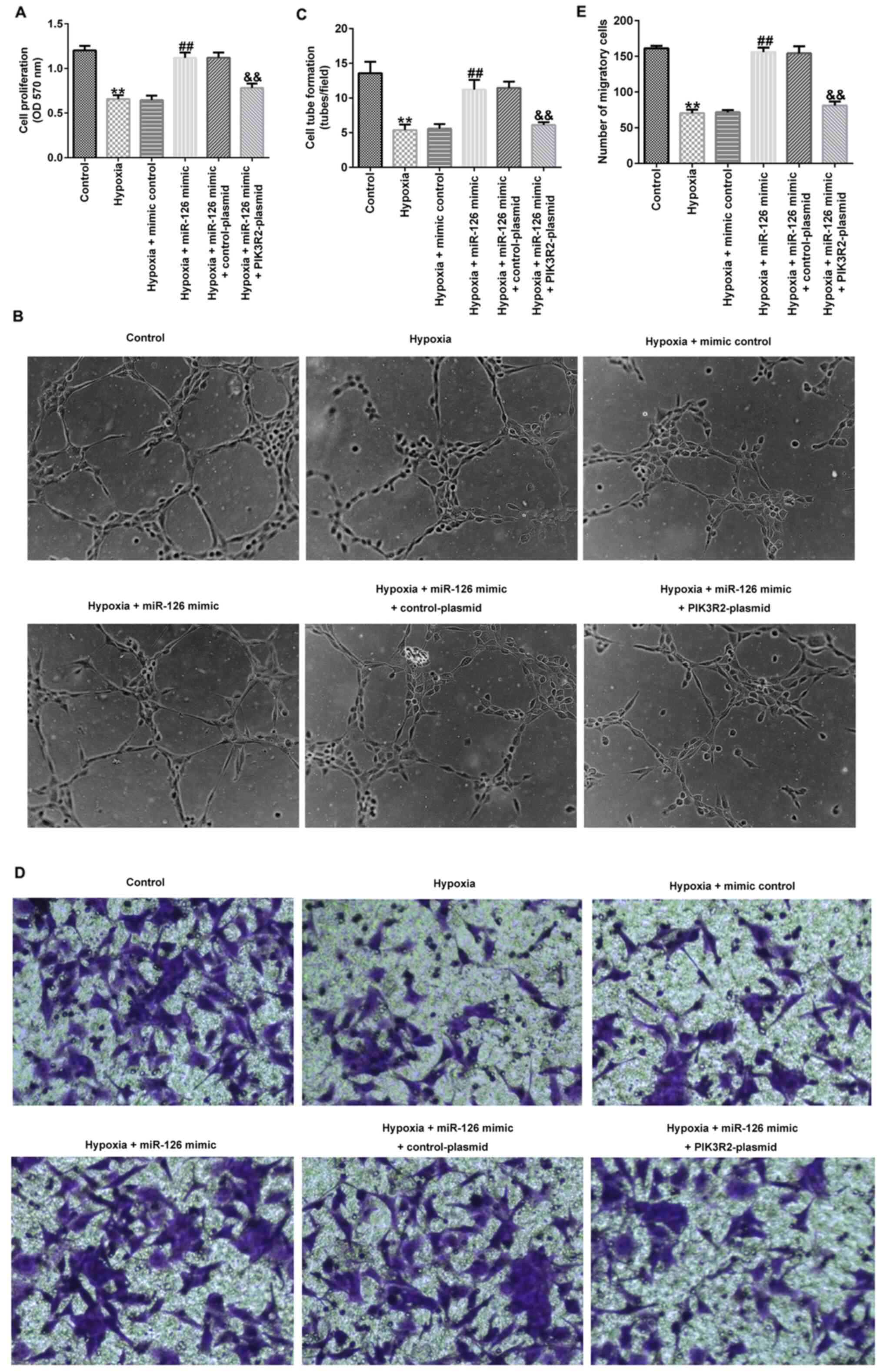 | Figure 3Effects of miR-126 mimic and
PIK3R2-plasmid transfection on the viability, tube-forming ability
and migration of EPCs under hypoxic conditions. EPCs were
transfected with control-plasmid, PIK3R2-plasmid, mimic control,
miR-126 mimic, miR-126 mimic + control-plasmid or miR-126 mimic +
PIK3R2-plasmid under hypoxic conditions for 72 h. (A) MTT assay was
performed to evaluate the cell viability of transfected EPCs. (B)
Tube formation assay was used to measure the tube-forming ability
of transfected EPCs. Magnification, x100. (C) Tube formation assay
was quantified. (D) Transwell assay was used to measure the
migration ability of transfected EPCs. Magnification, x200. (E)
Transwell assay was quantified. **P<0.01 vs. Control.
##P<0.01 vs. hypoxia + mimic control.
&&P<0.01 vs. hypoxia + miR-126 mimic +
control-plasmid. miR, microRNA; PIK3R2, PI3K regulation subunit 2;
OD, optical density; EPCs, endothelial progenitor cells. |
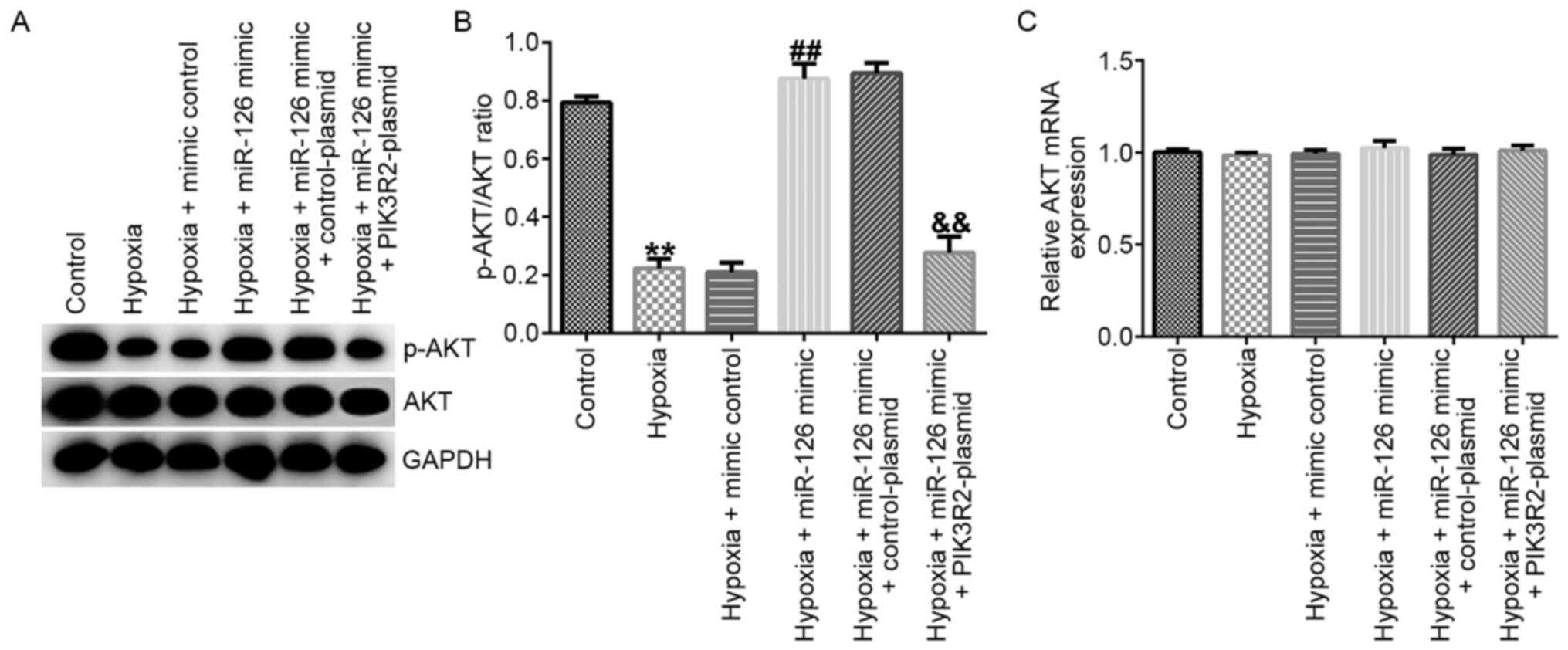
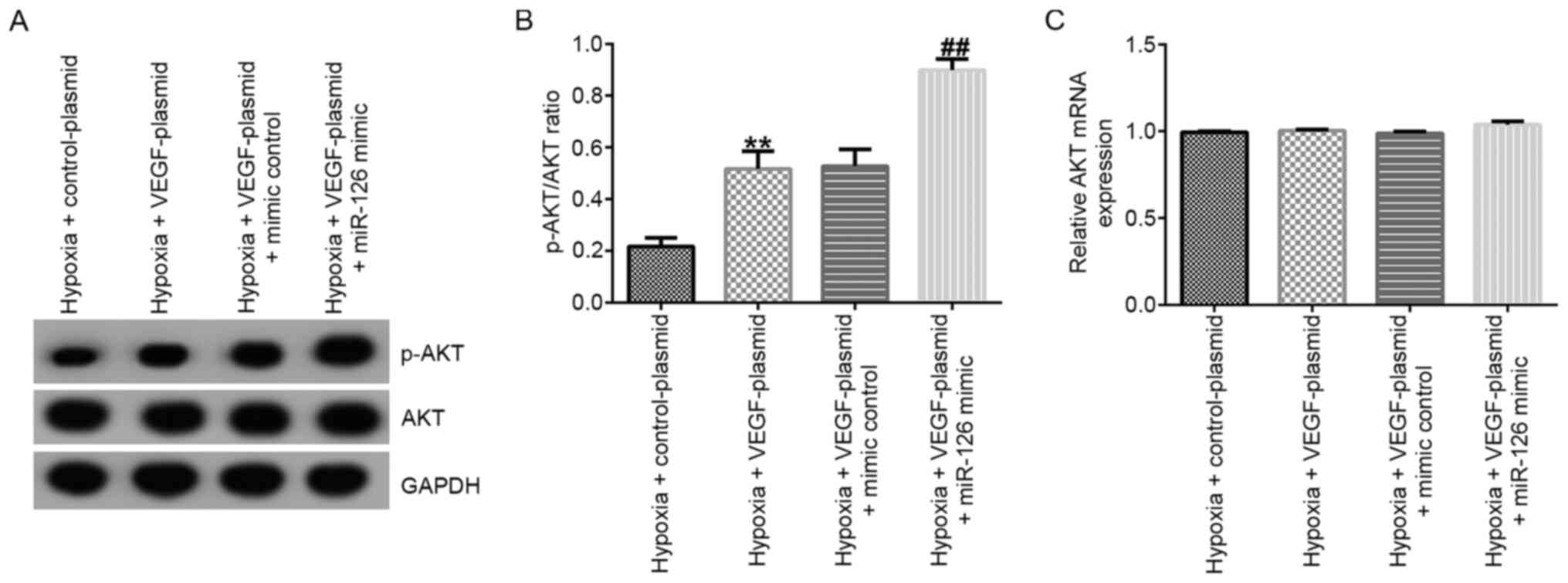
Western blotting and RT-qPCR assays were performed
to measure the related protein and mRNA expression of AKT in EPCs.
The results demonstrated that, compared with those in the control
group, the protein levels of p-AKT (Fig. 4A) and the p-AKT/AKT ratio (Fig. 4B) were significantly decreased in
EPCs of the hypoxia group. In comparison with those in the hypoxia
+ mimic control group, the protein levels of p-AKT (Fig. 4A) and the p-AKT/AKT ratio (Fig. 4B) were significantly increased
hypoxia + miR-126 mimic group, which were all significantly
reversed by PIK3R2-plasmid co-transfection (Fig. 4A and B). However, the mRNA expression of AKT did
not differ significantly among the six groups (Fig. 4C).
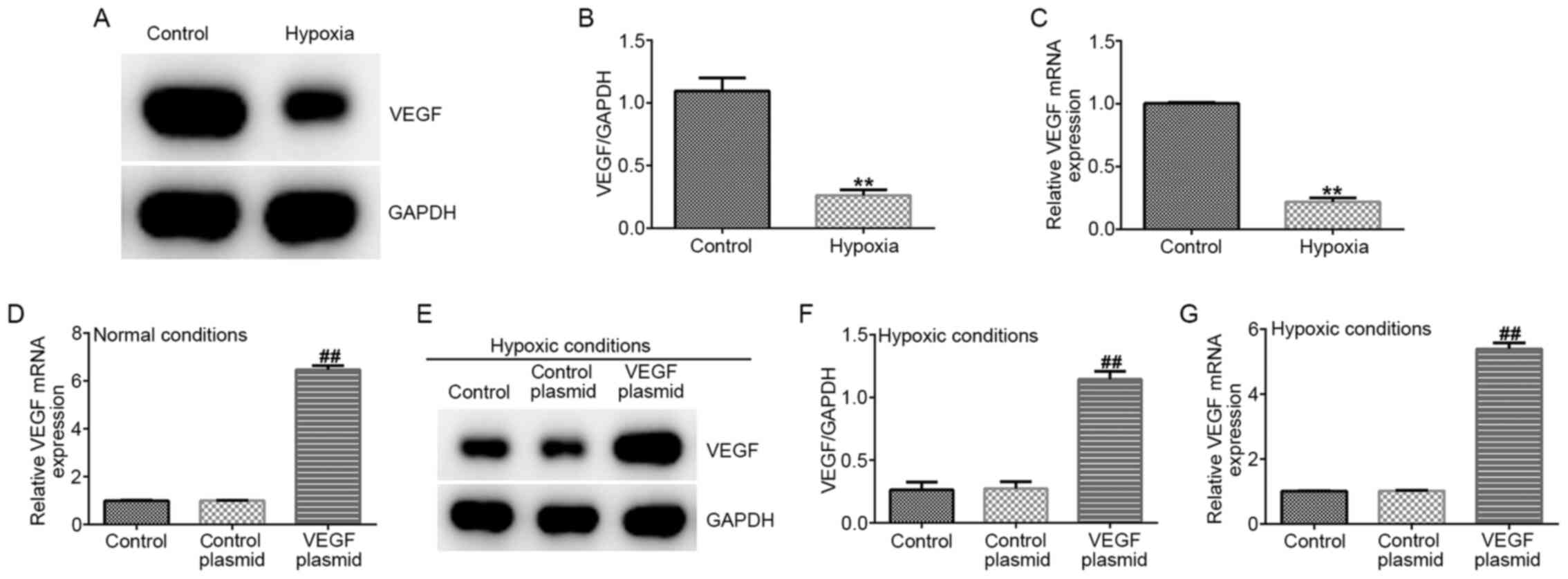
VEGF expression is reduced by hypoxia
in EPCs
To further explore the relationship between miR-126
and VEGF in EPCs under hypoxic conditions, the protein and mRNA
expression of VEGF were measured. VEGF expression was found to be
significantly lower in the hypoxia group compared with that in the
control group on both protein and mRNA levels (Fig. 5A-C). In addition, EPCs were
transfected with either the control-plasmid or VEGF-plasmid under
normal conditions, following which it was observed that
VEGF-plasmid transfection significantly increased VEGF mRNA levels
in EPCs under normal conditions compared with that in cells
transected with the control plasmid (Fig. 5D). In addition, EPCs were
transfected with either the control-plasmid or VEGF-plasmid under
hypoxic conditions, where it was observed that VEGF-plasmid
transfection increased the expression of VEGF on both protein and
mRNA levels in EPCs under hypoxic conditions compared with that in
cells transected with the control plasmid (Fig. 5E-G).
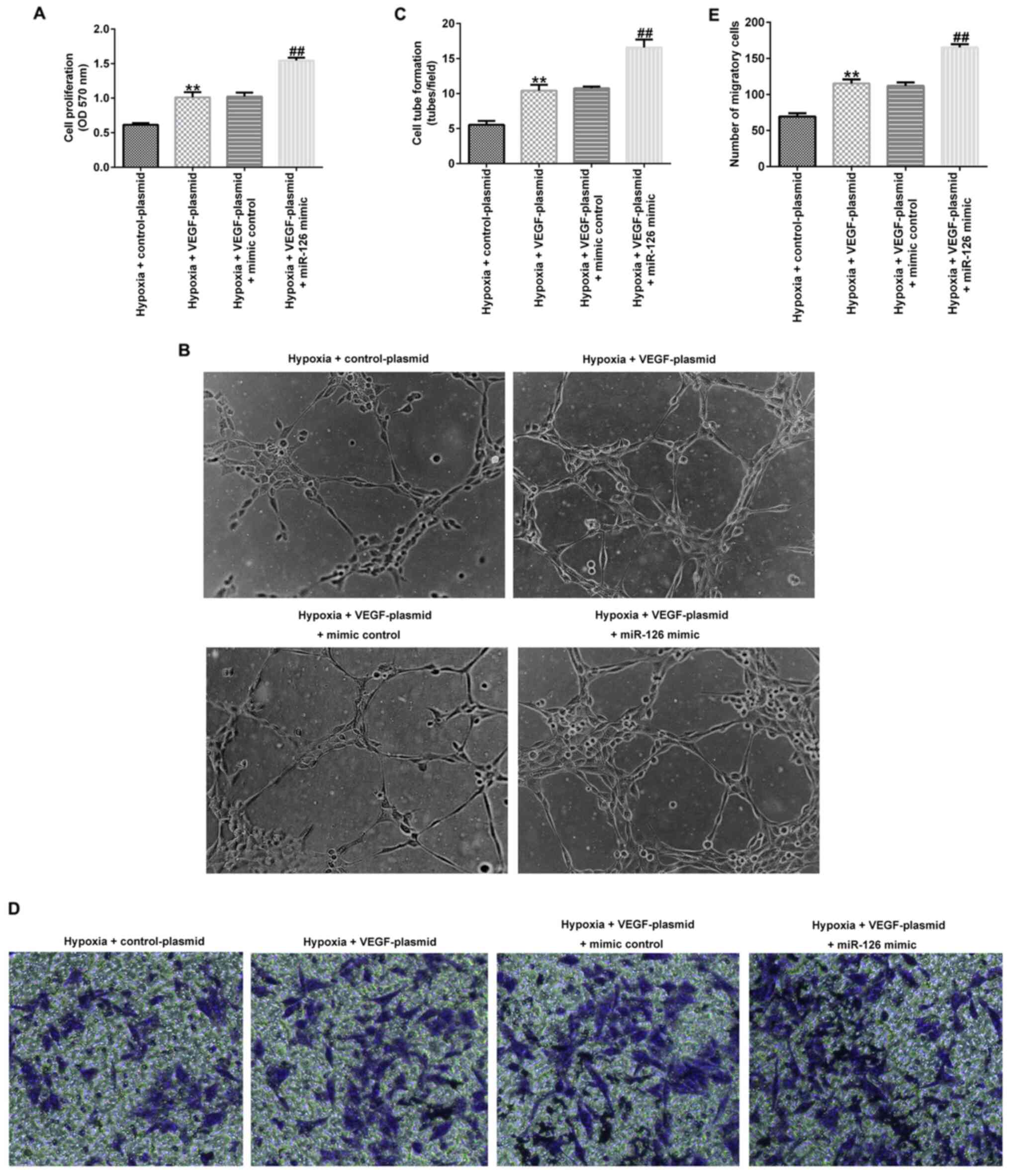
VEGF overexpression enhances the
effects of miR-126 on EPCs under hypoxic conditions
Several experiments were performed after the cells
were transfected for 72 h under hypoxic conditions. As shown in
Fig. 6A-E, VEGF-plasmid
transfection significantly increased the cell viability (Fig. 6A), tube-forming ability (Fig. 6B and C) and migration (Fig. 6D and E) of EPCs under hypoxic conditions
compared with those in cells transfected with the control-plasmid.
However, co-transfection with the miR-126 mimic significantly
potentiated all of these aforementioned effects.
Furthermore, it was observed that transfection with
the VEGF-plasmid significantly enhanced the protein levels of p-AKT
(Fig. 7A) and the p-AKT/AKT ratio
(Fig. 7B) in EPCs under hypoxic
conditions compared with those in cells transfected with the
control-plasmid. These aforementioned effects were significantly
potentiated by co-transfection with the miR-126 mimic. However,
VEGF overexpression did not affect the mRNA expression of AKT
(Fig. 7C).
Discussion
AMI is one of the main causes of morbidity and
mortality worldwide (3,5,32).
EPCs are also known as angioblasts and belong to a family of
precursor cells in the vascular endothelium (33). Previous studies have shown that EPCs
serve an important role in cardiovascular and cerebrovascular
diseases, peripheral vascular diseases, tumor angiogenesis and
wound healing, where they can potentially provide novel approaches
for the treatment of ischemic diseases (34,35).
There have also been an increasing number of studies on the
biological characteristics and therapeutic effects of EPCs
(36,37). The proliferation and migration of
endothelial cells serve an important role in maintaining vascular
integrity, regeneration and wound repair (38). In particular, miR-126 was previously
demonstrated to mediate a key function in maintaining the integrity
of endothelial cells, inflammation, angiogenesis and vascular
repair (39). EPCs were used to
establish an in vitro hypoxia model in the present study,
where it was verified that miR-126 expression was reduced in EPCs
under hypoxic conditions, which is consistent with previous finding
(40). However, the phenotypic
confirmation of the EPCs by FACS/flow cytometry was not performed
in this study, which was a limitation of the present study.
It has been previously reported that PIK3R2, which
encodes the p85β regulatory subunit of PI3K, is a target of miR-126
(27,28,41,42).
In the present study, it was confirmed that miR-126 can directly
target PIK3R2. Therefore, it was hypothesized that miR-126 may
regulate the proliferation of EPCs by targeting PIK3R2 expression.
To further study the molecular mechanisms through which miR-126
regulates the proliferation of EPCs, the cells were transfected for
72 h and divided into the following groups: Control, hypoxia,
hypoxia + mimic control, hypoxia + miR-126 mimic, hypoxia + miR-126
mimic + control-plasmid and hypoxia + miR-126 mimic +
PIK3R2-plasmid. MTT, Transwell and tube formation assays were used
to detect cell proliferation, migration and tube-forming ability,
respectively. It was observed that upregulating miR-126 could
promote the viability, migration and tube-forming capabilities of
EPCs under hypoxic conditions by downregulating the expression of
PIK3R2. Previous studies have shown that miR-126 can upregulate the
response of cells to VEGF by directly inhibiting a number of its
inhibitors, including Spred-1 and PIK3R2 (43-45).
However, whether miR-126 regulates angiogenesis through VEGF during
myocardial ischemia remains poorly understood. The relationship
between miR-126 and VEGF in the regulation of EPC physiology under
hypoxic conditions was investigated in the present study, where the
results suggest that VEGF expression was reduced in EPCs under
hypoxic conditions and VEGF upregulation enhanced cellular
functions (cell proliferation, migration, and tube formation
ability) by combining with miR-126.
The PI3K/AKT pathway appears to serve an important
role in improving the function of EPCs by regulating the migration
and angiogenesis of EPCs (46,47).
Previous studies have also reported that miR-126 can regulate
PI3K/AKT pathway activation in cancer cells such as non-small cell
lung cancer and bladder cancer cells (28,41).
However, the possible effects on the PI3K/AKT pathway exerted by
changes in miR-126 expression in EPCs under hypoxic conditions
remain unclear. Therefore, activities of AKT was measured in this
study. It was found that miR-126 overexpression enhanced AKT
activation in EPCs under hypoxic conditions, which was reversed by
PIK3R2 overexpression. In addition, it was confirmed that VEGF and
miR-126 mediate a synergistic role in promoting the activation of
AKT.
However, there were some limitations in the present
study. For example, the effects of miR-126 and VEGF overexpression
on apoptosis and/or necrosis in EPCs under hypoxic conditions were
not investigated. In addition, since the differences in cell
viability, tube forming abilities and migration of EPCs were
already between the normoxic control and hypoxic condition were
already observed, the normoxic control group was not designated in
the miR-126 and VEGF-plasmid co-transfection experiments, which
would've made the results more credible. In addition, the role of
miR-126/VEGF on EPC function in AMI should be studied in
vivo.
In conclusion, the present study demonstrated that
miR-126 overexpression promoted the functions of EPCs under hypoxic
conditions by downregulating PIK3R2 expression, where the
combination of miR-126 and VEGF overexpression can potentiate these
effects. These findings suggest that targeting miR-126 and VEGF may
provide novel directions for the clinical treatment of AMI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Basic and
Frontier Projects Of Science And Technology Plan in Yuzhong
District, Chongqing (grant no. 20160122).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JX contributed to study design, data
collection and interpretation and statistical analysis. YX, KZ and
GK contributed to performing the experiments and statistical
analysis. YZ and JX confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Emergency Medical Center (Fourth People's
Hospital of Chongqing; Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nichols M, Townsend N, Scarborough P and
Rayner M: Cardiovascular disease in Europe 2014: Epidemiological
update. Eur Heart J. 35:2950–2959. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yeh RW, Sidney S, Chandra M, Sorel M,
Selby JV and Go AS: Population trends in the incidence and outcomes
of acute myocardial infarction. N Engl J Med. 362:2155–2165.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tibaut M, Mekis D and Petrovic D:
Pathophysiology of Myocardial Infarction and Acute Management
strategies. Cardiovasc Hematol Agents Med Chem. 14:150–159.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anderson JL and Morrow DA: Acute
myocardial infarction. N Engl J Med. 376:2053–2064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gulati R, Behfar A, Narula J, Kanwar A,
Lerman A, Cooper L and Singh M: Acute myocardial infarction in
young individuals. Mayo Clin Proc. 95:136–156. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Arora G and Bittner V: Chest pain
characteristics and gender in the early diagnosis of acute
myocardial infarction. Curr Cardiol Rep. 17(5)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shah AH, Puri R and Kalra A: Management of
cardiogenic shock complicating acute myocardial infarction: A
review. Clin Cardiol. 42:484–493. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abed MA, Ali RM, Abu Ras MM, Hamdallah FO,
Khalil AA and Moser DK: Symptoms of acute myocardial infarction: A
correlational study of the discrepancy between patients'
expectations and experiences. Int J Nurs Stud. 52:1591–1599.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ndrepepa G, Keta D, Schulz S, Byrne RA,
Mehilli J, Pache J, Seyfarth M, Schömig A and Kastrati A:
Prognostic value of minimal blood flow restoration in patients with
acute myocardial infarction after reperfusion therapy. Clin Res
Cardiol. 99:13–19. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Peters EB: Endothelial progenitor cells
for the vascularization of engineered tissues. Tissue Eng Part B
Rev. 24:1–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Naito H, Iba T and Takakura N: Mechanisms
of new blood-vessel formation and proliferative heterogeneity of
endothelial cells. Int Immunol. 32:295–305. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X, Xue X, Sun Y, Chen L, Zhao T, Yang
W, Chen Y and Zhang Z: MicroRNA-326-5p enhances therapeutic
potential of endothelial progenitor cells for myocardial
infarction. Stem Cell Res Ther. 10(323)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang H, Xu Z, Qi Y, Zhang W, Zhang C,
Jiang M, Deng S and Wang H: Exosomes from SIRT1-Overexpressing
ADSCs restore cardiac function by improving angiogenic function of
EPCs. Mol Ther Nucleic Acids. 21:737–750. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou W, Zhou W, Zeng Q and Xiong J:
MicroRNA-138 inhibits-hypoxia-induced proliferation of endothelial
progenitor cells via inhibition of HIF-1α-mediated MAPK and AKT
signaling. Exp Ther Med. 13:1017–1024. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Juźwik CA, S Drake S, Zhang Y,
Paradis-Isler N, Sylvester A, Amar-Zifkin A, Douglas C, Morquette
B, Moore CS and Fournier AE: MicroRNA dysregulation in
neurodegenerative diseases: A systematic review. Prog Neurobiol.
82(101664)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun IO and Lerman LO: Urinary microRNA in
kidney disease: Utility and roles. Am J Physiol Renal Physiol.
316:F785–F793. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wojciechowska A, Braniewska A and
Kozar-Kamińska K: MicroRNA in cardiovascular biology and disease.
Adv Clin Exp Med. 26:865–874. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Johnson JL: Elucidating the contributory
role of microRNA to cardiovascular diseases (a review). Vascul
Pharmacol. 114:31–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun J, Zhang Z, Ma T, Yang Z, Zhang J, Liu
X, Lu D, Shen Z, Yang J and Meng Q: Endothelial progenitor
cell-derived exosomes, loaded with miR-126, promoted deep vein
thrombosis resolution and recanalization. Stem Cell Res Ther.
9(223)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meng S, Cao JT, Zhang B, Zhou Q, Shen CX
and Wang CQ: Downregulation of microRNA-126 in endothelial
progenitor cells from diabetes patients, impairs their functional
properties, via target gene Spred-1. J Mol Cell Cardiol. 53:64–72.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fu R and Tong JS: MiR-126 reduces
trastuzumab resistance by targeting PIK3R2 and regulating AKT/mTOR
pathway in breast cancer cells. J Cell Mol Med. 24:7600–7608.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D
and Peng LP: MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell
proliferation, migration, and invasion by regulation of
PTEN/PI3K/AKT pathway. Clin Lung Cancer. 17:e65–e75.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
MiR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ye P, Liu J, He F, Xu W and Yao K:
Hypoxia-induced deregulation of miR-126 and its regulative effect
on VEGF and MMP-9 expression. Int J Med Sci. 11:17–23.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ratajska A, Jankowska-Steifer E,
Czarnowska E, Olkowski R, Gula G, Niderla-Bielińska J, Flaht-Zabost
A and Jasińska A: Vasculogenesis and its cellular therapeutic
applications. Cells Tissues Organs. 203:141–152. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yuan JJ, Yang J, Sun SL, Zhang R and Xu
YM: Endothelial progenitor cells' classification and application in
neurological diseases. Tissue Eng Regen Med. 14:327–332.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma Y, Jiang L, Wang L, Li Y, Liu Y, Lu W,
Shi R, Zhang L, Fu Z, Qu M, et al: Endothelial progenitor cell
transplantation alleviated ischemic brain injury via inhibiting
C3/C3aR pathway in mice. J Cereb Blood Flow Metab. 40:2374–2386.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Steinle H, Golombek S, Behring A,
Schlensak C, Wendel HP and Avci-Adali M: Improving the angiogenic
potential of EPCs via engineering with synthetic modified mRNAs.
Mol Ther Nucleic Acids. 13:387–398. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zeng YC, Peng LS, Zou L, Huang SF, Xie Y,
Mu GP, Zeng XH, Zhou XL and Zeng YC: Protective effect and
mechanism of lycopene on endothelial progenitor cells (EPCs) from
type 2 diabetes mellitus rats. Biomed Pharmacother. 92:86–94.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klein-Soyer C, Beretz A, Cazenave JP,
Driot F and Maffrand JP: Behavior of confluent endothelial cells
after irradiation. Modulation of wound repair by heparin and acidic
fibroblast growth factor. Biol Cell. 68:231–238. 1990.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen G, Li P, Liu Z, Zeng R, Ma X, Chen Y,
Xu H, Li Z and Lin H: Enrichment of miR-126 enhances the effects of
endothelial progenitor cell-derived microvesicles on modulating
MC3T3-E1 cell function via Erk1/2-Bcl-2 signalling pathway. Prion.
13:106–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pan Q, Zheng J, Du D, Liao X, Ma C, Yang
Y, Chen Y, Zhong W and Ma X: MicroRNA-126 priming enhances
functions of endothelial progenitor cells under physiological and
hypoxic conditions and their therapeutic efficacy in cerebral
ischemic damage. Stem Cells Int. 2018(2912347)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xiao J, Lin HY, Zhu YY, Zhu YP and Chen
LW: MiR-126 regulates proliferation and invasion in the bladder
cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt
signaling pathway. Onco Targets Ther. 9:5181–5193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang WZ, Yang J, Xue LP, Xiao LB and Li Y:
MiR-126 overexpression inhibits high glucose-induced migration and
tube formation of rhesus macaque choroid-retinal endothelial cells
by obstructing VEGFA and PIK3R2. J Diabetes Complications.
31:653–663. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li SN, Li P, Liu WH, Shang JJ, Qiu SL,
Zhou MX and Liu HX: Danhong injection enhances angiogenesis after
myocardial infarction by activating MiR-126/ERK/VEGF pathway.
Biomed Pharmacother. 120(109538)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang L, Ouyang P, He G, Wang X, Song D,
Yang Y and He X: Exosomes from microRNA-126 overexpressing
mesenchymal stem cells promote angiogenesis by targeting the
PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med.
25:2148–2162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nammian P, Razban V, Tabei SMB and
Asadi-Yousefabad SL: MicroRNA-126: Dual role in angiogenesis
dependent diseases. Curr Pharm Des. 26:4883–4893. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen X, Chen Q, Wang L and Li G: Ghrelin
induces cell migration through GHSR1a-mediated PI3K/Akt/eNOS/NO
signaling pathway in endothelial progenitor cells. Metabolism.
62:743–752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li WD, Zhou DM, Sun LL, Xiao L, Liu Z,
Zhou M, Wang WB and Li XQ: LncRNA WTAPP1 promotes migration and
angiogenesis of endothelial progenitor cells via MMP1 through
MicroRNA 3120 and Akt/PI3K/autophagy pathways. Stem Cells.
36:1863–1874. 2018.PubMed/NCBI View Article : Google Scholar
|