Introduction
Despite the great achievements obtained in the early
detection of hepatocellular carcinoma (HCC) through screening
programs and application of targeted therapies with protease
inhibitors such as sorafenib, the incidence and the poor survival
rate reported are still high, especially in endemic areas for
hepatotropic viruses (HBV, HCV and HDV) such as southeastern Europe
(1-4).
Panels of biomarkers are standardized to help clinicians in their
efforts to improve knowledge in terms of better HCC
characterization for more efficient therapies. A single biomarker
still used for HCC diagnosis or follow-up is α-fetoprotein (AFP),
which is considered as the gold standard of care. Yet, clinical
evidence suggests that it does not help facilitate improvement in
HCC progression, prognosis, or survival rates (5). In general, tumor cells present
metabolic signatures compared to healthy cells, both at the tissue
and bio-humoral levels. The detection of new tumor cell biomarkers,
and their validation has presented new research goals for HCC
characterization. Viral infections, alcohol abuse, dysmetabolic
states [obesity, type 2 diabetes mellitus (T2DM), nonalcoholic
steatohepatitis (NASH)], and other rare conditions causing
subsequent chronic liver damage promote liver tumorigenesis through
different mechanisms and this situation makes it difficult to
standardize a panel of predictive biomarkers for HCC progression
(6-9).
Survivin-1, an anti-apoptotic protein modulated by the p53 gene,
presents overexpression in 70% of Asian HCC patients with chronic
viral hepatitis in whom mutations of p53-gene are apparent
(10). The overexpression of this
tissue biomarker has not been studied in other research. It
represents an opportunity to study Survivin-1 due to the similarity
between risk factors for HCC occurrence in our patients and Asian
patients-a vast majority being infected by hepatitis B, C, and D
viruses. Tumor-associated glycoprotein 72 (TAG-72) is a mucin-like
membrane complex considered to be a feasible biomarker for
unfavorable prognosis of adenocarcinomas in general, with potential
applicability in HCC (11,12). HECT and RLD domain containing E3
ubiquitin protein ligase 5 (HERC5), a protein with a ligand role,
activates the chemotaxis and the local infiltration with T
lymphocytes, being considered a biomarker for predicting HCC
recurrence, as well as the poor survival rates even for early stage
HCC (13,14). In this scenario, we aimed to
evaluate and validate the overexpression of Survivin-1, TAG-72, and
HERC5 as tissue biomarkers for HCC characterization in patients
from our geographical region and to standardize a local biomarker
panel to be introduced in the current management guideline.
Materials and methods
Materials
Thirty liver specimens with a histopathological
diagnosis of HCC (study group) and a similar number of liver tissue
specimens of benign liver tumors (adenomas, HNF, regenerative
nodules, and hemangiomas-control group) were selected from the
Gastroenterology file database and Pathology Clinic registries from
St. Apostle Andrew Emergency Clinical Hospital, Constanta and
Fundeni Institute, Bucharest and compared in terms of Survivin-1,
Tag-72, and HERC5 overexpression. All cases were registered in our
databases in the last 3 years and 6 months (January 2017 to June
2020).
All cases histologically confirmed by pathologists
from both clinics, were reinterpreted for the current study during
the interval December 2019 to June 2020, and the
immunohistochemistry study was conducted at the Research and
Development Centre for the Morphologic and Genetic Study of
Malignant Pathology (CEDMOG) and founded by ‘Ovidius’ University.
The morphological features of the tumors were noted, establishing
the histological type, the grade, and the stage of HCC based on the
World Health Organization (WHO) Histological Classification for
digestive tumors (15).
Demographic data of all patients (study and control
group) providing the liver specimens, including age, sex,
provenence, medical history, liver disease background-chronic viral
infections with B, C, or D viruses, co-morbidities and laboratory
parameters recorded at the time of hospital admission were obtained
from the clinical files and are noted in Table I.
 | Table IDemographic, clinical, imaging and
laboratory features of the HCC and control (benign tumor)
group. |
Table I
Demographic, clinical, imaging and
laboratory features of the HCC and control (benign tumor)
group.
| | Benign tumors
(control group) (N=30) n (%) | HCC group (study
group) (N=30) n (%) | P-value | r-value |
|---|
| Age, mean ± SD,
years | 42.09±9.4 | 59.2±5.9 | 0.002 | 0.96 |
| Sex | | | | |
|
Male | 17 (56.66) | 25 (83.33) | 0.023 | 0.24 |
|
Female | 13 (43.33) | 5 (16.66) | 0.033 | -0.32 |
| Urban | 12 (40.00) | 15 (50.00) | 0.051 | 0.70 |
| Risk factors | | | | |
|
Viral
infection | 3 (10.00) | 21 (70.00) | 0.001 | 0.98 |
|
Alcohol
abuse | 15 (30.00) | 20 (66.66) | 0.048 | 0.33 |
|
NASH/NAFLD | 5 (16.66) | 11 (36.66) | 0.042 | 0.34 |
|
Metabolic
disease | 2 (6.66) | 3 (10.00) | 0.7 | 0.11 |
| Clinical
features | | | | |
|
Hepatomegaly | 3 (10.00) | 22 (73.33) | 0.005 | 0.91 |
|
Jaundice | 11 (36.66) | 20 (66.66) | 0.044 | 0.59 |
|
Ascites | 1 (3.33) | 16 (53.33) | <0.001 | 0.99 |
|
Weight
loss | 12 (40.00) | 24 (80.00) | 0.003 | 0.94 |
|
UDB | - | 7 (23.33)) | - | - |
| Imaging features | | | | |
|
Multifocal | 6 (20.00) | 10 (33.33) | 0.037 | 0.28 |
|
Portal vein
thrombosis | 1 (3.33) | 6 (20.00) | 0.032 | 0.26 |
|
Ascites | 1 (3.33) | 9 (30.00) | 0.027 | 0.21 |
| Laboratory
results | | | | |
|
ALT UI/ml,
mean ± SD↑ | 30±11.67 10
(33.33) | 37.3±14.11 23
(76.66) | 0.067 | 0.58 |
|
GGT UI/ml
↑ | 34.33±8.22 16
(53.33) | 71.54±6.99 24
(80.00) | 0.029 | 0.30 |
|
Albumin
g/dl↓ | 4.3±1.2 1
(3.33) | 2.3±1.4 22
(73.33) | <0.001 | 0.99 |
|
Bilirubin
mg/dl↑ | 1.9±0.5 11
(16.66) | 4.3±2.7 24
(80.00) | 0.002 | |
|
INR↑ | 1.4±0.8 10
(33.33) | 1.9±1.0 12
(40.00) | 0.026 | 0.44 |
|
AFP ng/ml
↑ | 12 (40.00) | 23 (76.66) | 0.011 | 0.29 |
|
≥6
ng/ml | 6.92±2.6 11
(18.60) | 8.22±3.1 14
(46.66) | 0.051 | 0.70 |
|
>180
ng/ml | 204.11±17.77 1
(4.80) | 308.56±44.01 9
(30.00) | 0.015 | 0.18 |
| BCLC
classification | | | | |
|
A | - | 13 | - | - |
|
B | - | 11 | - | - |
|
C | - | 6 | - | - |
The Ethics Committee of Emergency Clinical Hospital
St. Apostle Andrew of Constanta approved the study following
European and local regulations (no. 32/22.11.2019).
Methods
For the immunohistochemical (IHC) assessment, the
representative samples were chosen, and 4-µm sections of
formalin-fixed, paraffin-embedded tissue blocks were obtained for
each case enrolled. Epitope retrieval was conducted prior to
incubation of tissue sections with a panel of three primary
antibodies (ready-to-use) from Novus Biological: Survivin-1
(NB100-911 clone), Tag-72 (CC49 clone), and HERC5 (NBP-91985
clone). The immunostaining protocol for each antibody used was
provided by the manufacturer. As chromogen, we used
3,3'diaminobenzidine (DAB), and brown staining was obtained. The
final step was represented by counterstaining all slides with
Mayer's Hematoxylin. Positive control was used for each antibody:
Human testis for HERC5 antibody, malign melanoma for Survivin-1
antibody, and human prostate carcinoma for the Tag-72 antibody.
Comparisons of the studied biomarker overexpression from HCC tissue
samples with a matched non-HCC group of normal liver tissue
specimens were made.
Statistical analysis
Quantitative variables such as mean ± standard
deviation (SD) and categorical variables are presented as
percentages. The Student t-test and Mann-Whitney U test were used
to identify differences between the two studied groups. The
Chi-square test facilitated the comparison of categorical
variables. The correlation between Survivin-1, Tag-72, and HERC5
overexpression and different HCC variables was performed using the
Spearman rank correlation test. A multivariate logistic regression
analysis detected the independent variables of HCC. The
discriminative power of Survivin-1, Tag-72, and HERC5
overexpression was assessed using ROC curves. The predictive
performance of biomarker overexpression was classically evaluated
by the area under the ROC curve (AUC), sensitivity (Se),
specificity (Sp), positive, and negative predictive values (PPV,
NPV). SPSS 16.0 software (SPSS, Inc.) was used for statistical
analysis. P-values <0.05 were considered statistically
significant.
Results
Demographic, clinical and laboratory
features of the HCC patients
According to demographics, we noted an evident
predominance of male gender in the study group compared to the
control group (17 vs. 25 male patients, P=0.023). The age of the
HCC patients was significantly older than that of the control group
(42.09±9.4 vs. 59.2±5.9 years, P=0.002). Laboratory results were
various and without any statistical significance related to
cytolysis (30±11.67 vs. 37.3±14.11 UI/ml, P=0.067) but
significantly different related to parameters of liver
insufficiency such as albumin (4.3±1.2 vs. 2.3±1.4 g/dl,
P<0.001), bilirubin (1.9±0.5 vs. 4.3±2.7 mg/dl, P=0.002), and
INR (International Normalized Ratio) (1.4±0.8 vs. 1.9±1.0,
P=0.026). AFP had values slightly above the normal upper limit in
both HCC and controls, but levels >180 ng/ml were more
frequently encountered in HCC patients compared to the controls
(204.11±17.77 vs. 308.56±44.01 ng/ml, P=0.015). Jaundice was more
regularly present in the HCC patients compared with the control (11
vs. 20 patients, P=0.044) (Table
I).
Comparison of IHC overexpression of
Survivin-1, Tag-72, and HERC5 in liver tissue samples between HCC
and controls
Statistical analysis using a multivariate linear
regression tool was conducted to correlate the IHC overexpression
of Survivin-1, Tag-72, and HERC5 and independent variables as age,
sex, laboratory results, imaging and clinical features. The
multivariate linear regression analysis revealed that Survivin-1,
Tag-72, and HERC5 were significantly overexpressed upon IHC
analysis in HCC samples in patients older than 50 years (P=0.003,
P=0.006, P=0.004, respectively), male gender (P=0.031, P=0.004,
P=0.020, respectively) patients with increased AFP over 180 ng/dl
(P=0.012, P=0.004, P=0.029, respectively) and with low serum
albumin <3 mg/dl (P=0.031, P=0.021, P=0.003, respectively) as
well as in patients with imaging features of portal thrombosis
(P=0.004, P=0.020, P=0.004, respectively) and ascites (P=0.002,
P=0.004, P=0.019, respectively) and in BCLC B class (P=0.045,
P=0.036, P=0.045, respectively) and C (P=0.033, P=0.001, P=0.027,
respectively) classified patients (Table II). Overexpression of the studied
biomarkers did not correlate positively with cytolysis and other
cholestasis tests or with the remaining clinical or imaging
features of HCC (Table II).
 | Table IIMultivariate linear regression
analysis to identify the correlation between overexpression of
liver biomarkers and clinical, laboratory and imaging variables in
HCC. |
Table II
Multivariate linear regression
analysis to identify the correlation between overexpression of
liver biomarkers and clinical, laboratory and imaging variables in
HCC.
| | Liver tissue
biomarker overexpression |
|---|
| Patient
variables | Survivin-1 P-value
(r) | Tag-72 P-value
(r) | HERC5 P-value
(r) |
|---|
| Age, mean ± SD,
>50 years | 0.003 (0.94) | 0.006 (0.92) | 0.004 (0.94) |
| Male gender | 0.031 (0.55) | 0.004 (0.96) | 0.020 (0.70) |
| Bilirubin | 0.076 (0.24) | 0.064 (0.41) | 0.060 (0.44) |
| INR | 0.055 (0.54) | 0.088 (0.27) | 0.059 (0.52) |
| AFP >180
ng/ml | 0.012 (0.83) | 0.004 (0.96) | 0.029 (0.80) |
| Albumin <3
mg/dl | 0.031 (-0.66) | 0.021 (0.72) | 0.003 (0.94) |
| GGT | 0.059 (0.72) | 0.055 (0.51) | 0.030 (0.48) |
| Portal
thrombosis | 0.004 (0.96) | 0.020 (0.70) | 0.004 (0.96) |
| Ascites | 0.002 (0.98) | 0.004 (0.96) | 0.019 (0.82) |
|
Hepatosplenomegaly | 0.060 (0.74) | 0.003 (0.94) | 0.039 (0.59) |
| BCLC | | | |
|
A | 0.076 (0.33) | 0.086 (0.25) | 0.055 (0.54) |
|
B | 0.045 (0.67) | 0.036 (0.24) | 0.045 (0.67) |
|
C | 0.033 (0.60) | 0.001 (0.99) | 0.027 (0.78) |
Diagnostic accuracy of Survivin-1,
Tag-72 and HERC5 for HCC
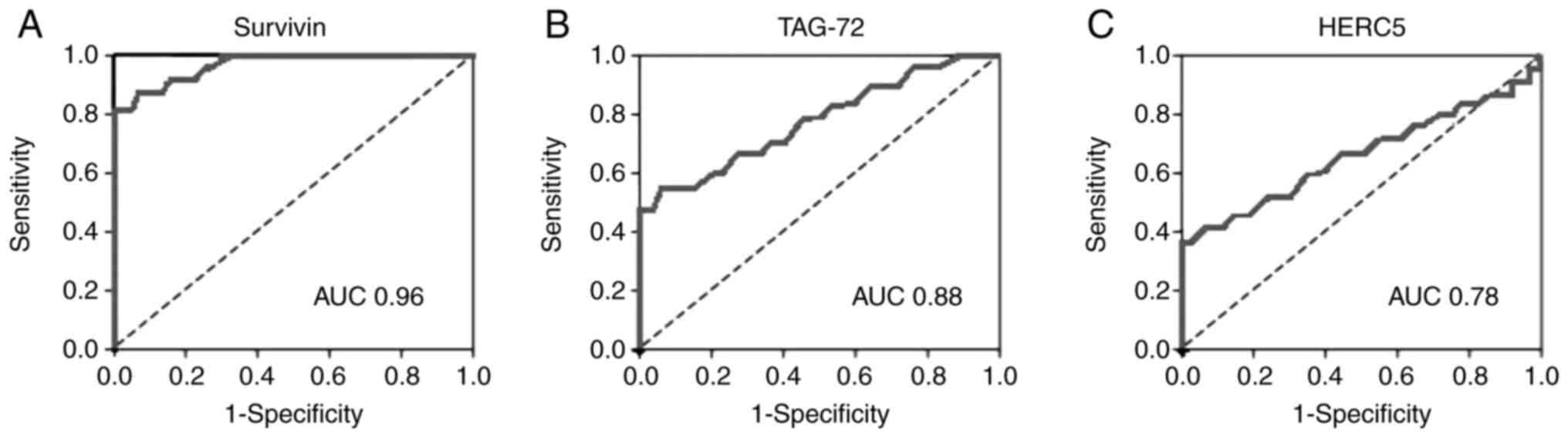
Survivin-1 tissue biomarker had an AUC of 0.96 [95%
confidence interval (CI), 0.90-1.00] for the diagnosis of HCC, with
90.0% sensitivity, 100% specificity, 100% PPV, and 94.2% NPV for an
optimal cut-off value of 0.3 (Fig.
1A and Table III). Tag-72
tissue biomarker had an AUC of 0.88 (95% CI, 0.78-0.94), with 72.8%
sensitivity, 95.6% specificity, 82.2% PPV, and 84% NPV for an
optimal cut-off value of 0.3 (Fig.
1B and Table III). HERC5 had
an AUC of 0.78 (95% CI, 0.66-0.84), with 65% sensitivity, 86%
specificity, 77.4% PPV, and 72.3% NPV (Fig. 1C and Table III) for an optimal cut-off value
of 0.3. AFP, still considered the gold standard biomarker used in
clinical settings and recommended by the international guidelines
for HCC management, had an AUC of 0.34 (95% CI, 0.28-48) for the
diagnosis of HCC, with 38.0% sensitivity, 66% specificity, 64% PPV,
and 68.8% NPV for an optimal cut-off value of 180 ng/dl (Fig. 2). The diagnostic performance of
Survivin-1, Tag-72 and HER-C5 tissue biomarkers for HCC
characterization was superior to that of AFP, considered the gold
standard biomarker used in clinical guidelines (Survivin-1: Z
statistic=2.911, P=0.0039; Tag-72: Z statistic=2.789, P=0.0049,
respectively; HERC5: Z statistic=2.844, P=0.0043) and AFP assay
alone (Z statistic=5.022, P<0.0001) (Table IV).
 | Table IIIAccuracy parameters of Survivin-1,
Tag-72 and HERC5 for HCC diagnosis. |
Table III
Accuracy parameters of Survivin-1,
Tag-72 and HERC5 for HCC diagnosis.
| | Survivin-1 | Tag-72 | HERC5 |
|---|
| AUC (95% CI) | 0.96
(0.90-1.00) | 0.88
(0.78-0.94) | 0.78
(0.66-0.84) |
| Accuracy (%) | 97.2 | 80.4 | 75 |
| Sn (%) | 90 | 72.8 | 65 |
| Sp (%) | 100.0 | 96.6 | 86 |
| PPV (%) | 100.0 | 82.2 | 77.4 |
| NPV (%) | 94.2 | 84.0 | 72.3 |
 | Table IVDiagnostic performance of Survivin-1,
Tag-72 and HER-C5 tissue biomarkers for HCC compared to gold
standard AFP. |
Table IV
Diagnostic performance of Survivin-1,
Tag-72 and HER-C5 tissue biomarkers for HCC compared to gold
standard AFP.
| Diagnostic
performance | Survivin-1 z
statistic (P-value) | Tag-72 z statistic
(P-value) | HERC5 z statistic
(P-value) |
|---|
| AFP | 2.911 (0.0039) | 2.789 (0.0049) | 2.844 (0.0043) |
Discussion
Immunohistochemistry represents a research tool with
large applicability of monoclonal and polyclonal antibodies in
detecting a specific antigen with a high potential for a positive
diagnosis (16). This method is
largely used to diagnose malignant tumors, but it presents an area
of interest for numerous applications useful not only for positive
and differential diagnosis but also to provide a research field in
the attempt to identify prognostic markers for cancer evolution, to
confirm the positive diagnosis of tumors with uncertain
histogenesis, to predict the response to treatment and to identify
or confirm certain infections (7,17,18).
Such biomarkers facilitate the efforts of researchers for a better
understanding of HCC pathogeny, to provide more efficient
therapies, and to improve disease prognosis. Epidemiology data
confirm the persistent high global morbi-mortality rates for HCC,
which are more consistent in endemic areas for chronic viral
infections, such as Asia, the Middle East, Mediterranean countries,
South America, and Africa (19-22).
The current guidelines use a single biomarker for HCC follow-up,
this being α-fetoprotein (AFP). The literature reports a low
accuracy for AFP, and declines its role as a potent prognostic
tool, thus new liver tissue biomarkers are being explored worldwide
to improve HCC management (5). The
difference between various etiopathogenic mechanisms involved in
liver primary carcinogenesis makes it difficult to standardize a
global panel of liver tissue biomarkers to simplify disease
management. Using this scenario, having literature models from
other studies conducted in different populations, we evaluated the
expression of three liver tissue markers, aiming to confirm their
overexpression in HCC tissue in patients from the Dobrogea area and
to provide the background for further research for prognostic
predictability or patient classification in risk groups. Our study
demonstrated the same pattern of demographic, clinical, and
laboratory features as the majority of published data, with a high
prevalence of viral infections and alcohol abuse leading the risk
factor background, and male gender and middle age, being the main
characteristics of our patients. All liver tissue biomarkers
explored provided a good accuracy for HCC diagnosis: 97.2% for
Survivin-1, 80.4% for Tag-72, and 75% for HERC5, similar to the
literature data (10,12,13).
AFP over the upper limit did not prove to have diagnostic or
predictive value for HCC, similar to other literature articles
(23-26).
Still, values over 180 ng/dl were highly predictive, and they were
found to be positively correlated with all biomarkers studied,
Survivin-1, Tag-72, and HERC5 (r=0.83, r=0.96, r=0.80,
respectively). Our study results confirm the background hypothesis,
indicating the overexpression of Survivin-1, Tag-72, and HERC5 as
feasible biomarkers with which to diagnose HCC. Larger IHC studies
should sustain the accuracy of the proposed tools before their
introduction to international HCC management guidelines.
Comparative study with other methods of diagnosis and prognostic
evaluation used and other types of carcinomas, such as the study of
neoangiogenesis or nuclear morphometry, can bring new useful data
both in regards to diagnosis and in the prognosis of the disease
(27,28). Despite the importance of our study
results, our work had limits due to the increased costs of study
materials, a fact that influenced the number of samples evaluated
by immunohistochemistry, a problem partially solved by the funds
gained through university research grant competition.
In conclusion, our study results validate the
overexpression of Survivin-1, Tag-72, and HERC5 as tissue
biomarkers for HCC characterization in patients from our
geographical region and could be standardized in the current HCC
management guideline.
Acknowledgements
The present research was performed at the Research
and Development Centre for the Morphologic and Genetic Study of
Malignant Pathology from the ‘Ovidius’ University of Constanta,
with the precious support of Professor Dr Mariana Aschie.
Funding
Financial support was obtained after attaining the
‘Ovidius’ University contest-July 2019 for research grants [POSCCE
2.2.1; Project ID: 1844; code SMIS: 48750; CEDMOG, contract
627/11.03.2014].
Availability of data and materials
The data were obtained from the HIPOCRATE file
archive of the Emergency Hospital St. ‘Apostle Andrew’ of Constanta
and CEDMOG archive (http://www.spitalulconstanta.ro/). Further information
about the present study is available from the corresponding author
upon reasonable request.
Authors' contributions
AIS, APS, GIB, VH, CN, LCP, FV, ISM and LM conceived
and designed the study; ND, LCP, CB and FB collected the data. LCP,
ND, CB, and FV analyzed the data; AIS, APS, CN and ISM validated
the results. FB, CN, LCP and ND were responsible for original draft
preparation; AIS, GIB, LCP, ND, ISM and VH were responsible for the
final manuscript editing. AIS, APS, GIB and CN supervised the
manuscript publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Emergency Clinical Hospital
St Apostle Andrei Constanta approved the study following European
and local regulations. Approval no. 32/22.11.2020. Emergency
Clinical Hospital St. Apostle Andrew from Constanta is a university
hospital and all admitted patients sign an informed consent by
which they agree that their data are available for academic and
scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baecker A, Liu X, La Vecchia C and Zhang
ZF: Worldwide incidence of hepatocellular carcinoma cases
attributable to major risk factors. Eur J Cancer Prev. 27:205–212.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liang Y, Chen J, Yu Q, Ji T, Zhang B, Xu
J, Dai Y, Xie Y, Lin H, Liang X and Cai X: Phosphorylated ERK is a
potential prognostic biomarker for Sorafenib response in
hepatocellular carcinoma. Cancer Med. 6:2787–2795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kudo M: Systemic Therapy for
hepatocellular carcinoma: 2017 Update. Oncology. 93 (Suppl
1):S135–S146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kudo M: Immuno-oncology in hepatocellular
carcinoma: 2017 Update. Oncology. 93 (Suppl 1):S147–S159.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Farinati F, Marino D, De Giorgio M, Baldan
A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA,
Benvegnù L, et al: Diagnostic and prognostic role of
alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am
J Gastroenterol. 101:524–532. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nishimoto S, Fukuda D, Higashikuni Y,
Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M,
Yagi S, et al: Obesity-induced DNA released from adipocytes
stimulates chronic adipose tissue inflammation and insulin
resistance. Sci Adv. 2(e1501332)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH,
Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ and Chen CJ: Hepatitis C
virus seromarkers and subsequent risk of hepatocellular carcinoma:
Long-term predictors from a community-based cohort study. J Clin
Oncol. 28:4587–4593. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jee SH, Ohrr H, Sull JW and Samet JM:
Cigarette smoking, alcohol drinking, hepatitis B, and risk for
hepatocellular carcinoma in Korea. J Natl Cancer Inst.
96:1851–1856. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kannangai R, Wang J, Liu QZ, Sahin F and
Torbenson M: Survivin-1 overexpression in hepatocellular carcinoma
is associated with p53 dysregulation. Int J Gastrointest Cancer.
35:53–60. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin B, Wang X, Jin Y, Xia W, Chen B, Liu
L, Chen Z, Hong L, Du W, Yan K, et al: Detection of serum gastric
cancer-associated MG7-Ag from gastric cancer patients using a
sensitive and convenient ELISA method. Cancer Invest. 27:227–233.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chauhan SC, Vinayek N, Maher DM, Bell MC,
Dunham KA, Koch MD, Lio Y and Jaggi M: Combined staining of TAG-72,
MUC1, and CA125 improves labeling sensitivity in ovarian cancer:
Antigens for multi-targeted antibody-guided therapy. J Histochem
Cytochem. 55:867–875. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xue F, Higgs BW, Huang J, Morehouse C, Zhu
W, Yao X, Brohawn P, Xiao Z, Sebastian Y, Liu Z, et al: HERC5 is a
prognostic biomarker for post-liver transplant recurrent human
hepatocellular carcinoma. J Transl Med. 13(379)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bernassola F, Karin M, Ciechanover A and
Melino G: The HECT family of E3 ubiquitin ligases: Multiple players
in cancer development. Cancer Cell. 14:10–21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Torbenson MS, Ng IOL, Park YN, Roncalli M
and Sakamoto M: Hepatocellular Carcinoma. In: WHO Classification of
Tumours Editorial Board. Digestive system tumours. 5th edition.
International Agency for Research on Cancer, Lyon, pp229-239,
2019.
|
|
16
|
Ramos-Vara JA and Miller MA: When tissue
antigens and antibodies get along: Revisiting the technical aspects
of immunohistochemistry-the red, brown, and blue technique. Vet
Pathol. 51:42–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Torlakovic EE, Cheung CC, D'Arrigo C,
Dietel M, Francis GD, Gilks CB, Hall JA, Hornick JL, Ibrahim M,
Marchetti A, et al: Evolution of quality assurance for clinical
immunohistochemistry in the era of precision medicine-part 2:
Immunohistochemistry test performance characteristics. Appl
Immunohistochem Mol Morphol. 25:79–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bolocan A, Păduraru DN, Nițipir C,
Hainăroșie R, Pițuru SM, Diaconu C, Suceveanu A and Stoian CD:
Mixed adenoneuroendocrine carcinoma of the gastrointestinal
tract-features, diagnosis, management and prognosis. Rom Biotechnol
Lett. 23:14193–14202. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gurtsevitch VE: Human oncogenic viruses:
Hepatitis B and hepatitis C viruses and their role in
hepatocarcinogenesis. Biochemistry (Mosc). 73:504–513.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Donato F, Tagger A, Gelatti U, Parrinello
G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML,
Martelli C, et al: Alcohol and hepatocellular carcinoma: The effect
of lifetime intake and hepatitis virus infections in men and women.
Am J Epidemiol. 155:323–331. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World gastroenterology organisation guideline.
Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
22
|
Suceveanu AI, Pantea Stoian A, Mazilu L,
Voinea F, Hainăroșie R, Diaconu CC, Pițuru S, Nițipir C, Badiu DC,
Ceaușu I and Suceveanu AP: Interferon-free therapy is not a trigger
for hepatocellular carcinoma in patients with chronic infection
with hepatitis C virus. Farmacia. 66:904–908. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carr BI, Akkiz H, Üsküdar O, Yalçın K,
Guerra V, Kuran S, Karaoğullarından Ü, Altıntaş E, Özakyol A,
Tokmak S, et al: HCC with low- and normal-serum alpha-fetoprotein
levels. Clin Pract (Lond). 15:453–464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang SL, Liu LP, Yang S, Liu L, Ren JW,
Fang X, Chen GG and Lai PB: Preoperative serum α-fetoprotein and
prognosis after hepatectomy for hepatocellular carcinoma. Br J
Surg. 103:716–724. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
An SL, Xiao T, Wang LM, Rong WQ, Wu F,
Feng L, Liu FQ, Tian F and Wu JX: Prognostic significance of
preoperative serum alpha-fetoprotein in hepatocellular carcinoma
and correlation with clinicopathological factors: A single-center
experience from China. Asian Pac J Cancer Prev. 16:4421–4427.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-Pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ardeleanu V, Georgescu C, Frîncu LD,
Frâncu LL and Vesa D: Angiogenesis as prospective molecular biology
technique for cancer study. Rom Biotehnol Lett. 19:9637–9648.
2014.
|
|
28
|
Ardeleanu V, Francu L and Georgescu C:
Neoangiogenesis. Assessment in esophageal adenocarcinomas. Indian J
Surg. 77 (Suppl 3):S971–S976. 2015.PubMed/NCBI View Article : Google Scholar
|