Introduction
Pain is comprised of both physiological and
pathological pain (1). Pathological
pain can be further sub-divided into inflammatory, neuropathic and
cancerous pain (2). Among them, as
one of the common clinical symptoms, inflammatory pain affects
25-35% of adults in major European countries (3,4).
Current treatment strategies for pain combined with inflammation
mainly consists of nonsteroidal anti-inflammatory drugs (NSAIDs)
(5). However, long-term use of
NSAIDs may cause gastrointestinal bleeding and other side effects,
including acute myocardial infarction, heart failure and acute
kidney injury (5). Therefore, it is
necessary to discover novel safe and effective medication for
inflammatory pain.
Lidocaine has been previously reported to improve
tumor-free survival and overall survival by inhibiting the growth
and metastasis of breast tumor cells (6,7). In
addition, lidocaine is a commonly used local anesthetic that also
confers analgesic effects, resists hyperalgesia and
anti-inflammatory properties (8,9), which
is conducive to postoperative analgesia and controlling the
inflammatory response. Accumulating evidence has shown that
lidocaine serves an important regulatory role in numerous aspects
of the inflammatory response (10-12).
It was also reported that the NF-κB signaling pathway is activated
during inflammation (13), such
that lidocaine has been found to exert anti-inflammatory effects by
inhibiting the Toll-like receptor 4/NF-κB pathway (14).
Complete Freund's adjuvant (CFA) is a mixed oil that
is often used to study the pathology and mechanism underlying
inflammatory pain (15,16). Nagakura et al (15) suggested that CFA can cause
mechanical hyperalgesia in rats, where its inflammatory pain course
can last for >2 weeks, making it a superior model for chronic
pain research. In addition, Bai et al (16) previously established a rat model of
inflammatory muscle pain by injecting CFA into the rat masseter
muscle.
To the best of our knowledge, the effects of
lidocaine on MAPK/ERK/NF-κB signaling in CFA-induced chronic
inflammation has not been reported previously. Therefore, in the
present study, CFA was injected into Sprague-Dawley rats to
establish a rat model of inflammatory pain, following which the
role of lidocaine in inflammatory pain was assessed.
Materials and methods
Animals
A total of 50 healthy male Sprague-Dawley rats (age,
4-6 weeks; weight, 150-200 g; Beijing Vital River Laboratory Animal
Technology Co., Ltd.) were fed in a standard animal room with a
12-h light/dark cycle at a temperature of 22-25˚C with 40-50%
humidity and free access to food and water. All protocols strictly
followed the Institutional Animal Care and Use of Laboratory
Animals by the National Institutes of Health (17). The experimental protocols were
approved by the Animal Care and Use Committee of the First
Affiliated Hospital of Xinjiang Medical University (Urumqi,
China).
Establishment of a rat model and study
groups
To establish a rat model of inflammatory pain, the
plantar surface of the right hind paw of the rats was
subcutaneously injected with 100 µl CFA (Sigma-Aldrich; Merck
KGaA). All rats were randomly assigned into five groups (n=10). In
the control group, 100 µl normal saline was injected into the tail
vein of rats once a day for 4 days (18). In the CFA group, 100 µl CFA was
subcutaneously injected into rats. In the CFA + lidocaine group,
rats were firstly treated with 100 µl CFA, followed by injected
with 1, 3 or 5 mg/kg lidocaine (Sigma-Aldrich, Merck KGaA) into the
tail vein 1 h later; lidocaine injections were performed once a day
for 4 days. In the CFA + lidocaine + human epidermal growth factor
(rh-EGF) group, rats were firstly injected with 100 µl CFA, then 5
mg/kg lidocaine and 10 µg/kg rh-EGF were injected into the tail
vein 1 h later. Lidocaine/rh-EGF injections were performed once a
day for 4 days.
In total, 4 days after CFA injection, rats (4 rats
from each group) were anesthetized with an intraperitoneal
injection of 50 mg/kg pentobarbital and sacrificed by cervical
dislocation. The peripheral blood and spinal cord tissues were
subsequently harvested following euthanasia. Other rats (6 rats
from each group) were subjected to behavioral tests (mechanical
withdrawal threshold, thermal withdrawal latency and frequency
responses to cold stimulation) at 0 and 4 h, 1, 4, 7 or 14 days
after CFA injection.
Behavioral tests
Rat behavioral tests assessed the mechanical
withdrawal threshold (MWT), thermal withdrawal latency (TWL) and
frequency responses to cold stimulation.
Mechanical hyperalgesia was evaluated using Von Frey
monofilaments (Stoelting Co.). Von Frey monofilaments includes a
set of 8 nylon filaments. The stimulus generated by the tip when it
is bent is ~0.5, 1, 2, 4, 6, 8, 10 and 12 g (g, intensity unit).
These eight stimuli were used to stimulate different positions on
the plantar surface of the rats. The back paws of the rats were
first put under increasing pressure lasting 5-6 sec, where the
minimum force required to induce paw withdrawal was termed as the
MWT.
For the thermal preference test, the response of
each back paw was evaluated at intervals of 3 min after a radiant
heater (-3-65˚C; cat. no. BME-410A; Institute of Biomedical
Engineering, Chinese Academy of Medical Sciences) was placed under
the plantar surface of the back paw. The TWL was defined as when
the hind paws were removed from the thermal plate.
For the cold allodynia testing, a syringe was
connected to a thin polyethylene tube to smear a drop of acetone to
each back paw. A brisk paw withdrawal response was considered as a
sign of cold hyperalgesia. The test was performed for three times
and there were intervals of 5-10 min between each test.
All tests were performed after CFA injection for 0
and 4 h, 1, 4, 7 or 14 days.
ELISA
After 4 days of CFA injection, ELISA kits (Beyotime
Institute of Technology) were used to measure TNF-α (cat. no.
PT519), IL-1β (cat. no. PI303) and IL-6 (cat. no. PI328) levels in
rat serum according to the manufacturer's protocols. Antibodies
were purchased from Beyotime Institute of Biotechnology. Each set
of experiments was performed in triplicate.
Western blotting assay
The rats were euthanized after 4 days of CFA
injection. RIPA buffer (Beyotime Institute of Technology)
containing PMSF was used to extract total protein from the L4-L6
spinal cord tissues. Protein concentration was determined using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (40 µg per lane) were separated using
10% SDS-PAGE. Separated proteins were then transferred onto PVDF
membranes. Subsequently, membranes were blocked with 5% skimmed
milk for 1 h at room temperature. Membranes were then incubated
with primary antibodies: Erk1/2 (cat. no. 4695; dilution 1:1,000;
Cell Signaling Technology, Inc.), phosphorylated (p)-Erk1/2 (cat.
no. 4370; dilution 1:1,000; Cell Signaling Technology, Inc.), p65
(cat. no. 8242; dilution 1:1,000; Cell Signaling Technology, Inc.),
p-p65 (cat. no. 3033; dilution 1:1,000; Cell Signaling Technology,
Inc.) and GAPDH (cat. no. 5174; dilution 1:1,000; Cell Signaling
Technology, Inc.) overnight at 4˚C. After three washes, membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (cat. no. 7074; dilution 1:2,000; Cell Signaling
Technology, Inc.) for 1 h at 37˚C. Protein bands were visualized
using Clarity Western ECL Substrate (Bio-Rad Laboratories, Inc.).
Band densities were quantified using the Gel-Pro Analyzer
densitometry software (version 6.3; Media Cybernetics, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Spinal cord tissues were used to extract total RNA
using an MagMAX™-96 Total RNA Isolation Kit according to
manufacturer's protocols (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized using a PrimeScript™ RT reagent kit
(Takara Bio, Inc.). The reaction conditions were as follows: 70˚C
for 5 min, 37˚C for 5 min and 42˚C for 60 min. Power SYBR™-Green
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to quantify mRNA expression. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
5 min; followed by 40 cycles of 15 sec at 95˚C, 1 min at 60˚C and
30 sec at 72˚C; and a final extension for 10 min at 72˚C. GAPDH was
used as the internal control. The following primer sequences were
used: GAPDH forward 5'-TTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; Erk forward
5'-GGCACCAACCATTGAGCAGA-3' and reverse,
5'-GATCATTGCTGAGGTGCTGTGTC-3'; p65 forward
5'-CGCGGATCCGCCACCATGGACGAACTG-3' and reverse,
5'-CCGCTCGAGTTAGGAGCTGATCTG-3'. Gene expression was calculated
using the 2-ΔΔCq method (19). Experiments were performed in
triplicate.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. Statistical analysis was performed using
SPSS 18.0 (SPSS, Inc.). One-way ANOVA with Tukey's post hoc test
was used for all comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
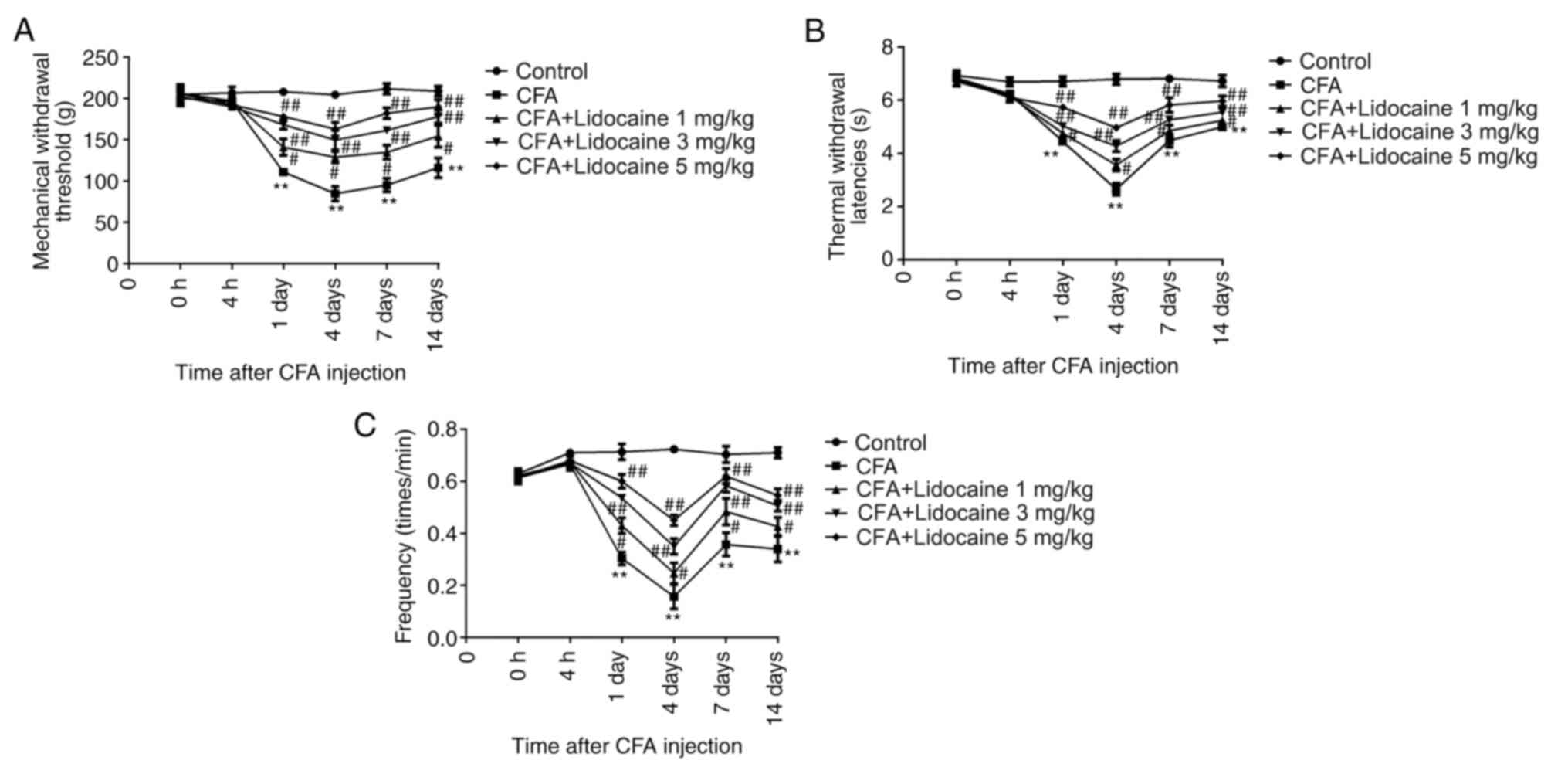
Effects of lidocaine on the behavior
of rats treated with CFA
Rats were first treated with either CFA alone or CFA
+ lidocaine. The TWL, MWT and frequency responses to cold
stimulation were then assessed. The results showed that CFA
significantly reduced TWL, MWT and the frequency responses to cold
stimulation by rats after treatment for 1, 4, 7 and 14 days.
However, 1, 3 and 5 mg/kg lidocaine significantly reversed these
effects (Fig. 1A-C).
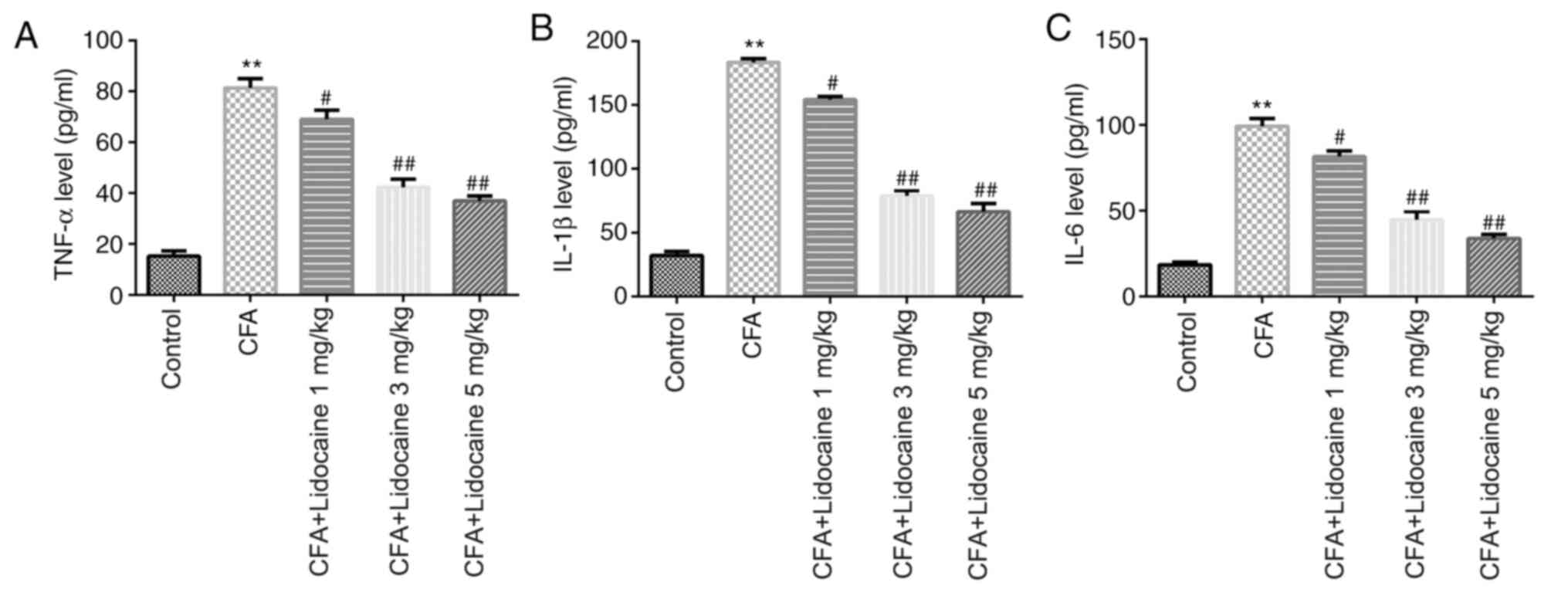
Lidocaine affects the levels of
pro-inflammatory factors in the serum of CFA-induced rats and
inhibits MAPK/ERK/NF-κB pathway activation
After 4 days of CFA treatment, ELISA was used to
evaluate the levels pro-inflammatory mediators in the rat serum.
The results showed that CFA injection significantly promoted TNF-α,
IL-1β and IL-6 levels compared with those in the control group, all
of which were significantly reversed by lidocaine (1, 3 or 5 mg/kg)
(Fig. 2).
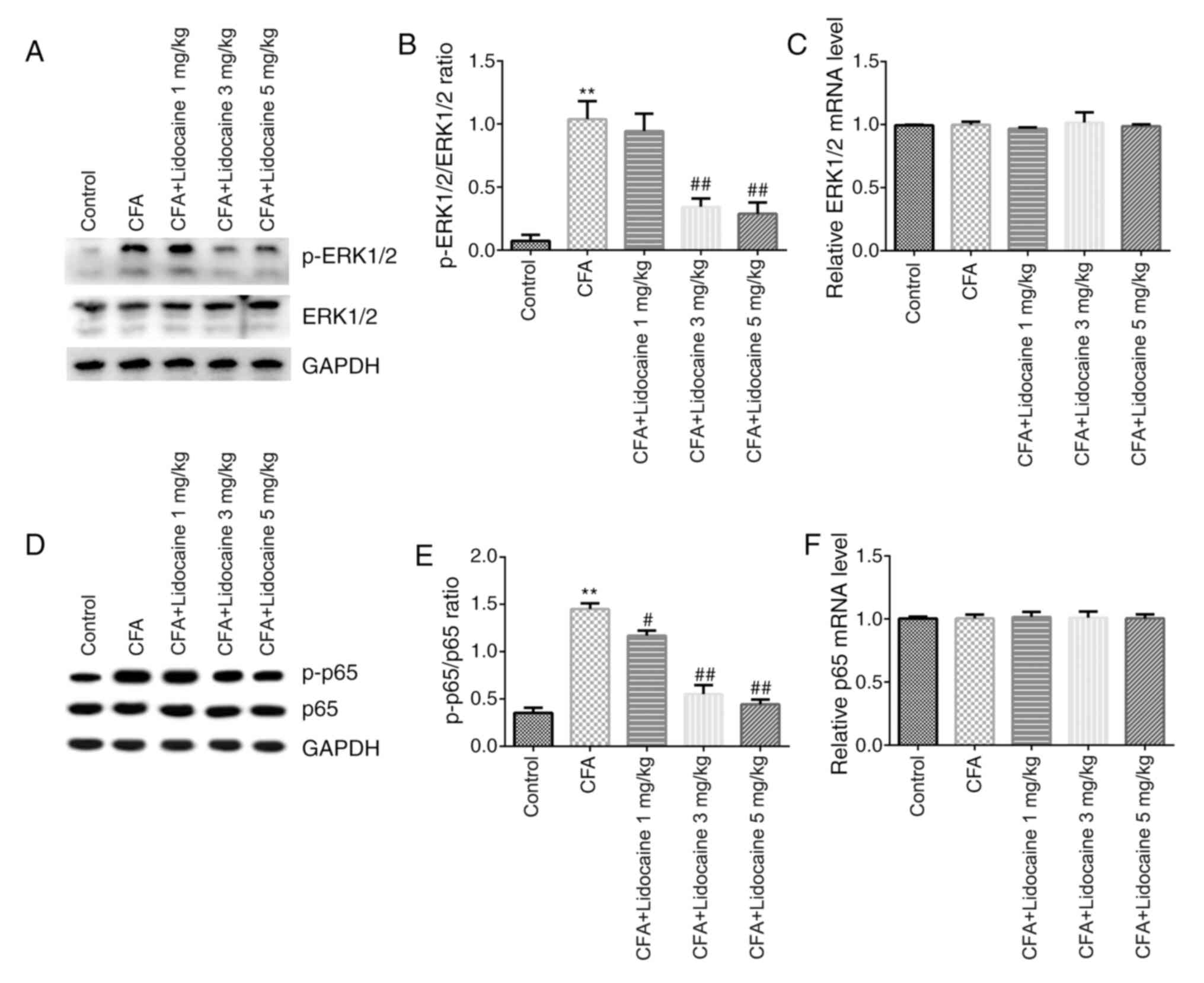
Western blotting and RT-qPCR were then performed to
assess the signaling pathways in rat spinal cord tissues. The
results showed that protein levels of p-ERK1/2 (Fig. 3A) and p-p65 (Fig. 3D) in addition to the ratios of
p-ERK1/2/total-ERK1/2 (Fig. 3B) and
p-p65/total-p65 (Fig. 3E) in the
CFA group were significantly elevated. However, lidocaine (3 or 5
mg/kg) significantly reversed the effects of CFA on ERK1/2
phosphorylation. Lidocaine (1, 3 or 5 mg/kg) significantly reversed
the effects of CFA on p65 phosphorylation. Furthermore, the mRNA
expression of ERK1/2 and p65 did not significantly differ after CFA
treatment (Fig. 3C and F).
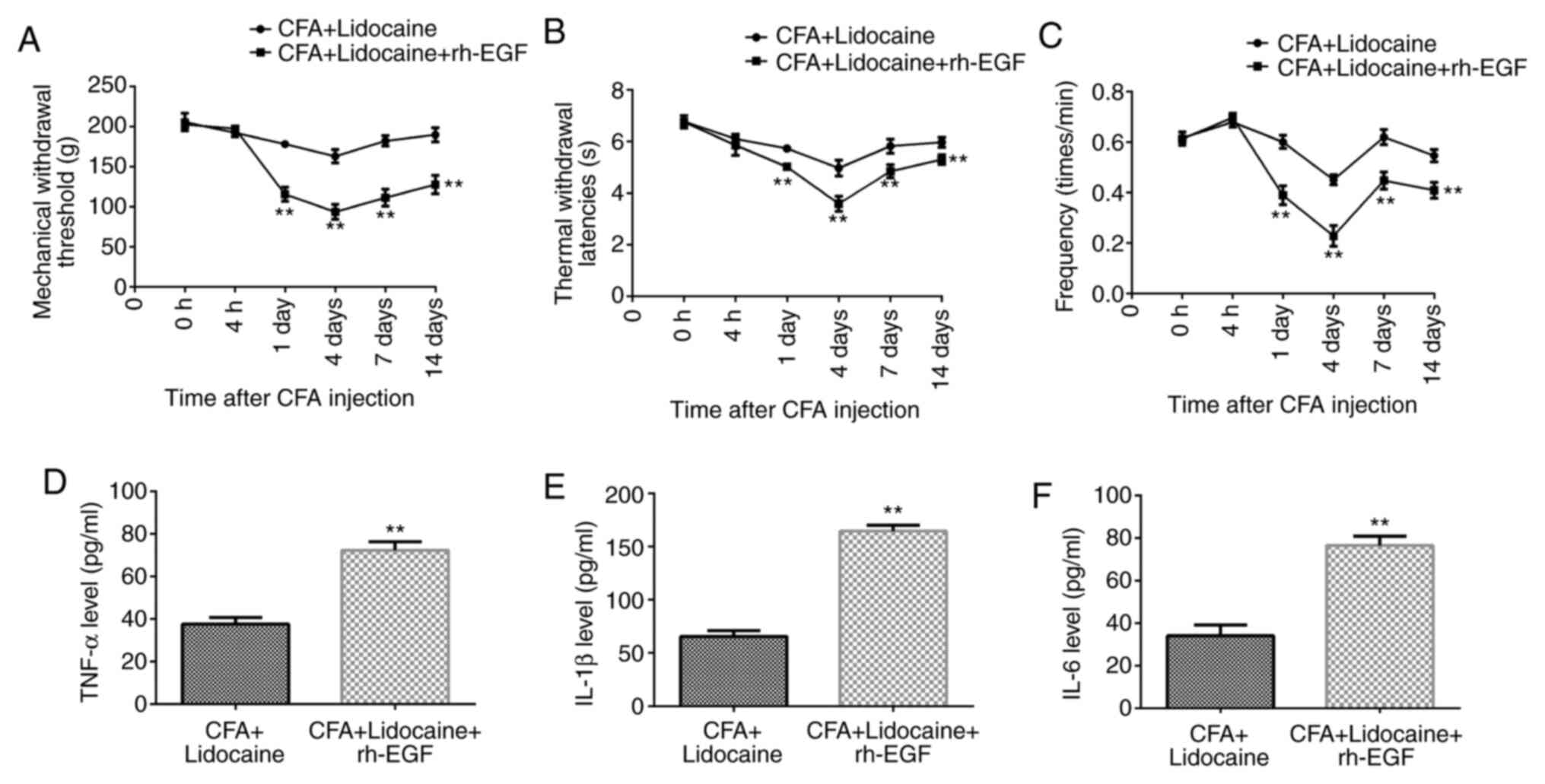
Lidocaine reduces inflammatory pain
following CFA injection by affecting the MAPK/ERK-NF-κB
pathway
Compared with those after lidocaine treatment alone,
in the presence of CFA, lidocaine + rh-EGF significantly reduced
TWL, MWT and frequency responses to cold stimulation at days 1, 4,
7 and 14 (Fig. 4A-C) and
significantly increased TNF-α, IL-1β and IL-6 levels in the rat
serum at 4 days after CFA treatment (Fig. 4D-F).
In addition, in the presence of CFA, lidocaine +
rh-EGF treatment significantly increased the levels of p-ERK1/2 and
the p-ERK1/2/total-ERK1/2 ratio when compared with those after
lidocaine treatment alone (Fig. 5A
and B). There were no significant
differences in ERK1/2 mRNA expression between the lidocaine and
lidocaine + rh-EGF groups (Fig.
5C). In addition, lidocaine + rh-EGF significantly increased
the levels of p-p65 and the p-p65/total-p65 ratio compared with
those after lidocaine treatment alone (Fig. 5D and E). There were no significant differences
in p65 mRNA expression between either treatment groups (Fig. 5F). The results suggested that
lidocaine relieved inflammatory pain caused by CFA, which was
reversed by rh-EGF in rats, suggesting that lidocaine relieved
inflammatory pain caused by CFA via inhibiting the MAPK/ERK/NF-κB
pathway.
Discussion
Lidocaine is a commonly used anesthetic in clinical
practice (20). In recent years,
studies have previously shown that lidocaine exerts beneficial
pharmacological effects for treating inflammatory reactions, such
that it has been successfully applied to a variety of inflammatory
diseases, including acute thyroiditis and inflammatory pain
(21,22). The present study found that
lidocaine reduced inflammatory pain in rats induced by CFA based on
the results of behavioral tests.
Lidocaine is known to have a short half-life when
given intravenously (23,24). Studies have previously investigated
the potential use of lidocaine for many headache disorders,
primarily via injection or infusion (24). A previous study reported that
intravenous systemic lidocaine infusion is beneficial for patients
with pain uncontrolled by opioid medications and can improve pain
scores whilst reducing the need for opioid treatment (25). Systemic administration of lidocaine
is antinociceptive in both chronic and acute pain states,
especially in acute postoperative and chronic neuropathic pain
(26). In the present study, 1, 3
and 5 mg/kg lidocaine were used to treat CFA-induced inflammatory
pain in rats via tail vein injection based on a previous study
(27).
TNF-α is a widely bioactive peptide substance that
is secreted by activated macrophages, endothelial cells,
neutrophils, B lymphocytes, monocytes, dendritic cells, neurons and
astrocytes (28). TNF-α activates
neutrophils and macrophages, promoting the production of IL-1β,
IL-6 and other Th2 cytokines, such as IL-4 and IL-5 to serve a key
role in the inflammatory response (29). IL-1β and IL-6 levels has been used
to objectively reflect the degree of inflammation and are usually
highly expressed during inflammatory reactions (30). It has also been reported to be
positively correlated with the severity of the inflammatory pain
(31-34).
In the present study, the ELISA results were consistent with a
previous study (35), which showed
that CFA injection significantly increased inflammation-associated
factors TNF-α, IL-1β and IL-6. The present results also showed that
lidocaine reversed the inflammatory effects of CFA in rats.
MAPKs are located in eukaryotic cytoplasm that serve
as key signal transductors to transduce extracellular signals to
intracellular responses in eukaryotic cells. MAPKs can connect key
regulatory factors and cell surface receptors, mediating a variety
of biological responses to serve key roles in physiological and
pathological processes (36,37).
ERK is a major member of the MAPK pathway family. There are two
types of ERK: ERK1 and ERK2. After phosphorylation, p-ERK1/2 enters
the nucleus and regulates the expression of transcription factors,
including cyclin D and cyclin E, to promote cell proliferation
(38). NF-κB is a protein with
transcriptional activation function, with the p65/p50 complex being
the main form (39). Inactivated
NF-κB is located in the cytoplasm, but when cells are stimulated by
cytokines, NF-κB then translocates into the nucleus to activate
gene transcription (40). Studies
have previously shown that NF-κB can promote the expression of
inflammatory factors IL-1, TNF-α and IL-6 during hepatic fibrosis
(41,42). In the present study, western
blotting results showed that injection of CFA increased ERK1/2 and
p65 protein phosphorylation but lidocaine treatment reversed this.
However, no significant differences in total ERK1/2 and p65 mRNA
expression was observed by either CFA and/or lidocaine
treatment.
Next, to further confirm that lidocaine inhibits the
MAPK/ERK/NF-κB pathway to relieve inflammatory pain caused by CFA,
CFA-induced rats were treated with rh-EGF (an ERK agonist) based on
data from the present study. The results suggested that compared
with those after lidocaine treatment alone, lidocaine + rh-EGF
treatment significantly reduced TWL, MWT and frequency responses to
cold stimulation whilst significantly increasing TNF-α, IL-1β and
IL-6 levels. In addition, the levels of ERK1/2 and p65
phosphorylation and the ratios of p-ERK1/2/total-ERK1/2 and
p-p65/total-p65 were significantly increased, whilst the mRNA
expression of ERK1/2 and p65 were not significantly different
between these two groups. These findings indicated that the effects
of lidocaine on CFA-induced rats were significantly reversed by
rh-EGF. Since rh-EGF is an ERK agonist (43), data from the present study suggest
that rh-EGF + lidocaine significantly reduced TWL, MWT and
frequency responses to cold stimulation but significantly increased
TNF-α, IL-1β and IL-6 levels through activating the ERK
pathway.
In summary, these results indicated that lidocaine
inhibited the activation of the MAPK/ERK-NF-κB pathway, to inhibit
the inflammatory response and alleviate inflammatory pain caused by
CFA. This study provides more theoretical basis for the use of
lidocaine for the clinical treatment of inflammatory pain.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ designed the current study, in addition to
performing all experiments and analyzing the data. YL contributed
to performing ELISA, western blot assay, reverse
transcription-quantitative PCR and analyzing the data. YT
contributed to establishing the rat model conduction and behavioral
tests and analyzing the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All protocols strictly followed the Institutional
Animal Care and Use of Laboratory Animals by the National
Institutes of Health. The experimental protocols were approved by
the Animal Care and Use Committee of the First Affiliated Hospital
of Xinjiang Medical University (approval no. IACUC-20200113-01;
Urumqi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swieboda P, Filip R, Prystupa A and Drozd
M: Assessment of pain: Types, mechanism and treatment. Ann Agric
Environ Med. 1:2–7. 2013.PubMed/NCBI
|
|
2
|
Grace PM, Hutchinson MR, Maier SF and
Watkins LR: Pathological pain and the neuroimmune interface. Nat
Rev Immunol. 14:217–231. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Fang JQ, Du JY, Liang Y and Fang JF:
Intervention of electroacupuncture on spinal p38 mapk/atf-2/vr-1
pathway in treating inflammatory pain induced by CFA in rats. Mol
Pain. 9(13)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Del Giorno R, Frumento P, Varrassi G,
Paladini A and Coaccioli S: Assessment of chronic pain and access
to pain therapy: A cross-sectional population-based study. J Pain
Res. 10:2577–2584. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang B, He XL, Ding Y and Du GH:
Gaultherin, a natural salicylate derivative from gaultheria
yunnanensis: Towards a better non-steroidal anti-inflammatory drug.
Eur J Pharmacol. 530:166–171. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chamaraux-Tran TN and Tobias P: The amide
local anesthetic lidocaine in cancer surgery-potential
antimetastatic effects and preservation of immune cell function? A
narrative review. Front Med (Lausanne). 4(235)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johnson MZ, Crowley PD, Foley AG, Xue C,
Connolly C, Gallagher HC and Buggy DJ: Effect of perioperative
lidocaine on metastasis after sevoflurane or ketamine-xylazine
anaesthesia for breast tumour resection in a murine model. Br J
Anaesth. 121:76–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lauretti GR: Mechanisms of analgesia of
intravenous lidocaine. Rev Bras Anestesiol. 58:280–286.
2008.PubMed/NCBI View Article : Google Scholar : (In En,
Portuguese).
|
|
9
|
Kawamata M, Takahashi T, Kozuka Y, Nawa Y,
Nishikawa K, Narimatsu E, Watanabe H and Namiki A: Experimental
incision-induced pain in human skin: Effects of systemic lidocaine
on flare formation and hyperalgesia. Pain. 100:77–89.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang L, Wang M, Li S, Wu H, Shen Q, Zhang
S, Fang L and Liu R: Nebulized lidocaine ameliorates allergic
airway inflammation via downregulation of TLR2. Mol Immunol.
97:94–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chiu KM, Lu CW, Lee MY, Wang MJ, Lin TY
and Wang SJ: Neuroprotective and anti-inflammatory effects of
lidocaine in kainic acid-injected rats. Neuroreport. 27:501–507.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van der Wal SEI, van den Heuvel SAS,
Radema SA, van Berkum BFM, Vaneker M, Steegers MAH, Scheffer GJ and
Vissers KCP: The in vitro mechanisms and in vivo efficacy of
intravenous lidocaine on the neuroinflammatory response in acute
and chronic pain. Eur J Pain. 20:655–674. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Liu LM, Liang DY, Ye CG, Tu WJ and Zhu T:
The UII/UT system mediates upregulation of proinflammatory cytokines
through p38MAPK and NF-κB pathways in LPS stimulated Kupffer cells.
PLoS One. 10(e0121383)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang HL, Xing YQ, Xu YX, Rong F, Lei WF
and Zhang WH: The protective effect of lidocaine on septic rats via
the inhibition of high mobility group box 1 expression and NF-κB
activation. Mediators Inflamm. 2013(570370)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagakura Y, Okada M, Kohara A, Kiso T,
Toya T, Iwai A, Wanibuchi F and Yamaguchi T: Allodynia and
hyperalgesia in adjuvant-induced arthritic rats: Time course of
progression and efficacy of analgesics. J Pharmacol Exp Ther.
306:490–497. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai G, Ambalavanar R, Wei D and Dessem D:
Downregulation of selective microRNAs in trigeminal ganglion
neurons following inflammatory muscle pain. Mol Pain.
3(15)2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bayne K: Revised guide for the care and
use of laboratory animals available American physiological society.
Physiologist. 39:208–211. 1996.PubMed/NCBI
|
|
18
|
Tan SS, Liu H, Wang YZ and Zhu SS: The
molecular mechanisms associated with the effects of propofol in a
rat model of pain due to inflammation following injection with
complete freund's adjuvant. Med Sci Monit. 25:10190–10197.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klein JA and Jeske DR: Estimated maximal
safe dosages of tumescent lidocaine. Anesth Analg. 122:1350–1359.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang X, Yang LX, Wu J, Guo ML, Zhang Y and
Ma SG: Treatment of lidocaine on subacute thyroiditis via
restraining inflammatory factor expression and inhibiting
pyroptosis pathway. J Cell Biochem: Apr 8, 2019 (Epub ahead of
print).
|
|
22
|
Leon-Constantin MM, Alexa-Stratulat T,
Luca A, Tamba BI, Trandafir LM, Harabagiu V and Cojocaru E: The
morphofunctional impact of topical lidocaine formulation in
inflammatory pain-experimental study. Rom J Morphol Embryol.
60:869–874. 2019.PubMed/NCBI
|
|
23
|
Soto G, Naranjo González M and Calero F:
Intravenous lidocaine infusion. Rev Esp Anestesiol Reanim.
65:269–274. 2018.PubMed/NCBI View Article : Google Scholar : (In En,
Spanish).
|
|
24
|
Berk T and Silberstein SD: The use and
method of action of intravenous lidocaine and its metabolite in
headache disorders. Headache. 58:783–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reeves DJ and Foster AE: Continuous
intravenous lidocaine infusion for the management of pain
uncontrolled by opioid medications. J Pain Palliat Care
Pharmacother. 31:198–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hermanns H, Hollmann MW, Stevens MF, Lirk
P, Brandenburger T, Piegeler T and Werdehausen R: Molecular
mechanisms of action of systemic lidocaine in acute and chronic
pain: A narrative review. Br J Anaesth. 123:335–349.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ,
Li AZ, Li MC, Shi CX, Du J and Zhou HD: The protective effect of
lidocaine on lipopolysaccharide-induced acute lung injury in rats
through NF-κB and p38 MAPK signaling pathway and excessive
inflammatory responses. Eur Rev Med Pharmacol Sci. 22:2099–2108.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Caminero A, Comabella M and Montalban X:
Tumor necrosis factor alpha (TNF-α), anti-TNF-α and demyelination
revisited: An ongoing story. J Neuroimmunol. 234:1–6.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo Y, Pang Z, Zhu Q, Cai X, Yin Y, Wang
M, Zhu J, Chen J, Zeng K, Zhang C and Zhang J: Locally instilled
tumor necrosis factor-α antisense oligonucleotide inhibits allergic
inflammation via the induction of Tregs. J Gene Med. 14:374–383.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Slaats J, Ten Oever J, van de Veerdonk FL
and Netea MG: IL-1β/IL-6/CRP and IL-18/ferritin: Distinct
inflammatory programs in infections. PLoS Pathog.
12(e1005973)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dai T, Shi KQ, Chen G, Shen YM and Pan T:
Malvidin attenuates pain and inflammation in rats with
osteoarthritis by suppressing NF-κB signaling pathway. Inflamm Res.
66:1075–1084. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dokumaci A: Performing pain and
inflammation: Rendering the invisible visible. AMA J Ethics.
19:834–838. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Barrientos RM, Hein AM, Frank MG, Watkins
LR and Maier SF: Intracisternal interleukin-1 receptor antagonist
prevents postoperative cognitive decline and neuroinflammatory
response in aged rats. J Neurosci. 32:14641–14648. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippocampus. Anesthesiology. 106:436–443. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yuan Y, Zhang Y, He X and Fan S:
Protective effects of sinomenine on CFA-induced inflammatory pain
in rats. Med Sci Monit. 24:2018–2024. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brown MD and Sacks DB: Compartmentalised
MAPK pathways. Handb Exp Pharmacol. 186:205–235. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aouadi M, Binetruy B, Caron L, Le
Marchand-Brustel Y and Bost F: Role of MAPKs in development and
differentiation: Lessons from knockout mice. Biochimie.
88:1091–1098. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhong W, Shen WF, Ning BF, Hu PF, Lin Y,
Yue HY, Yin C, Hou JL, Chen YX, Zhang JP, et al: Inhibition of
extracellular signal-regulated kinase 1 by adenovirus mediated
small interfering RNA attenuates hepatic fibrosis in rats.
Hepatology. 50:1524–1536. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Giridharan S and Srinivasan M: Mechanisms
of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm
Res. 11:407–419. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sunami Y, Leithäuser F, Gul S, Fiedler K,
Güldiken N, Espenlaub S, Holzmann KH, Hipp N, Sindrilaru A, Luedde
T, et al: Hepatic activation of IKK/NFκB signaling induces liver
fibrosis via macrophage-mediated chronic inflammation. Hepatology.
56:1117–1128. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen X, Liu C, Lu Y, Yang Z, Lv Z, Xu Q,
Pan Q and Lu L: Paeoniflorin regulates macrophage activation in
dimethylnitrosamine-induced liver fibrosis in rats. BMC Complement
Altern Med. 12(254)2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gao Y, Xi B, Li J, Li Z, Xu J, Zhong M, Xu
Q, Lian Y, Wei R, Wang L, et al: Scoparone alleviates hepatic
fibrosis by inhibiting the TLR-4/NF-κB pathway. J Cell Physiol: Oct
8, 2020 (Epub ahead of print).
|
|
43
|
Liu CH, Hua N, Fu X, Pan YL, Li B and Li
XD: Metformin regulates atrial SK2 and SK3 expression through
inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats.
BMC Cardiovasc Disord. 18(236)2018.PubMed/NCBI View Article : Google Scholar
|