1. Introduction
Parkinson's disease (PD) is the second most frequent
neurodegenerative disorder following Alzheimer's disease, affecting
almost 1% of the population over 60 years of age and 5% in
individuals up to 85 years (1).
Advanced stages of PD, 4 or 5 of the Hoehn and Yahr Scale, are
characterized by severe motor complications, limited mobility
without assistance, risk of falling, and non-motor complications
(1). PD affects individuals
differently and not all the patients may experience the full range
of non-motor symptoms. Patients with stage 4 are unable to live an
independent life and require permanent assistance throughout the
day and are not capable of walking or standing unassisted, but they
frequently use a walker to help them. When patients reach the final
stage of PD, stage five, they require a wheelchair or may be
immobilized. An around-the-clock care team is mandatory to help the
patient which is overwhelmed by critical non-motor symptomatology.
Furthermore, the intensification of critical symptomatology is
frequently a consequence of insufficient optimization of
levodopa-carbidopa therapy. Since dementia also occurs in up to 75%
of individuals with stage 5 and the side effects of pharmacological
agents outweigh their benefits, caring for these patients
represents a real challenge for their families and for the
healthcare personnel (1-3).
The number of patients with advanced stages of PD
has increased as a direct consequence of a greater life expectancy
and an improved clinical management. Although in the last decade
considerable progress has been made in PD pharmacological therapy,
particularly in the advanced stages, over the years, the treatment
loses its efficacy, thereby reducing the quality of life (QoL) and
lifespan of the patient (1).
Pharmacological therapy of the advanced stages of PD is completely
different from treatment of the earlier stages and is focused on a
multidisciplinary team that could help overcome the critical
non-motor symptomatology consisting of hallucinations, psychosis,
dysphagia, urinary incontinence, orthostatic hypotension, postural
instability, and multiple fractures (1-3).
Notwithstanding the fact that current therapeutic
guidelines are comprehensive and accurate, tailored therapy is
still recommended for each individual patient (1-3).
This approach implies a careful assessment of a considerable number
of resources, including professional healthcare systems,
specialized personnel, interdisciplinary collaborations and
synchronized medical procedures. Unfortunately, the unprecedented
shortage of healthcare workers is worsening constantly worldwide,
with artificial intelligence (AI)-based medical technologies
emerging as one of the most promising solutions to this problem
(4,5).
At present, only a limited range of specific
AI-based medical systems are available in clinical practice,
including the automated detection of atrial fibrillation, seizures,
or automated imaging computer-aided diagnosis (4). Additionally, as the global population
ages, the economic burden of neurodegenerative disorders is
significantly increasing, thereby any measures or technologies that
prevent, delay, or improve PD evolution may contribute to reduced
healthcare system costs (5). The
present review therefore aimed to provide a practical overview of
specific AI systems for the management of advanced stages of PD and
to discuss future directions of implementation of AI systems in
clinical practice, as well as relevant technologic limitations.
2. Methods
To identify potentially eligible observational
studies evaluating AI applications and robotic systems used in the
management of PD patients with advanced stages, the authors
conducted a systematic search of PubMed and EMBASE from inception
until the 4th of December 2020 without restrictions. The search
strategy applied in these two databases included the following
search string for PubMed [(‘advanced PD’(MeSH)] or (‘AI’) or
[‘AI’(MeSH)] or (‘automated’) or [‘telemedicine’(MeSH)] or
(‘machine learning’) or (‘deep learning’) or [‘home-based
telemedicine’(MeSH)] and the following search string for EMBASE
(‘advanced PD’/exp or ‘AI’ or ‘AI’/exp or ‘telemedicine’ or ‘deep
learning’/exp or ‘home-based telemedicine’).
Furthermore, to minimize results bias, the reference
list was manually searched for pertinent articles to identify any
additional relevant missed publications. Exclusion criteria
included the following: Other stages of PD than 4 and 5 of the
Hoehn and Yahr Scale, case reports, reviews, practice guidelines,
commentaries, opinions, letters, editorials, short surveys,
articles in press, conference abstracts, conference papers, and
abstracts published without a full article.
According to the aforementioned eligibility
criteria, two investigators (LPD and LCP) performed a screening
evaluation independently by scrutinizing titles and abstracts
excluding any apparently irrelevant studies. Subsequently, selected
articles fulfilling the inclusion and exclusion criteria were
further evaluated by carefully reviewing the entire text. A mutual
consensus was reached by discussing any discrepancies regarding
study eligibility. One investigator (LPD) extracted the data
through an electronic spreadsheet, and then the other investigator
(LCP) reviewed the extracted data for accuracy. Discrepancies
regarding the results of extracted data were settled by discussion.
Extracted data was then entered into three tables, while final data
was collated and presented in the following sections of the
review.
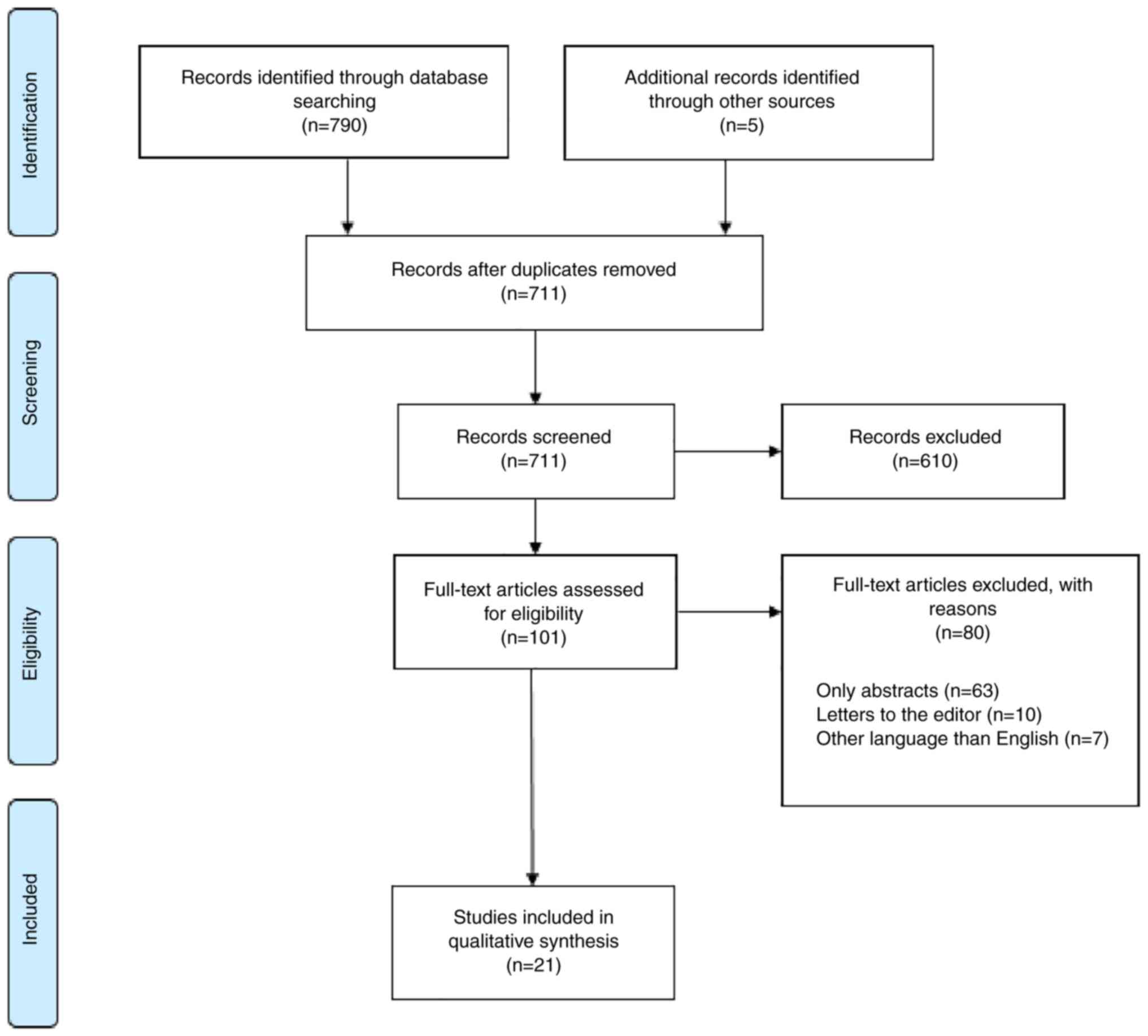
The search algorithm identified 21 studies that were
included in the qualitative synthesis (Fig. 1).
3. AI applications for autonomous management
of pharmacologic therapy
The search identified 6 articles regarding
autonomous management of pharmacologic PD therapy (Table I). A study performed by Li et
al revealed how extracting movement information from 2D videos
using deep-learning-based pose estimation algorithm (Convolution
Pose Machines) could detect disease severity or levodopa-induced
dyskinesia (LID) (6). They used
nine participants diagnosed with PD and LID and evaluated those
regularly using standardized scales such as the Unified Dyskinesia
Rating Scale (UDysRS) and Unified Parkinson's Disease Rating Scale
(UPDRS). This information was corroborated with movement
trajectories of individual joints extracted from videos. Specific
features of movement trajectories were used to train random forests
to detect and estimate PD severity and LID presence. The tapping
task presented satisfactory results for PD detection, while leg
agility detected PD severity. Based on the communication task,
authors were capable of estimating LID presence (6).
 | Table IArtificial intelligence applications
for autonomous management of pharmacologic therapy used in advanced
stages of PD. |
Table I
Artificial intelligence applications
for autonomous management of pharmacologic therapy used in advanced
stages of PD.
| First author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|
| Li et
al | 2018 | Observational
study | Extracting movement
information from 2D videos using a deep-learning-based pose
estimation algorithm (Convolution Pose Machines) | Levodopa | Communication
task-evaluation detected levodopa-induced dyskinesia. Toe-tapping
detected parkinsonism, while leg agility detected parkinsonism
severity | (6) |
| Shamir et
al | 2015 | Observational
study | Creation of a CDSS
that retrieves patient information, visualizes drug treatment, and
recommends deep brain stimulation and drug dosages based on 3
machine-learning methods (support vector machines, Naïve Bayes and
random forest) | Levodopa Deep brain
stimulation | A CDSS that uses
appropriate parameters and has the potential of improving the
clinical management of PD patients | (7) |
| Tucker et
al | 2015 | Clinical
observational study | Data mining using
non-wearable sensors to model and predict adherence to drug
treatment outside the hospital, based on gait variations | PD medication
Levodopa | Whole-body movement
data allow cost-effective, remote monitoring of drug treatment
adherence in PD | (8) |
| Turner et
al | 2016 | Clinical
observational study | EpiNet, a novel
artificial gene regulatory network, with dynamic analysis
transition between different states of PD | Levodopa | EpiNet can
discriminate between different movement patterns indicative of
levodopa including optimal, insufficient, or excessive
treatment | (9) |
| Yue et
al | 2017 | Case study | WGCNA was used to
identify two over-represented PD-specific gene co-expression
network modules and using drug-protein regulatory relationship
databases (DMAP), developed a DESS for candidate drugs that could
restore gene expression in the identified modules | Novel PD drugs that
could restore gene expression in the identified modules | Integrating gene
co-expression modules with biomolecular interaction network
analysis can lead to the identification of network modules
important in PD pathways This approach can help identify drugs
useful in polygenic diseases such as PD | (10) |
| Przybyszewski et
al | 2016 | Clinical
experimental study | Rough set theory
was used to integrate reflexive saccades (latency, amplitude, and
duration) to develop predictions of neurological symptoms in
patients | Deep brain
stimulation Levodopa | Reflexive saccades
can be a powerful biomarker in the assessment of symptom
progression in PD | (11) |
Shamir et al created a complex clinical
decision support system (CDSS) based on support vector machines,
Naïve Bayes and random forest machine learning methods (7). The CDSS was created using diagnosis
information and drug treatment from 10 patients following 89
postoperative visits post-deep brain stimulation (DBS) to make
information retrieval, visualization of treatment and
recommendations either for deep brain stimulation or for drug
treatment adjustments. The CDSS was capable of predicting 86% of
the motor improvement scores at one year following DBS (7).
Lack of adherence to drug treatment is a widespread
problem, but in PD it is even more prevalent (more than 61% of
patients) due to the cognitive impairment associated with old-age
and to disease progression, particularly in the lack of adherence
(8). As adherence to drug treatment
is usually assessed by self-reporting throughout short clinical
visits, non-adherence may be easily missed. Tucker et al
used non-wearable sensors to model and predict adherence to drug
treatment outside the hospital, based on gait variations, using
data mining (8). The authors used
seven voluntary patients first off their dopaminergic drugs
(overnight, >12 h) and subsequently on therapy (at least 1 h
following drug therapy administration). Each time, they were
subjected to a walking trial. The Microsoft Kinect multimodal
off-the-shelf sensor was used to capture 3D skeletal images.
Recorded data was processed and revealed a high correlation between
adherence and non-adherence identification and whole-body movement
patterns (8). Thus, remote
monitoring of PD adherence to drug treatment may increase drug
treatment effectiveness, disease control and patient QoL.
Apart from movement evaluation, Turner et al
used epiNet, a novel artificial gene regulatory network, with
dynamic analysis transition between different states of PD
(9). First, they acquired data from
25 patients with confirmed PD diagnosis that were being treated
with levodopa (9). Patients were
subjected to movement monitoring using light-weight sensors and
UDysRS. Secondly, movement data was corroborated with gene
expression data to reveal patterns in LID or insufficient levodopa
administration. Authors revealed that epiNet was able to
differentiate between states of under- or over-dosage of levodopa
(9).
Regarding drug discovery for PD, Yue et al
used a weighted gene correlation network analysis (WGCNA) and
identified two over-represented PD-specific gene co-expression
network modules, namely the Brown module (comprising 544 genes) and
the Turquoise module (comprising 1,190 genes) (10). Furthermore, using drug-protein
regulatory relationship databases (DMAP) they developed a Drug
Effect Sum Score (DESS) for candidate drugs that could restore gene
expression in the identified modules. Authors identified 5
potential new treatments for PD and 6 drugs with potential
repositioning applications (10).
Thus, integrating gene co-expression modules with biomolecular
interaction network analysis may lead to the identification of
network modules important in PD pathways. This approach could help
identify drugs useful in polygenic diseases such as PD (10).
Furthermore, being able to assess symptom
progression in PD could mean rapid dosing changes or referral to
deep brain stimulation, resulting in a higher disease control and
QoL. Przybyszewski et al used a rough set theory to
integrate reflexive saccades (latency, amplitude and duration) to
develop predictions of neurological symptoms in patients (11). Authors evaluated 10 patients,
integrating the age of patients and reflexive saccades. They were
able to accurately predict neurological symptoms in ~80% of
patients (11).
4. Home-based telemedicine systems
The search strategy returned 5 articles related to
the management of advanced stages of PD using home-based
telemedicine systems, out of which 4 were population-based cohort
studies and 1 was a randomized controlled trial (Table II).
 | Table IIHome-based telemedicine systems used
in advanced stages of Parkinson's disease. |
Table II
Home-based telemedicine systems used
in advanced stages of Parkinson's disease.
| Author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|
| Willows et
al | 2017 | Observational
study | Nasojejunal tube
placed during fluoroscopic passive/active positioning, with
radiological confirmation of placement | LCIG delivery
initiated through telemedicine over a 16-h period: total morning
5-10 ml (100-200 mg levodopa) to 20 ml max; median time for
titration 2.8 days | Technically
achievable, a well-tolerated alternative method | (12) |
| Evans et
al | 2020 | Pilot Study | Phone consultations
in virtual clinic combined with a report from a Parkinson's
KinetiGraph | - | Acceptable for most
patients, timesaving, in need of further cost analysis | (13) |
| Hssayeni et
al | 2019 | Comparative
Study | Wearable sensors
combined with gradient tree boosting or with a deep learning model
based on LSTM networks | - | Highest correlation
for gradient tree boosting; solid approach for assessing tremor
severity | (14) |
| Cilia et
al | 2020 | Observational
Study | Remote telenursing
assistance service ‘Parkinson Care’ and video-consultations on
Microsoft teams® platform | - | Introduction of a
new element (case managers, not initially part of patient's care
team); development of triage algorithm | (15) |
| Beck et
al | 2017 | Randomized
controlled trial | Usual care by a
physician compared to 4 virtual consults by a remote neurologist
added to usual care | - | Virtual care was
achievable with no major differences in quality of life and
burden | (16) |
Home-based telemedicine systems are defined as the
process of providing healthcare remotely using communication
devices and AI applications for the diagnosis and management of a
particular disorder. Telemedicine systems follow a hierarchical
tiered structure which includes three distinct levels: Rural/remote
center; city/district hospital center; speciality center. The
system provides real-time, high quality audio-video interactions
between home-based patients and nurses, physicians and occasionally
a multidisciplinary team (12).
A study performed by Willows et al analyzed
the feasibility of telemedicine for levodopa-carbidopa intestinal
gel (LCIG) home titration, optimization and assessed patient,
physician, and nurse satisfaction (12). A total of 15 patients with advanced
stages of PD were enrolled from 4 clinics and telemedicine-assisted
LCIG home management period was defined as the start of the pump to
the decision for permanent percutaneous endoscopic transgastric
jejunostomy (PEG-J) surgery or termination of LCIG treatment
(12). To assess technical
feasibility and also procedural limits, all technical events were
reported and further analyzed. The results revealed that the system
was highly efficient, technically feasible and well-accepted
(12). Patients, neurologists and
nurses were satisfied and considered the system to consume less
time than required for a patient admitted to the hospital for the
management of LCIG (12). Technical
problems associated with the novel home-based telemedicine system
were extremely rare, correlated with internet outage and rapidly
resolved. No severe adverse events were present, with the exception
of only one event: A tube occlusion. The authors of the study
emphasized that the home-based telemedicine system for LCIG
management may reduce healthcare costs by reducing in-ward bed
occupancy as well as hospitalization and travel costs (12).
A pilot study by Evans et al on 61 PD
patients analyzed a new concept called PD virtual clinic to develop
an efficient system combining home-based telemedicine systems and
digital wearable technology. The Parkinson's KinetiGraph (a
wrist-worn device providing objective motor assessment) was used by
clinicians for management and optimization of medication (13). The results of the study demonstrated
that a PD virtual clinic is safe and efficient, and the majority of
consultations are equivalent to face-to-face clinic in terms of
treatment outcome (13).
Notwithstanding the fact that home-based
telemedicine systems are a feasible solution for the management of
advanced stages of PD, a constant difficulty is represented by the
absence of a precise motor dysfunction quantification method in
advanced stages of PD. However, a study by Hssayeni et al
(14) revealed that a novel
ensemble model based on gradient tree boosting with an automated
interpretation by a deep learning algorithm is able to provide a
full spectrum of motor dysfunction by analyzing the tremor of the
patients. The system performs a continuous monitoring of the
movement of individuals in their own homes, to record the results,
and finally to estimate total Parkinsonian tremor. The system
represents an efficient method for automated PD motor dysfunction
assessment and could be properly used in future home-based
telemedicine applications (14).
Since the outbreak of the COVID-19 pandemic in
December 2019, it was demonstrated that patients with pre-existing
neurodegenerative disorders and COVID-19 may develop an
exacerbation of the neurological symptomatology and severe,
potentially life-threatening forms of COVID-19 pneumonia. In this
new paradigm for managing crises throughout and following the
curfew, home-based telemedicine systems represent a safe,
cost-efficient solution for patients, physicians, nurses, and
healthcare systems. A study by Cilia et al proposes a
two-pronged model to optimize the management of PD patients, based
on a successful experience of telemedicine throughout the COVID-19
crisis in Italy (15), which was
operated in two distinct steps. The first step was a novel remote
telemedicine platform for nursing assistance service, named
‘ParkinsonCare’, which currently remains available in Milan since
February 2019(15). Notably, the
whole telemedicine service was made available free of charge for
all patients. A subset of data obtained between the 12th of March
and the 14th of May 2020 was analyzed and the results revealed that
the telemedicine system managed 2,021 interactions (telephone
calls) between nurses and 525 patients and only one third of PD
patients required a neurologist and out of the 194
video-consultations (using Zoom® platform) performed by
a neurologist, only 18 failed, mostly due to inability of the
patient to deal with the software (15). The second step was implemented using
Microsoft Teams® platform for regulation-compliant
video-consultations with experienced neurologists (15). Similar with the first step, between
the 30th of March and the 14th of May 2020,
video-consultations increased steadily to become over two-thirds of
the total number of outpatient assessments and only 21.8% of
patients required a visit to the hospital. The analysis of the
questionnaires of the patients revealed that more than two-thirds
of the patients had provided positive feedbacks, comments were not
provided in 28% of cases and 2% of PD patients had a negative
feedback (15). The authors
concluded that for the future a pre-existing multidisciplinary
medical team could overcome difficulties in treating non-motor
complications of PD using the aforementioned home-based
telemedicine applications (15).
A randomized controlled trial compared usual care
with usual care supplemented by 4 virtual visits (16) in order to determine whether
home-based telemedicine is a feasible and efficient method of
providing specialized neurologic care for PD patients. The results
revealed that each virtual house call saved patients a median of 88
min and 38 miles, demonstrating that virtual consultations are
feasible and provide substantial convenience. The virtual visit was
neither more nor less effective than usual in-person care visits
(16).
5. Robotic systems and AI applications for
gait management
The search identified 10 articles concerning robotic
systems and AI applications for gait management in PD (Table III).
 | Table IIIRobot-assisted gait training systems
and their outcomes. |
Table III
Robot-assisted gait training systems
and their outcomes.
| Author | Year | Evidence type | Method | Treatment | Outcome | (Refs.) |
|---|
| Lo et
al | 2010 | Prospective
study | Lokomat | 10 sessions of 30
min each | Reduction in FOG
(self-report and clinician-rated scoring) | (17) |
| Nardo et
al | 2014 | Prospective
study | Lokomat | Daily 45 min
sessions for 5 weeks | Possible
improvement of gait performance | (18) |
| Picelli et
al | 2012 | Randomized
controlled trial | Robotic stepper
training (using the Gait Trainer) vs. physiotherapy | 12 sessions of 45
min each (3/week, 4 consecutive weeks) | Statistically
significant improvement in walking speed and walking distance | (19) |
| Galli et
al | 2016 | Prospective
study | Commercially
available G-EO system vs. intensive treadmill therapy (Gait
trainer™) | 20 sessions of 45
min each (5 days a week for 4 weeks) | | (20) |
| Kang et
al | 2019 | Prospective,
single-blind, single-center, randomized controlled trial |
Walkbot-S™ vs. treadmill
training | 12 sessions in 4
weeks each with an actual training time of 30 min | Changes in gait
speed | (21) |
| Fundarò et
al | 2019 | Retrospective
study | Lokomat
System® vs. conventional training program under the
supervision of a physiotherapist | 20 sessions of 30
min each (5 days/week for 4 weeks) | Significant
improvement of total UPDRS | (22) |
| Capecci et
al | 2019 | Multicentre
single-blind prospective randomized controlled study | End-effector
robotic device G-EO system vs. treadmill training | 20 sessions of 45
min each (5 days/week for 4 weeks) | Significantly lower
frequency of daily episodes of gait freezing | (23) |
| Arami et
al | 2019 | Comparative
study | Two-class approach
vs. three class approach for predicting freezing of gait | - | Superior to the
conventional approach | (24) |
| Shalin et
al | 2020 | Observational
study | Use of foot plantar
pressure data for predicting freezing of gait | - | Removal of the need
for feature extraction and selection | (25) |
| Bevilacqua et
al | 2020 | Single-blinded
randomized controlled trial | Traditional therapy
vs. Tymo system vs. Walker view | 50 min traditional
training vs. 30 min traditional training + 20 min RAGT 10 sessions
(2 per week) | Still in
progress | (26) |
Maintenance of independent motor function and
ability to walk is the primary goal of therapy for the 4th stage of
PD. Such a personalized approach enables the patient to remain
independent for as long as possible, improves QoL and delays
entering the 5th stage of PD where the patient is immobilized, and
therefore, gait management is impossible. For this reason, in our
review only the articles referring to robotic systems and AI-based
applications for gait management exclusively for advanced stages of
PD were included.
A pilot study that investigated the influence of
robot-assisted gait training (RAGT) on patients diagnosed with PD
and suffering from gait freezing, revealed encouraging results
(17) and recommended further
follow-up evaluations of the long-term effects of RAGT (17). When performed on patients with
previous deep brain stimulation, therapy sessions with the same
system as in the aforementioned study, were considered possibly
constructive only regarding space-temporal gait parameters and
motor score (UPDRS), but not concerning kinetic and kinematic gait
parameters (18).
The starting point for trials comparing conventional
gait training with RAGT was encouraging, as the trial conducted by
Picelli et al identified a statistically significant
difference in favour of robotic stepper training (when compared to
physiotherapy with active joint mobilization) (19). The positive effect lasted up to 1
month. The authors suggested the future comparison between RAGT and
treadmill training or the same amount of overground walking
(19).
The first study to perform a quantitative comparison
of the effects of RAGT and treadmill training in PD, evaluating
gait kinematics as well as spatiotemporal parameters identified
improved gait kinematics, particularly in the frontal plane at the
pelvic and hip joint level in patients who underwent robotic
training (20). This study was
followed by further research concerning the comparison between the
two treatment options, such as the one by Kang et al
(21). This team designed a study
to specifically evaluate the effects of RAGT
(Walkbot-S™) on gait speed, compared with treadmill
training (21), in addition to also
revealing what pathways apply for these effects to occur, by
monitoring fluctuations in brain functional networks and gait
automaticity (21).
When it comes to the effect on self-selected speed
gait training, a previous study did not identify any differences
between RAGT (with the Lokomat plus VR), and conventional gait
training overground (22). The same
study raised the need to further investigate and adjust other
parameter settings such as body weight support and guidance force
for a tailored use of RAGT, which could significantly add to the
benefits of this type of treatment (22).
Another comparison of RAGT with treadmill training
took walking endurance, speed, and number of episodes of gait
freezing into consideration, but also the general attitude towards
the disease (23). The frequency of
daily episodes of gait freezing appeared to be significantly
decreased in the group using RAGT. Medium and long-term follow-up
could prove valuable, as motor learning retention may benefit from
prolonged exercise (23).
As freezing of gait is a frequently encountered
phenomenon in parkinsonian disorders certain studies have attempted
to predict its occurrence, such as the novel algorithm submitted by
Arami et al (24). It
inferred the use of a binary classification preceded by a feature
time series prediction, and it demonstrated an improvement compared
with the conventional three-class prediction approach. Therefore,
it was suggested as a standard (24).
Another method that proved useful for predicting the
aforementioned incident involves plantar pressure data represented
in the form of 2D images and evaluated by a convolutional neural
network (CNN) (25). The newest
research designed by Bevilacqua et al included 195 subjects
divided into three groups: A control group that followed
traditional therapy sessions with a duration of 50 min, and two
technological intervention groups, one that used the Tymo system,
and another that used the Walker View (in addition to a 30-min
traditional rehabilitation session, and another 20 min of treatment
with a robotic system) (26). Its
aim was to analyze the improvement of balance and gait in older PD
patients at 3 follow-ups (6 months, 1 year and 2 years following
the 5-week rehabilitation programme) (26).
The present study has several strengths. First, to
the best of our knowledge, this is the first systematic review to
evaluate the feasibility of the automatized management of advanced
stages of PD by AI-based technologies and robotic systems. Second,
the subject of this systematic review is of major relevance due to
the rapid increase of prevalence of PD worldwide (27), the coronavirus COVID-19 outbreak,
and the serious global human resources shortage (28). Third, AI algorithms are more
cost-efficient than conventional methods and the effect of complete
autonomous management on healthcare systems and on the QoL of PD
patients is underestimated (29).
In advanced stages of the disease, patients
frequently experience an enhanced sensitivity to small changes in
pharmacological therapy, particularly in levodopa-carbidopa and
present more severe adverse reactions to antiparkinsonian drugs
(30). Consequently, the number of
consultations, the time of transportation and the general economic
burden, further increase (31). All
this makes an integrated autonomous AI-based system unavoidable in
the near future.
Despite levodopa effectiveness in PD, long-term use
may lead to an either ‘on-off’ syndrome or to motor complications
such as LID (32-34).
Prompt detection of the lack of effectiveness or overexposure to
levodopa may have beneficial effects for patients and be
cost-effective for the healthcare system (35). The use of deep learning methods may
enable a faster and more accurate detection of drug therapy
problems as well as informing patients and physicians regarding
symptoms and disease evolution (6-9).
Using computer vision and deep learning, including
the deep-learning-based pose estimation algorithm (Convolution Pose
Machines) that detect disease severity or LID, may facilitate more
frequent patient-clinician interactions and an improved treatment
personalization and quality of care (6). In addition, complex CDSS may help the
clinician provide a DBS recommendation or drug treatment adjustment
(7-9).
Other sensor-based devices aid in identifying
non-adherence to drug treatment and help decrease its prevalence
(8). Movement assessment devices,
coupled with gene expression data from PD patients may help to
improve differentiation between different states of levodopa
treatment (under- or over-treatment) (9). As in advanced PD, levodopa treatment
may no longer be effective and DBS is not always an option for
every PD patient, drug discovery is an important part of PD
research and future perspectives of AI-based technologies focus on
new methods for identifying drug candidates, which are represented
by WGCNA systems that use gene expression analysis to identify key
dysregulated modules and suggest drugs that could restore
homeostasis in these systems (10).
This review has five major limitations. The first
limitation is the small number of participants in the majority of
the studies included in our review. Secondly, the limited number of
trials analyzing AI-based technologies exclusively for advanced
stages of PD. Thirdly, it was not possible to analyze every
possible lifestyle factor which alters the results, nor the way
those technologies were applied by patients, relatives and
healthcare professionals. Thereby, the observational nature of this
approach leaves the possibility of residual confounding. Fourth,
studies differed in their outcomes, design, and measurements, and
this heterogeneity reduced our capacity to formulate a precise
evaluation. Fifth, no studies which included all types of
technology available for the automatization process of the
management of advanced stages of PD were identified; therefore, it
was not possible to analyze the feasibility of all AI-based systems
applied as one integrated structure. Thus, international
cooperation is recommended between a multidisciplinary team of
healthcare professionals composed of neurologists,
gastroenterologists, psychiatrists, and nurses on one hand, and a
multidisciplinary team of hardware engineers and software
developers, on the other hand.
6. Conclusions
Significant evidence demonstrating that current
AI-based technologies are feasible for automatic management of
patients with advanced stages of PD was identified. Improving the
quality of care and reducing the cost for patients and healthcare
systems are the most important advantages.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LPD suggested the methodology, searched the
literature and made substantial contributions to the writing of the
manuscript. MB contributed to the writing of the robotic systems
and AI applications for gait management section. DCL made
contributions to the methodology of the manuscript. CP contributed
to the writing of the AI-based applications for autonomous
management of pharmacologic therapy section. LCP searched the
literature and revised the manuscript. MB and SLP confirm the
authenticity of all the raw data. NT revised the manuscript for
important intellectual content. SLP made contributions to the
writing of non-motor complications management and analyzed the
results. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pedersen SW, Suedmeyer M, Liu LW, Domagk
D, Forbes A, Bergmann L, Onuk K, Yegin A and van Laar T: The role
and structure of the multidisciplinary team in the management of
advanced Parkinson's disease with a focus on the use of
levodopa-carbidopa intestinal gel. J Multidiscip Healthc. 10:13–27.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grimes D, Fitzpatrick M, Gordon J,
Miyasaki J, Fon EA, Schlossmacher M, Suchowersky O, Rajput A,
Lafontaine AL, Mestre T, et al: Canadian guideline for Parkinson
disease. CMAJ. 191:E989–E1004. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amjad F, Bhatti D, Davis TL, Oguh O, Pahwa
R, Kukreja P, Zamudio J and Metman LV: Current practices for
outpatient initiation of levodopa-carbidopa intestinal gel for
management of advanced Parkinson's disease in the United States.
Adv Ther. 36:2233–2246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tran BX, Vu GT, Ha GH, Vuong QH, Ho MT,
Vuong TT, La VP, Ho MT, Nghiem KP, Nguyen HLT, et al: Global
evolution of research in artificial intelligence in health and
medicine: A bibliometric study. J Clin Med. 8(360)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang W, Hamilton JL, Kopil C, Beck JC,
Tanner CM, Albin RL, Ray Dorsey E, Dahodwala N, Cintina I, Hogan P
and Thompson T: Current and projected future economic burden of
Parkinson's disease in the U.S. NPJ Parkinsons Dis.
6(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li MH, Mestre TA, Fox SH and Taati B:
Vision-based assessment of parkinsonism and levodopa-induced
dyskinesia with pose estimation. J Neuroeng Rehabil.
15(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shamir RR, Dolber T, Noecker AM, Walter BL
and McIntyre CC: Machine learning approach to optimizing combined
stimulation and medication therapies for Parkinson's disease. Brain
Stimul. 8:1025–1032. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tucker CS, Behoora I, Nembhard HB, Lewis
M, Sterling NW and Huang X: Machine learning classification of
medication adherence in patients with movement disorders using
non-wearable sensors. Comput Biol Med. 66:120–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Turner AP, Lones MA, Trefzer MA, Smith SL,
Jamieson S, Alty JE, Cosgrove J and Tyrrell AM: Using epigenetic
networks for the analysis of movement associated with levodopa
therapy for Parkinson's disease. Biosystems. 146:35–42.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yue Z, Arora I, Zhang EY, Laufer V,
Bridges SL and Chen JY: Repositioning drugs by targeting network
modules: A Parkinson's disease case study. BMC Bioinformatics. 18
(Suppl 14)(S532)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Przybyszewski AW, Kon M, Szlufik S,
Szymanski A, Habela P and Koziorowski DM: Multimodal learning and
intelligent prediction of symptom development in individual
Parkinson's patients. Sensors (Basel). 16(1498)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Willows T, Dizdar N, Nyholm D, Widner H,
Grenholm P, Schmiauke U, Urbom A, Groth K, Larsson J, Permert J and
Kjellander S: Initiation of levodopa-carbidopa intestinal gel
infusion using telemedicine (video communication system)
facilitates efficient and well-accepted home titration in patients
with advanced Parkinson's disease. J Parkinsons Dis. 7:719–728.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Evans L, Mohamed B and Thomas EC: Using
telemedicine and wearable technology to establish a virtual clinic
for people with Parkinson's disease. BMJ Open Qual.
9(e001000)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hssayeni MD, Jimenez-Shahed J, Burack MA
and Ghoraani B: Wearable sensors for estimation of parkinsonian
tremor severity during free body movements. Sensors (Basel).
19(4215)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cilia R, Mancini F, Bloem BR and Eleopra
R: Telemedicine for parkinsonism: A two-step model based on the
COVID-19 experience in Milan, Italy. Parkinsonism Relat Disord.
75:130–132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beck CA, Beran DB, Biglan KM, Boyd CM,
Dorsey ER, Schmidt PN, Simone R, Willis AW, Galifianakis NB, Katz
M, et al: National randomized controlled trial of virtual house
calls for Parkinson disease. Neurology. 89:1152–1161.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lo AC, Chang VC, Gianfrancesco MA,
Friedman JH, Patterson TS and Benedicto DF: Reduction of freezing
of gait in Parkinson's disease by repetitive robot-assisted
treadmill training: A pilot study. J Neuroeng Rehabil.
7(51)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nardo A, Anasetti F, Servello D and Porta
M: Quantitative gait analysis in patients with Parkinson treated
with deep brain stimulation: The effects of a robotic gait
training. NeuroRehabilitation. 35:779–788. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Picelli A, Melotti C, Origano F, Waldner
A, Fiaschi A, Santilli V and Smania N: Robot-assisted gait training
in patients with Parkinson disease: A randomized controlled trial.
Neurorehabil Neural Repair. 26:353–361. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Galli M, Cimolin V, De Pandis MF, Le Pera
D, Sova I, Albertini G, Stocchi F and Franceschini M:
Robot-assisted gait training versus treadmill training in patients
with Parkinson's disease: A kinematic evaluation with gait profile
score. Funct Neurol. 31:163–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang MG, Yun SJ, Shin HI, Kim E, Lee HH,
Oh BM and Seo HG: Effects of robot-assisted gait training in
patients with Parkinson's disease: Study protocol for a randomized
controlled trial. Trials. 20(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fundarò C, Maestri R, Ferriero G, Chimento
P, Taveggia G and Casale R: Self-selected speed gait training in
Parkinson's disease: Robot-assisted gait training with virtual
reality versus gait training on the ground. Eur J Phys Rehabil Med.
55:456–462. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Capecci M, Pournajaf S, Galafate D, Sale
P, Le Pera D, Goffredo M, De Pandis MF, Andrenelli E, Pennacchioni
M, Ceravolo MG and Franceschini M: Clinical effects of
robot-assisted gait training and treadmill training for Parkinson's
disease. A randomized controlled trial. Ann Phys Rehabil Med.
62:303–312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arami A, Poulakakis-Daktylidis A, Tai YF
and Burdet E: Prediction of gait freezing in parkinsonian patients:
A binary classification augmented with time series prediction. IEEE
Trans Neural Syst Rehabil Eng. 27:1909–1919. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shalin G, Pardoel S, Nantel J, Lemaire ED
and Kofman J: Prediction of freezing of gait in Parkinson's disease
from foot plantar-pressure arrays using a convolutional neural
network. Annu Int Conf IEEE Eng Med Biol Soc. 2020:244–247.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bevilacqua R, Maranesi E, Di Rosa M, Luzi
R, Casoni E, Rinaldi N, Baldoni R, Lattanzio F, Di Donna V,
Pelliccioni G and Riccardi GR: Rehabilitation of older people with
Parkinson's disease: An innovative protocol for RCT study to
evaluate the potential of robotic-based technologies. BMC Neurol.
20(186)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dorsey ER, Elbaz A, Nichols E, Abd-Allah
F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi JYJ,
Collado-Mateo D, et al: Global, regional, and national burden of
Parkinson's disease,1990-2016: A systematic analysis for the Global
Burden of Disease Study 2016. Lancet Neurol. 17:939–953.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
De Raeve P, Adams E and Xyrichis A: The
impact of the COVID-19 pandemic on nurses in Europe: A critical
discussion of policy failures and opportunities for future
preparedness. Int J Nurs Stud Adv. 3(100032)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cesario A, D'Oria M, Calvani R, Picca A,
Pietragalla A, Lorusso D, Daniele G, Lohmeyer FM, Boldrini L,
Valentini V, et al: The role of artificial intelligence in managing
multimorbidity and cancer. J Pers Med. 11(314)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Prakash N and Simuni T: Infusion therapies
for Parkinson's disease. Curr Neurol Neurosci Rep.
20(44)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Keränen T, Kaakkola S, Sotaniemi K,
Laulumaa V, Haapaniemi T, Jolma T, Kola H, Ylikoski A, Satomaa O,
Kovanen J, et al: Economic burden and quality of life impairment
increase with severity of PD. Parkinsonism Relat Disord. 9:163–168.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Grandas F, Galiano ML and Tabernero C:
Risk factors for levodopa-induced dyskinesias in Parkinson's
disease. J Neurol. 246:1127–1133. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fabbrini G, Brotchie JM, Grandas F, Nomoto
M and Goetz CG: Levodopa-induced dyskinesias. Mov Disord.
22:1379–1389. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nutt JG: Motor fluctuations and dyskinesia
in Parkinson's disease. Parkinsonism Relat Disord. 8:101–108.
2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Olanow CW: Levodopa: Effect on cell death
and the natural history of Parkinson's disease. Mov Disord.
30:37–44. 2015.PubMed/NCBI View Article : Google Scholar
|