Introduction
During pregnancy, the fetus not only evades but also
provokes immune responses in the uterus, maternal peripheral
tissues and immune system, which are essential for the success of
pregnancy (1). Lymph nodes are
secondary lymphoid organs that participate in inducing and
regulating adaptive immunity (2).
Pregnancy induces an increase in the weights of lumbar, renal and
inguinal lymph nodes in mice (3),
and the weights of iliac lymph nodes also increase progressively
during pregnancy in rats (4).
It has been reported that early pregnancy induces
increases in the expression levels of progesterone (P4) receptor,
P4-induced blocking factor (5),
cyclooxygenase 1 (COX-1), COX-2, prostaglandin E synthase,
Aldo-keto reductase family 1 member B1(6), interleukin (IL)-5 and IL-10, but
TNF-β and IL-2 are decreased in the maternal lymph nodes during
early pregnancy in sheep (7).
Furthermore, there are increases in the protein expression levels
of melatonin receptor 1, cluster of differentiation 4, signal
transducer and activator of transcription 1, 2',5'-oligoadenylate
synthetase, myxovirus resistance protein 1, C-X-C motif chemokine
10 and gonadotropin releasing hormone and its receptor in the
maternal lymph nodes during early pregnancy in sheep (8-10).
Early pregnancy induces changes in maternal lymph nodes, which may
be associated with maternal immune tolerance to paternal
alloantigens.
The complement system plays important roles in
innate immune defense, which are associated with shaping adaptive
immune responses (11). The major
effector fragments of the complement system that are associated
with the innate and adaptive immune systems include complement
components C1, C3, C5 and C9, and the C1 complex (C1qC1r2s2), while
C2 and C4 are implicated in C3 convertase formation (12).
Complement activation can promote inflammation and
facilitate macrophage phagocytosis to placenta-derived particles
and apoptotic cells, and the complement system also serves as a
regulator of complex tolerance in healthy pregnancy (13). Complement components are located in
placental tissue, and are associated with protecting the
semiallogenic conceptus against the maternal immune system in
humans (14). However, a
complement-mediated immune attack against semiallogeneic fetal
tissues can occur, which leads to adverse pregnancy outcomes in
humans and animals (15). Early
pregnancy regulates expression of complement components in the
liver and thymus in the ovine (16,17).
At present, it is unclear whether the expression of complement
components is changed in maternal lymph nodes during early
pregnancy in sheep.
During early gestation in ruminants, the
trophectoderm of the conceptus secretes interferon-tau (IFNT),
which participates in the maternal recognition of pregnancy and
prevents luteolysis (18). IFNT
increases the expression of interferon stimulated genes (ISGs) in
the corpus luteum (CL) in an endocrine manner (19), and enhances the expression of ISGs
in immune organs, including bone marrow (20), the thymus (21), the spleen (22,23)
and lymph nodes (6,9) during early pregnancy in sheep.
It is hypothesized that early pregnancy affects the
expression of complement components in ovine lymph nodes. The
objective of the present study was to explore the expression of the
complement components C1q, C1r, C1s, C2, C3, C4a, C5b and C9 in
maternal lymph nodes, which may be helpful for understanding the
immune regulation of maternal lymph nodes during early pregnancy in
sheep.
Materials and methods
Animals and experimental design
A total of 24 small-tail Han ewes (Ovis
aries) ~18 months old and average weight of 41 kg were observed
daily for estrus in the presence of vasectomized rams at a farm in
Handan, China. The ewes were housed under a condition of average
atmospheric pressure of 1,027 hectopascals, a 11 h light/13 h dark
cycle, temperature of 3-18˚C and free access to food and water. The
ewes were randomly divided into four groups (n=6 for each group),
and at estrus (day 0), the animals were mated to either intact or
vasectomized rams. The females were necropsied on days 13, 16 and
25 of pregnancy, and day 16 of the estrous cycle as described
previously (9). Euthanasia of the
ewes was performed by an experienced person to cut both the carotid
arteries and jugular veins to bleed out the animals after
electrical stunning. The uterus was flushed, and pregnancy was
confirmed by the presence of a normal conceptus in the uterine
lumen flushing. Transverse pieces of the inguinal lymph nodes were
collected immediately after slaughter and snap-frozen in liquid
nitrogen (-196˚C) until subsequent mRNA and protein analyses. In
addition, longitudinal cross sections of the lymph nodes were cut
into pieces (0.3 cm3), and fixed with fresh 4% buffered
paraformaldehyde for 12 h at room temperature for subsequent
immunohistochemical analysis. The study protocol was reviewed and
approved by the Hebei University of Engineering Animal Care and Use
Committee (approval no. 2019-017), and humane animal care and
handling procedures were followed throughout the experiment.
RNA extraction and reverse
transcription-quantitative PCR assay
The samples (transverse pieces of the inguinal lymph
nodes) were crushed in liquid nitrogen, and total RNA was isolated
using TRNzol Universal Reagent (DP424; Tiangen Biotech Co., Ltd.)
according to the manufacturer's recommendations. A FastQuant RT kit
with gDNase (cat. no. KR106; Tiangen Biotech Co., Ltd.) was used to
remove genomic DNA and synthesize cDNA according to the
manufacturer's instructions. The specific primers (Table I) were designed based on the
sequences in the NCBI database (http://www.ncbi.nlm.nih.gov/) for the ovine C1q, C1r,
C1s, C2, C3, C4a, C5b and C9 genes, and were synthesized by
Shanghai Sangon Biotech Co., Ltd. Primer matrix experiments were
performed to determine the optimal primer concentrations. The
amplification efficiencies of the primer sequences were evaluated
before quantification, and were in an acceptable range (between 0.9
and 1.1). A CFX96 real-time PCR system (Bio-Rad Laboratories, Inc.)
was used for quantitative PCR in a total volume of 20 µl in
triplicate using a SuperReal PreMix Plus kit (Tiangen Biotech Co.,
Ltd.). Cycling conditions of PCR included an initial denaturation
at 95˚C for 10 min followed by 40 cycles of denaturation (95˚C for
10 sec), annealing (60-62˚C for 20 sec) and extension (72˚C for 25
sec) followed by one cycle of final extension (72˚C for 7 min). The
annealing temperatures were 60˚C for C1q, C1s,
C2, C3 and C9, or 62˚C for C1r,
C4a and C5b. Mean threshold cycle values (Cq) for the
target genes and CT values for the reference gene (GAPDH) of
each sample were calculated from triplicate wells, and the relative
transcript abundances for the target genes were calculated using
the 2-ΔΔCq analysis method (24). Mean Cq values of estrous cycle of
day 16 were used to normalize the relative levels of mRNA
transcripts.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5'-3') | Size, bp | Accession
numbers |
|---|
| C1qA | Forward |
CAGGAGAACGTGTACCAGAGCAAC | 122 | XM_012152629.2 |
| | Reverse |
CTCCGAGAGGACCTGATGGACAG | | |
| C1r | Forward |
CCCAGACTACCGCCAGGAAGAG | 109 | XM_012175492.2 |
| | Reverse |
TGGGAGGCAGATTGGCAGGAG | | |
| C1s | Forward |
CCTGGCAAGTCTTCTTCTCGAACC | 130 | XM_004006917.4 |
| | Reverse |
ACCACTGAGGAGGACCCAACATAC | | |
| C2 | Forward |
CCACCAATCCCATCCAGCAGAAG | 95 | XM_027958809.1 |
| | Reverse |
GGCGTCCAGGAGCAGGTAGAG | | |
| C3 | Forward |
CGCCACCAGCAGACTATAACGATC | 105 | XM_027969774.1 |
| | Reverse |
AGCAGCCTTGACCTCCACCTC | | |
| C4α | Forward |
TTCAGGACAGGTGGTGAGAGGATC | 167 | XM_027958803.1 |
| | Reverse |
GGAGGAGATGGAGGCGACAGAG | | |
| C5 | Forward |
GCTACGCTGGTGTTACTCTGGATC | 157 | XM_004003966.3 |
| | Reverse |
GCAGACATGACCTCGCCTATAAGC | | |
| C9 | Forward |
GCCGCAACAGAGTGGTGGAAG | 138 | XM_004017026.3 |
| | Reverse |
TGCCATCCCTAACTCGGTCACAG | | |
| GAPDH | Forward |
GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| | Reverse |
GGTCATAAGTCCCTCCACGA | | |
Western blotting
The samples (transverse pieces of the inguinal lymph
nodes) were homogenized using RIPA lysis buffer (Tiangen Biotech
Co., Ltd.) to isolate total protein, and a BCA protein assay kit
(Tiangen Biotech Co., Ltd.) was used to determine the
concentrations of the total proteins. Total protein samples (10
µg/lane) were separated by 12% SDS-PAGE gel, and transferred
electrophoretically to polyvinylidene fluoride membranes
(MilliporeSigma). The membranes were blocked with 5% (w/v) skim
milk powder at 4˚C overnight, and then incubated with a mouse
anti-C1q monoclonal antibody (cat. no. sc-53544), a mouse anti-C1r
monoclonal antibody (cat. no. sc-514105), a mouse anti-C1s
monoclonal antibody (cat. no. sc-365273), a mouse anti-C2
monoclonal antibody (cat. no. sc-373809), a mouse anti-C3
monoclonal antibody (cat. no. sc-28294), a mouse anti-C4a
monoclonal antibody (cat. no. sc-271181), a mouse anti-C5b
monoclonal antibody (cat. no. sc-398247) and a mouse anti-C9
monoclonal antibody (cat. no. sc-390000) (all 1:1,000; Santa Cruz
Biotechnology, Inc.) at 4˚C overnight. After washing, the membranes
were incubated with horseradish peroxidase (HRP) conjugated
anti-mouse secondary antibody at a 1:2,000 dilution (cat. no.
BL001A; Biosharp Life Sciences). The blots were detected using a
pro-light HRP chemiluminescence kit (Tiangen Biotech Co., Ltd.).
Densitometric analysis of the relative intensities of the blots was
performed using a Quantity One v452 (Bio-Rad Laboratories, Inc.),
and normalized to a reference protein (GAPDH) using an anti-GAPDH
antibody (1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology,
Inc.).
Immunohistochemical analysis
The paraffin-embedded sections (5-µm thick) were
deparaffinized and rehydrated in a series of xylene and ethanol.
Some sections (transverse sections of the inguinal lymph nodes)
were stained by hematoxylin for 30 sec and eosin for 20 sec at room
temperature before antigen retrieval and staining with antibodies.
The sections were boiled in citrate solution for 12 min at 100˚C
for antigen retrieval, washed with phosphate-buffered saline and
then treated with 3% hydrogen peroxide to remove endogenous
peroxidase activity. Blocking non-specific binding site in the
sections was performed using 5% goat serum for 1 h at room
temperature, and then incubated with the mouse anti-C3 monoclonal
antibody (cat. no. sc-28294; Santa Cruz Biotechnology, Inc.) at a
final dilution of 1:200 at 4˚C overnight. The tissue sections were
further treated with the secondary antibody (cat. no. BL001A;
Biosharp Life Sciences) for 45 min at room temperature. For
negative control sections, the primary antibody was replaced with
antiserum-specific isotype at the same dilution. A DAB kit
(Enhanced HRP-DAB Chromogenic kit; Tiangen Biotech Co., Ltd.) was
used to detect the specific binding sites according to the
manufacturer's instructions. Digital images (magnification, x400)
were captured using a light microscope (Nikon Eclipse E800; Nikon
Corporation) with a DP12 digital camera. The intensities of
staining digital images were examined independently by four
observers. The immunostaining intensities of the samples from
different ewes (n=6 for each group) were analyzed through the
images in a blind manner. Staining intensities for C3 protein were
calculated by assigning an immunoreactive intensity on a scale of 0
to 3, as described previously (25). The staining intensity was as
follows: 0=negative; 1+=weak; 2+=strong.
Statistical analysis
Statistical analysis was performed using
least-squares ANOVA in mixed and general linear model procedures of
the Statistical Analysis System v9.2 (SAS Institute, Inc.). Day and
status (cyclic or pregnant), and interaction between day and status
on expression of mRNA and protein were tested using repeated
measure for multivariate analysis of variance. Numerical data were
presented as least squares means with standard errors, and
comparison of means was tested by Tukey HSD test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Relative expression levels of C1q,
C1r, C1s, C2, C3, C4a, C5b and C9 mRNA in lymph nodes
The relative expression levels of C1q, C1s
and C5b mRNA were significantly higher during pregnancy
compared with day 16 of the estrous cycle (P<0.05), and the
level of C1q mRNA was the highest at day 16 of pregnancy
among the four groups (Fig. 1).
The relative expression levels of C1r and C4a mRNAs were increased
at day 25 of pregnancy compared with those at day 16 of the estrous
cycle, and at days 13 and 16 of pregnancy (P<0.05). The levels
of C2 and C9 mRNAs were the highest at day 16 of
pregnancy among the four groups (P<0.05). In addition, the
C3 mRNA level was the highest at day 16 of pregnancy, and
was the lowest at day 25 of pregnancy among the four groups
(P<0.05).
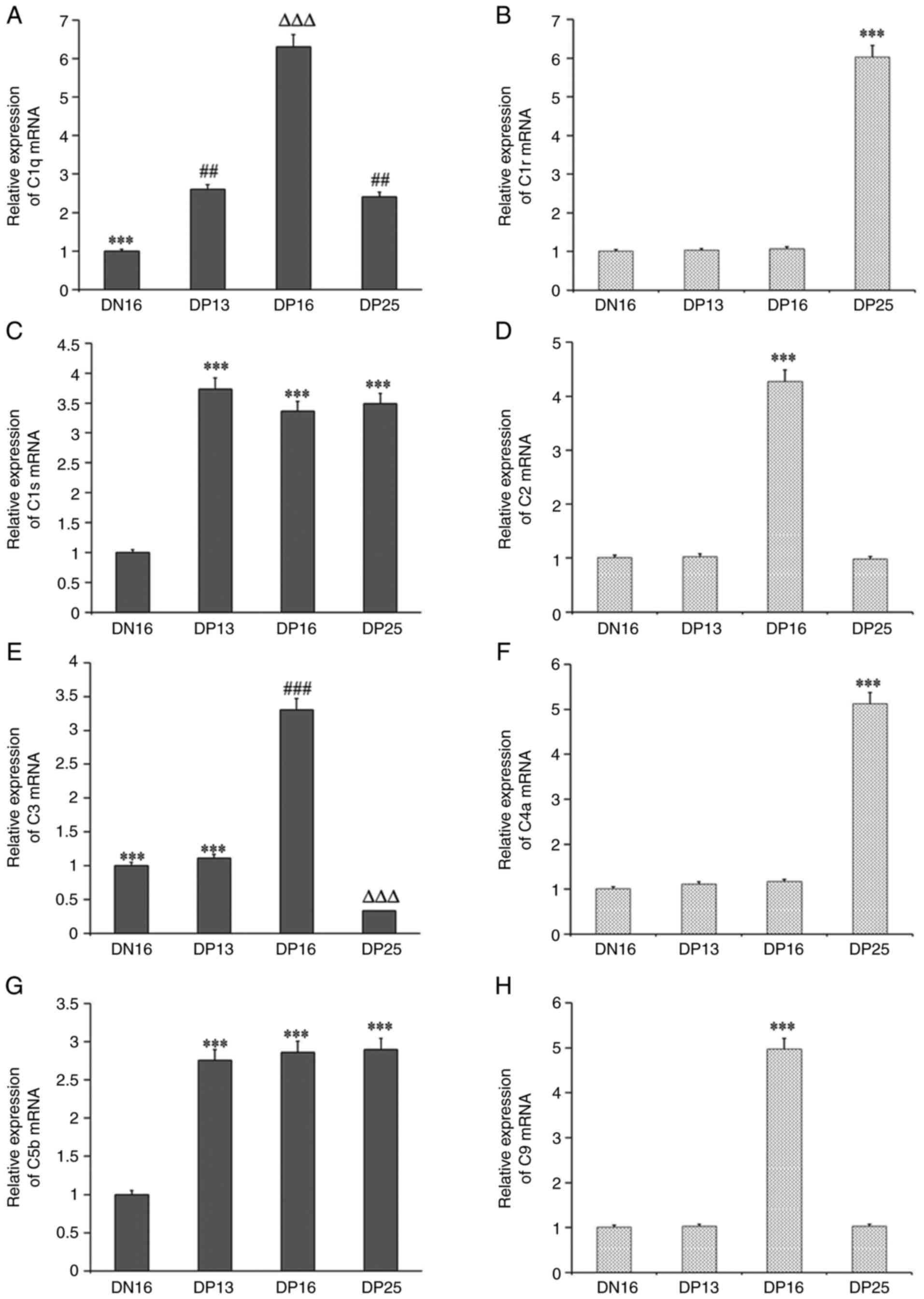 | Figure 1Relative expression values of C1q,
C1r, C1s, C2, C3, C4, C5 and C9 mRNAs in the lymph nodes
from the non-pregnant and pregnant ewes measured by reverse
transcription-quantitative PCR. (A) Relative expression of C1q
mRNA. ***P<0.001. DN16 vs. DP13, DP16 and DP25;
##P<0.01. DP13 and DP25 vs. DN16 and DP16;
∆∆∆P<0.001. DP16 vs. DN16, DP13 and DP25. (B)
Relative expression of C1r mRNA. ***P<0.001. DP25 vs.
DN16, DP13 and DP16. (C) Relative expression of C1s mRNA.
***P<0.001. DP13, DP16 and DP25 vs. DN16. (D)
Relative expression of C2 mRNA. ***P<0.001. DP16 vs.
DN16, DP13 and DP25. (E) Relative expression of C3 mRNA.
***P<0.001. DN16 and DP13 vs. DP16 and DP25;
###P<0.001. DP16 vs. DN16, DP13 and DP25;
∆∆∆P<0.001. DP25 vs. DN16, DP13 and DP16; (F)
Relative expression of C4a mRNA. ***P<0.001. DP25 vs.
DN16, DP13 and DP16. (G) Relative expression of C5b mRNA.
***P<0.001. DP13, DP16 and DP25 vs. DN16. (H)
Relative expression of C9 mRNA. ***P<0.001. DP16 vs.
DN16, DP13 and DP25. DN16, day 16 of the estrous cycle; DP13, day
13 of pregnancy; DP16, day 16 of pregnancy; DP25, day 25 of
pregnancy. |
Expression levels of C1q, C1r, C1s,
C2, C3, C4a, C5b and C9 proteins in lymph nodes
Western blotting indicated that there was almost no
expression of C1s protein on day 16 of the estrous cycle, but
pregnancy induced the expression of C1q, C1s and C5b proteins in
lymph nodes compared with day 16 of the estrous cycle (P<0.05;
Fig. 2). In addition, the C1q
protein level was the highest at day 16 of pregnancy, and the C1s
protein level was the highest at day 13 of pregnancy among the four
groups (P<0.05). There was very low expression of C1r and C4a
proteins at day 16 of the estrous cycle, and days 13 and 16 of
pregnancy, but the expression levels of C1r and C4a proteins were
significantly upregulated at day 25 of pregnancy compared with the
other three groups (P<0.05). C2 and C9 proteins were highly
expressed at day 16 of pregnancy, but their expression levels were
very low at day 16 of the estrous cycle, and days 13 and 25 of
pregnancy. Furthermore, the C3 protein level at day 16 of pregnancy
was the highest among the four groups, and was very low at day 25
of pregnancy (P<0.05).
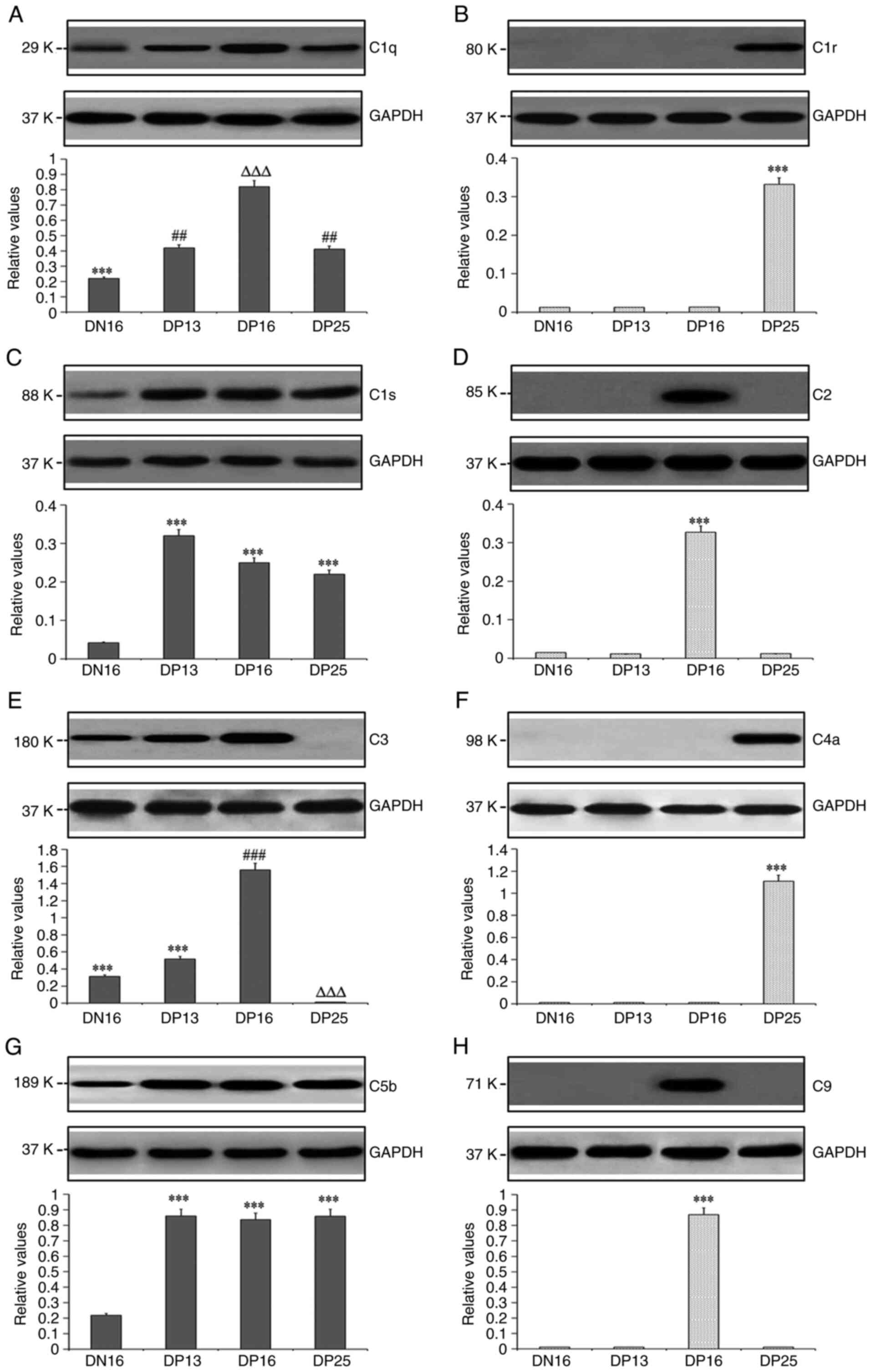 | Figure 2Expression levels of C1q, C1r, C1s,
C2, C3, C4, C5 and C9 proteins in the lymph nodes from the
non-pregnant and pregnant ewes analyzed using western blotting. (A)
Relative expression of C1q protein. ***P<0.001. DN16
vs. DP13, DP16 and DP25; ##P<0.01. DP13 and DP25 vs.
DN16 and DP16; ∆∆∆P<0.001. DP16 vs. DN16, DP13 and
DP25. (B) Relative expression of C1r protein.
***P<0.001. DP25 vs. DN16, DP13 and DP16. (C)
Relative expression of C1s protein. ***P<0.001. DP13,
DP16 and DP25 vs. DN16. (D) Relative expression of C2 protein.
***P<0.001. DP16 vs. DN16, DP13 and DP25. (E)
Relative expression of C3 protein. ***P<0.001. DN16
and DP13 vs. DP16 and DP25; ###P<0.001. DP16 vs.
DN16, DP13 and DP25; ∆∆∆P<0.001. DP25 vs. DN16, DP13
and DP16; (F) Relative expression of C4a protein.
***P<0.001. DP25 vs. DN16, DP13 and DP16. (G)
Relative expression of C5b protein. ***P<0.001. DP13,
DP16 and DP25 vs. DN16. (H) Relative expression of C9 protein.
***P<0.001. DP16 vs. DN16, DP13 and DP25. DN16, day
16 of the estrous cycle; DP13, day 13 of pregnancy; DP16, day 16 of
pregnancy; DP25, day 25 of pregnancy. |
Immunohistochemistry for C3 protein in
the lymph nodes
The C3 protein was located in the subcapsular
sinuses and lymph sinuses, but there was almost no immunostaining
in lymphoid nodules and medullary cords (Fig. 3). The staining intensities for C3
protein were 0, 1+, 1+, 2+ and 0 for the negative control, the
lymph nodes from day 16 of the estrous cycle and lymph nodes from
days 13, 16 and 25 of pregnancy, respectively (Fig. 3).
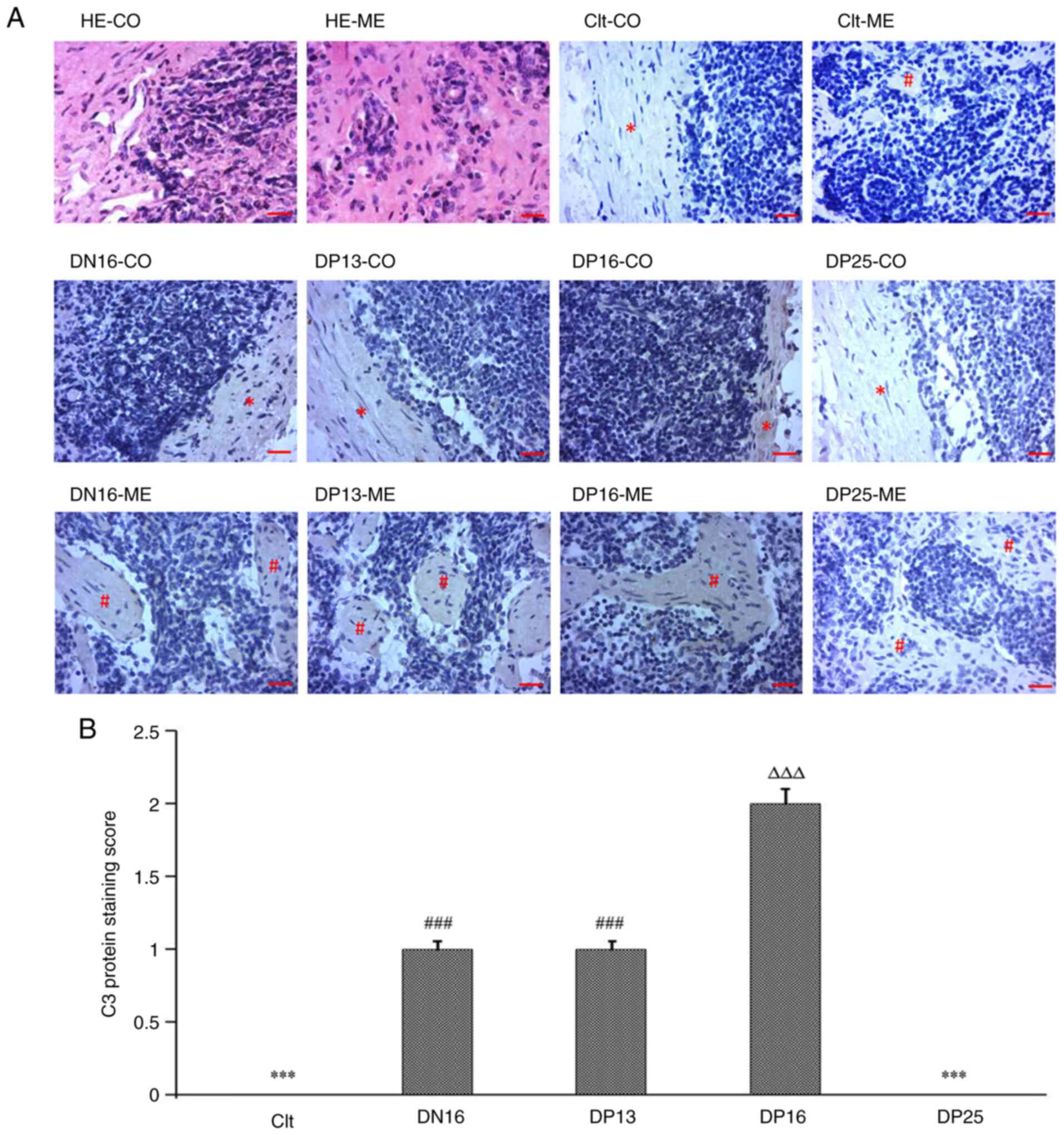 | Figure 3Immunohistochemical localization of
C3 protein in the lymph nodes from non-pregnant and pregnant ewes.
(A) Immunohistochemical localization of C3 protein. Lymph node is
divided into the CO and the ME. Scale bar=20 µm. (B) Staining
intensities for C3 protein. ***P<0.001. Clt and DP25
vs. DN16, DP13 and DP16; ###P<0.001. DN16 and DP13
vs. Clt, DP16 and DP25; ∆∆∆P<0.001. DP16 vs. Clt,
DN16, DP13 and DP25. CO, cortex; ME, medulla; HE, hematoxylin and
eosin; Clt, control; DN16, day 16 of the estrous cycle; DP13, day
13 of pregnancy; DP16, day 16 of pregnancy; DP25, day 25 of
pregnancy. |
Discussion
The present study demonstrated that C1q mRNA
and protein levels were increased during early pregnancy, and
peaked at day 16 of gestation in the maternal lymph node. C1q is a
complex glycoprotein with a C-terminal globular head region and an
N-terminal collagen-like tail that mediates a variety of
immunoregulatory functions (26).
C1q participates in feto-maternal tolerance, trophoblast migration
and spiral artery remodeling, and the transcription factor PU.1
regulates decidual C1q expression during early pregnancy in humans
(27). Complement C1q can modulate
the functions of immune and non-immune cells, and its deficiency
and dysregulation result in preeclampsia, missed abortion,
miscarriage or spontaneous loss (28). Paternal deficiency of complement
component C1q results in a preeclampsia-like pregnancy, and
wild-type female mice exhibit renal dysfunction, fetal growth
restriction and reduced placental efficiency during mid- and late
gestation (29). Upregulation of
anti-C1q antibody levels and thyroid-stimulating hormone levels are
associated with to autoimmune thyroid disorders during pregnancy in
women (30). Mouse C1q is located
in various tissues, including lymph nodes (31). Therefore, it is hypothesized that
the upregulation of C1q in the maternal lymph node may be important
for embryo implantation during early pregnancy in ewes.
The present study revealed that C1r was upregulated
in the maternal lymph node at day 25 of gestation. As a modular
serine protease, C1r is the autoactivating component of the C1
complex during activation of the next complement components
(32). The complement C1r
subcomponent protein exists in the maternal serum during pregnancy
and can be quantified using isotype tagging in humans (33). Amnion tissue explants and
amnion-derived epithelial cells synthesize C1r, which indicates
that the amnion synthesizes complement C1r (34). C1q is responsible for the
prevention of pregnancy loss, and the immune functions of C1q are
mediated by autoactivation of C1r during pregnancy (35). There is an upregulation of the
C1R gene in peripheral blood polymorphonuclear cells,
suggesting that C1R is implicated in the early immune
response to conceptus presence during the pre-attachment period of
pregnancy in heifers (36).
Therefore, the upregulation of C1r in maternal lymph nodes at day
25 of pregnancy may be associated with embryonic development in
ewes.
The results of the present study demonstrated that
early pregnancy induced the expression of C1s in the maternal lymph
node, with a peak at day 13 of gestation. The complement component
C1 complex is composed of target-recognition subcomponent C1q and
modular proteases C1r and C1s, and C1s executes the catalytic
function of the C1 complex to cleave C2 and C4(37). C1s is present in the circulating
immune complexes during the first trimester of normal pregnancy,
and the C1s level decreases during the following weeks of gestation
in women (38). C1s is detectable
in the placenta, and IFN-γ stimulates the synthesis of C1s in
chorion-derived cells (39). The
IFN-γ protein is downregulated in bovine peripheral blood
mononuclear cells during early pregnancy (40), which may be associated with the
decline in C1s, and the loss of immune response to allograft fetus.
Complement C1s protein is elevated in maternal plasma of the women
with preeclampsia, and is implicated in the remodeling process of
the spiral arteries before the manifestation of clinical disease
(41). C1s protein is
significantly enhanced in the plasma of women carrying Turner
syndrome fetuses compared with pregnant women with normal fetuses
in the second trimester of pregnancy (42). Therefore, the upregulation of C1s
at day 13 of gestation may be associated with initiating
implantation, and the downregulation of C1s in maternal lymph nodes
at day 25 of gestation may be helpful for pregnancy maintenance in
ewes.
The results of the current study also revealed that
C2 mRNA and protein levels were upregulated in the maternal
lymph node at day 16 of gestation. Deficiency of C2 leads to
autoimmunity, and C2 protein participates in both the classical and
lectin pathways of the complement cascade, though C2 is not
required for activation of the complement system by the classical
or lectin pathway (43). C2 does
not participate in activation of the complement system on
trophoblastic basement membranes through the classical pathway of
complement activation, suggesting that C2 may not be associated
with materno-fetal communication during normal human pregnancy
(44). C2 deficiency and systemic
lupus erythematosus lead to thrombocytopenia and renal
abnormalities during the first trimester of pregnancy (45). Interferon stimulates the synthesis
of C2 in human monocytes in vitro (46). IFNT exerts its effects on maternal
lymph nodes (6). IFNT (Protein X)
and additional proteins are detected between days 14 and 21, and
are produced by conceptus trophoblasts in sheep as previously
reported (47). It is suggested
that the peak of C2 expression at day 16 of pregnancy in the
current study may have been associated with the effects of IFNT,
which may not be associated with the activation of complement
pathways in the lymph nodes of ewes.
In the present study, C3 peaked in maternal lymph
nodes at day 16 of gestation, and then decreased at day 25 of
gestation. Component C3 acts as a point of convergence of
activation pathways to amplify the complement response, and helps
to coordinate downstream immune responses (48). A high level of maternal serum C3 in
the first trimester is associated with an increased risk of preterm
birth, which can be used for the early diagnosis and prognosis of
preterm birth in pregnant women (49). C3 is implicated in the development
of preeclampsia through bioinformatics-based identification
(50). The serum concentration of
C3 is elevated in women with preeclampsia compared with normal
pregnant women (51). Human lymph
nodes, peripheral blood leucocytes and monocytes can synthesize C3
using an in vitro culture technique (52). It is suggested that the decline of
C3 in maternal lymph nodes at day 25 of pregnancy may be required
for pregnancy maintenance in sheep.
In the present study, C4a mRNA and protein
were only expressed in maternal lymph nodes at day 25 of pregnancy.
C4a is an isoform of C4, and plays key roles in innate immune
surveillance, cellular activation and endothelial permeability
(53). C4a protein expression is
lower in JAR cells under hypoxic conditions compared with normoxic
conditions, indicating that preeclampsia is associated with low C4a
and hypoxia (54). Maternal plasma
C4a concentrations are determined by enzyme-linked immunoassay, and
there is a lower C4a level in women with gestational diabetes
compared with women with normal glucose tolerance at the time of
term delivery (55). A low
concentration of maternal plasma C4a is associated with
preeclampsia and small-for-gestational age fetuses during pregnancy
(56). Pregnant patients with
primary anti-phospholipid syndrome or undifferentiated connective
tissue disease have significantly lower levels of serum C4 compared
with healthy women in each trimester (57). A low amount of C4 mRNA can
be detected in normal human lymph nodes using slot blot
hybridization (58). Therefore, it
is hypothesized that the upregulation of C4a in maternal lymph
nodes at day 25 of pregnancy may be beneficial for successful
pregnancy in ewes.
The results of the present study demonstrated that
C5b mRNA and protein levels were upregulated in maternal
lymph nodes during early gestation compared with day 16 of the
estrous cycle. C5 protein cleaves into C5a and C5b, and C5b
participates in the formation of the membrane attack complex (MAC)
associated with C6, C7, C8 and C9(59). C5 convertase cleaves C5 to C5b to
result in C5b-9 assembly as the MAC pore on the cell surface
(60). C5b-9 is detectable in all
placentae, and localized in the surface of syncytiotrophoblasts,
intervillous fibrin and decidual vessels (61), which contribute to placental
formation. There is an increase in C5b-9 staining within villous
trophoblasts of placentas from normal controls compared with
patients with preeclampsia in humans (62). In addition, a lack of C5 is
associated with embryonic death in Crry-deficient mice, which
suggests that C5 plays a key role in preventing embryonic lethality
during early pregnancy (63).
Therefore, it is reasonable to hypothesize that the upregulation of
C5b in maternal lymph nodes may be helpful for pregnancy
maintenance during early pregnancy.
The present study demonstrated that early pregnancy
induced the expression of C9 mRNA and protein in maternal
lymph nodes at day 16 of gestation. C9 is the final component of
the MAC, and the only component of the assembly (64). MACs are involved in pore formation
in the plasma membrane of target cells, which is associated with
innate and adaptive immune responses (65). Serum C9 levels are significantly
higher in healthy pregnant women compared with in non-pregnant
women (66). A Japanese woman with
C9 deficiency suffered three mid-trimester miscarriages and one
early spontaneous miscarriage, suggesting that C9 deficiency is a
potential cause of undiagnosed recurrent miscarriage (67). The deposition of C9 is increased in
placentae with preeclampsia compared with normal tissues, which is
associated with the chorionic villus immunopathology of
preeclampsia in humans (68). C9
deposition increases at the implantation sites in pregnant mice
with fetal loss (69). C9
concentration is enhanced in the umbilical cord blood of term
infants, which is associated with its role in immunity in
prematurity (70). Therefore, it
is suggested that the upregulation of C9 in maternal lymph nodes at
day 16 of pregnancy may be associated with placentation, and the
downregulation of C9 at day 25 of pregnancy may be beneficial for
pregnancy maintenance in sheep.
Lymph enters the convex system through afferent
lymphatic vessels with several branched sinus systems, including
subcapsular sinuses, and flows into the blood circulation through
efferent lymphatic vessels, including lymph sinuses (71). The immunohistochemistry results of
the present study indicated that the immunostaining for C3 protein
was located in the subcapsular sinuses and lymph sinuses. Component
C3 regulates its receptor expression in B cells from the spleen and
lymph nodes, which is implicated in innate and adaptive immune
responses in mice (72).
Therefore, it was suggested that the downregulation of C3 in the
maternal lymph node may be involved in immune tolerance of the
maternal lymph node at day 25 of pregnancy in sheep.
In summary, the expression levels of C1q, C1s and
C5b were increased during early pregnancy, and the expression
levels of C1r and C4a were increased at day 25 of pregnancy only.
There were peaks in the expression levels of C2 and C9 at day 16 of
pregnancy. C3 was downregulated at day 25 of pregnancy, and C3
protein was located in the subcapsular sinuses and lymph sinuses in
the maternal lymph nodes. Therefore, the expression profiles of
complement components were changed, indicating that complement
pathways may be involved in regulating immune responses of the
maternal lymph node during early pregnancy in sheep.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science
Foundation of Hebei Province, China (grant no. C2021402019), and
the Science and Technology R&D Project of Hebei Province, China
(grant no. 21326601D).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY conceived and designed the experiments. LC and LZ
performed the experiments. PF and XH analyzed and interpreted the
data. LY and LZ wrote the manuscript. LY and LZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Hebei University of Engineering Animal Care and Use Committee
(approval no. 2019-017), and humane animal care and handling
procedures were followed throughout the experiment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ott TL: Immunological detection of
pregnancy: Evidence for systemic immune modulation during early
pregnancy in ruminants. Theriogenology. 150:498–503.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gasteiger G, Ataide M and Kastenmüller W:
Lymph node-an organ for T-cell activation and pathogen defense.
Immunol Rev. 271:200–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hetherington CM and Humber DP: The effect
of pregnancy on lymph node weight in the mouse. J Immunogenet.
4:271–276. 1977.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McLean JM, Mosley JG and Gibbs AC: Changes
in the thymus, spleen and lymph nodes during pregnancy and
lactation in the rat. J Anat. 118(Pt 2):223–229. 1974.PubMed/NCBI
|
|
5
|
Yang L, Zang S, Bai Y, Yao X and Zhang L:
Effect of early pregnancy on the expression of progesterone
receptor and progesterone-induced blocking factor in ovine lymph
node. Theriogenology. 93:78–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang L, Wang Q, Liu Y, Zhang L, Lv W and
Liu B: Expression profiles of interferon-stimulated gene 15 and
prostaglandin synthases in the ovine lymph nodes during early
pregnancy. Mol Reprod Dev. 86:100–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang L, Wang P, Mi H, Lv W, Liu B, Du J
and Zhang L: Comparison of Th1 and Th2 cytokines production in
ovine lymph nodes during early pregnancy. Theriogenology.
123:177–184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bai J, Zhang L, Zhao Z, Li N, Wang B and
Yang L: Expression of melatonin receptors and CD4 in the ovine
thymus, lymph node, spleen and liver during early pregnancy.
Immunology. 160:52–63. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang L, Cao L, Yang F, Han X, Wang Y, Cao
N and Yang L: Relative abundance of interferon-stimulated genes
STAT1, OAS1, CXCL10 and MX1 in ovine lymph nodes during early
pregnancy. Anim Reprod Sci. 214(106285)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cao N, Cao L, Gao M, Wang H, Zhang L and
Yang L: Changes in mRNA and protein levels of gonadotropin
releasing hormone and receptor in ovine thymus, lymph node, spleen,
and liver during early pregnancy. Domest Anim Endocrinol.
76(106607)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lubbers R, van Essen MF, van Kooten C and
Trouw LA: Production of complement components by cells of the
immune system. Clin Exp Immunol. 188:183–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lo MW and Woodruff TM: Complement:
Bridging the innate and adaptive immune systems in sterile
inflammation. J Leukoc Biol. 108:339–351. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Teirilä L, Heikkinen-Eloranta J, Kotimaa
J, Meri S and Lokki AI: Regulation of the complement system and
immunological tolerance in pregnancy. Semin Immunol.
45(101337)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bulla R, Bossi F, Fischetti F, De Seta F
and Tedesco F: The complement system at the fetomaternal interface.
Chem Immunol Allergy. 89:149–157. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Girardi G and Salmon JB: The role of
complement in pregnancy and fetal loss. Autoimmunity. 36:19–26.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feng P, Yang G, Zhang W, Zhang L, Wu J and
Yang L: Early pregnancy regulates expression of complement
components in ovine liver. Anim Sci J. 92(e13660)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang L, Zhang Q, Wang H, Feng P, Yang G
and Yang L: Effects of early pregnancy on the complement system in
the ovine thymus. Vet Res Commun: Sep 24, 2021 (Epub ahead of
print).
|
|
18
|
Forde N and Lonergan P: Interferon-tau and
fertility in ruminants. Reproduction. 154:F33–F43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hansen TR, Sinedino LDP and Spencer TE:
Paracrine and endocrine actions of interferon tau (IFNT).
Reproduction. 154:F45–F59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang L, Liu B, Yan X, Zhang L, Gao F and
Liu Z: Expression of ISG15 in bone marrow during early pregnancy in
ewes. Kafkas Univ Vet Fak Derg. 23:767–772. 2017.
|
|
21
|
Zhang L, Xue J, Wang Q, Lv W, Mi H, Liu Y
and Yang L: Changes in expression of ISG15, progesterone receptor
and progesterone-induced blocking factor in ovine thymus during
early pregnancy. Theriogenology. 121:153–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang L, Liu Y, Lv W, Wang P, Wang B, Xue J
and Zhang L: Expression of interferon-stimulated gene 15-kDa
protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family
1, member B1, and prostaglandin E synthase in the spleen during
early pregnancy in sheep. Anim Sci J. 89:1540–1548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Han X, Zhang L, Cao N, Cao L and
Yang L: Early pregnancy induces expression of STAT1, OAS1 and
CXCL10 in ovine spleen. Animals (Basel). 9(E882)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kandil D, Leiman G, Allegretta M, Trotman
W, Pantanowitz L, Goulart R and Evans M: Glypican-3
immunocytochemistry in liver fine-needle aspirates: A novel stain
to assist in the differentiation of benign and malignant liver
lesions. Cancer. 111:316–322. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thielens NM, Tedesco F, Bohlson SS,
Gaboriaud C and Tenner AJ: . C1q: A fresh look upon an old
molecule. Mol Immunol. 89:73–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Madhukaran SP, Kishore U, Jamil K, Teo BH,
Choolani M and Lu J: Transcriptional factor PU.1 regulates decidual
C1q expression in early pregnancy in human. Front Immunol.
6(53)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kouser L, Madhukaran SP, Shastri A, Saraon
A, Ferluga J, Al-Mozaini M and Kishore U: Emerging and novel
functions of complement protein C1q. Front Immunol.
6(317)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sutton EF, Gemmel M, Brands J, Gallaher MJ
and Powers RW: Paternal deficiency of complement component C1q
leads to a preeclampsia-like pregnancy in wild-type female mice and
vascular adaptations postpartum. Am J Physiol Regul Integr Comp
Physiol. 318:R1047–R1057. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vitkova H, Jiskra J, Springer D, Limanova
Z, Telicka Z, Bartakova J, Trendelenburg M and Potlukova E:
Anti-C1q autoantibodies are linked to autoimmune thyroid disorders
in pregnant women. Clin Exp Immunol. 186:10–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
McManus LM and Nakane PK: Mouse c1q: Light
and electron microscopic immunohistochemical localization. J
Immunol. 126:1421–1427. 1981.PubMed/NCBI
|
|
32
|
Kardos J, Harmat V, Palló A, Barabás O,
Szilágyi K, Gráf L, Náray-Szabó G, Goto Y, Závodszky P and Gál P:
Revisiting the mechanism of the autoactivation of the complement
protease C1r in the C1 complex: structure of the active catalytic
region of C1r. Mol Immunol. 45:1752–1760. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Scholl PF, Cole RN, Ruczinski I, Gucek M,
Diez R, Rennie A, Nathasingh C, Schulze K, Christian P, Yager JD,
et al: Maternal serum proteome changes between the first and third
trimester of pregnancy in rural southern Nepal. Placenta.
33:424–432. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Katz Y, Gur S, Aladjem M and Strunk RC:
Synthesis of complement proteins in amnion. J Clin Endocrinol
Metab. 80:2027–2032. 1995.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Oberkersch R, Attorresi AI and Calabrese
GC: Low-molecular-weight heparin inhibition in classical complement
activation pathway during pregnancy. Thromb Res. 125:e240–5.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rocha CC, da Silva Andrade SC, de Melo GD,
Motta IG, Coutinho LL, Gonella-Diaza AM, Binelli M and Pugliesi G:
Early pregnancy-induced transcripts in peripheral blood immune
cells in Bos indicus heifers. Sci Rep. 10(13733)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gál P, Ambrus G and Závodszky P: C1s, the
protease messenger of C1. Structure, function and physiological
significance. Immunobiology. 205:383–394. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schena FP, Manno C, Selvaggi L, Loverro G,
Bettocchi S and Bonomo L: Behaviour of immune complexes and the
complement system in normal pregnancy and pre-eclampsia. J Clin Lab
Immunol. 7:21–26. 1982.PubMed/NCBI
|
|
39
|
Goldberg M, Luknar-Gabor N, Keidar R and
Katz Y: Synthesis of complement proteins in the human chorion is
differentially regulated by cytokines. Mol Immunol. 44:1737–1742.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang L, Wang Y, Li S, Zhu M, He K, Yao X
and Zhang L: Differential expression of interferon-gamma, IL-4 and
IL-10 in peripheral blood mononuclear cells during early pregnancy
of the bovine. Reprod Biol. 18:312–315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim SM, Cho BK, Kang MJ, Norwitz ER, Lee
SM, Lee J, Park CW, Kim BJ, Jun JK, Park JS, et al: Expression
changes of proteins associated with the development of preeclampsia
in maternal plasma: A case-control study. Proteomics. 16:1581–1589.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kolialexi A, Anagnostopoulos AK,
Papantoniou N, Vougas K, Antsaklis A, Fountoulakis M, Mavrou A and
Tsangaris GT: Potential biomarkers for Turner in maternal plasma:
possibility for noninvasive prenatal diagnosis. J Proteome Res.
9:5164–5170. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Miller EC and Atkinson JP: Overcoming C2
deficiency. Clin Immunol. 144:269–271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Faulk WP, Jarret R, Keane M, Johnson PM
and Boackle RJ: Immunological studies of human placentae:
Complement components in immature and mature chorionic villi. Clin
Exp Immunol. 40:299–305. 1980.PubMed/NCBI
|
|
45
|
Dixit R, Krieg AM and Atkinson JP:
Thrombotic thrombocytopenic purpura developing during pregnancy in
a C2-deficient patient with a history of systemic lupus
erythematosus. Arthritis Rheum. 28:341–344. 1985.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lappin DF, Birnie GD and Whaley K:
Modulation by interferons of the expression of monocyte complement
genes. Biochem J. 268:387–392. 1990.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Godkin JD, Bazer FW, Moffatt J, Sessions F
and Roberts RM: Purification and properties of a major, low
molecular weight protein released by the trophoblast of sheep
blastocysts at day 13-21. J Reprod Fertil. 65:141–150.
1982.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ricklin D, Reis ES, Mastellos DC, Gros P
and Lambris JD: Complement component C3-The ‘Swiss Army Knife’ of
innate immunity and host defense. Immunol Rev. 274:33–58.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huang S, Tian J, Liu C, Long Y, Cao D, Wei
L, Zhu X, Tang R, Liu W, Zeng D, et al: Elevated C-reactive protein
and complement C3 levels are associated with preterm birth: A
nested case-control study in Chinese women. BMC Pregnancy
Childbirth. 20(131)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jiang R, Wang T, Zhou F, Yao Y, He J and
Xu D: Bioinformatics-based identification of miRNA-, lncRNA-, and
mRNA-associated ceRNA networks and potential biomarkers for
preeclampsia. Medicine (Baltimore). 99(e22985)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kestlerová A, Feyereisl J, Frisová V,
Měchurová A, Šůla K, Zima T, Běláček J and Madar J: Immunological
and biochemical markers in preeclampsia. J Reprod Immunol.
96:90–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lai A, Fat RF and van Furth R: In vitro
synthesis of some complement components (C1q, C3 and C4) by
lymphoid tissues and circulating leucocytes in man. Immunology.
28:359–368. 1975.PubMed/NCBI
|
|
53
|
Wang H, Ricklin D and Lambris JD:
Complement-activation fragment C4a mediates effector functions by
binding as untethered agonist to protease-activated receptors 1 and
4. Proc Natl Acad Sci USA. 114:10948–10953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang H, Zhang Y, Yang F, Li L, Liu S, Xu
Z, Wang J and Sun S: Complement component C4A and apolipoprotein
A-I in plasmas as biomarkers of the severe, early-onset
preeclampsia. Mol Biosyst. 7:2470–2479. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lappas M: Lower circulating levels of
complement split proteins C3a and C4a in maternal plasma of women
with gestational diabetes mellitus. Diabet Med. 28:906–911.
2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Soto E, Romero R, Richani K, Espinoza J,
Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF,
et al: Preeclampsia and pregnancies with small-for-gestational age
neonates have different profiles of complement split products. J
Matern Fetal Neonatal Med. 23:646–657. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Reggia R, Ziglioli T, Andreoli L, Bellisai
F, Iuliano A, Gerosa M, Ramoni V, Tani C, Brucato A, Galeazzi M, et
al: Primary anti-phospholipid syndrome: any role for serum
complement levels in predicting pregnancy complications?
Rheumatology (Oxford). 51:2186–2190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Feucht HE, Zwirner J, Bevec D, Lang M,
Felber E, Riethmüller G and Weiss EH: Biosynthesis of complement C4
messenger RNA in normal human kidney. Nephron. 53:338–342.
1989.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Laursen NS, Magnani F, Gottfredsen RH,
Petersen SV and Andersen GR: Structure, function and control of
complement C5 and its proteolytic fragments. Curr Mol Med.
12:1083–1097. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Regal JF, Burwick RM and Fleming SD: The
complement system and preeclampsia. Curr Hypertens Rep.
19(87)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chighizola CB, Lonati PA, Trespidi L,
Meroni PL and Tedesco F: The complement system in the
pathophysiology of pregnancy and in pystemic autoimmune rheumatic
diseases during pregnancy. Front Immunol. 11(2084)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Matrai CE, Rand JH and Baergen RN: Absence
of distinct immunohistochemical distribution of annexin A5, C3b,
C4d, and C5b-9 in placentas from patients with antiphospholipid
antibodies, preeclampsia, and systemic lupus erythematosus. Pediatr
Dev Pathol. 22:431–439. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mao D, Wu X, Deppong C, Friend LD, Dolecki
G, Nelson DM and Molina H: Negligible role of antibodies and C5 in
pregnancy loss associated exclusively with C3-dependent mechanisms
through complement alternative pathway. Immunity. 19:813–822.
2003.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Spicer BA, Law RHP, Caradoc-Davies TT,
Ekkel SM, Bayly-Jones C, Pang SS, Conroy PJ, Ramm G, Radjainia M,
Venugopal H, et al: The first transmembrane region of complement
component-9 acts as a brake on its self-assembly. Nat Commun.
9(3266)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xie CB, Jane-Wit D and Pober JS:
Complement membrane attack complex: New roles, mechanisms of
action, and therapeutic targets. Am J Pathol. 190:1138–1150.
2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Derzsy Z, Prohászka Z, Rigó J Jr, Füst G
and Molvarec A: Activation of the complement system in normal
pregnancy and preeclampsia. Mol Immunol. 47:1500–1506.
2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Watanabe N, Suzuki T, Kitano E, Kitamura
H, Hatanaka M and Sago H: Successful pregnancy in a patient
suffering from recurrent mid-trimester miscarriage with C9
deficiency after receiving cervical cerclage followed by
clindamycin and progesterone: A case report. J Obstet Gynaecol Res.
38:562–566. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sinha D, Wells M and Faulk WP:
Immunological studies of human placentae: Complement components in
pre-eclamptic chorionic villi. Clin Exp Immunol. 56:175–184.
1984.PubMed/NCBI
|
|
69
|
Agostinis C, Biffi S, Garrovo C, Durigutto
P, Lorenzon A, Bek A, Bulla R, Grossi C, Borghi MO, Meroni P and
Tedesco F: In vivo distribution of β2 glycoprotein I under various
pathophysiologic conditions. Blood. 118:4231–4238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Gródecka-Szwajkiewicz D, Ulańczyk Z,
Zagrodnik E, Łuczkowska K, Rogińska D, Kawa MP, Stecewicz I,
Safranow K, Ustianowski P, Szymański S and Machaliński B:
Comparative analysis of global gene expression and complement
components levels in umbilical cord blood from preterm and term
neonates: Implications for significant downregulation of immune
response pathways related to prematurity. Int J Med Sci.
17:1840–1853. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jalkanen S and Salmi M: Lymphatic
endothelial cells of the lymph node. Nat Rev Immunol. 20:566–578.
2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Jacobson AC, Roundy KM, Weis JJ and Weis
JH: Regulation of murine splenic B cell CR3 expression by
complement component 3. J Immunol. 183:3963–3970. 2009.PubMed/NCBI View Article : Google Scholar
|

















