1. Introduction
Endometriosis is one of the most painful and
frequent chronic gynecological diseases that is characterized by
the presence and growth of endometrial ectopic tissue outside the
uterus (1). The incidence and
prevalence of endometriosis are difficult to quantify, considering
that some patients with this pathology are asymptomatic. Hsiao
et al (2) mentioned a
prevalence rate of 10-15% among all women of reproductive age
worldwide. In particular, ovarian endometriosis is one of the most
frequent subtype that appears in 17-44% women diagnosed with
endometriosis (3).
Sampson (4) in 1925,
was the first to present with histological details the complex
mechanism of the malignant transformation of endometriosis and its
association with epithelial ovarian cancer. Subsequently, Jiang
et al (5) indicated that
progression of endometriosis to ovarian cancer was corroborated by
molecular data. At present, the two pathologies are frequently
regarded as a single histological entity, which is known as
endometriosis-associated ovarian cancer (EAOC) (6). The association between ovarian
endometriosis and ovarian cancer is supported by epidemiological
data debated in several studies (1,5-14).
Ovarian cancer represents a complex and
heterogeneous malignant gynecological pathology (15) with a high mortality rate, with and a
5-year survival rate <45% (16),
which is considered to be the second most common gynecologic
malignancy in developed countries (17). The risk of ovarian cancer
development is ~1% among patients during lifetime in developed
countries (18).
In concordance with the histological modifications,
the dynamics in coding and non-coding gene expression at cellular
level is a decisive factor regarding the cellular phenotype
(19). Among these transcripts,
microRNAs (miRNAs/miRs), which are small sequences of 19-22
nucleotides in length without coding capacity, have been implicated
in post-transcriptional regulation of protein expression (19). miRNAs are capable of regulating
complex signaling networks due to their ability to target and
influence the translation of coding genes (20). In the case of cancer, miRNAs are
classified as tumor suppressors or oncogenes depending on their
target genes and also their level of expression (21). The profile of differentially
expressed miRNAs in patients with ovarian cancer and endometriosis
was analyzed (22). The expression
of miRNAs between ectopic and eutopic endometrium in patients with
and without endometriosis offered information with diagnostic,
prognostic or even therapeutic implications (23,24).
The present review focused on emphasizing the main
histological aspects, gene expression and miRNAs alterations in
ovarian endometriosis and cancer and their possible association,
based on the latest published literature.
2. Histological aspects
Histology represents the study of the arrangement
for various tissues, which requires a microscopic knowledge of the
microanatomy (25). Histopathology
is a branch of histology that involves the microscopic
identification and study of diseased tissue that not only establish
the diagnosis but is also crucial for providing prognostic in data
clinical management (26).
Ovarian endometriosis
In a histopathological perspective, endometriosis is
defined as the presence of endometrial-like glandular epithelium
and stroma outside the uterus. At the immunohistochemical level,
the phenotype of endometrial glands is similar to that of normal
endometrial tissue (27). However,
there are certain microscopic alterations specific of ovarian
endometriosis, which are listed in Table I and illustrated in Fig. 1.
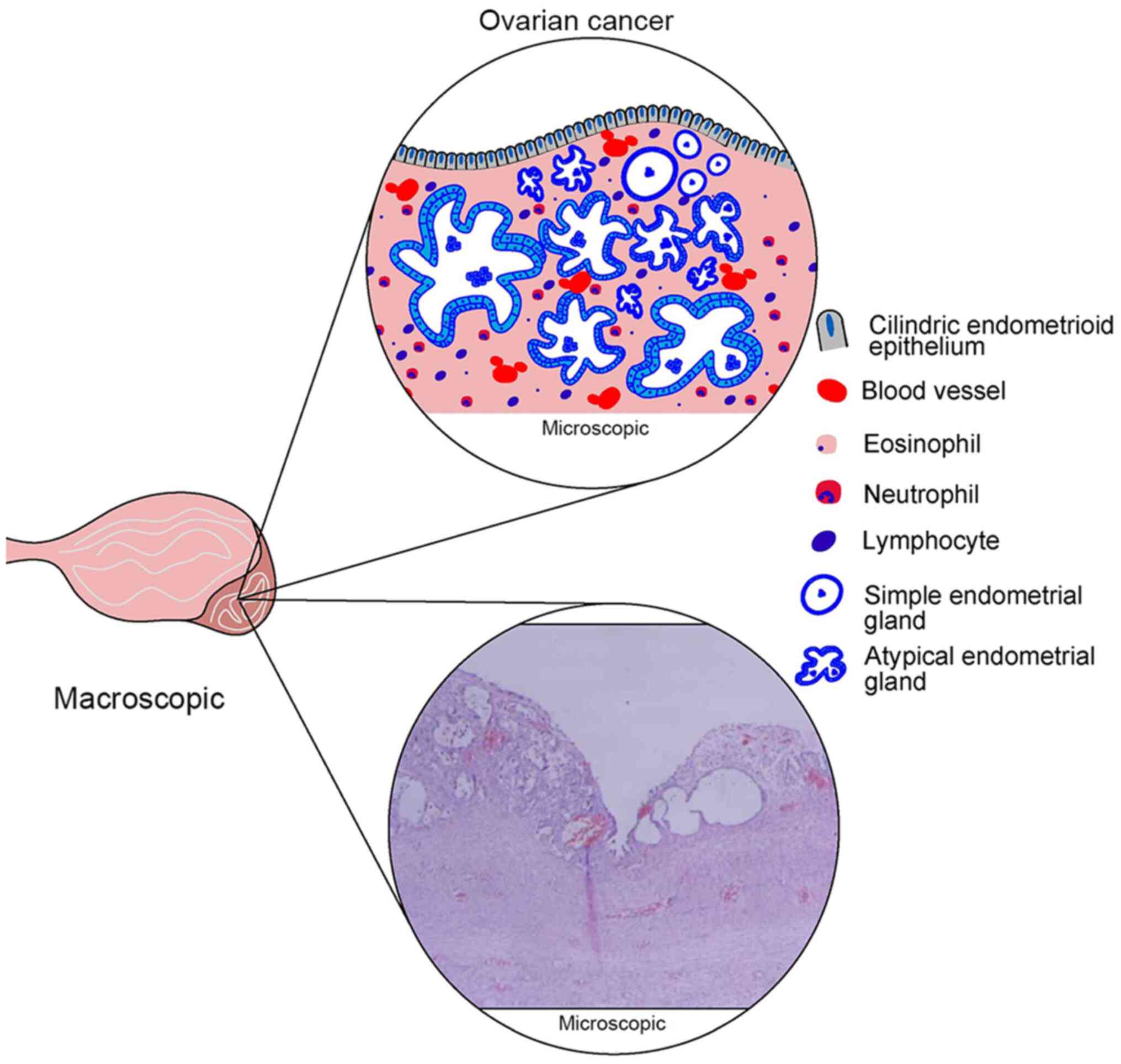
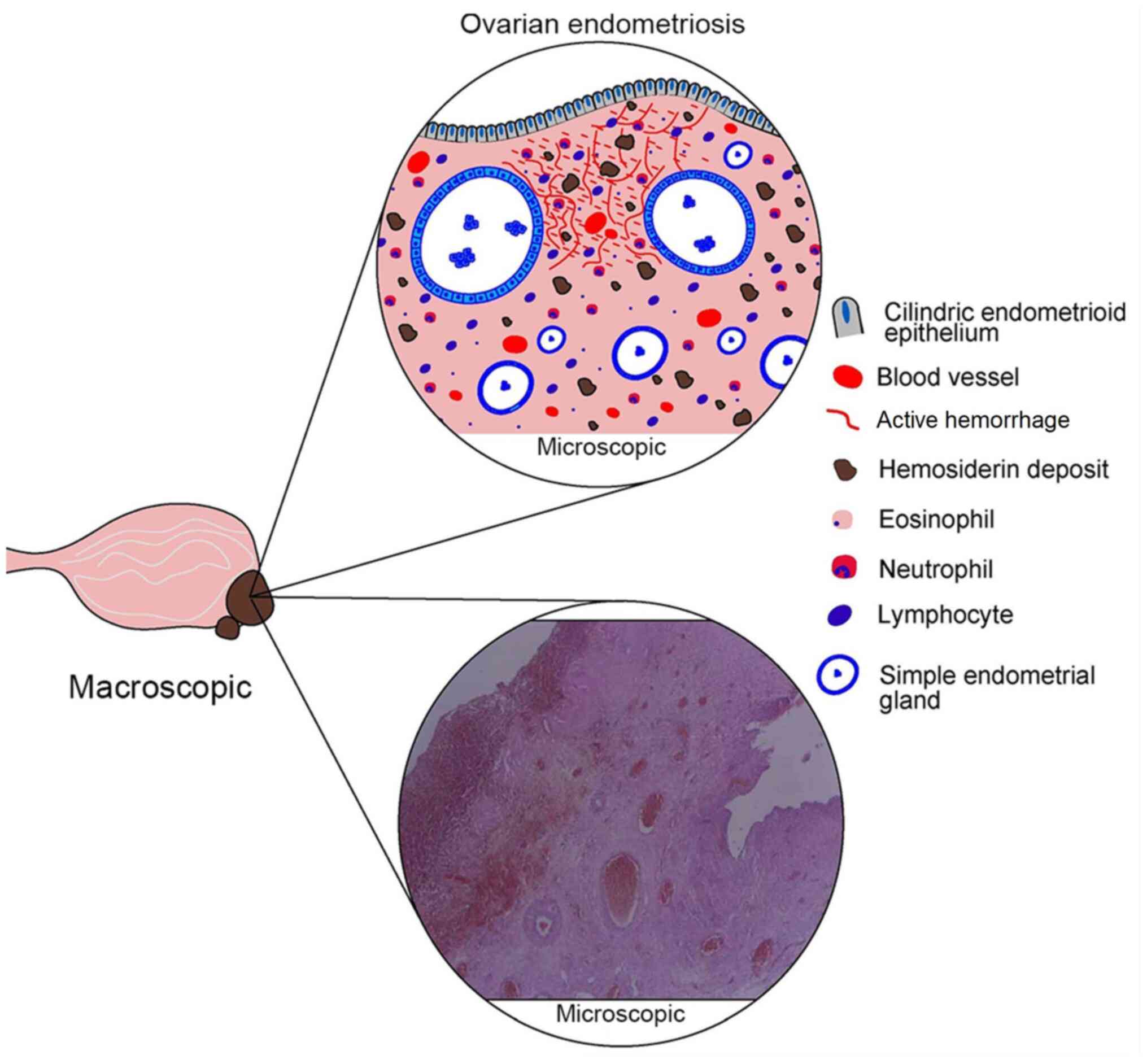 | Figure 1Histological aspects of ovarian
endometriosis. In terms of macroscopic features, ovarian
endometriosis usually takes the form of a cyst, well defined,
unilocular or multilocular, varying in size, with chocolate cysts.
In terms of microscopic features, schematic representation (upper
image) with subsequent identification of important characteristics
in the lower microscopic image outlines the defining endometriosis
elements, which include cylindrical endometrial epithelium,
endometrial-type glands (simple and/or sometimes atypical),
endometrial stroma containing hemosiderin deposits, active
bleeding, blood vessels and inflammatory infiltrates (eosinophils,
neutrophils and lymphocytes). |
 | Table IMicroscopic criteria for the
diagnosis of endometriosis (27). |
Table I
Microscopic criteria for the
diagnosis of endometriosis (27).
| Type | Criteria |
|---|
| Endometrial type
glandular epithelium and stroma with associated constellation of
findings | Granulation tissue
(macrophages) |
| | Hemosiderin
deposit |
| | Fibrosis |
| | Pseudoxanthoma
cells |
| | Island of residual
glandular epithelium or endometrial stroma |
| Endometrioid
cylindrical epithelium | Hyperchromatic
nucleus |
| | Smudgy
chromatin |
Regarding the presence of ectopic endometrial
epithelium, several studies have documented the presence of
hyperplasia and/or atypia in cytologic endometrial lesions
(25-27).
Atypical endometriosis has been indicated to confer a higher
increase risk of malignant transformation to ovarian cancer
(28). A total of ~8% of ovarian
endometriosis exhibits histopathological features of atypical
endometriosis (4). The presence of
hyperplastic lesions in the glandular epithelium is less frequent
compared with that in atypia but may be present in some ovarian
endometriosis cysts (28).
Ovarian cancer
Ovarian cancer is classified in two distinct
subgroups, type I and II, taking into account the clinical
features, histopathological characteristics and gene expression
pattern (24,27). Type I subgroup is characterized by a
low rate of proliferation, while type II subgroup exhibits a higher
proliferation rate and is more aggressive (24,27).
In addition, type I subgroup includes low-grade serous, borderline
serous, mucinous, endometrioid and clear cell carcinoma, while type
II subgroup is comprises high-grade serous carcinoma, mixed
malignant mesodermal carcinosarcomas and undifferentiated carcinoma
(28,29).
Two subcategories of type I epithelial ovarian
cancer, endometrioid and clear cell carcinoma, appear to be more
often derived from or coexist with ovarian endometriosis (9,10,18,30).
These two subcategories are the most frequent types after serous
carcinoma. Two previous population-based studies which reviewed the
pathology using modern diagnostic criteria presented an estimated
frequency of 12-13% for clear cell and 9-11% for endometrioid
carcinoma, among 20% of all epithelial ovarian cancer (18,31).
The main histological characteristics of these two
ovarian cancer subtypes are presented in Table II and Fig. 2.
 | Table IIMicroscopic criteria for the
diagnosis of endometrioid and clear cell ovarian cancer (27). |
Table II
Microscopic criteria for the
diagnosis of endometrioid and clear cell ovarian cancer (27).
| Endometrioid
ovarian carcinoma | Clear cell ovarian
carcinoma |
|---|
| Glandular,
cribriform or solid architecture and morular or squamous
differentiation | Tubulocystic,
papillary or solid architecturea |
| Cuboid to columnar
cells with grading similar to the uterine counterpart | Hobnail or
polyhedral cells with abundant eosinophilic granular/clear
cytoplasm, signet/ring-type cells |
| Nuclear grade
determined by the variation in nuclear size, chromatin distribution
and size of the nucleoli | Round/angular,
hyperchromatic nuclei, marked nuclear pleomorphism |
| Mitoses
present | Mitoses
present |
| Endometriosis often
present | Endometriosis often
present |
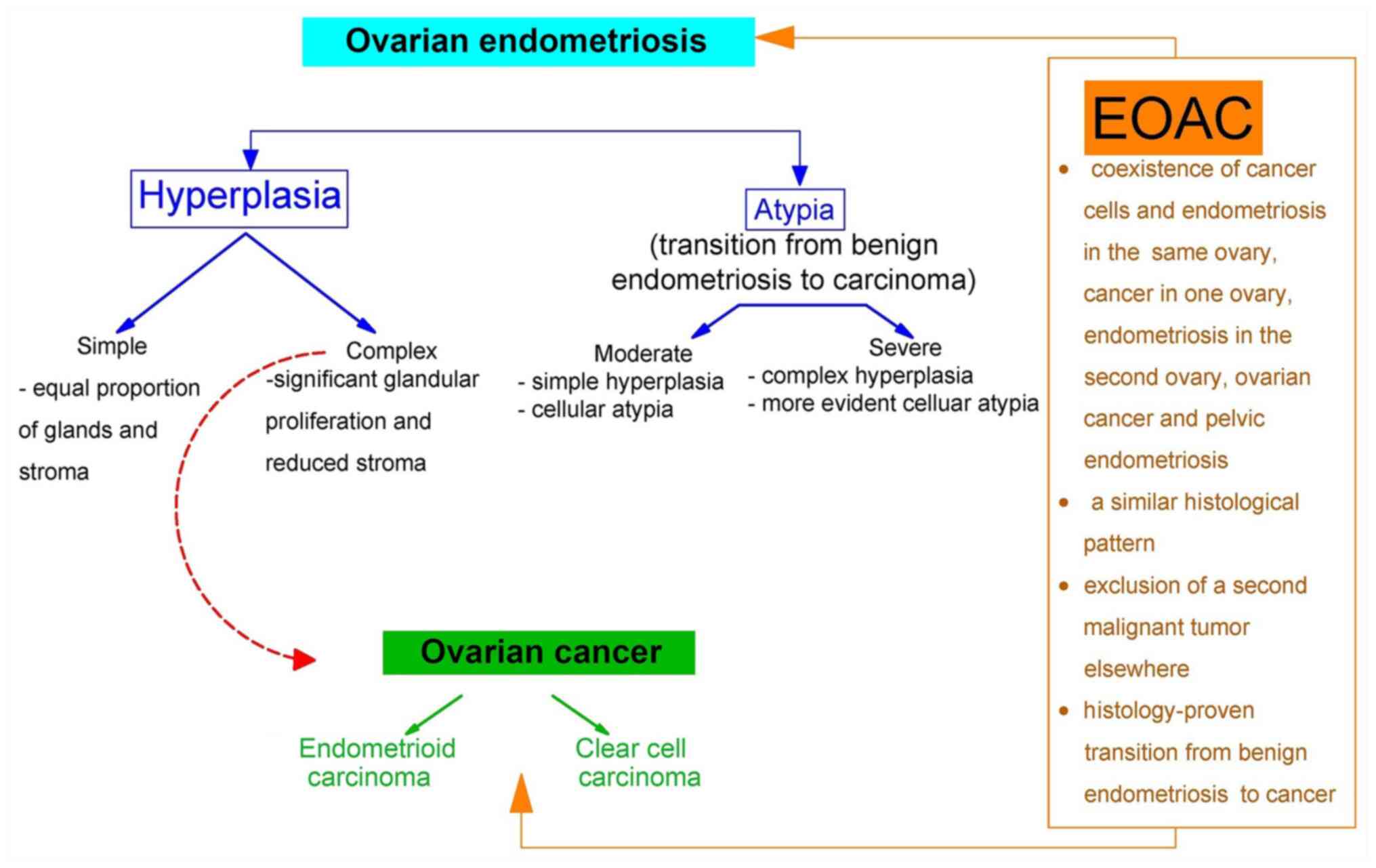
EAOC
EAOC is defined as one of the following three
conditions: i) Detection in the same ovary of endometriosis and
ovarian cancer; ii) detection of endometriosis in one ovary and of
ovarian cancer in the other; iii) coinciding identification of
ovarian cancer in any of the ovaries and pelvic endometriosis
(28). Considering the initial
assumption of Sampson regarding the malignant transformation of
endometriosis to ovarian cancer (2), when currently EAOC is considered as a
single entity, it has been defined as cumulative histological
features characteristic to benign endometriosis, endometriosis
contiguous with or associated with an ovarian malignancy and
malignant lesions intercalated with several intermediary lesions
(Fig. 3) (32).
Several studies reporting histopathological
characteristics of EAOC have been performed. Of these, Fukunaga
et al (33) reported
atypical endometriosis for 54% of clear cell and 42% of
endometrioid carcinoma. Ballouk et al (34) indicated that half of the endometrial
cysts demonstrated severe atypia and presented with an invasive
capacity of malignancies.
3. Gene expression
Abnormal cell transformations, such as endometriosis
and ovarian cancer, are sustained at molecular level via
alterations in homeostatic gene expression profiles and signaling
pathways (22). These changes often
result in pathologically expressed proteins that finally lead to
the alteration of cellular processes (5,32).
Ovarian endometriosis
From a molecular point of view, ovarian
endometriosis is characterized by a broad and important genetic
variety which can result in a wide genetic instability (32). Histologically, molecular
abnormalities can be concealed in benign endometriosis, which
subsequently may lead to a malignant transformation (28).
In ovarian endometriosis, several studies have
reported genetic mutations with important contributions in this
pathology and its possible malignant transformation (Table III).
 | Table IIIEarly abnormal molecular events in
ovarian endometriosis. |
Table III
Early abnormal molecular events in
ovarian endometriosis.
| Gene name | Function | (Refs.) |
|---|
| ARID1A | Tumor suppressor
gene inactivation | (28) |
| PTEN | Tumor suppressor
gene inactivation | (79) |
| PIK3CA | Oncogene
activation | (80) |
| BRCA1/BRCA2 | Tumor suppressor
gene inactivation | (22,39) |
| CTNNB1 | Oncogene
activation | (38) |
ARID1A is a gene identified to exhibit a tumor
suppressor role, and the loss of ARID1A expression is considered to
be responsible for the activation of early carcinogenic mechanisms
(28). Mutations of ARID1A have
been indicated to be directly connected with atypical endometriosis
(35). However, no alterations in
the ARID1A expression level have been identified in paired samples
of distal nonatypical endometriotic tissue (27).
PTEN, which is another tumor suppressor gene, has
been reported to be present in endometriotic lesions, while it has
been indicated that PTEN inactivation exhibits a significant role
in the malignant evolution of endometriosis (36).
PIK3CA has been recognized for its oncogenic role.
Mutations in this gene have been evaluated in nonatypical and
atypical endometriosis, and are considered to be an early event in
carcinogenesis, possibly at the beginning of malignant
transformation of endometriosis (37).
CTNNB1 gene also exhibits an oncogenic function,
which has been highlighted by its important role in the diagnosis
of nonatypical and atypical endometriosis (38). CTNNB1 has been indicated to exhibit
a prominent function in the early events of the transformation of
endometriosis to ovarian cancer (38).
BRCA1 and BRCA2 are important early onset tumor
suppressors genes. Mutations in the BRCA1 and BRCA2 genes have been
reported in the evolution of various human cancers, including
ovarian tumors. However, regarding their role in endometriosis,
there are fewer reports (18,39).
The presence of TP53 tumor suppressor gene mutations
in atypical endometriosis is debated. Certain studies have
indicated that alterations in the TP53 gene were present in
atypical and low levels or absent in nonatypical endometriosis
(37-39).
It has been speculated, according to microarray results, that TP53
cancer-related pathways may participate in endometriosis
progression (40).
KRAS is an oncogene, whose activation was detected
in de novo endometriosis in mice. This suggested that
activation of KRAS is an important pathway in the initiation and
progression of this disease (18).
KRAS mutations have also been observed at an important level in the
eutopic endometrium of patients with endometriosis (40).
Ovarian cancer
The classification of ovarian cancer based on
histopathological and clinical features is accompanied by the gene
expression pattern and mutational determination, which reflect the
main factors responsible for the malignant transformation of
ovarian cancer. These genes include KRAS, BRAF, PTEN, PIK3CA,
CTNNB1 (the gene encoding β-catenin), ARID1A, PPP2R1A and rarely
TP53 (18,28,29,41).
Considering the differentiation of ovarian cancers in two subtypes
(I and II) as artificial and limiting in managing the complex
biology of the disease, another classification consisting of five
different subcategories has been proposed, based on clinical,
morphological and molecular abnormalities (28).
Among all subcategories of ovarian cancer introduced
in routine classification, the two subcategories associated with
endometriosis, endometrioid and clear cell carcinoma, are
extensively studied at the molecular level (42). In these two subcategories, gene
mutation patterns have been identified in a different frequency,
which conferred heterogeneous reports on the progression of
malignant ovarian lesions (Table
IV). According to the experimental data, the inactivation of
ARID1A and PTEN tumor suppressor gene pathways and the activation
of the oncogenic pathways regulated by PI3KCA, KRAS and CTNNB1 are
responsible for initiating complex processes, which have been
suggested as potential mechanisms associated with the early
carcinogenesis and transformation of ovarian cancer (28,38).
Regarding the inactivating mutations in BRCA1/2 in endometrioid and
clear cell carcinoma, there is no unanimously accepted opinion that
BRCA gene mutations contribute to the pathology of the two cancers
(29,43). BRCA mutations or inactivation of
gene expression have been indicated to occur more frequently in
high grade serous carcinoma (29).
 | Table IVFrequency of genetic alterations in
endometrioid and clear cell ovarian carcinoma. |
Table IV
Frequency of genetic alterations in
endometrioid and clear cell ovarian carcinoma.
| A, Endometrioid
ovarian carcinoma |
|---|
| Gene name | Frequency (%) | (Refs.) |
|---|
| ARID1A | 30 | (81) |
| PTEN | 20 | (29) |
| PIK3CA | 31 | (38) |
| TP53 | 30 | (18) |
| BRCA1 | 33 | (43) |
| BRCA2 | 29 | (43) |
| KRAS | 29 | (47) |
| CTNNB1 | 40 | (29) |
| B, Clear cell
ovarian carcinoma |
| Gene name | Frequency (%) | (Refs.) |
| ARID1A | 46 | (81) |
| PTEN | 25 | (44) |
| PIK3CA | 35 | (38) |
| TP53 | 10 | (18) |
| BRCA1 | 10 | (43) |
| BRCA2 | 4 | (43) |
| KRAS | 7 | (82) |
| CTNNB1 | 3 | (82) |
EAOC
The molecular pathways associated with the malignant
transformation of EAOC remain to be fully elucidated. Endometriosis
has been indicated to comprise mutations highlighted in coexisting
tumors, which may designate a primary stage of evolution towards
ovarian malignant transformation (44).
EAOC has been reported to exhibit a high percentage
of PIK3CA and KRAS activating mutations and ARID1A and PTEN
inactivating mutations (35,37,38),
whilst a reduced percentage of cases has been indicated to comprise
TP53 and BRCA1/2 mutations (45,46). A
previous study has identified KRAS mutations in 29% of EAOC cases
and only in 3% of tumors with no endometriosis (47). Lakhani et al (43) reported a smaller, but significant
association between BRCA1/2 and endometrioid and clear cell
carcinoma, namely for endometrioid: 33% of BRCA1, 29% of BRCA2 and
for clear cell: 10% of BRCA; 1.4% of BRCA2.
4. miRNA alterations
Dysregulated miRNAs have been associated with a
large number of human diseases, including cancer (48), cardiovascular disease (49), neurodegenerative disease (50,51)
and diabetes mellitus (52). They
comprise information identified as molecular signatures, which are
represented by specific panels of upregulated and downregulated
molecules. The altered molecules define different profiles of gene
or protein expression in various diseases (53). The differential transcriptional
profiles of miRNAs between normal and pathological tissue samples
may be used as diagnostic and prognostic tools (24). The interaction of miRNAs with
different genes and their expression profiles is commonly specific
for certain cell types and different stages of each disease
(54). The present chapter presents
the miRNAs that have been directly associated with the gene
expression patterns previously reviewed in endometriosis and
ovarian cancer and reported to be dysregulated in the two
pathologies (Fig. 4).
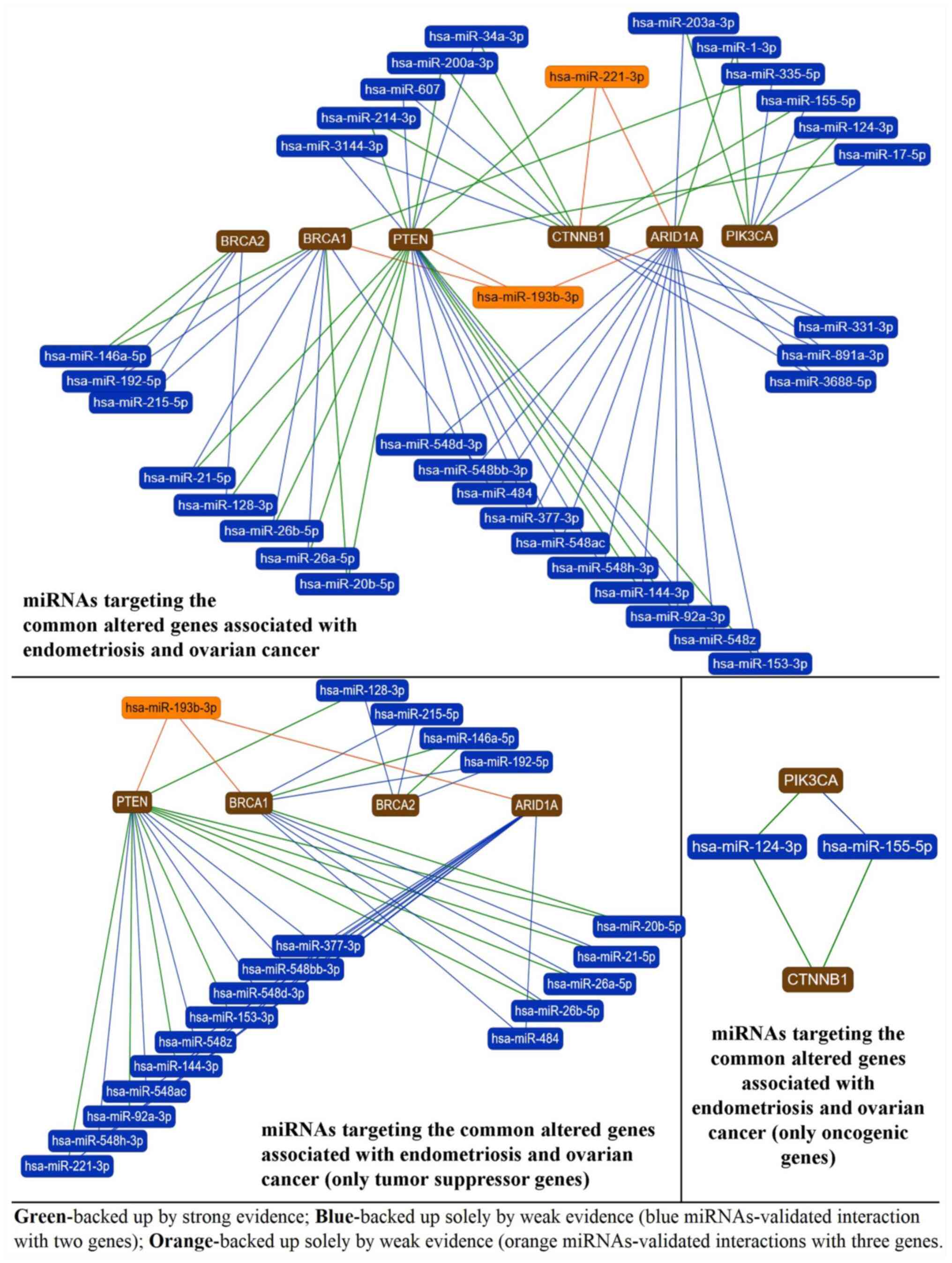 | Figure 4miRNAs targeting common altered genes
in endometriosis and ovarian cancer. BRCA1, BRCA2, PTEN, CTNNB1,
ARID1A and PIK3CA are altered in terms of mutation or expression in
both endometriosis and ovarian cancer and are targeted by multiple
miRNAs. The first graphical representation highlights miRNAs that
have >1 of these genes in their target profile (miRNAs linked to
the gene via green, blue or orange lines), with a special focus on
miR-221-3p and miR-193b-3p that target simultaneously PTEN, CTNNB1,
ARID1A (miR-221-3p) and BRCA1, PTEN and ARID1A (miR-193b-3p). The
remaining miRNAs target simultaneously only two genes. The second
graph illustrates the miRNAs that have >1 of these genes in
their target profile, but only illustrates the tumor suppressor
genes that are common between endometriosis and ovarian cancer
(PTEN, BRCA1, BRCA2 and ARID1A). miR-193b-3p targets concomitantly
three tumor suppressor genes and may become an important prognostic
marker or therapeutic target in endometriosis and ovarian cancer or
a marker of endometriosis transition toward ovarian malignancy,
which requires validation. The third graph highlights the miRNAs
that target simultaneously the two oncogenic genes (PIK3CA and
CTNNB1) associated with endometriosis and ovarian cancer,
miR-124-3p and miR-155-5p. The data were generated with
miRTargetLink online software (22). miRNA/miR, microRNA. |
Ovarian endometriosis
Endometriosis has been regarded as an ideal target
for genomic sciences, since it lacks an efficient diagnostic and
therapeutic management, as it represents a heterogeneous disease
with multiple phenotypes and a complex pathophysiology (55). Several studies have indicated that
miRNAs were implicated in the progression of endometriosis
(2,20,56).
However, at present there is no specific clinical biomarker to be
used in patients with endometriosis.
miR-200 family is one of the most widely studied
miRNA families in endometriosis, and comprises miR-200a, miR-200b,
miR-200c, miR-141 and miR-429(57).
miR-200b has been demonstrated to be the most downregulated
transcript in previous studies on the molecular regulation of
endometriosis (20). miRNAs of this
family have been indicated to target PTEN and CTNNB1 genes
(22).
miR-20a and miR-20b have been found to be
dysregulated in ovarian endometriosis (55,58).
miR-20b has been indicated to contributes to the process of
neovascularization in endometriosis (55) and target PTEN and BRCA1 genes.
miR-17-5p was reported by Jia et al (59) to be upregulated in patients with
endometriosis compared with those without the disease, and has been
indicated to target the PIK3CA gene.
Other miRNAs associated with endometriosis include
miR-34a/b/c, which were demonstrated by Burney et al
(23) to be downregulated in
ovarian endometriosis and target CTNNB1 and PTEN genes. miR-1-3p
has been also reported to be upregulated in patients with
endometriosis (20), and target the
PIK3CA gene. The CTNNB1 gene has been indicated to be a target of
miR-155-5p, which has been demonstrated to be dysregulated in
endometriosis (60). miR-21-5p has
been revealed to be involved in the pathogenesis of endometriosis
exhibiting aberrant expression profiles (60) has been indicated to directly target
BRCA1 and PTEN affecting their expression. PIK3CA, BRCA1 and BRCA2
have also been indicated to be targets of miR-335(22).
Ovarian cancer
miRNAs present regulatory functions, certain of
which exhibit important implications in carcinogenesis (61). Transcriptional microarray data have
demonstrated that there is a different expression level of miRNAs
between normal and tumor tissue (60). The up- and downregulation of miRNA
expression have been associated with cancer development and
progression (62). Alterations in
miRNAs expression have been implicated in invasion and migration in
ovarian carcinogenesis (63).
miR-200 family has been indicated to be involved in
the metastasis of ovarian cancer (19). Notch signaling is considered to be a
regulator of cell invasion in tumors (59). Notch signaling blockade via the
miR-200 family has been indicated to represent a promising
therapeutic approach for ovarian cancer (64).
miR-335 has been demonstrated to be downregulated in
primary ovarian cancer tissue compared with normal tissue (65). Cao et al (65) also reported that patients with
primary ovarian cancer who exhibited a low expression of miR-335
had a shorter survival period. In addition, miR-335 level has been
revealed to be an important prediction factor of tumor recurrence
(65). miR-335 has been indicated
to target PIK3CA, BRCA1 and BRCA2 genes (22).
miR-17-5p has been reported to be overexpressed in
ovarian cancer. Liu et al (66) demonstrated that miR-17 stimulated
the proliferation rate, accelerated cell cycle progression and
affected the invasion capacity of the ovarian cancer cells in
vitro. miR-17 has been also indicated to be involved in the
transition from low to high degree ovarian cancer (66) and directly target the PIK3CA gene
(22).
miR-34a-3p has been revealed to be frequently
downregulated in ovarian cancer, being directly transactivated by
the TP53 tumor suppressor gene, which is frequently dysregulated in
ovarian epithelial cancer (67).
miR-34 has been indicated to affect the motility, proliferation and
migration of ovarian cancer cells (67) and directly target the CTNNB1 gene
(22).
miR-1 level has been demonstrated to be decreased in
ovarian cancer compared with normal ovarian tissue, indicating the
potential tumor suppressor role of this gene (68).
miR-155-5p was revealed by Gulei et al
(69) to exhibit increased
expression in endometriosis, which was further accentuated in
ovarian cancer samples but without statistical significance.
miR-155 has been indicated to affect apoptosis and target the
CTNNB1 gene (22).
miR-21-5p upregulation has been associated with
ovarian cancer, where Liu et al (70) revealed that suppression of miR-21
reduced cell proliferation and promoted cell apoptosis by
increasing PTEN expression. Apart from PTEN, miR-21 has been
indicated to target the BRCA1 gene (22).
miR-148a is part of the miR-148/152 family.
Downregulation of miR148/152 family members has been associated
with unfavorable prognostic outcomes in ovarian cancer (71). miR-148a-5p targets the BRCA1 and
BRCA2 genes (22).
Another miRNA associated with ovarian cancer is
miR-20 which has been indicated to target the PIK3CA gene (22).
EAOC
Endometriosis presents a biological behavior with
increased invasiveness similar to that of tumors. The invasion
process is mediated by the downregulation of E-cadherin and
alterations in the cell phenotype following
epithelial-to-mesenchymal transition (72). This comprises a complex process
converting the immotile epithelial cells into motile ones, as a
response to injury (73).
Therefore, endometriosis may be induced by
epithelial-to-mesenchymal transition.
Fig. 4 presents the
network of miRNAs-target genes in the context of ovarian
endometriosis, EAOC and ovarian cancer. The three pathological
states depicted in Fig. 4 have
different common denominators of altered genes and miRNAs. However,
large patient cohorts need to be employed before establishment of
specific clinical signatures, such as a miRNA signature for the
prediction of the evolution of endometriosis towards
malignancy.
A synthesis of histological and molecular aspects
encountered in ovarian endometriosis and ovarian cancer are
highlighted in Fig. 5.
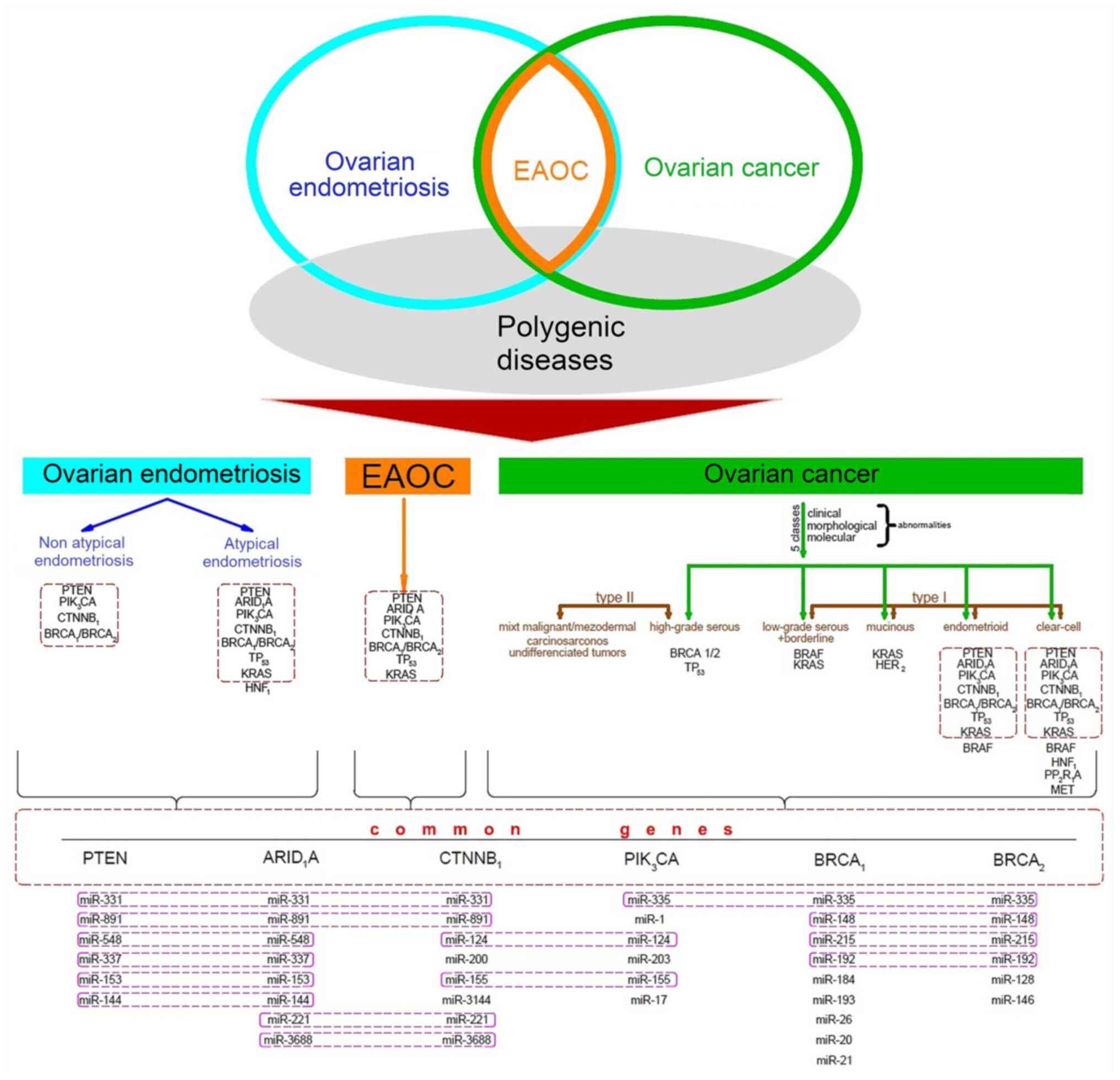 | Figure 5Overview of the histological and
molecular association between ovarian endometriosis and ovarian
malignant transformation. Molecular analysis of ovarian
endometriosis focused on atypical and non-atypical endometriosis
and ovarian cancer with focus on endometrioid ovarian carcinoma,
clear cell ovarian carcinoma and EOAC lesions has identified the
presence of certain genetic mutations and dysregulated miRNAs.
Their comparison has demonstrated the presence of certain mutated
genes in all three pathologies (highlighted with a red frame),
which include PTEN, ARID1A, PIK3CA, CTNNB1, BRCA1/BRCA2, TP53,
KRAS. These genes are targeted by certain commonly dysregulated
miRNAs, such as miR-331, miR-335, miR-891, miR-548, miR-124,
miR-148, miR-215, miR-192, miR-337, miR-153, miR-155, miR-144,
miR-221, miR-3688 and other miRNAs dysregulated in both
endometriosis and ovarian cancer, such as miR-200, miR-17, miR-34,
miR-1, miR-155 and miR-21. EAOC, endometriosis-associated ovarian
cancer; miRNA/miR, microRNA. |
5. Impact of endometriosis on the prognosis
of ovarian cancer
Endometriosis is considered by some specialists in
the field as a pathology, although proof for its implications in
ovarian cancer remain absent (18,74);
however, there are studies that demonstrate the contrary.
Braicu et al (19) performed a meta-analysis
demonstrating that patients with endometriosis presented an
increased risk of developing ovarian cancer. The data indicated an
increased ovarian cancer risk by 27% in case-control or two-cohort
studies, which included 314,421 females with or without
endometriosis, and by 80% in single-arm cohort studies, which
included 79,388 females with endometriosis (30). The results are in accordance with
those of previous studies (8,11) and
they constitute epidemiological data that endometriosis may be
associated with an increased risk of ovarian cancer (30).
Barreta et al (75) performed a database analysis between
1995 and 2016, aiming to clarify whether the clinicopathologies
features and prognosis of patients with clear cell carcinoma and
endometrioid carcinoma were associated with endometriosis.
According to the original pathology report, 29 cases from a total
of 55 cases, mentioned the presence of endometriosis. A second
revision by an expert pathologist identified another 11 cases with
endometrial foci. From the remaining 50 cases after exclusion
criteria, 40 cases (80%) were diagnosed as ovarian cancer
associated with endometriosis (75).
Park et al (76) conducted a retrospective study
between 1991 and 2012 that included 155 patients with clear cell
carcinoma, 78 of which presented associated ovarian
endometriosis.
Cases of EAOC in the aforementioned studies included
young females with an early-stage disease, low-grade disease and
specific histology of endometrioid and clear cell carcinoma.
Ovarian endometriosis was strongly associated with an increased
risk for ovarian cancer. In spite of favorable characteristics of
EAOC, the findings indicated that endometriosis did not affect
ovarian cancer prognosis after its onset, although a better overall
survival was reported, primarily in clear cell carcinoma (30,75,76).
6. Conclusion
Endometriosis, which is one of the most frequent
gynecological diseases, requires a more comprehensive
understanding. In addition, considering the possibilities of
evolution of the endometrial lesions, a need for accuracy in
diagnosis and a therapy plan is required (54).
The presence and increased number of cases of
atypical endometriosis, considered by several studies as an
intermediate lesion between endometriosis and ovarian malignancy,
may allow the identification of endometrial lesions at risk for
malignant transformation (28,77,78).
Since endometriosis is frequently observed in association with
endometrioid and clear cell ovarian carcinoma, or in another
perspective ovarian cancer arises from endometriosis, it can be
hypothesized that endometriosis may be viewed as a preneoplastic or
neoplastic process (36).
Moreover, the frequency of an aberrant mutation
pattern seems to increase in cases of endometriosis adjacent or
contiguous to ovarian cancer (28).
Analyzing the presented aspects, it can be observed that all the
gene mutations discussed (ARID1A, PI3KCA, PTEN, BRCA1/2, TP53 and
KRAS) as being present in ovarian endometriosis (18,28,36,39,40,79,80)
are also present in endometrioid and clear cell carcinoma (18,29,43,44,81,82),
although certain among them (BRCA1/2, KRAS and TP53) are debated
(39,40). Ovarian endometriosis and cancer tend
to share the same genetic mutations, which also support the model
of endometriosis as a malignant precursor. Development of genetic
analyses to detect these mutations may represent a tool in the
early detection of patients at risk to develop ovarian cancer
(54).
miRNAs, which are involved in the regulation of gene
expression, serve an important role in understanding endometriosis
evolution and are currently extensively investigated (2). Increasing evidence suggests their role
as biomarkers in endometriosis. miRNAs, such as miR-200, miR-17,
miR-34, miR-1, miR-155 and miR-21, have been reported as being
dysregulated in both endometriosis (23,57,59,60)
and ovarian cancer (58,62,64-66,68),
demonstrating the molecular association between these
pathologies.
There are few studies that have indicated an
association between ovarian endometriosis and ovarian cancer, but
these data do exist (1). Ovarian
endometriosis has been associated with an increased risk of ovarian
cancer (36); however, no
difference has been reported regarding prognosis in females with
and without EAOC (18).
To elucidate the implications of the association of
ovarian endometriosis and cancer, larger studies are required
(1,18). As a perspective, every group
managing endometriosis and ovarian cancer should publish their data
regarding this association. Further research on this topic with
extensive validation is needed. Novel molecular technologies
investigating epigenetic, transcriptomic, proteomic and
post-translational splicing alterations and complex chromosomal
rearrangements may revolutionize the management of endometriosis
(2). The goal of these innovative
efforts is the development of more sensitive diagnostic and
preventive tests. miRNAs may represent an important tool in the
management of endometriosis (21).
Their potential use in diagnosis and treatment implications of
endometriosis is a challenging and important step in its
management. The early detection of the possible malignant lesions
in females with endometriosis and the differentiation between women
at risk of EAOC and those who will continue to present a benign
disease is important in order to improve the preventive and
diagnostic methods, as managing the situation after cancer has
developed is not as efficient (36,56).
Until the materialization of these ideas, the caregivers in the
clinics should pay attention in handling these cases.
Acknowledgements
Not applicable.
Funding
The present review was funded by PhD research
projects of the Iuliu Hatieganu University of Medicine and
Pharmacy, Cluj-Napoca, Romania (grant nos. 7690/46/15.04.2016 and
5200/41/01.03.2017).
Availability of data and materials
Not applicable.
Authors' contributions
AIGO conceived the study, performed the
histological, molecular and miRNA literature search and edited the
manuscript. CB and DG performed the molecular and miRNA literature
search and edited the manuscript. RC, DM and HR performed the
clinical and histological literature search for endometriosis. AI
performed the clinical and histological literature search for
ovarian cancer. IBN supervised and critically revised the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
AIGO is M.D. in obstetrics-gynecology and Ph.D.
student at Iuliu Hatieganu University of Medicine and Pharmacy,
Cluj-Napoca, Romania. Her research topic is focused on
endometriosis and the possibility of its malignant transformation.
The field of research includes molecular and serological markers of
this pathology. Another topic of interest is reproductive
medicine.
CB is associate professor at the Research Center
for Functional Genomics, Biomedicine and Translational Medicine,
Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca,
Romania. Her main activities and responsibilities comprise
functional genomics studies (miRNA and mRNA), molecular
characterization and targeted therapies and genetic and genomic
methods applied in molecular diagnosis.
DG is a biologist and Ph.D. student at the Research
Center for Functional Genomics, Biomedicine and Translational
Medicine, Iuliu Hatieganu University of Medicine and Pharmacy,
Cluj-Napoca, Romania. Her main activities and responsibilities are
focused on recombinant DNA technology, DNA and RNA extraction,
genome editing and non-coning RNAs in cancer.
RC is M.D. in obstetrics-gynecology and Associate
Professor Ph.D. at the Department of Obstetrics-Gynecology, Iuliu
Hatieganu University of Medicine and Pharmacy, Cluj-Napoca,
Romania. Among his topics of interest are endometrial cancer and
endometriosis, with reference to clinical and research issues.
DM is M.D. in obstetrics-gynecology, Professor
Ph.D. and Chef of the Department of Obstetrics-Gynecology, Iuliu
Hatieganu University of Medicine and Pharmacy, Cluj-Napoca,
Romania. Among his topics of interest is endometriosis, with
reference to clinical and research issues.
HR is M.D. in gynecology, surgeon of deep
endometriosis at the Center of Endometriosis, Clinique Tivoli
Ducos, Bordeaux, France and Professor Ph.D. at University of
Aarhus, Denmark. Endometriosis represents his main topic of
interest with an impressive number of publications in this field
and >100 presentations and conferences in international and
French meetings.
AI is M.D. in oncological surgery, Professor Ph.D.
at the Department of Oncological Surgery, Iuliu Hatieganu
University of Medicine and Pharmacy, Cluj-Napoca, Romania. Among
his topics of interest is endometriosis, with reference to the
possibility of malignant transformation of endometriotic
lesions.
IBN is Professor Ph.D. and Director of the Research
Center of Functional Genomics, Biomedicine and Translational
Medicine, Director of the Research Center for Advanced Medicine
MedFuture at the University of Medicine and Pharmacy, Professor of
Immunology-Department of Oncology and Head of the Functional
Genomics Platform for Cancer, Iuliu Hatieganu University of
Medicine and Pharmacy, Cluj-Napoca, Romania. She presents an
impressive number of publications, international and national
projects.
References
|
1
|
Munksgaard PS and Blaakaer J: The
association between endometriosis and gynecological cancers and
breast cancer: A review of epidemiological data. Gynecol Oncol.
123:157–63. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hsiao KY, Wu MH and Tsai SJ: Epigenetic
regulation of the pathological process in endometriosis. Reprod Med
Biol. 16:314–319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Busacca M and Vignali M: Ovarian
endometriosis: From pathogenesis to surgical treatment. Curr Opin
Obstet Gynecol. 15:321–326. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into the venous circulation. Am J Pathol. 3:93–110.43.
1927.PubMed/NCBI
|
|
5
|
Jiang X, Hitchcock A, Bryan EJ, Watson RH,
Englefield P, Thomas EJ and Campbell IG: Microsatellite analysis of
endometriosis reveals loss of heterozygosity at candidate ovarian
tumor suppressor gene loci. Cancer Res. 56:3534–3539.
1996.PubMed/NCBI
|
|
6
|
Scarfone G, Bergamini A, Noli S, Villa A,
Cipriani S, Taccagni G, Vigano' P, Candiani M, Parazzini F and
Mangili G: Characteristics of clear cell ovarian cancer arising
from endometriosis: A two center cohort study. Gynecol Oncol.
133:480–484. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kvaskoff M, Mu F, Terry KL, Harris HR,
Poole EM, Farland L and Missmer SA: Endometriosis: A high-risk
population for major chronic diseases? Hum Reprod Update.
21:500–516. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee AW, Templeman C, Stram DA, Beesley J,
Tyrer J, Berchuck A, Pharoah PP, Chenevix-Trench G and Pearce CL:
Ovarian Cancer Association Consortium. Evidence of a genetic link
between endometriosis and ovarian cancer. Fertil Steril.
105:35–43.e1-e10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu Y, Cuellar-Partida G, Painter JN and
Nyholt DR: Australian Ovarian Cancer Study; International Endogene
Consortium (IEC). Morris AP, Fasching PA, Hein A, Burghaus S, et
al: Shared genetics underlying epidemiological association between
endometriosis and ovarian cancer. Hum Mol Genet. 24:5955–5964.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sayasneh A, Tsivos D and Crawford R:
Endometriosis and ovarian cancer: A systematic review. ISRN Obstet
Gynecol. 2011(140310)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heidemann LN, Hartwell D, Heidemann CH and
Jochumsen KM: The relation between endometriosis and ovarian
cancer-a review. Acta Obstet Gynecol Scand. 93:20–31.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nezhat FR, Apostol R, Nezhat C and Pejovic
T: New insights in the pathophysiology of ovarian cancer and
implications for screening and prevention. Am J Obstet Gynecol.
213:262–267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zafrakas M, Grimbizis G, Timologou A and
Tarlatzis BC: Endometriosis and ovarian cancer risk: A systematic
review of epidemiological studies. Front Surg. 1(14)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sauriol A, Simeone K, Portelance L,
Meunier L, Leclerc-Desaulniers K, de Ladurantaye M, Chergui M,
Kendall-Dupont J, Rahimi K, Carmona E, et al: Modeling the
diversity of epithelial ovarian cancer through ten novel well
characterized cell lines covering multiple subtypes of the disease.
Cancers (Basel). 12(2222)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Webb PM and Jordan SJ: Epidemiology of
epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol.
41:3–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gray S, Khor XY and Yiannakis D: Niraparib
as maintenance therapy in a patient with ovarian cancer and brain
metastases. BMJ Case Rep. 12(e230738)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo SW: Endometriosis and ovarian cancer:
Potential benefits and harms of screening and risk-reducing
surgery. Fertil Steril. 104:813–830. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Braicu OL, Budisan L, Buiga R, Jurj A,
Achimas-Cadariu P, Pop LA, Braicu C, Irimie A and Berindan-Neagoe
I: miRNA expression profiling in formalin-fixed paraffin-embedded
endometriosis and ovarian cancer samples. Onco Targets Ther.
10:4225–4238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saare M, Rekker K, Laisk-Podar T,
Rahmioglu N, Zondervan K, Salumets A, Götte M and Peters M:
Challenges in endometriosis miRNA studies-From tissue heterogeneity
to disease specific miRNAs. Biochim Biophys Acta Mol Basis Dis.
1863:2282–2292. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Braicu C, Catana C, Calin GA and
Berindan-Neagoe I: NCRNA combined therapy as future treatment
option for cancer. Curr Pharm Des. 20:6565–6574. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hamberg M, Backes C, Fehlmann T, Hart M,
Meder B, Meese E and Keller A: MiRTargetLink-miRNAs, genes and
interaction networks. Int J Mol Sci. 17(564)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Burney RO, Hamilton AE, Aghajanova L, Vo
KC, Nezhat CN, Lessey BA and Giudice LC: MicroRNA expression
profiling of eutopic secretory endometrium in women with versus
without endometriosis. Mol Hum Reprod. 15:625–631. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Forte A, Cipollaro M and Galderisi U:
Genetic, epigenetic and stem cell alterations in endometriosis: New
insights and potential therapeutic perspectives. Clin Sci (Lond).
126:123–138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Verma GP: Fundamentals of histology. New
Delhi, New Age, 2001.
|
|
26
|
Cameron RI and Allen DC: Histopathology
specimens clinical, pathological and laboratory aspects. London,
Springer, 2005.
|
|
27
|
Nucci MR and Oliva E, (eds): Gynecologic
pathology: Edinburgh: Churchill Livingstone, (Foundations in
diagnostic pathology), pp710, 2009.
|
|
28
|
Grandi G, Toss A, Cortesi L, Botticelli L,
Volpe A and Cagnacci A: The association between endometriomas and
Ovarian Cancer: Preventive effect of inhibiting ovulation and
menstruation during reproductive life. Biomed Res Int.
2015(751571)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kurman RJ and Shih IM: Molecular
pathogenesis and extraovarian origin of epithelial ovarian
cancer-shifting the paradigm. Hum Pathol. 42:918–931.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim HS, Kim TH, Chung HH and Song YS: Risk
and prognosis of ovarian cancer in women with endometriosis: A
meta-analysis. Br J Cancer. 110:1878–1890. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
McCluggage WG: Morphological subtypes of
ovarian carcinoma: A review with emphasis on new developments and
pathogenesis. Pathology. 43:420–432. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Worley MJ, Welch WR, Berkowitz RS and Ng
SW: Endometriosis-associated ovarian cancer: A review of
pathogenesis. Int J Mol Sci. 14:5367–5379. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fukunaga M, Nomura K, Ishikawa E and
Ushigome S: Ovarian atypical endometriosis: Its close association
with malignant epithelial tumours. Histopathology. 30:249–255.
1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ballouk F, Ross JS and Wolf BC: Ovarian
endometriotic cysts. An analysis of cytologic atypia and DNA ploidy
patterns. Am J Clin Pathol. 102:415–419. 1994.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chene G, Ouellet V, Rahimi K, Barres V,
Provencher D and Mes-Masson AM: The ARID1A pathway in ovarian clear
cell and endometrioid carcinoma, contiguous endometriosis, and
benign endometriosis. Int J Gynaecol Obstet. 130:27–30.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Worley MJ Jr, Liu S, Hua Y, Kwok JS,
Samuel A, Hou L, Shoni M, Lu S, Sandberg EM, Keryan A, et al:
Molecular changes in endometriosis-associated ovarian clear cell
carcinoma. Eur J Cancer. 51:1831–1842. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pavlidou A and Vlahos NF: Endometriosis
and ovarian cancer: Clinical and molecular aspects. Minerva
Endocrinol. 39:155–165. 2014.PubMed/NCBI
|
|
38
|
Matsumoto T, Yamazaki M, Takahashi H,
Kajita S, Suzuki E, Tsuruta T and Saegusa M: Distinct β-catenin and
PIK3CA mutation profiles in endometriosis-associated ovarian
endometrioid and clear cell carcinomas. Am J Clin Pathol.
144:452–463. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Govatati S, Challa K, Reddy SB, Pramod K,
Deenadayal M, Chakravarty B, Shivaji S and Bhanoori M: BRCA1
alterations are associated with endometriosis, but BRCA2
alterations show no detectable endometriosis risk: A study in
Indian population. J Assist Reprod Genet. 32:277–285.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sáinz de la Cuesta R, Izquierdo M,
Cañamero M, Granizo JJ and Manzarbeitia F: Increased prevalence of
p53 overexpression from typical endometriosis to atypical
endometriosis and ovarian cancer associated with endometriosis. Eur
J Obstet Gynecol Reprod Biol. 113:87–93. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dawson A, Fernandez ML, Anglesio M, Yong
PJ and Carey MS: Endometriosis and endometriosis-associated
cancers: Aew insights into the molecular mechanisms of ovarian
cancer development. Ecancermedicalscience. 12(803)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wei JJ, William J and Bulun S:
Endometriosis and ovarian cancer: A review of clinical, pathologic,
and molecular aspects. Int J Gynecol Pathol. 30:553–568.
2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lakhani SR, Manek S, Penault-Llorca F,
Flanagan A, Arnout L, Merrett S, McGuffog L, Steele D, Devilee P,
Klijn JG, et al: Pathology of ovarian cancers in BRCA1 and BRCA2
carriers. Clin Cancer Res. 10:2473–2481. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
King CM, Barbara C, Prentice A, Brenton JD
and Charnock-Jones DS: Models of endometriosis and their utility in
studying progression to ovarian clear cell carcinoma. J Pathol.
238:185–196. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bayramoğlu H and Düzcan E: Atypical
epithelial changes and mutant p53 gene expression in ovarian
endometriosis. Pathol Oncol Res. 7:33–38. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Aviel-Ronen S, Soriano D, Shmuel E,
Schonman R, Rosenblatt K, Zadok O, Vituri A, Seidman D, Barshack I
and Cohen Y: Surgically treated ovarian endometriosis association
with BRCA1 and BRCA2 mutations. Pathol Res Pract. 210:250–255.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Stewart CJ, Leung Y, Walsh MD, Walters RJ,
Young JP and Buchanan DD: KRAS mutations in ovarian low-grade
endometrioid adenocarcinoma: Association with concurrent
endometriosis. Hum Pathol. 43:1177–1183. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang F, Chen C and Wang D: Circulating
microRNAs in cardiovascular diseases: From biomarkers to
therapeutic targets. Front Med. 8:404–418. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Absalon S, Kochanek DM, Raghavan V and
Krichevsky AM: MiR-26b, upregulated in Alzheimer's disease,
activates cell cycle entry, tau-phosphorylation, and apoptosis in
postmitotic neurons. J Neurosci. 33:14645–14659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Miñones-Moyano E, Porta S, Escaramís G,
Rabionet R, Iraola S, Kagerbauer B, Espinosa-Parrilla Y, Ferrer I,
Estivill X and Martí E: MicroRNA profiling of Parkinson's disease
brains identifies early downregulation of miR-34b/c which modulate
mitochondrial function. Hum Mol Genet. 20:3067–3078.
2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Beuzelin D and Kaeffer B: Exosomes and
miRNA-Loaded biomimetic nanovehicles, a focus on their potentials
preventing type-2 diabetes linked to metabolic syndrome. Front
Immunol. 9(2711)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sung J, Wang Y, Chandrasekaran S, Witten
DM and Price ND: Molecular signatures from omics data: From chaos
to consensus. Biotechnol J. 7:946–957. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Coutinho LM, Ferreira MC, Rocha ALL,
Carneiro MM and Reis FM: New biomarkers in endometriosis. Adv Clin
Chem. 89:59–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ahn SH, Singh V and Tayade C: Biomarkers
in endometriosis: Challenges and opportunities. Fertil Steril.
107:523–532. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rekker K, Saare M, Roost AM, Kaart T,
Sõritsa D, Karro H, Sõritsa A, Simón C, Salumets A and Peters M:
Circulating miR-200-family micro-RNAs have altered plasma levels in
patients with endometriosis and vary with blood collection time.
Fertil Steril. 104:938–946.e2. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Agrawal S, Tapmeier T, Rahmioglu N,
Kirtley S, Zondervan K and Becker C: The miRNA Mirage: How close
are we to finding a non-invasive diagnostic biomarker in
endometriosis? A systematic review. Int J Mol Sci.
19(599)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jia SZ, Yang Y, Lang J, Sun P and Leng J:
Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women
with endometriosis. Hum Reprod. 28:322–330. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nisenblat V, Sharkey DJ, Wang Z, Evans SF,
Healey M, Ohlsson Teague EMC, Print CG, Robertson SA and Hull ML:
Plasma miRNAs display limited potential as diagnostic tools for
endometriosis. J Clin Endocrinol Metab. 104:1999–2022.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tomuleasa C, Braicu C, Irimie A, Craciun L
and Berindan-Neagoe I: Nanopharmacology in translational hematology
and oncology. Int J Nanomedicine. 9:3465–3479. 2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ross JS, Carlson JA and Brock G: miRNA:
The new gene silencer. Am J Clin Pathol. 128:830–836.
2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cancer Genome Atlas Research Network.
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhang M, Wang S, Tang L, Wang X, Zhang T,
Xia X and Fang X: Downregulated circular RNA hsa_circ_0067301
regulates epithelial-mesenchymal transition in endometriosis via
the miR-141/Notch signaling pathway. Biochem Biophys Res Commun.
514:71–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cao J, Cai J, Huang D, Han Q, Chen Y, Yang
Q, Yang C, Kuang Y, Li D and Wang Z: miR-335 represents an
independent prognostic marker in epithelial ovarian cancer. Am J
Clin Pathol. 141:437–442. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Liu T, Qin W, Hou L and Huang Y:
MicroRNA-17 promotes normal ovarian cancer cells to cancer stem
cells development via suppression of the LKB1-p53-p21/WAF1 pathway.
Tumour Biol. 36:1881–1893. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H and Nikitin AY: Frequent downregulation of miR-34
family in human ovarian cancers. Clin Cancer Res. 16:1119–1128.
2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Qu W, Chen X, Wang J, Lv J and Yan D:
MicroRNA-1 inhibits ovarian cancer cell proliferation and migration
through c-Met pathway. Clin Chim Acta. 473:237–244. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gulei D, Raduly L, Broseghini E, Ferracin
M and Berindan-Neagoe I: The extensive role of miR-155 in malignant
and non-malignant diseases. Mol Aspects Med. 70:33–56.
2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H,
Zhang HY and Zhou B: miR-21 regulates the proliferation and
apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev
Med Pharmacol Sci. 23:4149–4155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Miao C, Zhang J, Zhao K, Liang C, Xu A,
Zhu J, Wang Y, Hua Y, Tian Y, Liu S, et al: The significance of
microRNA-148/152 family as a prognostic factor in multiple human
malignancies: A meta-analysis. Oncotarget. 8:43344–43355.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Xiong Y, Liu Y, Xiong W, Zhang L, Liu H,
Du Y and Li N: Hypoxia-inducible factor 1α-induced
epithelial-mesenchymal transition of endometrial epithelial cells
may contribute to the development of endometriosis. Hum Reprod.
31:1327–1338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Du Y, Zhang Z, Xiong W, Li N, Liu H, He H,
Li Q, Liu Y and Zhang L: Estradiol promotes EMT in endometriosis
via MALAT1/miR200s sponge function. Reproduction. 157:179–188.
2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ren T, Wang S, Sun J, Qu JM, Xiang Y, Shen
K and Lang JH: Endometriosis is the independent prognostic factor
for survival in Chinese patients with epithelial ovarian carcinoma.
J Ovarian Res. 10(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Barreta A, Sarian L, Ferracini AC, Eloy L,
Brito ABC, de Angelo Andrade L and Derchain S:
Endometriosis-associated ovarian cancer: Population characteristics
and prognosis. Int J Gynecol Cancer. 28:1251–1257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Park JY, Kim DY, Suh DS, Kim JH, Kim YM,
Kim YT and Nam JH: Significance of ovarian endometriosis on the
prognosis of ovarian clear cell carcinoma. Int J Gynecol Cancer.
28:11–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Czernobilsky B and Morris WJ: A histologic
study of ovarian endometriosis with emphasis on hyperplastic and
atypical changes. Obstet Gynecol. 53:318–323. 1979.PubMed/NCBI
|
|
78
|
Ñiguez Sevilla I, Machado Linde F, Marín
Sánchez MDP, Arense JJ, Torroba A, Nieto Díaz A and Sánchez Ferrer
ML: Prognostic importance of atypical endometriosis with
architectural hyperplasia versus cytologic atypia in
endometriosis-associated ovarian cancer. J Gynecol Oncol.
30(e63)2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A,
Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ and Shih IeM:
Frequent activating mutations of PIK3CA in ovarian clear cell
carcinoma. Am J Pathol. 174:1597–1601. 2009.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Sato N, Tsunoda H, Nishida M, Morishita Y,
Takimoto Y, Kubo T and Noguchi M: Loss of heterozygosity on 10q23.3
and mutation of the tumor suppressor gene PTEN in benign
endometrial cyst of the ovary: Possible sequence progression from
benign endometrial cyst to endometrioid carcinoma and clear cell
carcinoma of the ovary. Cancer Res. 60:7052–7056. 2000.PubMed/NCBI
|
|
82
|
Pavone ME and Lyttle BM: Endometriosis and
ovarian cancer: Links, risks, and challenges faced. Int J Womens
Health. 7:663–672. 2015.PubMed/NCBI View Article : Google Scholar
|