Introduction
Circadian rhythm in mammals is associated with the
periodic oscillation of clock genes. The principal pacemaker is
SCN, which can express circadian clock genes autonomously. Brain
and muscle ARNT-like 1 (BMAL1) is the core promoter of circadian
rhythm, which binds to circadian locomotor output cycles protein
kaput (CLOCK) to form a BMAL1/CLOCK complex, which then initiates
the transcription of the PER and CRY genes. A negative feedback
loop is then activated by the increased numbers of PER/CRY
complexes which inhibits the activity of BMAL1/CLOCK complexes
(1). An increasing body of evidence
has demonstrated that circadian clock genes regulate the
anti-oxidative stress mechanisms, especially the NRF2/ARE pathway
(2,3).
NRF2 is recognized as the core transcription factor
of anti-oxidative stress, able to activate a large number of
protective proteins, in which ARE-regulated antioxidant proteins
are most important, including NAD(P)H dehydrogenase [quinone] 1
(NQO1), glutamate-cysteine ligase modifier (GCLM) and heme
oxygenase 1 (HO1) (4). BMAL1 can
regulate the expression of NRF2 and its downstream antioxidant
stress protein by binding to the PPAR promoter through an E-BOX
element, aggregating the NRF2 protein together in a circadian
rhythm, which involves the transcription of ARE and other key
antioxidant proteins in a circadian rhythm (5). The cyclic activation of NRF2 plays a
vital role through its rhythmic recruitment of the promoter of the
targeted antioxidant gene, coordinating its ability to resist
oxidative stress in renal disease (6,7).
A previous study had confirmed that NRF2 plays an
important role in ischemia-reperfusion (IR) injury as a key
endogenous protective mechanism of oxidative stress (8). In addition, recent studies have found
that clock genes can act as endogenous molecular regulators of the
NRF2 redox pathway, participating in the pathological mechanism of
pulmonary fibrosis, and affecting anti-oxidative response
capability (9). However, the
internal mechanisms of the circadian clock genes that regulate the
NRF2-associated endogenous redox pathway or dysrhythmia of the
NRF2/ARE pathway that affect the circadian clock in renal IR injury
have not been defined. Thus, in the present study, the effect and
endogenous regulatory mechanism of dysrhythmia via the NRF2/ARE was
explored in the kidney for protection from oxidative stress induced
by IR.
Materials and methods
Materials
In total, 50 male adult SD rats (220-250 g; 6-8
weeks old) were purchased from The Animal Center of Renmin Hospital
of Wuhan University (Wuhan, China). Rats were housed in
specific-pathogen-free (SPF) conditions at 22-24˚C, a relative
humidity of 50±15%, receiving standard laboratory chow and water. A
total of ≥10 days prior to experimentation, the rats were housed in
a strict 12-h light/dark cycle [lights on at zeitgeber time (ZT)
0]. The experimental protocol of the present study was approved by
the Ethics Committee of Renmin Hospital of Wuhan University and in
accordance with the principles of Laboratory Animal Care by the
National Institutes of Health (permit no. 8023).
Antibodies for BMAL1 and NRF2 were purchased from
Abcam. Antibodies for NQO1, GCLM and HO1 were obtained from
Sigma-Aldrich. β-actin and LaminB were purchased from Cell
Signaling Technology, Inc. (cat. nos. 4970 and 13435,
respectively), and horseradish peroxidase (HRP)-conjugated
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. Blood urea nitrogen (BUN) and serum creatinine (Scr) were
measured using an Olympus automatic analyzer and neutrophil
gelatinase associated lipocalin (NGAL) levels were quantified using
the corresponding enzyme-linked immunosorbent assay (ELISA) kit
purchased from Elabscience, Inc. Superoxide dismutase (SOD) and
malondialdehyde (MDA) assay kits were purchased from Nanjing
Jiancheng Biochemicals Ltd.
Renal ischemia-reperfusion model
All rats were anesthetized by intraperitoneal
injection of 2% pentobarbital sodium (40 mg/kg). Rats were
immobilized and subsequently connected to an ECG monitor, after
whom the trachea was cut and the animals mechanically ventilated.
The IR model was established by bilateral renal pedicle occlusion
for ischemia (45 min), followed by removal of the microvascular
clip for 24 h reperfusion (10).
Except for occlusion, the other surgical procedures in the S Group
were the same. The procedure was successful if the kidney turned
from red to black after the pedicle occlusion, then black to red
after gradual removal the clip. The surgery was considered
successful if the rats regained consciousness after 1-3 h.
Histopathology of kidney tissue
The left kidney was sectioned, then fixed with 4%
formaldehyde for 24 h at room temperature, then embedded in
paraffin, from which 4-µm sections were cut and stained with
hematoxylin for 3 min and eosin for 60 sec at room temperature. The
slides were evaluated using light microscopy (original
magnification, x200; Olympus BX50; Olympus Corporation). Renal
histological assessment was conducted using a semi-quantitative
scale, as described by Spandou et al (11): For each kidney, ≥100 cortical
tubules from 10 different regions were scored. Higher scores
represented more severe damage, maximum score per tubule was 10,
scoring as follows: 0=Normal kidney; 1=minimal damage (<5%
involvement of the cortex or outer medulla); 2=mild damage (5-25%
involvement of the cortex or outer medulla); 3=moderate damage
(25-75% involvement of the cortex or outer medulla); 4=severe
damage (>75% involvement of the cortex or outer medulla)
(12).
Immunohistochemical assessment of NRF2
in the kidney
The streptavidin-biotin complex immunohistochemical
technique has been described previously (13). It was used to detect NRF2 protein in
paraffin-embedded kidney tissue sections by permeabilizing with
0.3% Triton X-100 (cat. no. P0096; Beyotime Institute of
Biotechnology) at room temperature for 10 min, then blocked with
10% goat serum (cat. no. C0265; Beyotime Institute of
Biotechnology) at 37˚C for 10 min, incubated overnight at 4˚C with
1:400 NRF2 antibody (cat. no. ab92946; Abcam), incubated 30 min at
37˚C with 1:500 Biotin-labeled secondary antibody (cat. no. A0277;
Beyotime Institute of Biotechnology), incubated 1 h at room
temperature with the 1:400 Streptavidin-HRP (cat. no. A0303;
Beyotime Institute of Biotechnology) and dyed 2-5 min at room
temperature with DAB + 30% H2O2. Positive
expression in the cytoplasm and/or nucleus was stained brown
(original magnification, x200; Olympus BX50; Olympus Corporation).
The optical density of positive staining was semi-quantitatively
evaluated using Image Pro®plus version 6.0 software
(Media Cybernetics, Inc.).
Measurement of Scr, BUN and NGAL
levels
After the end of IR, the right internal carotid
artery of the rats was isolated, 2 ml blood was collected from each
group. Blood samples were collected at the end of reperfusion,
centrifuged at 3,000 x g for 10 min at 4˚C and then serum was
separated and stored at -20˚C. Scr and BUN were measured using an
Olympus automatic analyzer (AU5400; Olympus Corporation), and NGAL
levels were measured using ELISA assay kits (cat. no. E-EL-R0662c;
Elabscience, Inc.) as described previously (13).
Measurement of SOD activity and MDA
levels in renal tissues
Renal tissues were harvested and immediately
homogenized on ice in 5 volumes of normal saline. The homogenates
were centrifuged at 1,200 x g for 10 min at 4˚C. SOD activity (cat.
no. A001-3-2) and MDA levels (cat. no. A003-1-2) were measured
using a chemical assay kit (cat. nos. A001-3-2 and A003-1-2,
respectively; Nanjing Jiancheng Biochemicals Ltd.) in accordance
with the manufacturer's protocol.
Western blot analysis
Cytoplasmic and nuclear proteins of the renal
tissues were extracted using nuclear and cytoplasmic protein
extraction kit (cat. no. P0028; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. After
measurement of the protein concentration using the bicinchoninic
acid method, an equal quantity of 50 µg protein was separated by
12% SDS-PAGE. After electrophoresis, proteins were transferred onto
polyvinylidene difluoride membranes. Each membrane was blocked with
5% nonfat milk for 2 h at room temperature, then incubated
overnight at 4˚C with an appropriate primary antibody: BMAL1 (cat.
nos. ab231793; Abcam), NRF2 (cat. nos. ab92946; Abcam), NQO1, GCLM
or HO1 (cat. nos. N5288, SAB2100907 and 374087, respectively; Merck
KGaA), each at 1:800 dilution). After repeated washing with TBS-T
(containing 0.05% Tween-20) the membranes were incubated with the
HRP-conjugated secondary antibodies (1:2,000; cat. no. sc2357;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. The
immunoreactive bands were visualized by enhanced chemiluminescence
(cat. no. NEL103E001EA; PerkinElmer, Inc.) and captured on X-ray
films. The optical density of the bands was measured with
Glyko® BandScan V4.0 imaging analysis system (http://bandscan.software.informer.com/).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was isolated from renal tissue using an
RNAeasy™ animal RNA isolation kit (Beyotime Institute of
Biotechnology). cDNA was synthesized at 42˚C for 60 min and 70˚C
for 15 min using a BeyoRT™ First Strand cDNA synthesis
kit (cat. no. D7166; Beyotime Institute of Biotechnology) according
to the manufacturer's protocols. Quantitative real-time PCR
(protocol: 50˚C for 2 min, 95˚C for 10 min; 40 cycles of 95˚C for
30 sec and 60˚C for 30 sec) was conducted using SYBR Green Master
mix (Vazyme Biotech, Co., Ltd.) and primers for CLOCK (forward,
5'-TCAAGGCCAGAGTTCATCGT-3' and reverse,
5'-GAGTTGGGCTGTGATCGAAC-3'), BMAL1 (forward,
5'-TGAACCAGACAATGAGGGCT-3' and reverse,
5'-TATGCCAAAATAGCCGTCGC-3'), and NRF2 (forward,
5'-CCCATTGAGGGCTGTGAT-3' and reverse, 5'-TTGGCTGTGCTTTAGGTC-3').
Results were quantified using the 2-ΔΔCq method
(14) and normalized against
β-actin (forward primer, 5'-CACGATGGAGGGGCCGGACTCATC-3'; reverse,
5'-TAAAGACCTCTATGCCAACACAGT-3').
Chromatin immunoprecipitation (ChIP)
assays
Following homogenization, the kidney tissue was
cross-linked with 1% (v/v) formaldehyde for 10 min at 37˚C and cell
nuclei lyzed using Bioruptor ultrasound (500 bp; 25% power; 4.5 sec
impact, 9 sec interval, 14 times in total) to obtain chromatin. The
chromatin was incubated with the following antibodies at 4˚C
overnight as follows: 1 µg/ml anti-NRF2 (cat. no. ab137550; Abcam),
4 µg/ml anti-BMAL1 (cat. no. ab231793; Abcam), and 1 µg/ml control
IgG (cat. no. ab172730; Abcam). Target DNA fragment were obtained
by protein G magnetic Dynabead (cat. no. 10003D; Life Technologies)
immunoprecipitation, then reverse chromatin cross-linking with 5 M
NaCl overnight at 65˚C and digestion by protease K (cat. no.
1.24568; Sigma-Aldrich; Merck KGaA), after which the DNA was
eluted. Using RT-qPCR technology, the E-BOX (forward
5'-GAGCCCAGGGCACGTGGGAGAAGTGG-3' and reverse,
5'-CCACTTCTCCCACGTGCCCTGGGCTC-3') of the promoter region was
amplified and quantified.
Statistical analysis
All outcome measurements were expressed as means ±
SD values (n=5) and analyzed using Graph Pad Prism 6.0 (GraphPad
Software, Inc.). Statistical significance of differences among
groups was determined by a one-way ANOVA with Tukey's post hoc
test. Comparison of two groups was evaluated using a t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Circadian rhythm of NRF2 gene in the
kidney
In order to explore the circadian rhythm of NRF2
protein expression in normal kidneys, total NRF2 protein expression
levels were evaluated in the normal rat kidneys that were collected
every 4 h after ZT0. Western blot analysis indicated that total
NRF2 protein expression levels in the S group displayed a strong
circadian rhythm (Fig. 1A). The
peak of NRF2 protein expression was between ZT0 and ZT4, with a
trough between ZT12 and ZT16 (Fig.
1A). NRF2 protein translocation into the nucleus initiates
activation of downstream antioxidant proteins. It was found that
the nuclear NRF2 protein expression in normal kidney tissue also
exhibited a circadian rhythm, with an amplitude and peak phase that
mirrored total NRF2 protein expression (Fig. 1B). In addition, immunostaining of
NRF2 protein indicated that the nuclear NRF2 protein expression in
renal tubular epithelial cells displayed clear diurnal variability
(Fig. 1C). Compared with ZT0, the
expression level of nuclear NRF2 protein in the ZT12 group was
weaker (P<0.05; Fig. 1C). In
order to further explore the circadian rhythm of the NRF2 gene
transcription, quantitative PCR was used to measure mRNA of the
core clock genes CLOCK and BMAL1 in the rat kidney, finding that
they displayed a strong endogenous circadian rhythm, corresponding
to that of NRF2 mRNA (Fig. 1D).
Together, these results strongly suggest that there is a
significant circadian rhythm in the NRF2 gene in the kidney that is
closely associated with the circadian clock of the body.
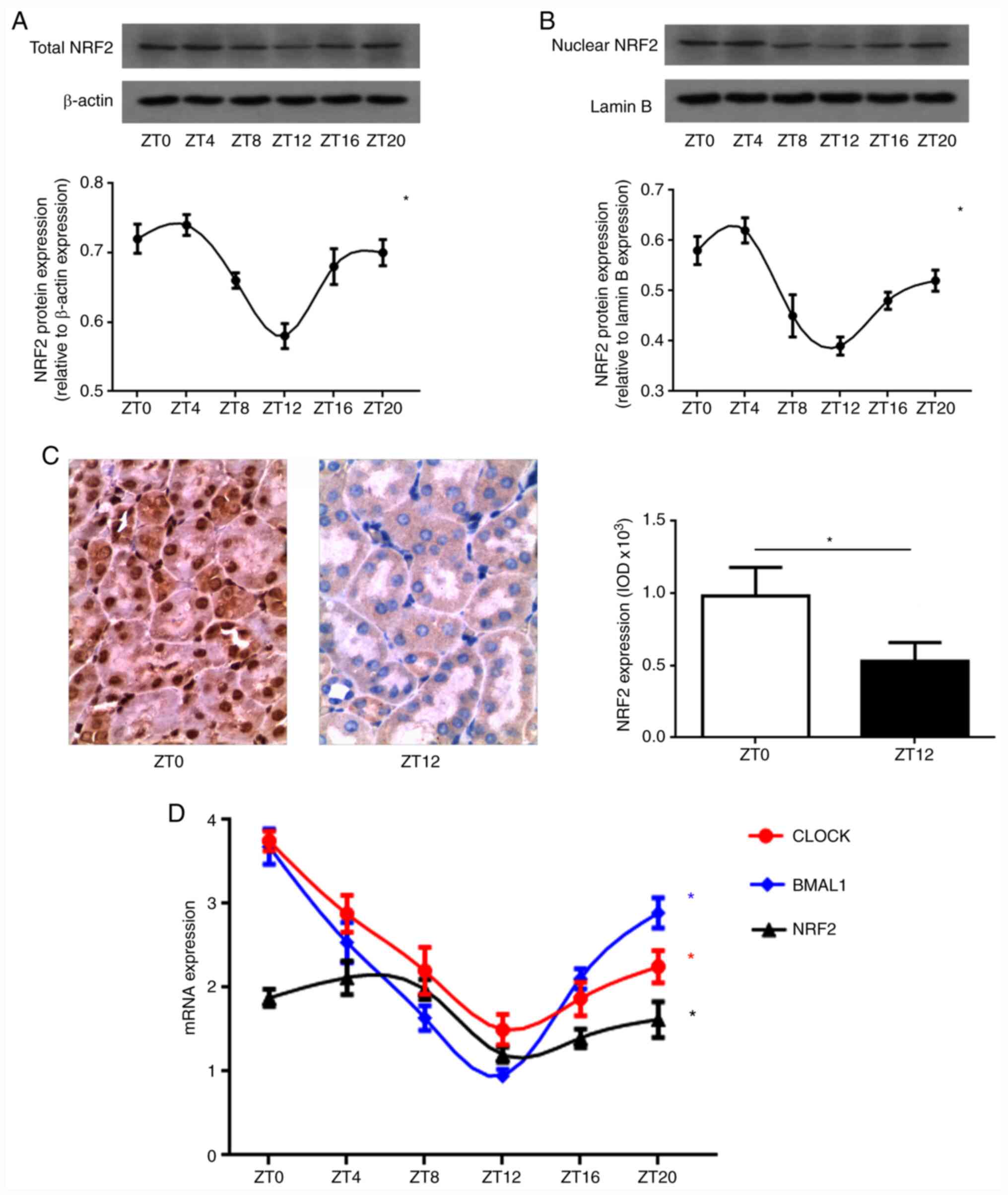 | Figure 1Circadian rhythm of NRF2 gene in the
kidney. (A) Total NRF2 protein expression levels by western blot
analysis. A strong circadian rhythm of total NRF2 protein
expression was revealed in normal kidney. The peak of NRF2 protein
expression was between ZT0 and ZT4, with a trough between ZT12 and
ZT16. (B) Nuclear NRF2 protein expression levels by western blot
analysis. The nuclear NRF2 protein expression in normal kidney
tissue also exhibited a circadian rhythm, with an amplitude and
peak phase that mirrored total NRF2 protein expression. NRF2
densitometry (mean ± SD; n=5) was normalized to β-actin or lamin B.
One-way ANOVA for the effect of time, *P<0.05. (C)
NRF2 protein expression levels by immunostaining. Positive
expression in the cytoplasm and/or nucleus was stained brown
(original magnification, x200). The nuclear NRF2 protein expression
in renal tubular epithelial cells displayed clear diurnal
variability. Compared with ZT0, the expression of nuclear NRF2
protein at ZT12 group was weaker. Data presented as mean ± SD, n=5.
*P<0.05. (D) The CLOCK, BMAL1 and NRF2 mRNA
expression by quantitative PCR. CLOCK and BMAL1 mRNA expression
displayed a strong endogenous circadian rhythm, corresponding to
that of NRF2 mRNA in normal kidney. Data (mean ± SD; n=5) were
normalized to GAPDH. One-way ANOVA for the effect of time,
*P<0.05. NRF2, nuclear factor erythroid 2-related
factor 2. |
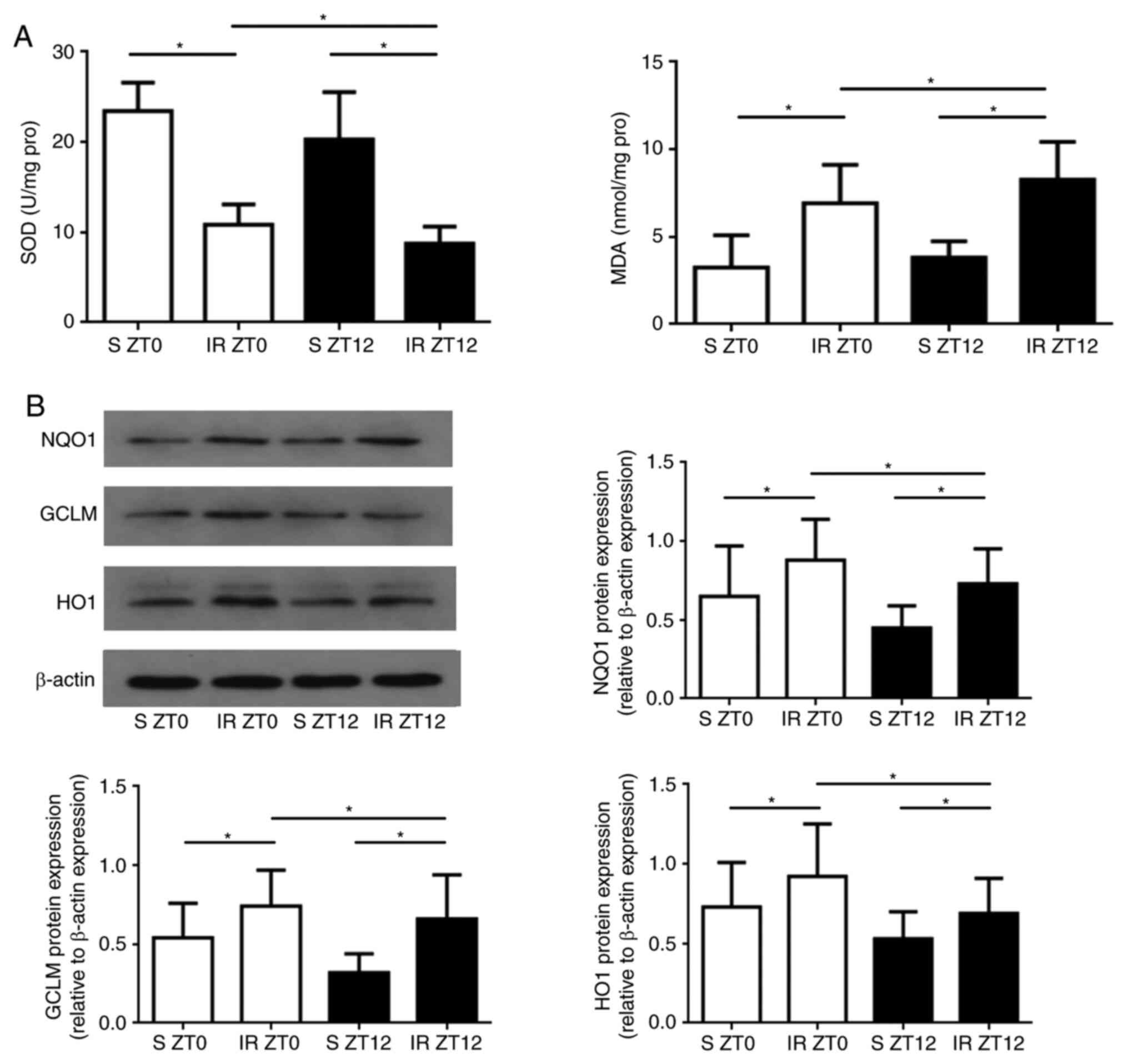
Diurnal variability of oxidative
stress in the kidney following IR injury
The present study investigated whether the rhythmic
expression of the oxidative stress and core protein NRF2 induced
diurnal variability in the anti-oxidant stress capability in renal
IR injury. It was hypothesized that the rhythmic expression of NRF2
may regulate the downstream ARE-regulated genes affecting IR
injury, which has diurnal variability. In order to verify this, the
rat renal IR model was established at a time point close to the
peak or trough of NRF2 protein expression. The peak of NRF2 protein
expression was between ZT0 and ZT4, and a trough between ZT12 and
ZT16. In order to fit into a 12-h light/12-h dark cycle, ZT0 and
ZT12 were selected as the two time points for sampling. As shown in
Fig. 2A, following 45 min of
ischemia, reperfusion significantly decreased SOD activity and
increased MDA levels of renal tissues in the ZT12 group compared
with those in the ZT0 Group (P<0.05). The protein expression
levels of NQO1, GCLM and HO1 exhibited clear diurnal variability,
and the expression in the ZT0 group was significantly higher
compared with that of the ZT12 group (P<0.05 for all three
genes; Fig. 2B).
Diurnal variability of renal injury
induced by IR
As expected, and displayed in Fig. 3A, following 24-h reperfusion,
characteristic histological changes to renal tubules, including
tubular epithelial edema and swelling, lumen dilation, epithelial
simplification, nuclear necrosis, cytoplasmic translucency, and
vacuolation were observed in the IR groups. Histological changes in
ZT12 were significantly increased compared with the ZT0 IR group.
Compared with the normal kidney tissue, renal histology scores in
the IR group were significantly higher (P<0.05). Compared with
ZT0, the score was significantly higher when the IR model was
established at ZT12 (P<0.05). Furthermore, Scr, BUN and NAGL
levels were higher in the ZT12 IR group compared with the ZT0 IR
group, indicating a higher induction of acute kidney injury
(Fig. 3B). Overall, the results
confirm the hypothesis that rhythmic activation of ARE-regulated
antioxidant protein induces diurnal variability of the anti-oxidant
stress capability in renal IR injury.
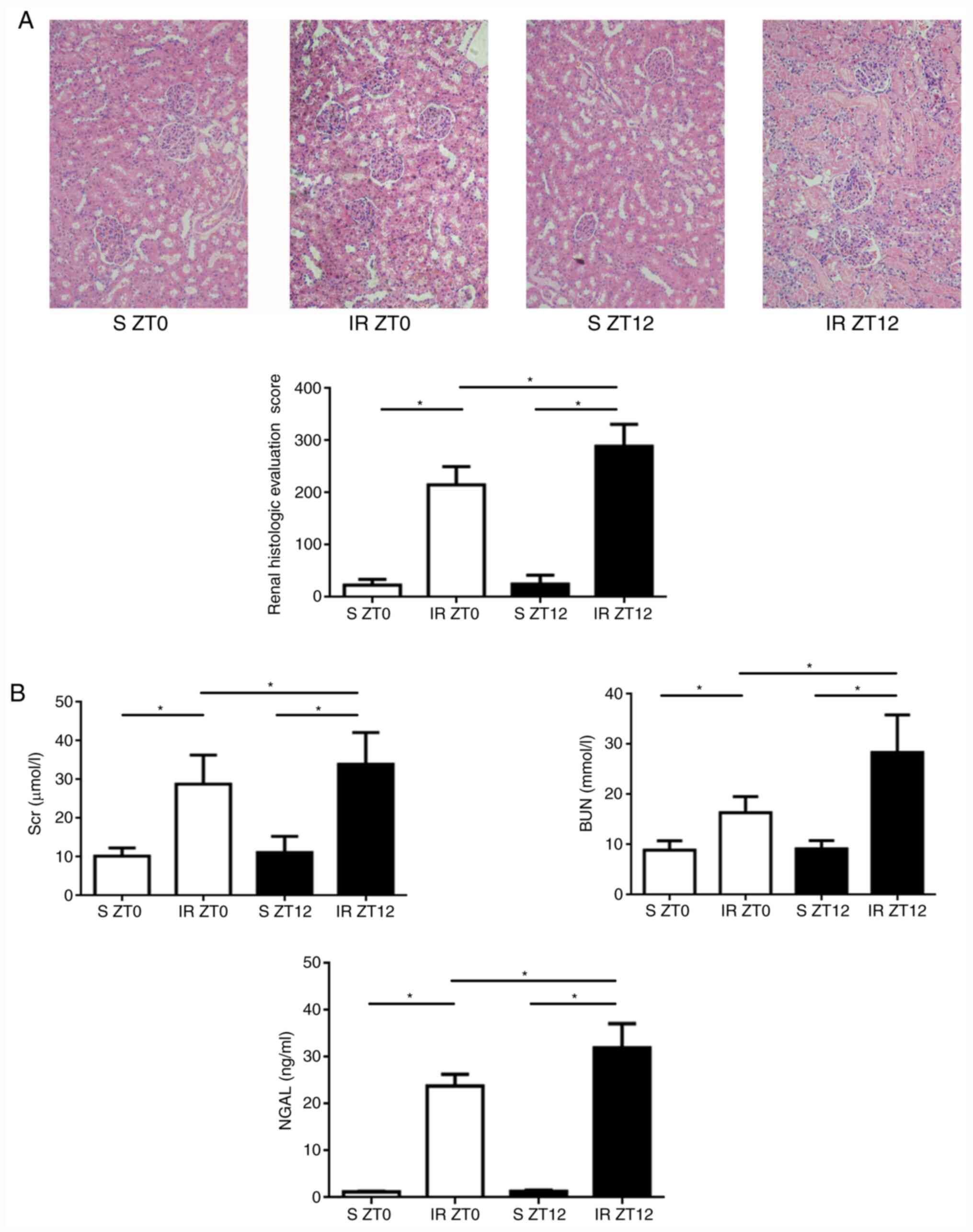 | Figure 3Diurnal variability of renal injury
induced by IR. (A) Renal IR injury assessment (original
magnification, x200). Following 24-h reperfusion, characteristic
histological changes in renal tubule, including tubular epithelial
edema and swelling, lumen dilation, epithelial simplification,
nuclear necrosis, cytoplasmic translucency and vacuolation were
observed in IR groups. Histological changes of ZT12 increased
significantly compared with those of ZT0 in the IR groups. Renal
histologic evaluation score was higher when the IR model was
established at ZT12 (data presented as mean ± SD; n=5;
*P<0.05). (B) The Scr, BUN and NAGL levels determined
by enzyme-linked immunosorbent assay. The levels of ZT0 group were
significantly higher compared with those of the ZT12 group (data
presented as mean ± SD; n=5; *P<0.05 for all three
indicators). IR, ischemia-reperfusion; Scr, serum creatinine; BUN,
blood urea nitrogen; NAGL, neutrophil gelatinase associated
lipocalin. |
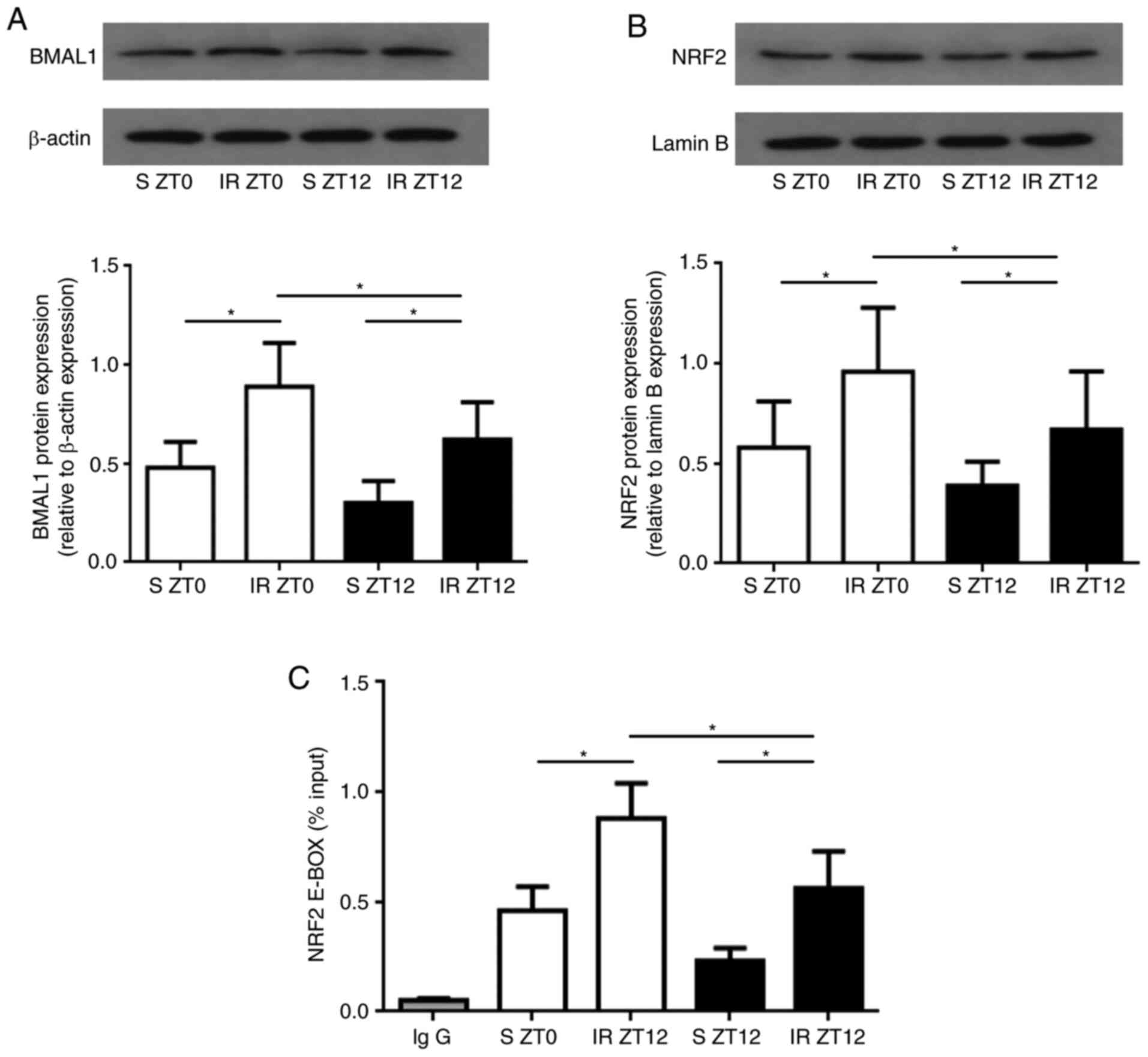
Binding of BMAL1 to the NRF2 gene
through an E-BOX region in the kidney following IR injury
The rhythmic recruitment and activation of NRF2 and
downstream antioxidant proteins led to the probing of whether and
how the circadian clock regulated the NRF2/ARE pathway in renal IR
injury. To explore the role of BMAL1 rhythms in coupling the
rhythmic activation of the NRF2 gene to diurnal variability of IR
injury in the kidney, the expression of BMAL1 and nuclear NRF2
protein were measured. After reperfusion, BMAL1 and nuclear NRF2
protein expression were both significantly higher when IR was
established at ZT0 compared with ZT12 (P<0.05; Fig. 4A and B).
In order to further verify the hypothesis that the
core clock gene may bind rhythmically to the promoter of the NRF2
gene, we conducted temporal ChIP assays in the rat kidney tissue.
The results demonstrated that the BMAL1 gene bound to the promoter
of the NRF2 gene through the E-BOX region. It was observed that the
binding of BMAL1 to the NRF2 gene at ZT0 was significantly higher
compared with that of ZT12 in both S and IR groups (P<0.05;
Fig. 4C), corresponding to the
diurnal variability of the NRF2 expression. Together, the results
indicate that the core clock gene BMAL1 controls the rhythmic
expression of the NRF2 gene directly through the E-BOX region of
the kidney following IR injury.
Discussion
In the present study, it was found that the
circadian rhythm of the NRF2/ARE pathway controlled by the
circadian clock is essential for the regulation of antioxidant
stress in renal IR injury. Strong rhythmic binding of BMAL1
specifically to an E-BOX region was detected in the gene promoters
of NRF2. Subsequently, the rhythmic recruitment and activation of
NRF2 protein played a critical role in the rhythmic expression of
downstream antioxidant proteins (such as NQO1, GLCM and HO1), which
are involved in renal IR injury. The results identified a pivotal
role for the circadian rhythm of the NRF2/ARE pathway controlled by
the circadian clock, which is essential in protecting against
oxidative stress injury in renal IR.
As a peripheral organ, the kidney has an independent
biological clock system, that regulates its physiological function
and exhibits diurnal variability, including its blood pressure,
glomerular filtration rate, and urinary sodium excretion. Recent
studies have confirmed that the circadian rhythm of kidney function
and the phase of clock gene expression (such as those of CLOCK,
BMAL1 and Per1/2) are coordinated, a critical molecular mechanism
that maintains the physiological function of the kidney (15). Furthermore, dysregulation of
circadian gene expression can damage renal function and
significantly influence systemic diseases, such as hypertension,
sleep cycle disorder and cancer (16). As shown in Fig. 1, mRNA expression levels of core
clock genes, including CLOCK and BMAL1, exhibited robust endogenous
circadian rhythm in the normal kidney.
A report in the Lancet demonstrated that patients
undergoing cardiac surgery during the daytime exhibit a different
tolerance to IR injury and degree of myocardial injury compared
with those during the night, due to the rhythmic expression of
clock genes (17). A similar
observation has been reported in animals in models of IR (18-20),
suggesting that the rhythmic expression of the clock gene plays an
important role in oxidative stress injury following IR, although
the underlying mechanism is unclear. In the present study, it was
found that the rhythm of clock genes and indicators of oxidative
stress within the kidney were disordered following IR, which was
accompanied by the pathological and functional impairment of the
kidney. Therefore, it was speculated that IR may lead to the
disorder of the circadian rhythm of the renal clock gene, impairing
the consistency between clock genes, which may represent the
endogenous molecular mechanism causing renal oxidative stress
injury. IR injury is an inevitable pathological process in renal
transplantation. Clinical observation has shown that melatonin
secretion, blood pressure, and fluctuation in body temperature,
time of sleep onset, total duration of sleep time and its depth did
not improve in renal transplant patients, and the rhythmic
variations in urinary hormone excretion were also disturbed
(15,21). This may also be closely associated
with the incidence of cardiovascular disease, deterioration in
renal allograft function and long-term survival in patients
undergoing renal transplant.
In previous research, it was confirmed that the
NRF2/ARE pathway plays a vital role in defense against oxidation
that balances oxidative stress induced by ROS in renal IR injury
(13,22). Cyclic activation and rhythmic
recruitment of NRF2 protein are controlled by the core clock genes
CLOCK and BMAL1. The rhythmic activation of the Nrf2/ARE pathway
may be a key process for the downstream expression of antioxidant
proteins for effective removal of ROS and inhibition of tissue
damage (23). The results of the
present study indicate that activation of NRF2 results in a change
in the amplitude of circadian rhythm and periodicity, with
associated variation in the rhythmic expression of CLOCK and BMAL1
in both normal and IR kidney tissue. The transactivation of NRF2 is
regulated by the binding of BMAL1 to the NRF2 promoter in the E-BOX
region, contributing to the rhythmic activation of the NRF2/ARE
pathway and rhythmic expression of downstream antioxidant proteins
(24). Thus ChIP assay was used to
identify that, under physiological conditions, the recruitment and
activation of NRF2 protein is especially controlled by the core
clock protein BMAL1 in the kidney through its binding to NRF2. The
data highlight further evidence that the dysrhythmia of NRF2 in the
kidney has wide implications for renal pathological and functional
impairment induced by IR and together with ARE redox-mediated
regulation of antioxidant proteins, by detecting which can
indirectly reflect the activation of NRF2/ARE pathway, including of
NQO1, GCLM and HO1. From these observations, it appears that NRF2
may be a vital mechanism between the disorder of clock gene rhythm
and diurnal oscillation of redox balance in renal IR injury. In the
circadian pathway, IR-induced upregulation of NRF2 via
BMAL1/CLOCK-mediated transactivation results in the transactivation
of ARE-associated antioxidant proteins.
In conclusion, it was demonstrated that endogenous
circadian clock genes regulating the NRF2/ARE pathway are
associated with an anti-oxidative stress protection mechanism in
the rat kidney following IR. The data indicate that the core clock
gene BMAL1 plays a vital role in regulating the recruitment and
activation of the NRF2 gene in the kidney. In addition, dysrhythmia
of the NRF2/ARE pathway may affect the expression of downstream
antioxidant proteins (such as NQO1, GLCM and HO1) conferring the
rhythmic regulation to alter susceptibility to oxidative stress
induced by IR in the kidney.
Limitation and future direction
The role of NRF2/ARE pathway in renal I/R injury has
been confirmed by using NRF2 agonists and inhibitors in our
previous study (25). The present
study focused on the effect of the clock gene on NRF2 and its
downstream substrates. Since the clock gene has no specific
agonists or antagonists, future studies should employ specific gene
knockout or gene mutations in mice in vivo, and gene
silencing in primary cells in vitro, in order to establish
that the inhibition of core clock genes such as CLOCK and BMAL1
affect the expression of NRF2, and so represent as potential
therapeutic targets.
Supplementary Material
Original images of protein bands and
sizes from western blotting. A) Total NRF2 protein expression level
in the kidney. B) Nuclear NRF2 protein expression level in the
kidney. C) NQO1, GCLM and HO1 protein expression levels in the
kidney following IR injury. D) BMAL1 protein expression level in
the kidney following IR injury. E) Nuclear NRF2 protein expression
level in the kidney following IR injury.
Acknowledgements
Not applicable.
Funding
This work was supported by the Independent Research
Projects of Wuhan University (grant no. 2042018kf0100) and National
Natural Science Foundation of China (grant no. 82072140).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS and CD designed the study; CZ performed the
majority of experiments; LD analyzed the data; QS wrote the
manuscript; Illustrations and proofreading was performed by CD. QS
and CZ can authenticate the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experimental protocol of the present study was
approved by the Ethics Committee of Renmin Hospital of Wuhan
University (Wuhan, China) and in accordance with the principles of
Laboratory Animal Care by the National Institutes of Health (permit
no. 8023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bollinger T and Schibler U: Circadian
rhythms-from genes to physiology and disease. Swiss Med Wkly.
144(w13984)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilking M, Ndiaye M, Mukhtar H and Ahmad
N: Circadian rhythm connections to oxidative stress: Implications
for human health. Antioxid Redox Signal. 19:192–208.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pei JF, Li XK, Li WQ, Gao Q, Zhang Y, Wang
XM, Fu JQ, Cui SS, Qu JH, Zhao X, et al: Diurnal oscillations of
endogenous H2O2 sustained by
p66Shc regulate circadian clocks. Nat Cell Biol.
21:1553–1564. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tonelli C, Chio IIC and Tuveson DA:
Transcriptional regulation by Nrf2. Antioxid Redox Signal.
29:1727–1745. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tamaru T, Hattori M, Ninomiya Y, Kawamura
G, Varès G, Honda K, Mishra DP, Wang B, Benjamin I, Sassone-Corsi
P, et al: ROS stress resets circadian clocks to coordinate
pro-survival signals. PLoS One. 8(e82006)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Desvergne A, Ugarte N, Radjei S, Gareil M,
Petropoulos I and Friguet B: Circadian modulation of proteasome
activity and accumulation of oxidized protein in human embryonic
kidney HEK 293 cells and primary dermal fibroblasts. Free Radic
Biol Med. 94:195–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wible RS, Ramanathan C, Sutter CH, Olesen
KM, Kensler TW, Liu AC and Sutter TR: NRF2 regulates core and
stabilizing circadian clock loops, coupling redox and timekeeping
in Mus musculus. Elife. 7(e31656)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Q, Shen ZY, Duan WN, Meng QT and Xia
ZY: Mechanism of myocardial ischemia/reperfusion-induced acute
kidney injury through DJ-1/Nrf2 pathway in diabetic rats. Exp Ther
Med. 14:4201–4207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pekovic-Vaughan V, Gibbs J, Yoshitane H,
Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D,
Loudon A, et al: The circadian clock regulates rhythmic activation
of the NRF2/glutathione-mediated antioxidant defense pathway to
modulate pulmonary fibrosis. Genes Dev. 28:548–560. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Firsov D and Bonny O: Circadian rhythms
and the kidney. Nat Rev Nephrol. 14:626–635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Spandou E, Tsouchnikas I, Karkavelas G,
Dounousi E, Simeonidou C, Guiba-Tziampiri O and Tsakiris D:
Erythropoietin attenuates renal injury in experimental acute renal
failure ischaemic/reperfusion model. Nephrol Dial Transplant.
21:330–336. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen R, Zeng Z, Zhang YY, Cao C, Liu HM,
Li W, Wu Y, Xia ZY, Ma D and Meng QT: Ischemic postconditioning
attenuates acute kidney injury following intestinal
ischemia-reperfusion through Nrf2-regulated autophagy,
anti-oxidation, and anti-inflammation in mice. FASEB J.
34:8887–8901. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Q, Meng QT, Jiang Y, Liu HM, Lei SQ,
Su WT, Duan WN, Wu Y and Xia ZY and Xia ZY: Protective effect of
ginsenoside Rb1 against intestinal ischemia-reperfusion induced
acute renal injury in mice. PLoS One. 8(e80859)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Solocinski K and Gumz ML: The circadian
clock in the regulation of renal rhythms. J Biol Rhythms.
30:470–486. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johnston JG and Pollock DM: Circadian
regulation of renal function. Free Radic Biol Med. 119:93–107.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Montaigne D, Marechal X, Modine T, Coisne
A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, et al:
Daytime variation of perioperative myocardial injury in cardiac
surgery and its prevention by Rev-Erbα antagonism: A single-centre
propensity-matched cohort study and a randomized study. Lencet.
391:59–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Durgan DJ, Pulinilkunnil T,
Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow
CW, Dyck JR and Young ME: Short communication: Ischemia/reperfusion
tolerance is time-of-day-dependent: Mediation by the cardiomyocyte
circadian clock. Circ Res. 106:546–550. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rotter D, Grinsfelder DB, Parra V, Pedrozo
Z, Singh S, Sachan N and Rothermel BA: Calcineurin and its
regulator, RCAN1, confer time-of-day changes in susceptibility of
the heart to ischemia/reperfusion. J Mol Cell Cardiol. 74:103–111.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Beker MC, Caglayan B, Yalcin E, Caglayan
AB, Turkseven S, Gurel B, Kelestemur T, Sertel E, Sahin Z, Kutlu S,
et al: Time-of-day dependent neuronal injury after ischemic stroke:
Implication of circadian clock transcriptional factor Bmal1 and
survival kinase AKT. Mol Neurobiol. 55:2565–2576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Russcher M, Nagtegaal JE, Nurmohamed SA,
Koch BC, van der Westerlaken MM, van Someren EJ, Bakker SJ, Ter Wee
PM and Gaillard CA: The effects of kidney transplantation on sleep,
melatonin, circadian rhythm and quality of life in kidney
transplant recipients and living donors. Nephron. 129:6–15.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun Q, Meng QT, Jiang Y and Xia ZY:
Ginsenoside Rb1 attenuates intestinal ischemia reperfusion induced
renal injury by activating Nrf2/ARE pathway. Molecules.
17:7195–7205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang G, Wright CJ, Hinson MD, Fernando AP,
Sengupta S, Biswas C, La P and Dennery PA: Oxidative stress and
inflammation modulate Rev-erbα signaling in the neonatal lung and
affect circadian rhythmicity. Antioxid Redox Signal. 21:17–32.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wende AR, Young ME, Chatham J, Zhang J,
Rajasekaran NS and Darley-Usmar VM: Redox biology and the interface
between bioenergetics, autophagy and circadian control of
metabolism. Free Radic Biol Med. 100:94–107. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng Z, Qian S, Qingtao M, Zhongyuan X
and Yeda X: Effects of ATRA on diabetic rats with renal
ischemia-reperfusion injury. Acta Cir Bras.
35(e202000106)2020.PubMed/NCBI View Article : Google Scholar
|