Introduction
Osteosarcoma (OS), a type of malignant tumour that
is common in teenagers with a worldwide incidence of 3.4 per
million people per year (1),
typically originates from mesenchymal stem cells (2,3). In
the past, amputation was primarily utilised to treat OS, but the
curative effect of this remains limited (1). At present, various treatments for OS
exist, including systemic chemotherapy, targeted drug therapy,
immunotherapy and radiotherapy (4).
However, many side effects occur from these processes, such as
leukopenia and thrombocytopenia and the survival rate of OS
patients remains less than 25% (5,6). Thus,
understanding the mechanisms that underlie OS is critical for
developing a new treatment strategy.
Long non-coding RNAs (lncRNAs) are RNAs that do not
code for a protein but do serve important roles in cellular
processes by regulating specific genes (7). Previous studies have shown that many
lncRNAs are involved in the pathogenesis of OS, such as taurine
upregulated 1 (TUG1), X-inactive specific transcript (XIST), long
intergenic non-protein coding RNA 152 and FOXD2 adjacent opposite
strand RNA 1 (FOXD2-AS1) (8-11).
Zhang et al (8) found that
downregulation of lncRNA TUG1 significantly inhibits OS cell
proliferation and promotes apoptosis. Li et al (9) reported that XIST inhibition suppresses
the proliferation and invasion of OS cells. Zhang et al
(11) showed that FOXD2-AS1
downregulation limits the proliferation, migration and invasion of
OS cells. The aforementioned lncRNAs serve as oncogenes in OS.
Furthermore, lncRNA small nucleolar RNA host gene 1 (SNHG1) has
been demonstrated to facilitate the progression of OS (12-14).
Jiang et al (13) determined
that upregulation of SNHG1 promotes OS cell proliferation and
migration and inhibits apoptosis. In agreement with this, Wang
et al (14) found that SNHG1
silencing restrains the proliferation, migration and invasion of OS
cells. However, the detailed mechanisms of action of SNHG1 on OS
still need to be deciphered.
MicroRNAs (miRNAs) are a kind of small endogenous
RNA that can influence the post-transcriptional regulation of
specific genes (15). Increasing
attention has been paid to the anti-tumoral roles of miRNAs in OS,
such as miRNA (miR)-206(16),
miR-137(9), and miR-193-3p
(10). miR-424-5p is widely
considered to be a suppressor in several types of human cancers,
such as glioma (17),
cholangiocarcinoma (18) and
ovarian cancer (19). Notably, the
inhibitory effect of miR-424 on the metastasis of OS cells has also
been confirmed (20). LncRNAs can
act as competitive endogenous RNAs or sponges of miRNAs. SNHG1 has
been reported to facilitate the progression of OS by regulating
many miRNAs, including miR-101-3p (12), miR-577(13) and miR-326(14). However, the regulatory relationship
between SNHG1 and miR-424 remains unclear.
In the present study, the influence of SNHG1
inhibition on the viability, migratory ability and invasive ability
of OS cells as well as the potential regulatory mechanisms of
SNHG1/miR-424-5p/FGF2 were investigated with the goal of developing
a new treatment strategy for OS.
Materials and methods
Sample collection
Between January 2016 and January 2018, 61 pairs of
OS tissue samples and adjacent normal tissues were obtained from
patients with OS (average age, 18.6 years old) at the ZhouPu
Hospital Affiliated to Shanghai University of Medicine & Health
Sciences. These patients did not receive radiotherapy or
chemotherapy before the operation. The protocols of this study were
reviewed and approved by the Ethical Committee of ZhouPu Hospital
Affiliated to Shanghai University of Medicine & Health
Sciences. All participants provided signed informed consent.
Cell grouping and transfection
The OS cell lines Saos-2, MG63, HOS and U2OS, as
well as the human osteoblast cell line hFOB1.19 were purchased from
Tongpai (Shanghai) Biotechnology Co., Ltd. The cells were cultured
in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal
bovine serum at 5% CO2, 37˚C and 95% humidity. Small
interfering (si)RNA-negative control (si-NC;
5'-UUCUCCGAACGUGUCACGUTT-3'), siRNA-SNHG1-1 (si-SNHG1-1,
5'-CAGCAGTTGAGGGTTTGCTGTGTAT-3') and si-SNHG-2
(5'-TTCAACAGCTAGGTTGTCCTT-3') were purchased from Sangon Biotech
Co., Ltd. Overexpression vectors pcDNA-FGF2, pcDNA-SNHG1 and empty
vector (pcDNA-NC), along with miR-424-5p mimics, miRNA mimics-NC
(miR-NC), miR-424-5p inhibitor and inhibitor NC were all procured
from Guangzhou RiboBio Co., Ltd. The aforementioned agents (all, 50
nM) were transfected into the cells (6x105 cells/well)
using a Lipofectamine RNAiMAX kit (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h at 37˚C. Following transfection, the
cells were harvested to perform the following experiments.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract the total RNA from
tissues or cell lines. The GoScript reverse transcription system
(Promega Corporation) was used to reverse transcribe the extracted
RNA into cDNA. qPCR was performed using the SYBR Green PCR Master
mix (Takara Biotechnology Co., Ltd.). The reaction conditions were
as follows: 95˚C for 10 min; followed by 40 cycles at 94˚C for 10
sec, 60˚C for 20 sec and 72˚C for 34 sec. The data were analysed by
the 2-ΔΔCq method (21).
For normalization, GAPDH was used as endogenous control to
normalize lncRNA SNHG1 expression level and U6 was used as
endogenous control to normalize miR-424-5p expression level. The
sequences of the primers are as follows: SNHG1 forward,
5'-ACGTTGGAACCGAAGAGAGC-3' and reverse, 5'-GCAGCTGAATTCCCCAGGAT-3';
miR-424-5p forward, 5'-GGCTAGTCAGCAGCAATTCATGT-3' and reverse,
5'-GTGCAGGGTCCGAGGT-3'; FGF2 forward, 5'-AGGAGAGCGACCCACACATCAA-3'
and reverse, 5'-AGCCAGCAGTCTTCCATCTTCC-3'; U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3';
GAPDH forward, 5'-CCAGGTGGTCTCCTCTGACTT-3' and reverse,
5'-GTTGCTGTAGCCAAATTCGTTGT-3'.
MTT assay
Transfected MG63 and U2OS cells were seeded
(2x105 cells/well) into a 96-well plate and incubated
for 24, 48, 72 and 96 h. MTT (5 mg/ml; 20 µl; Sigma-Aldrich; Merck
KGaA) was added at different time points. After 2 h incubation at
37˚C, cell viability (optical density at 450 nm) was analysed using
a SpectraMax microplate spectrophotometer (Molecular Devices,
LLC).
Wound healing assay
The transfected MG63 and U2OS cells
(2x105 cells/well) were seeded into 6-well plates. When
the cells grew to a 100% confluence, wounds on the cell monolayer
were created using a sterile p200 pipette tip, and the cells were
incubated for 24 h in a serum-free medium. Subsequently, the cells
were washed three times with PBS to wash away the floating cells.
Images were captured at 0 and 24 h under a light microscope
(magnification, x400; Olympus Corporation) and analysed with ImageJ
software [version 1.46, National Institutes of Health (NIH)].
Transwell invasion assay
Cell invasion was assessed using Transwell chambers
(Corning, Inc.) that were pre-coated (at 37˚C for 30 min) with
Matrigel® (BD Biosciences). Transfected MG63 and U2OS
cells (2x105 cells/well) were resuspended in serum-free
medium and seeded into the Matrigel-coated upper chamber. A total
of 600 µl DMEM containing 10% FBS was added into the lower chamber.
After 24 h of culturing, the invasive cells were stained with 0.5%
crystal violet. Invasive ability was evaluated by counting the
number of invasive cells under a light microscope (magnification,
x400; Olympus Corporation) in five randomly selected views.
Target prediction
StarBase version 2.0 (http://starbase.sysu.edu.cn), a software that decodes
miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from
large-scale CLIP-Seq data, was used to predict the miRNA targets of
SNHG1. A total of 144 putative targets was predicted. Among these
miRNA targets, miR-424-5p was selected for the following assays
owing to its important role in OS and the unknown regulatory
relationship. In addition, TargetScan (http://www.targetscan.org), a software that predicts
effective microRNA target sites in mammalian mRNAs, was used to
predict the mRNA targets of miR-424-5p. Among the 1,515 target
mRNAs, FGF2 was selected for the following assays owing to its
important role in OS.
RNA-binding protein
immunoprecipitation assay (RIP)
RIP was conducted using the EZ-Magna RIP RNA-Binding
Protein Immunoprecipitation Kit (EMD Millipore). MG63 and U2OS
cells (5x105 cells/well) were lysed with RIPA lysis
buffer (Beyotime Institute of Biotechnology). Subsequently, the
cell extracts were incubated with RIPA buffer magnetic beads as
well as anti-Argonaute2 (AGO2) and anti-immunoglobulin G (IgG)
(Shanghai Kanglang Biotechnology Co., Ltd.) at 4˚C overnight and
then washed with RIPA buffer (Beyotime Institute of Biotechnology).
The eluates were collected, and the expression levels of SNHG1 and
miR-424-5p were detected by RT-qPCR, aforementioned.
Dual-luciferase reporter gene (DLR)
assay
The predicated binding sequences of SNHG1 (binding
sites, CCAGUGAUGAAUUGCUGCU) and corresponding mutation sequences
(GCUGUGUACUUAACGACGA) were inserted into the pGL3 vector to
establish the SNHG1-wild-type (WT)/SNHG1-mutant-type (Mut).
Similarly, The predicated binding sequences of FGF2 (binding sites,
AAAAUAUUUUGCUGCU) and corresponding mutation sequences
(UUUUUUAUAACGACGA) were inserted into pGL3 vector to construct the
FGF2-WT/FGF2-Mut. MG63 and U2OS cells (1x105 cells/well)
were then co-transfected with SNHG1-Mut/FGF2-Mut or
SNHG1-WT/FGF2-WT (80 ng) and miR-424-5p mimics/miR-NC (50 nM) at
37˚C. After 48 h of culture, a Dual-Luciferase Reporter Assay
System (Promega Corporation) was used to detect the luciferase
activity. The activity of firefly luciferase was normalized to that
of Renilla luciferase.
Western blot assay
The total protein from U2OS cells was extracted in
RIPA buffer (Beyotime Institute of Biotechnology) containing 10
mmol/l phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology); the protein concentration was detected by the BCA
Protein Assay Kit (Abcam). All steps were conducted on ice. A total
of ~30 µg protein was separated by 10% SDS-PAGE and then
transferred to a polyvinylidene fluoride membrane (EMD Millipore).
Membrane blocking was performed using 5% bovine serum albumin
(Thermo Fisher Scientific, Inc.) at room temperature. Next, the
membrane was incubated overnight at 4˚C with primary antibodies
against FGF2 (1:1,000; cat. no. ab208687; Abcam) and β-actin
(1:1,000; cat. no. ab8226; Abcam). The membrane was washed with TBS
+ Tween-20 (0.05%) three times followed by incubation with the
HRP-conjugated rabbit anti-mouse IgG secondary antibody (1:3,000;
cat. no. ab6728; Abcam) for 1 h at 37˚C. β-actin served as the
internal loading control. Chemiluminescence was examined using the
SuperSignal™ West Femto Maximum Sensitivity Substrate
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. ImageJ software (version 1.46; NIH) was utilised to
semi-quantify the image.
Statistical analysis
SPSS Statistics 22.0 software (IBM Corp.) was used
to analyse the data. The data are presented as the mean ± SD. The
comparisons between two groups were analysed by unpaired t-tests,
matched samples were compared by paired t-test, and the one-way
ANOVA was measured for more than two groups. After ANOVA analysis,
pairwise comparisons were assessed using Tukey's multiple
comparisons test. Pearson's correlation analysis was used to
determine the correlations between the expression of SNHG1 and
miR-424-5p, FGF2 and miR-424-5p, as well as SNHG1 and FGF2 in OS
tissues. P<0.05 was considered to indicate a statistically
significant difference. All experiments were conducted in
triplicate in at least three independent experiments.
Results
lncRNA SNHG1 expression is
significantly increased in OS tissues and cell lines
To evaluate whether SNHG1 influences the development
of OS, samples from 61 patients with OS were obtained and compared
with the adjacent normal tissues. The expression levels of SNHG1 in
OS tissues was found to be significantly higher compared with the
adjacent tissues (P<0.001; Fig.
1A). In addition, it was also determined that SNHG1 expression
in OS is related to its clinical stages. The expression of SNHG1 in
stage III/IV of OS was significantly higher compared with the
expression levels in stage I/II, which indicated that SNHG1
expression was related to the severity of OS (P<0.001; Fig. 1B). As presented in Table I, the patients were divided into two
groups: High and low lncRNA SNHG1 expression, using the median
expression level as the cut-off point. High and low expression
levels of SNHG1 were speculated to exhibit distinct differences
based on tumour stage. SNHG1 expression levels were also detected
in hFOB1.19 and a number of OS cell lines. SNHG1 was found to be
expressed in the four OS cell lines at significantly higher levels
compare with expression in the hFOB1.19 cells (P<0.01; Fig. 1C). MG63 and U2OS cell lines were
selected for the further experiments due to their relatively high
expression of SNHG1. These results indicated that SNHG1 may be an
onco-lncRNA in OS.
 | Table IClinicopathological characteristics
of patients with OS, and lncRNA SNHG1 expression levels in OS
tissues. |
Table I
Clinicopathological characteristics
of patients with OS, and lncRNA SNHG1 expression levels in OS
tissues.
| | lncRNA SNHG1
expression | |
|---|
| Clinicopathological
characteristic | n=61 | Low (n=30) | High (n=31) | P-value |
|---|
| Age, years | | | | 0.699 |
|
<20 | 31 | 16 | 15 | |
|
≥20 | 30 | 14 | 16 | |
| Sex | | | | 0.885 |
|
Male | 27 | 13 | 14 | |
|
Females | 34 | 17 | 17 | |
| Diameter, cm | | | | 0.683 |
|
<3 | 26 | 12 | 14 | |
|
≥3 | 35 | 18 | 17 | |
| Resection
degree | | | | 0.699 |
|
Total
resection | 31 | 16 | 15 | |
|
Subtotal
resection | 30 | 14 | 16 | |
| WHO Grade | | | | <0.001 |
|
I + II | 35 | 24 | 11 | |
|
III +
IV | 26 | 6 | 20 | |
Silencing lncRNA SNHG1 inhibits the
proliferation, migration and invasion of OS cells
Following transfection of si-SNHG1-1, si-SNHG1-2 and
si-NC into OS cells, SNHG1 expression level was detected by
RT-qPCR. The results indicated that SNHG1 expression in MG63 and
U2OS cells were downregulated after transfection with si-SNHG1-1
and SNHG1-2 compared with the si-NC group (P<0.01; Fig. 2A). Using the MTT assay, it was
confirmed that the viability was significantly inhibited 96 and 72
h after si-SNHG1-1 transfection in MG63 and U2OS cells,
respectively (P<0.05; Fig. 2B).
The migratory ability of the OS cell lines was also significantly
inhibited after SNHG1 knockdown compared with si-NC (P<0.01;
Fig. 2C). The Transwell invasion
assay showed similar results; the invasive ability of OS cells was
significantly inhibited after SNHG1 interference (P<0.01;
Fig. 2D). Together, these data
demonstrated that SNHG1 silencing may limit the proliferation,
migration and invasion of OS cells.
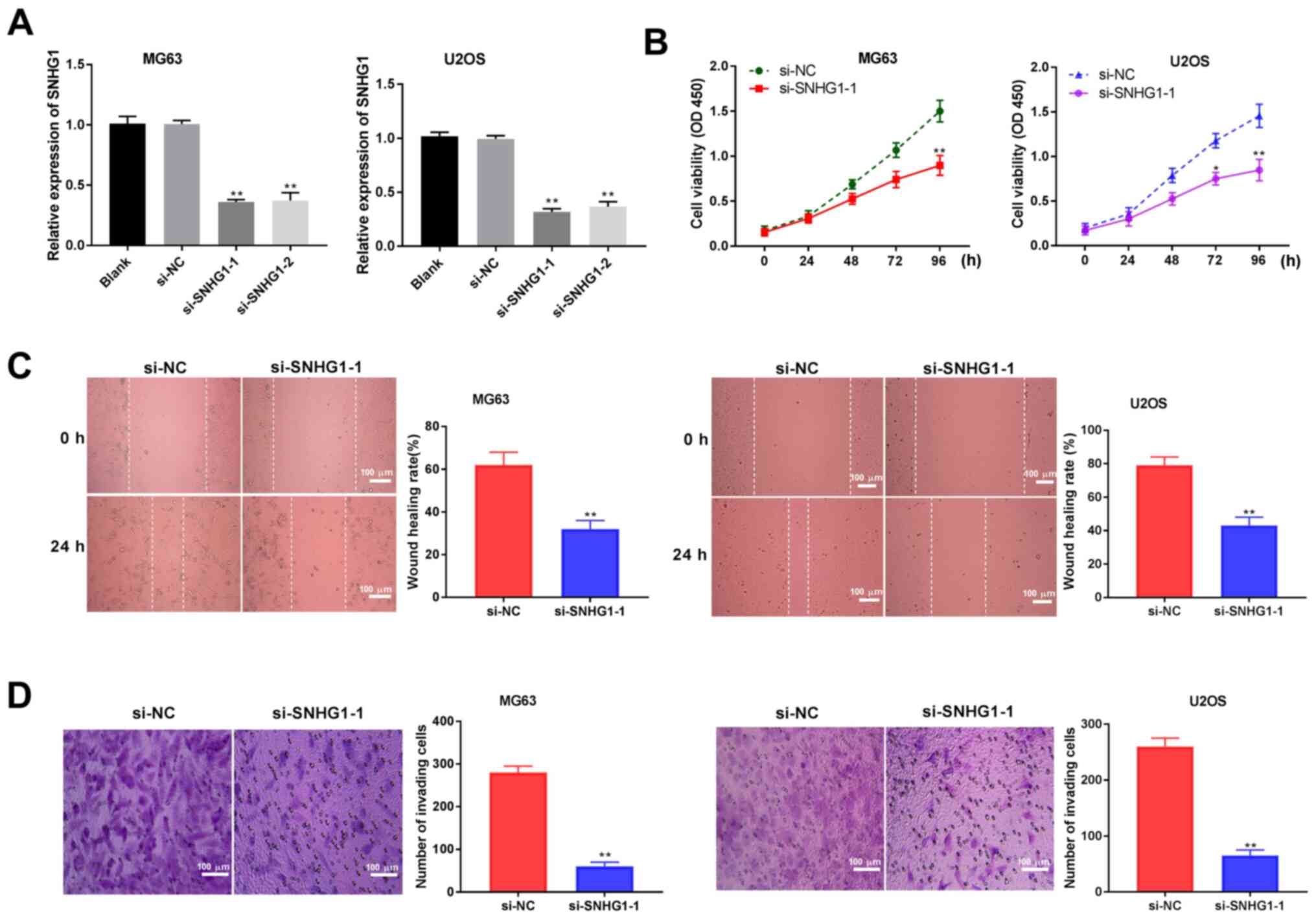 | Figure 2lncRNA SNHG1 knockdown inhibits
proliferation, migration and invasion of OS cells. (A) Reverse
transcription-quantitative PCR was used to detect the expression of
lncRNA SNHG1 after transfection of si-SNHG1-1, si-SNHG1-2 and si-NC
into MG63 and U2OS OS cells. (B) The viability of OS cells was
detected by MTT assay. (C) The migratory ability of OS cells was
determined by wound healing assay. (D) The invasive ability of OS
cells was analyzed by Transwell invasion assay. Scale bar, 100 µm;
magnification, x400. The data are expressed as the mean ± SD.
*P<0.05, **P<0.01 vs. si-NC. LncRNA,
long non-coding RNA; NC, negative control; OD, optical density; OS,
osteosarcoma; si-; small interfering RNA; SNHG1, small nucleolar
RNA host gene 1. |
lncRNA SNHG1 targets miR-424-5p
Using starBase software, the binding region between
miR-424-5p and SNHG1 was predicted (Fig. 3A). miR-424-5p expression levels were
detected after transfection of si-SNHG1-1 into MG63 and U2OS OS
cells, and the results revealed that the expression of miR-424-5p
was significantly increased in the si-SNHG1-1 group compared with
the si-NC group (P<0.01; Fig.
3B). The RIP assay demonstrated that in OS cell lines, SNHG1
was enriched with anti-AGO2 compared with those of the anti-IgG
control and that miR-424-5p exhibited similar results (P<0.01;
Fig. 3C). The DLR assays showed a
marked decrease in luciferase activity in the SNHG1 WT + miR-424-5p
mimics group compared with that of the SNHG1 WT + miR-NC group
(P<0.01; Fig. 3D). miR-424-5p
expression in the patient tumour and adjacent tissues were also
detected; RT-qPCR results demonstrated that miR-424-5p expression
levels in OS tissues decreased significantly compared with
expression levels in the adjacent tissues (P<0.001; Fig. 3E). Correlation analysis between
SNHG1 and miR-424-5p expression levels revealed that there was a
negative correlation (r=-0.3545; P=0.0051; Fig. 3F). Finally, the expression of
miR-424-5p was detected in OS and normal human osteoblast cell
lines. RT-qPCR results indicated that miR-424-5p expression levels
in the OS cells were significantly reduced compared with that of
hFOB1.19 (P<0.01; Fig. 3G).
These data indicated that miR-424-5p was the target of, and
negatively modulated by, SNHG1.
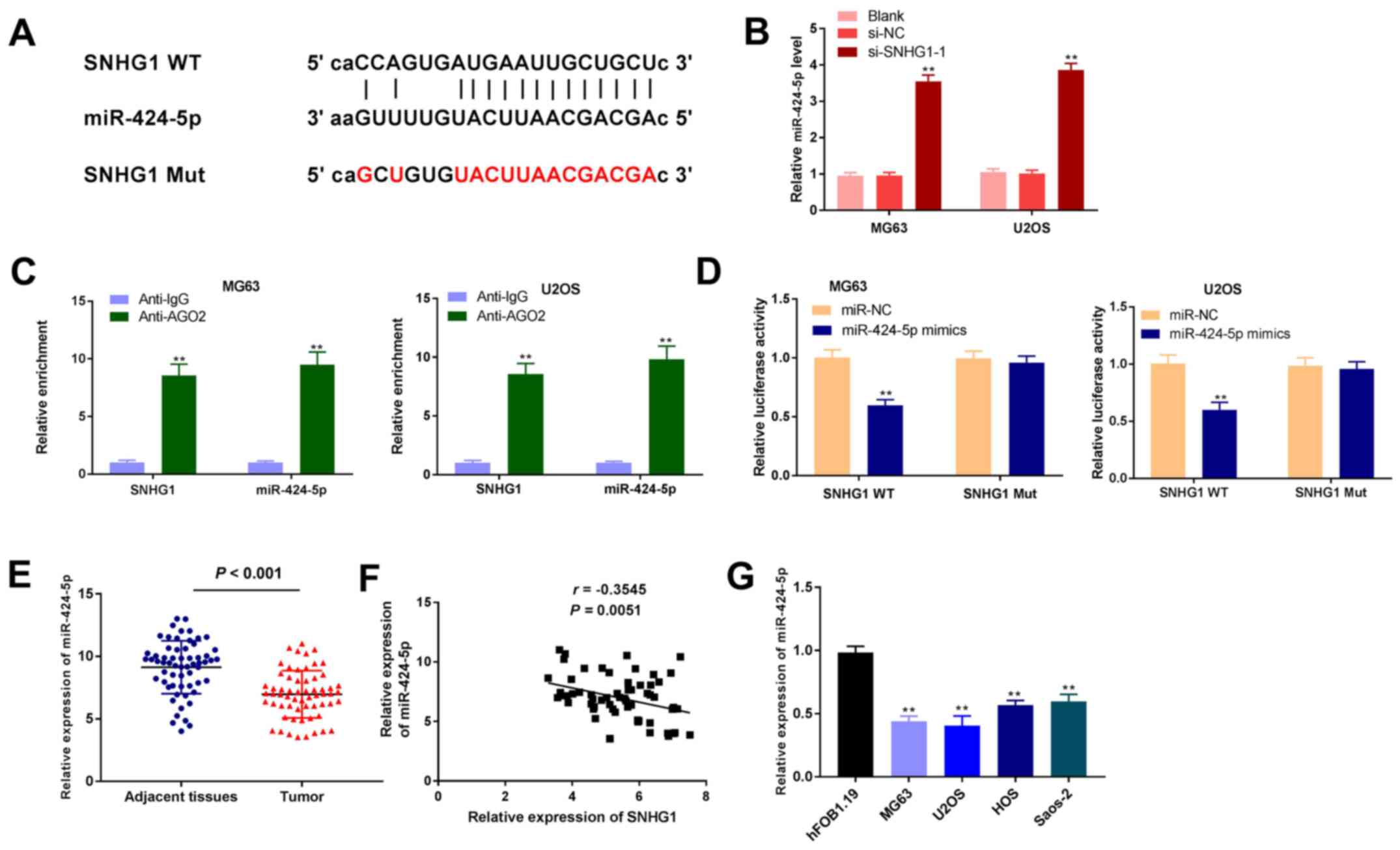 | Figure 3lncRNA SNHG1 targets miR-424-5p. (A)
starBase was used to predict the binding between lncRNA SNHG1 and
miR-424-5p. (B) RT-qPCR was used to detect the expression levels of
miR-424-5p after transfection of si-SNHG1-1 into MG63 and U2OS OS
cells. **P<0.01 vs. si-NC. (C) RNA-binding protein
immunoprecipitation assay was performed in OS cells, and the
expression of SNHG1 and miR-424-5p was detected by RT-qPCR.
**P<0.01 vs. Anti-IgG. (D) Dual-luciferase reporter
gene assays were used to confirm the targeting relationship between
SNHG1 and miR-424-5p. **P<0.01 vs. miR-NC. (E)
RT-qPCR was used to detect the expression of miR-424-5p in patient
OS and adjacent normal tissues. (F) Correlation analysis between
SNHG1 and miR-424-5p. (G) RT-qPCR was used to detect the expression
of miR-424-5p in hFOB1.19 and OS cell lines. **P<0.01
vs. hFOB1.19. The data are expressed as the mean ± SD. AGO2,
argonaute2; IgG, immunoglobulin G; lncRNA, long non-coding RNA;
miR, microRNA; Mut, mutant; NC, negative control; OS, osteosarcoma;
RT-qPCR, reverse transcription-quantitative PCR; si, small
interfering RNA; SNHG1, small nucleolar RNA host gene 1; WT,
wild-type. |
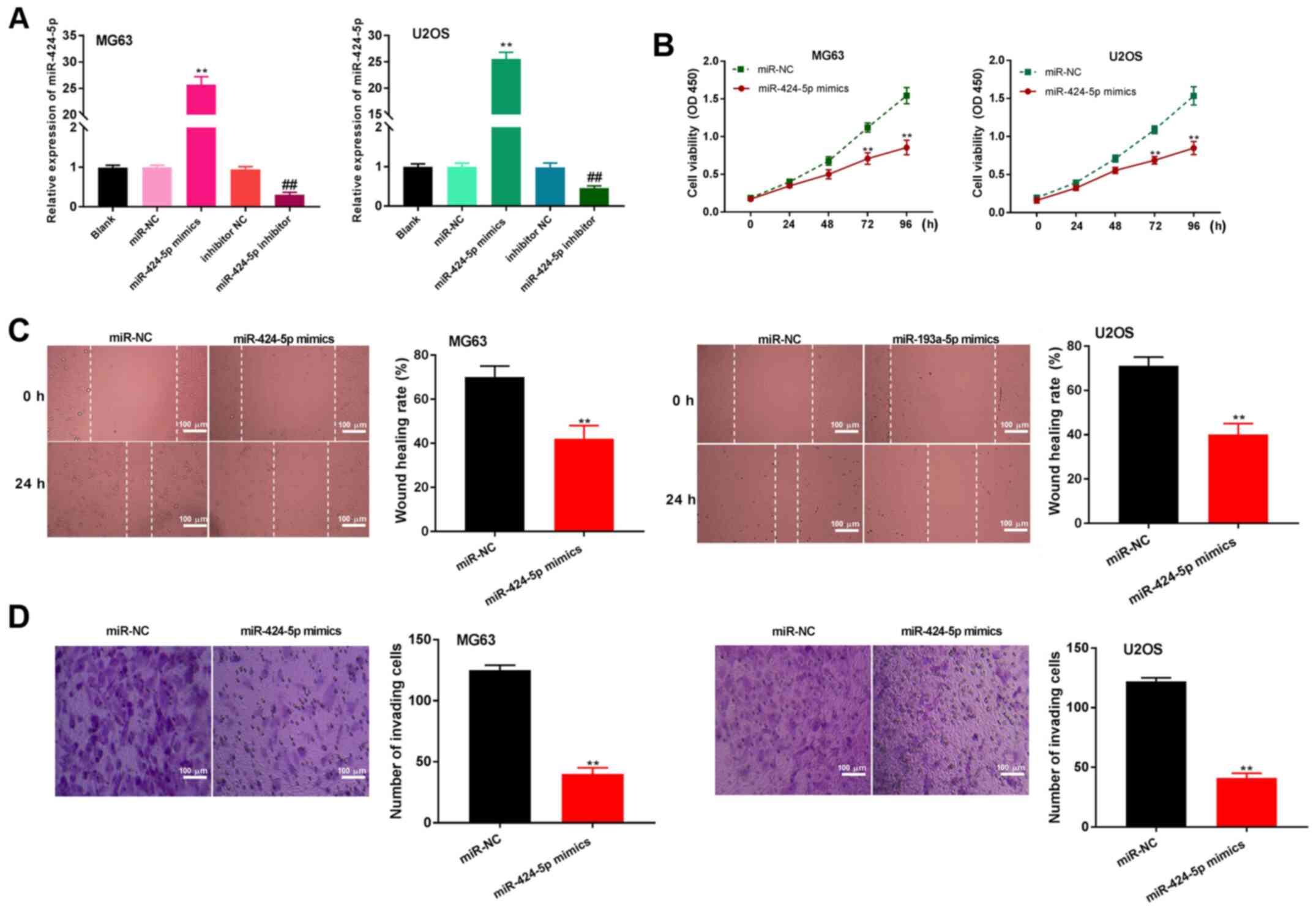
miR-424-5p upregulation limits the
proliferation, migration and invasion of OS cells
After transfection of miR-424-5p mimics, miR-NC,
miR-424-5p inhibitor or inhibitor NC into MG63 and U2OS OS cells,
miR-424-5p expression was detected. The results demonstrated that
the expression of miR-424-5p was upregulated after transfection of
miR-424-5p mimics and downregulated after transfection of the
miR-424-5p inhibitor, compared with the respective controls
(P<0.01; Fig. 4A). The MTT assay
revealed that miR-424-5p overexpression significantly suppressed
the viability of OS cells at 72 h (P<0.01; Fig. 4B). Furthermore, the wound healing
assay confirmed that upregulated miR-424-5p expression
significantly inhibited the migratory ability of OS cells
(P<0.01; Fig. 4C). miR-424-5p
overexpression also limited the number of invasive OS cells
(P<0.01; Fig. 4D). These results
indicated that overexpression of miR-424-5p inhibits the
proliferation, migration and invasion of OS cells.
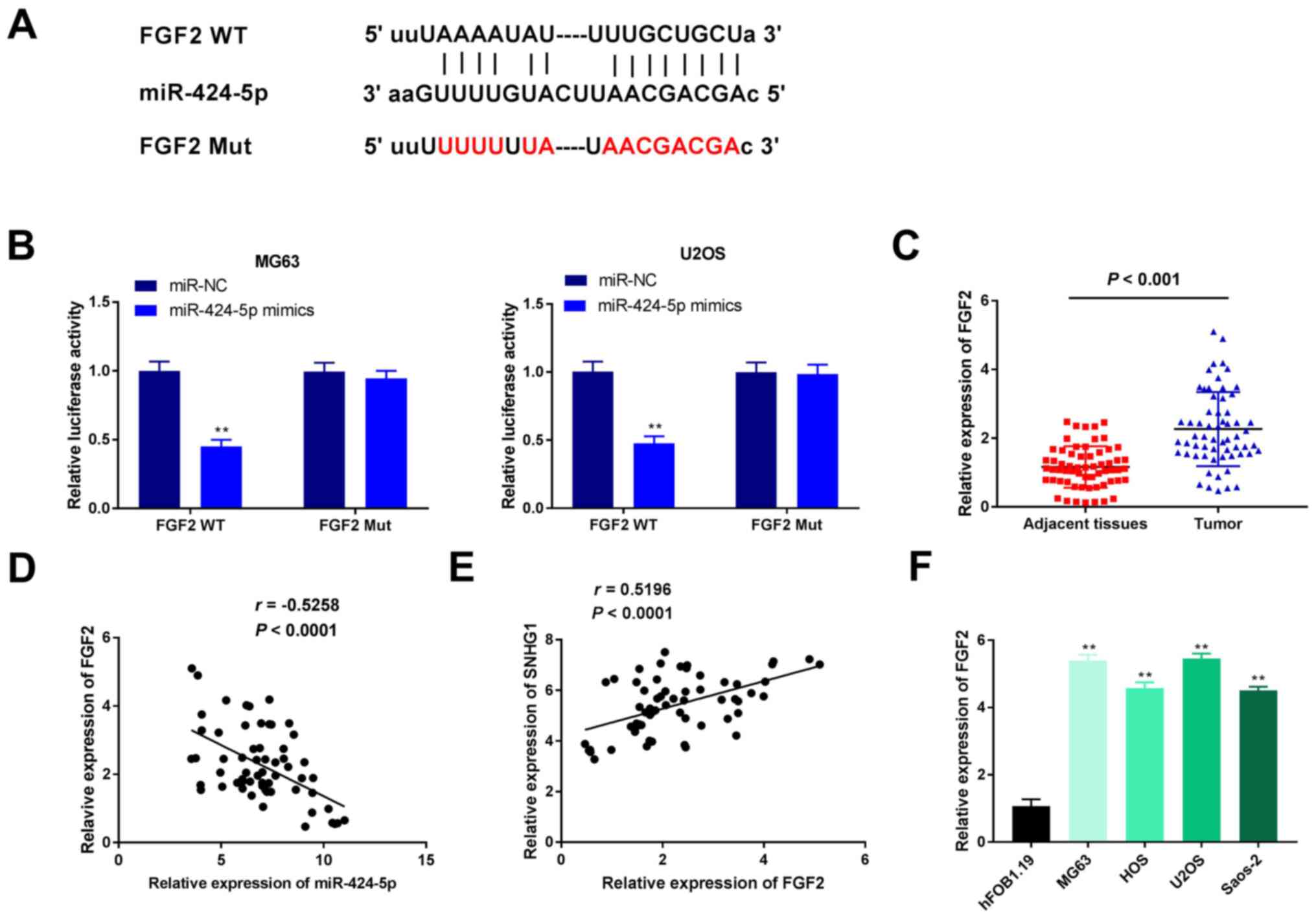
miR-424-5p targets FGF2
Using TargetScan software, FGF2 was predicted to be
a downstream target of miR-424-5p (Fig.
5A). The DLR assay revealed that in MG63 and U2OS OS cell
lines, the luciferase activity in the FGF2 WT + miR-424-5p mimics
group was significantly decreased compared with that of the FGF2 WT
+ miR-NC group, an effect that was partially reversed when SNHG1
was overexpressed (P<0.01; Fig.
5B). In addition, the expression levels of FGF2 in patient
tissue samples were examined; FGF2 expression levels in the OS
tissues were significantly higher compared with expression in the
adjacent normal tissues (P<0.001; Fig. 5C). Correlation analysis indicated
that FGF2 expression was negatively correlated with miR-424-5p
expression (r=-0.5258; P<0.0001; Fig. 5D) and positively correlated with
SNHG1 expression (r=0.5196; P<0.0001; Fig. 5E). Finally, compared with the
hFOB1.19 cells group, the expression of FGF2 in the OS cell lines
were found to be significantly higher (P<0.01; Fig. 5F). The aforementioned data suggested
that FGF2, which was highly expressed in OS, is a target of
miR-424-5p.
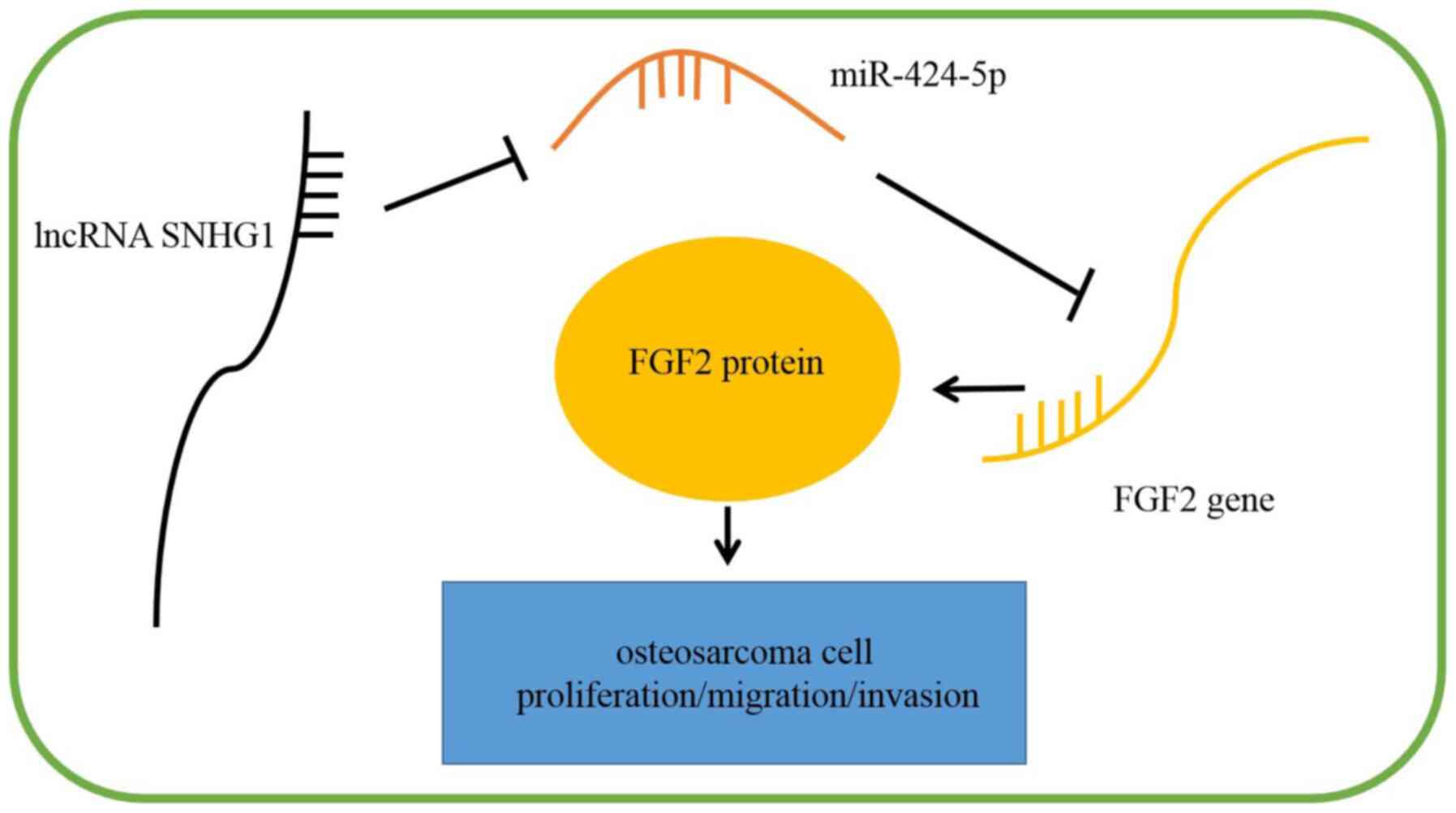
SNHG1 knockdown inhibits the
proliferation, migration and invasion of OS cells by regulating
miR-424-5p/FGF2 in vitro
The transfection efficiency of pcDNA-SNHG1 or
pcDNA-FGF2 in U2OS cells was initially determined; the expression
levels of SNHG1 were significantly increased by transfection of
pcDNA-SNHG1 in U2OS cells (P<0.01; Fig. 6A), and FGF2 expression levels were
significantly upregulated in U2OS cells transfected with pcDNA-FGF2
(P<0.01; Fig. 6B). Western blot
analysis results demonstrated that miR-424-5p overexpression in
U2OS cells significantly inhibited FGF2 expression, which could be
partially reversed by overexpression of SNHG1 (P<0.01; Fig. 6C). The MTT assay revealed that cell
viability was inhibited by depletion of SNHG1 at 96 h, but this
effect could be partly reversed by miR-424-5p inhibition or
overexpression of FGF2 (P<0.01; Fig.
6D). Similarly, the wound healing and Transwell invasion assays
demonstrated that inhibition of SNHG1 suppressed the migratory and
invasive abilities of U2OS cells, both of which could be reversed
by miR-424-5p downregulation and FGF2 upregulation (P<0.01;
Fig. 6E and F). These data indicated that SNHG1
knockdown may suppress the proliferation, migration and invasion of
OS cells by regulating the miR-424-5p/FGF2 axis (Fig. 7).
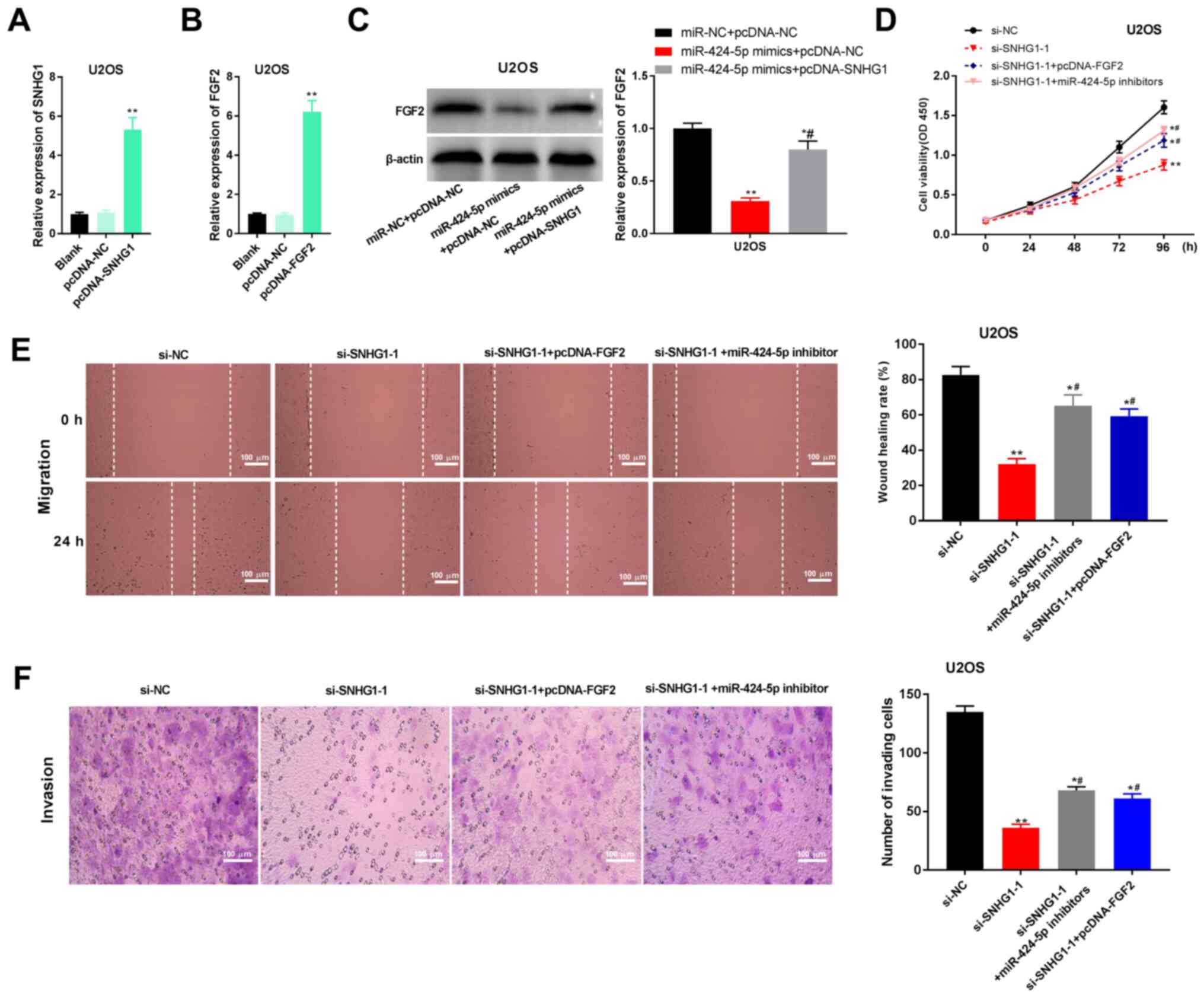 | Figure 6lncRNA SNHG1 knockdown inhibits
proliferation, migration and invasion of U2OS cells by regulating
miR-424-5p/FGF2. (A) RT-qPCR was used to detect the expression
levels of SNHG1 after transfection of pcDNA-SNHG1.
**P<0.01 vs. pcDNA-NC. (B) RT-qPCR was used to detect
the expression of FGF2 after transfection of pcDNA-FGF2.
**P<0.01 vs. pcDNA-NC. (C) Western blotting was used
to detect the protein expression levels of FGF2 in transfected U2OS
cells. *P<0.05, **P<0.01 vs. miR-NC +
pcDNA-NC; #P<0.05 vs. miR-424-5p mimics + pcDNA-NC.
(D) MTT assays were used to detect the viability of transfected
U2OS cells. *P<0.05, **P<0.01 vs.
si-NC; #P<0.05 vs. si-SNHG1-1. (E) Wound healing
assays were used to determine the migratory ability of transfected
U2OS cells. *P<0.05, **P<0.01 vs.
si-NC; #P<0.05 vs. si-SNHG1-1. (F) Transwell invasion
assays were used to analyze the invasive ability of transfected
U2OS cells. Scar bar, 100 µm; magnification, x400.
*P<0.05, **P<0.01 vs. si-NC;
#P<0.05 vs. si-SNHG1-1. The data are expressed as the
mean ± SD. FGF2, FGF2, fibroblast growth factor-2; lncRNA, long
non-coding RNA; miR, microRNA; NC, negative control; OD, optical
density; OS, osteosarcoma; si-, small interfering RNA; SNHG1, small
nucleolar RNA host gene 1. |
Discussion
OS is one of the most common malignant bone tumours
in adolescents (22). Several
lncRNAs have been reported to be involved in the regulation of OS.
For example, Fei et al (16)
discovered that expression of the lncRNA regulator of reprogramming
(ROR) is significantly increased in OS tissues and in OS cell
lines, and that upregulation of ROR appears strongly related to
tumour stage. Yang et al (23) found that the expression of lncRNA
XIST is dramatically elevated in OS tissues and also strongly
associated with tumour stage. Similarly, results from the present
study demonstrated that SNHG1 expression is elevated in OS tissues
and cell lines, and presents a notable correlation with tumour
stage. Thus, it was hypothesised that SNHG1 may act as a pathogenic
factor in OS.
In the past decade, researchers have determined that
lncRNAs function as crucial regulators in the progression of OS.
For example, Li et al (9)
reported that suppression of XIST limits the proliferation and
invasion of OS cells. Zhao et al (24) discovered that ASBEL interference
decreases viability and the migratory and invasive abilities of OS
cells. Xu et al (25) found
that SNHG4 inhibition suppresses the proliferation of OS cells. The
present study found that knockdown of SNHG1 inhibited the
viability, migratory and invasive abilities of OS cells. Similar
findings in another study demonstrated that OS cell viability,
migration and invasion is regulated by silencing SNHG1, thereby
eventually halting the progression of OS (14). Therefore, it was hypothesized that
SNHG1 inhibition may attenuate the development of OS.
miR-424 has been shown to attenuate the progression
of several cancers, including endometrial cancer (EC) (26), breast cancer (BC) (27) and colorectal cancer(CRC) (28). Dong et al (26) reported that miR-424 expression is
decreased in EC tissues and cell lines, and that upregulation of
miR-424 suppresses EC cell invasion. Wang et al (27) found that miR-424 is minimally
expressed in BC tissues and cell lines, and miR-424 upregulation
inhibits the development of BC. Fang et al (28) demonstrated that miR-424 expression
in CRC tissues and cell lines is low, and overexpression of miR-424
eventually suppresses the growth of CRC. The present study
discovered that miR-424-5p expression is significantly lower in OS
tissues and cell lines. Furthermore, OS cell viability, migration
and invasion are inhibited by miR-424-5p overexpression. Consistent
with these results, previous studies have reported that miR-424
expression is decreased in OS tissues and that miR-424
overexpression limits the viability (29), as well as the migratory and invasive
abilities (17) of OS cells. In
addition, the present study further determined that miR-424-5p is
the target of and negatively modulated by SNHG1. Therefore, it was
hypothesized that miR-424-5p may be regulated by SNHG1 to inhibit
the development of OS.
FGF2 is a member of the FGF family and has been
thought to take part in the development of various cancers
(30-32).
Zhang et al (33) found that
FGF2 is highly expressed in cervical carcinoma tissues, and Cheng
et al (34) reported that
FGF2 expression is elevated in non-small cell lung cancer tissues
and cells. The present study showed that FGF2 expression is
upregulated in OS tissues and cell lines. Similarly, Sun et
al (35) observed that FGF2
expression in OS tissues is significantly upregulated. In addition,
the present study demonstrated that miR-424-5p targeted FGF2, and
there is a notable inverse correlation between their expressions.
The results indicated that FGF2 may be negatively modulated by
miR-424-5p. The present study also found that high expression of
miR-424-5p and low expression of FGF2 both reverse the suppressive
effects of SNHG1 knockdown on the viability, migratory and invasive
abilities of OS cells. These data indicated that SNHG1 knockdown
may inhibit the viability, migration and invasion of OS cells by
regulating miR-424-5p/FGF2.
However, there are some limitations within the
present study. For instance, only miR-424-5p mimics were used in
cell culture; miR-424-5p inhibitor should be added in future
studies to further support these results. Secondly, the regulatory
effects of the SNHG1/miR-424-5p/FGF2 axis on the apoptosis of OS
cells was not determined. Furthermore, the present study was
limited to in vitro experiments, thus, additional research
using animal models is required. Finally, the detailed mechanisms
of the SNHG1/miR-424-5p/FGF2 axis on OS, such as upstream factors
and related signalling pathways, need to be further explored.
Collectively, results from the presented study
suggested that SNHG1 knockdown may suppress the progression of OS
by regulating miR-424-5p/FGF2 in vitro. Thus, the
SNHG1/miR-424-5p/FGF2 axis may present a new potential target for
treating OS.
Acknowledgements
Not applicable.
Funding
Funding: The present work is supported by the Featured Clinical
Discipline Project of Shanghai Pudong (grant no. PWYts2018-2) and
the construction of key discipline group of Sanitary System of
Shanghai Pudong New District (grant no. PWZxq2017 12).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL made substantial contributions to the conception
and design of the study. ZL, XW, and SL made substantial
contributions to the acquisition, analysis and interpretation of
data, as well as the drafting and revision of the manuscript. All
authors confirmed the authenticity of all the raw data, gave final
approval of the version to be published, and agreed to be
accountable for all aspects of the work.
Ethics approval and consent to
participate
The present study was conducted after obtaining
local Ethical Committee approval of Zibo Zhoucun People's Hospital.
Written informed consent was obtained from patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumar R, Kumar M, Malhotra K and Patel S:
Primary osteosarcoma in the elderly revisited: Current concepts in
diagnosis and treatment. Curr Oncol Rep. 20(13)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17(506)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang B and Zhang Y, Li R, Li J, Lu X and
Zhang Y: The efficacy and safety comparison of first-line
chemotherapeutic agents (high-dose methotrexate, doxorubicin,
cisplatin, and ifosfamide) for osteosarcoma: A network
meta-analysis. J Orthop Surg Res. 15(51)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP
and Yao J: Down-regulation of long non-coding RNA TUG1 inhibits
osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J
Cancer Prev. 14:2311–2315. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li H, Cui J, Xu B, He S, Yang H and Liu L:
Long non-coding RNA XIST serves an oncogenic role in osteosarcoma
by sponging miR-137. Exp Ther Med. 17:730–738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu P, He W, Lu Y and Wang Y: Long
non-coding RNA LINC00152 promotes tumorigenesis via sponging
miR-193b-3p in osteosarcoma. Oncol Lett. 18:3630–3636.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang H, Lu Y, Wang J, Zhang T, Dong C, Li
X, Wang X, Ma Q, Yang T and Zhou Y: Downregulation of the long
non-coding RNA FOXD2-AS1 inhibits cell proliferation, migration and
invasion in osteosarcoma. Mol Med Rep. 20:292–302. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Deng R, Zhang J and Chen J: lncRNA SNHG1
negatively regulates miRNA-101-3p to enhance the expression of
ROCK1 and promote cell proliferation, migration and invasion in
osteosarcoma. Int J Mol Med. 43:1157–1166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang Z, Jiang C and Fang J: Up-regulated
lnc-SNHG1 contributes to osteosarcoma progression through
sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin
pathway. Biochem Biophys Res Commun. 495:238–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fei D, Sui G, Lu Y, Tan L, Dongxu Z and
Zhang K: The long non-coding RNA-ROR promotes osteosarcoma
progression by targeting miR-206. J Cell Mol Med. 23:1865–1872.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng Z, Shu H, Cui Y, Zhang Q, Zhao B,
Pan D, Chao Q and Wang D: MiR-424-5p inhibits proliferation,
invasion and promotes apoptosis and predicts good prognosis in
glioma by directly targeting BFAR. Pathol Oncol Res. 26:2327–2335.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu JB, Yang B, Zhang Y, Feng X, He B, Xie
H, Zhou L, Wu J and Zheng S: miR-424-5p represses the metastasis
and invasion of intrahepatic cholangiocarcinoma by targeting ARK5.
Int J Biol Sci. 15:1591–1599. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang K, Zhu G, Bao S and Chen S: Long
non-coding RNA LINC00511 mediates the effects of ESR1 on
proliferation and invasion of ovarian cancer through miR-424-5p and
miR-370-5p. Cancer Manag Res. 11:10807–10819. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Long XH, Mao JH, Peng AF, Zhou Y, Huang SH
and Liu ZL: Tumor suppressive microRNA-424 inhibits osteosarcoma
cell migration and invasion via targeting fatty acid synthase. Exp
Ther Med. 5:1048–1052. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang Y, Liu W, He B, Wang L, Zhang F, Shu
H and Sun L: Exosomes derived from bone marrow mesenchymal stem
cells promote osteosarcoma development by activating oncogenic
autophagy. J Bone Oncol. 21(100280)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang C, Wu K, Wang S and Wei G: Long
non-coding RNA XIST promotes osteosarcoma progression by targeting
YAP via miR-195-5p. J Cell Biochem. 119:5646–5656. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao J, Zhang C, Gao Z, Wu H, Gu R and
Jiang R: Long non-coding RNA ASBEL promotes osteosarcoma cell
proliferation, migration, and invasion by regulating microRNA-21. J
Cell Biochem. 119:6461–6469. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu R, Feng F, Yu X, Liu Z and Lao L:
LncRNA SNHG4 promotes tumour growth by sponging miR-224-3p and
predicts poor survival and recurrence in human osteosarcoma. Cell
Prolif. 51(e12515)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dong P, Xiong Y, Yue J, Hanley SJB and
Watari H: miR-34a, miR-424 and miR-513 inhibit MMSET expression to
repress endometrial cancer cell invasion and sphere formation.
Oncotarget. 9:23253–23263. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J, Wang S, Zhou J and Qian Q:
miR-424-5p regulates cell proliferation, migration and invasion by
targeting doublecortin-like kinase 1 in basal-like breast cancer.
Biomed Pharmacother. 102:147–152. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fang Y, Liang X, Xu J and Cai X: miR-424
targets AKT3 and PSAT1 and has a tumor-suppressive role in human
colorectal cancer. Cancer Manag Res. 10:6537–6547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shekhar R, Priyanka P, Kumar P, Ghosh T,
Khan MM, Nagarajan P and Saxena S: The microRNAs miR-449a and
miR-424 suppress osteosarcoma by targeting cyclin A2 expression. J
Biol Chem. 294:4381–4400. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamanaka Y, Friess H, Buchler M, Beger HG,
Uchida E, Onda M, Kobrin MS and Korc M: Overexpression of acidic
and basic fibroblast growth factors in human pancreatic cancer
correlates with advanced tumor stage. Cancer Res. 53:5289–5296.
1993.PubMed/NCBI
|
|
31
|
Berger W, Setinek U, Mohr T, Kindas-Mügge
I, Vetterlein M, Dekan G, Eckersberger F, Caldas C and Micksche M:
Evidence for a role of FGF-2 and FGF receptors in the proliferation
of non-small cell lung cancer cells. Int J Cancer. 83:415–423.
1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gazzaniga P, Gandini O, Gradilone A,
Silvestri I, Giuliani L, Magnanti M, Gallucci M, Saccani G, Frati L
and Agliano AM: Detection of basic fibroblast growth factor mRNA in
urinary bladder cancer: Correlation with local relapses. Int J
Oncol. 14:1123–1127. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang QH, Xu P, Lu YX and Dou HT: Acidic
and basic fibroblast growth factor expression levels in cervical
cancer and their effects on tumor cell proliferation. Genet Mol
Res. 15:2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cheng Z, Ma R, Tan W and Zhang L: MiR-152
suppresses the proliferation and invasion of NSCLC cells by
inhibiting FGF2. Exp Mol Med. 46(e112)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun XH, Geng XL, Zhang J and Zhang C:
miRNA-646 suppresses osteosarcoma cell metastasis by downregulating
fibroblast growth factor 2 (FGF2). Tumour Biol. 36:2127–2134.
2015.PubMed/NCBI View Article : Google Scholar
|