Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumor types and is responsible for ~700,000 deaths
annually worldwide (1). With the
advances in diagnostic and therapeutic methods, the prognosis of
liver cancer has improved, but the frequent recurrence and high
metastasis rates of HCC adversely affect patient outcomes.
Therefore, the identification of tumor markers is crucial for early
diagnosis, treatment and outcome prediction in HCC.
Semaphorin 3B (SEMA-3B), which belongs to the
semaphorin family, is a secreted molecule that contains a highly
conserved Sema domain in the amino terminus. SEMA-3B was initially
discovered as an inhibitory axonal guidance molecule, but recent
studies revealed that SEMA-3B also functions as a tumor suppressor
in lung, renal, gastric, breast and prostate cancers (2-7).
SEMA-3B forms a complex with neuropilins (NPs) and plexins on the
cell surface. Vascular endothelial growth factor (VEGF)-A also
binds to NPs and this complex promotes cellular migration and
promotes tumor growth (4,8-10).
SEMA-3B family proteins may competitively inhibit the function of
VEGF in promoting tumor angiogenesis, as they share the same
transmembrane receptors for NP-1 and NP-2(4). SEMA-3B inhibits the migration and
proliferation of cancer cells; therefore, it was hypothesized that
its expression levels may affect the prognosis of patients with
HCC. A previous study by our group demonstrated the suppressive
effect of the SEMA-3B protein on the migration and invasion ability
of cancer cells in vitro (11).
Aberrant expression and methylation of SEMA-3B may
be of value as a marker in patients with lung and renal cancer
(12). The previous study by our
group revealed that SEMA-3B is downregulated in tumor tissues and
has a tumor suppressor role in HCC (11). The aim of the present study was to
examine the clinical value of serum SEMA-3B as a tumor marker and
determine whether it may be used as a prognostic marker.
Materials and methods
Sample collection
A total of 132 patients (73 males and 59 females,
aged 37-78 years) with HCC who underwent curative surgery and were
diagnosed pathologically were hospitalized at the Department of
General Surgery of Qilu Hospital, Shandong University (Jinan,
China) between May 2013 and December 2014, and all cases who
presented during this period were enrolled. Serum samples were also
collected during this period from 57 subjects who had no liver
disease (healthy controls; 31 males and 26 females, aged 42-80
years). All subjects and their families agreed to participate in
the present study and signed informed consent. The protocol was
approved by the Ethics Committee of Qilu Hospital (Jinan, China).
All patients who had a visit were contacted by telephone every
month and had ultrasound or CT scan every 2-3 months. The follow-up
was terminated in November 2019.
ELISA
Peripheral blood (5 ml) was collected from each of
the subjects, centrifuged at 3000 x g for 15 min at 4˚C and the
serum was separated and stored in the refrigerator at -80˚C until
use. The serum SEMA-3B was examined by ELISA (DLdevelop; cat. no.
DL-SEMA3B-Hu) according to the manufacturer's protocol.
Statistical analysis
For statistical comparison of count data between
SEMA-3B expression and clinical indicators, the χ2 test
was used. The levels of SEMA-3B between patients with HCC and
controls were compared using Student's t-test. Survival curves were
constructed using the Kaplan-Meier survival analysis method and the
differences in survival curves were compared with the log-rank
test. All the statistical analyses were performed with SPSS 18.0
statistical software (SPSS Inc.) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum SEMA-3B is downregulated in
HCC
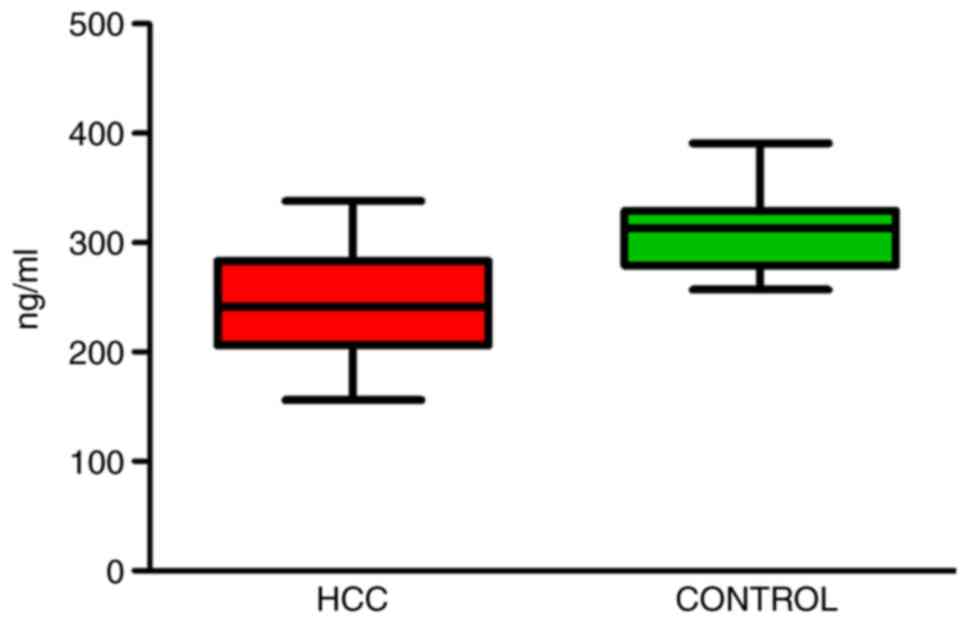
The expression of SEMA-3B in the serum of patients
with HCC and healthy controls was measured to determine whether
there was any difference between the two groups. ELISA was used to
monitor the expression of serum SEMA-3B. The serum levels of
SEMA-3B were indicated to be significantly downregulated in
patients with HCC compared with those in the controls (242.7±44.06
vs. 310.7±32.62 ng/ml, respectively; P<0.05; Fig. 1).
Association of clinicopathological
variables with different expression patterns of serum SEMA-3B
HCC cases were divided into two groups according to
the mean expression of serum SEMA-3B (cut-off, 242.7 ng/ml). The
expression of serum SEMA-3B was high in 65 and low in 67 HCC
patients. Serum SEMA-3B was negatively associated with the number
of tumors, largest tumor size, presence of tumor capsule, Cancer of
the Liver Italian Program (CLIP) score (13) and the TNM stage (P<0.05; Table I).
 | Table IClinicopathological variables
associated with different expression patterns of serum SEMA-3B. |
Table I
Clinicopathological variables
associated with different expression patterns of serum SEMA-3B.
| | Number of
patients | |
|---|
| Variable | High | Low | P-value |
|---|
| Sex | | | 0.382 |
|
Male | 33 | 40 | |
|
Female | 32 | 27 | |
| Age (years) | | | 0.229 |
|
≤60 | 28 | 36 | |
|
>60 | 37 | 31 | |
| HBsAg status | | | 0.374 |
|
Negative | 22 | 28 | |
|
Positive | 43 | 39 | |
| AFP (ng/ml) | | | 0.163 |
|
≤20 | 30 | 25 | |
|
>20 | 35 | 42 | |
| Liver cirrhosis | | | 0.209 |
|
Negative | 20 | 28 | |
|
Positive | 45 | 39 | |
| Cell
differentiation | | | 0.475 |
|
Moderate/poor | 43 | 40 | |
|
High | 22 | 27 | |
| Number of tumors | | | 0.008 |
|
Solitary | 41 | 25 | |
|
Multiple | 24 | 40 | |
| Largest tumor size
(cm) | | | 0.039 |
|
≤5 | 39 | 28 | |
|
>5 | 26 | 40 | |
| Tumor capsule | | | 0.002 |
|
Absent | 40 | 22 | |
|
Present | 25 | 45 | |
| CLIP score
(points) | | | 0.025 |
|
0-1 | 41 | 29 | |
|
≥2 | 24 | 38 | |
| TNM stage | | | 0.034 |
|
I | 36 | 23 | |
|
II/III | 29 | 42 | |
Serum SEMA-3B may be used as a
prognostic marker for HCC
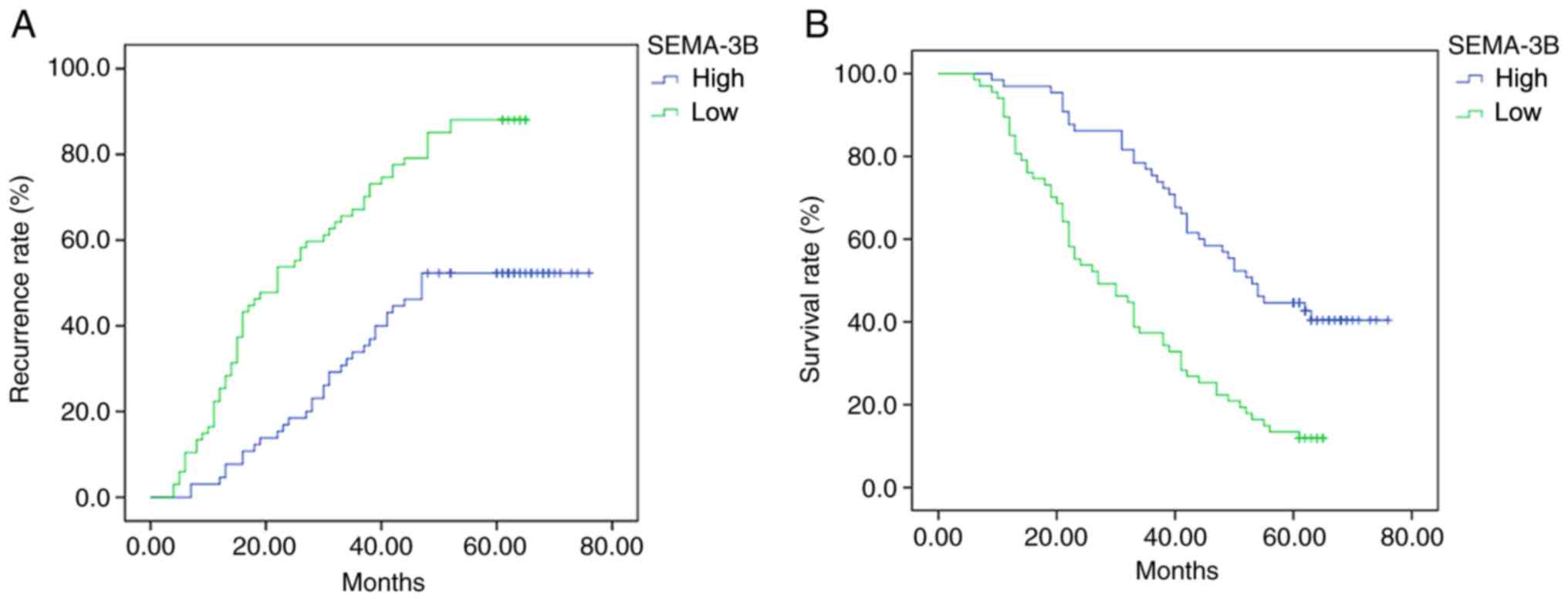
Clinical follow-up of the patients revealed that the
cancer recurrence rate in the low-expression group was
significantly lower compared with that in the high-expression group
(P<0.001; Fig. 2A). The
recurrence rates of the high-expression group at 1, 3 and 5 years
were 4.62, 29.23 and 60.00%, respectively. The recurrence rates of
the low-expression groups were 25.37, 67.16 and 88.06%,
respectively. Furthermore, the survival rate in the high-expression
group was significantly higher compared with that in the
low-expression group (P<0.001; Fig.
2B): 1-year survival rate 96.92 vs. 83.58%, respectively;
3-year survival rate 72.308 vs. 53.73%, respectively; and 5-year
survival rate 38.46 vs. 31.34%, respectively.
Discussion
As HCC is characterized by low sensitivity to
radiotherapy and chemotherapy, the prognosis of the patients with
HCC is poor (14). Despite advances
in the development of early detection methodologies, the
questionable effectiveness and high cost of the procedures
available for the treatment of HCC pose a challenge for disease
management. Therefore, it is crucial to identify novel therapeutic
targets and diagnostic biomarkers to ensure timely treatment and
improve survival rates. In the present study, the clinical value of
serum SEMA-3B was assessed and it was discussed whether it may be
of value in the treatment of HCC.
SEMA-3B, encoded at chromosome 3p21.3, is a secreted
molecule that contains a highly conserved Sema domain in the amino
terminus. SEMA-3B was initially discovered as an inhibitory axonal
guidance molecule and it was also recently recognized as a
candidate tumor suppressor gene (15-17).
Loss of SEMA-3B expression in cells of the tumor microenvironment,
as well as tumor cells themselves, may increase the aggressiveness
of breast cancer (7). In a previous
study by our group, the expression of SEMA-3B protein was
investigated in HCC and normal tissues and the expression of
SEMA-3B was indicated to be significantly downregulated in HCC
tissues (11). To the best of our
knowledge, no previous studies have reported the serum levels of
SEMA-3B and SEMA-3F in patients with HCC to date; therefore, ELISA
was used to monitor the expression of serum SEMA-3B and SEMA-3F,
but only the expression of serum SEMA-3B was observed to be
decreased compared with that in healthy controls. Several
mechanisms are involved in decreasing the expression of SEMA-3B.
Methylation participates in the downregulation of SEMA-3B (18). Introduction of exogenous p53 into a
glioblastoma cell line lacking wild-type p53, U373MG, markedly
induced the expression of SEMA-3B mRNA, as SEMA-3B is a direct
target of p53(19).
Based on the expression of serum SEMA-3B in patients
with HCC, the patients were followed-up. A χ2 test for
the association of clinicopathological parameters with the serum
levels of SEMA-3B (high vs. low) revealed that the expression
levels of SEMA-3B were significantly associated with the formation
of a capsule, possibly via the inhibitory effect of SEMA-3B on the
formation of vessels (20). SEMA-3B
is able to inhibit the expression and activity of MMP-2 and MMP-9
(21,22). SEMA-3B family proteins may
competitively inhibit the function of VEGF in promoting tumor
angiogenesis, as they share the same transmembrane receptors of
NP-1 and NP-2(4). The expression of
NP-1 was reported to be associated with the clinicopathologic
parameters of patients with cholangiocarcinoma (23). The positive rates of NP-1 in HCC
tissues and adjacent tissues were 63.0 and 4.1%, and a study by our
group also indicated that the expression of NP-1 in HCC is
associated with clinicopathological parameters (24). The previous study (11) by our group demonstrated a negative
association between the level of SEMA-3B protein expression and
microvascular density, which may reflect the fact that SEMA-3B has
an important role in angiogenesis. In addition, serum SEMA-3B was
indicated to be negatively correlated with the number of tumors,
largest tumor size, tumor capsule, CLIP score and TNM stage in the
present study. As the number of tumors, largest tumor size, tumor
capsule, CLIP score and TNM stage are closely associated with the
prognosis of the patients (25), it
was inferred that serum SEMA-3B is a tumor suppressor gene.
In order to determine the possible influence of the
serum levels of SEMA-3B on prognosis, patients were divided into
two groups, namely a high- and a low-expression group. Analysis of
the follow-up data of the patients of the present study revealed
that the recurrence rate in the high-expression group was markedly
lower compared with that in the low-expression group, while the
cumulative survival rate was significantly higher compared with
that of the low-expression group. Suppression of miR-221 was
previously reported to inhibit glioma cells by targeting SEMA-3B,
and SEMA-3B inhibited cell proliferation and invasion (26). SEMA-3B was also indicated to be
associated with a favorable survival prognosis for patients with
esophageal squamous cell carcinoma due to upregulating p53 and
p21(27). A previous study by our
group confirmed that SEMA-3B inhibits angiogenesis and cell
migration, and SEMA-3B levels in HCC tissue may be used as a marker
to predict prognosis (11).
Therefore, serum SEMA-3B may be a valuable indicator for predicting
the prognosis of patients with HCC.
In conclusion, serum SEMA-3B may be easily detected
in the peripheral blood and may be used for the diagnosis and
prediction of prognosis of patients with HCC. The exact role of
SEMA-3B in cancer development and underlying mechanisms of action
require further elucidation, but the results of the present study
indicated the potentially important role of serum SEMA-3B in the
diagnosis and prediction of prognosis of patients with HCC.
Acknowledgements
Not applicable.
Funding
This study was supported by Shandong Provincial
Natural Science Foundation of China (grantno. Z R2020QH203and
26010105201516).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZLZ and YCG conceived the study and drafted the
manuscript. GZL enrolled subjects and compared their data. DS, GHL,
MW, LJZ, ZLL, RQS and SJZ performed ELISA and collected data. All
authors read and approved the final manuscript. ZLZ and YCG
confirmed the authenticity of the raw data.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Qilu Hospital (Jinan, China). Patients who participated in this
study, provided written informed consent and had complete clinical
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sekido Y, Bader S, Latif F, Chen JY, Duh
FM, Wei MH, Albanesi JP, Lee CC, Lerman MI and Minna JD: Human
semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer
deletion region and demonstrate distinct expression patterns. Proc
Natl Acad Sci USA. 93:4120–4125. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tse C, Xiang RH, Bracht T and Naylor SL:
Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3
suppresses tumor formation in an adenocarcinoma cell line. Cancer
Res. 62:542–546. 2002.PubMed/NCBI
|
|
4
|
Castro-Rivera E, Ran S, Thorpe P and Minna
JD: Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast
cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci
USA. 101:11432–11437. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Beuten J, Garcia D, Brand TC, He X, Balic
I, Canby-Hagino E, Troyer DA, Baillargeon J, Hernandez J, Thompson
IM, et al: Semaphorin 3B and 3F single nucleotide polymorphisms are
associated with prostate cancer risk and poor prognosis. J Urol.
182:1614–1620. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pignata A, Ducuing H and Castellani V:
Commissural axon navigation: Control of midline crossing in the
vertebrate spinal cord by the semaphorin 3B signaling. Cell Adhes
Migr. 10:604–617. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shahi P, Wang CY, Chou J, Hagerling C,
Gonzalez Velozo H, Ruderisch A, Yu Y, Lai MD and Werb Z: GATA3
targets semaphorin 3B in mammary epithelial cells to suppress
breast cancer progression and metastasis. Oncogene. 36:5567–5575.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bernatchez PN, Rollin S, Soker S and
Sirois MG: Relative effects of VEGF-A and VEGF-C on endothelial
cell proliferation, migration and PAF synthesis: Role of
neuropilin-1. J Cell Biochem. 85:629–639. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Castro-Rivera E, Ran S, Brekken RA and
Minna JD: Semaphorin 3B inhibits the phosphatidylinositol
3-kinase/Akt pathway through neuropilin-1 in lung and breast cancer
cells. Cancer Res. 68:8295–8303. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabag AD, Smolkin T, Mumblat Y, Ueffing M,
Kessler O, Gloeckner CJ and Neufeld G: The role of the plexin-A2
receptor in Sema3A and Sema3B signal transduction. J Cell Sci.
127:5240–5252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li G, Gao Y, Ma D and Zhang Z: Downgraded
expression of SEMA3B indicates an unfavorable prognosis in patients
of resectable hepatocellular carcinoma. Int J Clin Exp Pathol.
9:841–853. 2016.
|
|
12
|
Loginov VI, Dmitriev AA, Senchenko VN,
Pronina IV, Khodyrev DS, Kudryavtseva AV, Krasnov GS, Gerashchenko
GV, Chashchina LI, Kazubskaya TP, et al: Tumor Suppressor Function
of the SEMA3B Gene in Human Lung and Renal Cancers. PLoS One.
10(e0123369)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Llovet JM and Bruix J: Prospective
validation of the Cancer of the Liver Italian Program (CLIP) score:
A new prognostic system for patients with cirrhosis and
hepatocellular carcinoma. Hepatology. 32:679–680. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Braga EA, Kashuba VI, Maliukova AV,
Loginov VI, Senchenko VN, Bazov IV, Kiselev LL and Zabarovskiĭ ER:
New tumor suppressor genes in hot spots of human chromosome 3: New
methods of identification. Mol Biol (Mosk). 37:194–211.
2003.PubMed/NCBI(In Russian).
|
|
16
|
Falk J, Bechara A, Fiore R, Nawabi H, Zhou
H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Püschel AW, et al:
Dual functional activity of semaphorin 3B is required for
positioning the anterior commissure. Neuron. 48:63–75.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tischoff I, Markwarth A, Witzigmann H,
Uhlmann D, Hauss J, Mirmohammadsadegh A, Wittekind C, Hengge UR and
Tannapfel A: Allele loss and epigenetic inactivation of 3p21.3 in
malignant liver tumors. Int J Cancer. 115:684–689. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen R, Zhuge X, Huang Z, Lu D, Ye X, Chen
C, Yu J and Lu G: Analysis of SEMA3B methylation and expression
patterns in gastric cancer tissue and cell lines. Oncol Rep.
31:1211–1218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ochi K, Mori T, Toyama Y, Nakamura Y and
Arakawa H: Identification of semaphorin3B as a direct target of
p53. Neoplasia. 4:82–87. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Neufeld G, Lange T, Varshavsky A and
Kessler O: Semaphorin signaling in vascular and tumor biology. Adv
Exp Med Biol. 600:118–131. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Halbersztadt A, Haloń A, Pajak J,
Robaczyński J, Rabczynski J and St Gabryś M: The role of matrix
metalloproteinases in tumor invasion and metastasis. Ginekol Pol.
77:63–71. 2006.PubMed/NCBI(In Polish).
|
|
22
|
Patel S, Sumitra G, Koner BC and Saxena A:
Role of serum matrix metalloproteinase-2 and -9 to predict breast
cancer progression. Clin Biochem. 44:869–872. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhu H, Zhai B, He C, Li Z, Gao H, Niu Z,
Jiang X, Lu J and Sun X: LncRNA TTN-AS1 promotes the progression of
cholangiocarcinoma via the miR-320a/neuropilin-1 axis. Cell Death
Dis. 11(637)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao Y: Expression of Semaphorin-3B,
Vascular endothelial growth factor,and their common receptor
Neuropilin-1 inHepatocellular carcinoma. China Doctoral Thesis
Library 04, 2014.
|
|
25
|
Huang YH, Chen CH, Chang TT, Chen SC, Wang
SY, Lee HS, Lin PW, Huang GT, Sheu JC, Tsai HM, et al: Evaluation
of predictive value of CLIP, Okuda, TNM and JIS staging systems for
hepatocellular carcinoma patients undergoing surgery. J
Gastroenterol Hepatol. 20:765–771. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cai G, Qiao S and Chen K: Suppression of
miR-221 inhibits glioma cells proliferation and invasion via
targeting SEMA3B. Biol Res. 48(37)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang H, Wu Y, Liu M, Qin Y, Wang H, Wang
L, Li S, Zhu H, He Z, Luo J, et al: SEMA3B improves the survival of
patients with esophageal squamous cell carcinoma by upregulating
p53 and p21. Oncol Rep. 36:900–908. 2016.PubMed/NCBI View Article : Google Scholar
|