Introduction
Acute pancreatitis (AP) is the third most familiar
gastrointestinal disease with a rising morbidity, with 275,000
cases admitted in the States (1,2). The
clinical manifestations are mild, self-limited local inflammation,
or severe systemic inflammatory reaction. Severe AP (SAP) patients
may require intensive care (3).
Additionally, 15-25% AP patients are likely to progress to SAP,
which is manifested as pancreatic necrosis that can lead to various
complications, and its mortality is as high as 10-20% (4). Early intervention for SAP patients is
helpful to improve their survival. However, the assessment system
of AP severity and imaging diagnosis requires at least 48 h to
ensure accuracy. Furthermore, the detection of laboratory
indicators cannot effectively cope with the clinical heterogeneity
of SAP patients (5). Therefore,
exploring new markers for the early diagnosis of SAP is crucial. At
present, the treatment methods for AP include liquid therapy,
nutrition therapy, antibiotic therapy and surgical intervention
(6). The determination of a
clinical treatment plan is generally based on the etiology of
patients and possible complications to control the disease
(6). The pathological mechanism of
AP involves a series of comprehensive processes such as immune
system mediation, and complex cascade reaction of inflammatory
activation (7). Based on the
clinical characteristics and pathological mechanism of AP patients,
examination of the therapeutic targets, as well as relevant
diagnostic, therapeutic and prognostic evaluation markers is
imperative.
Glutamine (Glu) is a functional essential amino acid
that can regulate key biological functions, and it is helpful in
regulating intestinal mucosal barrier function and relieving
immunosuppression (8,9). A study of Glu in acute necrotizing
pancreatitis (ANP) rats by Alhan et al explained that using
Glu alone significantly reduced the abnormal pathological indexes
of rats, suggesting that it could treat AP (10). As an immune nutrient, Glu can be
used for nutritional intervention during AP attacks to relieve the
inflammatory state of the disease. Its intravenous infusion reduces
infection complications and the mortality of SAP patients (11,12).
Ulinastatin (UTI) is a trypsin inhibitor obtained by separation and
purification of human urine, which can stimulate intestinal mucosal
dysfunction and immune function recovery in SAP patients (13,14).
Previous findings have shown that UTI also reduces the level of
inflammatory cytokines and inhibits oxidative stress, which can
balance the permeability of vascular endothelial cells through the
RhoA/ROCK signaling pathway to start an anti-inflammatory mechanism
(15,16). Soluble intercellular adhesion
molecule-1 (sICAM-1) is a member of the immunoglobulin superfamily.
Its high expression abnormality is related to human diseases such
as gastric cancer and pancreatic cancer, and it has certain
predictive value for pancreatic necrosis and death in AP patients
(17,18). Soluble receptor for advanced
glycosylation end products (sRAGE) is a soluble splice variant of
the full-length receptor RAGE. It participates in the pathological
mechanism of acute inflammation, and can be used as an effective
marker for early identification of SAP patients (19,20).
SICAM-1 and sRAGE are soluble molecules involved in
pancreatic-related diseases, but there are few studies on the
diagnosis, efficacy and prognosis evaluation of AP patients. This
study was to determine the diagnostic, therapeutic and prognostic
value of the two on AP with Glu and UTI by detecting their serum
expression.
Materials and methods
General data
Totally 134 AP patients treated in the Department of
Gastroenterology of the Yidu Central Hospital of Weifang from March
2017 to June 2019 were selected as AP group (APG), comprising 74
male and 60 female patients. All of them were treated with Glu
combined with UTI. Inclusion criteria: AP was diagnosed according
to the diagnostic criteria of clinical practice guidelines, and
further classified as mild acute pancreatitis (MAP) and SAP
(21); Acute Physiology and Chronic
Health Evaluation (APACHE) II Score (22) was not less than 8; patients were
informed and willing to cooperate with this research; patients had
no history of allergy to this therapeutic drug. Exclusion criteria:
Patients had malignancy, infectious or allergic diseases; patients
had taken drugs that influenced the relevant indicators for 6
months; patients had communication or cognitive disorders; patients
died within 7 days after admission.
The 134 patients were subdivided into the MAP and
SAP groups, each group comprising 34 and 40 male, and 20 and 40
female patients, respectively. Another 60 healthy individuals who
came to the Yidu Central Hospital of Weifang for physical
examination during the same period were selected as the normal
group (NG), comprising 35 males and 25 females. They were
(45.76±5.84) years old on average. We then divided AP patients into
the effective (EG) and ineffective (IG) groups in light of the
different therapeutic effect of patients; 103 were effective and 31
were ineffective. Based on whether SAP patients died within 30
days, 65 survivals were included in the good prognosis group (GPG)
and the dead (15 individuals) in the poor prognosis group
(PPG).
All the subjects and their families agreed to
participate in the study and signed the written informed consent
form. The study was approved by the Hospital Ethics Committee of
Yidu Central Hospital (Weifang, China).
Treatment methods
Patients in the APG were treated with Glu combined
with UTI. Glu (KS-01055, Shanghai Keshun Biotechnology Co., Ltd.)
(0.4 g/kg) was injected intravenously once a day, while UTI
(Shanghai Yubo Biotechnology Co., Ltd.; cat. no. CP-140785) of 105
units was dissolved in 0.5 liter glucose injection for intravenous
drip once a day for two weeks. Edaravone combined with UTI was used
to treat any patients who did not respond to the Glu combined with
UTI treatment.
Assessment of efficacy
Effectiveness was assessed as indicated below:
Markedly effective: After treatment, the clinical symptoms of
patients were improved significantly; CT examination showed that
morphology of abdominal pancreas was normal, blood/urine amylase
and white blood cell count and other laboratory indicators returned
to normal; effective: After treatment, the clinical symptoms of
patients were relieved; CT examination showed that the abdominal
pancreatic edema was relieved, and the laboratory indexes such as
blood/urine amylase and white blood cell count were improved;
ineffective: There was no marked improvement in clinical symptoms
after treatment; CT examination showed no obvious improvement in
abdominal pancreatic edema, and no marked decrease in laboratory
indicators such as blood/urine amylase and white blood cell count.
The total effective rate was the percentage of patients with marked
effective and effective efficacy in the total number.
Index detection
Altogether 5 ml venous blood was collected from
patients in the APG on an empty stomach in the morning of the next
day after admission (for efficacy evaluation) and 7 days after
treatment (for prognosis evaluation), centrifuged 10 min at 1,500 x
g at 4˚C and the serum and plasma were separated and placed in a
refrigerator at -70˚C for standby. Serum sICAM-1 and sRAGE levels
were determined by enzyme-linked immunosorbent assay (ELISA)
(23), and this step strictly
adhered to the protocol of the human sICAM-1 and sRAGE ELISA kits
(China Shanghai Guandao Bioengineering Co., Ltd., GD-E001264474,
GD-E001271747). Serum D-lactic acid and diamine oxidase (DAO)
levels were determined by spectrophotometry (D-lactic acid, DAO
spectrophotometric kits were purchased from Shanghai Yaji
Biotechnology Co., Ltd., YS-1132K, YS-1358K), and plasma endotoxin
levels were tested via tachypleus amebocyte lysate (endotoxin
limulus test kits were provided by Shanghai Xinfan Biotechnology
Co., Ltd., XFNDS001). The peripheral blood T-lymphocyte subsets
(CD3+, CD4+, CD8+ and
CD4+/CD8+) were measured via flow cytometry
(China Changzhou Beamdiag Biotechnology Co., Ltd.; cat. no.
1026).
Statistical analysis
The experimental data were statistically analyzed
via SPSS20.0 [Bioeasy (Beijing) Technology Co., Ltd.], and the
pictures were drawn via GraphPad Prism 6. The counting data were
expressed by number of cases/percentage [n (%)] and the comparison
between two groups was analyzed via Chi-square test. The
measurement data were represented as mean ± standard deviation and
compared via unpaired t-test. The comparison before and after
treatment was assessed via paired t-test, and univariate analysis
was employed for comparison between multiple groups. The risk
factors for poor prognosis in SAP patients were analyzed via
Logistic regression analysis. P<0.05 denotes there are
statistical differences.
Results
General data
There was no statistical difference in sex, age,
average age, body mass index (BMI), systolic blood pressure,
diastolic blood pressure, history of drinking or smoking, place of
residence and other aspects between the three groups (P>0.05).
Thereinto, the average APACHE II scores of the MAP group (MAPG) and
the SAP group (SAPG) were (10.87±2.38) and (20.36±4.13),
respectively (Table I).
 | Table IComparison of general data [n (%),
mean ± SD]. |
Table I
Comparison of general data [n (%),
mean ± SD].
| Factors | Normal group
(n=60) | MAP group (n=54) | SAP group (n=80) | χ2 | F | t | P-value |
|---|
| Sex | | | | 1.878 | | | 0.296 |
|
Male | 35 (58.33) | 34 (55.22) | 40 (50.00) | | | | |
|
Female | 25 (41.67) | 20 (44.78) | 40 (50.00) | | | | |
| Age (years) | | | | 3.089 | | | 0.187 |
|
<50 | 26 (43.33) | 25 (52.24) | 45 (56.25) | | | | |
|
≥50 | 34 (56.67) | 29 (47.76) | 35 (43.75) | | | | |
|
Average
age | 44.55±5.37 | 44.98±4.15 | 46.16±4.62 | | 2.181 | | 0.116 |
| BMI
(kg/m2) | 21.87±2.55 | 21.78±2.10 | 22.45±1.98 | | 1.901 | | 0.152 |
| Systolic blood
pressure (mmHg) | 125.09±10.13 | 127.88±8.26 | 126.84±9.73 | | 1.280 | | 0.280 |
| Diastolic blood
pressure (mmHg) | 79.62±7.10 | 82.03±8.20 | 81.56±7.16 | | 1.759 | | 0.175 |
| History of
drinking | | | | 1.878 | | | 0.296 |
|
No | 35 (58.33) | 27 (44.78) | 33 (41.25) | | | | |
|
Yes | 25 (41.67) | 27 (55.22) | 47 (58.75) | | | | |
| History of
smoking | | | | 3.390 | | | 0.170 |
|
No | 36 (60.00) | 26 (47.76) | 38 (47.50) | | | | |
|
Yes | 24 (40.00) | 28 (52.24) | 42 (52.50) | | | | |
| Place of
residence | | | | 1.103 | | | 0.437 |
| Countryside | 21 (35.00) | 24 (43.28) | 34 (42.50) | | | | |
| Cities and
towns | 39 (65.00) | 30 (56.72) | 46 (57.50) | | | | |
| APACHE II
scores | - | 10.87±2.38 | 20.36±4.13 | | | 15.251 | <0.001 |
Expression of serum sICAM-1 and
sRAGE
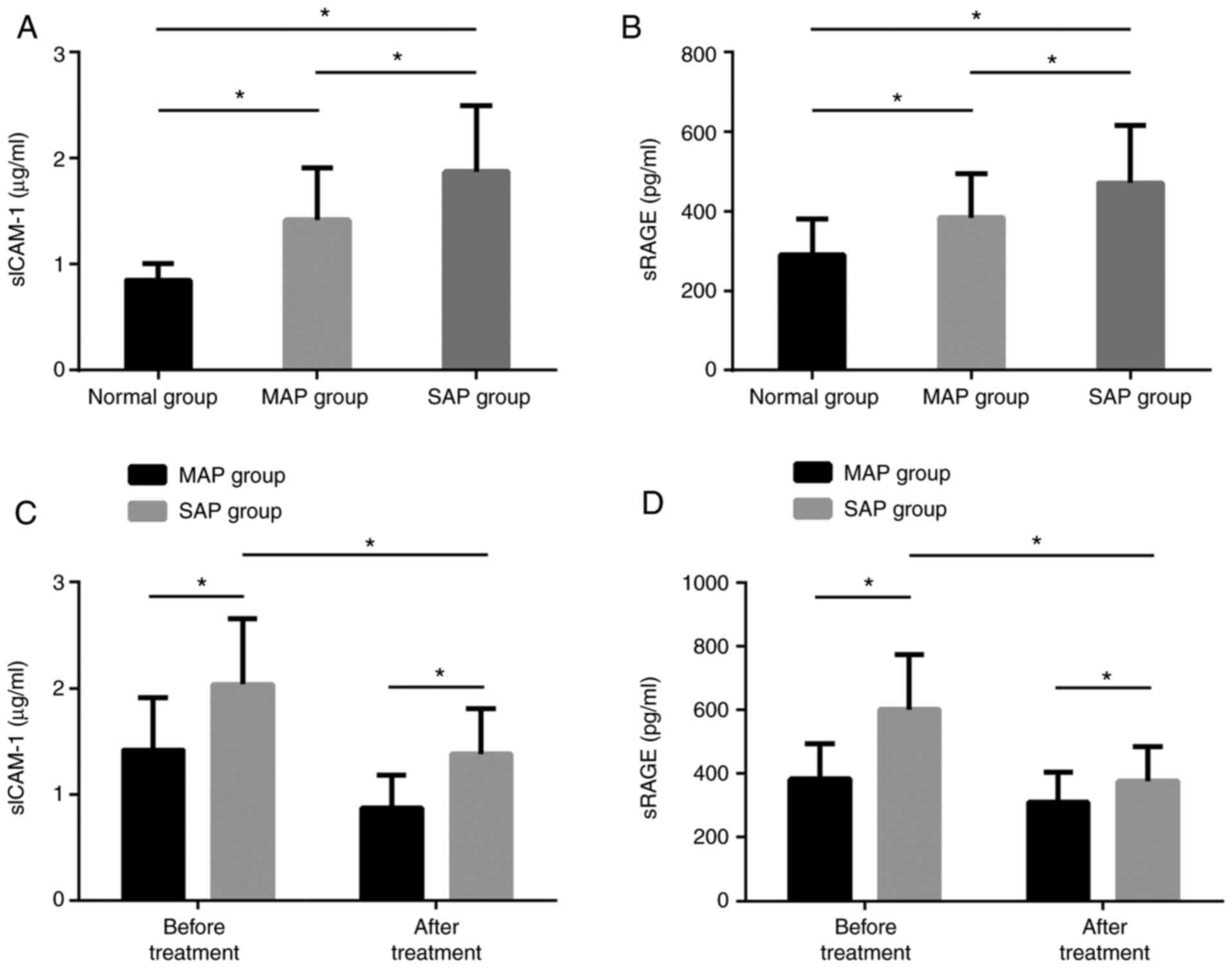
The serum sICAM-1 and sRAGE levels in the SAPG were
markedly higher than those in the MAPG and the NG, and the levels
in the MAPG were markedly higher than those in the NG, with
statistically marked differences (P<0.05). Before treatment, the
two levels in the the SAPG were markedly higher than those in the
MAPG. After treatment, the levels in the SAPG were dramatically
lower than those before treatment, but still higher than those in
the MAPG, with statistically marked differences (P<0.05)
(Fig. 1).
Diagnostic value of serum sICAM-1 and
sRAGE
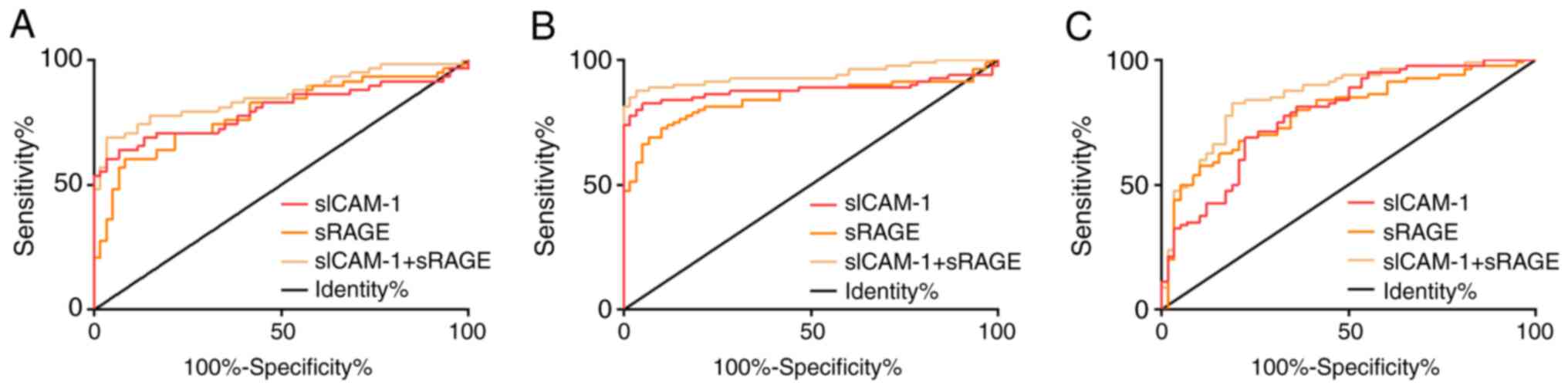
After conducting ROC curve analysis, we found that
the AUC value of serum sICAM-1 in diagnosing NG and MAPG was 0.799,
and the best cut-off value was 1.12 µg/ml; the AUC value of serum
sRAGE in diagnosing NG and MAPG was 0.786, and the best cut-off
value was 393.10 pg/ml; the AUC value of serum sICAM-1 combined
with sRAGE in diagnosing NG and MAPG was 0.857, and the best
cut-off value was 0.61; AUC value of serum sICAM-1 in diagnosing NG
and SAPG was 0.879, and the best cut-off value was 1.17 µg/ml; AUC
value of serum sRAGE in diagnosing NG and SAPG was 0.844, and the
best cut-off value was 372.00 pg/ml; AUC value of serum sICAM-1
combined with sRAGE in diagnosing NG and SAPG was 0.939, and the
best cut-off value was 0.55; AUC value of serum sICAM-1 in
diagnosing MAPG and SAPG was 0.783, and the best cut-off value was
1.70 µg/ml; AUC value of serum sRAGE in diagnosing MAPG and SAPG
was 0.790, and the best cut-off value was 494.70 pg/ml; AUC value
of serum sICAM-1 combined with sRAGE in diagnosing MAPG and SAPG
was 0.856, and the best cut-off value was 0.57 (Fig. 2, Table
II).
 | Table IIROC parameters of each group. |
Table II
ROC parameters of each group.
| Group | Indicators | AUC | 95% CI | Standard error | Cut-off value | Sensitivity
(%) | Specificity
(%) |
|---|
| NG and MAPG | sICAM-1 | 0.799 | 0.714-0.885 | 0.044 | 1.12 µg/ml | 63.79 | 93.33 |
| | sRAGE | 0.786 | 0.702-0.871 | 0.043 | 393.10 pg/ml | 60.34 | 91.67 |
| | sICAM-1+sRAGE | 0.857 | 0.787-0.927 | 0.036 | 0.61 | 68.97 | 96.67 |
| NG and SAPG | sICAM-1 | 0.879 | 0.815-0.944 | 0.033 | 1.17 µg/ml | 82.50 | 95.00 |
| | sRAGE | 0.844 | 0.777-0.912 | 0.035 | 372.00 pg/ml | 72.50 | 90.00 |
| | sICAM-1+sRAGE | 0.939 | 0.897-0.981 | 0.021 | 0.55 | 87.50 | 96.67 |
| MAPG and SAPG | sICAM-1 | 0.783 | 0.706-0.861 | 0.040 | 1.70 µg/ml | 68.75 | 77.59 |
| | sRAGE | 0.790 | 0.714-0.866 | 0.039 | 494.70 pg/ml | 57.50 | 89.66 |
| | sICAM-1+sRAGE | 0.856 | 0.791-0.920 | 0.033 | 0.57 | 82.50 | 81.03 |
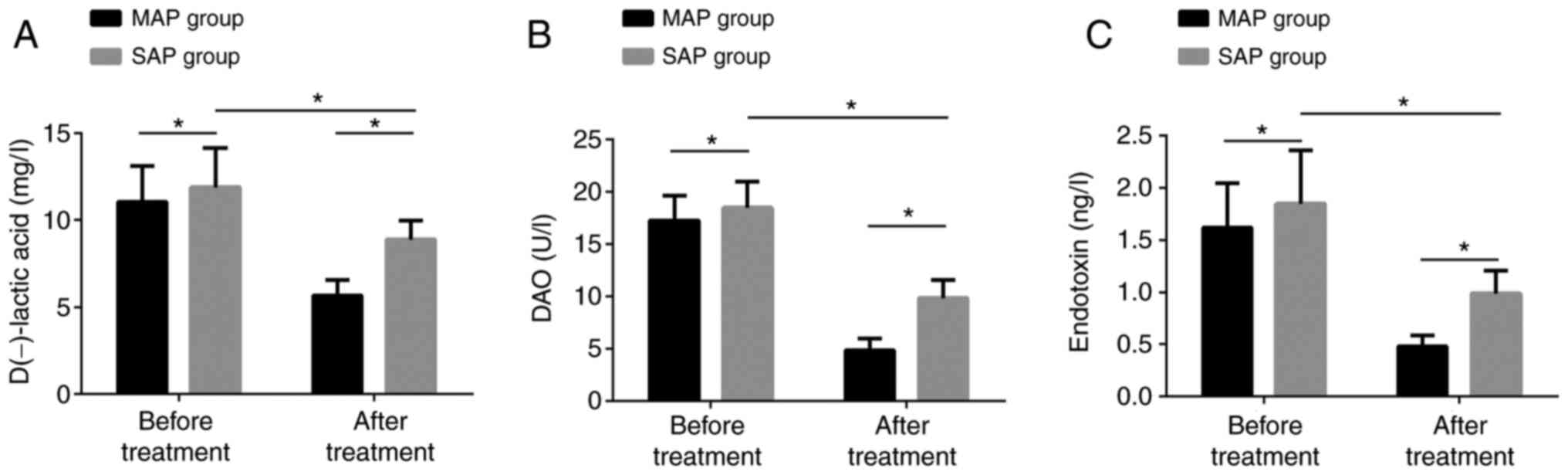
Changes of intestinal mucosal barrier
function before and after treatment
Before treatment, the concentrations of D-lactic
acid, DAO and endotoxin in the SAPG were dramatically higher than
those in the MAPG. After treatment, the concentrations in the SAPG
were obviously lower than those before treatment, but still higher
than those in the MAPG. The differences were statistically obvious
(P<0.05) (Fig. 3).
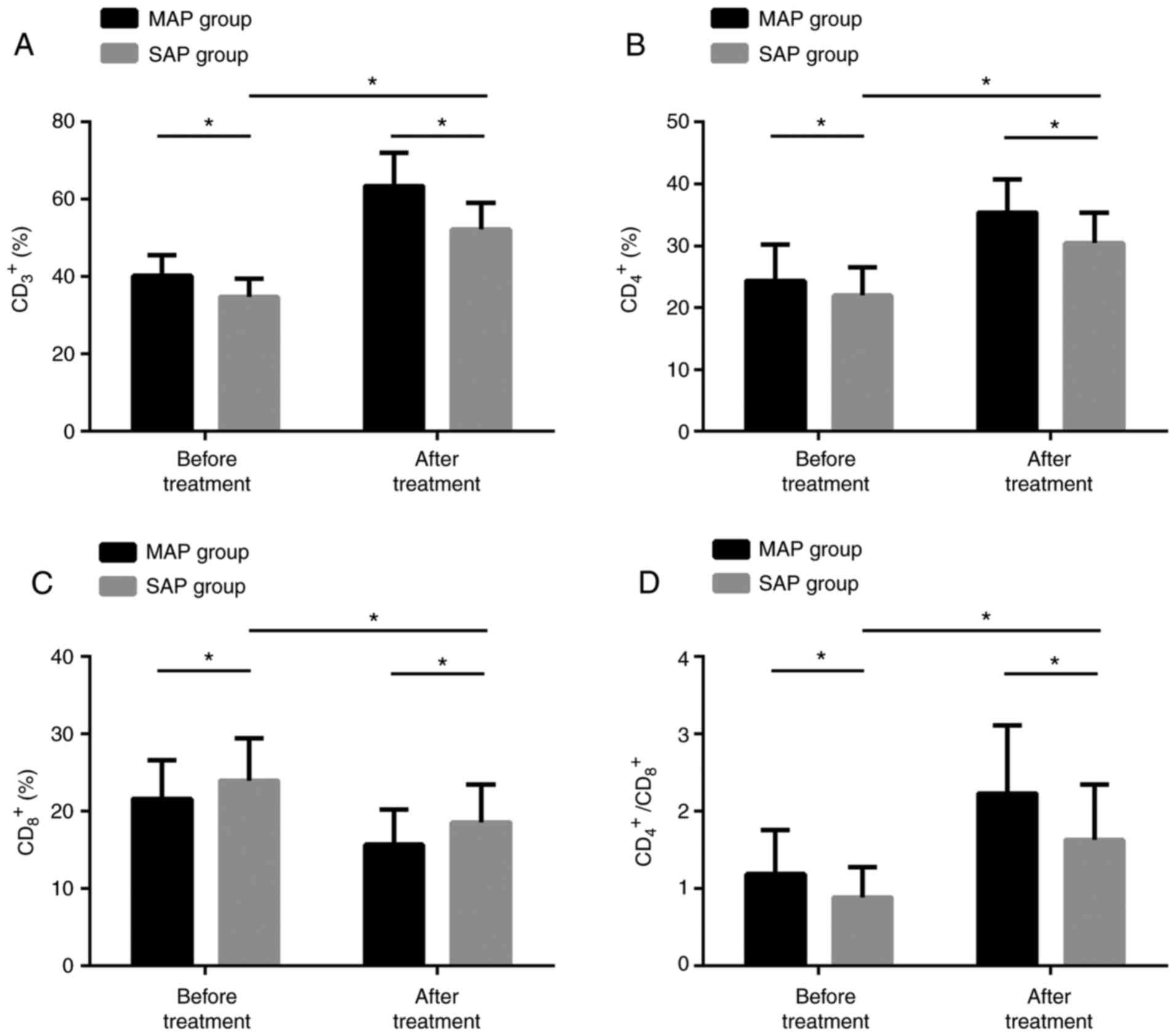
Changes of T-lymphocyte subsets before
and after treatment
Prior to treatment, CD3+,
CD4+, CD4+/CD8+ in the SAPG were
remarkably lower than those in the MAPG. After treatment, the three
in the SAPG were dramatically higher than those before treatment,
but still lower than those in the MAPG, while CD8+
results were contrary to the other three. The difference was
statistically marked (P<0.05) (Fig.
4).
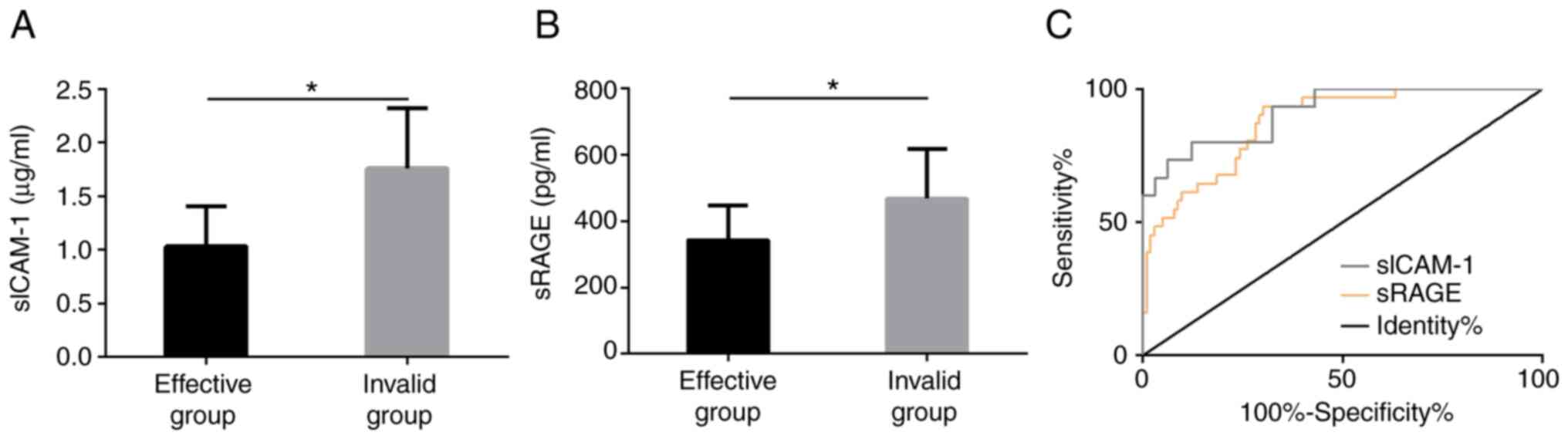
Predictive value of serum sICAM-1 and
sRAGE on efficacy
We divided AP patients into the EG and IG according
to different efficacy of patients, including 103 cases with
effective treatment (43 markedly effective and 60 effective cases)
and 31 with ineffective treatment. The results showed that the
sICAM-1 and sRAGE levels in serum of patients in EG were obviously
lower than those in IG before treatment, and the difference was
statistically marked (P<0.05). ROC analysis manifested that the
predicted AUC of sICAM-1 for ineffective treatment was 0.920, and
the predicted AUC of sRAGE for ineffective treatment was 0.874, all
of which had good predictive value (Fig. 5).
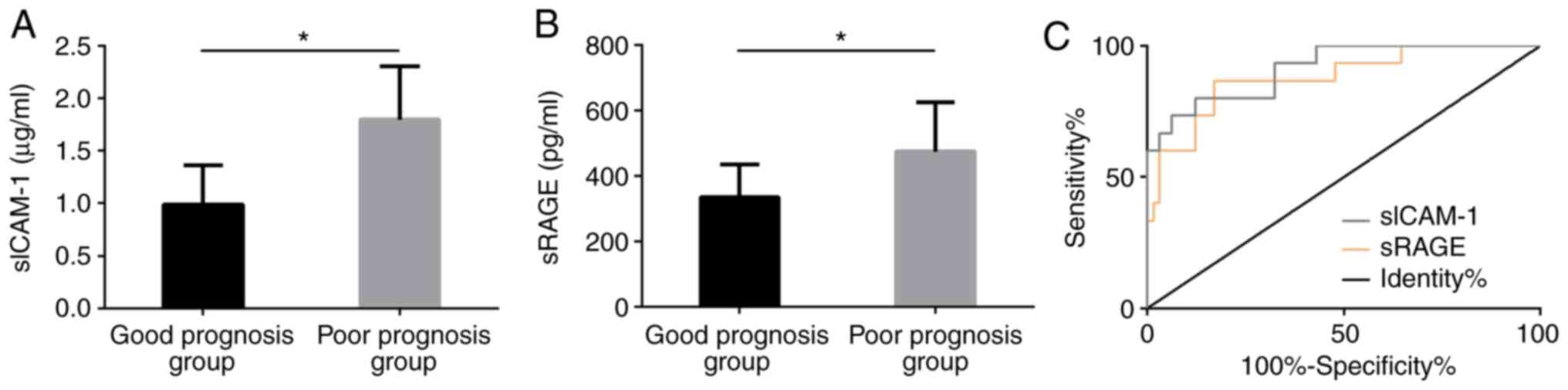
Prognostic value of serum sICAM-1 and
sRAGE in SAP patients
Based on whether SAP patients died within 30 days,
65 survivals were included in the GPG and the dead (15 individuals)
were included in the PPG. After detecting the sICAM-1 and sRAGE
levels in the serum of patients in both groups, we found that the
levels in the serum of the GPG after treatment were markedly lower
than those in the PPG, with statistically significant differences
(P<0.05). After drawing ROC curve, we found that sICAM-1 had a
good predictive value (0.914) for poor therapeutic prognosis in SAP
patients and sRAGE also had a good predictive value (0.879) for
their poor therapeutic prognosis (Fig.
6).
Univariate analysis of poor prognosis
in SAP patients
After univariate analysis of patients in the GPG and
the PPG, we found that there was no obvious difference in the
average age, sex, etiology and other aspects (P>0.05). However,
there were marked differences in multiple organ failure (MOF),
acute respiratory distress syndrome (ARDS), acute renal failure
(ARF), shock, liver function damage, sICAM-1, and sRAGE (P<0.05)
(Table III).
 | Table IIIUnivariate analysis of poor prognosis
in SAP patients [n (%), mean ± SD]. |
Table III
Univariate analysis of poor prognosis
in SAP patients [n (%), mean ± SD].
| Factor | Good prognosis
group (GPG) (n=65) | Poor prognosis
group (PPG) (n=15) |
χ2/t | P-value |
|---|
| Average age
(years) | | | 3.096 | 0.079 |
|
<45 | 38 (58.46) | 5 (33.33) | | |
|
≥45 | 27 (41.54) | 10 (66.67) | | |
| Sex | | | 1.141 | 0.286 |
|
Male | 48 (73.85) | 9 (60.00) | | |
|
Female | 17 (26.15) | 6 (40.00) | | |
| Etiology | | | 2.881 | 0.237 |
|
Biliary
pancreatitis | 17 (26.15) | 7 (46.67) | | |
|
Alcoholic
pancreatitis | 12 (18.46) | 3 (20.00) | | |
|
Rests | 36 (55.39) | 5 (33.33) | | |
| MOF | | | 13.396 | <0.001 |
|
No | 10 (15.38) | 9 (60.00) | | |
|
Yes | 55 (84.62) | 6 (40.00) | | |
| ARDS | | | 5.970 | 0.015 |
|
No | 59 (90.77) | 10 (66.67) | | |
|
Yes | 6 (9.23) | 5 (33.33) | | |
| ARF | | | 19.704 | <0.001 |
|
No | 62 (95.38) | 8 (53.33) | | |
|
Yes | 3 (4.62) | 7 (46.67) | | |
| Shock | | | 4.867 | 0.027 |
|
No | 58 (89.23) | 10 (66.67) | | |
|
Yes | 7 (10.77) | 5 (33.33) | | |
| Liver function
damage | | | 12.062 | <0.001 |
|
No | 54 (83.08) | 6 (40.00) | | |
|
Yes | 11 (16.92) | 9 (60.00) | | |
| sICAM-1
(µg/ml) | 0.99±0.37 | 1.80±0.51 | 7.091 | <0.001 |
| sRAGE (pg/ml) | 334.84±100.98 | 475.16±150.39 | 4.394 | <0.001 |
Multivariate analysis of poor
prognosis in SAP patients
We analyzed MOF, ARDS, ARF, shock, liver function
damage, sICAM-1, and sRAGE, and listed them as dependent variables
for assignment. Whether there was a poor prognosis was taken as
dependent variables, and logistic regression model was used for
multivariate analysis. The results revealed that MOF, ARDS, ARF,
shock, liver function damage, sICAM-1, and sRAGE were independent
risk factors for poor prognosis (Tables IV and V).
 | Table IVMultivariate logistic regression
analysis assignment. |
Table IV
Multivariate logistic regression
analysis assignment.
| Factor | Variable | Assignment |
|---|
| MOF | X1 | No=0, yes=1 |
| ARDS | X2 | No=0, yes=1 |
| ARF | X3 | No=0, yes=1 |
| Shock | X4 | No=0, yes=1 |
| Liver function
damage | X5 | No=0, yes=1 |
| sICAM-1
(µg/ml) | X6 | The data belong to
continuous variables and are analyzed with original data. |
| sRAGE (pg/ml) | X7 | The data belong to
continuous variables and are analyzed with original data. |
 | Table VMultivariate analysis of poor
prognosis in patients with ASP. |
Table V
Multivariate analysis of poor
prognosis in patients with ASP.
| Factor | β | SE | Wald | P-value | OR | 95% CI |
|---|
| MOF | 0.169 | 0.077 | 4.997 | 0.023 | 1.962 | 1.087-1.347 |
| ARDS | 0.621 | 0.252 | 6.835 | 0.016 | 1.832 | 1.103-2.874 |
| ARF | 1.161 | 0.507 | 4.768 | 0.019 | 2.013 | 1.348-5.371 |
| Shock | 0.338 | 0.108 | 9.935 | 0.032 | 1.399 | 1.137-1.736 |
| Liver function
damage | 0.020 | 0.066 | 3.692 | 0.020 | 1.389 | 1.021-1.710 |
| sICAM-1
(µg/ml) | 1.247 | 0.507 | 4.768 | 0.001 | 3.201 | 1.235-8.672 |
| sRAGE (pg/ml) | 0.756 | 0.298 | 6.584 | 0.012 | 2.217 | 1.231-3.816 |
Discussion
AP is an acute abdominal disease that can affect the
function of multiple organs from local pancreatic damage. It is
caused by pancreatic duct blockage, alcoholism, cationic
trypsinogen gene mutation and other factors (24,25).
The minimal organ dysfunction accompanying MAP causes frequent
attacks of AP, which may eventually progress to SAP or even
pancreatic cancer (26). SAP is a
severe acute inflammatory reaction of AP. The onset symptom is
severe acute abdominal pain, and the prognosis is often poor
(27). The study aimed to
ameliorate the poor prognosis of SAP.
AP patients were treated by Glu combined with UTI.
We collected the serum of patients after admission (before
treatment) to evaluate the predictive value of sICAM-1 and sRAGE on
efficacy, which had important guiding value for the matching degree
and adaptability of patients before treatment. In addition, we
collected the serum levels of sICAM-1 and sRAGE 7 days after
treatment to analyze their predictive value for the prognosis of
SAP patients under this treatment, which was of great significance
to evaluate their potential as prognostic indicators for those
under the treatment of Glu combined with UTI. Glu is a crucial free
amino acid for maintaining intestinal mucosal barrier function and
regulating immune response in human body. Based on SAP's large loss
of UTI in vivo (28), we
used Glu to supplement exogenous nutrition for patients. It was
reported that UTI could repair SAP tissue damage and maintain
immune homeostasis by regulating regulatory T cells (29). However, for AP patients, prediction
of the severity of the disease and the efficacy of drug therapy
still had timeliness and heterogeneity. It was necessary to detect
other indicators to predict the condition, efficacy and prognosis
of patients, so as to timely change the treatment plan and improve
their prognosis. ICAM-1 is a cell surface protein that can
participate in cell adhesion, inflammation, immune response and
biological processes related to cancer. sICAM-1 is a soluble form
of ICAM-1 and plays an immunomodulatory role due to its binding
with antigen 1 molecule tied to lymphocyte function (30,31).
RAGE is a member of the pattern recognition receptor
family, which can activate the inflammation regulation pathway by
recognizing its ligand, and mediate cell migration, adhesion and
production of pro-inflammatory molecules. As its soluble receptor,
sRAGE's high expression is relevant to ligand accumulation at the
injured site (32). Zhao et
al (20) confirmed that the
sRAGE expression in SAP patients was obviously higher than that of
moderate MAP group (MSAPG), MAPG and healthy control group (HCG).
AUC of sRAGE for predicting AP severity was 0.8304, and the
sensitivity and specificity were 91 and 81%, respectively,
suggesting that sRAGE had potential as an early diagnostic
marker.
This research revealed that the serum sICAM-1 and
sRAGE expression in the SAPG, MAPG and NG decreased markedly in
turn. After treatment, the expression in the SAPG decreased
compared with that before treatment, but was still higher than that
in the MAPG, indicating that the two had potential value for early
identification of AP disease. AUC values of serum sICAM-1 and sRAGE
for combined diagnosis of NG and MAPG, NG and SAPG, MAPG and SAPG
were 0.857, 0.939 and 0.856, respectively, suggesting that serum
sICAM-1 and sRAGE had higher predictive value for the combined
diagnosis of AP patients and had the highest discriminating ability
for NG and SAPG. After treatment, the injury indexes of intestinal
mucosal barrier such as D-lactic acid, diamine oxidase (DAO) and
endotoxin in the SAPG and the MAPG decreased obviously compared
with those before treatment, but the indexes in the SAPG were
higher than those in the MAPG, which indicated that Glu combined
with UTI had different repair abilities for intestinal mucosal
barrier in AP patients. Our immune function index test manifested
that CD3+, CD4+, and
CD4+/CD8+ in the SAPG and the MAPG were
remarkably higher after treatment than before treatment, but the
index in the SAPG was lower than that in the MAPG, while
CD8+ was opposite, indicating that Glu combined with UTI
had improved immune function of AP patients to varying degrees.
Research on efficacy prediction indicated that the sICAM-1 and
sRAGE levels in the serum of patients in the EG were markedly lower
than those in the IG before treatment. AUC of sICAM-1 and sRAGE for
predicting ineffective treatment were 0.920 and 0.874,
respectively, which meant that the two had high predictive value
for efficacy. Research on prognosis represented that the levels of
serum sICAM-1 and sRAGE of patients in the GPG after treatment were
dramatically lower than those in the PPG. AUC of sICAM-1 and sRAGE
for predicting poor prognosis after treatment were 0.914 and 0.879,
respectively, which indicated that the two had high predictive
value. Park et al (33)
suggested that the AUC of APACHE II score for differentiating MAP
from SAP and predicting the adverse prognosis of AP were 0.800 and
0.870, respectively. We believe that serum sICAM-1 and sRAGE can
play a great role in this aspect if they are used as biological
complementary indicators of APACHE II. Finally, we performed
univariate and multivariate analysis on poor prognosis, and found
that MOF, ARDS, ARF, shock, liver function damage, sICAM-1, and
sRAGE were independent risk factors.
In summary, although results of the present study
confirmed that serum sICAM-1 and sRAGE had high predictive value
for the early diagnosis, efficacy and prognosis of SAP patients
treated with Glu and UTI; elevation of sICAM-1 and sRAGE were
independent predictive factors for poor prognosis; but there is
still some room for improvement. Firstly, we can increase the
sample size of patients and improve the accuracy and universality
of research. Secondly, we can increase sICAM-1 and sRAGE's
mechanism in SAP and MAP in our basic experiments in the
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and SB conceived and designed the study. LS and
MZ were responsible for the collection and analysis of the
experimental data. LS and SB interpreted the data and drafted the
manuscript. LS wrote the manuscript. SB and MZ revised the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yidu Central Hospital of Weifang, China. Patients who participated
in this research signed the informed consent and had complete
clinical data. Signed written informed consents were obtained from
the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Dijk SM, Hallensleben NDL, van
Santvoort HC, Fockens P, van Goor H, Bruno MJ and Besselink MG:
Dutch Pancreatitis Study Group. Acute pancreatitis: Recent advances
through randomised trials. Gut. 66:2024–2032. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vege SS, DiMagno MJ, Forsmark CE, Martel M
and Barkun AN: Initial medical treatment of acute pancreatitis:
American gastroenterological association institute technical
review. Gastroenterology. 154:1103–1139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mole DJ, Webster SP, Uings I, Zheng X,
Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B,
et al: Kynurenine-3-monooxygenase inhibition prevents multiple
organ failure in rodent models of acute pancreatitis. Nat Med.
22:202–209. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Maheshwari R and Subramanian RM: Severe
acute pancreatitis and necrotizing pancreatitis. Crit Care Clin.
32:279–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nesvaderani M, Eslick GD and Cox MR: Acute
pancreatitis: Update on management. Med J Aust. 202:420–423.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shah AP, Mourad MM and Bramhall SR: Acute
pancreatitis: Current perspectives on diagnosis and management. J
Inflamm Res. 11:77–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shamoon M, Deng Y, Chen YQ, Bhatia M and
Sun J: Therapeutic implications of innate immune system in acute
pancreatitis. Expert Opin Ther Targets. 20:73–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang B, Wu G, Zhou Z, Dai Z, Sun Y, Ji Y,
Li W, Wang W, Liu C, Han F and Wu Z: Glutamine and intestinal
barrier function. Amino Acids. 47:2143–2154. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cruzat V, Macedo Rogero M, Noel Keane K,
Curi R and Newsholme P: Glutamine: Metabolism and immune function,
supplementation and clinical translation. Nutrients.
10(1564)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alhan E, Usta A, Türkyılmaz S, Kural BV
and Erçin C: Effects of glutamine alone on the acute necrotizing
pancreatitis in rats. J Surg Res. 193:161–167. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan LL, Li J, Shamoon M, Bhatia M and Sun
J: Recent advances on nutrition in treatment of acute pancreatitis.
Front Immunol. 8(762)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yong L, Lu QP, Liu SH and Fan H: Efficacy
of glutamine-enriched nutrition support for patients with severe
acute pancreatitis: A meta-Analysis. JPEN J Parenter Enteral Nutr.
40:83–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu B, Huang W, Xiao X, Xu Y, Ma S and Xia
Z: Neuroprotective effect of ulinastatin on spinal cord
ischemia-reperfusion injury in rabbits. Oxid Med Cell Longev.
2015(624819)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu R, Jiang S, Zhou H and Jin W: Clinical
efficacy of ulinastatin combined with somatostatin for treatment of
severe acute pancreatitis and effects on immune function. Int J
Clin Exp Med. 12:11333–11341. 2019.
|
|
15
|
Li C, Ma D, Chen M, Zhang L, Zhang L,
Zhang J, Qu X and Wang C: Ulinastatin attenuates LPS-induced human
endothelial cells oxidative damage through suppressing JNK/c-Jun
signaling pathway. Biochem Biophys Res Commun. 474:572–578.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wei F, Liu SY, Luo L, Gu N, Zeng Y, Chen
X, Xu S and Zhang D: Anti-inflammatory mechanism of ulinastatin:
Inhibiting the hyperpermeability of vascular endothelial cells
induced by TNF-α via the RhoA/ROCK signal pathway. Int
Immunopharmacol. 46:220–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schellerer VS, Langheinrich MC, Zver V,
Grützmann R, Stürzl M, Gefeller O, Naschberger E and Merkel S:
Soluble intercellular adhesion molecule-1 is a prognostic marker in
colorectal carcinoma. Int J Colorectal Dis. 34:309–317.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Staubli SM, Oertli D and Nebiker CA:
Laboratory markers predicting severity of acute pancreatitis. Crit
Rev Clin Lab Sci. 52:273–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bopp C, Hofer S, Weitz J, Bierhaus A,
Nawroth PP, Martin E, Büchler MW and Weigand MA: sRAGE is elevated
in septic patients and associated with patients outcome. J Surg
Res. 147:79–83. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao B, Chen Y, Sun WW, Chen WW, Ma L,
Yang ZT, Huang J, Chen EZ, Fei J and Mao EQ: Effect of S100A12 and
soluble receptor for advanced glycation end products on the
occurrence of severe acute pancreatitis. J Dig Dis. 17:475–482.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Greenberg JA, Hsu J, Bawazeer M, Marshall
J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E and McLeod
RS: Clinical practice guideline: Management of acute pancreatitis.
Can J Surg. 59:128–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akavipat P, Thinkhamrop J, Thinkhamrop B
and Sriraj W: Acute physiology and chronic health evaluation
(APACHE) II score-the clinical predictor in neurosurgical intensive
care unit. Acta Clin Croat. 58:50–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hornbeck PV: Enzyme linked immunosorbent
assays. Curr Protoc Immunol. 110:2.1.1–2.1.23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang ZW, Meng XX and Xu P: Central role of
neutrophil in the pathogenesis of severe acute pancreatitis. J Cell
Mol Med. 19:2513–2520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Manohar M, Verma AK, Venkateshaiah SU,
Sanders NL and Mishra A: Pathogenic mechanisms of pancreatitis.
World J Gastrointest Pharmacol Ther. 8:10–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saluja A and Maitra A: Pancreatitis and
pancreatic cancer. Gastroenterology. 156:1937–1940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shen X and Li WQ: High-mobility group box
1 protein and its role in severe acute pancreatitis. World J
Gastroenterol. 21:1424–1435. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang C, Wen J, Xia M and Wang SR: Effect
of glutamine nutrition support on the intestinal mucosal barrier
function and inflammatory response in patients with severe acute
pancreatitis. J Hainan Med Univ. 23:17–21. 2017.
|
|
29
|
Pan Y, Fang H, Lu F, Pan M, Chen F, Xiong
P, Yao Y and Huang H: Ulinastatin ameliorates tissue damage of
severe acute pancreatitis through modulating regulatory T cells. J
Inflamm (Lond). 14(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kuessel L, Wenzl R, Proestling K,
Balendran S, Pateisky P, Yotova I, Yerlikaya G, Streubel B and
Husslein H: Soluble VCAM-1/soluble ICAM-1 ratio is a promising
biomarker for diagnosing endometriosis. Hum Reprod. 32:770–779.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Budnik A, Grewe M, Gyufko K and Krutmann
J: Analysis of the production of soluble ICAM-1 molecules by human
cells. Exp Hematol. 24:352–359. 1996.PubMed/NCBI
|
|
32
|
Scavello F, Zeni F, Tedesco CC, Mensà E,
Veglia F, Procopio AD, Bonfigli AR, Olivieri F and Raucci A:
Modulation of soluble receptor for advanced glycation end-products
(RAGE) isoforms and their ligands in healthy aging. Aging (Albany
NY). 11:1648–1663. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park JY, Jeon TJ, Ha TH, Hwang JT, Sinn
DH, Oh TH, Shin WC and Choi WC: Bedside index for severity in acute
pancreatitis: Comparison with other scoring systems in predicting
severity and organ failure. Hepatobiliary Pancreat Dis Int.
12:645–650. 2013.PubMed/NCBI View Article : Google Scholar
|